Abstract
Objective
To determine whether the modified Glasgow prognostic score (mGPS) and high-sensitivity mGPS (HS-mGPS) could predict outcomes among patients with hypopharyngeal squamous cell carcinoma (HSCC).
Study Design
Retrospective cohort study.
Setting
Affiliated university hospital.
Methods
We reviewed the records of 115 patients with histologically confirmed HSCC between March 2007 and December 2019. Univariate and multivariable Cox proportional hazard analyses were performed for overall survival (OS) and disease-free survival (DFS).
Results
The 5-year OS rates were 84.0% for the mGPS0 group, 47.8% for the mGPS1 group, and 17.9% for the mGPS2 group (P < .0001), while the 5-year OS rates were 86.7% for the HS-mGPS0 group, 69.0% for the HS-mGPS1 group, and 22.2% for the HS-mGPS2 group (P < .001). The mGPS and HS-mGPS were both associated with OS in the univariate analyses, although only the HS-mGPS was independently associated with OS (hazard ratio, 2.68 [95% CI, 1.19-6.05]; P < .05). The 5-year DFS rates were 75.8% for the mGPS0 group, 53.0% for the mGPS1 group, and 13.8% for the mGPS2 group (P < .001), while the 5-year DFS rates were 79.8% for the HS-mGPS0 group, 56.8% for the HS-mGPS1 group, and 11.6% for the HS-mGPS2 group (P < .001). The mGPS and HS-mGPS were both associated with DFS in the univariate analyses, although only the HS-mGPS was independently associated with DFS (hazard ratio, 2.35 [95% CI, 1.03-5.37]; P < .05).
Conclusion
Our study suggests that the HS-mGPS is useful as prognostic factor in HSCC.
Keywords: modified Glasgow prognostic score, high-sensitivity modified Glasgow prognostic score, head and neck cancer, C-reactive protein, survival
Despite improvements in the treatment of many head and neck cancers, outcomes for hypopharyngeal squamous cell carcinoma (HSCC) have remained relatively poor and exhibited only marginal improvements in survival. 1 Recurrence is also quite common, as nearly 50% of patients experience recurrence within the first year after diagnosis and are frequently diagnosed with distant metastasis. 2 The available treatment options for HSCC include surgery, radiotherapy, and/or chemotherapy, which are based on the clinical stage and comorbid conditions.
The TNM staging system is used to differentiate between patients with early-stage and more advanced disease; it also provides reliable prognostic information. However, the TNM system is less accurate for predicting the prognosis of patients with an intermediate extent of tumor invasion. 3 Therefore, a more accurate method for predicting prognosis is needed to provide appropriate preoperative counseling and guide treatment selection.
Several inflammation-based scoring systems have been devised, and they are strongly associated with prognoses among patients with various neoplasms. One common system is the Glasgow prognostic score (GPS), 4 which considers elevated serum C-reactive protein (CRP) concentrations and hypoalbuminemia. Blood-based changes during acute-phase reactions can also be evaluated by the neutrophil-to-lymphocyte ratio 5 and platelet-to-lymphocyte ratio. 6 The modified GPS (mGPS) can be used to evaluate systemic inflammation and nutritional status in patients with cancer based on serum concentrations of CRP and albumin. The mGPS has been validated as an independent prognostic factor in various malignancies, including hypopharyngeal cancer, 7 and Iuchi et al reported that combining the mGPS with conventional TNM staging provided more accurate prognostic predictions. 7 Recent research has established that the high-sensitivity mGPS (HS-mGPS) is an even more sensitive prognostic marker for several cancers.8,9
Few studies have evaluated these systems in cases of head and neck cancers, which involve different primary lesions, treatment methods, and expected outcomes. 8 Therefore, the present study aimed to evaluate whether the mGPS and HS-mGPS could predict outcomes among patients with HSCC and to compare the prognostic values of these 2 systems.
Methods
Patient Inclusion and Exclusion Criteria
This retrospective study evaluated 142 consecutive patients who underwent initial treatment at the Department of Otolaryngology–Head and Neck Surgery at Kagoshima University between March 2007 and December 2019. This retrospective study was approved by the institutional review board of Kagoshima University (180238). Other inclusion criteria were as follows: primary head and neck cancer in the hypopharynx, no history of treatment for other head and neck cancer, pathologic diagnosis as squamous cell carcinoma, and cases in which the CRP and albumin levels had been measured at the initial diagnosis. The following cases were excluded: 5 duplicate cases, 7 with recurrence, 3 with distant metastasis, and 12 in which the CRP and albumin concentrations were not measured at the initial diagnosis. Based on these criteria, the study ultimately included 115 patients with HSCC. Clinical stages were diagnosed according to the TNM staging system of the American Joint Committee on Cancer, seventh edition.
Treatment Protocol
Induction therapy is defined as chemotherapy that facilitates subsequent local therapy, such as definitive concurrent chemoradiotherapy (CCRT) or surgery. Induction chemotherapy involved docetaxel (60 mg/m2, day 1) plus cisplatin (60 mg/m2, day 1) and fluorouracil (600 mg/m2, days 1-4). CCRT consisted of a standard regimen with cisplatin (100 mg/m2; days 1, 22, and 43) or carboplatin (area of curve, 5; every week) plus radiotherapy (conventional fractionation, 1.8-2.0 Gy; once daily, 5 d/wk, until a total tumor dose of 70 Gy). Postoperative adjuvant concurrent CCRT was performed for high-risk patients based on positive surgical margins or extranodal/perineural invasion near the cervical lymph nodes.
Treatment Assessment and Follow-up
Clinical response was assessed according to the Response Evaluation Criteria in Solid Tumors (version 1.1) at 4 to 6 weeks after the completion of treatment. Follow-up evaluations included physical examination, blood tests, endoscopy, and enhanced computed tomography of the neck and chest. Patients were followed up every 3 months for the first year, every 6 months for the second year, and then annually thereafter.
Inflammation-Based Prognostic Scores
Laboratory testing had been performed on the day of admission, and the results were searched to determine the patients’ serum CRP and albumin concentrations. The mGPS was calculated as previously reported 10 : a score of 2 was assigned to patients with an elevated CRP concentration (>1.0 mg/dL) and a reduced albumin concentration (<3.5 g/dL); a score of 1 was assigned to patients with an elevated CRP concentration (>1.0 mg/dL) and a nondecreased albumin concentration (≥3.5 g/dL); and a score of 0 was assigned to patients without an elevated CRP concentration (≤1.0 mg/dL), regardless of their albumin concentration. The cutoff value for the CRP concentration was reduced to 0.3 mg/dL for the HS-mGPS.
Statistical Analyses
All statistical analyses were performed with SPSS for Windows software (version 27.0; IBM Corp). Overall survival (OS) was defined as the primary endpoint and calculated as the interval between the first admission (the same time as the blood test) and the first instance of the date of death due to any cause or the last follow-up. Disease-free survival (DFS) was defined as no evidence of disease, whether local or systemic, from the date of diagnosis to the date of documented death or loss of follow-up. Survival outcomes were estimated with the Kaplan-Meier method and compared with the log-rank test. Subjects were treated as censored if they were lost to follow-up. The relationships between survival outcomes and the mGPS or the HS-mGPS were evaluated with multivariate Cox proportional hazard models, and the results were reported as the hazard ratio (HR) and 95% CI. All tests were 2-sided, and differences were considered significant at P values <.05.
Results
Patient Characteristics
Table 1 shows the baseline characteristics of the 115 included patients. The median follow-up period was 62 months (range, 3-149 months). The median age at diagnosis was 68 years (range, 48-88 years), and the majority of patients were male (97.4%). Most patients had a performance status of 0 (72.2%) and stage IV disease (63.5%). The primary tumors were located at the pyriform sinus (78.2%), postcricoid (15.7%), and posterior wall (6.1%). The mGPS scores were 0 for 63 patients, 1 for 39 patients, and 2 for 13 patients. The mGPS was significantly associated with T classification (P = .011) and TNM stage (P = .04). The HS-mGPS scores were 0 for 57 patients, 1 for 38 patients, and 2 for 20 patients. The HS-mGPS was significantly associated with T classification (P = .002) and TNM stage (P = .001).
Table 1.
Baseline Patient Characteristics (N = 115). a
| mGPS | HS-mGPS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | 0 | 1 | 2 | P value | 0 | 1 | 2 | P value | |
| Age, y | |||||||||
| <68 | 54 (47.0) | 32 | 20 | 2 | .053 | 30 | 17 | 7 | .378 |
| ≥68 | 61 (53.0) | 31 | 19 | 11 | 27 | 21 | 13 | ||
| Sex | |||||||||
| Male | 112 (97.4) | 61 | 38 | 13 | >.999 | 55 | 37 | 20 | >.999 |
| Female | 3 (2.6) | 2 | 1 | 0 | 2 | 1 | 0 | ||
| Performance status b | |||||||||
| 0 | 83 (72.2) | 52 | 26 | 8 | .173 | 47 | 27 | 12 | .207 |
| 1 | 24 (20.1) | 9 | 12 | 4 | 8 | 10 | 7 | ||
| 2 | 8 (7.7) | 2 | 1 | 1 | 2 | 1 | 1 | ||
| Tumor depth c | |||||||||
| T2 | 43 (37.4) | 30 | 10 | 3 | .011 | 29 | 10 | 4 | .002 |
| T3 | 46 (40.0) | 26 | 14 | 6 | 23 | 13 | 10 | ||
| T4 | 26 (22.6) | 7 | 15 | 4 | 5 | 15 | 6 | ||
| Lymph node metastasis c | |||||||||
| N0 | 38 (33.0) | 24 | 10 | 4 | .067 | 24 | 9 | 5 | .074 |
| N1 | 15 (13.0) | 9 | 2 | 4 | 8 | 2 | 5 | ||
| N2 | 58 (50.0) | 29 | 25 | 4 | 24 | 25 | 9 | ||
| N3 | 4 (4.0) | 1 | 2 | 1 | 1 | 2 | 1 | ||
| TNM stage c | |||||||||
| II | 22 (19.1) | 17 | 5 | 0 | .04 | 17 | 4 | 1 | .001 |
| III | 20 (17.4) | 11 | 3 | 6 | 11 | 2 | 7 | ||
| IV | 73 (63.5) | 35 | 31 | 7 | 29 | 32 | 12 | ||
| Tumor location | |||||||||
| Pyriform sinus | 90 (78.2) | 48 | 33 | 9 | .692 | 44 | 32 | 14 | .529 |
| Postcricoid | 18 (15.7) | 10 | 5 | 3 | 8 | 5 | 5 | ||
| Posterior wall | 7 (6.1) | 5 | 1 | 1 | 5 | 1 | 1 | ||
| Treatment | |||||||||
| Induction chemotherapy | 56 (58.7) | 27 | 21 | 8 | .920 | 23 | 23 | 10 | .597 |
| CCRT | 84 (73.0) | 45 | 29 | 10 | 42 | 28 | 14 | ||
| TPLE + neck dissection | 31 (27.0) | 18 | 10 | 3 | 15 | 10 | 6 | ||
Abbreviations: CCRT, concurrent chemoradiotherapy HS-mGPS, high-sensitivity modified Glasgow prognostic score; mGPS, modified Glasgow prognostic score; TPLE, total pharyngolaryngoesophagectomy.
The groups were compared with the chi-square test or Fisher’s exact test.
Per the Eastern Cooperative Oncology Group.
Staging based on the guidelines of the Union for International Cancer Control, seventh edition.
Treatment Characteristics
Table 1 shows the treatment characteristics of the 115 included patients. On the basis of treatment intents, 84 (73%) patients received CCRT and 31 (27%) underwent surgery. In addition, 56 (58.7%) patients received induction chemotherapy. In patients who received CCRT, 40.0% (n = 31) underwent induction chemotherapy. In patients who received surgery, 80.1% (n = 25) received induction chemotherapy. The regimen for CCRT was triweekly cisplatin for 64% and weekly carboplatin for 36%. The median dose of irradiation was 69.2 Gy (range, 66-70). There was no significant difference in treatment content between the mGPS group and the HS-mGPS group.
Relationship Between OS and the mGPS or HS-mGPS
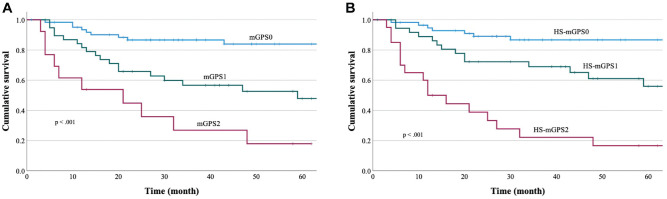
The Kaplan-Meier curves for OS were compared according to the mGPS ( Figure 1A ), which revealed 5-year OS rates of 84.0% for the mGPS0 group, 47.8% for the mGPS1 group, and 17.9% for the mGPS2 group. Significant differences in the OS outcomes were observed among the mGPS0, mGPS1, and mGPS2 groups (HR, 2.96 [95% CI, 1.94-4.55]; P < .0001). The Kaplan-Meier curves for OS were also compared according to the HS-mGPS ( Figure 1B ), which revealed 5-year OS rates of 86.7% for the HS-mGPS0 group, 69.0% for the HS-mGPS1 group, and 22.2% for the HS-mGPS2 group. Significant differences in the OS outcomes were observed among the HS-mGPS0, HS-mGPS1, and HS-mGPS2 groups (HR, 3.17 [95% CI, 2.10-4.89]; P < .0001). Univariate analyses revealed that OS was associated with T classification (P < .001), N classification (P = .018), clinical stage (P = .01), the mGPS (P < .001), and the HS-mGPS (P < .001; Table 2 ). Multivariable analysis revealed that only the HS-mGPS independently predicted OS (HR, 2.68 [95% CI, 1.19-6.05]; P < .05).
Figure 1.
Overall survival was significantly associated with (A) the modified Glasgow prognostic score (log-rank P < .001) and (B) the high-sensitivity modified Glasgow prognostic score (log-rank P < .001).
Table 2.
Univariate and Multivariable Analyses of Overall Survival.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | P value | HR (95% CI) | P value | HR (95% CI) |
| Age: <68 vs ≥68 y | .947 | 1.022 (0.542-1.935) | ||
| Sex: male vs female | .985 | 1.019 (0.057-4.715) | ||
| Performance status a : 0 vs 1-2 | .24 | 1.408 (0.834-2.249) | ||
| Smoking status: non vs ex or current | .196 | 0.725 (0.445-1.181) | ||
| Drink: non vs ex or current | .736 | 0.898 (0.481-1.678) | ||
| Tumor stage: 1, 2, 3, 4 | <.001 | 2.111 (1.402-3.233) | .053 | 1.748 (0.983-3.108) |
| Nodal stage: 0, 1, 2, 3 | .018 | 1.754 (1.22-2.609) | .086 | 1.766 (0.890-3.502) |
| AJCC stage: I, II, III, IV | .01 | 2.101 (1.306-3.768) | .756 | 0.839 (0.276-1.192) |
| Tumor location: PS, PC, PW | .147 | 1.343 (0.818-2.063) | ||
| mGPS | <.001 | 2.964 (1.938-4.533) | .552 | 1.272 (0.568-2.850) |
| HS-mGPS | <.001 | 3.179 (2.089-4.838) | .023 | 2.683 (1.189-6.053) |
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; HS-mGPS, high-sensitivity modified Glasgow prognostic score; mGPS, modified Glasgow prognostic score; PC, postcricoid; PS, pyriform sinus; PW, posterior wall.
Per the Eastern Cooperative Oncology Group.
Relationship Between DFS and the mGPS or HS-mGPS
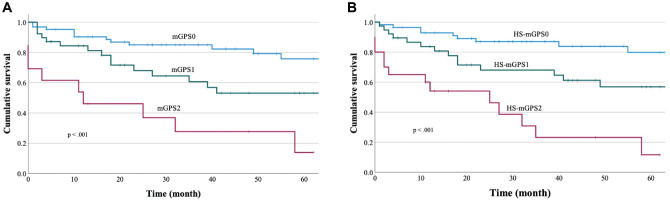
The Kaplan-Meier curves for DFS were compared according to the mGPS ( Figure 2A ), which revealed 5-year DFS rates of 75.8% for the mGPS0 group, 53.0% for the mGPS1 group, and 13.8% for the mGPS2 group. Significant differences in DFS outcomes were observed among the mGPS0, mGPS1, and mGPS2 groups (HR, 2.664 [95% CI, 1.741-4.076]; P < .001). The Kaplan-Meier curves for DFS were also compared according to the HS-mGPS ( Figure 2B ), which revealed 5-year DFS rates of 79.8% in the HS-mGPS0 group, 56.8% in the HS-mGPS1 group, and 11.6% in the HS-mGPS2 group. Significant differences in the DFS outcomes were observed among the HS-mGPS0, HS-mGPS1, and HS-mGPS2 groups (HR, 2.896 [95% CI, 1.893-4.429]; P < .001). Univariate analyses revealed that DFS was associated with performance status (P = .034), N classification (P = .028), the mGPS (P < .001), and the HS-mGPS (P < .001; Table 3 ). Multivariable analysis revealed that DFS was independently predicted only by performance status (HR, 1.89 [95% CI, 1.07-3.33]; P < .05) and the HS-mGPS (HR, 2.35 [95% CI, 1.03-5.37]; P < .05).
Figure 2.
Disease-free survival was significantly associated with (A) the modified Glasgow prognostic score (log-rank P < .001) and (B) the high-sensitivity modified Glasgow prognostic score (log-rank P < .001).
Table 3.
Univariate and Multivariable Analyses of Disease-Free Survival.
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Variable | P value | HR (95% CI) | P value | HR (95% CI) |
| Age: <68 vs ≥68 y | .625 | 1.174 (0.618-2.229) | ||
| Sex: male vs female | .385 | 1.881 (0.452-7.837) | ||
| Performance status a : 0 vs 1-2 | .034 | 1.730 (1.042-2.872) | .027 | 1.893 (1.076-3.329) |
| Smoking status: non vs ex or current | .585 | 0.865 (0.515-1.454) | ||
| Drink: non vs ex or current | .161 | 1.629 (0.823-3.227) | ||
| Tumor stage: 1, 2, 3, 4 | .142 | 1.364 (0.901-2.063) | ||
| Nodal stage: 0, 1, 2, 3 | .028 | 1.516 (1.047-2.196) | .151 | 1.336 (0.900-1.984) |
| AJCC stage: I, II, III, IV | .157 | 1.362 (0.888-2.088) | ||
| Tumor location: PS, PC, PW | .944 | 0.981 (0.571-1.685) | ||
| mGPS | <.001 | 2.664 (1.741-4.076) | .448 | 1.398 (0.589-3.319) |
| HS-mGPS | <.001 | 2.896 (1.893-4.429) | .041 | 2.356 (1.034-5.367) |
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; HS-mGPS, high-sensitivity modified Glasgow prognostic score; mGPS, modified Glasgow prognostic score; PC, postcricoid; PS, pyriform sinus; PW, posterior wall.
Per the Eastern Cooperative Oncology Group.
Discussion
The present study showed that the mGPS and HS-mGPS were significantly associated with outcomes among patients with HSCC in the univariate analyses. However, just the HS-mGPS was independently associated with OS and DFS in the multivariable analysis. Therefore, the results from this study showed that the HS-mGPS is superior to the mGPS for predicting outcomes in cases of HSCC.
The inflammation-based GPS system is based on elevated serum CRP concentrations and hypoalbuminemia. 11 Elevated serum CRP concentrations reflect a state of systemic inflammation and are generally associated with a higher cancer risk and poorer prognosis. 12 Hypoalbuminemia reflects the hypercatabolic state of cancer cachexia, which is caused by cytokine activation, and it is commonly observed in patients with cancer. 13 Previous studies have indicated that the GPS was superior to white blood cell count, neutrophil count, platelet count, the neutrophil-lymphocyte ratio, and the Edinburgh Clinical Risk Score for predicting survival among patients with cancer.14,15 Several studies have also investigated the association between the mGPS and outcomes among patients with head and neck cancer. Nakayama et al were the first to report the prognostic value of the mGPS in this setting, 16 and Iuchi et al subsequently found that combining the mGPS and conventional TNM staging provided more accurate prognostication. 7 In addition, Chen et al noted that the GPS may have prognostic value and guide personalized treatment among patients with metastatic nasopharyngeal carcinoma who received cisplatin-based palliative chemotherapy. 17 The present study revealed that the mGPS was useful for predicting outcomes among patients with HSCC. Therefore, when considered together, our findings and previous findings suggest that the mGPS is a significant and independent predictor of survival outcomes.
Several studies have recently suggested that the HS-mGPS is superior to the mGPS as a prognostic marker for many cancer types.18,19 Proctor et al reported that the HS-mGPS provided better prognostic value than the GPS and mGPS in a large cohort of patients with cancer. 18 Furthermore, Hanai et al demonstrated that the HS-mGPS is superior to the mGPS as a prognostic predictor for head and neck cancer, and that study included patients with HSCC. 8 However, there is limited evidence regarding the prognostic value of the HS-mGPS among patients with HSCC, and the present study revealed that the HS-mGPS was an independent prognostic marker in cases of HSCC. A large retrospective study of patients with resectable gastric cancer also suggested that the mGPS and HS-mGPS provided good preoperative prediction of OS outcomes, although the HS-mGPS was superior per multivariable Cox regression analysis. 20 Another study compared the prognostic values of the GPS, mGPS, HS-mGPS, and other inflammation-based markers among patients with resectable non–small cell lung cancer, which revealed that the HS-mGPS provided better ability to predict OS (vs the GPS and mGPS). 21 Therefore, when considered together, our findings and previous findings suggest that the HS-mGPS is a significant and independent predictor of survival outcomes and superior to the mGPS.
In this study, HS-mGPS is more useful than the mGPS among patients with HSCC. The high-sensitivity test for CRP, termed low-reactive protein, measures very low levels of CRP more accurately and is even more reliable than standard CRP. 22 Technological advances have permitted very accurate measurements of inflammatory markers, including CRP, even at relatively small values. 23 In addition, there is increasing evidence that CRP concentrations >0.3 mg/dL can predict poor prognosis among patients with and without cancer, 24 and high-sensitivity CRP (hs-CRP) level is positively associated with cancer. 22 Two possibilities have been pointed out for this. First, elevated hs-CRP levels are suggested to be the result of cancer, and chronic inflammation and elevated hs-CRP may play a role in carcinogenesis. 22 These findings could explain the ability of the HS-mGPS to independently predict survival outcomes.
This study has 2 important limitations. First, a small single-center retrospective study is prone to various sources of bias, including selection bias. Second, the mGPS and HS-mGPS were calculated retrospectively, not by the clinicians who were making the treatment decisions, which is a potential source of information bias. Thus, large prospective studies are needed to validate our findings.
Conclusion
The present study showed that the mGPS and HS-mGPS have prognostic value among patients with HSCC, although only the HS-mGPS was independently associated with OS and DFS in the multivariable analysis. The greater prognostic value of the HS-mGPS may be related to it being a more sensitive index of inflammation. Therefore, we suggest using the HS-mGPS for prognostication among patients with HSCC. However, further studies are required to confirm our results.
Author Contributions
Hiroyuki Iuchi, acquired and organized the data, prepared the initial draft of the manuscript, and revised the manuscript; Junichiro Ohori, acquired, organized, and interpreted the data, prepared the initial draft of the manuscript, and revised the manuscript; Hisahiro Matsuzaki, interpreted the data and revised the manuscript; Satoshi Kiyama, analyzed the data and revised the manuscript; Masaru Yamashita, formulated the research question, collected the data, and drafted and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
References
- 1. Newman JR, Connolly TM, Illing EA, Kilgore ML, Locher JL, Carroll WR. Survival trends in hypopharyngeal cancer: a population-based review. Laryngoscope. 2015;125(3):624-629. [DOI] [PubMed] [Google Scholar]
- 2. Jonathan CG, Richard LB, Brett AM. Hypopharyngeal cancer: a state of the art review. Oral Oncol. 2018;86:244-250. [DOI] [PubMed] [Google Scholar]
- 3. Lewin NL, Lewin F, Andersson BA, Lofgren S, Rutqvist LE. The use of rapid and cost-effective blood-based biomarkers in combination with tumour TNM stage for individual head and neck cancer patient treatment selection. Med Oncol. 2017;34(4):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown DJ, Milroy R, Preston R, McMillan DC. The relationship between an inflammation based prognostic score (GPS) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J Clin Pathol. 2007;60:705-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16(23):5805-5813. [DOI] [PubMed] [Google Scholar]
- 6. Xu Z, Xu W, Cheng H, Ying J, Cheng F, Xu W. The prognostic role of the platelet-lymphocytes ratio in gastric cancer: a meta-analysis. PLoS One. 2016;11(9):e163719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iuchi H, Kyutoku T, Ito K, Matsumoto H, Ohori J, Yamashita M. Impacts of inflammation-based prognostic scores on survival in patients with hypopharyngeal squamous cell carcinoma. OTO Open. 2020;4(4):2473974X20978137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanai N, Sawabe M, Kimura T, et al. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor for head and neck cancer. Oncotarget. 2018;9(97):37008-37016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng C, Min F, Qiuyan W, Zhang X, Song T, Wu S. High-sensitivity modified Glasgow prognostic score (HS-mGPS) is superior to the mGPS in esophageal cancer patients treated with chemoradiotherapy. Oncotarget. 2017;8(59):99861-99870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22(8):881-886. [DOI] [PubMed] [Google Scholar]
- 11. Al Murri AM, Bartlett JM, Canney PA, Doughty JC, Wilson C, McMillan DC. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer. 2006;94(2):227-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27(13):2217-2224. [DOI] [PubMed] [Google Scholar]
- 13. Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34(1):94-104. [DOI] [PubMed] [Google Scholar]
- 14. Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109(2):205-212. [DOI] [PubMed] [Google Scholar]
- 15. Leitch EF, Chakrabarti M, Crozier JE, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007;97(9):1266-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakayama M, Tabuchi K, Hara A. Clinical utility of the modified Glasgow prognostic score in patients with advanced head and neck cancer. Head Neck. 2015;37(12):1745-1749. [DOI] [PubMed] [Google Scholar]
- 17. Chen C, Sun P, Dai QS, Weng HW, Li HP, Ye S. The Glasgow prognostic score predicts poor survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. PLoS One. 2014;9(11):e112581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Proctor MJ, Horgan PG, Talwar D, Fletcher CD, Morrison DS, McMillan DC. Optimization of the systemic inflammation-based Glasgow prognostic score: a Glasgow Inflammation Outcome Study. Cancer. 2013;119(12):2325-2332. [DOI] [PubMed] [Google Scholar]
- 19. Takeno S, Hashimoto T, Shibata R, et al. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor in patients with resectable gastric cancer. Oncology. 2014;87(4):205-214. [DOI] [PubMed] [Google Scholar]
- 20. Takeno S, Hashimoto T, Shibata R, et al. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor in patients with resectable gastric cancer. Oncology. 2014;87(4):205-214. [DOI] [PubMed] [Google Scholar]
- 21. Osugi J, Muto S, Matsumura Y, Higuchi M, Suzuki H, Gotoh M. Prognostic impact of the high-sensitivity modified Glasgow prognostic score in patients with resectable non-small cell lung cancer. J Cancer Res Ther. 2016;12(2):945-951. [DOI] [PubMed] [Google Scholar]
- 22. Brendon JC, Martin LA, Michael AQ, Svetomir NM, Steven LY, Andrew PR. CRP identifies homeostatic immune oscillations in cancer patients: a potential treatment targeting tool? J Transl Med. 2009;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45(12):2136-2141. [PubMed] [Google Scholar]
- 24. Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality: a population-based, prospective study. Thromb Haemost. 2006;95(03):511-518. [DOI] [PubMed] [Google Scholar]