Abstract
A plethora of neurological manifestations are associated with the 2019 coronavirus infectious disease (COVID-19). We hereby report the first case of a patient infected with SARS-CoV-2 who acutely presented with autonomic dysfunction preceding the onset of complete clinical picture of Miller Fisher syndrome. She was finally diagnosed to be a case of anti-ganglioside antibody positive post-COVID-19 Miller Fisher syndrome with dysautonomia and treated with intravenous immunoglobulin with an excellent response. We also discuss the plausible pathogenic mechanisms of COVID-19 induced Miller Fisher syndrome and furnish a review of the post-COVID-19 Miller Fisher syndrome cases reported.
Keywords: Autonomic dysfunction, COVID-19, Miller Fisher syndrome (MFS), SARS-CoV-2
Introduction
A plethora of neurological manifestations are associated with the 2019 coronavirus infectious disease (COVID-19). 1 Apart from central nervous system manifestations, Guillain–Barré syndrome and its variants are considered as the most common peripheral nervous system disorders in COVID-19.2-4 Dysautonomia has also been reported as an emerging complication of COVID-19. 4 Amidst the spectrum of Guillain–Barré syndrome, only a few cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induced Miller Fisher syndrome (MFS) have been reported.3,5-14 On the other hand, autonomic dysfunction preceding the development of full blown MFS is exceedingly rare, 15 unlike other variants of Guillain–Barré syndrome.
We hereby report a unique case of a patient infected with SARS-CoV-2 who acutely presented with autonomic dysfunction. She was finally diagnosed to be a case of anti-ganglioside antibody positive post-COVID-19 MFS with dysautonomia and treated with intravenous immunoglobulin with an excellent response. We have also discussed the plausible pathogenic mechanisms of COVID-19 induced MFS and furnish a review of literature of the post-COVID-19 MFS cases reported.
Case Presentation
A 55-year-old woman presented to the emergency department with history of intermittent extreme dizziness, excessive dryness of mouth, squeezing sensation in central chest, recent-onset constipation and excessive sweating, for last six days. This was followed by sudden onset unsteadiness of gait, double vision, and “pins and needles” sensation over bilateral fingertips, distal legs and circumoral areas. Her past medical history was unremarkable except that 15 days prior to admission, she had suffered from COVID-19, but as the symptoms had been mild, she was advised to stay in vigilant home isolation without any specific therapy. General systemic examination was remarkable for slight nasal intonation, tachycardia (108 bpm) and arterial hypertension (supine blood pressure was 178/92 mm of Hg) and postural hypotension (blood pressure after standing from supine position for 3 minutes was 136/74 mm of Hg and heart rate was 114 bpm). Respiratory rate and room-air oxygen saturation were normal. Emergency electrocardiographic monitoring revealed absence of respiratory sinus arrhythmia without any signs of ischemic changes or high degree atrio-ventricular block. Urgent cardiac troponins tested by kit were negative. Neurological examination revealed normal cognitive functions, hyposmia, bilateral symmetrical abducens palsy, generalized areflexia, broad-based ataxic gait, and autonomic dysfunction as demonstrated by Valsalva manoeuvre (Valsalva ratio <1.2), absence of reflex blood pressure changes, abnormally decreased heart rate variability with respiration (5 bpm) and posture change (30:15 ratio <1), inadequate rise of blood pressure, nearly fixed heart rate during isometric handgrip exercise and abnormal bedside thermoregulatory sweat testing. Motor strength, sensory system and most of cerebellar functions (except broad-based ataxic gait) were normal. Visual acuity, pupillary reflexes, color perception, intraocular pressures and fundoscopic examination were otherwise normal. No ptosis, orbicularis weakness or diurnal variations were noted. Signs of meningeal irritation were absent.
Complete blood cell count revealed neutrophilic leucocytosis and lymphopenia. Thyroid, hepatic and renal function tests were normal. Pre-prandial and post-prandial plasma glucose levels were also normal. Serologies for hepatitis B, C, HIV (1, 2), Campylobacter jejuni, Cytomegalovirus, Haemophilus influenzae and Epstein Barr virus were all negative. Cerebrospinal fluid analysis revealed albumino-cytologic dissociation (white blood cell count = 3/µl and protein = 245 mg/dl). Serum ganglioside antibody profile by immunoblot method revealed positive results for anti-GQ-1b-IgG and anti-GD-1b-IgG. Gadolinium-enhanced magnetic resonance imaging (MRI) of the brain and spinal cord as well as computed tomography scan of the thorax revealed no abnormalities. The electromyography study of both abductor pollicis brevis, adductor digiti minimi, first dorsal interosseous, extensor digitorum communis, biceps brachii, triceps brachii, deltoids, extensor digitorum brevis, tibialis anterior, medial gastrocnemius, rectus femoris and vastus medialis was normal. Sensory and motor nerve conduction studies, F waves, and H reflexes were otherwise normal. In addition, on repetitive stimulation, no decremental or incremental response was observed.
Based on the Brighton criteria, 16 the diagnosis of MFS was established with level 1 of diagnostic certainty. She was treated with intravenous immunoglobulin 0.4 g/kg/day for five days on the third day of hospital stay. The cranial neuropathies and the ataxia improved significantly over the succeeding days and she was discharged home three weeks after admission, with a resolution of the neurological features, except generalized hyporeflexia.
Discussion
Modern medicine is facing the evolution of COVID-19 over one year and has acquainted with several neurological conditions involving the entire cranio-spinal axis, 1 caused by a primary respiratory pathogen. Spectrum of neurological disorders encompass central,1,17 peripheral2-4 and autonomic nervous systems. 4 To date, nearly 80 cases of Guillain-Barré syndrome spectrum have been reported. 2 Among these, MFS has been expectedly reported on rare occasions (Table 1).3,5-14 In the six out of 12 reported cases, including the current one, the neurological symptoms started two weeks after the onset of first symptoms of COVID-19 and in the remaining, the gap was typically less than five days.3,5-13 Most the patients responded favourably to intravenous immunoglobulin with minimal or no persisting neurological deficits.3,5-13 Only one patient died unexpectedly. 14 Classical albumino-cytological dissociation in cerebrospinal fluid analysis was found in seven out of 12 cases, meanwhile in the rest of them, three had normal findings and, in two cases, it was not reported.3,5-14 Contrast-enhanced neuroimaging was available in 10 out of 12 cases;5,6,8-11,13,14 in three patients, cranial, peripheral nerve root or radicular thickening, suggestive of leptomeningeal invasion by the virus or immune mediated radiculitis, was found.5,9,10 Search for viral RNA in cerebrospinal fluid was reported only in five out of 12 cases and those conveyed negative results,3,11,12,14 but one which was positive. 13 Eleven out of 12 patients were tested for anti-ganglioside antibodies,3,5,6,8-14 of whom five had a positive result, including the current case.3,5,10,13
Table 1.
Clinical Profile of Reported Patients With Post-COVID-19 Miller Fisher Syndrome.
| Author(s) | Age/sex | Neurological findings | Gap from the COVID-19 onset (in days) | EMG/Nerve conduction studies | Neuroimaging | SARS-CoV-2 in cerebrospinal fluid | Anti-ganglioside antibody testing | Specific therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 50/M | Anosmia, ageusia, internuclear ophthalmoparesis, right fascicular oculomotor palsy ataxia and areflexia | 5 | Not reported | Normal unenhanced CT scan of the brain | Negative | Positive (anti-GD1b–IgG) | Intravenous immunoglobulin | Resolution within two weeks |
| 5 | 36/M | Left eye drooping, blurry vision, reduced sensation, paresthesia in both legs to all modalities below knee level, partial left third nerve, bilateral sixth nerve palsy and areflexia | 4 | Not reported | Enlargement and T2 hyperintensity, and enhancement of the affected third cranial nerve from the cavernous sinus through the orbit on MRI | Not reported | Positive | Intravenous immunoglobulin | Resolution after one week |
| 6 | 51/F | Intense root-type pain in all four limbs, dorsal and lumbar back pain, weakness in her lower limbs, double binocular vision (left sixth cranial nerve palsy), bilateral facial paresis, symmetrical paraparesis, global areflexia and autonomic dysfunction | 15 | Guillain-Barre type demyelinating polyneuropathy | Normal | Not reported | Negative | Intravenous immunoglobulin | Progressive improvement |
| 7 | 63/M | Perioral and fingertips paresthesias, diplopia, divergent squint, areflexia and ataxic gait | 1 | Not reported | Not reported | Not reported | Not reported | Intravenous immunoglobulin | Self-limiting illness |
| 8 | 50/F | Ataxia, ophthalmoplegia, left upper arm cerebellar dysmetria, generalized areflexia, mild lower facial defects and mild hypoesthesia over face | 16 | Not reported | Normal | Not reported | Negative | Intravenous immunoglobulin | Complete resolution within 14 days |
| 9 | 36/M | Partial left oculomotor palsy, bilateral abducens palsies, lower limbs hyporeflexia, hypoesthesia and gait ataxia | 4 | Not reported | Enhancement and T2-hyperintensity and enlargement of the left oculomotor nerve on MRI | Not reported | Negative | Intravenous immunoglobulin | Partially complete resolution |
| 10 | 45/M | Ataxia, ophthalmoplegia, areflexia and tetraparesis | 16 | Not reported | Intrathecal cauda-equina enhancement on MRI | Not reported | Positive | Intravenous immunoglobulin | During report writing still needed respiratory support. Partially improvement of neurological picture |
| 11 | 74/F | Lower limbs areflexia with patent gait ataxia | 15 | Slight F-wave delay in upper limbs | Normal MRI | Negative | Negative | Intravenous immunoglobulin | Improved |
| 12 | 61/M | Ophthalmoplegia, generalized areflexia and sensory ataxia | 20 | F-wave was not detectable | Not reported | Negative | Negative | Intravenous immunoglobulin | Improved |
| 13 | 31/F | Ophthalmoplegia, ataxia, areflexia and dysmetria | 3 | Not reported | Normal | Positive | Positive | Convalescent plasma, tocilizumab and intravenous immunoglobulin | Mild improvement |
| 14 | 50/M | Dysmetria, dysdiadochokinesia and absent upper limbs reflexes | 1 | Not performed | Normal | Negative | Negative | Intravenous immunoglobulin | Some improvement, before developing ventricular arrhythmia and cardiac arrest unexpectedly. |
| Current case | 55/F | Dysautonomia, ataxia, ophthalmoplegia, areflexia, and paresthesias involving perioral areas and fingers/toes | 15 | Normal | Normal MRI | Not reported | Positive | Intravenous immunoglobulin | Complete resolution, except generalized hyporeflexia |
The pathogenesis of MFS following SARS-CoV-2 infection may be either mediated by neurotropism or by aberrant immune mediated injury, the latter being the most likely. Specifically, there are several pieces of evidence to support an aberrant immune mediated injury. First, the gap between the onset of first COVID-19 symptoms and the first neurological symptoms was in most cases more than two weeks, which may be suggestive of a post-infectious autoimmune process. Second, as seen in most cases of previously reported COVID-19 induced Guillain–Barré syndrome spectrum, 2 the cerebrospinal fluid was devoid of virus itself or its RNA, similarly to the cases of MFS.3,11,12,14 This suggests that there is no intrathecal viral replication or direct neuroinvasion. Third, spectrum of Guillain–Barré syndrome, that also includes MFS, is a prototype for post-infectious immune-mediated neuropathy with known infectious triggers (i.e., Campylobacter jejuni, enterovirus, hepatitis virus, Zika virus, Hemophilus influenzae, H1N1 virus, West Nile virus, SARS-CoV, and MERS-CoV, among others). 18 Fourth, in a few cases of post-COVID-19 MFS, antibodies against ganglioside comprising of disialosyl moiety (i.e., GQ-1b, GD-1b and GT-1b) were detected.3,5,10,13 Fifth, apart from ACE-2 receptors, there is robust evidence that SARS-CoV-2 also uses sialic-acid containing gangliosides/glycoproteins on cell surfaces. 19 Hence, cross-reactivity and molecular mimicry between SARS-CoV-2 antigenic (spike proteins) epitopes and carbohydrate moieties of surface cranial nerve glycoproteins may bring about immune-driven neuropathy in COVID-19 induced MFS. 19 Finally, last but not the least, dramatic response to intravenous immunoglobulin in all cases of MFS points towards an underlying immune driven process. On the other hand, direct neurotropism as a pathogenic process did not lie far behind. First, unlike rapidly reversible anosmia/hyposmia and ageusia/dysgeusia following viral infection to non-neural olfactory epithelial cells, in COVID-19 patients, anosmia/hyposmia tends to persist longer, implying this to be a result of viral neurotropism targeting olfactory neurons. 20 Second, gaining access to the olfactory bulb and later to the brainstem cranial nuclei may ensue in COVID-19 similarly to SARS-CoV infection in animal models. 20 Specifically, SARS-CoV can also enter the central nervous system via retrograde axonal transport through branches of trigeminal and glossopharyngeal nerves widely spread in oro-nasal cavity, corneal plexus and peripheral nerves. 20 The finding of enhancement and thickening of cranial nerve roots and peripheral nerve radicles in three cases of SARS-CoV-2 induced MFS further supports this notion.5,9,10 Theoretically, in the upcoming years, direct viral neurotropism might also catch attention as an additional pathogenic mechanism for SARS-CoV-2 induced MFS.
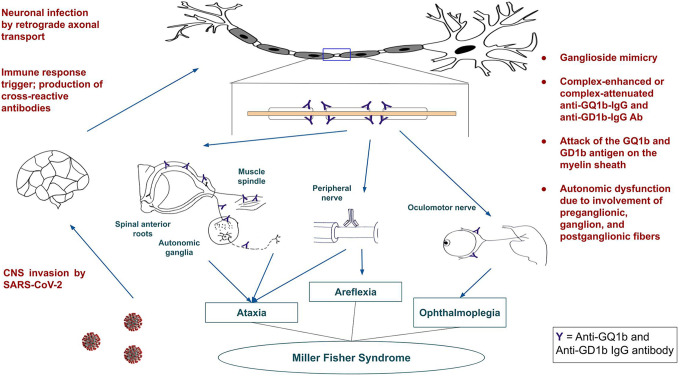
Figure 1 shows our proposed pathogenic pathway for the development of MFS in COVID-19. SARS-CoV-2 could gain entry to the central nervous system through retrograde axonal transport. Thereafter, it could affect the myelin sheath through ganglioside mimicry. The antibodies formed could be either complex-enhanced or complex-attenuated anti-GQ1b-IgG or anti-GD1b-IgG antibodies acting against the GQ1b and GD1b antigens in the myelin sheath. This in turn may cause the characteristic triad of areflexia, ataxia and ophthalmoplegia in MFS.
Figure 1.
Proposed pathogenic pathway for the development of Miller Fisher syndrome in COVID-19.
The main limitation of our case was that we could not search for viral RNA of SARS-CoV-2 in cerebrospinal fluid because of the extreme circumstances in our hospitals at this pandemic.
Conclusions
Surveillance is needed to detect and manage COVID-19 associated immune-driven disorders. For cases of likely SARS-CoV-2 induced MFS, anti-ganglioside antibodies, along with targeted high-resolution contrast enhancing MRI, should be done to muster more information regarding natural history and pathogenesis of the disease. To improve outcome, facilitate early recovery and lessen the hospital stay, intravenous immunoglobulin should be initiated as soon as possible. Our case adds to the tally of novel cases of post-SARS-CoV-2 infection related neurological manifestations as well as to the exceedingly rare list of cases in whom frank autonomic dysfunction preceded the fully developed clinical triad of MFS.
Supplemental Material
Supplemental Material, sj-pdf-1-nho-10.1177_19418744211016709 for COVID-19 Induced Miller Fisher Syndrome Presenting With Autonomic Dysfunction: A Unique Case Report and Review of Literature by Subhrajyoti Biswas, Ritwik Ghosh, Arpan Mandal, Alak Pandit, Dipayan Roy, Samya Sengupta, Kaustav De, Bikash Chandra Swaika and Julián Benito-León in The Neurohospitalist
Acknowledgments
Subhrajyoti Biswas, Ritwik Ghosh, Arpan Mandal, Alak Pandit, Dipayan Roy, Samya Sengupta, Kaustav De, and Bikash Chandra Swaika report no relevant disclosures. J. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967 -1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451).
Footnotes
Authors’ Note: Written informed consent was obtained from the patient participating in the study (consent for research). Informed written consent was obtained from the patient involvedin this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Ritwik Ghosh  https://orcid.org/0000-0002-8192-0807
https://orcid.org/0000-0002-8192-0807
Julián Benito-León  https://orcid.org/0000-0002-1769-4809
https://orcid.org/0000-0002-1769-4809
References
- 1.Roy D, Ghosh R, Dubey S, Dubey MJ, Benito-León J, Kanti Ray B. Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can J Neurol Sci. 2021;48(1):9–24. doi:10.1017/cjn.2020.173 Epub 2020 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2021;268(4):1133–1170. doi:10.1007/s00415-020-10124-x Epub 2020 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, et al. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi:10.1212/wnl.0000000000009619 [DOI] [PubMed] [Google Scholar]
- 4.Ghosh R, Roy D, Sengupta S, Benito-León J. Autonomic dysfunction heralding acute motor axonal neuropathy in COVID-19. J Neurovirol. 2020;26(6):964–966. doi:10.1007/s13365-020-00908-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantos JE, Strauss SB, Lin E. COVID-19-associated Miller Fisher syndrome: MRI findings. AJNR Am J Neuroradiol. 2020;41(7):1184–1186. doi:10.3174/ajnr.A6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyes-Bueno JA, García-Trujillo L, Urbaneja P, et al. Miller-Fisher syndrome after SARS-CoV-2 infection. Eur J Neurol. 2020;27(9):1759–1761. doi:10.1111/ene.14383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray A. Miller Fisher syndrome and COVID-19: is there a link? BMJ Case Rep. 2020;13(8):e236419. doi:10.1136/bcr-2020-236419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manganotti P, Pesavento V, Buoite Stella A, et al. Miller fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. J Neurovirol. 2020;26(4):605–606. doi:10.1007/s13365-020-00858-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinkin M, Gao V, Kahan J, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020;95(5):221–223. doi:10.1212/wnl.0000000000009700 [DOI] [PubMed] [Google Scholar]
- 10.Lowery MM, Taimur Malik M, Seemiller J, Tsai CS. Atypical variant of Guillain Barre syndrome in a patient with COVID-19. J Crit Care Med (Targu Mures). 2020;6(4):231–236. doi:10.2478/jccm-2020-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Domínguez J, Ameijide-Sanluis E, García-Cabo C, García-Rodríguez R, Mateos V. Miller-Fisher-like syndrome related to SARS-CoV-2 infection (COVID 19). J Neurol. 2020;267(9):2495–2496. doi:10.1007/s00415-020-09912-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senel M, Abu-Rumeileh S, Michel D, et al. Miller-Fisher syndrome after COVID-19: neurochemical markers as an early sign of nervous system involvement. Eur J Neurol. 2020;27(11):2378–2380. doi:10.1111/ene.14473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopscik MR, Giourgas BK, Presley BC. A case report of acute motor and sensory polyneuropathy as the presenting symptom of SARS-CoV-2. Clin Pract Cases Emerg Med. 2020;4(3):352–354. doi:10.5811/cpcem.2020.6.48683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajani S, Kajani R, Huang CW, Tran T, Liu AK. Miller Fisher syndrome in the COVID-19 Era - a novel target antigen calls for novel treatment. Cureus. 2021;13(1):e12424. doi:10.7759/cureus.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Mannan O, D’Argenzio L, Pitt M, et al. Two cases of Guillain-Barré syndrome variants presenting with dysautonomia. Child Neurol Open. 2019;6:2329048x19856778. doi:10.1177/2329048x19856778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137(Pt 1):33–43. doi:10.1093/brain/awt285 [DOI] [PubMed] [Google Scholar]
- 17.Ghosh R, Lahiri D, Dubey S, Ray BK, Benito-León J. Hallucinatory palinopsia in COVID-19-induced posterior reversible encephalopathy syndrome. J Neuroophthalmol. 2020;40(4):523–526. doi:10.1097/wno.0000000000001135 [DOI] [PubMed] [Google Scholar]
- 18.Gwathmey KG, Smith AG. Immune-mediated neuropathies. Neurol Clin. 2020;38(3):711–735. doi:10.1016/j.ncl.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 19.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55(5):105960. doi:10.1016/j.ijantimicag.2020.105960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi:10.3390/v12010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-nho-10.1177_19418744211016709 for COVID-19 Induced Miller Fisher Syndrome Presenting With Autonomic Dysfunction: A Unique Case Report and Review of Literature by Subhrajyoti Biswas, Ritwik Ghosh, Arpan Mandal, Alak Pandit, Dipayan Roy, Samya Sengupta, Kaustav De, Bikash Chandra Swaika and Julián Benito-León in The Neurohospitalist