Abstract
Over the last several years multiple studies, primarily from European centers have demonstrated the clinical and outcomes benefits of multidisciplinary endocarditis teams. Despite this literature, adoption of this approach to patient care has been slower in the United States. While there is literature outlining the optimal composition of an endocarditis team, there is little information to guide providers as they attempt to transform practice from a fragmented, disjointed process to an efficient, collaborative care model. In this review, the authors will outline the steps they took to create and implement a successful multidisciplinary endocarditis team at the University of Michigan. In conjunction with existing data, this piece can be used as a resource for clinicians seeking to improve the care of patients with endocarditis at their institutions.
Keywords: endocarditis, multidisciplinary care, quality improvement, valve surgery
Introduction
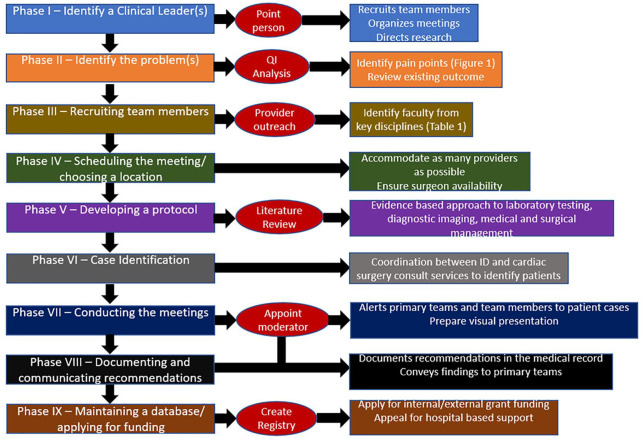
Over the last several years, there has been an increasing interest in the clinical implementation of multidisciplinary endocarditis teams. 1 Since the first publication in 2009 demonstrating the mortality benefits associated with such groups, they have become the standard of care for the diagnosis and management of endocarditis at European tertiary care medical centers.2,3 Adoption of this coordinated approach has been slower in North America, although one recent study from the University of Michigan demonstrated a dramatic reduction in in-hospital endocarditis mortality after the implementation of a multidisciplinary endocarditis team. 4 In addition, the increase in endocarditis associated with the opioid epidemic has sparked interest in developing a similar model of care in the United States.5,6 Several publications have discussed the basic organization and structure of such groups. These papers have included direction regarding which specialists should be involved, how referrals from transferring hospitals should be coordinated and the role of the team in participating in clinical research.1,7 However, there has been little discussion about the logistics behind how a large institution transforms its endocarditis practice from a fragmented, disjointed process to an efficient, collaborative care model. Moreover, the majority of previous publications on this topic have come from European centers. While there are undoubtedly many areas of overlap, the American and European systems of health care have substantial differences that impact the effective development of these groups. In this piece, the authors will discuss the step-by-step approach they took to creating a successful multidisciplinary endocarditis team at the University of Michigan (Figure 1). 4
Figure 1.
Graphic overview of the authors’ step-by-step approach to developing a multidisciplinary endocarditis team.
Phase I: identify the clinical leader(s)
One of the most important components of this process is identifying who will coordinate and lead a newly formed multidisciplinary endocarditis team. Having designated leadership is crucial for recruitment of team members, reviewing existing outcomes (see Phase II), organizing and moderating meetings and directing research. It is also helpful to have a ‘point person’ for other providers and administrators at an institution to contact with questions about endocarditis. The leadership can also apply for institutional review board (IRB) approval for review of retrospective data and future team research projects. Depending on the hospital, there may be funding available for such a position. However, in practice these, efforts often develop without financial support. Ideally, the multidisciplinary team will be led by 1–2 individuals who are principally involved in the care of patients with endocarditis at the institution. Infectious disease (ID) providers are well suited for this role as every patient with endocarditis requires antibiotic therapy, and ID consultation has been shown to improve mortality in multiple types of blood stream infections.8,9 However, cardiac surgeons, cardiologists and hospitalists are also good candidates for this position. In some circumstances, medical residents or fellows can play a significant role as they are well-versed in hospital workflow and may have protected research time they can devote to the project. 10 If the development of an endocarditis team is driven by the hospital administration, then the team leadership may be appointed. In the absence of such a directive, then the clinical leads are often the individuals who are most passionate about improving the care of the patients with endocarditis or have clinical or research interests in this domain.
Phase II: identify the problem(s)
A crucial early step is understanding the population of patients with endocarditis that an individual institution cares for and what their clinical outcomes are relative to national numbers. In the United States, the in-hospital mortality for infectious endocarditis is reported as ranging from 15% to 20%, but theses outcomes may vary between regions and medical centers. 11 Utilizing an internal data collection tool, the authors performed a retrospective chart review of all endocarditis admissions to their institution over a 1-year period in order to help better understand the patient population and its needs. 4 A developing endocarditis team should understand its own institution’s mortality outcomes and attempt to identify which variables contribute to these results. For example, it is important to determine whether a significant proportion of the hospital’s patients with endocarditis are individuals who inject drugs. This will help inform the future composition of the team as addiction medicine/psychiatry and social work play critical roles in caring for this population. 12 If these providers are not already available at an institution, endocarditis team members can advocate for their hospital to acquire more addiction treatment resources.
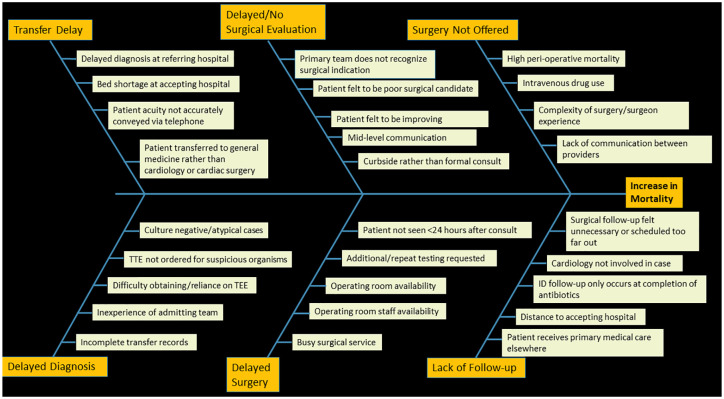
There are myriad challenges associated with caring for all patients with endocarditis with many opportunities for interruptions in care. During preliminary meetings, the authors used a quality improvement informed approach to create a ‘fishbone diagram’ that identified factors contributing to delays in care or adverse outcomes (Figure 2). 13 As highlighted in Figure 2, delays may occur in obtaining certain diagnostic testing, such as transesophageal echocardiography, magnetic resonance imaging, or coronary angiography. 14 At the authors institution most patients with endocarditis are admitted as outside hospital referrals for surgical evaluation. However, many of these transfers happen on Friday evenings due to bed availability. Endocarditis admissions occurring over the weekend have notably been associated with adverse outcomes. 15 Consequently, the authors’ endocarditis team scheduled meetings on Monday mornings to ensure that all patients presenting over the weekends were discussed by the group in a timely fashion. In addition, medical providers may not always be aware of endocarditis surgical indications or may feel that patients are unlikely to be offered surgery. This may result in delays in or the absence of cardiac surgical consultation which can in-turn serve to increase in-hospital mortality as early surgical intervention is associated with improved clinical outcomes.16–18 At our institution, these obstacles were overcome by hiring an advanced practice provider (APP) to increase the capacity of the cardiac surgery department to see new consults and by development of a best practice advisory in the electronic medical record that suggested a cardiac surgery consultation to providers whenever a diagnosis of endocarditis was made.
Figure 2.
Fishbone diagram created by members of the University of Michigan Multidisciplinary Endocarditis Team outlining potential factors contributing to increased mortality for patients with endocarditis.
Phase III: recruiting team members
After a leadership structure has been identified and existing outcomes are reviewed, the next phase involves recruitment of the other key team members. Previous studies have highlighted that an effective multidisciplinary endocarditis team should include representatives from ID, cardiac surgery, cardiology (including cardiologists with expertise in echocardiography and electrophysiology), neurology and/or neurosurgery, and pharmacy (Table 1).1,2 Inclusion of addiction medicine/psychiatry and social work is also imperative as almost all referral centers will care for patients with injection drug use associated endocarditis. 12 If the hospital has an outpatient antimicrobial treatment program, it may be beneficial to include these providers as well. Although not every patient with endocarditis will have an indication for valve surgery initially, they may develop indications subsequently. In addition, cardiac surgeons provide valuable experience and perspective when considering complex valvular pathology as evidenced by their inclusion in heart valve teams. 19 Neurology and neurosurgery participation could be limited to instances when patients have a neurologic complication of endocarditis.
Table 1.
Roles and recommended attendance of the specialties comprising a multidisciplinary endocarditis team.
| Specialty | Role | Recommended attendance |
|---|---|---|
| Infectious diseases | 1. Case ascertainment 2. Screen patients for substance use disorder 3. Selection of antibiotic regimen and duration 4. Coordinate outpatient follow-up |
All meetings |
| Cardiology | 1. Review echocardiographic and angiographic imaging 2. Provide recommendations for and interpretation of advanced cardiac imaging modalities (i.e., PET) 3. Recommendations regarding follow-up imaging |
All meetings |
| Cardiac Surgery | 1. Evaluate and select patients for valve surgery 2. Recommendations regarding pre-operative testing 3. Recommendations regarding follow-up |
All meetings |
| Pharmacy | 1. Recommendations about optimal dosing of antimicrobial | All meetings |
| Neurology | 1. Recommendations about neurologic imaging 2. Recommendations about the timing of anti-coagulation in patients undergoing valve surgery |
As needed – for patients with known neurologic complications of endocarditis |
| Neurosurgery | 1. Screening and management of mycotic aneurysms | As needed – for patients with known neurologic complications of endocarditis |
| Addiction medicine/psychiatry | 1. Initiation of medication assisted treatment for substance use disorder 2. Link patients to outpatient programs for ongoing treatment |
As needed – for patients with substance use disorder complicating the diagnosis of endocarditis |
PET, positron-emission tomography.
The identification of team members may be challenging, given that in most circumstances, there is no financial reimbursement to providers for the time commitment associated with participation. There can be multiple effective strategies for recruiting providers from the various necessary specialties. If the endocarditis team leader(s) are well known in the hospital, then direct communication with a colleague is a straightforward and expedient approach. However, if the leadership is relatively new to the medical center, then it may be prudent to have these individuals present the existing endocarditis data and the benefits of multidisciplinary endocarditis teams to the various specialties at a regularly scheduled departmental meeting. The authors both directly contacted providers with whom they had existing relationships and presented at the monthly faculty meetings for the divisions of cardiology, infectious diseases, and the department of cardiac surgery. If possible, teams should seek to involve 2–3 specialists from each department to ensure that consistent representation is not dependent on the availability of one person. The authors would also recommend including any APPs that work closely with physicians in the designated specialties.
Phase IV: scheduling the meetings/choosing a location
Scheduling a meeting may seem like a mundane, straightforward task that should not require much discussion. However, an effective endocarditis team is comprised of upwards of 10 medical providers who have numerous competing clinical and research responsibilities. While all providers are important to the success of the team, cardiac surgery is the most specialized of the involved disciplines and is closely associated with improved endocarditis survival.16–18 In addition, surgeons often have limited availability for such interdisciplinary work, given their responsibilities in the operating room. Therefore, the authors would recommend scheduling the meetings to accommodate as many cardiac surgeons as possible. This will require other team members to also acknowledge the prominent role of cardiac surgery. The day(s) of the week the meeting is held can also have significant impact on the team’s effectiveness. As alluded to previously, at the authors institution many patients are admitted over the weekend, prompting the scheduling of one team meeting on Monday mornings. The authors would recommend against holding conferences on Fridays as it may be challenging for team recommendations to be implemented prior to and over the weekend. Depending on the clinical volume some hospitals may find it necessary to meet for 30 or 60 minutes and to hold more than 1 meeting per week. Ad hoc meetings can be considered but may be hard to implement, given the busy practices of the involved clinicians. One advantage of the multidisciplinary endocarditis team is that it fosters familiarity between providers which can allow for increased communication regarding patient care outside of scheduled meetings, such as via phone call or e-mail.
With respect to choosing a designated meeting place, again it may be practical to select a location in close proximity to the surgery departmental offices. The ideal space has the capacity to seat 15–20 people with audio-visual capabilities, including access to echocardiographic and coronary angiographic images so that they may be reviewed by team specialists. Ultimately, the authors elected to hold their meeting in the cardiac surgery conference room adjacent to the surgeons’ offices. Generally, the authors recommend that the endocarditis team meet in-person to allow providers to build rapport with one another and to avoid miscommunications or interpersonal conflict. However, providing a virtual meeting option may also allow for increased attendance and social distancing if necessary.
Phase V: developing a protocol
Once the key team members have been selected and a designated meeting time and location are chosen, initial discussions would ideally focus on developing an institutional protocol for management of endocarditis cases. Although there are consensus endocarditis management guidelines published by the American Heart Association and the European Society of Cardiology (ESC), there are also notable gaps in these resources.3,20 Among these are issues surrounding the management of patients with injection drug use associated endocarditis. In addition, the broader medical endocarditis literature is limited by a dearth of randomized controlled trial in this patient population. Consequently, there is significant provider-dependent variability with respect to management recommendations, particularly surrounding decisions regarding echocardiographic imaging, surgery, and neurologic complications of endocarditis. Such protocols have correlated with the improvement in clinical outcomes described in previous studies. 2 While there are some aspects of endocarditis patient care that are consistent among hospitals, each institution has its own unique patient population as well as strengths and weaknesses. For example, positron-emission tomography (PET) is endorsed by the ESC guideline as an important adjunctive tool for diagnosing endocarditis. 3 However, many centers do not have clinical cardiac PET programs available to them. The multidisciplinary endocarditis team, supported by internal data (see Phase II), is best equipped to tailor a protocol unique to their hospital. After creation of their endocarditis team, the authors spent the first month of their group’s meetings developing a protocol which provided guidance about initial laboratory and imaging evaluation in suspected endocarditis, when to pursue advanced cardiac imaging modalities, how to screen for substance use disorders and when to involve addiction treatment providers, recommendations about neurologic imaging and when to pursue medical or surgical management. The authors’ full protocol has been previously published elsewhere. 4 Once a consensus document is created and approved by team members, the authors would recommend that the group makes the algorithm publicly available to all health care providers within their institution.
Phase VI: case identification
In general, all cases of definite endocarditis as defined by the modified Duke Criteria should be presented for discussion by the multidisciplinary endocarditis team. 21 The authors would also suggest that the group present patients with possible endocarditis, particularly if a valve vegetation is present, given the relative insensitivity of the Duke Criteria. 22 It is also reasonable for the team to consider cases where the diagnosis is uncertain at the request of the patient’s primary medical providers. The process of ascertaining endocarditis cases for presentation at regular gatherings of the endocarditis team may vary considerably between hospitals. Team leaders can consider different approaches based on their center’s internal workflow. Methods for identifying patients can include utilizing the ID consult service (the authors’ approach) as the majority of, if not all, patients with endocarditis will receive ID consultation. Depending on the volume of patients, the ID division may consider dedicating one of their consult services specifically to patients with endocarditis. The cardiac surgery consult team may also capture patients who are not identified by ID. Alternatively, patients can be found using ICD diagnosis codes. However, this approach may result in detection of an excess number of patients including those with a history of endocarditis that is not relevant to their current presentation. If this strategy is selected, the endocarditis team would then have to review each case to ensure they are appropriate for discussion. A formalized referral process could be considered but would require the presence of individuals who can review and triage patient cases.
Phase VII: conducting the meetings/case presentations
After recruiting members, agreeing to a standardized protocol and determining a method of case identification, the team is now positioned to begin presenting patient cases. There is no literature to support the best method for how to review patients. However, the authors have found from personal experience that certain practices may improve the caliber of the team’s discussion and the effectiveness of the meetings. First, the team leads should contact the group with a list of patients to be presented approximately 24 hours before the meeting. This will allow team members who are not directly involved in a patient’s care to review the case details in advance. For example, a neurologist can review patient imaging and provide recommendations via email about timing of anti-coagulation prior to the meeting. The authors also recommend that the endocarditis team invite providers from patients’ primary teams to the conferences as well as any consultants that may not be team members but whose input will directly impact management (for example, including a hepatologist for a patient with cirrhosis). It may be helpful to have a designated physician or APP moderator who facilitates the conversation and calls on specialists to give their input on pertinent clinical questions. This individual can also prepare a brief visual presentation that includes background on the patient’s medical, surgical and social history as well as the results of validated surgical and embolism risk calculators.23,24 This presentation would ideally also include pertinent radiologic, echocardiographic, and angiographic images. After hearing input from team members, the moderator can then help summarize the discussion and push the group toward making a final recommendation. The team can also use its meetings as an internal morbidity and mortality conference to review adverse events or poor outcomes, thereby continuing process improvements on a regular basis.
Phase VIII: documenting and communicating recommendations
A critical component of a successful endocarditis team is efficient and clear documentation of recommendations. Although providers cannot bill for their involvement with these meetings, the communication of recommendations through the medical record allows providers who are unable to attend the meetings to reference the discussion. Documentation is ideally completed by one of the team leads or the meeting moderator. A previous survey of providers at the authors’ institution found that a majority of health care providers felt the implementation of a multidisciplinary endocarditis team led to improved documentation of clinical recommendations. 25 Since the note is not being utilized for billing purposes, it can focus explicitly on the medical decision-making. For future research purposes, it may be useful for the team to document in their note important features of the case including the Duke Criteria, type and location of the involved valve, etiologic organism, echocardiographic and radiologic findings as well as surgical and embolism risk calculations. Ideally, primary team members will attend the multidisciplinary discussion. However, if they are unable due to their other clinical responsibilities the authors suggest that an endocarditis team member convey the recommendations either in-person or by phone.
Phase IX: maintaining a database/applying for funding
In order to demonstrate its clinical benefit, the multidisciplinary endocarditis team should longitudinally follow their patients with an institutional registry. As discussed earlier, the team leadership can apply for IRB approval to store patient information and review mortality. Collection of a broad range of demographic, clinical, microbiologic, echocardiographic, and outcome-related variables will help the team understand how it is affecting patient care and creates substantial research opportunities. 4 Although dedicated funding to support an endocarditis team may not be initially available, if the group can demonstrate decreases in mortality, length of stay, readmissions, or antibiotic usage then they can petition their hospital for financial support as a cost-saving measure. Assessment of clinicians’ perceptions of the endocarditis team can also help guide future team decisions. A survey of providers at the authors’ institution demonstrated that over 85% of respondents agreed that the group influenced diagnostic evaluation, reduced management errors, increased access to surgery, and decreased in-hospital mortality for patients with endocarditis. 25 In addition, once the team has collected sufficient data it can apply for external research grant funding. With dedicated resources the multidisciplinary endocarditis team could expand its reach, by offering virtual consultation to providers at smaller hospitals within a given healthcare network, as recommend by the ESC guidelines. 3
Conclusion
While the clinical benefits of multidisciplinary endocarditis teams are well-established, creation of an effective, consistent collaboration between multiple specialties can be challenging to accomplish. There are numerous variables to consider, any of which can derail this promising initiative. Despite the inclusion of endocarditis teams in the ESC guidelines, there has been little direction provided to clinicians about how to best construct and operate these groups. In this paper, the authors have used their own data-supported personal experiences as well as available data to outline a step-by-step guide to creation of a successful multidisciplinary endocarditis team. In conjunction with existing literature, this piece can be used as a resource for clinicians seeking to improve the care of patients with endocarditis at their institutions.
Footnotes
Author contributions: Sami El-Dalati: Conceptualization; Writing – original draft; Writing – review & editing.
Daniel Cronin: Writing – review & editing.
James Riddell: Writing – review & editing.
Michael Shea: Writing – review & editing.
Richard Weinberg: Writing – review & editing.
Emily Stoneman: Writing – review & editing.
Twisha Patel: Writing – review & editing.
Kirra Ressler: Writing – review & editing.
George Michael Deeb: Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our study did not require an ethical board approval because it did not contain human or animal trials.
ORCID iD: Sami El-Dalati  https://orcid.org/0000-0003-2603-2951
https://orcid.org/0000-0003-2603-2951
Contributor Information
Sami El-Dalati, Division of Infectious Diseases, Department of Internal Medicine, University of Kentucky, 740 South Limestone Street, Lexington, KY 40536, USA.
Daniel Cronin, Division of Hospital Medicine, Department of Internal Medicine, Michigan Medicine – University of Michigan, Ann Arbor, MI, USA.
James Riddell, IV, Division of Infectious Diseases, Department of Internal Medicine, Michigan Medicine – University of Michigan, Ann Arbor, MI, USA.
Michael Shea, Division of Cardiology, Department of Internal Medicine, Michigan Medicine – University of Michigan, Ann Arbor, MI, USA.
Richard L. Weinberg, Division of Cardiology, Department of Internal Medicine, Northwestern University, Chicago, IL, USA
Emily Stoneman, Division of Infectious Diseases, Department of Internal Medicine, Michigan Medicine – University of Michigan, Ann Arbor, MI, USA.
Twisha Patel, College of Pharmacy, Michigan Medicine – University of Michigan, Ann Arbor, MI, USA.
Kirra Ressler, Department of Cardiac Surgery, Michigan Medicine – University of Michigan, Ann Arbor, MI, USA.
George Michael Deeb, Department of Cardiac Surgery, Michigan Medicine – University of Michigan, Ann Arbor, MI, USA.
References
- 1. Davierwala PM, Marin-Cuartas M, Misfeld M, et al. The value of an ‘Endocarditis Team’. Ann Cardiothorac Surg 2019; 8: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis–related mortality with a management-based approach. Arch Intern Med 2009; 169: 1290–1298. [DOI] [PubMed] [Google Scholar]
- 3. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J 2015; 36: 3075–3128. [DOI] [PubMed] [Google Scholar]
- 4. El-Dalati S, Cronin D, Riddell JIV, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. Epub ahead of print 1 March 2021. DOI: 10.1016/j.athoracsur.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 5. Schranz AJ, Fleischauer A, Chu VH, et al. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med 2019; 170: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serota DP, Barocas JA, Springer SA. Infectious complications of addiction: a call for a new subspecialty within infectious diseases. Clin Infect Dis 2020; 70: 968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart 2014; 100: 524–527. [DOI] [PubMed] [Google Scholar]
- 8. Lahey T, Shah R, Gittzus J, et al. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore) 2009; 88: 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chesdachai S, Kline S, Helmin D, et al. The effect of infectious diseases consultation on mortality in hospitalized patients with methicillin-resistant Staphylococcus aureus, Candida, and Pseudomonas bloodstream infections. Open Forum Infect Dis 2020; 7: ofaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El-Dalati S, Cronin D. The importance of being earnest. Acad Med 2021; 96: 18–20. [DOI] [PubMed] [Google Scholar]
- 11. Bor DH, Woolhandler S, Nardin R, et al. Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS ONE 2013; 8: e60033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weimer MB, Falker CG, Seval N, et al. The need for multidisciplinary hospital teams for injection drug use-related infective endocarditis. J Addict Med. Epub ahead of print 10 September 2021. DOI: 10.1097/ADM.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 13. Harel Z, Silver SA, McQuillan RF, et al. How to diagnose solutions to a quality of care problem. Clin J Am Soc Nephrol 2016; 11: 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Dalati S, Shea M, Fukuhara S, et al. The role of coronary catheterization with angiography in surgically managed infectious endocarditis. Am J Med 2020; 133: 1101–1104. [DOI] [PubMed] [Google Scholar]
- 15. Guidi JL, Golbus JR, Thomas MP, et al. A Friday night transfer. Circulation 2019; 12: e005856. [DOI] [PubMed] [Google Scholar]
- 16. Bishara J, Leibovici L, Gartman-Israel D, et al. Long-term outcome of infective endocarditis: the impact of early surgical intervention. Clin Infect Dis 2001; 33: 1636–1643. [DOI] [PubMed] [Google Scholar]
- 17. Nadji G, Goissen T, Brahim A, et al. Impact of early surgery on 6-month outcome in acute infective endocarditis. Int J Cardiol 2008; 129: 227–232. [DOI] [PubMed] [Google Scholar]
- 18. Kang D, Kim Y, Kim S, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366: 2466–2473. [DOI] [PubMed] [Google Scholar]
- 19. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 143: e35–e71. [DOI] [PubMed] [Google Scholar]
- 20. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals From the American Heart Association. Circulation 2015; 132: 1435–1486. [DOI] [PubMed] [Google Scholar]
- 21. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30: 633–638. [DOI] [PubMed] [Google Scholar]
- 22. Shrestha N, Shakya S, Hussain S, et al. Sensitivity and specificity of Duke Criteria for diagnosis of definite infective endocarditis: a cohort study. Open Forum Infect Dis 2017; 4: S550–S551. [Google Scholar]
- 23. Wang TK, Oh T, Voss J, et al. Comparison of contemporary risk scores for predicting outcomes after surgery for active infective endocarditis. Heart Vessels 2015; 30: 227–234. [DOI] [PubMed] [Google Scholar]
- 24. Hubert S, Thuny F, Resseguier N, et al. Prediction of symptomatic embolism in infective endocarditis: construction and validation of a risk calculator in a multicenter cohort. J Am Coll Cardiol 2013; 62: 1384–1392. [DOI] [PubMed] [Google Scholar]
- 25. El-Dalati S, Khurana I, Soper N, et al. Physician perceptions of a multidisciplinary endocarditis team. Eur J Clin Microbiol Infect Dis 2020; 39: 735–739. [DOI] [PubMed] [Google Scholar]