Abstract
Scrub typhus, an acute febrile infectious disease prevalent in the ‘tsutsugamushi triangle’, is a mite-born rickettsial zoonosis, caused by Orientia tsutsugamushi. The clinical presentation is protean and involves multiple organ systems of the body, including central and peripheral nervous systems. We report a 22-year-old previously healthy Indian woman who presented with clinical (confusion, excessive sleepiness, cognitive dysfunction and focal seizures) and neuroimaging features of limbic encephalitis. After exclusion of common infectious, autoimmune and paraneoplastic causes, she was diagnosed with scrub typhus associated encephalitis, which responded to doxycycline and azithromycin therapy.
Keywords: scrub typhus, limbic encephalitis
Introduction
Scrub typhus, an acute febrile infectious disease prevalent in the ‘tsutsugamushi triangle’, is a mite-born rickettsial zoonosis, caused by Orientia tsutsugamushi. 1 The clinical presentation is protean and involves multiple organ systems of the body, including central and peripheral nervous systems.2-4 Acute infective encephalitis is well established as an emerging complication of scrub typhus with common neurological features, such as headache, altered sensorium, seizure, focal weakness, and nuchal rigidity.2-4 Although encephalitis is a quite common neurological manifestation of scrub typhus, neuroimaging characteristics are not well established.4-7 White matter lesions, particularly involving the subcortical, periventricular deep white matter, corpus callosum, and cerebellar peduncles, as well as brainstem and basal ganglia, are the most frequently affected areas.5-7 In some cases, micro-hemorrhages, as well as discrete, gray matter lesions, have been reported.5-7 Meningeal enhancement with gadolinium contrast is well recognized.4-7 However, bilaterally symmetrical medial temporal lobe hyperintense lesions, which are commonly seen in limbic encephalitis, have not been previously reported in scrub typhus.
We report a 22-year-old previously healthy Indian woman who presented with clinical (confusion, excessive sleepiness, cognitive dysfunction, and focal seizures) and neuroimaging features of limbic encephalitis. After exclusion of common infectious, autoimmune and paraneoplastic causes, she was diagnosed with scrub typhus- associated encephalitis, which responded to doxycycline and azithromycin therapy.
Case Report
A previously healthy right-handed 22-year-old college student, from a southern district area of West-Bengal, India, was brought to the emergency room in May 2020 by her family members, with a history of fever, intermittent confusion, increased somnolence and short-term memory loss for the last 10 days. Her past medical, travel and surgical histories were normal. During initial examination in the emergency room, she had a focal seizure with dyscognitive features (epigastric sensation with unpleasant smell followed by right upper limb dystonic posturing in conjunction with automatisms (lip-smacking) and head-turning/version towards the right side), which lasted for four minutes, followed by two minutes of post-ictal nose-wiping and confusion. Her airway, breathing and circulatory support were swiftly managed and she was given intravenous lorazepam (4 mg), which aborted the attack. After stabilization of vital signs and giving a loading dose of levetiracetam, general examination only revealed fever (103.0 ºF). Neurological examination showed cognitive impairment (Folstein Mini mental state examination ([MMSE], performed after two days of admission, revealed a score of 21/30 [2/3 in registration, 3/5 in counting backwards, 2/5 in spelling the word “WORLD” backwards and 0/3 in recall]). The remaining neurological examination including cranial nerves, motor, sensory, cerebellar, autonomic functions and signs of meningeal irritation was normal.
SARS-CoV-2 infection was ruled out with qualitative real-time reverse-transcriptase–polymerase-chain-reaction assay from nasopharyngeal and oropharyngeal swabs. Complete hemogram, electrolytes, renal, hepatic, thyroid function tests, arterial blood gas analysis and urinalysis were normal except mild thrombocytopenia (platelets 96000 per µL) and mild rise in transaminases levels (ALT 60 IU/L and AST 56 IU/L). Diagnostic tests for malaria, dengue, hepatitis B, C and HIV were negative. Interictal electroencephalogram revealed intermittent rhythmic slowing in frontal montages suggestive of focal encephalopathy (Figure 1). Brain magnetic resonance imaging (MRI) revealed hyperintense signal changes on T2-weighted and fluid-attenuated inversion recovery (FLAIR)-weighted images with diffusion restriction in diffusion-weighted imaging, in both medial temporal lobes, particularly involving hippocampi, without any contrast enhancement (Figure 2). Summarizing the clinical-radiological findings (fever, intermittent confusion, acute onset seizure, increased sleepiness, short-term memory loss with bilateral medial temporal involvement on neuroimaging), a diagnosis of limbic encephalitis was made. Cerebrospinal fluid (CSF) analysis revealed raised protein (80 mg/dL) with lymphocytic pleocytosis (52 cells/μL, 98% lymphocytes) with normal glucose (70 mg/dL) level. Paired sera for scrub typhus was sent on day three of admission and diagnosis of scrub typhus was made as Weil-Felix test titer for OXK was ≥ 1:160 and IgM antibodies to Orientia tsutsugamushi were detected by immunochromatographic card. Staining and culture of CSF for bacterial and fungal etiologies were negative (including testing for Cryptococcosis by antigen and India ink preparation). CSF cartridge-based nucleic acid amplification test for Mycobacterium tuberculosis was otherwise negative. Paired sera were sent for relevant neurovirus panel (adenovirus, enterovirus, Epstein Barr virus, human herpes virus 6, 7, human parechovirus, parvovirus B19, varicella zoster virus, herpes simplex virus 1, 2, cytomegalovirus, Japanese encephalitis virus and dengue virus) testing (Dr Lal PathLabs) and all were negative. Subsequently, antibody panel for autoimmune encephalitis (anti-glutamate receptor against NR1 subunit, anti-glutamate-GluR1, anti-glutamate-GluR2, γ-aminobutyric acid [GABA]-B receptor antibody, leucine-rich glioma inactivated 1 antibody and contactin associated protein 2 antibody) and for anti-neuronal antibody (paraneoplastic) encephalitis (amphiphysin CV2.1, PNMA2 [Ma2/Ta], ANNA-1/Hu, ANNA-2/Ri and PCA-1/Yo) were searched (Dr Lal PathLabs). However, they were also negative in serum and CSF. Contrast-enhanced CT scan of the thorax, abdomen and pelvis were normal. Paired sera for scrub typhus were tested and became positive with a 5-fold rise in antibody titer taken on day three and day 21 after admission.
Figure 1.
Electroencephalogram showing prominent frontal intermittent rhythmic delta activity (1-4 Hz slow waves) seen in FP1 and FP2 leads.
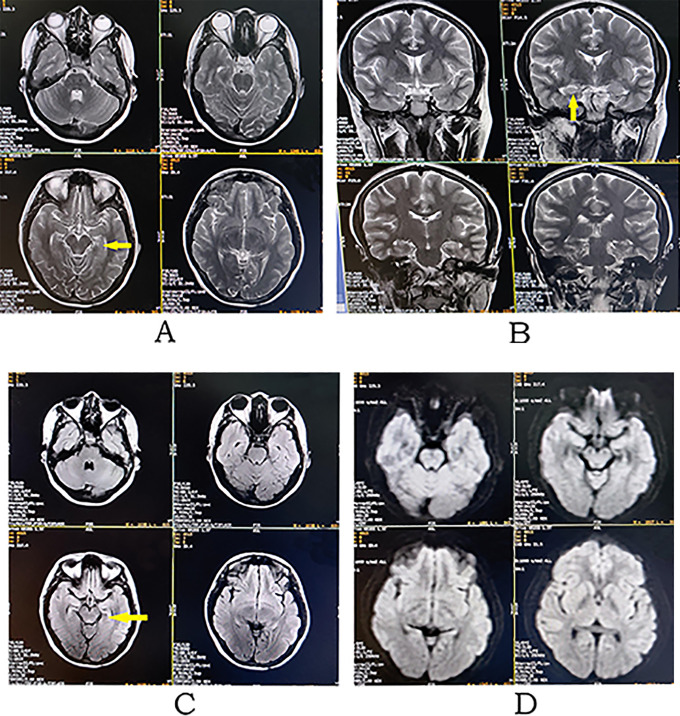
Figure 2.
Pre-treatment. Brain MRI revealing hyperintense signal changes on axial T2-weighted (A), coronal T2-weighted (B) and axial T2-FLAIR (C), images in both medial and mesial temporal lobes, especially involving the hippocampi, with diffusion restriction (D, axial diffusion-weighted image) and without contrast enhancement (E, axial contrast-enhanced T1-weighted image).
She needed two drugs for good control of seizure initially (levetiracetam 2 g/day in divided doses and lacosamide 200 mg/day in divided doses). Acetaminophen (on as need basis for fever), oral doxycycline (200 mg on the first day, then 100 mg/day for two weeks) and azithromycin (500 mg/day for one week) were administered. After four days of treatment, she became afebrile and seizures free; three weeks after completion of therapy with doxycycline, her MMSE improved to 30/30 and there was no demonstrable neurological deficit. Levetiracetam and lacosamide were gradually taken off one by one within three months. In the fourth month of follow-up, her neurological examination was normal and she did not have any seizure or cognitive dysfunction. Brain MRI and an electroencephalogram (1-20 Hz), performed at twelfth month of follow-up, revealed marked resolution of the lesions (Figure 3) and no epileptic discharges, respectively.
Figure 3.
Post-treatment. Brain MRI revealing marked resolution of the hyperintense signal changes (marked by yellow arrows) on axial T2-weighted (A), coronal T2-weighted (B) and axial T2-FLAIR (C), images in both medial and mesial temporal lobes, especially involving the hippocampi, without diffusion restriction (D, axial diffusion-weighted image).
Discussion
Our patient presented with a clinical picture that included confusion, excessive sleepiness, cognitive dysfunction and focal seizures, and a brain MRI with bilateral lesions in the medial temporal lobes, particularly involving hippocampi. Hence, it was compatible with the diagnosis of limbic encephalitis. On the other hand, an eschar (a dark, scab-like region at the site of the bite), found in 7-80% of scrub typhus cases 8 and in 30.8% of those with scrub typhus meningoencephalitis, 4 was absent. However, paired sera for scrub typhus were positive in acute and convalescent phases. She was also a resident of a southern district area of West Bengal, in which some scrub typhus cases have been reported recently. 9
Orientia tsutsugamushi invades both the endothelial and macrophage-monocyte systems in the periphery and gains entry to the central nervous system by hematogenous spread. 10 Host reactions to this neuroinvasion result in the activation of the nuclear factor-κB (NF-κB) (in macrophages) and NF-κB and AP-1 (in endothelial cells), leading to subsequent expression of chemokine genes for macrophage inflammatory protein 1α/β (MIP-1α/β), MIP-2 and monocyte chemoattractant protein-1 (MCP-1) in macrophages and MCP-1, IL-8, and RANTES/CCL5 in endothelial cells.2,10 All of this contributes to parenchymal inflammation, leptomeningeal infiltration, perivasculitis, infarction, demyelination and typhus nodule formation,2,10 and, therefore, neurological manifestations. Central nervous system manifestations are varied (i.e. meningitis, meningoencephalitis, cranial neuropathies, plexopathies, acute disseminated encephalomyelitis, Guillain-Barré syndrome, transverse myelitis, cerebral infarction, subarachnoid hemorrhage, cerebral venous sinus thrombosis, seizures, parkinsonian symptoms, coma, cerebellitis, psychiatric symptoms and cerebral salt wasting syndrome, among others).2-5,10,11 Clinical features may result either from direct damage to an area leading to altered functioning of that region or may be immune-mediated. As in our case, the focal seizures with dyscognitive features and the cognitive decline were due to the affection of medial temporal lobes. Sleep dysfunction, especially in the context of autoimmune encephalitis, deserves a bit of attention here. The projections from the cell groups of the ascending arousal pathway, namely the neurons located in the locus ceruleus, pedunculopontine and laterodorsal tegmental nuclei, tuberomammary nucleus, and dorsal and median raphe nucleus, regulate the sleep/wake cycle through the inhibitory actions of the GABAergic and galaninergic neurons of the ventrolateral preoptic nucleus (VLPO).12,13 The interactions between the ascending arousal pathway and the VLPO system functions like an “on-off” switch, thus keeping the balance between sleep and wakefulness.12,13 This balance also promotes the rapid transition between the two states.12,13 In case of sleep disorders, this switch is disrupted, with wakefulness intruding into sleep and vice versa.12,13 The specific pathological mechanisms in autoimmune encephalitis are mediated by intracellular antigens or cell-surface antibodies that target the neuronal structures, such as brainstem, thalamus, hypothalamus, as well as the limbic system and neurotransmitter systems.14,15 Insomnia, hypersomnia and REM sleep behavior disorders have been documented in LGI1 limbic encephalitis where the antibodies are active against the voltage-gated potassium channels at the neuronal surface. 14 The disruption of sleep architecture has been implicated in infectious diseases, e.g. trypanosomiasis, 16 and demyelinating conditions, e.g neuromyelitis optica spectrum disorder. 17
Immune response against rickettsial infections has been widely studied. In the central nervous system, rickettsial meningoencephalitis causes the formation of “glial nodules” in the subarachnoid space, which consist of perivascular infiltration of CD4 and CD8 T lymphocytes and macrophages. 18 Again, our patient presented with clinical features of limbic system involvement, which is commonly associated with paraneoplastic or non-paraneoplastic immune-mediated encephalitis.19,20 Limbic encephalitis and its variants have rarely been seen with rickettsial infections. 19 Differentials diagnoses of bilaterally symmetrical lesions, involving medial temporal lobes in adults, are mesial temporal sclerosis, herpes simplex encephalitis, autoimmune encephalitis, paraneoplastic limbic encephalitis, extra-pontine myelinolysis, hypoglycemic encephalopathy, mitochondrial cytopathies, hypoxic-ischemic encephalopathy, status epilepticus, basilar tip/bilateral posterior cerebral artery occlusive stroke and degenerative dementias.20,21 Arguably, in this case, the non-enhancing symmetrical intensity changes in T2, FLAIR and diffusion-weighted imaging in both medial temporal lobes could have resulted from ongoing severe seizure activity for which she needed two anti-epileptic drugs. However, post-ictal imaging changes tend to be transient, but may last up to five months. 22 Further, partial resolution with residual gliosis with or without volume loss have also been documented. 22 In the current case, after a prolonged asymptomatic seizure-free follow-up period of eight months, despite early withdrawal of anti-epileptic drugs, intensity was still faintly persisting without evidence of gliosis or volume loss. This argues in favor of infective or immune-mediated encephalitis as the cause of the characteristic neuroimaging.
Scrub typhus may present with features of limbic encephalitis. Particularly because scrub typhus is easily diagnosed and eminently treatable, it should be considered in the workup of patients with clinical and neuroimaging data suggestive of limbic encephalitis.
Acknowledgments
Julián Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967 -1, NetMD—platform for the tracking of movement disorder) and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451).
Authors’ Note: Written informed consent was obtained from the patient participating in the study (consent for research). Informed written consent was obtained from the patient involved in this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Ritwik Ghosh  https://orcid.org/0000-0002-8192-0807
https://orcid.org/0000-0002-8192-0807
Julián Benito-León  https://orcid.org/0000-0002-1769-4809
https://orcid.org/0000-0002-1769-4809
References
- 1.Farrar J, Manson P. Manson’s tropical diseases. Twenty-third edition / ed. Elsevier Saunders;2014:xxiv, 1337. [Google Scholar]
- 2.Rana A, Mahajan SK, Sharma A, Sharma S, Verma BS, Sharma A. Neurological manifestations of scrub typhus in adults. Trop Doct. 2017;47(1):22–25. doi:10.1177/0049475516636543 [DOI] [PubMed] [Google Scholar]
- 3.Sood AK, Chauhan L, Gupta H. CNS Manifestations in Orientia tsutsugamushi disease (Scrub Typhus) in North India. Indian J Pediatr. 2016;83(7):634–639. doi:10.1007/s12098-015-2001-2 [DOI] [PubMed] [Google Scholar]
- 4.Misra UK, Kalita J, Mani VE. Neurological manifestations of scrub typhus. J Neurol Neurosurg Psychiatry. 2015;86(7):761–766. doi:10.1136/jnnp-2014-308722 [DOI] [PubMed] [Google Scholar]
- 5.Neyaz Z, Bhattacharya V, Muzaffar N, Gurjar M. Brain MRI findings in a patient with scrub typhus infection. Neurol India. 2016;64(4):788–792. doi:10.4103/0028-3886.185397 [DOI] [PubMed] [Google Scholar]
- 6.Sood S, Sharma S, Khanna S. Role of advanced MRI brain sequences in diagnosing neurological complications of scrub typhus. J Clin Imaging Sci. 2015;5:11. doi:10.4103/2156-7514.152340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yum KS, Na SJ, Lee KO, Ko JH. Scrub typhus meningo-encephalitis with focal neurologic signs and associated brain MRI abnormal findings: literature review. Clin Neurol Neurosurg. 2011;113(3):250–253. doi:10.1016/j.clineuro.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 8.Rajapakse S, Rodrigo C, Fernando D. Scrub typhus: pathophysiology, clinical manifestations and prognosis. Asian Pac J Trop Med. 2012;5(4):261–264. doi:10.1016/S1995-7645(12)60036-4 [DOI] [PubMed] [Google Scholar]
- 9.Sarma N, Chakraborty S. Scrub typhus in southern districts of west Bengal. Indian J Dermatol. 2017;62(5):512–514. doi:10.4103/ijd.IJD_382_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahajan SK, Mahajan SK. Neuropsychiatric manifestations of scrub typhus. J Neurosci Rural Pract. 2017;8(3):421–426. doi:10.4103/jnrp.jnrp_44_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soundararajan S, Viswanathan S, Jain D, Krishnamurthy V, Gayathri MS. Acute parkinsonism and cerebral salt-wasting-related hyponatremia in scrub typhus. Cureus. 2020;12(1):e6706. doi:10.7759/cureus.6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz JR, Roth T. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol. 2008;6(4):367–378. doi:10.2174/157015908787386050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726–731. doi:10.1016/s0166-2236(00)02002-6 [DOI] [PubMed] [Google Scholar]
- 14.Iranzo A. Sleep and neurological autoimmune diseases. Neuropsychopharmacology. 2020;45(1):129–140. doi:10.1038/s41386-019-0463-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz-Lopetegi A, Graus F, Dalmau J, Santamaria J. Sleep disorders in autoimmune encephalitis. Lancet Neurol. 2020;19(12):1010–1022. doi:10.1016/s1474-4422(20)30341-0 [DOI] [PubMed] [Google Scholar]
- 16.Lundkvist GB, Kristensson K, Bentivoglio M. Why trypanosomes cause sleeping sickness. Physiology (Bethesda). 2004;19:198–206. doi:10.1152/physiol.00006.2004 [DOI] [PubMed] [Google Scholar]
- 17.Song Y, Pan L, Fu Y, et al. Sleep abnormality in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e94. doi:10.1212/nxi.0000000000000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahni A, Fang R, Sahni SK, Walker DH. Pathogenesis of rickettsial diseases: pathogenic and immune mechanisms of an endotheliotropic infection. Annu Rev Pathol. 2019;14(1):127–152. doi:10.1146/annurev-pathmechdis-012418-012800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tüzün E, Dalmau J. Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13(5):261–271. doi:10.1097/NRL.0b013e31813e34a5 [DOI] [PubMed] [Google Scholar]
- 20.Budhram A, Leung A, Nicolle MW, Burneo JG. Diagnosing autoimmune limbic encephalitis. CMAJ. 2019;191(19):E529–E534. doi:10.1503/cmaj.181548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eran A, Hodes A, Izbudak I. Bilateral temporal lobe disease: looking beyond herpes encephalitis. Insights Imaging. 2016;7(2):265–274. doi:10.1007/s13244-016-0481-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cianfoni A, Caulo M, Cerase A, et al. Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur J Radiol. 2013;82(11):1964–1972. doi:10.1016/j.ejrad.2013.05.020 [DOI] [PubMed] [Google Scholar]