Abstract
Objective
Heart failure (HF) is a common and highly morbid cardiovascular disorder. Oxidative stress worsens HF, and uric acid (UA) is a useful oxidative stress marker. The novel anti-hyperuricemic drug febuxostat is a potent non-purine selective xanthine oxidase inhibitor. The present study examined the UA-lowering and prognostic effects of febuxostat in patients with HF compared with conventional allopurinol.
Methods
This multicenter, randomized trial included 263 patients with chronic HF who were randomly assigned to two groups and received allopurinol or febuxostat (UA >7.0 mg/dL). All patients were followed up for 3 years after enrollment.
Results
There were no significant differences in baseline clinical characteristics between the two groups. The UA level was significantly decreased after 3 years of drug administration compared with the baseline in both groups. Urine levels of the oxidative stress marker 8-hydroxy-2′-deoxyguanosine were lower in the febuxostat group than in the allopurinol group (11.0 ± 9.6 vs. 22.9 ± 15.9 ng/mL), and the rate of patients free from hospitalization due to worsening HF tended to be higher in the febuxostat group than in the allopurinol group (89.0% vs. 83.0%).
Conclusions
Febuxostat is potentially more effective than allopurinol for treating patients with chronic HF and hyperuricemia.
This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (https://www.umin.ac.jp/ctr/; ID: 000009817).
Keywords: Heart failure, uric acid, febuxostat, oxidative stress, worsening heart failure, prognosis
Introduction
Despite advances in our understanding of its pathology and improvements in its management, heart failure (HF) remains a common disease with high morbidity and mortality and is a significant public health burden on healthcare systems.1,2 Renin–angiotensin–aldosterone systems, sympathetic nervous systems, and inflammatory responses are activated in patients with HF, subsequently leading to oxidative stress.1–5 Inflammatory systems and oxidative stress are involved in the development and progression of HF.2–6 Oxidative stress is defined as dysregulation between the production of reactive oxygen species (ROS) and the endogenous antioxidant protection mechanisms. Previously, various oxidative stress markers, such as 8-hydroxy-2′-deoxyguanosine (8-OHdG), advanced glycation end products, nicotinamide adenine dinucleotide phosphate oxidase, and xanthine oxidase (XO), were investigated to evaluate HF severity.4,5,7,8 During purine metabolism, XO catalyzes the final two steps (from hypoxanthine to xanthine and xanthine to uric acid [UA]). XO produces oxygen-derived free radicals 9 and is a major source of ROS in human physiology. The final product UA is a non-specific marker for oxidative stress. Excessive activation of XO induces hyperuricemia, and XO activity is upregulated in patients with HF. 10 Furthermore, a high UA level has been reported to be a strong and independent marker for impaired prognosis in patients with HF and acute myocardial infarction.11–13
XO inhibitors are used for treating hyperuricemia and have been shown to improve myocardial energetic efficiency, left ventricular ejection fraction (LVEF), and plasma B-type natriuretic peptide (BNP) levels in patients with HF.14–16 However, a previous double-blind, multicenter randomized placebo-controlled trial did not reveal improvements after allopurinol administration in clinical status, quality of life, or LVEF in high-risk patients with HF with reduced LVEF and hyperuricemia. 17 Allopurinol has long been regarded as a first-line drug for the treatment of hyperuricemia.18,19 It is a purine analog and a structural isomer of hypoxanthine, which is a naturally occurring purine derivative. In contrast, febuxostat is a potent non-purine selective XO inhibitor. Several in vitro studies have reported that febuxostat was superior in inhibiting the production of XO-derived ROS compared with allopurinol.20,21The purpose of the present study was to compare the UA-lowering and prognostic effects of febuxostat compared with allopurinol in patients with chronic HF and hyperuricemia.
Patients and methods
Study design
This trial was a multicenter, randomized study designed to determine the efficacy and safety of febuxostat compared with allopurinol in patients with chronic HF. This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (https://www.umin.ac.jp/ctr/; ID: 000009817). The first patient was enrolled in February 2013, and 263 patients were enrolled and randomized into febuxostat and allopurinol groups by March 2015. Written informed consent was obtained from all study subjects. The study protocol was approved by the institutional review board at Fukushima Medical University (approval number: 1430, date of approval: 24 May 2012) and each participating institution and complied with the Declaration of Helsinki. The reporting of this study conforms to the CONSORT statements. 22
Study participants
Patients were eligible for enrollment if they had chronic HF with hyperuricemia (UA >7.0 mg/dL). Patients with acute myocardial infarction, acute decompensated HF, severe hypotension (cardiogenic shock), and renal dysfunction (serum creatinine level >2.0 mg/dL) and those who were receiving medication for hyperuricemia other than febuxostat or allopurinol were excluded. Symptomatic HF diagnosis was determined by well-trained cardiologists using the Framingham criteria and/or current guidelines.1,2,23
All patients were randomly assigned to two groups: the febuxostat group and allopurinol group. In detail, someone unrelated to this study created several envelopes containing papers with the name of either allopurinol or febuxostat. The inside of the envelopes was not visible from the outside. When patients were included in this study, one envelope was randomly selected by the attending physician, and the patient was assigned the specified drug listed on the paper in the envelope.
Drug doses
The drug dose of allopurinol or febuxostat was adjusted to achieve a UA level ≤6 mg/dL based on past reports in patients with gout24,25 and the Japanese guidelines for the treatment of hyperuricemia and gout. 19 The initial dose of allopurinol in most patients was 200 mg/day, and it was reduced according to their renal function or UA level. The initial dose of febuxostat was 10 mg/day, and it was increased 2 weeks after depending on the UA level. These drugs were continued after enrollment unless there were adverse events.
Evaluation items for comparisons between two groups
Data on age, sex, body weight, vital signs, New York Heart Association (NYHA) functional class, comorbidities, laboratory tests, and echocardiographic exams were collected from medical records before the administration of allopurinol or febuxostat. Regarding laboratory data, UA, blood urea nitrogen, serum creatinine, estimated glomerular filtration rate (GFR), serum sodium, potassium, lipid, hemoglobin A1c, and plasma BNP were measured before the administration of allopurinol or febuxostat at baseline and 3 years after enrollment. GFR was estimated from the Modification of Diet in Renal Disease formula for Japanese patients. 26 Plasma BNP concentrations were measured using a commercially available radioimmunoassay specific to human BNP (Shionoria BNP kit, Shionogi, Osaka, Japan). Echocardiography was blindly performed by experienced echocardiographers using standard techniques. Two-dimensional echocardiographic images were acquired from the parasternal long and short axis, apical long axis, and apical four-chamber views. The following echocardiographic parameters were investigated: interventricular septum thickness, left ventricular end-diastolic diameter (LVEDD), LVEF, tricuspid valve regurgitation pressure gradient, and inferior vena cava diameter. 27 LVEF was calculated using a modified Simpson’s method. Moreover, we divided all study patients into two groups based on the LVEF. Patients with LVEF ≥50% were assigned to the heart failure with preserved ejection fraction (HFpEF) group, and those with LVEF <50% were allocated to the heart failure with reduced ejection fraction (HFrEF) group as an additional analysis.
Estimation of oxidative stress
We measured urine 8-OHdG levels as an oxidative stress marker using a commercially available enzyme-linked immunosorbent assay kit (8-OHdG check high sensitivity; Japan Institute for the Control of Aging, Fukuroi, Japan) at baseline and 3 years after enrollment. This kit measures serum 8-OHdG concentrations linearly between 0.5 and 200 ng/mL with previously established monoclonal antibody specificity.7,28
Prognostic investigation
All patients with HF visited each hospital for the treatment of HF every month or bimonthly and were followed up after enrollment (mean: 644.6 days, range: 3–1255 days). The endpoints evaluated independently by researchers were (1) cardiovascular events, including cardiovascular death defined as death from worsening HF or sudden cardiac death or acute myocardial infarction, and (2) hospitalization due to worsening HF.
Adverse events
All related adverse events were evaluated during the follow-up periods. Adverse events were unfavorable reactions that required allopurinol or febuxostat discontinuation. Drug discontinuation was decided by the attending physician.
Sample size estimation
We hypothesized that the incidence of cardiovascular events was 10% in the febuxostat group and 25% in the allopurinol group based on the flowing reports. In the multicenter, randomized, double-blind trial EXACT-HF, cardiovascular events, including death or re-hospitalization, occurred in almost 40% of patients with HF in the allopurinol group. 17 In that study, the inclusion criteria were relatively strict, such as requiring an acute HF event (hospitalization or emergency department visit) within 12 months, severe LV dysfunction (LVEF ≤25%), or an elevated natriuretic peptide level. Because the inclusion criteria in our study were not as strict, we predicted that the incidence of cardiovascular events in the allopurinol group would be 25%. When planning our study, there were few appropriate reports to predict the prognostic effect of febuxostat on patients with HF. However, White et al. reported a multicenter, double-blind trial using febuxostat in patients with gout and cardiovascular disease in 2018. 29 In that study, the primary endpoint, including cardiovascular death, occurred in 10.8% of patients in the febuxostat group during the median follow-up period of 32 months. Therefore, the prognosis prediction of 10% is considered valid in the febuxostat group in this study. The sample size was calculated based on these hypotheses, and a two-sided test with a 0.05 significance level (α = 0.05, β = 0.10) was used. We set the sample size to 135 in the febuxostat group and 120 in the allopurinol group.
Statistical analysis
The results are expressed as the mean ± standard deviation, and skewed variables are presented as the median and inter-quartile range. A P value of less than 0.05 was considered statistically significant. Significance between the two groups was determined by an unpaired Student’s t-test for continuous variables and a chi-square test for discrete variables. The changes in blood pressure, laboratory data, and echocardiographic data between baseline and 3 years in each group were determined by a paired t-test. If the data were not distributed normally, the Mann–Whitney U test was used. Missing data were excluded from the analysis. Kaplan–Meier survival curves determined the time-dependent cumulative event-free rates in patients between the allopurinol and febuxostat groups, and differences were compared by a log-rank test. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Comparisons of baseline clinical characteristics between the allopurinol and febuxostat groups
Two hundred and sixty-three patients were enrolled in this study and randomly assigned to allopurinol (n = 135) or febuxostat (n = 128) groups. Enrollment, randomization, and follow up were performed according to the CONSORT guideline (Figure 1). 22 Comparisons of baseline clinical characteristics, including age, sex, body weight, HF severity defined according to NYHA functional class, blood pressure, comorbidities, HF etiologies, blood samples, echocardiographic data, and medications, between the allopurinol and febuxostat groups are shown in Table 1. There were no significant differences in these data between the two groups. The UA levels were 8.70 ± 1.40 vs. 8.59 ± 1.39 mg/dL in the allopurinol and febuxostat groups, respectively. The median plasma BNP levels were 95.8 vs. 129.3 pg/mL, and the LVEDD and LVEF were 49.8 ± 8.7 vs. 50.4 ± 8.7 mm and 56.1% ± 14.3% vs. 53.4% ± 14.0% in the allopurinol and febuxostat groups, respectively.
Figure 1.
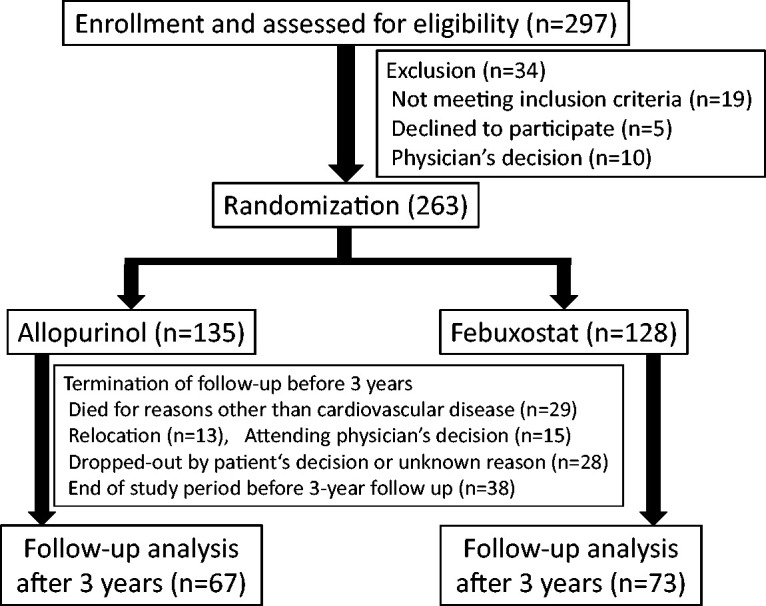
Patient selection flowchart according to CONSORT guidelines in this study.
Table 1.
Comparisons of baseline clinical characteristics between the allopurinol and febuxostat groups.
| Allopurinol | Febuxostat | ||
|---|---|---|---|
| (n = 135) | (n = 128) | P value | |
| Age (years) | 71.0 ± 13.2 | 71.0 ± 13.2 | 0.996 |
| Sex (Men, %) | 85 (63.0) | 90 (70.3) | 0.207 |
| Body weight (kg) | 62.9 ± 16.5 | 60.9 ± 15.4 | 0.764 |
| NYHA I/II/III/IV | 82/48/5/0 | 64/57/6/0 | 0.311 |
| Systolic BP (mmHg) | 129.8 ± 24.0 | 124.6 ± 18.4 | 0.059 |
| Diastolic BP (mmHg) | 73.5 ± 16.9 | 71.9 ± 12.7 | 0.414 |
| Comorbidities (n, %) | |||
| Hypertension | 102 (75.6) | 89 (69.5) | 0.273 |
| Dyslipidemia | 83 (61.5) | 69 (53.9) | 0.214 |
| Diabetes mellitus | 43 (31.9) | 34 (26.6) | 0.346 |
| Atrial fibrillation | 30 (22.2) | 40 (31.3) | 0.232 |
| Etiologies of heart failure (n, %) | 0.505 | ||
| Ischemic heart disease | 49 (36.3) | 36 (28.1) | |
| Dilated cardiomyopathy | 14 (10.4) | 16 (12.5) | |
| Valvular heart disease | 27 (20.0) | 30 (23.4) | |
| Hypertensive heart disease | 29 (21.5) | 23 (18.0) | |
| Hypertrophic cardiomyopathy | 6 (4.4) | 11 (8.6) | |
| Others | 10 (7.4) | 12 (9.4) | |
| Blood sample data | |||
| Uric acid (mg/dL) | 8.70 ± 1.40 | 8.59 ± 1.39 | 0.558 |
| Blood urea nitrogen (mg/dL) | 21.8 ± 9.4 | 21.5 ± 7.7 | 0.738 |
| Serum creatinine (mg/dL) | 1.15 ± 0.42 | 1.13 ± 0.30 | 0.555 |
| Estimated GFR (mL/min/1.73 m2) | 49.7 ± 18.2 | 50.1 ± 15.4 | 0.853 |
| Serum sodium (mEq/L) | 141.7 ± 3.0 | 141.3 ± 3.0 | 0.314 |
| Serum potassium (mEq/L) | 4.36 ± 0.44 | 4.37 ± 0.48 | 0.827 |
| HbA1c (%) | 6.16 ± 0.99 | 6.01 ± 0.70 | 0.212 |
| LDL-cholesterol (mg/dL) | 99.2 ± 38.5 | 101.0 ± 31.8 | 0.704 |
| HDL-cholesterol (mg/dL) | 51.3 ± 27.4 | 51.0 ± 24.2 | 0.950 |
| Triglyceride (mg/dL) | 142.9 ± 95.7 | 131.4 ± 78.0 | 0.321 |
| BNP (pg/mL) | 95.8 (222.5) | 129.3 (233.7) | 0.500 |
| Echocardiographic data | |||
| IVST (mm) | 11.0 ± 2.5 | 11.1 ± 3.3 | 0.866 |
| LVEDD (mm) | 49.8 ± 8.7 | 50.4 ± 8.7 | 0.584 |
| LVEF (%) | 56.1 ± 14.3 | 53.4 ± 14.0 | 0.123 |
| TR-PG (mmHg) | 25.7 ± 10.2 | 27.9 ± 10.8 | 0.163 |
| IVC diameter (mm) | 14.0 ± 4.3 | 14.3 ± 4.6 | 0.612 |
| Medication (n, %) | |||
| Diuretics | 85 (63.0) | 91 (71.1) | 0.190 |
| MRA | 39 (28.9) | 50 (39.1) | 0.091 |
| ACE inhibitor | 39 (28.9) | 42 (32.8) | 0.507 |
| ARB | 69 (51.1) | 61 (47.7) | 0.575 |
| Β-blocker | 103 (76.3) | 101 (78.9) | 0.346 |
NYHA, New York Heart Association classification; BP, blood pressure; GFR, glomerular filtration rate; Hb1Ac, hemoglobin 1Ac; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BNP, B-type natriuretic peptide; IVST, interventricular septum thickness; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; TR-PG, tricuspid regurgitation pressure gradient; IVC, inferior vena cava, MRA, mineralocorticoid receptor antagonist; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Skewed data are reported as median (inter-quartile range).
Comparisons of clinical characteristics between the two groups at 3 years after enrollment
The final results at 3 years after enrollment are shown in Table 2. No significant differences were observed in HF severity, blood samples, echocardiographic data, and UA level (5.02 ± 1.41 vs. 5.20 ± 1.09 mg/dL) between the allopurinol and febuxostat groups. UA levels were significantly decreased at 3 years compared with the baseline in both groups (8.70 ± 1.40 to 5.02 ± 1.41 mg/dL in the allopurinol group, P < 0.001; 8.59 ± 1.39 to 5.20 ± 1.09 mg/dL in the febuxostat group, P < 0.001).
Table 2.
Comparisons of clinical characteristics between the allopurinol and febuxostat groups at 3 years.
| Allopurinol | Febuxostat | ||
|---|---|---|---|
| (n = 135) | (n = 128) | P value | |
| NYHA I/II/III/IV | 42/19/4/0 | 35/34/2/0 | 0.688 |
| Systolic BP (mmHg) | 124.4 ± 17.2 | 123.2 ± 17.3 | 0.071 |
| Blood samples data | |||
| Uric acid (mg/dL) | 5.02 ± 1.41# | 5.20 ± 1.09# | 0.405 |
| Blood urea nitrogen (mg/dL) | 19.9 ± 8.4 | 21.7 ± 8.2 | 0.200 |
| Serum creatinine (mg/dL) | 1.14 ± 0.45 | 1.18 ± 0.46 | 0.575 |
| Estimated GFR (mL/min/1.73 m2) | 51.3 ± 21.0 | 49.6 ± 18.3 | 0.623 |
| Serum sodium (mEq/L) | 141.4 ± 3.0 | 140.8 ± 3.0 | 0.206 |
| Serum potassium (mEq/L) | 4.33 ± 0.55 | 4.32 ± 0.50 | 0.908 |
| HbA1c (%) | 6.13 ± 0.74 | 6.06 ± 0.79 | 0.621 |
| LDL-cholesterol (mg/dL) | 97.4 ± 30.1 | 95.8 ± 33.7 | 0.790 |
| Triglyceride (mg/dL) | 129.8 ± 60.9 | 151.6 ± 97.0 | 0.160 |
| BNP (pg/mL) | 66.9 (202.9) | 75.8 (149.8) | 0.528 |
| Echocardiographic data | |||
| IVST (mm) | 10.5 ± 2.1 | 10.3 ± 2.6 | 0.629 |
| LVEDD (mm) | 47.9 ± 8.7 | 50.6 ± 7.4 | 0.073 |
| LVEF (%) | 60.8 ± 11.5 | 58.0 ± 12.6 | 0.204 |
| TR-PG (mmHg) | 24.8 ± 10.3 | 23.7 ± 8.9 | 0.609 |
| IVC diameter (mm) | 13.8 ± 4.1 | 14.9 ± 5.1 | 0.194 |
NYHA, New York Heart Association classification; BP, blood pressure; GFR, glomerular filtration rate; Hb1Ac, hemoglobin 1Ac; LDL, low-density lipoprotein; BNP, B-type natriuretic peptide; IVST, interventricular septum thickness; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; TR-PG, tricuspid regurgitation pressure gradient; IVC, inferior vena cava.
Skewed data are reported as median (inter-quartile range).
#P < 0.001 vs. uric acid level at baseline in each respective group.
Regarding the oxidative stress marker, we measured the patient’s urine 8-OHdG levels at 3 years after treatment. This level was significantly lower in the febuxostat group than in the allopurinol group (11.0 ± 9.6 vs. 22.9 ± 15.9 ng/mL, P < 0.001) (Figure 2).
Figure 2.
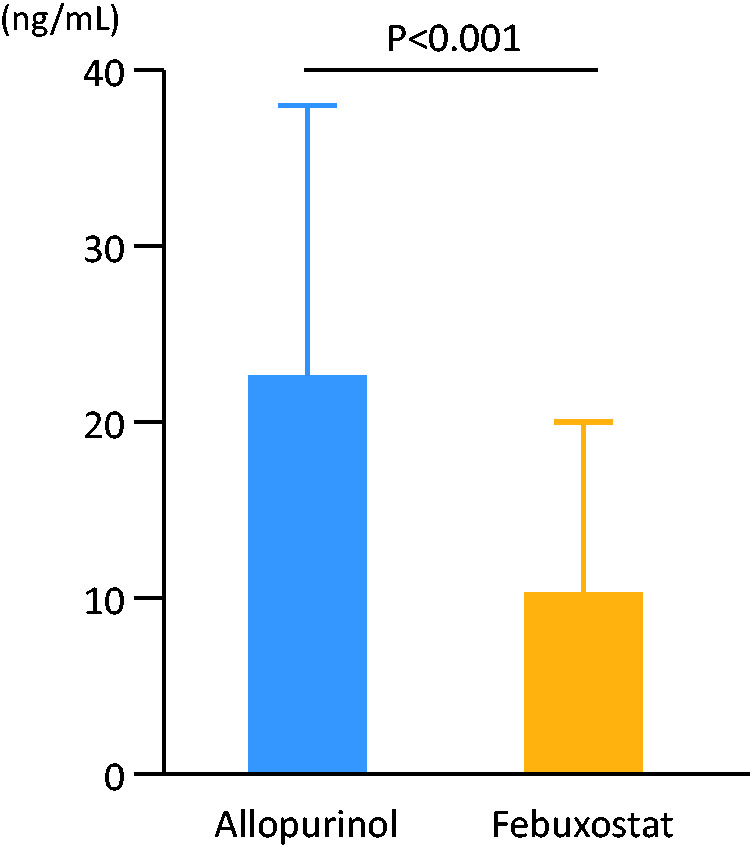
Comparison of urine 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels at 3 years between the allopurinol (n = 135) and febuxostat groups (n = 128) (22.9 ± 15.9 vs. 11.0 ± 9.6 ng/mL, P < 0.001). 8-OHdG levels were measured using a commercially available enzyme-linked immunosorbent assay kit.
Prognostic analysis
There were 12 cardiovascular deaths, including five cardiac deaths, and 37 hospitalizations due to worsening HF during the follow-up period. Cardiovascular event-free rates were not significantly different between the allopurinol and febuxostat groups (82.2% vs. 82.7%, respectively) (Figure 3). There were five cardiovascular deaths in the allopurinol group and seven in the febuxostat group (no statistical significance). However, the event-free rate of hospitalization due to worsening HF tended to be higher in the febuxostat group than in the allopurinol group (89.0% vs. 83.0%, P = 0.055) (Figure 4).
Figure 3.
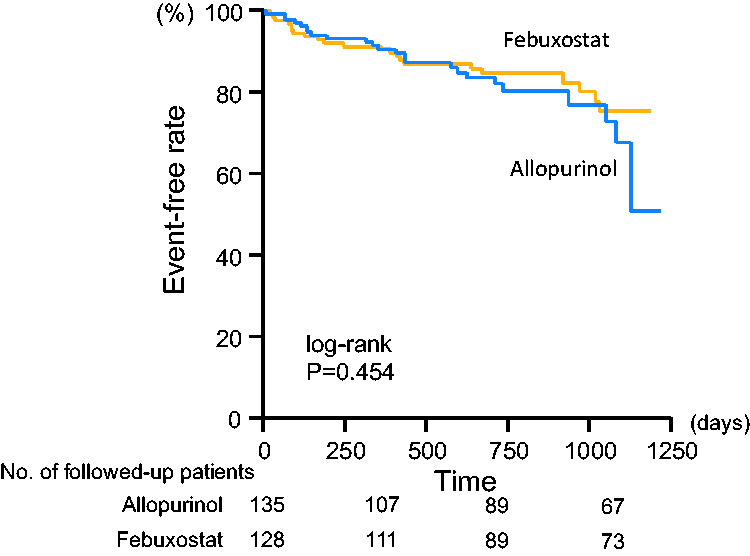
Kaplan–Meier analyses for patients free from cardiovascular events in the febuxostat and allopurinol groups. During this study period, 12 cardiovascular deaths and 37 hospitalizations due to worsening heart failure occurred. Numbers of followed-up patients in respective groups were indicated at the bottom of the figure.
Figure 4.
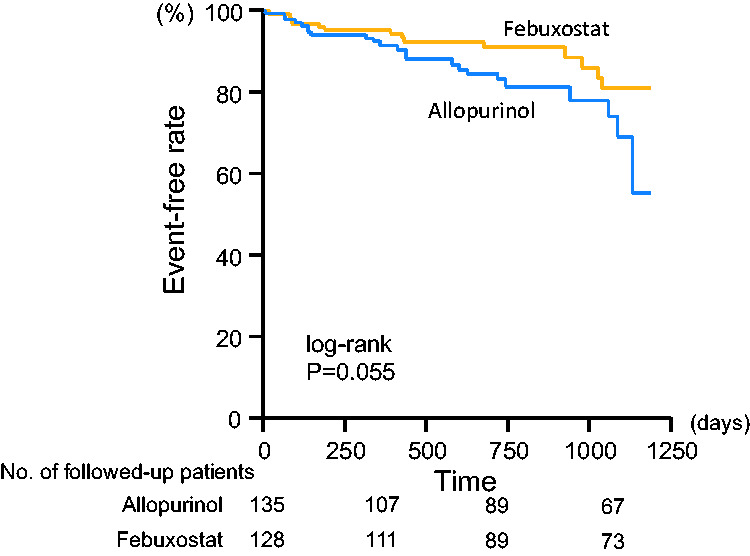
Kaplan–Meier analyses for patients free from hospitalization due to worsening heart failure in the febuxostat and allopurinol groups. Numbers of followed-up patients in respective groups were indicated at the bottom of the figure.
We evaluated the clinical safety of allopurinol and febuxostat in both groups. There were 10 adverse events requiring study drug discontinuation (five in the allopurinol and five in the febuxostat group), and there was no statistical significance between the two groups.
Comparisons based on left ventricular systolic function
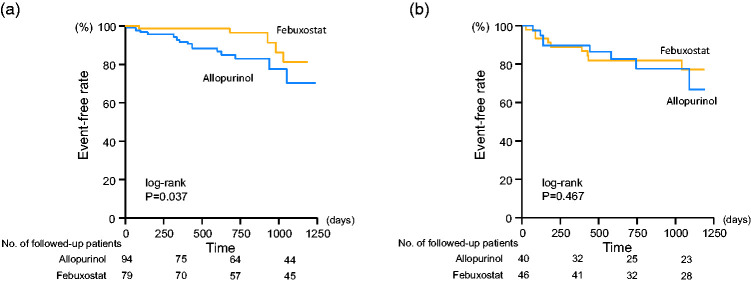
We divided all study patients into two groups based on the LVEF: the HFpEF (n = 173) and HFrEF (n = 86) groups. Four patients were excluded because the LVEF was not evaluated at baseline. The HFpEF group included 94 patients treated with allopurinol and 79 patients treated with febuxostat, whereas the HFrEF group included 40 patients who received allopurinol and 46 patients who received febuxostat. Baseline clinical characteristics did not show significant differences between the allopurinol and febuxostat groups in patients with HFpEF or HFrEF. UA levels were decreased in both HFpEF and HFrEF groups at 3 years after enrollment, but there were no significant differences between the allopurinol and febuxostat groups in patients with HFpEF (4.77 ± 1.27 vs. 4.99 ± 0.97 mg/dL) or HFrEF (5.74 ± 1.57 vs. 5.48 ± 1.23 mg/dL). Interestingly, the urine 8-OHdG level at 3 years after enrollment was significantly lower in the febuxostat group than in the allopurinol group in patients with HFpEF (10.9 ± 8.9 vs. 24.1 ± 15.6 ng/mL, P < 0.001) (Figure 5a); however, there was no statistical significance between these two groups in patients with HFrEF (11.4 ± 10.5 vs. 17.9 ± 12.2 ng/mL) (Figure 5b). Kaplan–Meier curves revealed that the event-free rate of hospitalization due to worsening HF was significantly higher in the febuxostat group than in the allopurinol group for patients with HFpEF (93.6% vs. 85.1%, P = 0.037) (Figure 6a), but there was no significant difference between these two groups in patients with HFrEF (80.4% vs. 77.5%) (Figure 6b). Cardiovascular event-free rates did not show a significant difference between the allopurinol and febuxostat groups in patients with HFpEF (85.9% vs. 84.0%) or HFrEF (78.3% vs. 77.5%).
Figure 5.
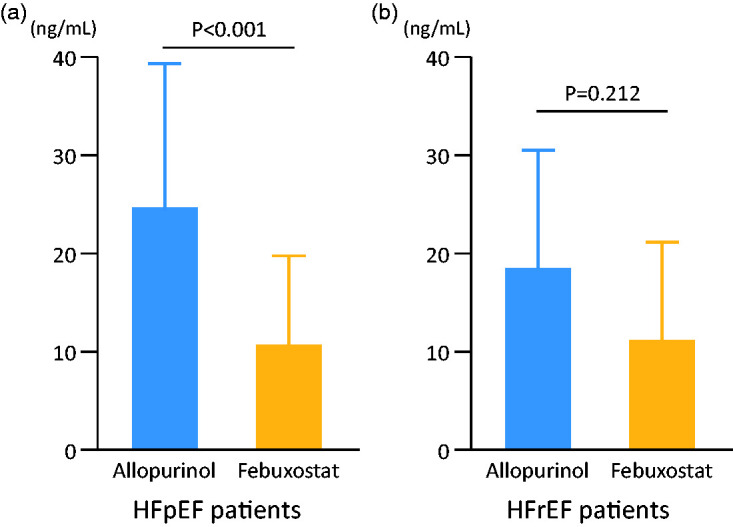
Comparison of urine 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels at 3 years between allopurinol and febuxostat users divided into two groups based on the left ventricular ejection fraction. 8-OHdG levels were measured using a commercially available enzyme-linked immunosorbent assay kit. a) Heart failure with preserved ejection fraction (HFpEF) (n = 173) (24.1 ± 15.6 vs. 10.9 ± 8.9 ng/mL, P < 0.001). b) Heart failure with reduced ejection fraction (HFrEF) (n = 86) (17.9 ± 12.2 vs. 11.4 ± 10.5 ng/mL, P = 0.212).
Figure 6.
Kaplan–Meier analyses for patients free from hospitalization due to worsening heart failure in the febuxostat and allopurinol groups. Numbers of followed-up patients in respective groups were indicated at the bottom of the figure. (a) Heart failure with preserved ejection fraction (HFpEF) (log-rank, P = 0.037). (b) Heart failure with reduced ejection fraction (HFrEF) (log-rank, P = 0.467).
Discussion
In the present multicenter, randomized controlled trial, we demonstrated the clinical safety and efficacy of febuxostat in patients with HF and hyperuricemia compared with allopurinol. Moreover, the rate of patients free from hospitalization due to worsening HF at 3 years tended to be higher, and the oxidative stress marker level was significantly lower in the febuxostat group than in the allopurinol group.
Several studies have reported the association between hyperuricemia and various cardiovascular diseases. Large-scale studies have revealed that the serum UA level was an independent predictor of mortality in patients with acute myocardial infarction.30,31 In addition, an elevated UA level is associated with low LVEF, stroke volume, and cardiac output and cardiac remodeling15,32,33 and is a risk factor for atrial fibrillation. 34 These hemodynamic and electrophysiological influences on the heart lead to HF, and several studies have reported that a high UA level is a strong and independent predictor of mortality in patients with not only mild to moderate chronic HF11,35 but also acute HF.12,36 In particular, Anker et al. reported that the mortality risk increased 6.3- and 18.5-fold in patients with HF with UA levels >600 μmol/L and >800 μmol/L, respectively, compared with those with normal UA levels (<400 μmol/L). 11 Based on these results, a reduction in UA levels may contribute to the treatment of HF. According to the study by Borghi et al. with a mean age equivalent to our study, the serum UA level is inversely related to EF% in elderly male patients with HF, but not in women. 37
Allopurinol is widely used to treat gout or hyperuricemia, and a number of clinical studies have reported the effects of allopurinol in patients with cardiovascular disease, including HF. In a large-scale clinical study targeting more than 2000 older adult patients with hypertension, allopurinol use was associated with lower rates of cardiovascular events, particularly at higher doses, compared with patients without allopurinol. 38 Another large-scale retrospective case-control analysis of over 25,000 patients with HF revealed that complications with gout were associated with an increased risk of HF readmissions or death, and continuous allopurinol use (>30 days) improved outcomes in terms of readmissions or death and all-cause mortality. 39 However, a randomized controlled trial in patients with symptomatic HF with low LVEF and hyperuricemia (EXACT-HF study) was unable to demonstrate improvement in prognosis (decreased rates of death or re-hospitalization due to HF) in the allopurinol group compared with the placebo group. 17
Febuxostat inhibits XO through a different mechanism from allopurinol and was approved in January 2011 in Japan for the treatment of gout or hyperuricemia. Unlike allopurinol, febuxostat does not inhibit nucleic acid metabolizing enzymes other than XO and is therefore recognized as a selective XO inhibitor.40,41 The clinical usefulness of febuxostat has been widely reported. Chronic kidney disease (CKD) can worsen HF, and several randomized trials have revealed that febuxostat suppresses the worsening of CKD compared with placebo.42,43 Moreover, the clinical significance of febuxostat compared with allopurinol was investigated in patients with cardiovascular disease in several studies. The NU-FLASH trial revealed that febuxostat reduced the UA level, exhibited a renoprotective effect, and suppressed oxidative stress in patients with hyperuricemia at high risk for cardiac surgery. 18 The relationship between oxidative stress and worsening HF is intricate. ROS act as signaling molecules to trigger pro-inflammatory cytokine production. 4 Several animal models revealed that both increased oxidative stress and reduced antioxidants, including superoxide dismutase, catalase, and glutathione peroxidase, are associated with the worsening of HF. 44 Chronic release of ROS and persistent inflammation lead to cardiac hypertrophy, fibrotic changes, and myocyte apoptosis. 45
Regarding the comparison between allopurinol and febuxostat, a large-scale population-based cohort study demonstrated that febuxostat users had a significantly higher risk of HF hospitalization and cardiovascular deaths than allopurinol users, and febuxostat increased the risk of adverse cardiovascular events in a dose-dependent manner. 46 Moreover, the CARES trial was conducted in a study population of over 6000 patients with gout and cardiovascular disease who were randomly assigned to receive febuxostat or allopurinol. In this trial, febuxostat was non-superior to allopurinol in reducing the rates of adverse cardiovascular events. In addition, all-cause and cardiovascular mortalities were higher in febuxostat users than in allopurinol users. 29 In Japan, the FREED trial was conducted in patients with hyperuricemia at risk for cerebral, cardiovascular, or renal disease who were randomly separated into febuxostat and non-febuxostat groups. 47 That trial reported significantly lower incidences of primary composite events, including cerebral, cardiovascular, and renal events, and all-cause deaths in the febuxostat group compared with the non-febuxostat group. 47
The present study included patients with HF and hyperuricemia in a stable condition, regardless of their hospitalization history, cardiac function, and HF etiologies. Similar to the FREED trial, the present trial was a multicenter, prospective, randomized open-label study, and all participants were administered the UA-lowering agents febuxostat or allopurinol. Both groups in our study achieved a reduction in UA levels, but the non-febuxostat group (of which 27.2% received 100 mg allopurinol) in the FREED trial did not achieve a significant reduction in UA levels at the 3-year follow-up. 47 In the FREED trial, compared with the non-febuxostat group, the febuxostat group showed a significantly lower primary composite event rate, which included not only HF re-hospitalization but also death due to cerebral or cardiovascular disease. However, there was no significant difference in HF re-hospitalization alone between the febuxostat group and the non-febuxostat group in their study. 47 In the present study, HF hospitalization occurred in 23 patients in the allopurinol group (17%) and 14 in the febuxostat group (11%) over the 3-year follow-up period, and these rates were comparably higher than the rates of HF hospitalization in the FREED trial (1.7% in the febuxostat group and 2.3% in the non-febuxostat group). 47 In the present study, we were unable to demonstrate a statistically significant superiority of febuxostat to allopurinol regarding the cardiovascular event-free rate (Figure 3); however, the rate of patients free from hospitalization due to worsening HF tended to be higher in this group than in the allopurinol group (Figure 4). Moreover, we showed that the anti-oxidative effect evaluated by urine 8-OHdG levels was superior in the febuxostat group compared with the allopurinol group (Figure 2). In addition, the reduction in the oxidative stress marker and the superiority of event-free from hospitalization due to worsening HF in febuxostat users compared with allopurinol users were remarkable in the HFpEF group compared with the HFrEF group (Figure 5 and 6).
There were also some limitations to our study. In the present study, the UA-lowering effect of febuxostat and allopurinol might have been relatively mild because the UA level was not reduced at 3 years in either group. The target UA level was ≤6 mg/dL because it was considered relatively easy to achieve by febuxostat or allopurinol administration. The rates of patients with a UA level ≤6 mg/dL at the 3-year follow up were 82.2% in the febuxostat group and 74.6% in the allopurinol group. Moreover, the daily administration dose of febuxostat was 10 mg in 36.5%, 20 mg in 44.6%, and 40 mg in 18.9% of patients. However, a previous study reported that febuxostat increased the risk of adverse cardiovascular events in a dose-dependent manner, 46 indicating that increasing the drug dose might not lead to a better prognosis. Second, the study drug was randomly assigned but not in a blinded manner. Therefore, the results of the present study, including adverse events, might have been biased due to the lack of blinding. Third, we measured urine 8-OHdG levels at baseline, but there were a large number of un-measurable samples. Therefore, we could not obtain enough data to compare these values at baseline and 3 years after enrollment. Fourth, we did not evaluate coronary re-vascularization therapy as a prognostic event during the follow-up period. Fifth, the number of study subjects in the current study was relatively small. Future research will require a larger scale study to validate the clinical usefulness of febuxostat compared with allopurinol in Japan.
Conclusions
The present study indicated the clinical characteristics and usefulness of febuxostat compared with the current standard drug allopurinol in patients with HF and hyperuricemia.
Appendix: In addition to the authors, the following investigators participated in this trial: Hiroyuki Yamauchi, Satoshi Abe, Yuichi Nakamura, Takamasa Sato, Nobuo Sakamoto, and Koichi Sugimoto, Fukushima Medical University; Mitsunori Ishino and Akira Funayama, Yamagata University School of Medicine; Shoji Iwaya, Minamisoma Municipal General Hospital; Shigebumi Suzuki, Kazuyuki Yoshinari, and Yasuyuki Watanabe, Fukushima Accident Hospital; Keiji Kokubun, Kokubun Medical Clinic; Hideki Ohtake and Tomofumi Misaka, Saiseikai Fukushima General Hospital; Hiroshi Ohtani, Iwase General Hospital; Akihiko Sato, Takeshi Shimizu, Hoshi General Hospital; Kazuo Machii, Machii Cardiovascular Clinic; Kyoko Iwaya, Iwaya Clinic; Joji Shindo, Shindo Medical Clinic; Masahiko Sato, Public Soma General Hospital; Takayuki Ohwada, Fukushima Red Cross Hospital; Tomiyoshi Saito, Shirakawa Kosei General Hospital; Mitsuru Muto and Masayuki Sato, Southern Tohoku General Hospital; Morio Igarashi, Igarashi Medical Clinic; Yasuhumi Tsukahara, Tsukahara Medical Clinic; Shunsuke Watanabe, Ohta Nishinouchi Hospital; Yoshihiro Miyazaki, Miyazaki Cardiovascular Clinic; and Hiroyuki Mizukami; Jusendo General Hospital.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study was supported in part by a grant from the Japanese Heart Foundation (No. 12130004). No additional external funding was received for this study.
ORCID iD: Satoshi Suzuki https://orcid.org/0000-0002-3548-1604
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 3.Van Der Pol A, Van Gilst WH, Voors AA, et al. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 2019; 21: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayoub KF, Pothineni NVK, Rutland J, et al. Immunity, Inflammation, and Oxidative Stress in Heart Failure: Emerging Molecular Targets. Cardiovasc Drugs Ther 2017; 31: 593–608. [DOI] [PubMed] [Google Scholar]
- 5.Takeishi Y. Biomarkers in heart failure. Int Heart J 2014; 55: 474–481. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki S, Shishido T, Funayama A, et al. Long pentraxin PTX3 exacerbates pressure overload-induced left ventricular dysfunction. PLoS One 2013; 8: e53133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki S, Shishido T, Ishino M, et al. 8-Hydroxy-2′-deoxyguanosine is a prognostic mediator for cardiac event. Eur J Clin Invest 2011; 41: 759–766. [DOI] [PubMed] [Google Scholar]
- 8.Koyama Y, Takeishi Y, Arimoto T, et al. High serum level of pentosidine, an advanced glycation end product (AGE), is a risk factor of patients with heart failure. J Card Fail 2007; 13: 199–206. [DOI] [PubMed] [Google Scholar]
- 9.McCord JM andFridovich I.. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem 1968; 243: 5753–5760. [PubMed] [Google Scholar]
- 10.Landmesser U, Spiekermann S, Dikalov S, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 2002; 106: 3073–3078. [DOI] [PubMed] [Google Scholar]
- 11.Anker SD, Doehner W, Rauchhaus M, et al. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 2003; 107: 1991–1997. [DOI] [PubMed] [Google Scholar]
- 12.Niizeki T, Takeishi Y, Arimoto T, et al. Hyperuricemia associated with high cardiac event rates in the elderly with chronic heart failure. J Cardiol 2006; 47: 219–228. [PubMed] [Google Scholar]
- 13.Shimizu T, Yoshihisa A, Kanno Y, et al. Relationship of hyperuricemia with mortality in heart failure patients with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2015; 309: H1123–H1129. [DOI] [PubMed] [Google Scholar]
- 14.Cappola TP, Kass DA, Nelson GS, et al. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 2001; 104: 2407–2411. [DOI] [PubMed] [Google Scholar]
- 15.Cingolani HE, Plastino JA, Escudero EM, et al. The effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata Study. J Card Fail 2006; 12: 491–498. [DOI] [PubMed] [Google Scholar]
- 16.Gavin AD andStruthers AD.. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart 2005; 91: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Givertz MM, Anstrom KJ, Redfield MM, et al . Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: The xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation 2015; 131: 1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH Trial). Circ J 2013; 77: 2043–2049. [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka H; Japanese Society of Gout and Nucleic Acid Metabolism. Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids 2011; 30: 1018–1029. [DOI] [PubMed] [Google Scholar]
- 20.Malik UZ, Hundley NJ, Romero G, et al. Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production. Free Radic Biol Med 2011; 51: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamoto K, Eger BT, Nishino T, et al. An extremely potent inhibitor of xanthine oxidoreductase. Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition. J Biol Chem 2003; 278: 1848–1855. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 24.Shoji A Yamanaka H andKamatani N.. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum 2004; 51: 321–325. [DOI] [PubMed] [Google Scholar]
- 25.Li-Yu J, Clayburne G, Sieck M, et al. Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout? J Rheumatol 2001; 28: 577–580. [PubMed] [Google Scholar]
- 26.Matsuo S, Imai E, Horio M, et al . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 27. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of, Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 412. [DOI] [PubMed] [Google Scholar]
- 28.Kono Y, Nakamura K, Kimura H, et al. Elevated levels of oxidative DNA damage in serum and myocardium of patients with heart failure. Circ J 2006; 70: 1001–1005. [DOI] [PubMed] [Google Scholar]
- 29.White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018; 378: 1200–1210. [DOI] [PubMed] [Google Scholar]
- 30.Kaya MG, Uyarel H, Akpek M, et al. Prognostic value of uric acid in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 2012; 109: 486–491. [DOI] [PubMed] [Google Scholar]
- 31.Von Lueder TG, Girerd N, Atar D, et al . Serum uric acid is associated with mortality and heart failure hospitalizations in patients with complicated myocardial infarction: findings from the High-Risk Myocardial Infarction Database Initiative. Eur J Heart Fail 2015; 17: 1144–1151. [DOI] [PubMed] [Google Scholar]
- 32.Fang X, Pan C, Chen Y, et al. Assessment of subclinical left ventricular changes in essential hypertensive patients with hyperuricemia: A three-dimensional speckle-tracking echocardiography study. Clin Exp Hypertens 2017; 39: 93–99. [DOI] [PubMed] [Google Scholar]
- 33.Cicero AF, Rosticci M, Parini A, et al. Serum uric acid is inversely proportional to estimated stroke volume and cardiac output in a large sample of pharmacologically untreated subjects: data from the Brisighella Heart Study. Intern Emerg Med 2014; 9: 655–660. [DOI] [PubMed] [Google Scholar]
- 34.Kuwabara M, Niwa K, Nishihara S, et al. Hyperuricemia is an independent competing risk factor for atrial fibrillation. Int J Cardiol 2017; 231: 137–142. [DOI] [PubMed] [Google Scholar]
- 35.Jankowska EA, Ponikowska B, Majda J, et al. Hyperuricaemia predicts poor outcome in patients with mild to moderate chronic heart failure. Int J Cardiol 2007; 115: 151–155. [DOI] [PubMed] [Google Scholar]
- 36.Pascual-Figal DA, Hurtado-Martínez JA, Redondo B, et al. Hyperuricaemia and long-term outcome after hospital discharge in acute heart failure patients. Eur J Heart Fail 2007; 9: 518–524. [DOI] [PubMed] [Google Scholar]
- 37.Borghi C, Cosentino ER, Rinaldi ER, et al. Uricaemia and ejection fraction in elderly heart failure outpatients. Eur J Clin Invest 2014; 44: 573–578. [DOI] [PubMed] [Google Scholar]
- 38.MacIsaac RL, Salatzki J, Higgins P, et al. Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension 2016; 67: 535–540. [DOI] [PubMed] [Google Scholar]
- 39.Thanassoulis G, Brophy JM, Richard H, et al. Gout, allopurinol use, and heart failure outcomes. Arch Intern Med 2010; 170: 1358–1364. [DOI] [PubMed] [Google Scholar]
- 40.Burns CM andWortmann RL.. Gout therapeutics: new drugs for an old disease. Lancet 2011; 377: 165–177. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto K, Okamoto K, Ashizawa N, et al. FYX-051: a novel and potent hybrid-type inhibitor of xanthine oxidoreductase. J Pharmacol Exp Ther 2011; 336: 95–103. [DOI] [PubMed] [Google Scholar]
- 42.Sircar D, Chatterjee S, Waikhom R, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: A 6-Month, double-Blind, randomized, placebo-controlled trial. Am J Kidney Dis 2015; 66: 945–950. [DOI] [PubMed] [Google Scholar]
- 43.Kimura K, Hosoya T, Uchida S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: A randomized trial. Am J Kidney Dis 2018; 72: 798–810. [DOI] [PubMed] [Google Scholar]
- 44.Shiomi T, Tsutsui H, Matsusaka H, et al. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 2004; 109: 544–549. [DOI] [PubMed] [Google Scholar]
- 45.Sorescu D andGriendling KK.. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail 2002; 8: 132–140. [DOI] [PubMed] [Google Scholar]
- 46.Su CY, Shen LJ, Hsieh SC, et al. Comparing cardiovascular safety of febuxostat and allopurinol in the real world: A population-based cohort study. Mayo Clin Proc 2019; 94: 1147–1157. [DOI] [PubMed] [Google Scholar]
- 47.Kojima S, Matsui K, Hiramitsu S, et al. Febuxostat for cerebral and cardiorenovascular events prevention study. Eur Heart J 2019; 40: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]