This systematic review and meta-analysis reports the implications of various lifestyle interventions for gestational weight gain and maternal and neonatal outcomes.
Key Points
Question
Do different types of antenatal diet and physical activity interventions reduce gestational weight gain and improve maternal and neonatal outcomes?
Findings
In this systematic review and meta-analysis of 117 randomized clinical trials (involving 34 546 pregnancies), antenatal diet and physical activity–based lifestyle interventions were associated with less gestational weight gain. Structured diet, physical activity, and diet with physical activity were all associated with improved maternal outcomes; only diet was associated with improved neonatal outcomes.
Meaning
The findings support the implementation of structured diet and physical activity–based interventions in antenatal care programs and policies across the world.
Abstract
Importance
Excessive gestational weight gain (GWG) is common and associated with adverse pregnancy outcomes. Antenatal lifestyle interventions limit GWG; yet benefits of different intervention types and specific maternal and neonatal outcomes are unclear.
Objective
To evaluate the association of different types of diet and physical activity–based antenatal lifestyle interventions with GWG and maternal and neonatal outcomes.
Data Sources
A 2-stage systematic literature search of MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, and Health Technology Assessment Database was conducted from February 1, 2017, to May 31, 2020. Search results from the present study were integrated with those from a previous systematic review from 1990 to February 2017.
Study Selection
Randomized trials reporting GWG and maternal and neonatal outcomes.
Data Extraction and Synthesis
Data were extracted for random-effects meta-analyses to calculate the summary effect estimates and 95% CIs.
Main Outcomes and Measures
Outcomes were clinically prioritized, with mean GWG as the primary outcome. Secondary outcomes included gestational diabetes, hypertensive disorders of pregnancy, cesarean section, preterm delivery, large or small for gestational age neonates, neonatal intensive care unit admission, or fetal death.
Results
A total of 117 randomized clinical trials of antenatal lifestyle interventions (involving 34 546 women) were included. Overall lifestyle intervention was associated with reduced GWG (−1.15 kg; 95% CI, −1.40 to −0.91), risk of gestational diabetes (odds ratio [OR], 0.79; 95% CI, 0.70-0.89), and total adverse maternal outcomes (OR, 0.89; 95% CI, 0.84-0.94) vs routine care. Compared with routine care, diet was associated with less GWG (−2.63 kg; 95% CI, −3.87 to −1.40) than physical activity (−1.04 kg; 95% CI, −1.33 to −0.74) or mixed interventions (eg, unstructured lifestyle support, written information with weight monitoring, or behavioral support alone) (−0.74 kg; 95% CI, −1.06 to −0.43). Diet was associated with reduced risk of gestational diabetes (OR, 0.61; 95% CI, 0.45-0.82), preterm delivery (OR, 0.43; 95% CI, 0.22-0.84), large for gestational age neonate (OR, 0.19; 95% CI, 0.08-0.47), neonatal intensive care admission (OR, 0.68; 95% CI, 0.48-0.95), and total adverse maternal (OR, 0.75; 95% CI, 0.61-0.92) and neonatal outcomes (OR, 0.44; 95% CI, 0.26-0.72). Physical activity was associated with reduced GWG and reduced risk of gestational diabetes (OR, 0.60; 95% CI, 0.47-0.75), hypertensive disorders (OR, 0.66; 95% CI, 0.48-0.90), cesarean section (OR, 0.85; 95% CI, 0.75-0.95), and total adverse maternal outcomes (OR, 0.78; 95% CI, 0.71-0.86). Diet with physical activity was associated with reduced GWG (−1.35 kg; 95% CI, −1.95 to −0.75) and reduced risk of gestational diabetes (OR, 0.72; 95% CI, 0.54-0.96) and total adverse maternal outcomes (OR, 0.81; 95% CI, 0.69-0.95). Mixed interventions were associated with reduced GWG only.
Conclusions and Relevance
This systematic review and meta-analysis found level 1 evidence that antenatal structured diet and physical activity–based lifestyle interventions were associated with reduced GWG and lower risk of adverse maternal and neonatal outcomes. The findings support the implementation of such interventions in routine antenatal care and policy around the world.
Introduction
With an obesogenic environment, unhealthy lifestyle, and accelerating weight gain, obesity is now the most common medical condition in the world, projected to affect 21% of women globally by 2025.1 In the US, obesity prevalence is higher, affecting 25% of women who become pregnant.2 Preconception and pregnancy are priority life stages for healthy lifestyles and obesity prevention,3,4 with excess weight being associated with adverse pregnancy outcomes, long-term noncommunicable disease in women, and epigenetic consequences across generations.4,5,6 In meta-analyses of more than 1.3 million pregnancies worldwide, gestational weight gain (GWG) that exceeds international recommendations affected approximately half of pregnancies6,7 and was an independent risk factor in adverse maternal and neonatal pregnancy outcomes.5,6,8 The US Preventive Services Task Force has prioritized antenatal lifestyle interventions to limit excessive GWG,9 yet the optimal intervention type and specific associations with maternal and neonatal outcomes remain unclear.
Previous individual patient data meta-analyses across 36 randomized clinical trials (RCTs) in 12 526 women noted that antenatal lifestyle interventions were associated with reduced GWG by 0.7 kg (95% CI, −0.92 to −0.48 kg) and reduced cesarean section by 9% (odds ratio [OR], 0.91; 95% CI, 0.83- 0.99).10 In another systematic review of 68 studies and 25 789 participants, antenatal lifestyle interventions were associated with a decrease in GWG and emergency cesarean sections as well as improved neonatal outcomes.9 Interventions were broadly classified into active or counseling interventions, with statistical heterogeneity in pooled analyses associated with variability in components.9 Further insights into different intervention types are now needed.7
We aimed to evaluate the association of different types of diet and physical activity–based antenatal lifestyle interventions with GWG and maternal and neonatal outcomes. We classified the interventions into structured diet, structured physical activity, and diet with physical activity with at least 1 structured component. Other interventions were captured as mixed, which predominantly included unstructured lifestyle support, written information with weight monitoring, or behavioral support alone. We focused on clinically prioritized maternal and neonatal outcomes and aimed to generate level 1 evidence to underpin health economic analysis, public health guidelines, and implementation into policy and practice.11
Methods
Search Strategy and Selection Criteria
For this systematic review and meta-analysis, a 2-stage search of the literature was conducted across MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, and Health Technology Assessment Database between February 1, 2017, and May 31, 2020. Search results from the present study were integrated with those from a previous systematic review, which was performed from January 1990 to February 2017.10 Search terms and outcomes were clinically prioritized and have been previously published.10,12 Bibliographies of included studies were also reviewed to identify additional studies. There were no language restrictions. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. This study has been registered in PROSPERO (CRD42013003804).
Search methods from the 2017 systematic review were applied, guided by consistent authorship and the registered protocol.10 Updated search results to May 2020 were screened by title and abstract by 2 independent investigators (including E.R., for the 2017-2018 search; C.B. and M.B.K., for the 2017-2020 search) who screened the full text of eligible studies. Discrepancies were resolved by a third reviewer (H.J.T.). We included antenatal RCTs of interventions based on diet and/or physical activity, with or without behavioral modification. We excluded studies that targeted maternal conditions that are known to affect GWG (eg, gestational diabetes), involved animals, evaluated nonlifestyle-based interventions (GWG-monitoring RCTs alone), reported only nonclinical outcomes, included weight-reducing drugs or surgical interventions, or were published before 1990.12 The comparators were routine antenatal care with outcomes that were clinically prioritized.13
Two of us (L.J.M., a dietitian, and C.L.H., an exercise physiologist) independently classified the interventions, and discrepancies were resolved by a third reviewer (H.J.T.). The classifications were structured diet, structured physical activity, diet with physical activity, and mixed interventions. Structured diet interventions used dietary targets, either self-directed or facilitator-led (by researcher, instructor, trainer, or dietitian), with or without monitoring (logs, recalls, or diaries) or supply of food. Structured physical activity interventions involved specified physical activity programs conducted in controlled conditions (research facility, gym, or class) or a few physical activity interventions that were self-led (activity targets and equipment provided). We also extended the initial protocol by separating diet with physical activity interventions (with at least 1 having a structured component) from the original mixed interventions to create 4 intervention types. Residual mixed interventions did not meet the inclusion criteria for structured interventions and focused on unstructured lifestyle support, written information with weight monitoring, or behavioral support alone, or they inadequately described the structured diet and physical activity components. Behavioral strategies were heterogeneously applied across all intervention types, preventing a separate analysis.
The primary outcome was mean GWG. Secondary outcomes included adverse maternal (gestational diabetes; hypertensive disorders of pregnancy encompassing pregnancy-induced hypertension and preeclampsia; any cesarean section; and preterm delivery) and neonatal (large for gestational age [LGA] or small for gestational age [SGA] neonates; newborn admission to a neonatal intensive care unit [NICU]; or fetal death, encompassing intrauterine fetal death and stillbirth) outcomes. Composite outcomes could not be generated from aggregate data; hence, we evaluated total adverse outcomes. All outcomes were clinically prioritized in a previously published Delphi survey.13
We accepted the primary clinical trial10 definitions and reporting of GWG, gestational diabetes, hypertensive disorders of pregnancy, cesarean section, fetal death, and admission to NICU. We defined preterm delivery as birth before 37 weeks’ gestation, SGA as birth weight lower than the 10th percentile for gestational age, and LGA as birth weight at or more than the 90th percentile for the gestational age, adjusted for the mother’s body mass index, parity, and gestational age at delivery. When these definitions varied, we excluded the outcomes for that particular variable.
Statistical Analysis
Two researchers (including C.B.) assessed risk of bias using the Cochrane Risk of Bias Tool, version 1.0.14 Discrepancies were resolved by consensus with a third reviewer (C.L.H.). Methodological quality of 6 study domains was assessed using the Cochrane Handbook for Systematic Reviews of Interventions templates: randomization, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting.14 The nature of lifestyle interventions made blinding of participants generally not feasible given that selective reporting was rarely documented.15 Hence, we calculated risk of bias according to 4 study domains: randomization, allocation concealment, blinding of outcome assessment, and incomplete outcome data. We considered a study at high risk of bias if it scored as such in at least 1 domain. For low risk of bias, all domains had to be scored as low risk.
We assessed the association of interventions with primary and secondary outcomes by calculating the mean differences in continuous ratios and ORs for dichotomous outcomes using the intention-to-treat principle. Random-effects meta-analysis was used to calculate the summary effect estimates and 95% CIs for the intervention effects; the DerSimonian and Laird method was applied using the metan Stata command.16 Heterogeneity was assessed with the I2 statistic, and I2 greater than 50% indicated substantial heterogeneity.
We evaluated the differential implications of interventions by performing a subgroup meta-analysis by intervention type (diet, physical activity, diet with physical activity, and mixed). In addition, we conducted sensitivity analyses to bring the structured interventions (diet, physical activity, and diet with physical activity) together, repeating the meta-analyses that omitted mixed interventions. We analyzed primary outcomes for studies with a low or high risk of bias. When 10 or more studies were available, publication bias was assessed using Egger test plots. Statistical significance was defined as a 2-sided P < .05. All statistical analyses were performed using Stata, version 16 (StataCorp LLC).
Results
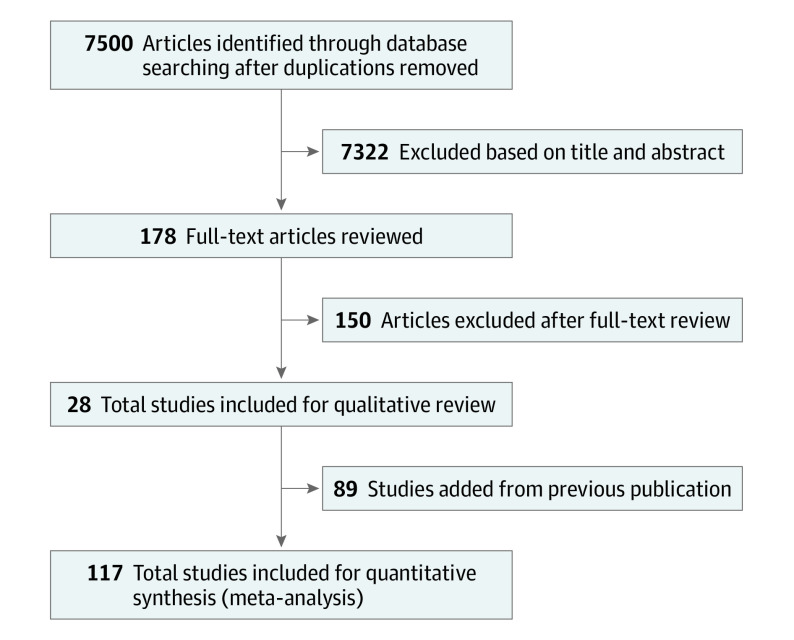
The search identified 7500 studies, of which 178 were retained for full-text review and 28 were included in the present analysis.17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44 In the 2017 systematic review, 103 studies were identified, of which 89 were eligible.10,12 The 28 new and 89 previous articles were combined for a total of 117 studies for the present meta-analysis.10,12,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44 The PRISMA diagram is presented in Figure 1.
Figure 1. PRISMA Diagram of the Systematic Search.
This sample consisted of RCTs (which involved 34 546 women) that examined diet (n = 14), physical activity (n = 53), diet with physical activity (n = 19), and mixed interventions (n = 31) (eTable in the Supplement). Forty-four studies were from Europe, 29 were from North America, 13 were from Australia or New Zealand, 9 were from the United Kingdom, 9 were from South America, 7 were from Asia (China, India, or Taiwan), and 6 were from the Middle East (Iran and Egypt). The studies reported on GWG (n = 99), gestational diabetes (n = 67), hypertensive disorders of pregnancy (n = 53), preterm delivery (n = 52), cesarean section (n = 76), fetal death (n = 12), SGA (n = 24) or LGA (n = 28) neonates, and admission to NICU (n = 17).
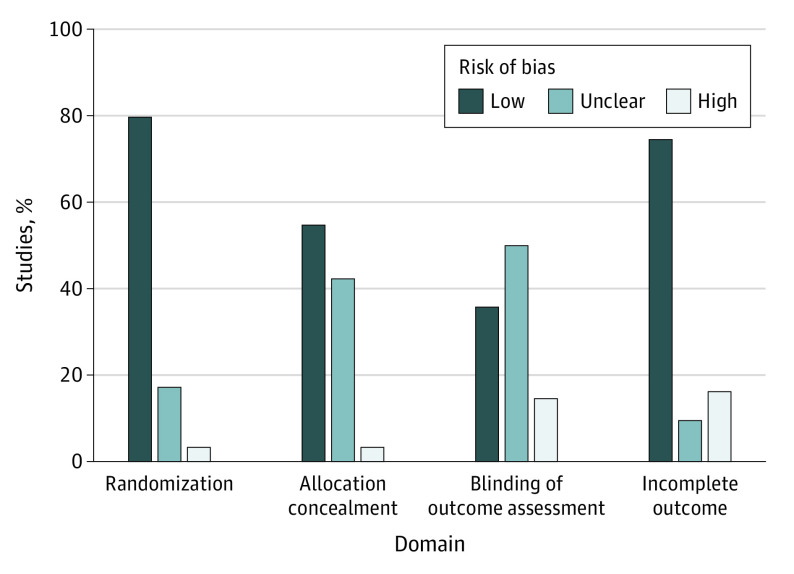
Assessment of quality using all 6 study domains (randomization, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting) showed that 73 studies (62.4%) had a high risk of bias and 44 (37.6%) had an unclear risk of bias. When evaluating the 4 study domains that were appropriate for lifestyle interventions (randomization, allocation concealment, blinding of outcome assessment, and incomplete outcome data), few studies had a high risk of bias: 22 (18.8%) had a low, 59 (50.4%) had an unclear, and 36 (30.8%) had a high risk of bias (Figure 2).
Figure 2. Assessment of Risk of Bias in 4 Domains.
Visual inspection of the funnel plot for GWG suggested a possible bias against small studies that favored better intervention group outcomes, which was confirmed by Egger test (−9.51; 95% CI, −12.98 to −6.03; P < .001) (eFigure 1A in the Supplement). Funnel plots for maternal and neonatal outcomes were largely symmetrical, suggesting a low risk of publication bias, which was supported by Egger test (eFigure 1B in the Supplement).
The GWG results are presented in Table 1 and in a forest plot (eFigure 2 in the Supplement). Overall lifestyle intervention was associated with reduced GWG compared with routine care (−1.15 kg; 95% CI, −1.40 to −0.91; I2 = 85.3%; 29 247 women). Diet (−2.63 kg; 95% CI, −3.87 to −1.40; I2 = 94.2%; 4928 women), diet with physical activity (−1.35 kg; 95% CI, −1.95 to −0.75; I2 = 53.6%; 2942 women), physical activity (−1.04 kg; 95% CI, −1.33 to −0.74; I2 = 56.2%; 8714 women), and mixed interventions (−0.74 kg; 95% CI, −1.06 to −0.43; I2 = 70.1%; 12 663 women) were associated with reduced GWG. Diet appeared to have greater implications for weight, with 95% CIs that did not overlap those of physical activity and mixed interventions but were inclusive of that for diet with physical activity. Sensitivity analysis showed that, when analyzed together, structured diet and physical activity interventions (excluding mixed interventions) were associated with reduced GWG of −1.31 kg (95% CI, −1.64 to −0.99; I2 = 79.0%; 16 584 women), compared with routine care. Analysis by risk of bias showed a mean difference in GWG of −1.23 kg (95% CI, −1.75 to −0.70) for studies with a high risk of bias and −1.13 kg (95% CI, −1.63 to −0.63) for studies with a low risk of bias, with overlapping 95% CIs and no clear differences between the risk of bias study groups.
Table 1. Diet and Physical Activity–Based Lifestyle Interventions and Associations With Gestational Weight Gain (GWG).
| Intervention | No. | Intervention | Routine care | % Differencea | GWG (95% CI), kg | I2, % | |||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Women | Mean weight (SD), kg | Total No. | Mean weight (SD), kg | Total No. | ||||
| Overall | 99 | 29 247 | 0.7 (3.0) | 14 861 | 11.9 (2.9) | 14 386 | 9.7 | −1.15 (−1.40 to −0.91) | 85.3 |
| Diet | 13 | 4928 | 8.9 (2.5) | 2447 | 11.6 (3.1) | 2481 | 22.7 | −2.63 (−3.87 to −1.40) | 94.2 |
| Physical activity | 42 | 8714 | 11.1 (3.2) | 4229 | 11.9 (3.1) | 4485 | 8.7 | −1.04 (−1.33 to −0.74) | 56.2 |
| Diet with physical activity | 16 | 2942 | 10.2 (2.9) | 1506 | 11.6 (2.5) | 1436 | 11.6 | −1.35 (−1.95 to −0.75) | 53.6 |
| Mixed | 28 | 12 663 | 11.0 (2.9) | 6679 | 12.0 (2.9) | 5984 | 6.2 | −0.74 (−1.06 to −0.43) | 70.1 |
Lower mean GWG in the intervention group compared with the control group.
For maternal outcomes, overall interventions were associated with reduced risk of gestational diabetes (OR, 0.79; 95% CI, 0.70-0.89; I2 = 38.3%; 24 371 women) and total adverse maternal outcomes (OR, 0.89; 95% CI, 0.84-0.94; I2 = 27.9%) compared with routine care (Tables 2 and 3). Diet interventions were associated with lower risk of gestational diabetes (OR, 0.61; 95% CI, 0.45-0.82; I2 = 25.7%; 3029 women), preterm delivery (OR, 0.43; 95% CI, 0.22-0.84; I2 = 47.2%; 3379 women), total adverse maternal outcomes (OR, 0.75; 95% CI, 0.61-0.92; I2 = 47.2%), and total adverse neonatal outcomes (OR, 0.44; 95% CI, 0.26-0.72; I2 = 48.4%). Physical activity interventions were associated with lower risk of gestational diabetes (OR, 0.60; 95% CI, 0.47-0.75; I2 = 21.4%; 7519 women), hypertensive disorders of pregnancy (OR, 0.66; 95% CI, 0.48-0.90; I2 = 23.4%; 5332 women), cesarean section (OR, 0.85; 95% CI, 0.75-0.95; I2 = 0.6%; 7528 women), and total adverse maternal outcomes (OR, 0.78; 95% CI, 0.71-0.86; I2 = 13.1%). Diet with physical activity interventions was associated with reduced risk of gestational diabetes (OR, 0.72; 95% CI, 0.54-0.96; I2 = 29.8%; 3154 women) and total adverse maternal outcomes (OR, 0.81; 95% CI, 0.69-0.95; I2 = 39.2%) (Tables 2 and 3). Mixed interventions were not associated with maternal or neonatal outcomes. Sensitivity analysis showed that, when analyzed together, structured diet and physical activity interventions (excluding mixed interventions) were associated with reduced risk of gestational diabetes (OR, 0.64; 95% CI, 0.55-0.74; I2 = 25.1%; 13 702 women), hypertensive disorders of pregnancy (OR, 0.72; 95% CI, 0.58-0.88; I2 = 32.1%; 10 795 women), and total adverse maternal outcomes (OR, 0.79; 95% CI, 0.73-0.85; I2 = 28.5%) as well as a pattern of fewer cesarean sections (OR, 0.91; 95% CI, 0.82-1.01; I2 = 16.4%; 13 138 women).
Table 2. Diet and Physical Activity–Based Lifestyle Interventions and Associations With Total Adverse Maternal and Total Adverse Neonatal Outcomesa.
| Intervention | Maternal outcome, OR (95% CI) | I2, % | Neonatal outcome, OR (95% CI) | I2, % |
|---|---|---|---|---|
| Overall | 0.89 (0.84-0.94) | 27.9 | 0.94 (0.86-1.04) | 17.1 |
| Diet | 0.75 (0.61-0.92) | 47.2 | 0.44 (0.26-0.72) | 48.4 |
| Physical activity | 0.78 (0.71-0.86) | 13.1 | 0.87 (0.67-1.12) | 0 |
| Diet with physical activity | 0.81 (0.69-0.95) | 39.2 | 0.92 (0.74-1.13) | 3.6 |
| Mixed | 1.02 (0.97-1.08) | 0 | 1.04 (0.95-1.13) | 0 |
Abbreviation: OR, odds ratio.
Total adverse maternal outcomes included gestational diabetes, hypertensive disorders of pregnancy, any cesarean section, or preterm delivery. Total adverse neonatal outcomes included large for gestational age or small for gestational age neonates, newborn admission to neonatal intensive care unit, or fetal death.
Table 3. Diet and Physical Activity–Based Lifestyle Interventions and Associations With Individual Adverse Maternal and Individual Adverse Neonatal Outcomes.
| Intervention | No. | Intervention | Routine care | % Differencea | OR (95% CI) | I2, % | |||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Women | No. of events | Total No. (%) | No. of events | Total No. (%) | ||||
| Overall | |||||||||
| Gestational diabetes | 67 | 24 371 | 1477 | 12 061 (12.2) | 1732 | 12 310 (14.1) | 1.8 | 0.79 (0.70-0.89) | 38.3 |
| Hypertensive disorders of pregnancy | 53 | 20 811 | 883 | 10 363 (8.5) | 936 | 10 448 (9.0) | 0.4 | 0.87 (0.75-1.01) | 37.5 |
| Preterm delivery | 52 | 20 083 | 546 | 9941 (5.5) | 632 | 10 142 (6.2) | 0.7 | 0.93 (0.80-1.07) | 11.4 |
| Cesarean section | 76 | 23 333 | 3053 | 11 664 (29.4) | 3164 | 11 669 (38.1) | 8.7 | 0.94 (0.88-1.01) | 14.7 |
| Fetal death | 12 | 7174 | 20 | 3558 (0.6) | 25 | 3616 (0.7) | 0.1 | 0.73 (0.40-1.32) | 0 |
| SGA neonate | 24 | 8747 | 379 | 4309 (8.8) | 382 | 4438 (8.6) | −0.2 | 1.02 (0.86-1.12) | 6.3 |
| LGA neonate | 28 | 11 432 | 589 | 5657 (10.4) | 684 | 5775 (11.8) | 1.4 | 0.83 (0.69-1.01) | 35.9 |
| NICU admission | 17 | 9613 | 762 | 4793 (15.9) | 754 | 4820 15.6) | −0.3 | 1.02 (0.89-1.17) | 9.8 |
| Diet | |||||||||
| Gestational diabetes | 7 | 3029 | 183 | 1490 (12.3) | 276 | 1539 (17.9) | 5.7 | 0.61 (0.45-0.82) | 25.7 |
| Hypertensive disorders of pregnancy | 6 | 2683 | 80 | 1316 (6.1) | 97 | 1367 (7.1) | 1.0 | 0.80 (0.48-1.32) | 48.8 |
| Preterm delivery | 6 | 3379 | 65 | 1658 (3.9) | 109 | 1721 (6.3) | 2.4 | 0.43 (0.22-0.84) | 47.2 |
| Cesarean section | 6 | 2426 | 358 | 1192 (30.0) | 354 | 1234 (28.7) | −1.3 | 1.07 (0.89-1.30) | 0 |
| Fetal death | 2 | 1389 | 1 | 674 (0.1) | 3 | 715 (0.4) | 0.3 | 0.46 (0.07-3.13) | 0 |
| SGA neonate | 2 | 974 | 10 | 484 (2.1) | 28 | 490 (5.7) | 3.6 | 0.54 (0.06-4.64) | 83.1 |
| LGA neonate | 2 | 974 | 6 | 484 (1.2) | 29 | 490 (5.9) | 4.7 | 0.19 (0.08-0.47) | 0 |
| NICU admission | 3 | 2092 | 65 | 1037 (6.3) | 94 | 1055 (8.9) | 2.6 | 0.68 (0.48-0.95) | 0 |
| Physical activity | |||||||||
| Gestational diabetes | 24 | 7519 | 219 | 3603 (6.1) | 374 | 3916 (9.6) | 3.5 | 0.60 (0.47-0.75) | 21.4 |
| Hypertensive disorders of pregnancy | 18 | 5332 | 116 | 2604 (4.5) | 182 | 2728 (6.7) | 2.2 | 0.66 (0.48-0.90) | 23.4 |
| Preterm delivery | 23 | 6299 | 154 | 3057 (5.0) | 177 | 3242 (5.5) | 0.4 | 1.03 (0.81-1.29) | 0 |
| Cesarean section | 34 | 7528 | 715 | 3697 (19.3) | 847 | 3831 (22.1) | 2.8 | 0.85 (0.75-0.95) | 0.6 |
| Fetal death | 2 | 140 | 1 | 66 (1.5) | 1 | 74 (1.4) | −0.2 | NAb | NA |
| SGA neonate | 9 | 1265 | 43 | 561 (7.7) | 65 | 704 (9.2) | 1.6 | 0.74 (0.48-1.15) | 0 |
| LGA neonate | 9 | 1236 | 66 | 542 (12.2) | 85 | 694 (12.2) | 0.1 | 1.07 (0.69-1.68) | 16.1 |
| NICU admission | 3 | 997 | 18 | 500 (3.6) | 25 | 497 (5.0) | 1.4 | 0.72 (0.39-1.35) | 0 |
| Diet with physical activity | |||||||||
| Gestational diabetes | 16 | 3154 | 177 | 1599 (11.1) | 215 | 1555 (13.8) | 2.8 | 0.72 (0.54-0.96) | 29.8 |
| Hypertensive disorders of pregnancy | 13 | 2780 | 127 | 1405 (9.0) | 165 | 1375 (12.0) | 3.0 | 0.74 (0.52-1.06) | 38.4 |
| Preterm delivery | 10 | 1934 | 53 | 973 (5.4) | 73 | 961 (7.6) | 2.1 | 0.75 (0.38-1.49) | 52.4 |
| Cesarean section | 17 | 3184 | 346 | 1622 (21.3) | 335 | 1562 (21.4) | 0.1 | 0.96 (0.75-1.23) | 38.8 |
| Fetal death | 1 | 0 | 0 | 0 | NA | NA | |||
| SGA neonate | 6 | 1417 | 78 | 708 (11.0) | 70 | 709 (9.9) | −1.1 | 1.15 (0.82-1.62) | 0 |
| LGA neonate | 8 | 1720 | 63 | 867 (7.3) | 86 | 853 (10.1) | 2.8 | 0.71 (0.45-1.11) | 28.7 |
| NICU admission | 4 | 1040 | 64 | 517 (12.4) | 68 | 523 (13.0) | 0.6 | 0.95 (0.66-1.38) | 0 |
| Mixed | |||||||||
| Gestational diabetes | 23 | 10 669 | 898 | 5369 (16.7) | 867 | 5300 (16.4) | −0.4 | 1.03 (0.93-1.15) | 0 |
| Hypertensive disorders of pregnancy | 17 | 10 016 | 560 | 5038 (11.1) | 492 | 4978 (9.9) | −1.2 | 1.14 (1.00-1.30) | 0 |
| Preterm delivery | 14 | 8471 | 274 | 4253 (6.4) | 273 | 4218 (6.5) | 0 | 1.00 (0.84-1.19) | 0 |
| Cesarean section | 20 | 10 195 | 1634 | 5153 (31.7) | 1628 | 5042 (32.3) | 0.6 | 0.98 (0.89-1.08) | 11.1 |
| Fetal death | 7 | 5645 | 18 | 2818 (0.6) | 21 | 2827 (0.7) | 0.1 | 0.78 (0.40-1.51) | 0 |
| SGA neonate | 9 | 5091 | 248 | 2556 (9.7) | 219 | 2535 (8.6) | −1.1 | 1.14 (0.94-1.38) | 0 |
| LGA neonate | 11 | 7502 | 454 | 3764 (12.1) | 484 | 3738 (12.9) | 0.9 | 0.92 (0.77-1.10) | 18.6 |
| NICU admission | 7 | 5484 | 615 | 2739 (22.5) | 567 | 2745 (20.7) | −1.8 | 1.12 (0.98-1.29) | 0 |
Abbreviations: LGA, large for gestational age; NA, not applicable (when there was only 1 article in a subcategory, the results were marked NA); NICU, neonatal intensive care unit; SGA, small for gestational age.
Absolute % difference.
More than 1 article, but the result was excluded by the meta-analysis process.
For neonatal outcomes, compared with routine care, overall interventions were not associated with the risk of an SGA or LGA neonate, fetal death, NICU admission, or total adverse neonatal outcomes. Diet interventions were associated with a lower risk of NICU admission (OR, 0.68; 95% CI, 0.48-0.95; I2 = 0%; 2092 women), LGA neonate (OR, 0.19; 95% CI, 0.08-0.47; I2 = 0%; 974 women), and total adverse neonatal outcomes (OR, 0.44; 95% CI, 0.26-0.72; I2 = 48.4%) and were not associated with fetal death or SGA neonate (Tables 2 and 3). Other intervention types were not associated with neonatal outcomes. Sensitivity analysis showed that structured diet and physical activity interventions, when analyzed together (excluding mixed interventions), were associated with a lower risk of NICU admission (OR, 0.78; 95% CI, 0.62-0.98; I2 = 0%; 4129 women) and total adverse neonatal outcomes (OR, 0.78; 95% CI, 0.66-0.92; I2 = 18.9%).
Data on potential harms of the intervention were limited. However, no association between lifestyle interventions and an SGA neonate was noted.
Discussion
Excessive GWG is common and associated with increased adverse maternal and neonatal pregnancy outcomes. In this study, we found level 1 evidence11 from 117 RCTs, which involved 34 546 women, more than 30 years of research, and 5 continents.10,12,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44 Compared with routine care, antenatal diet and physical activity–based lifestyle interventions were associated with reduced GWG. Lifestyle interventions were also associated with lower risk of gestational diabetes and total adverse maternal outcomes. Diet interventions seemed to have greater implications for GWG than physical activity alone or mixed interventions, whereas the implications of diet and diet with physical activity for GWG could not be differentiated. Compared with routine care, diet was associated with a reduced risk of gestational diabetes, preterm delivery, total adverse maternal outcomes, LGA neonate, NICU admission, and total adverse neonatal outcomes. Physical activity was associated with a lower risk of gestational diabetes, hypertensive disorders of pregnancy, cesarean section, and total adverse maternal outcomes, whereas diet with physical activity was associated with a reduced risk of gestational diabetes and total adverse maternal outcomes. Mixed interventions were not associated with maternal and neonatal outcomes.
Gestational weight gain that exceeds the recommendations occurs in approximately half of pregnancies and has been associated with adverse maternal and neonatal health outcomes.5,6,7,45 Antenatal lifestyle interventions were associated with reduced GWG by 0.7 kg in a previous systematic review and individual patient-level data meta-analysis, with similar efficacy regardless of the mother’s body mass index, age, parity, race and ethnicity, or preexisting medical conditions.10 In another systematic review and meta-analysis of 68 studies,9 lifestyle interventions were associated with 1.02 kg less GWG with a significant interaction with study intensity.9 We found that structured diet and physical activity–based interventions were associated with reduced GWG of 1.15 kg, but 1.13 kg in studies with a low risk of bias.
Regarding intervention types, based on mean GWG and nonoverlapping 95% CIs, diet interventions had greater implications for GWG than physical activity alone or mixed interventions but could not be differentiated from diet with physical activity interventions. The broader weight implications of diet over physical activity alone were consistent with the balance between energy intake and expenditure.46 Intake is diet dependent, whereas 60% to 70% of energy expenditure is resting (partly impacted by lean muscle mass) and the residual 30% is used in physical activity. Hence, substantial physical activity is required to achieve an energy deficit and weight loss.47,48,49 In the present study, antenatal diet intervention reduced GWG by approximately 23%, likely limiting longer-term obesity and noncommunicable disease risks given the evidence of postpartum weight benefits.4,9 Physical activity specifically declines in pregnancy,50 with barriers to engagement and improvement.51 The present study supports physical activity intervention in pregnancy to improve maternal health outcomes. Because most of these interventions were structured and delivered by trained health professionals alongside routine antenatal care practitioners, we highlight the strong public health argument for implementing structured diet with physical activity lifestyle interventions during pregnancy that are facilitated by trained professionals.
The GWG and public health benefits of pregnancy lifestyle interventions are enhanced by the associated improvement in clinically prioritized maternal outcomes.13 Previous meta-analyses inconsistently noted the association of lifestyle intervention with reduced risk of cesarean sections and gestational diabetes9,10 but not gestational hypertension. Reported intervention classification has varied, including active interventions with a structured physical element (eg, supervised exercise programs, prescribed exercise or dietary programs, or intensive weight management) or counseling alone. Only active and intensive interventions were associated with reduced risk of gestational hypertension.9 We found that lifestyle interventions overall were associated with a reduced risk of gestational diabetes and total adverse maternal outcomes that encompassed gestational diabetes, hypertensive disorders of pregnancy, preterm delivery, and cesarean section. Structured diet interventions were associated with reduced risk of gestational diabetes and preterm delivery, whereas physical activity interventions were associated with reduced risk of gestational diabetes, hypertensive disorders of pregnancy, and cesarean sections. Diet with physical activity interventions were associated with reduced risk of gestational diabetes. Such findings advance existing knowledge, showing broad maternal benefits and differences across intervention types.9,10
Lifestyle interventions were also associated with neonatal benefits, which varied across intervention types. The 2017 individual patient data did find neonatal benefits,10 but a more recent review found associations with a reduced risk of macrosomia and LGA.9 In the present study, diet was associated with reductions in the broadest range of adverse neonatal outcomes, including LGA, NICU admission, and total adverse neonatal outcomes. This current systematic review directly underpinned a cost-effectiveness analysis.52 When analyzed together and based on maternity outcomes alone, diet, physical activity, and diet with physical activity interventions appeared to be cost-saving. When NICU costs were incorporated, all except mixed interventions were cost-saving, supporting the implementation of structured lifestyle interventions in pregnancy.
Mixed lifestyle interventions did not include clearly articulated structured diet and physical activity components or encompassed passive lifestyle information or written resources with or without gestational weighing and with or without behavioral strategies. These interventions were associated with limited GWG benefit, with no associations with secondary outcomes. This finding highlights the need for evidence on the most effective intervention components, delivery modes, settings, staffing, and behavioral strategies to inform implementation.3,53
Implementation research is underway. A secondary analysis of these 117 interventions is being conducted to identify optimal intervention characteristics via the TIDieR (Template for Intervention Description and Replication) framework.54,55,56 Nationally and internationally funded research initiatives, including the Global Alliance of Chronic Disease and Horizon 2020 projects,3 are informing the development of an implementation tool kit. This systematic review supports the implementation of structured diet and physical activity interventions by trained staff. It does not support isolated monitoring of GWG and provision of passive lifestyle information by routine antenatal care staff, an approach that is akin to the control group in many of the RCTs we captured for this study. Barriers for routine antenatal care staff include inadequate training, time, resources,57 knowledge, skills, and confidence in delivery of lifestyle interventions, which all affect implementation.57,58 Antenatal care practitioners will need to be trained to support healthy lifestyle and healthy GWG, integrated with trained staff to deliver evidence-based, cost-effective lifestyle interventions during pregnancy. International, rigorous, evidence-based guidelines are also needed given the inadequacy of the current guidance.59 Furthermore, although the focus in pregnancy is on healthy lifestyle and prevention of excessive GWG and not on weight loss, weight stigma remains a major challenge and must be given consideration using appropriate language, resources, and health professional training.60
Strengths and Limitations
This study has some strengths. These included the comprehensive design and inclusion of studies in all languages; with a large sample; and with diverse racial and ethnic populations, settings, countries, and types of interventions. Moreover, the interventions were classified and analyzed by type, advancing the knowledge from previous systematic reviews.
This study also has some limitations. Reporting of lifestyle interventions has inconsistencies and inadequacies, affecting evidence synthesis and strengthening the need for standardization.61 Risk of bias was low in 18.8% of studies and unclear in 50.4%. Nine studies on the physical activity intervention, which were captured in the 2017 systematic review, provided limited details on the control group, although all studies included clear physical activity interventions over and above routine care. Framework analysis of behavioral strategies as well as intervention characteristics (ie, intensity, duration, delivery mode, facilitator, and setting), penetration, and participation was beyond the scope of this work, but it is underway. Some outcome definitions and criteria varied, including for gestational diabetes, cesarean section, and admission to NICU, although standardized definitions were applied for preterm birth and SGA and LGA. Aggregate data precluded the analysis of composite outcomes, with multiple outcomes possible in any 1 participant; hence, total adverse maternal and neonatal outcomes were assessed.
Conclusions
This systematic review and meta-analysis found that antenatal structured diet and physical activity–based lifestyle interventions were associated with reduced GWG and with maternal and neonatal benefits. Structured diet interventions appeared to have greater implications for GWG than physical activity alone or mixed interventions. Diet was associated with improved maternal and neonatal outcomes, whereas physical activity was associated with improved adverse maternal outcomes. Coupled with evidence of cost-effectiveness, this analysis of 117 RCTs involving more than 34 000 women strongly supports the integration of structured diet and physical activity interventions alongside routine antenatal care and policy to improve the health of mothers and their offspring around the world.
eTable. Author, Year, Country Sample Size, Population, Intervention, Comparator and Outcomes of Eligible Studies (By Year of Publication)
eFigure 1. Funnel Plots and Egger’s Tests Exploring Potential Publication Bias; Gestational Weight Gain (panel A), Maternal and Neonatal Outcomes Across Gestational Diabetes, Hypertensive Disorders of Pregnancy, Preterm Delivery, Cesarean Section, Fetal Death, Small for Gestational Age, Large for Gestational Age and Neonatal Intensive Care admission (panels B-I)
eFigure 2. Forest Plot of Randomized Controlled Trials and Impact on Gestational Weight Gain
eReferences
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) . Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377-1396. doi: 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogunwole SM, Zera CA, Stanford FC. Obesity management in women of reproductive age. JAMA. 2021;325(5):433-434. doi: 10.1001/jama.2020.21096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill B, Skouteris H, Boyle JA, et al. Health in Preconception, Pregnancy and Postpartum Global Alliance: international network pregnancy priorities for the prevention of maternal obesity and related pregnancy and long-term complications. J Clin Med. 2020;9(3):822. doi: 10.3390/jcm9030822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanson MA, McAuliffe FM, Killeen SL, Jacob CM, Hod M. New guidelines, position paper, and insights from the FIGO Pregnancy Obesity and Nutrition Initiative (PONI). Int J Gynaecol Obstet. 2020;151(suppl 1):1-3. doi: 10.1002/ijgo.13321 [DOI] [PubMed] [Google Scholar]
- 5.Goldstein RF, Abell SK, Ranasinha S, et al. Gestational weight gain across continents and ethnicity: systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018;16(1):153. doi: 10.1186/s12916-018-1128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207-2225. doi: 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein RF, Harrison CL, Teede HJ. Editorial: the importance of gestational weight gain. Obes Rev. 2020;21(10):e13073. doi: 10.1111/obr.13073 [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009. [PubMed] [Google Scholar]
- 9.Cantor AG, Jungbauer RM, McDonagh M, et al. Counseling and behavioral interventions for healthy weight and weight gain in pregnancy: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(20):2094-2109. doi: 10.1001/jama.2021.4230 [DOI] [PubMed] [Google Scholar]
- 10.International Weight Management in Pregnancy (i-WIP) Collaborative Group . Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ. 2017;358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackley BJ, Swan BA, Ladwig G, Tucker S. Evidence-Based Nursing Care Guidelines Medical-Surgical Interventions. Mosby Elsevier; 2008:7. [Google Scholar]
- 12.Ruifrok AE, Rogozinska E, van Poppel MNM, et al. ; i-WIP (International Weight Management in Pregnancy) Collaborative Group . Study protocol: differential effects of diet and physical activity based interventions in pregnancy on maternal and fetal outcomes—individual patient data (IPD) meta-analysis and health economic evaluation. Syst Rev. 2014;3(1):131. doi: 10.1186/2046-4053-3-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogozinska E, D’Amico MI, Khan KS, et al. ; International Weight Management in Pregnancy (iWIP) Collaborative Group . Development of composite outcomes for individual patient data (IPD) meta-analysis on the effects of diet and lifestyle in pregnancy: a Delphi survey. BJOG. 2016;123(2):190-198. doi: 10.1111/1471-0528.13764 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younge JO, Kouwenhoven-Pasmooij TA, Freak-Poli R, Roos-Hesselink JW, Hunink MM. Randomized study designs for lifestyle interventions: a tutorial. Int J Epidemiol. 2015;44(6):2006-2019. doi: 10.1093/ije/dyv183 [DOI] [PubMed] [Google Scholar]
- 16.Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JAC. Metan: fixed- and random-effects meta-analysis. Stata J. 2008;8(1):3-28. doi: 10.1177/1536867X0800800102 [DOI] [Google Scholar]
- 17.Abdel-Aziz SB, Hegazy IS, Mohamed DA, Abu El Kasem MMA, Hagag SS. Effect of dietary counseling on preventing excessive weight gain during pregnancy. Public Health. 2018;154:172-181. doi: 10.1016/j.puhe.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 18.H Al Wattar B, Dodds J, Placzek A, et al. ; ESTEEM study group . Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLoS Med. 2019;16(7):e1002857. doi: 10.1371/journal.pmed.1002857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anleu E, Reyes M, Araya B M, Flores M, Uauy R, Garmendia ML. Effectiveness of an intervention of dietary counseling for overweight and obese pregnant women in the consumption of sugars and energy. Nutrients. 2019;11(2):385. doi: 10.3390/nu11020385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arthur C, Di Corleto E, Ballard E, Kothari A. A randomized controlled trial of daily weighing in pregnancy to control gestational weight gain. BMC Pregnancy Childbirth. 2020;20(1):223. doi: 10.1186/s12884-020-02884-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assaf-Balut C, García de la Torre N, Durán A, et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): a randomized controlled trial: the St. Carlos GDM prevention study. PLoS One. 2017;12(10):e0185873. doi: 10.1371/journal.pone.0185873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacchi M, Mottola MF, Perales M, Refoyo I, Barakat R. Aquatic activities during pregnancy prevent excessive maternal weight gain and preserve birth weight: a randomized clinical trial. Am J Health Promot. 2018;32(3):729-735. doi: 10.1177/0890117117697520 [DOI] [PubMed] [Google Scholar]
- 23.Barakat R, Franco E, Perales M, López C, Mottola MF. Exercise during pregnancy is associated with a shorter duration of labor: a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:33-40. doi: 10.1016/j.ejogrb.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 24.Barakat R, Refoyo I, Coteron J, Franco E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes: a randomized controlled trial. Braz J Phys Ther. 2019;23(2):148-155. doi: 10.1016/j.bjpt.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brik M, Fernández-Buhigas I, Martin-Arias A, Vargas-Terrones M, Barakat R, Santacruz B. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? a randomized controlled trial. Ultrasound Obstet Gynecol. 2019;53(5):583-589. doi: 10.1002/uog.20147 [DOI] [PubMed] [Google Scholar]
- 26.Buckingham-Schutt LM, Ellingson LD, Vazou S, Campbell CG. The Behavioral Wellness in Pregnancy study: a randomized controlled trial of a multi-component intervention to promote appropriate weight gain. Am J Clin Nutr. 2019;109(4):1071-1079. doi: 10.1093/ajcn/nqy359 [DOI] [PubMed] [Google Scholar]
- 27.Cahill AG, Haire-Joshu D, Cade WT, et al. Weight control program and gestational weight gain in disadvantaged women with overweight or obesity: a randomized clinical trial. Obesity (Silver Spring). 2018;26(3):485-491. doi: 10.1002/oby.22070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan RS-M, Tam W-H, Ho IC-H, et al. Randomized trial examining effectiveness of lifestyle intervention in reducing gestational diabetes in high risk Chinese pregnant women in Hong Kong. Sci Rep. 2018;8(1):13849. doi: 10.1038/s41598-018-32285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao AM, Srinivas SK, Studt SK, Diewald LK, Sarwer DB, Allison KC. A pilot randomized controlled trial of a technology-based approach for preventing excess weight gain during pregnancy among women with overweight. Front Nutr. 2017;4:57. doi: 10.3389/fnut.2017.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark E, Isler C, Strickland D, et al. Influence of aerobic exercise on maternal lipid levels and offspring morphometrics. Int J Obes (Lond). 2019;43(3):594-602. doi: 10.1038/s41366-018-0258-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva SG, Hallal PC, Domingues MR, et al. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. Int J Behav Nutr Phys Act. 2017;14(1):175. doi: 10.1186/s12966-017-0632-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daley A, Jolly K, Jebb SA, et al. Effectiveness of a behavioural intervention involving regular weighing and feedback by community midwives within routine antenatal care to prevent excessive gestational weight gain: POPS2 randomised controlled trial. BMJ Open. 2019;9(9):e030174. doi: 10.1136/bmjopen-2019-030174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrara A, Hedderson MM, Brown SD, et al. A telehealth lifestyle intervention to reduce excess gestational weight gain in pregnant women with overweight or obesity (GLOW): a randomised, parallel-group, controlled trial. Lancet Diabetes Endocrinol. 2020;8(6):490-500. doi: 10.1016/S2213-8587(20)30107-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennelly MA, Ainscough K, Lindsay KL, et al. Pregnancy exercise and nutrition with smartphone application support: a randomized controlled trial. Obstet Gynecol. 2018;131(5):818-826. doi: 10.1097/AOG.0000000000002582 [DOI] [PubMed] [Google Scholar]
- 35.Kiani Asiabar A, Amin Shokravi F, Hajifaraji M, Zayeri F. The effect of an educational intervention in early pregnancy with spouse’s participation on optimal gestational weight gain in pregnancy: a randomized controlled trial. Health Educ Res. 2018;33(6):535-547. doi: 10.1093/her/cyy040 [DOI] [PubMed] [Google Scholar]
- 36.Kunath J, Günther J, Rauh K, et al. Effects of a lifestyle intervention during pregnancy to prevent excessive gestational weight gain in routine care—the cluster-randomised GeliS trial. BMC Med. 2019;17(1):5. doi: 10.1186/s12916-018-1235-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okesene-Gafa KAM, Li M, McKinlay CJD, et al. Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: Healthy Mums and Babies (HUMBA) randomized trial. Am J Obstet Gynecol. 2019;221(2):152.e1-152.e13. doi: 10.1016/j.ajog.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 38.Olson CM, Groth SW, Graham ML, Reschke JE, Strawderman MS, Fernandez ID. The effectiveness of an online intervention in preventing excessive gestational weight gain: the e-moms Roc randomized controlled trial. BMC Pregnancy Childbirth. 2018;18(1):148. doi: 10.1186/s12884-018-1767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parat S, Nègre V, Baptiste A, et al. Prenatal education of overweight or obese pregnant women to prevent childhood overweight (the ETOIG study): an open-label, randomized controlled trial. Int J Obes (Lond). 2019;43(2):362-373. doi: 10.1038/s41366-018-0205-z [DOI] [PubMed] [Google Scholar]
- 40.Pelaez M, Gonzalez-Cerron S, Montejo R, Barakat R. Protective effect of exercise in pregnant women including those who exceed weight gain recommendations: a randomized controlled trial. Mayo Clin Proc. 2019;94(10):1951-1959. doi: 10.1016/j.mayocp.2019.01.050 [DOI] [PubMed] [Google Scholar]
- 41.Phelan S, Wing RR, Brannen A, et al. Randomized controlled clinical trial of behavioral lifestyle intervention with partial meal replacement to reduce excessive gestational weight gain. Am J Clin Nutr. 2018;107(2):183-194. doi: 10.1093/ajcn/nqx043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez-Blanque R, Aguilar-Cordero MJ, Marín-Jiménez AE, Núñez-Negrillo AM, Sánchez-López AM, Sánchez-García JC. Influence of a water-based exercise program in the rate of spontaneous birth: a randomized clinical trial. Int J Environ Res Public Health. 2020;17(3):795. doi: 10.3390/ijerph17030795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rönö K, Grotenfelt NE, Klemetti MM, et al. Effect of a lifestyle intervention during pregnancy-findings from the Finnish gestational diabetes prevention trial (RADIEL). J Perinatol. 2018;38(9):1157-1164. doi: 10.1038/s41372-018-0178-8 [DOI] [PubMed] [Google Scholar]
- 44.Sewell DA, Hammersley VS, Robertson A, et al. A pilot randomised controlled trial investigating a Mediterranean diet intervention in pregnant women for the primary prevention of allergic diseases in infants. J Hum Nutr Diet. 2017;30(5):604-614. doi: 10.1111/jhn.12469 [DOI] [PubMed] [Google Scholar]
- 45.Cedergren MI. Optimal gestational weight gain for body mass index categories. Obstet Gynecol. 2007;110(4):759-764. doi: 10.1097/01.AOG.0000279450.85198.b2 [DOI] [PubMed] [Google Scholar]
- 46.Howell S, Kones R. “Calories in, calories out” and macronutrient intake: the hope, hype, and science of calories. Am J Physiol Endocrinol Metab. 2017;313(5):E608-E612. doi: 10.1152/ajpendo.00156.2017 [DOI] [PubMed] [Google Scholar]
- 47.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring). 2012;20(8):1628-1638. doi: 10.1038/oby.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amorim Adegboye AR, Linne YM. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database Syst Rev. 2013;(7):CD005627. doi: 10.1002/14651858.CD005627.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertz F, Brekke HK, Ellegård L, Rasmussen KM, Wennergren M, Winkvist A. Diet and exercise weight-loss trial in lactating overweight and obese women. Am J Clin Nutr. 2012;96(4):698-705. doi: 10.3945/ajcn.112.040196 [DOI] [PubMed] [Google Scholar]
- 50.Harrison CL, Thompson RG, Teede HJ, Lombard CB. Measuring physical activity during pregnancy. Int J Behav Nutr Phys Act. 2011;8:19. doi: 10.1186/1479-5868-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duncombe D, Wertheim EH, Skouteris H, Paxton SJ, Kelly L. Factors related to exercise over the course of pregnancy including women’s beliefs about the safety of exercise during pregnancy. Midwifery. 2009;25(4):430-438. doi: 10.1016/j.midw.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 52.Bailey C, Skouteris H, Harrison CL, et al. A comparison of the cost-effectiveness of lifestyle interventions in pregnancy. Value Health. Published online September 18, 2021. doi: 10.1016/j.jval.2021.07.013 [DOI] [Google Scholar]
- 53.Hill B, Skouteris H, Teede HJ, et al. Health in Preconception, Pregnancy and Postpartum Global Alliance: international network preconception research priorities for the prevention of maternal obesity and related pregnancy and long-term complications. J Clin Med. 2019;8(12):2119. doi: 10.3390/jcm8122119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 55.Lim S, Liang X, Hill B, Teede H, Moran LJ, O’Reilly S. A systematic review and meta-analysis of intervention characteristics in postpartum weight management using the TIDieR framework: a summary of evidence to inform implementation. Obes Rev. 2019;20(7):1045-1056. doi: 10.1111/obr.12846 [DOI] [PubMed] [Google Scholar]
- 56.Lim S, Hill B, Teede HJ, Moran LJ, O’Reilly S. An evaluation of the impact of lifestyle interventions on body weight in postpartum women: a systematic review and meta-analysis. Obes Rev. 2020;21(4):e12990. doi: 10.1111/obr.12990 [DOI] [PubMed] [Google Scholar]
- 57.Fealy SM, Taylor RM, Foureur M, et al. Weighing as a stand-alone intervention does not reduce excessive gestational weight gain compared to routine antenatal care: a systematic review and meta-analysis of randomised controlled trials. BMC Pregnancy Childbirth. 2017;17(1):36. doi: 10.1186/s12884-016-1207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity (Silver Spring). 2013;21(5):904-909. doi: 10.1002/oby.20163 [DOI] [PubMed] [Google Scholar]
- 59.Harrison CL, Teede H, Khan N, et al. Weight management across preconception, pregnancy, and postpartum: a systematic review and quality appraisal of international clinical practice guidelines. Obes Rev. 2021;22(10):e13310. doi: 10.1111/obr.13310 [DOI] [PubMed] [Google Scholar]
- 60.Incollingo Rodriguez AC, Smieszek SM, Nippert KE, Tomiyama AJ. Pregnant and postpartum women’s experiences of weight stigma in healthcare. BMC Pregnancy Childbirth. 2020;20(1):499. doi: 10.1186/s12884-020-03202-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heggie L, Mackenzie RM, Ells LJ, Simpson SA, Logue J. Tackling reporting issues and variation in behavioural weight management interventions: design and piloting of the standardized reporting of adult behavioural weight management interventions to aid evaluation (STAR-LITE) template. Clin Obes. 2020;10(5):e12390. doi: 10.1111/cob.12390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Author, Year, Country Sample Size, Population, Intervention, Comparator and Outcomes of Eligible Studies (By Year of Publication)
eFigure 1. Funnel Plots and Egger’s Tests Exploring Potential Publication Bias; Gestational Weight Gain (panel A), Maternal and Neonatal Outcomes Across Gestational Diabetes, Hypertensive Disorders of Pregnancy, Preterm Delivery, Cesarean Section, Fetal Death, Small for Gestational Age, Large for Gestational Age and Neonatal Intensive Care admission (panels B-I)
eFigure 2. Forest Plot of Randomized Controlled Trials and Impact on Gestational Weight Gain
eReferences