Abstract
Background:
Individuals with intellectual disability (ID) and autism spectrum disorder (ASD) often receive psychotropic medications such as antipsychotics and antidepressants to treat aberrant behaviors and mood symptoms, frequently resulting in polypharmacy and drug-related adverse effects. Pharmacogenomic (PGx) studies with ASD and/or ID (ASD/ID) have been scarce despite the promise of optimizing treatment outcomes. We reviewed the literature on PGx studies with antipsychotics and antidepressants (e.g., treatment response and adverse effects) in ASD/ID.
Methods:
We performed a systematic review using MEDLINE, Embase, and PsycINFO, including peer-reviewed original articles in English referring to PGx in the treatment of ASD/ID in any age groups (e.g., treatment response and adverse effects).
Results:
A total of 28 PGx studies using mostly candidate gene approaches were identified across age groups. Notably, only 3 studies included adults with ASD/ID while the other 25 studies focused specifically on children/adolescents with ASD/ID. Twelve studies primarily investigated treatment response, of which 5 and 6 studies included patients treated with antipsychotics and antidepressants, respectively. Most interesting results for response were reported for 2 sets of candidate gene studies, namely: (1) The DRD3 Ser9Gly (rs6280) polymorphism was examined in patients treated with risperidone in 3 studies, 2 of which reported an association with risperidone treatment response and (2) the SLC6A4 5-HTTLPR polymorphism and treatment response to antidepressants which was investigated in 4 studies, 3 of which reported significant associations. In regard to side effects, 9 of 15 studies focused on hyperprolactinemia in patients treated with risperidone. Among them, 7 and 5 studies examined the impact of CYP2D6 and DRD2 Taq1A polymorphisms, respectively, yielding mostly negative study findings.
Conclusions:
There is limited data available on PGx in individuals with ASD/ID and in particular in adults. Given the potential for PGx testing in improving treatment outcomes, additional PGx studies for psychotropic treatment in ASD/ID across age groups are warranted.
Keywords: autism spectrum disorder, antidepressants, antipsychotics, intellectual disabilities, pharmacogenomics
Abstract
Contexte:
Les personnes souffrant de déficience intellectuelle (DI) et du trouble du spectre de l’autisme (TSA) reçoivent souvent des médicaments psychotropes comme des antipsychotiques et des antidépresseurs pour traiter des comportements aberrants et des symptômes de l’humeur, ce qui se traduit fréquemment par des effets indésirables de polypharmacie et liés aux médicaments. Les études pharmacogénomiques (PGx) sur les TSA/DI se sont faites rares malgré la promesse d’optimiser les résultats des traitements. Nous avons examiné la littérature traitant des études PGx à l’égard des antipsychotiques et des antidépresseurs (p. ex., la réponse au traitement et les effets indésirables) dans les TSA/DI.
Méthodes:
Nous avons mené une revue systématique à l’aide de MEDLINE, Embase, et PsycINFO, et avons inclus des articles originaux révisés par les pairs en anglais qui mentionnaient les PGx dans le traitement des TSA/DI pour tout groupe d’âge (p. ex., la réponse au traitement et les effets indésirables).
Résultats:
Un total de 28 études PGx recourant surtout à des approches de gènes candidats ont été identifiées dans tous les groupes d’âge. Notablement, seulement trois études incluaient des adultes souffrant de TSA/DI alors que les 25 autres études se concentraient spécifiquement sur les enfants/adolescents souffrant de TSA/DI. Douze études investiguaient principalement la réponse au traitement, parmi lesquelles cinq et six études incluaient des patients traités par antipsychotiques et antidépresseurs, respectivement. Les résultats les plus intéressants pour la réponse au traitement étaient rapportés pour deux ensembles d’études de gènes candidats, notamment: 1) le polymorphisme DRD3 Ser9Gly (rs6280) était examiné chez les patients traités par rispéridone dans trois études, dont deux rapportaient une association avec la réponse au traitement par rispéridone; 2) le polymorphisme SLC6A4 5-HTTLPR et la réponse au traitement par antidépresseurs qui a été investiguée dans quatre études, dont trois rapportaient des associations significatives. En ce qui concerne les effets secondaires, neuf études sur 15 portaient sur l’hyperprolactinémie chez les patients traités par rispéridone. Parmi celles-ci, sept et cinq études examinaient l’impact des polymorphismes CYP2D6 et DRD2 Taq1A, respectivement, aboutissant surtout à des résultats d’étude négatifs.
Conclusions:
Les données disponibles sur les PGx sont limitées pour les personnes souffrant de TSA/DI et en particulier pour les adultes. Compte tenu du potentiel des tests de PGx pour améliorer les résultats des traitements, des études PGx additionnelles des traitements par psychotropes dans les TSA/DI dans tous les groupes d’âge sont justifiées.
Introduction
It is estimated that 1% to 2% of the population are affected by either intellectual disability (ID) or autism spectrum disorder (ASD), 1 –3 which are neurodevelopmental disorders according to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders. 4 Children/adolescents with ASD and/or ID (i.e., ASD/ID for short) often present with problem behaviors including aggression toward others, self-injurious behavior, and disruptive behavior. 5,6 Such challenging behaviors often persist into adulthood. 7 –9 In addition, individuals with ASD/ID have higher rates of comorbid psychiatric disorders than other individuals; approximately 30% of individuals with ID 10 and 70% with ASD have comorbid psychiatric disorders. 11,12
High rates of use of psychotropic medications for children/adolescents and adults with ASD/ID have been reported in many countries. 13 –21 Notably, psychotropic medication use increases with age 22,23 and is highest in individuals with both ASD and ID. 24 Furthermore, polypharmacy and excessive dosages are common in children/adolescents and adults with ASD/ID. 22,25,26 Polypharmacy is frequent in these populations, and rates among individuals with ID have been reported between 11% and 60%, depending on the study design and sample size. 22 Likewise, a recent systematic review reported that the rate of psychotropic polypharmacy in individuals with ASD was estimated between 5.4% and 54%. 25 In general, polypharmacy and high doses are commonly associated with increased adverse effects, medication nonadherence, functional decline, and cognitive impairment, in addition to increased health-care costs. 27 Furthermore, polypharmacy is associated with a highly increased risk for drug–drug interactions typically occurring at the pharmacokinetic level, that is, the Phase-I cytochrome P450 enzymes. 28 Individuals with ID have been reported to be more susceptible to movement side effects of antipsychotic medications than those without ID. 29
Pharmacogenomics (PGx) enables us the opportunity to remedy these treatment inadequacies in individuals with ASD/ID. In general, PGx represents a decision support tool based on well-established gene–drug interactions. 30 Such gene–drug interactions depend on interindividual variability in human DNA sequence, which can determine plasma levels of medications and metabolites and thereby tolerance and response to medications. For antidepressants and antipsychotics medications, which are predominantly metabolized by CYP2C19 and CYP2D6, assessing the genetic variation of these enzymes has enabled researchers and clinicians to estimate their activities; this strongly correlates with exposure to medications (i.e., parent compound and metabolites) and affects treatment outcome for depression and psychotic disorders, respectively. 31,32 For various nonpsychiatric medications, clinical utility of PGx testing compared to treatment as usual has been demonstrated resulting in a reduction of hospitalization rates, health-care costs, and polypharmacy. 33 –35 In psychiatry, favorable treatment outcomes (e.g., higher remission rates) have also been observed in patients receiving PGx-guided antidepressant and antipsychotic treatments compared to those receiving treatment as usual. 36 –41 Therefore, PGx testing is globally becoming increasingly implemented, which is further encouraged by expert recommendation guidelines for psychiatric medications. 31,42 –44
Taken together, in addition to avoiding polypharmacy, PGx testing could be extremely useful for optimizing pharmacological treatment in individuals with ASD/ID by optimizing likelihood for treatment response and minimizing risk for adverse events. However, to the best of our knowledge, no study has systematically reviewed the clinical utility of PGx testing in individuals with ASD or ID, and no reviews have focused specifically on particular in adults. While there is a literature review using only 1 search engine (i.e., PubMed) that focused on PGx studies in ASD, there was no discussion regarding the age of the participants. 45 We aimed to review the literature on PGx studies with psychotropic drugs including antipsychotics and antidepressants (e.g., treatment response and adverse effects) in individuals with ASD/ID across all age groups (i.e., adults and children/adolescents).
Methods
Literature Search
We have followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. 46 The search was performed with MEDLINE, Embase, and PsycINFO until February 29, 2020. The following search terms were applied: (neuroleptic* OR antipsychotic* OR amisulpride OR aripiprazole OR chlorpromazine OR fluphenazine OR haloperidol OR olanzapine OR quetiapine OR risperidone OR thioridazine OR ziprasidone OR antidepressant* OR SNRI OR SSRI OR citalopram OR duloxetine OR escitalopram OR fluoxetine OR fluvoxamine OR mirtazapine OR paroxetine OR sertraline OR venlafaxine OR “alpha agonist” OR stimulant* OR atomoxetine OR clonidine OR guanfacine OR methylphenidate OR benzodiazepine* OR “mood stabilizer*” OR valproate) and (variant* OR polymorphism* OR gene OR genetic OR genetics OR pharmacogenetic OR pharmacogenetics OR pharmacogenomic OR pharmacogenomics) and (autism OR ASD OR “Intellectual* disab*” OR “Intellectual* impair*” OR “Intellectual* retard*” OR “Intellectual* handicap*” OR “Intellectual* subnormal*” OR “Intellectual* deficien*” OR “Learning disab*” OR “Learning impair*” OR “Learning retard*” OR “Learning handicap*” OR “Learning subnormal*” OR “Learning deficien*” OR “Mental* disab*” OR “Mental* impair*” OR “Mental* retard*” OR “Mental* handicap*” OR “Mental* subnormal*” OR “Mental* deficien*” OR “Developmental* disab*” OR “Developmental* impair*” OR “Developmental* retard*” OR “Developmental* handicap*” OR “Developmental* subnormal*” OR “Developmental* deficien*” OR “Neurodevelopmental* disab*” OR “Neurodevelopmental* impair*” OR “Neurodevelopmental* retard*” OR “Neurodevelopmental* handicap*” OR “Neurodevelopmental* subnormal*” OR “Neurodevelopmental* deficien*” OR “down syndrome” OR “Fragile X Syndrome” OR “Prader-Willi Syndrome” OR “Smith-Magenis Syndrome” OR “22q11.2 Deletion Syndrome” OR “15q13.3 Deletion Syndrome”). Limit was set for “English language” and “humans.” References of relevant articles were manually searched and an additional hand search was performed using available citations by 2 authors (K.Y. and E.K.) independently. Candidate articles were independently screened and scrutinized by these authors. Discrepancies in study selection were resolved by discussion between them.
Inclusion Criteria
Studies were included if (1) they were peer-reviewed original articles; (2) they investigated the association between any gene variants and serum/plasma concentrations or dosages of any psychotropics, treatment response to any psychotropics, and adverse effects of any psychotropics; and (3) they were published in English until February 29, 2020. In addition, we included PGx studies meeting the inclusion criteria above across all age groups in order to perform the literature search as comprehensively as possible.
Data Extraction
The following data were extracted by the 2 authors (K.Y. and E.K.) independently for each study: author name, year of publication, diagnosis, age, presence/absence of ID, gene(s) and polymorphism(s), outcomes, study design, sample size, ethnicity or nationality, treatment duration, treatment medication, and main findings.
Results
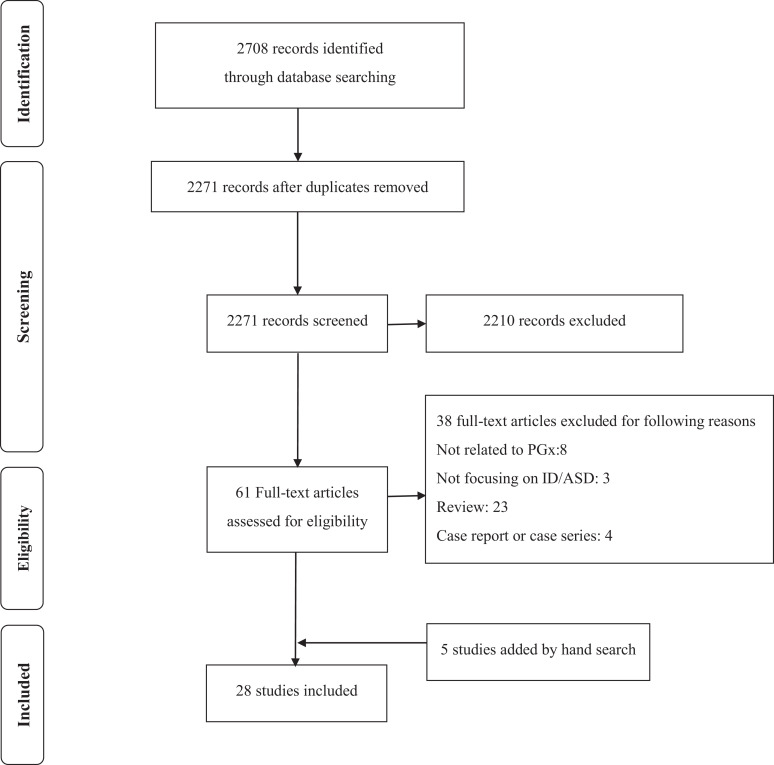
Twenty-eight studies were identified through the literature search (Figure 1). Identified studies were summarized in Table 1. We summarized those studies based on age category (i.e., adults and children/adolescents) and treatment outcomes, respectively.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram for review eligibility and inclusion. Note. ASD = autism spectrum disorder; ID = intellectual disabilities; PGx = pharmacogenomics.
Table 1.
Association between Genetic Polymorphisms and Treatment Outcomes.
| Study (First Author, Years) | Diagnoses | Age | Presence/Absence of Intellectual Disabilities | Gene(s) and Polymorphism(s) | Outcomes | Study Design | Number of Subjects | Ethnicity or Nationality | Treatment Duration | Treatment Medication | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AlOlaby et al., 2017 47 | Fragile X syndrome (58.8% of the subjects had ASD) | 46.1 ± 12.6 (range: 24.1 to 71.9) months | Patients with fragile X syndrome were included | SLC6A4 (5-HTTLPR, S or L alleles), BDNF (Val66Met [rs6265]), MAOA-VNTR (2, 3, 3.5, 4, 5 repeat alleles), CYP2C19 (*1, *2, *3, *17), CYP2D6 (*1, *2, *2, *4, *5, *6, *7, *8, *9, *10, *11, *15, * 17, *29, *35, *41) | Treatment response | DBRCT | 51 | Mainly Caucasian | 6 Months | Sertraline | Significant association between the BDNF polymorphism and improvements for several measures, including CGI-I (P = 0.008) and the cognitive T score (P = 0.017) for those treated with sertraline compared to placebo. MAOA, CYP2C19 and CYP2D6, and 5-HTTLPR also significantly correlated with secondary measures. |
| Anderson et al., 2007 48 | Autism (DSM-IV and ADI) | 8.4 ± 2.7 Years | Not reported (approximately 70% of patients treated with risperidone in the RUPP were with mild or more ID) | DRD2 Taq1A (rs1800497), -141C Ins/Del (rs1799732), and C957T (rs6277) | Adverse effect (hyperprolactinemia) | DBRCT (RUPP) | 78 | Various (mainly Caucasian) | 8Weeks | Risperidone | No significant association of the DRD2 variants with increases in prolactin. |
| Bishop et al., 2015 49 | ASD (autism, Asperger disorder, or PDD-NOS; DSM-IV-TR) | 136.7 ± 66.9 (range: 54 to 532) months | Mean ± SD (range)—nonverbal IQ (n = 89): 83.2 ± 31.7 (21 to 146), verbal IQ (n = 79): 76.7 ± 31.7 (11 to 141) | CYP2C19 (rs4244285, rs4986893, and rs12248560) | Treatment response | Prospective | 89 | Various (mainly Caucasian) | 6 Weeks | Escitalopram | No significant difference in the rate of improvement among metabolizer groups (UM, EM, and PM/IM) for the ABC-CV Irritability subscale. |
| Calarge et al., 2009 50 | Various diagnoses including ADHD, disruptive behavior, tic disorder, and PDD (a combination of a review of the psychiatric record and the National Institute of Mental Health Diagnostic Interview Schedule for Children) | 12.1 ± 2.8 Years | Not reported | DRD2 Taq1A (rs1800497), C957T (rs6277), -141C Ins/Del (rs1799732), and A-241G (rs1799978) | Adverse effect (hyperprolactinemia) | Cross-sectional | 90 | Non-Hispanic Caucasians | ≥6 Months | Risperidone | After controlling for risperidone concentration and the dose of psychostimulants, a synergistic effect of the TaqIA and the A-241G variants was found on prolactin concentration, using multiple regression analysis (P = 0.003). |
| Correia et al., 2010 51 | Patients who met the algorithm cutoff for the ADI-R and the ADOS | 8.67 ± 4.30 (range: 3 to 21) years | Absent (IQ ≥ 70): 37.8%, mild (69 ≥ IQ ≥ 50): 31.1%, moderate (49 ≥ IQ ≥ 35): 24.4%, severe (IQ < 35): 6.7% | CYP2D6*3, CYP2D6*4, CYP2D6*5, CYP2D6*6, CYP2D6 gene duplication, ABCB1 (rs1128503, rs1045642), HTR2A (rs6311), DRD2 (rs1800497), HTR2C (rs6318, rs3813928, rs3813929), BDNF (rs6265), HTR6 (rs9659997), DRD3 (rs6280) | Treatment response and adverse effect (AIWG and prolactin elevation) | Prospective | 45 | Various (mainly Caucasian) | Up to 1 year | Risperidone | The HTR2A rs6311 (P = 0.019), DRD3 rs6280
(P = 0.012), HTR2C rs3813928
(P = 0.035), and ABCB1
rs1128503 (P = 0.002) were significantly associated with the decline in the ATEC scores. The HTR2A rs6311 (P = 0.018), HTR2C rs6318 (P = 0.006), HTR6 rs9659997 (P = 9.5 × 10-5), and BDNF rs6265 (P = 0.016) significantly associated with prolactin elevation. The CYP2D6 and HTR2C rs6318 polymorphisms were significantly associated with increase in BMI or waist circumference (P < 0.05). |
| Cote et al., 2015 52 | Various diagnoses including anxiety disorder, mood disorder, ADHD, and PDD (DSM-IV-TR) | 13.1 ± 3.0 Years | Not reported | COMT Val158Met (rs4680) | Adverse effects (blood pressure and other cardiovascular risk factors) | Cross-sectional | 302 | Various (mainly European) | Median = 7 months | SGAs | SGA-treated children with the Met allele showed higher systolic and diastolic blood pressure (P = 0.014 and P = 0.034, respectively) and higher fasting glucose concentrations (P = 0.030) than those who with the Val/Val genotype. |
| dos Santos Júnior et al., 2015 53 | Various diagnoses including mild or moderate mental retardation (n = 36, 30%) and PDD (n = 26, 21.7%; ICD) | 13.0 ± 3.1 Years | Patients with mild or moderate ID were included | DRD2 (rs1799978 and rs6277), HTR2C (rs6318 and rs3813929), CYP2D6*10 (rs1065852), LEP (rs7799039), LEPR (rs1137101), MC4R (rs17782313), SCARB2 (rs3853188) | Adverse effect (hyperprolactinemia) | Cross-sectional | 120 | Various (mainly Caucasian) | 23.4 ± 28.6 Months (cases with hyperprolactinemia) and 30.9 ± 23.9 months (controls without hyperprolactinemia) | Risperidone | Hyperprolactinemia occurred with higher frequency in patients with the C allele of the HTR2C rs6318 polymorphism (P = 0.02). |
| Firouzabadi et al., 2017 54 | ASD (DSM-V) | 6.8 ± 1.3 (2.5 to 14) Years | Patients with severe ID were excluded | DRD3 Ser9Gly (rs6280) | Treatment response | Prospective | 56 | Persian | 8 Weeks | Risperidone | Responder rates (i.e., a 50% or greater decrease from baseline in ABC score) were significantly higher in carriers of Gly allele as well as carriers of Gly/Gly and Ser/Gly genotypes compared with carriers of Ser allele and Ser/Ser genotype (P = 0.027 and 0.014, respectively). |
| Hoekstra et al., 2010 55 | PDD (ADI-R) | 8.74 ± 2.83 (5 to 16) Years | Not reported | HTR2C (rs3813929 and rs1414334) | Adverse effect (AIWG) | Prospective | 32 | Dutch | 8 weeks | Risperidone | Presence of rs3813929T allele was significantly associated with a smaller weight gain (P < 0.001). |
| Hongkaew et al., 2018 56 | ASD (DSM-IV) | Median: 8.96 (quartile 1 to 3: 7.44 to 10.98) years | Not reported | 508 Drug-metabolizing enzymes and transporters SNPs | Adverse effect (hyperprolactinemia) | Observational- retrospective | 84 | Thai | Total sample duration: 43.57 months | Risperidone | Three UGT1A1 SNPs (UGT1A1*80 c.-364C > T [rs887829], UGT1A1*93 c.-3156G > A [rs10929302], and UGT1A1 c.-2950A > G [rs111741722]) showed a suggestive association with hyperprolactinemia (P = 0.0014). |
| Lit et al., 2012 57 | ASD (DSM-IV and ADI-R) | 112.7 ± 51.2 Months | Patients with nonverbal intelligence quotient < 55 were excluded | Exon expression levels in blood assessed using Affymetrix GeneChip Human Exon 1.0 ST Arrays | Gene expression and treatment response | Prospective | 42 | Caucasian (n = 24) and others (n = 18) | 8 Weeks | Risperidone | Expression of exons within 5 genes (GBP6, RABL5, RNF213, NFKBID, and RNF40) was significantly correlated with change in ABC Irritability subscale scores (GBP6, r = 0.78; RABL5, r = 0.72; RNF213, r = −0.73; NFKBID, r = 0.75; and RNF40, r = −0.74; P < 0.001). |
| McCracken et al., 2010 58 | PDD (PDD-NOS, Asperger disorder, or autistic disorder) with clinically significant symptoms of ADHD | 9.03 ± 3.14 Years | Not reported | MDR1 C3435T (rs1045642) | Treatment response | Prospective | 25 | Caucasian (n = 18), African American (n = 6), and Hispanic (n = 1) | 8 Weeks | Guanfacine | Patients with either C/T or C/C genotypes showed a significant greater improvement than T/T MDR1 C3435T genotype in the ABC hyperactivity scores (P < 0.03) and a greater improvement in the Swanson, Nolan, and Pelham (SNAP) scores (P = 0.05). |
| McCracken et al., 2014 59 | ASD (DSM-IV and ADI-R) | 6.90 ± 2.2 (range 5.0 to 13.0) years | Mean intelligence quotient: 65.0 ± 33.3 (range: 16 to 135) | DRD1 (rs4867798, rs5326, rs686), DRD2 (rs6277, rs6589377, rs4938019, rs7131056, rs1800498, rs2283265, rs6275, rs1800497), DRD3 (rs6280 [Ser9Gly], rs2134655, rs9880168, rs7633291, rs167771, rs3732790), DRD4 (rs11246226, rs3758653, Exon 3 VNTR), DRD5 (rs10033951), ADRA2A (rs1800544, rs12246561, rs3750625), SLC6A3 (3’UTR VNTR), SLC6A4 (rs12150214, rs4251417, rs11080121, 5HTT-LPR, STin2 VNTR), MAOA (rs1465108, rs3810709, rs3027399), MAOB (rs10521432, rs1799836), COMT (rs4680 [Val158Met]) | Treatment response and tolerability determined by adherence | Random-order, placebo-controlled, double-blind crossover | 58 | Caucasian (75.9%) | 4 Weeks | Methylphenidate | The DRD1 rs4867798 (P = 0.042), DRD1 rs5326 (P = 0.006), DRD3 rs6280 (P = 0.044), DRD4 rs11246226 (P = 0.038), SLC6A4 STin2 VNTR (P = 0.049), SLCA4 STin2 VNTR (P = 0.041), ADRA2A rs1800544 (P = 0.015), COMT rs4680 (P = 0.049) were significantly associated with responder status assessed using the CGI-I and ABC hyperactivity subscale. The DRD2 rs6275 (P < 0.001) and DRD3 rs6280 (P = 0.031) were significantly associated with tolerability. |
| Medhasi et al., 2016 60 | ASD (DSM-IV) | 8.8 (range: 3.4 to 18.6) years | Not reported | Exploratory analysis using Affymetrix DMETTM Plus Gene Chip microarray interrogating 1931 variants in 231 genes; 483 variants were included for final analysis | Plasma concentrations | Retrospective | 102 | Thai | Median duration of risperidone used: 41.62 months (range: 1.03 to 152.97) | Risperidone | ABCB11 (c.3084A>G, c.420A>G, c.368G>A, and c.236G>A) and ADH7 (c.690G>A and c.-5360G>A) were significantly associated with plasma concentrations of risperidone (P < 0.01). Two of the SCLO1B1 polymorphisms (c.-11187G>A and c.521T>C), SLCO1B3 (c.334G>T, c.699A>G, and c.1557G>A), and SLC7A5 c.438C>G were significantly associated with 9-hydroxyrisperidone and the total active-moiety levels (P < 0.01). |
| Najjar et al., 2015 61 | ASD (DSM-IV, Autism Diagnostic Interview–Revised, and Autism Diagnostic Observation Scale, second edition) | 161 ± 86 (range:61 to 532) months | Mean ± SD (range)—nonverbal IQ (n = 44): 80 ± 25 (35 to 130), verbal IQ (n = 38): 78 ± 25 (30 to 120), full scale (n = 38): 80 ± 25 (33 to 130) | SLC6A4 (5-HTTLPR) and HTR2A (rs7997012) | Treatment response | Prospective | 44 | Various (mainly Caucasian) | 6 Weeks | Escitalopram | No significant differences in the rate of symptom improvement assessed using the RBS-R CRS and the ABC-CV Irritability subscale scores over time across genotype groups. |
| Nuntamool et al., 2017 62 | Autistic disorder (91.36%), PDD-NOS (6.17%), Rett disorder (1.23%), and Asperger disorder (1.23%; DSM-IV) | Median:11 (IQR: 9.00 to 14.00) | Not reported | DRD2 (TaqIA [rs1800497], -241A>G [rs1799978]); DRD3 (25T>C [rs6280]); HTR2A (-1438G>A [rs6311]); ABCB1 (3435C>T [rs1045642], 2677G>T/A [rs2032582], 1236C>T [rs1128503]); CYP2D6 polymorphisms | Treatment response as the primary outcome | Cross-sectional | 82 | Thai | Median duration of risperidone used: 67.90 months (IQR: 52.53 to 90.93) | Risperidone | The nonstable symptom group assessed using CGI-I score and a 4-point scale for each of aggression, overactivity, and repetitive behaviors had DRD2 Taq1A non-wild-type (TT and CT) higher frequencies than the stable group (P = 0.048). Other gene polymorphisms showed no significant association. |
| Nurmi et al., 2013 63 | ASD (autism, Asperger disorder, PDD-NOS; DSM-IV) | 96.5 ± 32.3 Months | Not reported (approximately 70% and 40% of patients treated with risperidone in the RUPP and RUPP-PI were with mild or more ID) | FTO (rs1421085, rs6499640, rs1121980, rs17817449, rs8050136, rs9939609); MC4R (rs8087522, rs11872992, rs8093815, rs489693); LEP (rs7799039, rs10244329, rs12706832, rs2071045); CNR1 (rs806378, rs806377, rs1049353, rs806368); FAAH (rs324420) | Adverse effect (AIWG) | Data from 2 trials (RUPP: DBRCT, RUPP-PI: randomized, parallel-groups clinical trial) | 181 | Various (mainly Caucasian) | 8 Weeks | Risperidone | Three gene variants (LEP rs7799039, CNR1 rs806378, and rs1049353) were significantly associated with AIWG (P = 1.4 × 10-4, 1.0 × 10-6, and 9.6 × 10-5, respectively). |
| Owley et al., 2010 64 | ASD (autism, Asperger disorder, PDD-NOS; ADI-R and ADOS-2) | 117 ± 31 (range: 54 to 204) months | Mean ± SD (range); nonverbal IQ: 86 ± 34 (21 to 146), verbal IQ: 76 ± 35 (11 to 141) | SLC6A4 5-HTTLPR (S, LA, LG alleles, TT diplotype) | Treatment response | Prospective | 58 | Various (mainly Caucasian) | 10 Weeks | Escitalopram | A significant interaction between genotype group and time on the ABC Irritability subscale (linear maximum marginal likelihood estimation = −4.84, Z = −2.89, SE = 1.67, P = 0.004). |
| Prows et al., 2009 65 | Various diagnoses including mood disorders, disruptive behavior disorders, PDD (DSM-IV-TR) | 12.7 ± 3.2 Years | Patients with severe ID were excluded | CYP2D6*1, *3, *4, *5 and CYP2C19*1, *2 | Treatment response and adverse effects (the number of adverse effects) | Retrospective | 279 | Various (mainly Caucasian) | Not mentioned | Psychotropics including antidepressants and antipsychotics | Combined phenotype of CYP2D6 and CYP2C19 was significantly associated with the BIS (P = 0.01) and the number of adverse effects (P = 0.03). |
| Roke et al., 2013 66 | ASD or disruptive behavior disorder (no diagnostic tool was reported) | 14.7 ± 2.1 (range: 10 to 19) years | Patients with IQ above 85 were included | DRD2 Taq1A (rs1800497), CYP2D6*3 del A (rs35742686), CYP2D6*4G>A (rs3892097), CYP2D6*6 del T (rs5030655), CYP2D6 gene deletion (CYP2D6*5), and the gene duplication (CYP2D6xN) | Adverse effect (hyperprolactinemia) | Cross-sectional | 47 | Caucasian (97%) | Mean 52 months (range: 16 to 126 months) | Risperidone | No significant correlations between prolactin level and the presence of at least 1 Taq1A A1 allele of the DRD2 gene, using multiple regression analysis (P = 0.12). No significant difference in prolactin level between the CYP2D6 reduced activity group and the normal activity group, using an independent sample t test (P = 0.8). |
| Sherwin et al., 2012 67 | Mainly ASD | 9.6 ± 3.7 (3 to 18.3) Years | Some of the patients had ID (Aman et al., 2007 68 ) | CYP2D6 *2A, *3, *4, *5, *6, *7, *8, *9, *10, *11, *14, *15, *17, *18, *19, *20, *40, *41, *42, deletion, and duplication | Relative clearance of risperidone CL/F (liters/hour) | Prospective in original studies | 45 | Caucasian (93.3%) | Not mentioned. All patients started risperidone treatment prior to age 18 to treat neuropsychiatric disorder | Risperidone | Clearance estimates in the mixture model were 9.38 L/h (PM), 29.2 L/h (IM), and 37.4 L/h (EM). |
| Sugie et al., 2005 69 | Autism (DSM-IV) | Mean: 5 years and 4 months (n = 19) | Not reported | SLC6A4 (5-HTTLPR (S or L alleles) | Treatment response as the primary outcome | Crossover double-blind, placebo-controlled study | 19 (18 subjects were included in the analysis) | Japanese | 12 Weeks | Fluvoxamine | Fluvoxamine was significantly more effective in the L allele variant than the S allele variant when CGI was used as an assessment scale (P = 0.047). |
| Sukasem et al., 2016 70 | ASD (DSM: version was not reported) | 9.52 Years (inclusion criteria: 3 to 19 years) | Not reported | CYP2D6*4 (1846G>A, [rs3892097]), CYP2D6*10 (100C>T [rs1065852]), CYP2D6*41 (2988G>A [rs28371725]), CYP2D6 gene deletion (CYP2D6*5), and DRD2 Taq1A (rs1800497) | Adverse effect (hyperprolactinemia) | Retrospective cross-sectional | 147 | Thai | 46.06 Months | Risperidone | No significant correlation between the concentrations of prolactin among the CYP2D6 genotypes. There were statistically significant differences in prolactin level of patients among the DRD2 Taq1A A2A2, A1A2, and A1A1 groups (P = 0.033). |
| Sukasem et al., 2018 71 | ASD (DSM-IV) | Median: 10.00 (IQR: 8.90 to 13.40) years | Not reported | ABCB1 (2677G>T/A [rs2032582], 3435C>T [rs1045642]), DRD2 (Tag-SNP [T>C; rs4436578], Tag1A [C>T; [rs1800497]), BDNF (196G>A [rs6265]), LEP (-2548G>A [rs7799039]), GHRL (-604G>A [rs27647]), CYP2D6*4 (1846G>A [rs3892097]), CYP2D6*10 (100C>T [rs1065852]), and CYP2D6*41 (2988G>A [r s28371725]); CYP2D6*5 (gene deletion) | Adverse effect (insulin resistance) | Observational | 89 | Thai | 63.92 (40.40 to 83.49) Months | Risperidone | A significant association between insulin resistance and BDNF 196 G>A, using multiple regression analysis (P = 0.025). |
| Troost et al., 2007 72 | PDD (autistic disorder, Asperger disorder, or PDD-NOS; DSM-IV-TR) | 8.6 ± 2.2 Years | Not reported | CYP2D6*3, *4, *5, *6, and *7 | Adverse effect (prolactin elevation) | Prospective | 25 | Dutch | 8 Weeks | Risperidone | Significant positive correlations of serum prolactin level with dose per kilogram (r = 0.648, P < 0.001), number of functional CYP2D6 genes (J = 2.117, P = 0.034), and serum 9-hydroxyrisperidone concentration (r = 0.664, P = 0.001) and a negative correlation with the risperidone/9-hydroxyrisperidone ratio (r = 0.571, P = 0.004). |
| Vanwong et al., 2016 73 | ASD (DSM-IV) | Median: 10.00 (IQR: 6.83 to 11.55) years | Not reported | CYP2D6*1, *2, *3, * 4, *5, *6, *7, *8, * 9, *10, *11, *15, *17, *29, *41, *35, and duplications | Plasma concentrations | Prospective | 84 | Thai | >4 Weeks | Risperidone | IMs showed significantly higher plasma concentration of risperidone than EMs (P < 0.0001) but not UMs (P = 0.14), and significantly higher plasma concentration of risperidone/ 9-hydroxyrisperidone ratio than EMs (P < 0.0001) and UMs (P = 0.02) |
| Vanwong et al., 2017 74 | ASD (DSM-IV) | Median: 10.00 (IQR: 7.00 to 12.15) years | Not reported | CYP2D6*4 (1846G>A [rs3892097]), CYP2D6*10 (100C>T [rs1065852]), CYP2D6*41 (2988G>A [rs28371725]), CYP2D6*5 (CYP2D6 gene deletion), and CYP2D6*1 (absence of SNPs) | Plasma concentrations | Prospective | 97 | Thai | >4 Weeks | Risperidone | A significant association between high plasma levels of risperidone and CYP2D6*5/*10 (P = 0.02), CYP2D6*10/*10 (P = 0.04), and CYP2D6*10/*41 (P = 0.04). |
| Youngster et al., 2014 75 | ASD (autism, Asperger disorder, or PDD-NOS; DSM-IV, ADI-R, and Childhood Autism Rating Scale) | Median: 7 (range: 3 to 18) years | Not reported | CYP2D6*2, *3, *4, *5, *6, *8, *9, *10, *11, *14, *15, *17, *18, *19, *20, *25, *26, *29, *30, *31, *35, *36, *37, *40, *41, *43, *52, and a number of duplicated alleles | Treatment response and adverse effects (e.g., hyperprolactinemia, AIWG, and EPS) | Observational cohort study | 40 | Israeli | A median of 6 months (3 months minimum) | Risperidone | UMs (n = 2) were classified as nonresponders and had no adverse effects. In contrast, PMs (n = 2) were classified as responders and experienced adverse effects. PMs had significantly higher risperidone plasma levels (P = 0.03) and higher risperidone-to-9-OH-risperidone ratio (P = 0.02: as continuous variable, P = 0.004: as dichotomous with a cutoff ratio of 1) |
Note. ABC = Aberrant Behavior Checklist; ABC-CV = ABC–Community Version; ADHD = attention deficit hyperactivity disorder; ADI-R = Autism Diagnostic Interview–Revised; AIWG = antipsychotic-induced weight gain; ADOS = Autism Diagnostic Observation Scale: ASD = autism spectrum disorder; ATEC = Autism Treatment Evaluation Checklist; BIS = Behavioral Intervention Score; BMI = body mass index; CGI-I = Clinical Global Impression Scale–Improvement; DBRCT = double-blind randomized controlled trial; DSM-IV-TR = Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision; EM = extensive metabolizer; EPS = extrapyramidal symptom; ICD 10 = International Statistical Classification of Diseases and Related Health Problems, 10th revision; ID = intellectual disability; IM = intermediate metabolizer; IQ = intelligence quotient; IQR = interquartile range; PDD = pervasive developmental disorder; PDD-NOS = pervasive developmental disorder not otherwise specified; PM = poor metabolizer; RBS-R CRS = Repetitive Behavior Scale–Revised, Compulsive Behavior and Ritualistic/Sameness Behavior subscales; RUPP = Research Units on Pediatric Psychopharmacology; RUPP-PI = RUPP–Psychosocial Intervention; SD = standard deviation; SGAs = second-generation antipsychotics; SNP = single nucleotide polymorphism; UM = ultrarapid metabolizer.
PGx Studies Based on Age Groups
PGx studies in adults with ASD/ID
There were no PGx studies exclusively focusing on adults with ASD/ID although 3 PGx studies included adults with ASD/ID in their analyses. 49,51,61 One PGx study by Bishop et al. examined the association between metabolizer status for the CYP2C19 gene (i.e., ultrarapid metabolizer [UM; n = 26], extensive metabolizer [EM; n = 40], and poor [PM]/intermediate [IM] metabolizer; n = 23) and assessed treatment response to escitalopram using the Aberrant Behavior Checklist–Community Version (ABC-CV) in individuals with ASD. 49 Although adults were included, no breakdown by age was provided (mean ± standard deviation [SD] [range]: 136.7 ± 66.9 [54 to 532] months, N = 89), but this study included at least 1 adult patient (44.3 years old). A subgroup of individuals in this study had ID in addition to ASD, but no individuals were exclusively diagnosed with ID (nonverbal intelligence quotient [IQ]: 83.2 ± 31.7 [21 to 146], N = 89; verbal IQ: 76.7 ± 31.7 [11 to 141], N = 79). However, no information on response in adults versus children/adolescents or in those with or without concurrent ID was provided. Another study by Najjar et al. examined whether the SLC6A4 (5-HTTLPR) and HTR2A (rs7997012) polymorphisms were associated with response to escitalopram, using the Repetitive Behavior Scale–Revised, Compulsive Behavior and Ritualistic/Sameness Behavior Subscales (RBS-R-CRS) and ABC-CV Irritability subscale (ABC-CV-IRR) scores (N = 44). 61 Similar to the previous study, 49 this study also included adults but did not provide a breakdown by age (mean ± SD [range]: 161 ± 86 [61 to 532] months) while this study included at least 1 adult patient aged 44 years. 61 A subgroup of individuals had ASD with ID, but no individuals were exclusively affected with ID (nonverbal IQ: 80 ± 25 [35 to 130], N = 44; verbal IQ: 78 ± 25 [30 to 120], N = 38). A study by Correia et al. examined the relationship between treatment response to risperidone, which was assessed by the Autism Treatment Evaluation Checklist (ATEC), and 15 variants across 8 genes in autistic children and young adults who were receiving risperidone up to 1 year (N = 45). 51 Similar to the other 2 studies, 49,61 this study also included at least 1 adult with an age of 21 years (mean ± SD [range]: 8.67 ± 4.30 [3 to 21]). It reported patients’ IQ levels and included individuals with ID; however, it was not clear whether adults with ID were included or not (IQ ≥ 70, 37.8% of the patients; 69 ≥ IQ ≥ 50, 31.1%; 49 ≥ IQ ≥ 35, 24.4%; and IQ < 35, 6.7%). 51
PGx studies in children/adolescents with ASD/ID
In contrast to the 3 studies that included adults and children/adolescents, 49,51,61 the other 25 studies focused specifically on children/adolescents with ASD/ID. Among them, 1 PGx study by AlOlaby et al. included only children/adolescents with fragile X syndrome which is the most common inherited cause of ID. 47 In addition, 6 additional studies were conducted in children/adolescents with ASD, some of whom also had ID. 48,53,59,63,64,67 Three of the 6 studies clearly described that they included ASD with ID. 53,59,64 For the other 3 studies, 48,63,67 it was likely that they also included children/adolescents with ASD and ID, given their inclusion criteria. More specifically, Nurmi et al. investigated the association of key energy balance genes (i.e., FTO, MC4R, LEP, CNR1, FAAH) with antipsychotic-induced weight gain (AIWG) in children/adolescents with ASD treated with risperidone in the 2 National Institute of Mental Health Research Units on Pediatric Psychopharmacology (RUPP) Autism Network trials. 63 It was reported that approximately 70% and 40% of patients in the 2 trials had intellectual disabilities. 68,76 Likewise, Anderson et al. included individuals with ASD treated with risperidone from the RUPP trial, in which approximately 70% of the individuals were affected with mild or more ID. 48 The study by Sherwin et al. investigated the effect of the CYP2D6 phenotype on pharmacokinetic variability of risperidone in children and adolescents (majority of ASD; N = 45). 67 This study did not specify diagnoses but reported that the majority of individuals were affected by ASD. Forty-one of the 45 patients included in this study were from other studies, 1 of which included some individuals with ASD who had co-occurring ID. 68 Thus, it was possible that some individuals had ASD and possibly ID in this study. 67
There were 18 studies of children/adolescents with ASD, which either did not describe whether any of the participants also had ID or specifically mentioned including only individuals without ID. 50,52,54 –58,60,62,65,66,69,70 –75 For example, Roke et al. listed IQ above 85 in their inclusion criteria. 66 Three other studies specified excluding children/adolescents with IQs below 55 but did not provide information indicating whether individuals with IQs ranging from 56 to 75 participated in the studies. 54,57,65 The other 14 studies did not report if they included children/adolescents with ASD who also had ID.
PGx Studies Based on Treatment Outcomes
Response to psychotropics
Among the 28 studies included, twelve studies primarily investigated the association between treatment response and specific gene polymorphisms. 47,49,51,54,58,59,61,62,64,65,69,75
Response to antipsychotics
Five of the 12 studies focused on patients treated with antipsychotics. 51,54,62,65,75 Among them, 4 studies 51,54,62,75 included only patients treated with risperidone monotherapy and the other one used various antipsychotics. 65
Pharmacokinetic Genes: Three studies investigated the CYP polymorphisms, 51,62,75 among which 1 study 75 suggested an association of CYP2D6 metabolizer status with treatment response to risperidone. More specifically, an observational cohort study of 40 Israeli children by Youngster et al. evaluated the association between CYP2D6 genotypes (up to 34 CYP2D6 alleles and allele duplications) and treatment response to risperidone determined by parents and the treating neurologist, using a simple 3-point scale (i.e., improvement in disruptive behaviors, no change, or worsening). 75 This study reported that PMs (n = 2) were classified as responders whereas UMs (n = 2) were classified as nonresponders, while no serum levels of risperidone were taken. However, other studies reported no association of the CYP2D6 polymorphisms with treatment response. 51,62 Two studies examined the impact of the ABCB1 1236C>T polymorphism on treatment response to risperidone. 51,62 Correia et al. reported that the ABCB1 1236C>T (rs1128503) was significantly associated with clinical improvement assessed by the ATEC (P = 0.002; see also the “PGx Studies in Adults with ASD/ID” section) 51 whereas a cross-sectional study by Nuntamool et al., 62 where 82 Thai children/adolescents treated with risperidone for more than 1 year were included, reported no significant association with treatment response determined by the Clinical Global Impression Scale–Improvement (CGI-I) score and a 4-point scale for each of aggression, overactivity, and repetitive behaviors.
Pharmacodynamic Genes: Among the 4 studies investigating gene variants associated with treatment response to risperidone, 51,54,62,75 the DRD3 Ser9Gly (rs6280) polymorphism was examined in 3 studies, 51,54,62 2 of which reported significant findings. 51,54 More specifically, Correia et al. reported carriers of Gly allele showed greater treatment response to risperidone than noncarriers of Gly allele (i.e., Ser/Ser genotype; see also the “PGx Studies in Adults with ASD/ID” section). 51 An 8-week prospective study by Firouzabadi et al. reported that responder rates (i.e., a 50% or greater decrease of the ABC scores from baseline) were significantly higher in carriers of Gly allele as well as carriers of Gly/Gly and Ser/Gly genotypes compared with carriers of Ser allele and Ser/Ser genotype in Iranian children (N = 56; P = 0.027 and 0.014, respectively). 54 In contrast, a cross-sectional study by Nuntamool et al. 62 reported no significant association of the DRD3 rs6280 polymorphism with treatment response. The following other gene variants were also significantly associated with treatment response to risperidone in 1 study: the HTR2A c.-1438G>A (rs6311; P = 0.019), 51 HTR2C c.995G>A (rs3813928; P = 0.035), 51 and DRD2 Taq1A (rs1800497; P = 0.048). 62
Response to antidepressants
The association between response to antidepressants and gene variants was examined in 6 studies. 47,49,61,64,65,69 Among them, escitalopram was used in 3 studies (see also the “PGx Studies in Adults with ASD/ID” section), 49,61,64 sertraline in 1 study (see also the “Pharmacogenomic Studies in Children/Adolescents with ASD/ID” section), 47 fluvoxamine in 1 study, 69 and various antidepressants in 1 study. 65
Pharmacokinetic Genes: Bishop et al. found that there were no differences in the rate of improvement assessed using the ABC-CV across metabolizer groups for the CYP2C19 gene (i.e., UM [n = 26], EM [n = 40], and PM/IM metabolizer [n = 23]) in individuals with ASD treated with escitalopram in 6 weeks (P = 0.39). 49 However, the UM group exhibited a slower rate of dosing change compared to other groups when looking at titration trajectories, contrary to expectations. On the other hand, AlOlaby et al. reported that subjects with the PM/IM genotypes for the CYP2C19 gene showed a significant percentage in the very much improved/much improved CGI-I if they were treated with sertraline (n = 6) compared to those who were with placebo (n = 5) in patients with fragile X syndrome (P = 0.007). 47
Pharmacodynamic Genes: The association of the SLC6A4 (5-HTTLPR) gene and treatment response to antidepressants was investigated in 4 studies. 47,61,64,69 AlOlaby et al. reported that sertraline was associated with a significantly different change (i.e., symptom improvement) from baseline in the social participation raw score on the active arm compared to placebo in those with the L/L genotype (P = 0.005) whereas no significant difference was observed for the S/L (P = 0.422) or S/S (P = 0.997) genotypes (N = 51). 47 Likewise, Sugie et al. 69 found that the L allele conferred better response to fluvoxamine than the S allele in a 12-week double-blind crossover trial of fluvoxamine and placebo, in which treatment response was determined by CGI scores in Japanese patients (N = 18; P = 0.047). In addition, Owley et al. reported a significant interaction between genotype group of the 5-HTTLPR and time on the ABC-CV-IRR in patients treated with escitalopram for 10 weeks (N = 58; P = 0.004). 64 In contrast, Najjar et al. reported no significant differences in the rate of symptom improvement assessed using the RBS-R-CRS and ABC-CV-IRR across genotype groups in ASD treated with escitalopram over the 6 weeks (N = 44; P = 0.273 for RBS-R-CRS and P = 0.122 for ABC-CV-IRR). 61 Other results were summarized in Table 1.
Response to other medications
An 8-week open-label trial examined the effect of the MDR1 (ABCB1) C3435T polymorphism on treatment response to guanfacine in PDD patients with clinically significant symptoms of attention deficit hyperactivity disorder (ADHD; N = 25). 58 Patients with either C/T or C/C genotypes showed a significantly greater improvement than T/T genotype in the ABC Hyperactivity scores (P < 0.03) and Swanson, Nolan, and Pelham (SNAP) scores (P = 0.05). Another study reported a 4-week, placebo-controlled, double-blind crossover study with 58 children to evaluate the association between 36 variants across 10 genes and treatment response to methylphenidate defined by CGI and ABC hyperactivity subscale. 59 This study reported that the DRD1 rs4867798 (P = 0.042) and rs5326 (P = 0.006), DRD3 rs6280 (P = 0.044), DRD4 rs11246226 (P = 0.038), SLC6A3 VNTR (P = 0.049), SLC6A4 STin2 VNTR (P = 0.041), ADRA2A rs1800544 (P = 0.015), and COMT rs4680 (P = 0.049) among 36 variants tested were significantly associated with responder status; however, this significance in each variant did not remain after correction for multiple testing.
Adverse effects
Fifteen studies primarily investigated the association of side effects with gene polymorphisms. 48,50 –53,55,56,59,63,65,66,70 –72,75 Among them, 9 studies focused on prolactin elevation or hyperprolactinemia as primary outcome in patients treated with risperidone. 48,50,51,53,56,66,70,72,75 Other adverse effects were also investigated in 8 studies (e.g., AIWG, blood pressure, and insulin resistance). 51,52,55,59,63,65,71,75
Prolactin elevation or hyperprolactinemia
The most commonly investigated gene variant associated with prolactin elevation or hyperprolactinemia was CYP2D6 polymorphisms, 51,53,56,66,70,72,75 followed by the DRD2 Taq1A (rs1800497) polymorphism. 48,50,51,66,70
Pharmacokinetic Genes
Seven studies examined the impact of CYP2D6 polymorphisms on prolactin elevation or hyperprolactinemia. 51,53,56,66,70,72,75 Although 1 prospective study by Troost et al. reported a positive correlation of the number of functional CYP2D6 genes and serum prolactin level in 8 weeks (P = 0.034), 72 other studies reported no significant association of CYP2D6 polymorphisms, genotypes, or predicted phenotypes with prolactin elevation or hyperprolactinemia. 51,53,56,66,70,75
Pharmacodynamic Genes
Five studies investigated the association of the DRD2 Taq1A (rs1800497) polymorphism with hyperprolactinemia in patients treated with risperidone, 48,50,51,66,70 and 3 of them reported nonsignificant findings 48,51,66 whereas the others reported a synergistic effect of the DRD2 TaqIA and DRD2 A-241G variants on prolactin concentration using multiple regression analysis (P = 0.003) 50 and significant differences in prolactin level of patients among the DRD2 Taq1A A2A2, A1A2, and A1A1 groups (P = 0.033). 70
The presence of the C allele of HTR2C rs6318 polymorphism was significantly associated with prolactin elevation or hyperprolactinemia in 2 studies (P = 0.006 and 0.02, respectively). 51,53 Other results were summarized in Table 1.
Other adverse effects
Four studies investigated the association of gene variants with AIWG in patients treated with risperidone. 51,55,63,75 The HTR2C rs6318 51 and rs3813929, 55 LEP rs7799039, 63 and CNR1 rs806378 and rs1049353 polymorphisms 63 were significantly associated with AIWG (P < 0.05) whereas the findings of the association between the HTR2C rs3813929 polymorphism and AIWG yielded opposite findings in 2 studies. 51,55 In addition, UMs of CYP2D6 showed a 4.8% and 5.8% lower increase in body mass index and waist circumference compared to EMs. 51
Drug concentrations
Four studies investigated the association of drug concentrations with gene polymorphisms in patients treated with risperidone. 60,67,73,74 PMs/IMs of CYP2D6 showed significantly higher plasma concentration of risperidone and risperidone/9-hydroxyrisperidone ratio than EMs in 2 studies focusing on Thai patients (P < 0.05). 73,74 Other results were summarized in Table 1.
Discussion
The aim of this systematic review was to identify and review publications investigating the association between selected gene variants and treatment outcomes (e.g., treatment response and adverse effects) in individuals with ASD/ID and to review the clinical validity and utility of PGx. To achieve this aim, we included each identifiable study of individuals with ASD/ID regardless of age in our research. We found that although there were several PGx studies in children/adolescents with ASD, there were only very limited studies reported in adults with ASD/ID while not a single study focused exclusively on adults.
Similar to a recent review on PGx studies in ASD by Brown, 45 several gene variants associated with treatment response, and adverse effects of antipsychotics and antidepressants were reported exclusively in children/adolescents with ASD/ID. However, the number of PGx studies in ASD/ID across age groups, especially in adults with ASD/ID, was still very few. Also, it should be kept in mind that identified studies had relatively limited sample sizes and a variety of ethnicities and study designs. Furthermore, almost all studies applied a classic “candidate gene” approach. No genome-wide association studies were reported while there were 1 study investigating exonic expression levels using Affymetrix GeneChip Human Exon 1.0 ST Arrays (Affymetrix, Santa Clara, CA) 57 and 2 studies investigating several genetic variants in drug-metabolizing enzyme and transporter (DMET) genes using Affymetrix DMET arrays (Affymetrix Inc., Santa Clara, CA). 56,60
The majority of studies identified in this review have examined PGx associations with treatment response and/or adverse effects in patients treated with risperidone. The possible reasons for this finding are as follows: (1) Risperidone is one of the FDA-approved drugs for the treatment of challenging behavior in children/adolescents with ASD, 77 and (2) risperidone is similarly one of the most commonly studied medications for challenging behavior (i.e., repetitive, self-injurious, and aggressive behaviors) in adults with ASD/ID. 78 Although the DRD3 rs6280 polymorphism was the most investigated gene variant in regard to treatment response to risperidone in the 3 studies, 51,54,62 the results were still inconclusive. This inconclusive finding is consistent with a recent systematic review on the association between dopamine receptor gene polymorphisms and treatment response to risperidone assessed using the Positive and Negative Syndrome Scale, Brief Psychiatric Rating Scale, or CGI in schizophrenia. 79 Likewise, the association between pharmacokinetic gene variants (e.g., CYP and ABCB1 gene variants) and response to risperidone also remains controversial in ASD/ID, which is consistent with mixed findings in a recent review in patients with schizophrenia. 80
In regard to adverse effects, prolactin elevation or hyperprolactinemia was the most investigated adverse effect as a primary outcome in patients treated with risperidone in 9 studies, 48,50,51,53,56,66,70,72,75 examining the impact of several gene variants (e.g., CYP2D6 polymorphisms and DRD2 rs1800497). Most of the studies examining the impact of CYP2D6 polymorphisms on prolactin elevation or hyperprolactinemia reported negative findings. However, most of them were based on a cross-sectional or observational study design in relatively small sample sizes (n = 40 to 147). In regard to the DRD2 rs1800497, a recent meta-analysis that included 772 patients with schizophrenia, ASD, or disruptive behavior disorder from 8 studies showed no significant difference between the DRD2 Taq1A (rs1800497) A1 carriers and non-A1 carriers in risperidone-related prolactin level (P = 0.423); 81 this meta-analysis included 5 studies identified in our review. 48,50,51,66,70
Similar to PGx studies on antipsychotics, published PGx studies using antidepressants (e.g., selective serotonin reuptake inhibitors [SSRI]) are still limited in individuals with ASD/ID across age groups. We identified only 6 studies with relatively small sample sizes (n = 19 to 279) for whom the majority of individuals were of European ancestry. 47,49,61,64,65,69 For instance, although 3 studies reported a significant association of the 5-HTTLPR with treatment response to antidepressants (escitalopram, sertraline, and fluvoxamine) assessed using the ABC-CV-IRR or CGI, the small sample size in each study is a major limitation in each study (N = 58, 51, and 18, respectively). 47,64,69 It is of interest that this finding in individuals with ASD/ID is consistent with a meta-analysis of the association between 5-HTTLPR and treatment response to SSRIs (i.e., remission and response rates) in patients with major depressive disorder (MDD) and bipolar disorder (28 studies and 3,866 subjects). 82 In line with the findings of this meta-analysis, 82 the associations between 5-HTTLPR and treatment response to SSRIs were reported in patients with anxiety disorder in several studies although the findings were mixed. 83 However, it should be kept in mind that treatment response is assessed by depression and anxiety scales but not the ABC or CGI in the studies in patients with mood disorders and anxiety disorder. 82,83 Likewise, the findings of the association between CYP2C19 gene variants and response to antidepressants are mixed in ASD/ID, which is in line with previous studies showing inconsistent linking the CYP2D6 and CYP2C19 gene variants to antidepressant treatment response in patients with MDD. 84,85 Although antipsychotics are the most commonly prescribed medications in adults with ID in the United States, 24 a population-based study in UK (N = 33,016) reported the most common class of drugs to be prescribed was anxiolytics/hypnotics, followed by antidepressants in adults with ID between 1999 and 2013 and that the incidence rate of new antidepressants over the follow-up period was approximately 350 per 10,000 person years in 2013. 15 Nevertheless, the evidence for antidepressant use in individuals with ID across all age groups is sparse, and generally, low response rates and high rates of adverse events have been reported. 86 Although studies in ASD suggested that SSRIs might be better tolerated in adults than in children, most studies of SSRIs in individuals with ID included both adults and children/adolescents, and age-specific data were still limited. 86 Therefore, further investigation on PGx studies on antidepressants as well as antipsychotics is warranted.
While specific PGx guidelines have not been established for individuals with ASD/ID, several established PGx guidelines provided useful gene–drug information for medications used in ASD/ID across age groups. 87,88 For instance, the Clinical Pharmacogenetics Implementation Consortium Dosing Guidelines recommend SSRI dosing adjustment based on the metabolizer status of the CYP2C19 for citalopram, escitalopram, and sertraline and the CYP2D6 for fluvoxamine. 87 Likewise, the Royal Dutch Association for the Advancement of Pharmacy–Pharmacogenetics Working Group has also recommended the dosing adjustment of antipsychotics based on the CYP2D6 genotypes for 6 antipsychotics: aripiprazole, brexpiprazole, clozapine, haloperidol, olanzapine, risperidone, and zuclopenthixol. 89 These guidelines are also well summarized on the Pharmacogenomics Knowledgebase website, 88 which also provides levels of evidence for gene–drug associations. Given that those gene–drug pairs should be also relevant in ASD/ID across all age groups as regardless of diagnoses, minimal or excessive serum/plasma levels of antipsychotics and antidepressants are likely to affect treatment response and side effects in this population.
To the best of our knowledge, this is the first systematic review of PGx studies with psychotropic drugs including antipsychotics and antidepressants (i.e., treatment response, adverse effects, and drug concentrations) in adults and children/adolescents with ASD/ID. Nevertheless, the results of our study must be interpreted with caution, given several limitations. First, on a systematic level, despite that articles were systematically investigated through MEDLINE, Embase, and PsycINFO, some references may still have been missed, nonsignificant findings might not have been published (i.e. “publication bias”), and only articles written in English were included in this review. Second, although we included some studies that might have partly included adults with ASD/ID, the actual number of the adults included in each study was unclear. Third, although several studies reported significant associations between several gene variants and treatment outcomes (e.g., treatment response to antipsychotics and antidepressants, and adverse effects) prior to multiple testing, there were few studies that reported gene variants surviving correction for multiple testing (e.g., Nurmi et al. 63 ).
In conclusion, there is a limited number of PGx studies in individuals with ASD/ID in particular in adults. Given that psychotropic medication use increases with age, 22,23 accumulating evidence on PGx studies and further investigation focusing on the clinical validity and efficacy of PGx testing for psychotropic treatment in individuals with ASD/ID across age groups are warranted.
Footnotes
Author Contributions: These authors Pushpal Desarkar and Daniel J. Müller equally contributed to this work. Kazunari Yoshida and Emiko Koyama did the literature search, extracted the data, and wrote the first draft of the manuscript. All authors interpreted the data, wrote the report, and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Pushpal Desarkar is currently supported by Scottish Rite Charitable Foundation of Canada Research Grant, CAMH Discovery Fund, and the Academic Scholars Award from the Department of Psychiatry, University of Toronto. Kazunari Yoshida has received manuscript fees from Sumitomo Dainippon Pharma, fellowship grants from the Japan Research Foundation for Clinical Pharmacology and Azrieli Adult Neurodevelopmental Centre Postdoctoral Fellowship at CAMH, and consultant fees from Signant Health and VeraSci within the past 3 years. Emiko Koyama has received fellowship grants from the Discovery Fund Postdoctoral Fellowship at CAMH within the past 3 years. James L. Kennedy is a member of the Scientific Advisory Board of Myriad Neuroscience (unpaid) and holds several patents relating to pharmacogenetic tests for psychiatric medications. Daniel J. Müller was funded by the Canadian Institutes of Health Research (CIHR) and holds the Joanne Murphy Chair at CAMH. The other authors have nothing to disclose.
ORCID iDs: Kazunari Yoshida https://orcid.org/0000-0002-1965-9484
Yona Lunsky https://orcid.org/0000-0002-1866-9728
References
- 1. McKenzie K, Milton M, Smith G, Ouellette-Kuntz H. Systematic review of the prevalence and incidence of intellectual disabilities: current trends and issues. Curr Dev Disord Rep. 2016;3(2):104–115. [Google Scholar]
- 2. Christensen DL, Baio J, Van Naarden Braun K, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45(3):601–613. [DOI] [PubMed] [Google Scholar]
- 4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington (DC): American Psychiatric Publishing; 2013. [Google Scholar]
- 5. Gurney JG, McPheeters ML, Davis MM. Parental report of health conditions and health care use among children with and without autism: national survey of children’s health. Arch Pediatr Adolesc Med. 2006;160(8):825–830. [DOI] [PubMed] [Google Scholar]
- 6. Dekker MC, Koot HM, van der Ende J, Verhulst FC. Emotional and behavioral problems in children and adolescents with and without intellectual disability. J Child Psychol Psychiatry. 2002;43(8):1087–1098. [DOI] [PubMed] [Google Scholar]
- 7. Tsakanikos E, Costello H, Holt G, Sturmey P, Bouras N. Behaviour management problems as predictors of psychotropic medication and use of psychiatric services in adults with autism. J Autism Dev Disord. 2007;37(6):1080–1085. [DOI] [PubMed] [Google Scholar]
- 8. Cooper SA, Smiley E, Morrison J, Williamson A, Allan L. Mental ill-health in adults with intellectual disabilities: prevalence and associated factors. Br J Psychiatry. 2007;190(1):27–35. [DOI] [PubMed] [Google Scholar]
- 9. Crocker AG, Mercier C, Lachapelle Y, Brunet A, Morin D, Roy ME. Prevalence and types of aggressive behaviour among adults with intellectual disabilities. J Intellect Disabil Res. 2006;50(Pt 9):652–661. [DOI] [PubMed] [Google Scholar]
- 10. Mazza MG, Rossetti A, Crespi G, Clerici M. Prevalence of co-occurring psychiatric disorders in adults and adolescents with intellectual disability: a systematic review and meta-analysis. J Appl Res Intellect Disabil. 2020;33(2):126–138. [DOI] [PubMed] [Google Scholar]
- 11. Abdallah MW, Greaves-Lord K, Grove J, Nørgaard-Pedersen B, Hougaard DM, Mortensen EL. Psychiatric comorbidities in autism spectrum disorders: findings from a Danish Historic Birth Cohort. Eur Child Adolesc Psychiatry. 2011;20(11–12):599–601. [DOI] [PubMed] [Google Scholar]
- 12. Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):921–929. [DOI] [PubMed] [Google Scholar]
- 13. Lunsky Y, Khuu W, Tadrous M, Vigod S, Cobigo V, Gomes T. . Antipsychotic use with and without comorbid psychiatric diagnosis among adults with intellectual and developmental disabilities. Can J Psychiatry. 2018;63(6):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Kuijper GM, Hoekstra PJ. Physicians’ reasons not to discontinue long-term used off-label antipsychotic drugs in people with intellectual disability. J Intellect Disabil Res. 2017;61(10):899–908. [DOI] [PubMed] [Google Scholar]
- 15. Sheehan R, Hassiotis A, Walters K, Osborn D, Strydom A, Horsfall L. Mental illness, challenging behaviour, and psychotropic drug prescribing in people with intellectual disability: UK population based cohort study. BMJ. 2015;351:h4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deb S, Unwin G, Deb T. Characteristics and the trajectory of psychotropic medication use in general and antipsychotics in particular among adults with an intellectual disability who exhibit aggressive behaviour. J Intellect Disabil Res. 2015;59(1):11–25. [DOI] [PubMed] [Google Scholar]
- 17. Hsu SW, Chiang PH, Chang YC, Lin JD, Tung HJ, Chen CY. Trends in the use of psychotropic drugs in people with intellectual disability in Taiwan: a nationwide outpatient service study, 1997-2007. Res Dev Disabil. 2014;35(2):364–372. [DOI] [PubMed] [Google Scholar]
- 18. Tsiouris JA, Kim SY, Brown WT, Pettinger J, Cohen IL. Prevalence of psychotropic drug use in adults with intellectual disability: positive and negative findings from a large scale study. J Autism Dev Disord. 2013;43(3):719–731. [DOI] [PubMed] [Google Scholar]
- 19. Coury DL, Anagnostou E, Manning-Courtney P, et al. Use of psychotropic medication in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130 (Suppl. 2):S69–S76. [DOI] [PubMed] [Google Scholar]
- 20. Matson JL, Neal D. Psychotropic medication use for challenging behaviors in persons with intellectual disabilities: an overview. Res Dev Disabil. 2009;30(3):572–586. [DOI] [PubMed] [Google Scholar]
- 21. Holden B, Gitlesen JP. Psychotropic medication in adults with mental retardation: prevalence, and prescription practices. Res Dev Disabil 2004;25(6):509–521. [DOI] [PubMed] [Google Scholar]
- 22. Stortz JN, Lake JK, Cobigo V, Ouellette-Kuntz HM, Lunsky Y. Lessons learned from our elders: how to study polypharmacy in populations with intellectual and developmental disabilities. Intellect Dev Disabil. 2014;52(1):60–77. [DOI] [PubMed] [Google Scholar]
- 23. Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord. 2009;39(9):1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Esler A, Hewitt A, Hall-Lande J, Pettingell SL, Houseworth J. Psychotropic medication use for adults with autism spectrum disorder who receive services and supports through adult developmental disability services in the United States. J Autism Dev Disord. 2019;49(6):2291–2303. [DOI] [PubMed] [Google Scholar]
- 25. Jobski K, Höfer J, Hoffmann F, Bachmann C. Use of psychotropic drugs in patients with autism spectrum disorders: a systematic review. Acta Psychiatr Scand. 2017;135(1):8–28. [DOI] [PubMed] [Google Scholar]
- 26. Spencer D, Marshall J, Post B, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doan J, Zakrzewski-Jakubiak H, Roy J, Turgeon J, Tannenbaum C. Prevalence and risk of potential cytochrome P450-mediated drug-drug interactions in older hospitalized patients with polypharmacy. Ann Pharmacother. 2013;47(3):324–332. [DOI] [PubMed] [Google Scholar]
- 29. Sheehan R, Horsfall L, Strydom A, Osborn D, Walters K, Hassiotis A. Movement side effects of antipsychotic drugs in adults with and without intellectual disability: UK population-based cohort study. BMJ Open. 2017;7(8):e017406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bousman CA, Zierhut H, Müller DJ. Navigating the labyrinth of pharmacogenetic testing: a guide to test selection. Clin Pharmacol Ther. 2019;106(2):309–312. [DOI] [PubMed] [Google Scholar]
- 31. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cacabelos R, Hashimoto R, Takeda M. Pharmacogenomics of antipsychotics efficacy for schizophrenia. Psychiatry Clin Neurosci. 2011;65(1):3–19. [DOI] [PubMed] [Google Scholar]
- 33. Kim K, Magness JW, Nelson R, Baron V, Brixner DI. Clinical utility of pharmacogenetic testing and a clinical decision support tool to enhance the identification of drug therapy problems through medication therapy management in polypharmacy patients. J Manag Care Spec Pharm. 2018;24(12):1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elliott LS, Henderson JC, Neradilek MB, Moyer NA, Ashcraft KC, Thirumaran RK. Clinical impact of pharmacogenetic profiling with a clinical decision support tool in polypharmacy home health patients: a prospective pilot randomized controlled trial. PLoS One. 2017;12(2):e0170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brixner D, Biltaji E, Bress A, et al. The effect of pharmacogenetic profiling with a clinical decision support tool on healthcare resource utilization and estimated costs in the elderly exposed to polypharmacy. J Med Econ. 2016;19(3):213–228. [DOI] [PubMed] [Google Scholar]
- 36. Arranz MJ, Gonzalez-Rodriguez A, Perez-Blanco J, et al. A pharmacogenetic intervention for the improvement of the safety profile of antipsychotic treatments. Transl Psychiatry. 2019;9(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bousman CA, Arandjelovic K, Mancuso SG, Eyre HA, Dunlop BW. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37–47. [DOI] [PubMed] [Google Scholar]
- 38. Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111:59–67. [DOI] [PubMed] [Google Scholar]
- 39. Bousman C, Maruf AA, Müller DJ. Towards the integration of pharmacogenetics in psychiatry: a minimum, evidence-based genetic testing panel. Curr Opin Psychiatry. 2019;32(1):7–15. [DOI] [PubMed] [Google Scholar]
- 40. Jukic MM, Smith RL, Haslemo T, Molden E, Ingelman-Sundberg M. Effect of CYP2D6 genotype on exposure and efficacy of risperidone and aripiprazole: a retrospective, cohort study. Lancet Psychiatry. 2019;6(5):418–426. [DOI] [PubMed] [Google Scholar]
- 41. Jukić MM, Haslemo T, Molden E, Ingelman-Sundberg M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: a retrospective study based on 2,087 patients. Am J Psychiatry. 2018;175(5):463–470. [DOI] [PubMed] [Google Scholar]
- 42. Hicks JK, Sangkuhl K, Swen JJ, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hicks JK, Dunnenberger HM, Gumpper KF, Haidar CE, Hoffman JM. Integrating pharmacogenomics into electronic health records with clinical decision support. Am J Health Syst Pharm. 2016;73(23):1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leckband SG, Kelsoe JR, Dunnenberger HM, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and carbamazepine dosing. Clin Pharmacol Ther. 2013;94(3):324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brown JT, Eum S, Cook EH, Bishop JR. Pharmacogenomics of autism spectrum disorder. Pharmacogenomics. 2017;18(4):403–414. [DOI] [PubMed] [Google Scholar]
- 46. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(Jul 21 1):b2535–b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. AlOlaby RR, Sweha SR, Silva M, et al. Molecular biomarkers predictive of sertraline treatment response in young children with fragile X syndrome. Brain Dev. 2017;39(6):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anderson GM, Scahill L, McCracken JT, et al. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61(4):545–550. [DOI] [PubMed] [Google Scholar]
- 49. Bishop JR, Najjar F, Rubin LH, et al. Escitalopram pharmacogenetics: CYP2C19 relationships with dosing and clinical outcomes in autism spectrum disorder. Pharmacogenet Genomics. 2015;25(11):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Calarge CA, Ellingrod VL, Zimmerman B, Acion L, Sivitz WI, Schlechte JA. Leptin gene -2548G/A variants predict risperidone-associated weight gain in children and adolescents. Psychiatr Genet. 2009;19(6):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Correia CT, Almeida JP, Santos PE, et al. Pharmacogenetics of risperidone therapy in autism: association analysis of eight candidate genes with drug efficacy and adverse drug reactions. Pharmacogenomics J. 2010;10(5):418–430. [DOI] [PubMed] [Google Scholar]
- 52. Cote AT, Panagiotopoulos C, Devlin AM. Interaction between the Val158Met catechol-O-methyltransferase gene variant and second-generation antipsychotic treatment on blood pressure in children. Pharmacogenomics J. 2015;15(1):95–100. [DOI] [PubMed] [Google Scholar]
- 53. dos Santos Júnior A, Henriques TB, de Mello MP, et al. Hyperprolactinemia in children and adolescents with use of risperidone: clinical and molecular genetics aspects. J Child Adolesc Psychopharmacol. 2015;25(10):738–748. [DOI] [PubMed] [Google Scholar]
- 54. Firouzabadi N, Nazariat A, Zomorrodian K. DRD3 Ser9Gly polymorphism and its influence on risperidone response in autistic children. J Pharm Pharm Sci. 2017;20(1):445–452. [DOI] [PubMed] [Google Scholar]
- 55. Hoekstra PJ, Troost PW, Lahuis BE, et al. Risperidone-induced weight gain in referred children with autism spectrum disorders is associated with a common polymorphism in the 5-hydroxytryptamine 2C receptor gene. J Child Adolesc Psychopharmacol. 2010;20(6):473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hongkaew Y, Medhasi S, Pasomsub E, et al. UGT1A1 polymorphisms associated with prolactin response in risperidone-treated children and adolescents with autism spectrum disorder. Pharmacogenomics J. 2018;18(6):740–748. [DOI] [PubMed] [Google Scholar]
- 57. Lit L, Sharp FR, Bertoglio K, et al. Gene expression in blood is associated with risperidone response in children with autism spectrum disorders. Pharmacogenomics J. 2012;12(5):368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McCracken JT, Aman MG, McDougle CJ, et al. Possible influence of variant of the P-glycoprotein gene (MDR1/ABCB1) on clinical response to guanfacine in children with pervasive developmental disorders and hyperactivity. J Child Adolesc Psychopharmacol. 2010;20(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCracken JT, Badashova KK, Posey DJ, et al. Positive effects of methylphenidate on hyperactivity are moderated by monoaminergic gene variants in children with autism spectrum disorders. Pharmacogenomics J. 2014;14(3):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Medhasi S, Pinthong D, Pasomsub E, et al. Pharmacogenomic study reveals new variants of drug metabolizing enzyme and transporter genes associated with steady-state plasma concentrations of risperidone and 9-hydroxyrisperidone in Thai autism spectrum disorder patients. Front Pharmacol. 2016;7:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Najjar F, Owley T, Mosconi MW, et al. Pharmacogenetic study of serotonin transporter and 5HT2A genotypes in autism. J Child Adolesc Psychopharmacol. 2015;25(6):467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nuntamool N, Ngamsamut N, Vanwong N, et al. Pharmacogenomics and efficacy of risperidone long-term treatment in Thai autistic children and adolescents. Basic Clin Pharmacol Toxicol. 2017;121(4):316–324. [DOI] [PubMed] [Google Scholar]
- 63. Nurmi EL, Spilman SL, Whelan F, et al. Moderation of antipsychotic-induced weight gain by energy balance gene variants in the RUPP autism network risperidone studies. Transl Psychiatry. 2013;3(6):e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Owley T, Brune CW, Salt J, et al. A pharmacogenetic study of escitalopram in autism spectrum disorders. Autism Res. 2010;3(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Prows CA, Nick TG, Saldaña SN, et al. Drug-metabolizing enzyme genotypes and aggressive behavior treatment response in hospitalized pediatric psychiatric patients. J Child Adolesc Psychopharmacol. 2009;19(4):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roke Y, van Harten PN, Franke B, Galesloot TE, Boot AM, Buitelaar JK. The effect of the Taq1A variant in the dopamine D2 receptor gene and common CYP2D6 alleles on prolactin levels in risperidone-treated boys. Pharmacogenet Genomics. 2013;23(9):487–493. [DOI] [PubMed] [Google Scholar]
- 67. Sherwin CMT, Saldaña SN, Bies RR, Aman MG, Vinks AA. Population pharmacokinetic modeling of risperidone and 9-hydroxyrisperidone to estimate CYP2D6 subpopulations in children and adolescents. Ther Drug Monit. 2012;34(5):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aman MG, Vinks AA, Remmerie B, et al. Plasma pharmacokinetic characteristics of risperidone and their relationship to saliva concentrations in children with psychiatric or neurodevelopmental disorders. Clin Ther. 2007;29(7):1476–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sugie Y, Sugie H, Fukuda T, et al. Clinical efficacy of fluvoxamine and functional polymorphism in a serotonin transporter gene on childhood autism. J Autism Dev Disord. 2005;35(3):377–385. [DOI] [PubMed] [Google Scholar]
- 70. Sukasem C, Hongkaew Y, Ngamsamut N, et al. Impact of pharmacogenetic markers of CYP2D6 and DRD2 on prolactin response in risperidone-treated Thai children and adolescents with autism spectrum disorders. J Clin Psychopharmacol. 2016;36(2):141–146. [DOI] [PubMed] [Google Scholar]
- 71. Sukasem C, Vanwong N, Srisawasdi P, et al. Pharmacogenetics of risperidone-induced insulin resistance in children and adolescents with autism spectrum disorder. Basic Clin Pharmacol Toxicol. 2018;123(1):42–50. [DOI] [PubMed] [Google Scholar]
- 72. Troost PW, Lahuis BE, Hermans MH, et al. Prolactin release in children treated with risperidone: impact and role of CYP2D6 metabolism. J Clin Psychopharmacol. 2007;27(1):52–57. [DOI] [PubMed] [Google Scholar]
- 73. Vanwong N, Ngamsamut N, Hongkaew Y, et al. Detection of CYP2D6 polymorphism using Luminex xTAG technology in autism spectrum disorder: CYP2D6 activity score and its association with risperidone levels. Drug Metab Pharmacokinet. 2016;31(2):156–162. [DOI] [PubMed] [Google Scholar]
- 74. Vanwong N, Ngamsamut N, Medhasi S, et al. Impact of CYP2D6 polymorphism on steady-state plasma levels of risperidone and 9-hydroxyrisperidone in Thai children and adolescents with autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27(2):185–191. [DOI] [PubMed] [Google Scholar]
- 75. Youngster I, Zachor DA, Gabis LV, et al. CYP2D6 genotyping in paediatric patients with autism treated with risperidone: a preliminary cohort study. Dev Med Child Neurol. 2014;56(10):990–994. [DOI] [PubMed] [Google Scholar]
- 76. McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347(5):314–321. [DOI] [PubMed] [Google Scholar]
- 77. Xiong W. Pediatric pharmacologic management of autism-associated behavioral dysregulation. Am J Psychiatry Resid J. 2017;12(9):3–5. [Google Scholar]
- 78. Sawyer A, Lake JK, Lunsky Y, Liu SK, Desarkar P. Psychopharmacological treatment of challenging behaviours in adults with autism and intellectual disabilities: a systematic review. Res Autism Spectr Disord. 2014;8(7):803–813. [Google Scholar]
- 79. Ma L, Zhang X, Xiang Q, et al. Association between dopamine receptor gene polymorphisms and effects of risperidone treatment: a systematic review and meta-analysis. Basic Clin Pharmacol Toxicol. 2019;124(1):94–104. [DOI] [PubMed] [Google Scholar]
- 80. Yoshida K, Müller DJ. Pharmacogenetics of antipsychotic drug treatment: update and clinical implications. Mol Neuropsychiatry. 2018;5(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ma L, Xiang Q, Zhou S, et al. Association of dopamine D2 receptor gene polymorphisms with prolactin levels related to risperidone treatment: a systematic review and meta-analysis. J Clin Pharm Ther. 2019;44(4):543–552. [DOI] [PubMed] [Google Scholar]
- 82. Lueken U, Zierhut KC, Hahn T, et al. Neurobiological markers predicting treatment response in anxiety disorders: a systematic review and implications for clinical application. Neurosci Biobehav Rev. 2016;66:143–162. [DOI] [PubMed] [Google Scholar]
- 83. Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol. 2012;22(4):239–258. [DOI] [PubMed] [Google Scholar]
- 84. Solomon HV, Cates KW, Li KJ. Does obtaining CYP2D6 and CYP2C19 pharmacogenetic testing predict antidepressant response or adverse drug reactions? Psychiatry Res. 2019;271:604–613. [DOI] [PubMed] [Google Scholar]
- 85. Müller DJ, Kekin I, Kao ACC, Brandl EJ. Towards the implementation of CYP2D6 and CYP2C19 genotypes in clinical practice: update and report from a pharmacogenetic service clinic. Int Rev Psychiatry. 2013;25(5):554–571. [DOI] [PubMed] [Google Scholar]
- 86. Ji NY, Findling RL. Pharmacotherapy for mental health problems in people with intellectual disability. Curr Opin Psychiatry. 2016;29(2):103–125. [DOI] [PubMed] [Google Scholar]
- 87. Clinical Pharmacogenetics Implementation Consortium (CPIC). Genes-drugs. [accessed 2020 Mar 10]. https://cpicpgx.org/genes-drugs/
- 88. PharmGKB. [accessed 2020 Mar 10]. https://www.pharmgkb.org/
- 89. Royal Dutch association for the advancement of pharmacy–pharmacogenetics working group (DPWG). dosing guidelines. [accessed 2020 Mar 10]. https://www.pharmgkb.org/view/dosing-guidelines.do?source=DPWG#/