INTRODUCTION
Minimally invasive surgery has been introduced as a safe approach for repairing giant paraesophageal hernias (GPEH), without the rates of morbidity and mortality associated with an open approach (Video 1).1–4 Given existing studies on the feasibility of minimally invasive approach to GPEH repair, the laparoscopic approach remains at the forefront in managing this disease process. Decreases in blood loss, intraoperative complications, and length of stay have been attributed to the minimally invasive approach, despite an increase in comorbidity in the patient population.2,3
As of late, the robotic approach to GPEH has become increasingly popular and yields technically superior procedural aspects in comparison to the conventional laparoscopic approach, mainly in improved stable optics, degrees of freedom of motion, improved 3-dimensional high-definition view, intuitive movements, tremor filtering, and the ability to self-first assist.5,6 Despite these advantages, the learning curve must be considered given the technical demands of these procedures, requiring extensive adhesiolysis, hernia sac dissection, and esophageal mobilization within the mediastinum, where maintaining visualization for extended periods of time can be challenging.5
Data showing comparisons between robotic approaches and open and laparoscopic remain largely scarce. Gehrig and colleagues7,8 conducted a case control study displaying no differences in operative times in either approach, but the intraoperative and postoperative complication rates were lower for robotic and laparoscopic, 16.7% and 17.6%, respectively, when compared with the open approach (58%).
We have conducted a retrospective study assessing early outcomes in patients presenting with symptomatic GPEHs who underwent robotic-assisted GPEH repair over a 3-year period. The median age was 62 years and 63% of the patients (n = 15) underwent fundoplication and 37% (n = 9) for gastropexy. The median operative time was 277 minutes and decreased steadily over the experience. There were no intraoperative complications or surgical mortality, and there were no complaints of dysphagia in the early postoperative period.6
PREOPERATIVE ASSESSMENT
A full history and physical examination must be performed and the surgeon must elicit specific symptoms (regurgitation, heartburn, water-brash), aspiration, cough, abdominal pain, chest discomfort, and dysphagia. A barium esophagram is performed to assess esophageal motility, reflux, and severity of herniation and anatomic relationship between stomach, gastroesophageal junction, and diaphragmatic hiatus. A barium esophagram allows the surgeon to identify the anatomic contours of alimentary tract and presence of volvulus or endoluminal disease. Laboratory studies include hemoglobin and hematocrit to assess for anemia. A computed tomography scan is not routinely performed unless there is high suspicion for other pathology or to identify type IV GPEH. Manometry studies are not performed on every patient, because these studies have an inherent risk of placement difficulty, poor accuracy, and even esophageal perforation. Preoperative and intraoperative endoscopy should be performed to assess stomach viability, gastroesophageal junction location, occult malignancy, Barrett’s esophagus, diverticular disease, or other pathology. We advocate that the endoscopic evaluation is performed by the surgeon, at the very least, on the day of surgery.
Relative contraindications include an inability to tolerate general anesthesia, severe cardiopulmonary dysfunction, or an uncontrolled hematologic disorder. Patient age, the size of the hernia, and previous abdominal surgeries are not contraindications. When possible, a period of resuscitation is advisable before surgery to correct physiologic and laboratory derangements.
TECHNIQUE
The patient is placed in the supine position, followed by general anesthesia induction and subsequent orotracheal intubation. An arterial line is placed for hemodynamic monitoring. Careful endoscopic evaluation with minimal air insufflation is conducted by the surgeon. Excessive air insufflation would present technical challenges during laparoscopic visualization and robotic assistance. In obstructed patients, excessive air may cause gastric dilation, vagal stimulation, and possibly severe hemodynamic instability. During endoscopic assessment, the surgeon should thoroughly inspect the esophagus to rule out occult malignancy, confirm the level of the gastroesophageal junction, and decompress the stomach. A foot-board is placed to support reverse Trendelenburg positioning. We prefer keeping both arms out at 45°, but the left arm can be tucked at the surgeon’s discretion. A liver retractor (DiamondFlex, Snow-den Pencer, Vernon Hills, IL) is used; thus, the patient is placed to the right of the operating table. The operating table is positioned 90° and the robotic arms and cart (DaVinci Si, Intuitive Surgical, Sunnyvale, CA) are brought in over the midline and into position to allow entry and exit (Fig. 1).
Fig. 1.
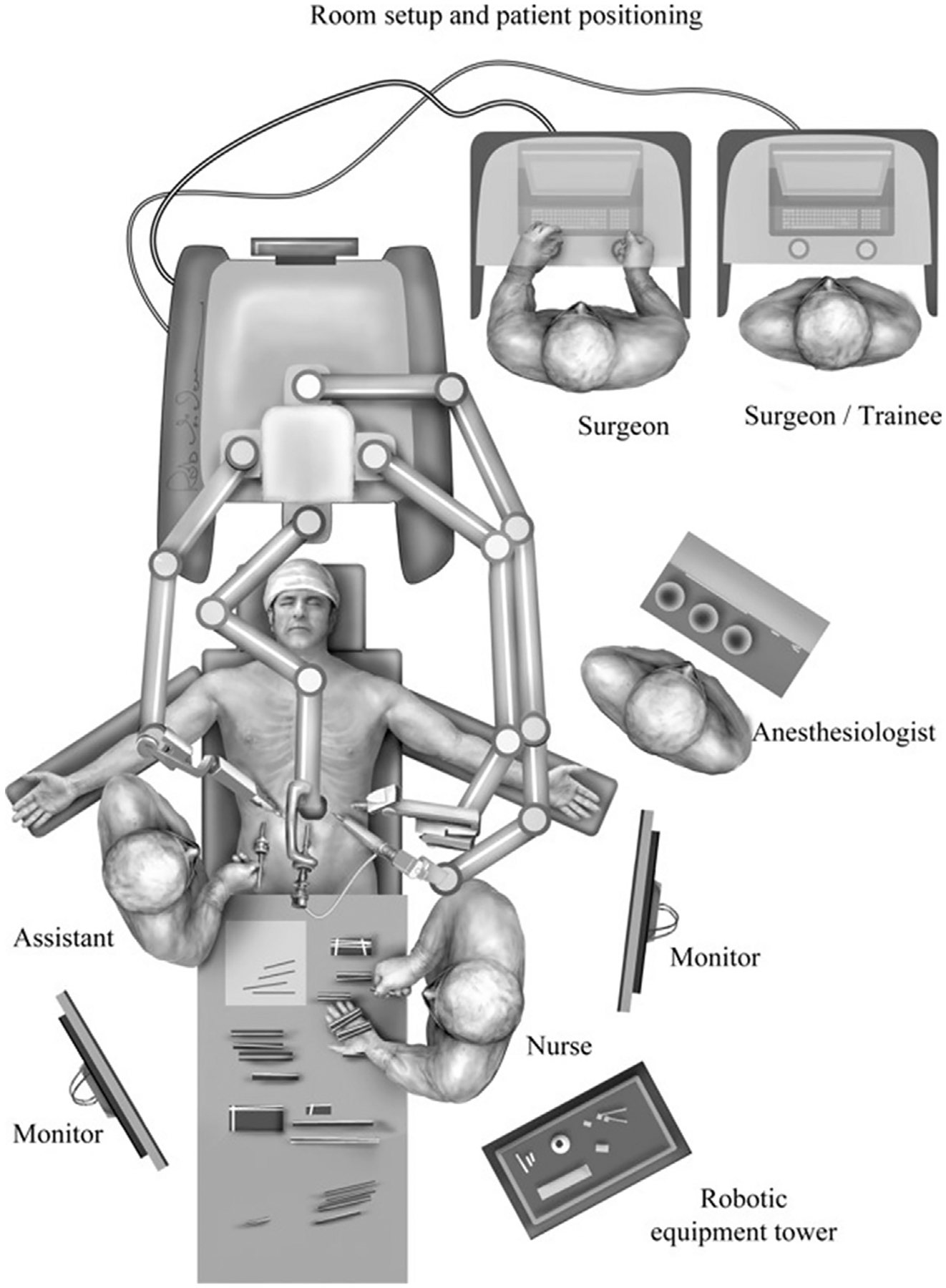
Operating room set up for robotically assisted GPEH repair. (From Karush J, Sarkaria IS. Robotic-assisted giant paraesophageal hernia repair and Nissen fundoplication. Oper Tech Thorac Cardiovasc Surg. 2013;18(3):205; with permission.)
The procedure is performed using 6 ports. Appropriate port placement is pivotal to a successful operation owing to the extensive mediastinal dissection necessary to reduce the hernia contents, excise the hernia sac, and adequately mobilize the esophagus. To facilitate this goal, the midline from the xiphoid to the level of the umbilicus is marked and divided in thirds. A camera incision is marked just left of the midline in the midabdomen, approximately half the distance from the xiphoid to the umbilicus. A left lateral subcostal 8-mm incision is marked for using the robotic atraumatic grasper for assistance. This may be a 5-mm incision on less recent versions of the robotic platform (DaVinci Si Platform, Intuitive Surgical, Sunnyvale, CA). Subsequently, left midclavicular 8-mm incision is marked at the left epigastrium, one fingerbreadth below the ribs. This port is used as the robotic right hand and for the robotic ultrasonic shears. An additional 8-mm right midclavicular port is marked at the epigastrium for bipolar fenestrated forceps (robotic left hand). This port is kept close to the midline to minimize the instrument angulation between the shaft and right crural pillar during transhiatal intrathoracic dissection. The liver retractor is inserted through a right lateral 5-mm subcostal port. A 12-mm port is placed at the level of the right periumbilical plane. Of note, we deem it critical to maintain at least an 8- to 10-cm distance between robotic ports to minimize collisions, and all ports are placed under visualization. More recent versions of the robotic platforms (DaVinci Xi Platform, Intuitive Surgical) allow closer placement of these ports without undue arm collisions, and the ability to advance the care independently of bed position. For optimal peritoneal distension and visualization, 15 mm Hg of CO2 insufflation is provided. Port placements are summarized in Fig. 2.
Fig. 2.
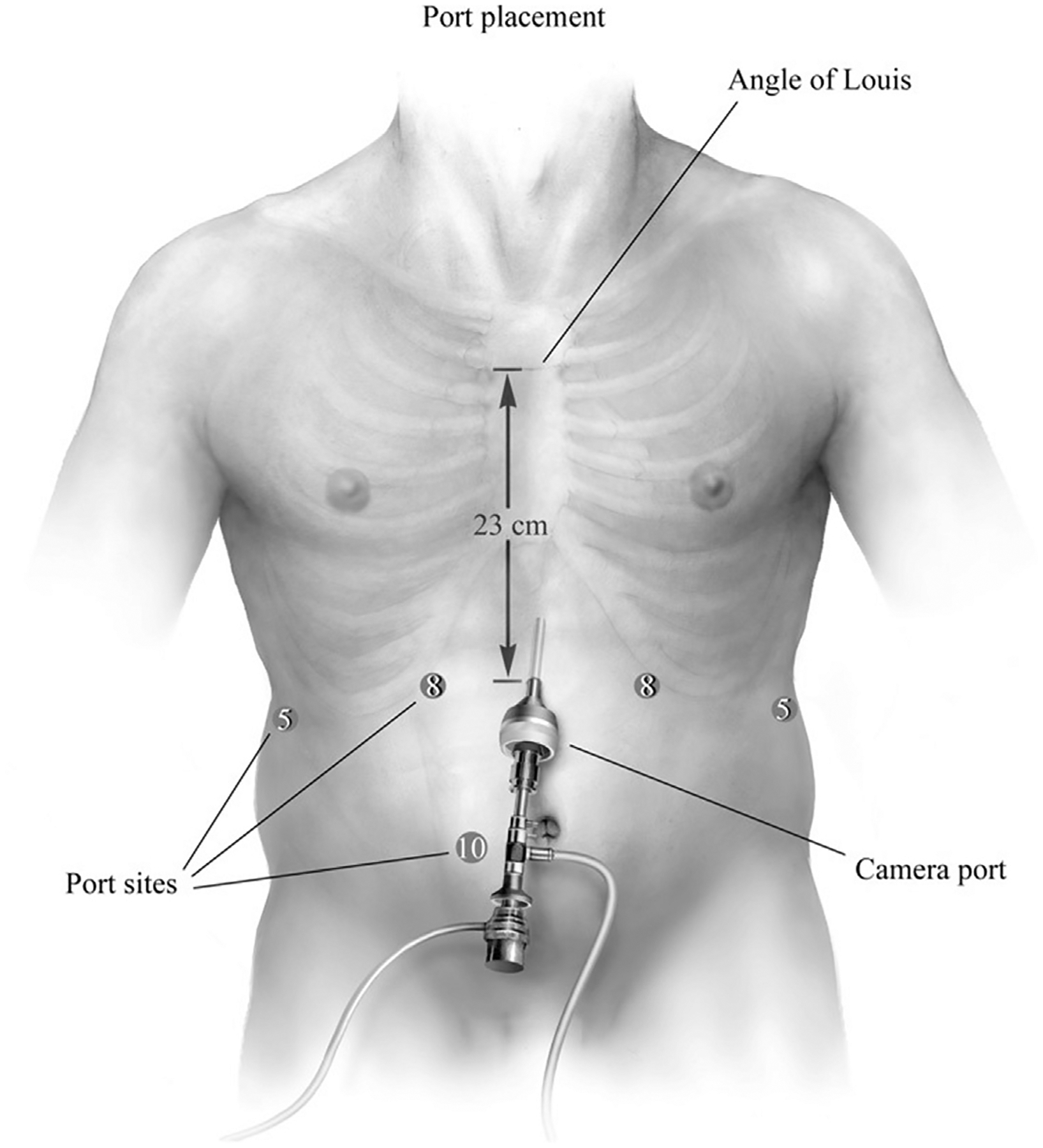
Port placement. An 8-mm robotic trocar is placed at the left lateral costal margin, in which a 5-mm atraumatic grasper is used as the robotic assistant. Second, a left 8-mm port for the harmonic device is placed at the midclavicular line, 1 to 2 cm below the costal margin. Third, a 5-mm port is placed in the right lateral subcostal margin for the liver retractor and a robotic 8-mm trocar is placed in the right upper quadrant at the level of the midclavicular region, for the bipolar fenestrated grasper. Last, a 12-mm assistant port is placed in the right periumbilical umbilical region. (From Karush J, Sarkaria IS. Robotic-assisted giant paraesophageal hernia repair and Nissen fundoplication. Oper Tech Thorac Cardiovasc Surg. 2013;18(3):206; with permission.)
HERNIA SAC REDUCTION
The patient is placed in reverse Trendelenburg to assist with adequate visualization of the hiatus. It is important to remember, many patients receive 1 L of polyethylene glycol electrolyte solution as bowel preparation and are prone to fluid shifts resulting in hemodynamic changes during positional changes at the beginning of the case. After reverse Trendelenburg is slowly achieved, we proceed to reduce the hernia contents (ie, omental fat, bowel) to achieve optimal visualization of the hiatus (Fig. 3). Sac reduction is achieved by grasping the hernia sac at 12 o’clock and atraumatically everting the hernia sac (Fig. 4). Using careful dissection, the layers of the weakened phrenoesophageal ligament and peritoneal reflection are divided with care to avoid injury to the widened anterior aspect of the crural pillars. After entering the posterior mediastinum, the surgeon can use the robotic ultrasonic shears to divide this largely areolar plane. Visualizing the arealor attachments is critical during this portion of the case, because it ensures that the surgeon is safely visualizing the posterior mediastinal boundaries and critical contents (bilateral pleurae, pericardium, esophagus, aorta, vertebrae, hemi-azygous vein, thoracic duct, pulmonary veins, and vagal nerves). During the sac dissection, the surgeon must visualize the pleural reflection to avoid causing an iatrogenic pneumothorax. If the pleural reflection is violated, the surgeon and anesthesiologist must communicate because a tension pneumothorax may quickly result in hemodynamic instability. Simply ceasing gas insufflation while evacuating the pneumothorax through the pleural defect with a laparoscopic suction device is the most effective immediate maneuver. Many patients tolerate these small rents without issue, despite the loss of visualization that may occur with collapse of the pleura into the mediastinal space. If this maneuver fails, insertion of a small bore pigtail and/or reducing abdominal CO2 insufflation may lessen the hemodynamic dysfunction. Extensive dissection is performed until the sac is reduced, with care to avoid injuring the anterior and posterior vagus nerve. Mediastinal dissection is continued until the inferior pulmonary veins are visualized, at minimum (Fig. 5).
Fig. 3.
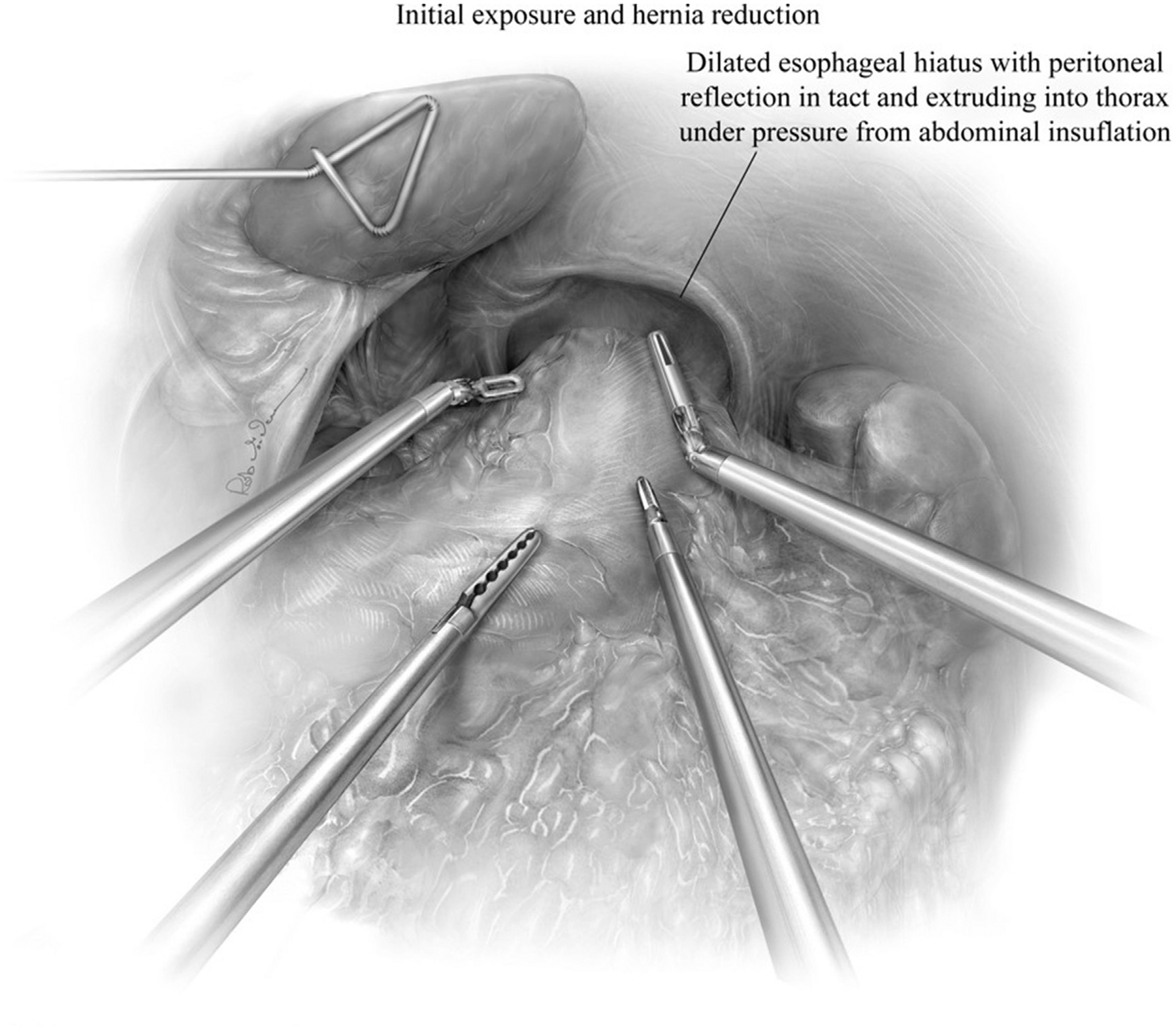
The patient is in reverse Trendelenburg to use gravity in reducing the hernia’s content. The remaining herniated contents are reduced from the mediastinum in the intraabdominal cavity with the use of robotic and bedside assistant ports. (From Karush J, Sarkaria IS. Robotic-assisted giant paraesophageal hernia repair and Nissen fundoplication. Oper Tech Thorac Cardiovasc Surg. 2013;18(3):207; with permission.)
Fig. 4.
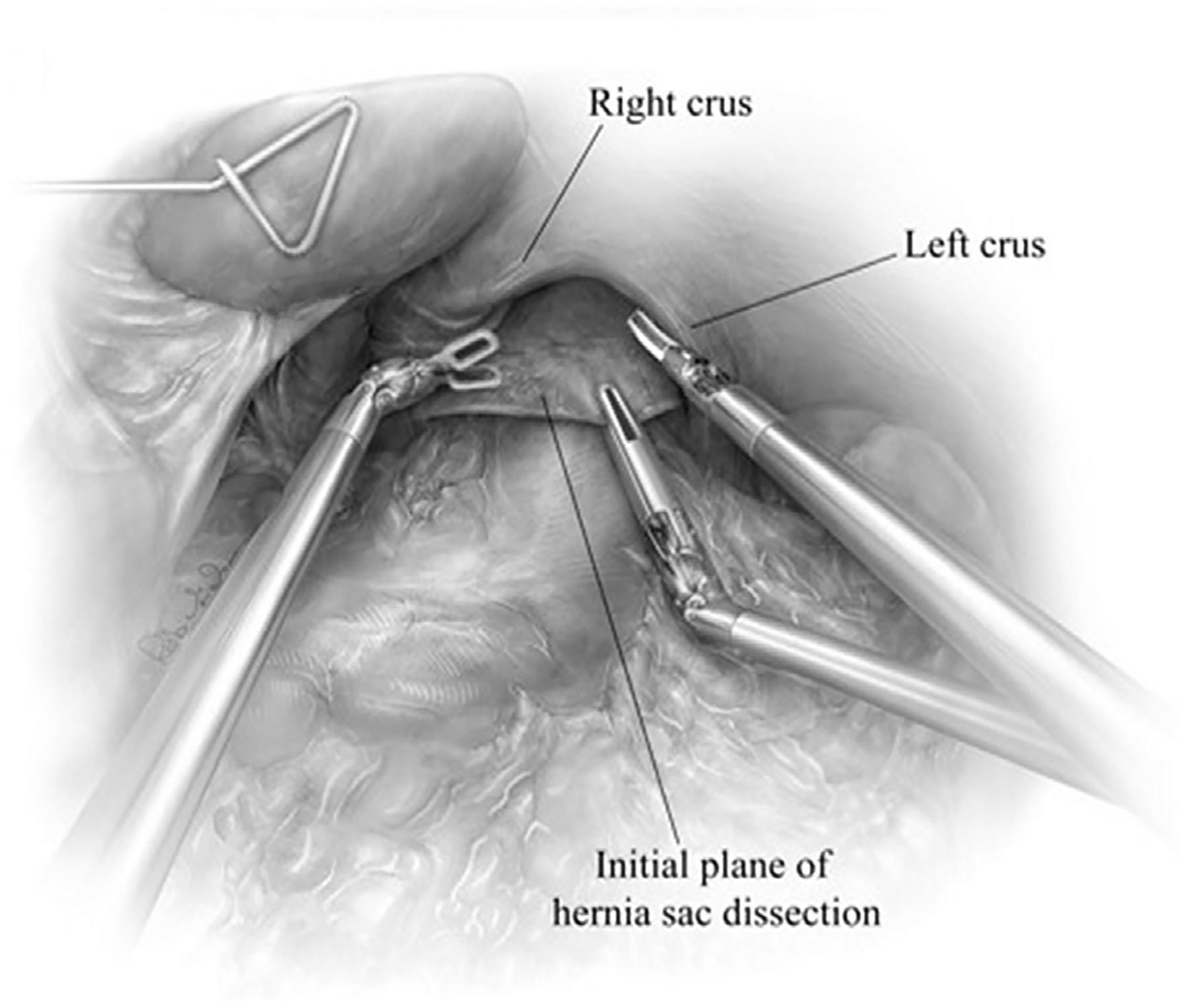
After reducing the herniated contents, the hernial defect can be well-visualized. The hernia sac along is grasped at 12 o’clock and retracted inferiorly therefore, exposing the initial line of dissection between the sac and anterior crura. Sac dissection is initiated by incising the sac just below the anterior crura and developing a plane posterior to the pericardium, with attention to the peritoneal reflection. In most hernias, the areolar plane is mobilized with blunt dissection using the ultrasonic shears. This plane is largely avascular; therefore, there should be minimal blood loss during dissection. (From Karush J, Sarkaria IS. Robotic-assisted giant paraesophageal hernia repair and Nissen fundoplication. Oper Tech Thorac Cardiovasc Surg. 2013;18(3):208; with permission.)
Fig. 5.
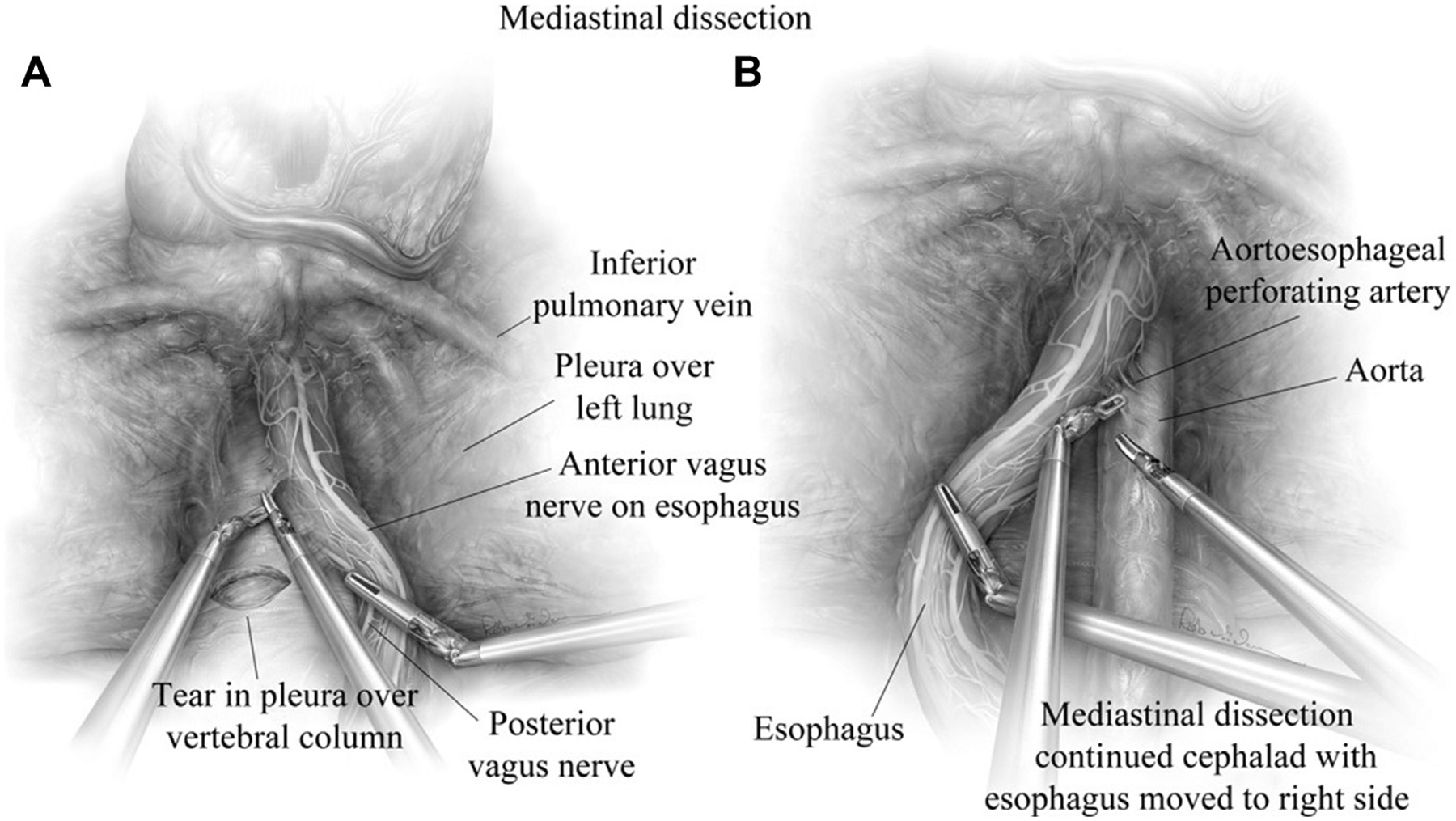
(A) Mediastinal dissection is continued until the inferior pulmonary vein is visualized. The left vagus nerve is readily identified and traced to the anterior vagus along the esophagus. (B) During anterior and posterior dissection, the mediastinal borders should always be well visualized to reduce injury to the aorta, vagus nerves, pleurae, vertebral column and the esophagus. (From Karush J, Sarkaria IS. Robotic-assisted giant paraesophageal hernia repair and Nissen fundoplication. Oper Tech Thorac Cardiovasc Surg. 2013;18(3):209; with permission.)
After successfully reducing the sac from the mediastinum, the sac must be dissected off the crural pillars bilaterally. While separating the sac from the crura, care must be taken to avoid damaging the peritoneal lining overlying the crura and exposing the muscle beneath. If the peritoneal lining is stripped from the underlying muscle, the integrity of the sutured crural reapproximation and closure may be at higher risk for dehiscence. We believe extensive mediastinal mobilization and adequate sac reduction is critical to establishing a tension-free repair and decreasing the risk of recurrence.
ESOPHAGEAL LENGTH ASSESSMENT AND REESTABLISHING INTRAABDOMINAL ESOPHAGEAL LENGTH
After reducing the hernia stomach and its associated contents with crural preservation, the esophagus is further mobilized to the level of the inferior pulmonary veins or further cephalad. Again, care is taken to visualize and preserve the vagus nerves. Extensive mobilization allows for accurate assessment of esophageal length.
Upon completing esophageal mobilization, we proceed with mobilizing the gastric fundus by ligating the gastrosplenic attachments and short gastric arteries using ultrasonic energy. Care is taken to avoid traction injury to the spleen. The gastric attachments to the left crus are completely divided to optimize fundus mobilization, again, with care to preserve the peritoneal lining overlying the left crus. Following fundus mobilization, the gastric fat pad is mobilized medially off the stomach (Fig. 6) and distal esophagus to adequately and accurately visualize the gastroesophageal junction. Assessing the gastroesophageal junction allows for adequate intraabdominal length in the neutral resting position. At least 2.5 cm of intraabdominal esophagus is recommended to achieve a tension-free repair. Additional mediastinal mobilization may be performed if more than 2 cm of an intraabdominal esophagus is not achieved. In the event esophageal length is inadequate despite extensive mediastinal esophageal mobilization, an esophageal lengthening procedure is performed. Our preferred approach is a modified (wedge) Collis gastroplasty.
Fig. 6.
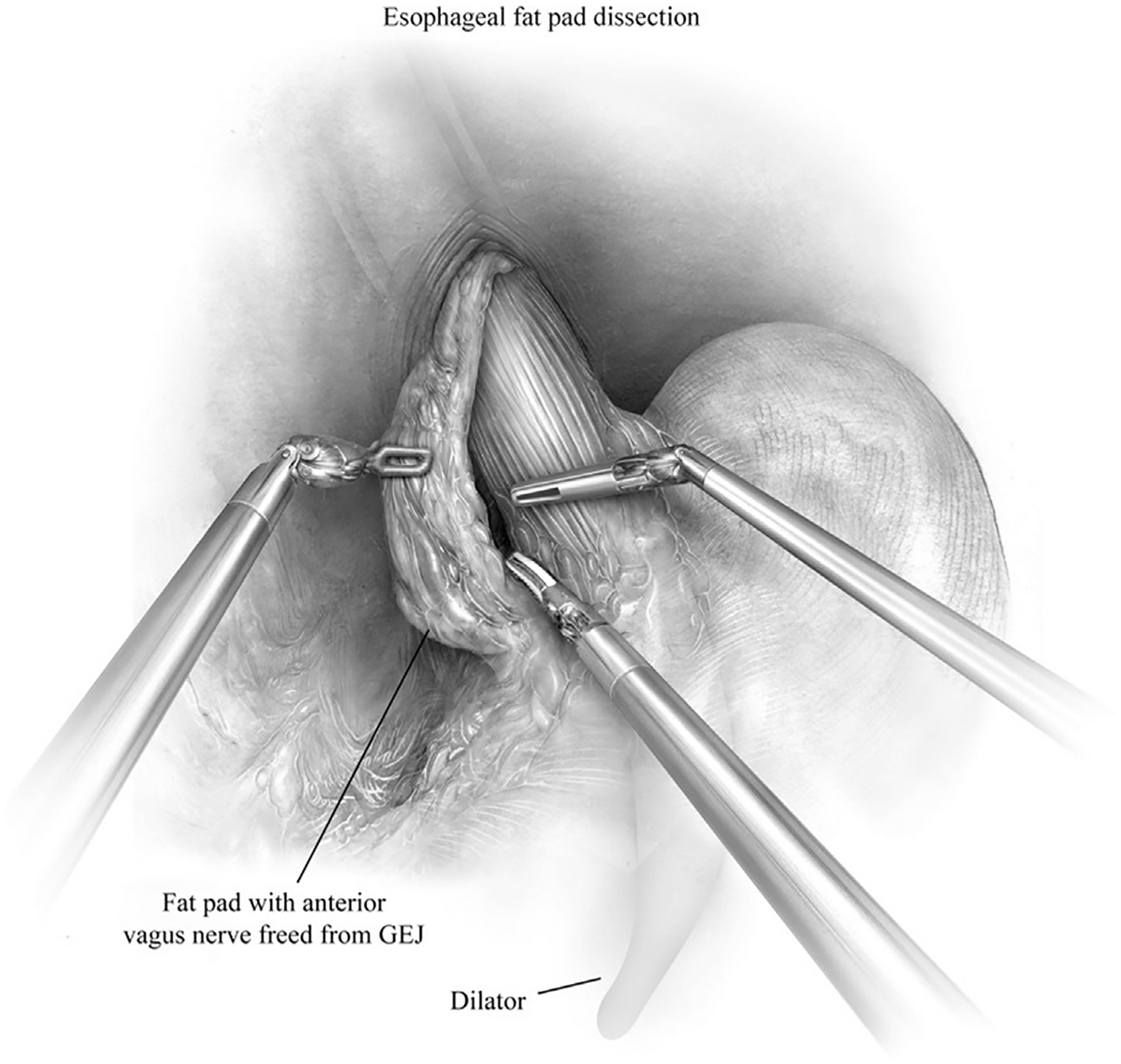
Esophageal fat pad is mobilized medially off the anterior esophagus. This maneuver facilitates visualization of the gastroesophageal junction (GEJ) and assessment of intraabdominal esophageal length. Care is taken to not injury the anterior or posterior vagus nerve. We do not routinely incorporate posterior vagus nerve in the fat pad dissection. (From Karush J, Sarkaria IS. Robotic-assisted giant paraesophageal hernia repair and Nissen fundoplication. Oper Tech Thorac Cardiovasc Surg. 2013;18(3):212; with permission.)
COLLIS GASTROPLASTY
A 54F bougie is inserted under direct visualization along the lesser curvature of the stomach to ensure safe gastric insertion. The right hand robotic working port is upsized to a 12-mm robotic stapler port. We use a robotic endostapler (45-mm EndoWrist Stapler with 3.5-mm blue staple loads) insert into this left subcostal robotic port (see Fig. 2). The first staple line is directly parallel to the line of the fundus and continued until the stapler closely approximates the bougie. The second staple line is carried horizontal to the axis of the esophagus and in tight apposition to the bougie. Care should be taken to avoid incorporating the esophageal body or vagus nerve into the stapler. Serial staple lines are performed in parallel with esophagus to complete a wedge resection of the stomach. The staple line establishes the new greater curve for the neoesophagus with the goal to obtain 2 to 3 cm of additional length.
HIATAL REPAIR
The hiatus is repaired in all patients. Care is taken to preserve the crural muscle fibers during mediastinal dissection to have adequate crural symphysis after closure. The robotic arm can be used to retract the crura laterally without grasping them during mediastinal dissection. To achieve a tension-free closure, additional dissection may be necessary if the crura are tethered by the phrenogastric or phrenosplenic ligament. Attention to identifying the pleural and peritoneal reflection is key to maintaining the integrity of both crural pillars. Inducing a left pneumothorax via a 5-mm intrathoracic port may provide significant laxity to the left hemidiaphragm and greatly aid in accomplishing a tension-free hiatal approximation. In this event, a pigtail catheter may be placed on the side of the pneumothorax after repair and removed at the surgeon’s discretion.
The freely mobilized left and right crural pillars are approximated using 2 to 3 interrupted 0-polyester permanent braided suture, placed posteriorly. If there is significant space anteriorly, additional crural sutures are placed. The robotic assistant arm helps to retract the esophagus superiorly and leftward, allowing for adequate exposure of the crura. Only the broad side of the retracting instrument should be used against the esophagus or stomach, especially while the bougie is in place, to avoid iatrogenic perforation. After closure, we evaluate the crura to ensure no excessive narrowing or impingement of the esophagus that would cause postoperative dysphagia.
Of note, bioprosthetic mesh or the use of pledget material can reinforce the crura or crural sutures in the event the pillars are severely denuded, or if there is concern for excessive crural tension. In our practice, the use of mesh is rare in primary cases.
ESTABLISHING AN ANTIREFLUX BARRIER
Owing to extensive esophageal dissection that would likely lead to significant gastroesophageal reflux, we typically create a new antireflux barrier. Surgeon preference, patient symptoms and preoperative physiologic testing and radiographic studies assessing esophageal motility dictate whether we perform a partial fundoplication or circumferential fundoplication (floppy Nissen).
NISSEN FUNDOPLICATION
The surgeon inserts a 54F bougie under visualization (Figs. 7 and 8). Use the atraumatic grasper in the 5-mm port to grasp the lateral of the staple line at the proximal fundus and pull through the retroesophageal window. The retractor in the robotic assistant arm port is able to retract the posterior aspect of the distal esophagus anteriorly and leftward, allowing passage of the proximal fundus through the retroesophageal window. If there is adequate fundus mobilization, the wrap will remain intact despite grasper release from the stomach, but further retrogastric mobilization is needed if this is not the case.
Fig. 7.
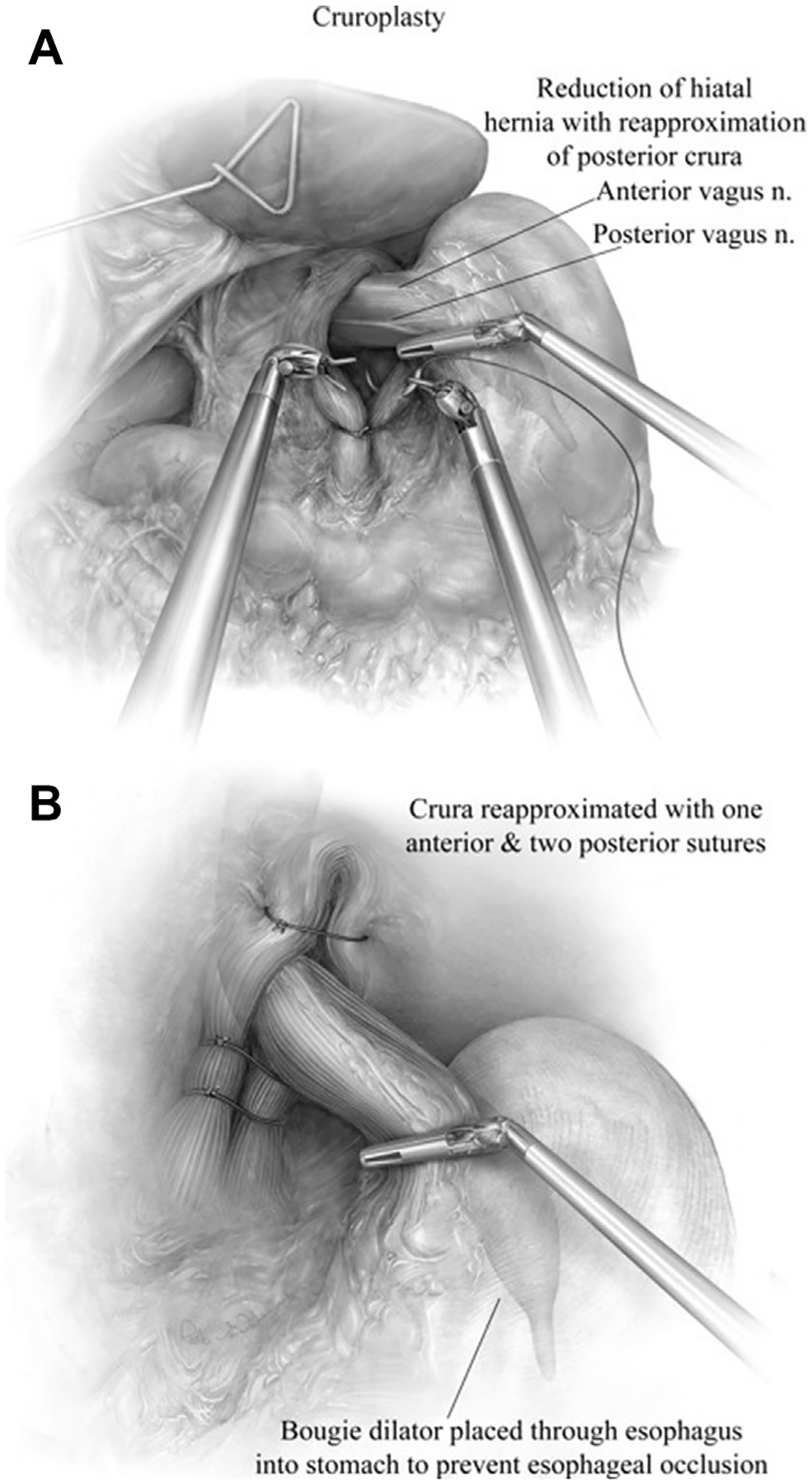
(A) After adequate mobilization of both crus, a 54F bougie is inserted and visualized robotically. The right and left crus are approximated using large 0 nonabsorbable suture in simple interrupted fashion. (B) We typically place 2 to 3 sutures to close the posterior aspect and 1 to 2 sutures to close the anterior aspect of the hiatus. (From Karush J, Sarkaria IS. Robotic-assisted giant paraesophageal hernia repair and Nissen fundoplication. Oper Tech Thorac Cardiovasc Surg. 2013;18(3):211; with permission.)
Fig. 8.
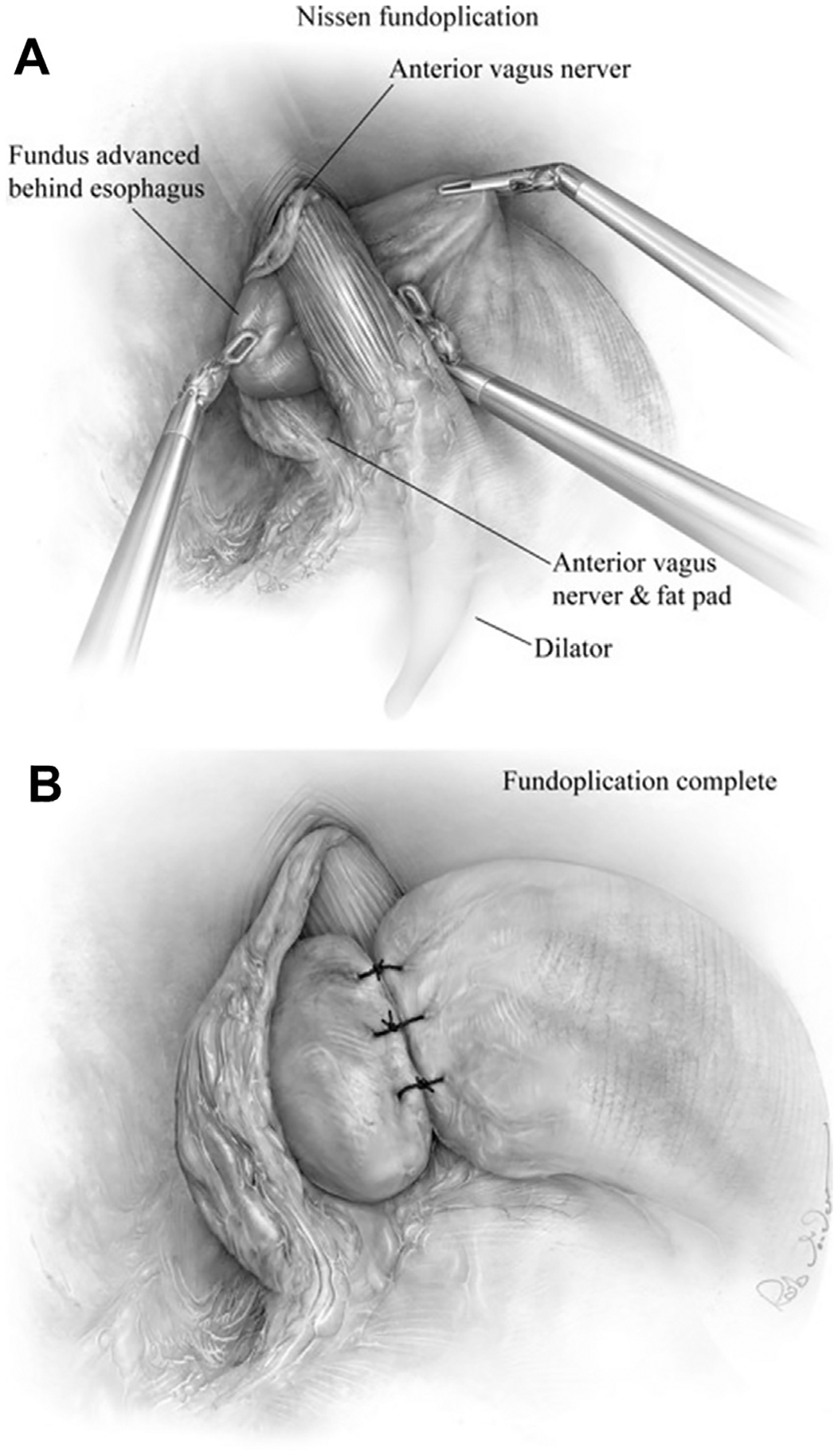
(A) After mobilizing the fundus, we perform a “floppy” Nissen fundoplication with a 54F bougie in place. The gastric fundus is grasped along the short gastric dissection border using the robotic assistant arm, while the bedside assistant holds gentle caudal retraction of the body of stomach. The robotic left-hand grasper is passed carefully behind the esophagus from right to left and the fundus brought through the retroesophageal window and kept in correct orientation, with the esophageal fat pad and anterior vagus kept outside the wrap. A “shoeshine” maneuver is performed to assure a tension-free fundus. (B) 3-stitch tension-free floppy wrap of 2 to 3 cm is centered and completed over the gastroesophageal junction with bougie in place. Nonabsorbable, 2–0 sutures are placed. The proximal and distal sutures fix the fundus to the esophagus and stomach, respectively. (From Karush J, Sarkaria IS. Robotic-assisted giant paraesophageal hernia repair and Nissen fundoplication. Oper Tech Thorac Cardiovasc Surg. 2013;18(3):213; with permission.)
The graspers in the 8-mm L robotic arm port and the 5-mm robotic port contain the staple line from the Collis and lateral border of the short gastric arteries, respectively. A shoe-shine maneuver is performed after the wrap is pulled through the window. To secure the wrap, 2–0 polyester suture is used, with care to ensure the superior aspect of the wrap is positioned over the esophagus or Collis segment. Only posterior aspects of the wrapped stomach should be visible if oriented and performed properly.
POSTOPERATIVE MANAGEMENT
The patient is typically extubated and transferred to the postoperative care unit or intensive care unit depending on the patient’s comorbidities, duration of the case, intraoperative complications, or urgency (elective vs emergent surgery). Most patients presenting with GPEHs are elderly with more associated comorbidities in comparison with patients presenting with gastroesophageal reflux disease in the absence of hiatal hernia. These patients are susceptible to aspiration pneumonia, myocardial ischemia, acute renal failure, postoperative leaks, atrial fibrillation, and pulmonary embolism.2 As in the literature describing the perioperative morbidity after laparoscopic approaches, the threshold to determine the presence or absence of rare postoperative leaks or hemorrhage from the Collis staple line must remain low in patients who undergo gastroplasty.
Patients ambulate and undergo a swallow esophagram on postoperative day 1. If the study is negative for a leak and postoperative anatomy is unremarkable, the nasogastric tube is removed and the patient’s diet is advanced to clear liquid diet (1–2 oz/h). If the patient is tolerating clear liquids without difficulty, we advance to full liquid diet, and a soft diet over 10 to 14 days as an outpatient. All patients are reevaluated 2 weeks after surgery and then yearly with an esophagram.
Common complaints include nausea and pain. Patients are managed with an intravenous antiemetic for nausea. Pain is commonly managed with acetaminophen and low-dose narcotics in intravenous form until the barium swallow is completed, after which they are graduated to liquid forms of pain medication and discharged on nonnarcotic analgesia. Patients may remain on proton pump inhibitor or H2-blocker therapy in the early postoperative period.
Patients are discharged 1 to 2 days postoperatively and evaluated 2 weeks after discharge.
SUMMARY
Currently, no prospective studies or long-term outcome data have been published comparing the robotic approach to laparoscopic approach for GPEHs. Studies on the robotic approach for giant paraesophageal repair are limited to mostly institution specific, retrospective studies. Ruurda and colleagues9 described their comprehensive robotic experience over a 4-year period, including type III or IV paraesophageal hernia repairs (n = 32). The median operating time was 130 minutes, the median blood loss was 50 mL, and 3 cases were converted to open technique (indication was not specified). The median hospital stay was 5 days. Draaisma and colleagues10 described 40 patients undergoing robotic-assisted GPEH repair, with the median operating time and blood loss reported at 127 minutes and 50 mL, respectively. The median hospital stay was 4.5 days, but 12.5% patients showed anatomic abnormalities upon follow-up esophagram.
Despite the scarcity of data, we believe the advantages of robotic yield optimal visualization and significant degree of control by the operating surgeon. We notice that the operating surgeon’s ability to self-first assist allows for efficient and coordinated exposure, particularly in the mediastinum. Additionally, the operator-controlled optics and robotic assistant arm allows for one assistant in comparison to the 2 additional bedside assistants commonly present with the laparoscopic approach.
Longer operative times, higher cost and technical expenses, and lack of tactile feedback are potential limitations of the robotic approach. Operative times and costs are likely to decrease with improved learning curves, and with introduction of additional industry competitors to the market. Limitations in haptic feedback do not seem to be a major factor, with adaptation to visual cues of tissue tension greatly augmenting the surgeon’s perception of feel. Although prospective studies would have to be conducted to truly compare differential outcomes with these techniques, our experience thus far has been encouraging.11,12
Supplementary Material
KEY POINTS.
For a successful robotic-assisted giant paraesophageal hernia repair, reduce the stomach and the associated intrathoracic contents including the hernia sac with extensive mediastinal dissection.
For a successful robotic-assisted giant paraesophageal hernia repair, reestablish adequate intraabdominal esophageal length and perform a Collis gastroplasty if needed.
For a successful robotic-assisted giant paraesophageal hernia repair, perform a diaphragmatic defect closure (with or without mesh).
For a successful robotic-assisted giant paraesophageal hernia repair, establish anti reflux barrier (or gastropexy, if needed).
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Video content accompanies this article at http://www.thoracic.theclinics.com.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.thorsurg.2019.06.001.
REFERENCES
- 1.Fullum TM, Oyetunji TA, Ortega G, et al. Open versus laparoscopic hiatal hernia repair. JSLS 2013;17(1):23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg 2010; 139:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferri LE, Feldman LS, Stanbridge D, et al. Should laparoscopic paraesophageal hernia repair be abandoned in favor of the open approach? Surg Endosc 2005;19(1):4–8. [DOI] [PubMed] [Google Scholar]
- 4.Pierre AF, Luketich JD, Fernando HC, et al. Results of laparoscopic repair of giant paraesophageal hernias: 200 consecutive patients. Ann Thorac Surg 2002;74(6):1909–15. [DOI] [PubMed] [Google Scholar]
- 5.Hance J, Rockall T, Darzi A. Robotics in colorectal surgery. Dig Surg 2004;21(5–6):339–43. [DOI] [PubMed] [Google Scholar]
- 6.Sarkaria IS, Latif MJ, Bianco VJ, et al. Early operative outcomes and learning curve of robotic assisted giant paraesophageal hernia repair. Int J Med Robot 2017;13(1):e1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehrig T, Mehrabi A, Fischer L, et al. Robotic-assisted paraesophageal hernia repair-case-control study. Langenbecks Arch Surg 2013;398(5):691–6. [DOI] [PubMed] [Google Scholar]
- 8.Nason KS, Luketich JD, Witteman BP, et al. The laparoscopic approach to paraesophageal hernia repair. J Gastrointest Surg 2012;16(2):417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruurda JP, Draaisma WA, van Hillegersberg R, et al. Robot-assisted endoscopic surgery: a four-year single-center experience. Dig Surg 2005;22(5):313–20. [DOI] [PubMed] [Google Scholar]
- 10.Draaisma WA, Gooszen HG, Consten EC, et al. Mid-term results of robot-assisted laparoscopic repair of large hiatal hernia: a symptomatic and radiological prospective cohort study. Surg Technol Int 2008; 17:165–70. [PubMed] [Google Scholar]
- 11.Latif MJ, Park BJ. Robotics in general thoracic surgery procedures. J Vis Surg 2017;3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karush J, Sarkaria IS. Robotic-assisted giant paraesophageal hernia repair and Nissen fundoplication. Oper Tech Thorac Cardiovasc Surg 2013;18(3): 204–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


