Abstract
Objective
To evaluate the antiviral activity of the oral disinfectant povidone-iodine (PVP-I) against severe acute respiratory syndrome-coronavirus-2 (SARS-CoV2) in vitro.
Methods
The cytotoxic effects of PVP-I were determined in Vero and Calu-3 cell lines using that by Cell Counting Kit-8 assay. Viral load in the cell culture medium above infected cells was quantitated using real-time polymerase chain reaction. The cytopathic effect (CPE) and viral infective rate were observed by immunofluorescence microscopy.
Results
PVP-I at a concentration >0.5 mg/ml in contact with SARS-CoV-2 for 30 s, 1 min, 2 min and 5 min showed up to 99% viral inhibition. For in vitro testing, upon exposure for 1 min, PVP-I showed a virucidal effect. PVP-I had no cytotoxic effects at the range of concentrations tested (0.125–1 mg/ml; CC50 > 2.75 mM) in Vero and Calu-3 cells.
Conclusion
These results demonstrate that the ideal contact time was 1 min and the optimal concentration was 1 mg/ml, which provides an experimental basis for the use of oral disinfectants in dental hospitals.
Keywords: Povidone-iodine, SARS-CoV-2, public health, prophylaxis, antiviral effect
Introduction
As of 23 October 2020, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV2) has infected more than 41.29 million people worldwide. 1 Previous research has shown that the oral cavity has a high viral load. 2 Thus, an oral disinfectant that has an anti-SARS-CoV-2 effect is urgently needed. At the end of 2019, the coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 first occurred in Wuhan, Hubei Province, China. 3 Since then, COVID-19 has spread globally via human-to-human transmission due to the high infectivity of SARS-CoV-2.3,4 According to the latest data, the virus has spread to more than 210 countries and regions, causing 41.29 million confirmed cases and more than 1.13 million deaths, 1 resulting in tremendous pressure on healthcare institutions and hospitals worldwide. On March 11th 2020, The World Health Organization (WHO) declared COVID-19 to be a novel pandemic. 5
By reviewing the available evidence and examining similar pandemics such as severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV), researchers have found that the increase in the number of infections is exponential.6,7 There are four common human coronaviruses, 229E, NL63, OC43 and HKU1 (229E, NL63 are antigenic group 1; OC43 and HKU1 are antigenic group 2), 8 but SARS-CoV-2 is more infectious and pathogenic than them. 9 With a positive and single-stranded RNA genome, SARS-CoV-2 is an enveloped virus and is a new member of the beta-coronaviruses in the phylogenetic tree, similar to MERS-CoV and SARS-CoV.3,7 SARS-CoV and MERS-CoV caused mass infections in 2002–2003 and continued intensive outbreaks in 2012–2020, respectively. 10 Furthermore, many studies have confirmed that the SARS-CoV-2 entry receptor is angiotensin-converting enzyme II, which is the same as SARS-CoV. 3
COVID-19 is so dangerous and infectious that many countries have chosen to temporarily close schools, social venues, public events and gathering places to contain the outbreak. Furthermore, more and more people have chosen to help limit the spread of the epidemic through self-isolation. However, even during this global pandemic, healthcare is still essential for people to shut down and quarantine completely. Accordingly, medical care personnel, especially those working in stomatology, are a high-risk group. Researchers and the WHO found that SARS-CoV-2 could infect healthy people though droplets, saliva and mucosa mucous emitted during coughing, speaking or sneezing.7,11–13 Therefore, oral healthcare professionals are at a higher risk of infection and more likely to transmit the virus to their peers, family members and other patients.14,15 This high risk of transmission has attracted the attention of national government departments and relevant medical institutions.
Accordingly, this current study investigated the common oral disinfectant povidone-iodine (PVP-I), which is widely used in stomatology. It was reported that PVP-I could inhibit bacterial growth without upsetting the balance of oral microorganisms. 16 Furthermore, PVP-I has been shown to be effective against Ebola and the modified vaccinia virus Ankara (a new European test virus used for enveloping viruses) in vitro. 17 PVP-I is also an attractive choice owing to its excellent anti-corrosion properties, safety, lack of resistance, accessibility and low cost. 18 Under these circumstances, in the face of the current severe pandemic, this current evaluated the inhibitive effect of PVP-I on SARS-CoV-2 in a Vero cell model. This current study provides theoretical guidelines and data support for the use of disinfectants in oral clinical environments during the COVID-19 pandemic.
Materials and methods
Virus and cell culture
SARS-CoV-2 (hCoV-19/Zhejiang/OS2/2020, GISAID, ID: 455692) was isolated from a patient in Zhejiang Provincial Centre for Disease Control and Prevention, Hangzhou, Zhejiang Province, China. Vero cells (ATCC CCL-81; Zhongyuan, Beijing, China) and Calu-3 cells (ATCC HTB-55; Zhongyuan) were grown in Modified Eagle Medium (Life Technologies, Gaithersburg, MD, USA) containing 2% fetal bovine serum (Life Technologies) and then kept in an incubator at 35°C under 5% CO2. The virus was propagated in Vero cells and Calu-3 cells that were cultured under standard conditions. At 48 h post-infection, the culture supernatants were collected, centrifuged at 485.63 g for 8 min at 24°C in a Velocity 18R centrifuge (Dynamica, Livingston, UK) and stored at –80°C to build virus stocks. All the experiments involving infectious viruses were conducted in the approved biosafety level III laboratory (CNAs BL0026, Zhejiang Provincial Centre for Disease Control and Prevention).
CCK-8 cell viability test
Vero cells and Calu-3 cells were inoculated into 96-well plates (5000 per well) and incubated at 35°C under 5% CO2 for 24 h and then treated with diluted (1:500) PVP-I solution (2, 1, 0.5, 0.25, 0.125 and 0.0625 mg/ml; prepared by 2-fold serial dilution) for 2 h. Then, 10 μl of the Cell Counting Kit-8 (CCK-8) solution (MedChemExpress, Monmouth Junction, NJ, USA) was added to each well and, after 3 h, the absorbance at 450 nm was measured using a microplate reader (iMark™ Microplate Absorbance Reader; Bio-Rad, Hercules, CA, USA). The data were used to calculate the toxic concentration and the maximum non-toxic concentration of PVP-I.
Antiviral effect of PVP-I disinfectant against SARS-CoV-2 virus
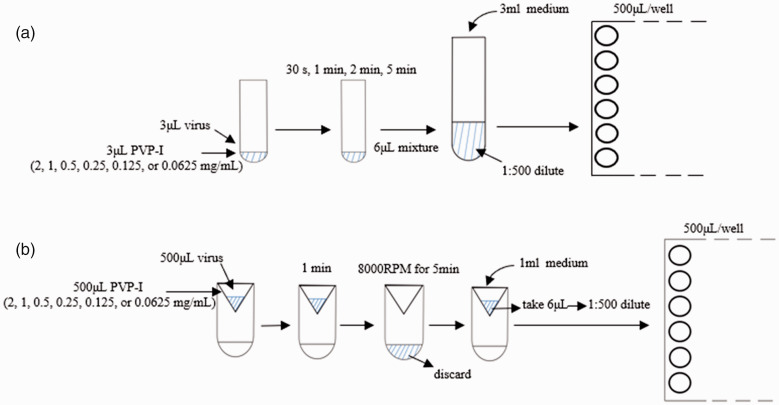
Vero cells were inoculated into 48-well plates (10 000 per well) and incubated at 35°C under 5% CO2 for 48 h. Then, 3 μl stock virus (106 IU/ml) was mixed with 3 μl PVP-I solution (2, 1, 0.5, 0.25, 0.125 and 0.0625 mg/ml) in test tubes for 30 s, 1 min, 2 min or 5 min. Then, each mixture was diluted 1:500 with culture medium and 500 μl aliquots of the diluted solutions were added to the wells containing Vero cells. For the control group, 500 μl of culture medium alone was added to the Vero cells (Figure 1a). After incubating at 35°C under 5% CO2 for 48 h, the cells were observed under a microscope (EVOS M7000 Imaging System; Thermo Fisher, Shanghai, China) as they became round, necrotic and detached from the tissue culture plastic surface, which was defined as the cytopathic effect (CPE). This part of the experiment demonstrates that SARS-COV-2 virus can cause Vero cell death.
Figure 1.
Schematic diagrams showing the two experimental procedures used to investigate the antiviral effect of povidone-iodine (PVP-I) against severe acute respiratory syndrome-coronavirus-2 (SARS-CoV2) in vitro: (a) stock virus (3 μl, 106 IU/ml) was mixed with 3 μl PVP-I solution (2, 1, 0.5, 0.25, 0.125, 0.0625 mg/ml) in test tubes. Contact times of 30 s, 1 min, 2 min and 5 min were used for each concentration. Each mixture was then diluted 500-fold with 3 ml culture medium and 500 μl aliquots of the diluted solutions were added to wells containing Vero cells (three replicates for each solution). After incubating at 35°C under 5% CO2 for 48 h, the cytopathic effect was observed under a microscope; (b) virus solution (0.5 ml) was mixed with different concentrations of PVP-I (2, 1, 0.5, 0.25, 0.125, 0.0625 mg/ml) for 1 min. The mixture was transferred to an ultrafiltration centrifuge tube, centrifuged at 485.63 g for 5 min, and then 1 ml of culture medium was added to resuspend the mixture. Subsequently, 6 μl of 1 ml mixture was diluted 1:500 with 3 ml culture medium and 500 μl of diluted solutions was added to the wells (three replicates for each solution). All plates were incubated at 35°C under 5% CO2 for 48 h. The 50% tissue culture infectious dose assay values were used to determine virus titre using the method of Reed and Muench. 19
Vero cells and Calu-3 cells were inoculated into 48-well plates (10 000 per well) and incubated at 35°C under 5% CO2 for 48 h. Then, 500 μl of viral stock (106 IU/ml) was mixed with 500 μl of different concentrations of PVP-I (2, 1, 0.5, 0.25, 0.125 and 0.0625 mg/ml) for 1 min. Then the mixture was transferred to an ultrafiltration centrifuge tube (Amicon Ultra 50K; Millipore, Billerica, MA, USA) and centrifuged at 4972.86 g for 5 min at 24°C in a Velocity 18R centrifuge (Dynamica) to concentrate the viral particles. 1 ml of culture medium was used to resuspend the mixture and 6 μl of the 1 ml solution was diluted 1:500 with an extra 3 ml of culture medium. The 1:500 dilution or ultrafiltration steps were used to eliminate the subsequent antiviral effect of PVP-I. Then 500 μl of diluted solution was added to 48-well plates (three replicates for each solution) containing Vero cells or Calu-3 cells (Figure 1b). For the control group, 500 μl of culture medium was added to the Vero cells and Calu-3 cells. All the plates were incubated at 35°C under 5% CO2 for 48 h. The 50% tissue culture infectious dose (TCID50) assay values were used to determine the virus titre using the method of Reed and Muench. 19
Viral RNA extraction and RT-PCR
The density of the cultured cells used to extract total RNA was 2.5 × 104 cells. Viral RNA was extracted from 200 μl of Vero cell culture supernatant (48 h post-infection) using an automated nucleic acid extraction system (MVR01; Liferiver, Shanghai, China). Real-time polymerase chain reaction (RT-PCR) was used to synchronously detect the RdRP, N and E genes of the virus and quantify the COVID-19 virus on a LightCycler® 480 System (Roche, Basel, Switzerland) with a One-Step RT-PCR kit (Z-RR-0479-02-50, Liferiver). The cycling programme involved preliminary denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 45 s, annealing at 60°C for 45 s and elongation at 72°C for 60 s, followed by a final elongation step at 72°C for 10 min. The relative expression between treatment and control groups was evaluated using the 2−△△Ct method.
Immunofluorescence microscopy
Vero cells and Calu-3 cells were washed with 10 mM phosphate-buffered saline (PBS; pH 7.4) at 48 h post-infection, fixed in 80% precooled acetone (Sigma-Aldrich, St Louis, MO, USA) for 30 min, incubated at room temperature in 1% bovine serum albumin in 10 mM PBS (pH 7.4), and then incubated with rabbit anti-Spike RBD polyclonal antibodies (1:1000 dilution; SARS-CoV-2 [2019-nCoV] Spike RBD Antibody, Rabbit PAb, Antigen Affinity Purified; cat no. 40592-T62; Sino Biological Inc., Beijing, China; Cat: 40592-T62) at 4°C overnight. Then, the cells were washed twice in 10 mM PBS (pH 7.4), incubated for 2 h at room temperature, and then incubated with Alexa Fluor488®-conjugated goat anti-rabbit secondary antibody (1:1500 dilution; cat no. ab150077; Abcam®, Cambridge, MA, USA) for 2 h. Cells were counterstained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride to stain the nuclei. The stained cells were observed by fluorescence microscopy and photographed (EVOS M7000 Imaging System; Thermo Fisher).
Statistical analyses
The EC50 values (concentration for 50% of maximal effect) were calculated using nonlinear regression and the data were analysed using GraphPad Prism 8.0.1 (Graphpad Software Inc., San Diego, CA, USA).
Results
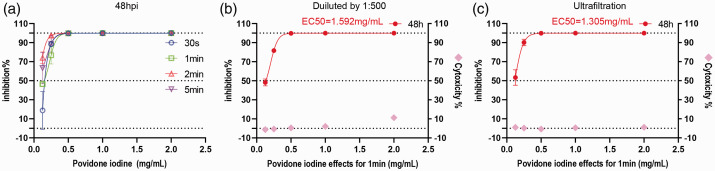
In this current study, CCK-8 cell viability analysis and cytopathic observations were used to determine the toxic effects of PVP-1 against Vero cells, Calu-3 cells and SARS-CoV-2 virus. In the first group of experiments, the viral samples were treated with PVP-I (2, 1, 0.5, 0.25, 0.125 and 0.0625 mg/ml) for 30 s, 1 min, 2 min or 5 min prior to incubation with Vero cells. The preliminary results showed that the viral inhibitory effect at the same concentration was highest when the contact time was 1 min (Figure 2a), so this contact time was used for all subsequent experiments. A second group of parallel experiments was conducted with 1:500 dilution and ultrafiltration, respectively. In these two groups of parallel experiments, viral samples were treated with PVP-I (2, 1, 0.5, 0.25, 0.125 and 0.0625 mg/ml) for an exposure time of 1 min. The supernatant from the cell cultures was collected for RT-PCR. Inhibition rate was calculated using ‘inhibition% = (1–2−△△Ct) × 100%’. The results show that PVP-I at a concentration of ≤1000 µg/ml had no viral inhibition on COVID-19 (CC50 > 2.75 mM) (Figures 2, 3 and 4). The CC50 is the minimum concentration of the drug needed to kill 50% of the host cells. The higher the value of CC50/EC50, the smaller the concentration required to suppress the pathogen and the less harmful it is to the host cell. After ultrafiltration, there was no viral inhibition at all the concentrations tested (CC50 > 100 µM; Figures 2, 3 and 4). The experimental results for ultrafiltration were approximately the same as those for dilution alone at 1:500. The results showed that the effect of PVP-I on the virus inhibition rate was mainly concentration dependent. In the Vero cell model and the Calu-3 cell model, the virucidal effect of PVP-I had an EC50 = 1.592 mg/ml and EC50 = 1.305 mg/ml, respectively (Figures 2 and 4).
Figure 2.
Antiviral activity and cytotoxicity of povidone-iodine (PVP-I) in vitro. (a) The inhibitory effect of PVP-1 on Vero cells was determined by CCK-8 analysis. (b) PVP-I treatment at different concentrations for 1 minute showing cytotoxicity and virus inhibition (experimental procedure as shown in Figure 1a). (c) PVP-I treatment at different concentrations for 1 minute showing cytotoxicity and virus inhibition (experimental procedure as shown in Figure 1b). The infected Vero cells in figures 2a, 2b and 2c exhibited different therapeutic outcomes that were mainly in response to different concentrations of PVP-I disinfectant. Viral RNA load was measured in the cell culture supernatant using real-time polymerase chain reaction and CCK-8 assays were used to measure the cytotoxicity of PVP-I to Vero cells. The left and right y-axes of the second two graphs show the mean inhibition rate (%) produced by the PVP-I on the virus and the cytotoxicity of the PVP-I on the Vero cells, respectively. The EC50 is shown at the top of the figure. hpi, hours post infection.
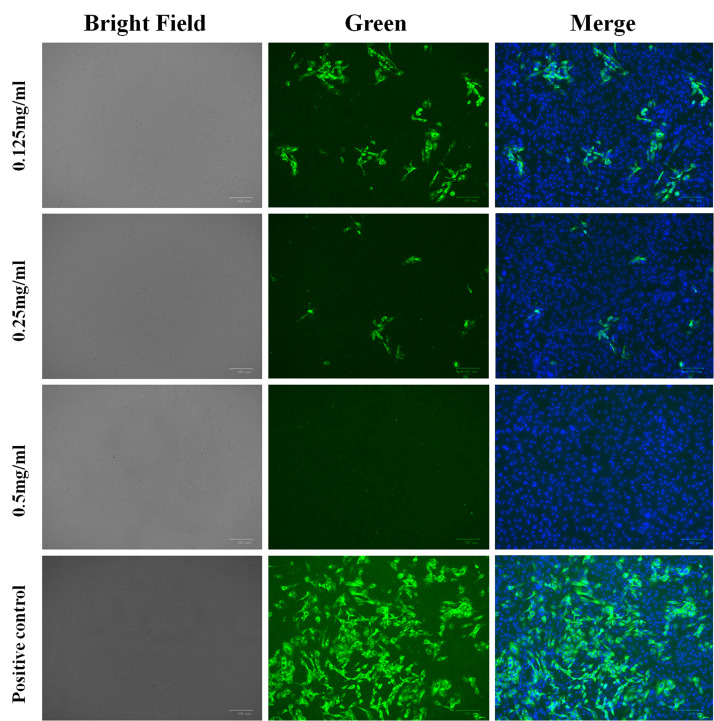
Figure 3.
Immunofluorescence microscopy of Vero cells infected with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV2) (multiplicity of infection 0.05) and disinfected with povidone-iodine (PVP-I) for 1 min at different concentrations. Cells were counterstained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride to stain the nuclei. Scale bar 100 μm.
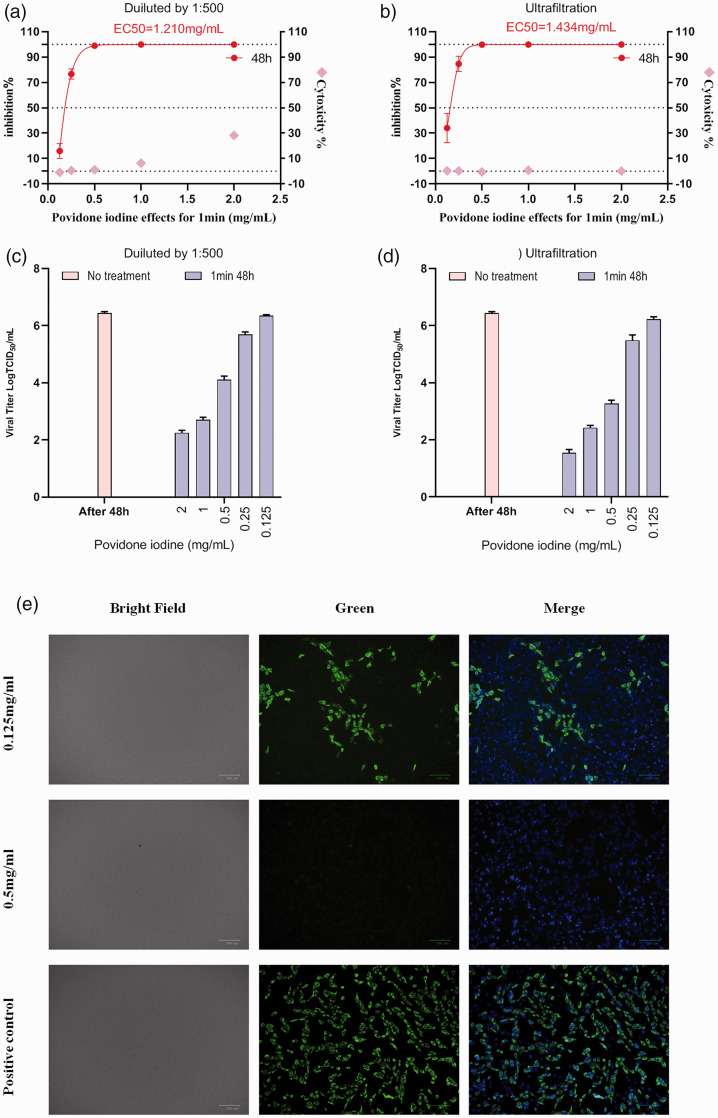
Figure 4.
Antiviral activity, cytotoxicity, viral titre data and immunofluorescence microscopy of Calu-3 cells infected with severe acute respiratory syndrome-coronavirus-2 treated with povidone-iodine (PVP-I) at different concentrations for 1 min. (a) Virus inhibition and cytotoxicity data obtained using different concentrations of PVP-I disinfectant on infected Calu-3 cells using the experimental procedure shown in Figure 1a. (b) Virus inhibition and cytotoxicity data obtained using different concentrations of PVP-I disinfectant on infected Calu-3 cells using the experimental procedure shown in Figure 1b. Real-time polymerase chain reaction was used to detect the viral RNA load in cell culture supernatant and CCK-8Continued.assays were used to detect the cytotoxicity of PVP-I to Calu-3 cells. The left and right y-axes of the first two graphs show the mean inhibition rate (%) produced by the PVP-I on the virus and the cytotoxicity of the PVP-I on the Calu-3 cells, respectively. (c, d) Calu-3 cells were treated with different concentrations of PVP-I for 1 min and the 50% tissue culture infectious dose assay (TCID50) values were used to determine virus titre using the method of Reed and Muench. 19 Data presented as mean ± SD for three repeated experiments. (e) Immunofluorescence microscopy of Calu-3 cells infected with severe acute respiratory syndrome-coronavirus-2 and disinfected with PVP-I for 1 min at different concentrations. Cells were counterstained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride to stain the nuclei. Scale bar 100 μm.
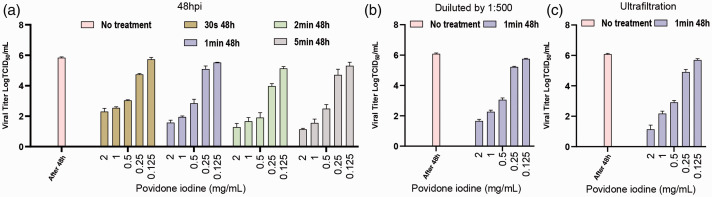
After treatment with PVP-I at preset concentrations (48 h post-infection), 200 μl aliquots of the culture supernatant were collected for RT-PCR and TCID50 assay. TCID50 assays were performed to help to identify the optimal concentration and contact time, as well as the virus titres for PVP-I at different defined concentrations or contact times against the same SARS-CoV-2 samples (106 UI/ml; Figures 4 and 5). The viral titres for the same contact time but different PVP-I concentrations (2, 1, 0.5, 0.25 and 0.125 mg/ml) decreased as the concentration of PVP-I increased (Figure 5a). The viral titres for different contact times (30 s, 1 min, 2 min or 5 min) at the same PVP-I concentration, revealing similar results at 2 and 1 mg/ml. Thus, the results of the TCID50 assays indicate that treatment with high concentrations of PVP-I for an appropriate contact time can inhibit viral infection and CPE was correspondingly diminished in such cases. However, although PVP-I exhibits the strongest antiviral effect at 2 mg/ml, decreasing viral titre by approximately 5 Log10 TCID50/ml compared with the control, there was no difference compared with the reduction observed for 1 mg/ml, which was over 4 Log10 TCID50/ml, thus meeting European standards. 20 For PVP-I at high concentrations, prolonging the contact time does not enhance virus suppression. Overall, oral disinfection with PVP-I at a clinical concentration (1 mg/ml) for 1 min is ideal and these parameters were used for subsequent tests (Figures 4 and 5).
Figure 5.
Severe acute respiratory syndrome-coronavirus-2 viral titre data for povidone-iodine (PVP-I) at different concentrations and contact times. (a) The cytopathic effect (CPE) on Vero cells 48 h post-infection treated with PVP-I under the conditions shown were determined by microscopy and the 50% tissue culture infectious dose assay (TCID50) values were used to determine virus titre using the method of Reed and Muench. 19 Data presented as mean ± SD for three repeated experiments. (b, c) Results for Vero cells treated with PVP-I for 1 min at different concentrations based on the results shown in (a). The colour version of this figure is available at: http://imr.sagepub.com.
Discussion
The COVID-19 pandemic has had a major impact on global public health and has not been effectively contained. Although there are many drugs that have some therapeutic effect against SARS-CoV-2, there is currently no effective treatment for SARS-COV-2 infection. 21 Therefore, to respond to the COVID-19 pandemic, personal hygiene and the use of personal protective equipment are crucial for the prevention of widespread infection. However, due to the unique working environment of healthcare workers, especially those involved in oral surgery or dental prosthetics, they are at a high risk of infection by aerosol transmission.7,11,22 Therefore, in order to reduce the risk of transmission of the virus, it is vital that stomatological hospitals perform effective oral disinfection of patients.
Povidone-iodine is a common disinfectant in dental clinics. Previous studies have shown that PVP-I has a certain therapeutic effect in the prevention and treatment of chronic respiratory tract infection. 11 A 0.23% solution of PVP-I has been proven to be very effective against SARS-CoV and MERS-CoV. 23 Both SARS-CoV-2 and SARS-CoV are beta-coronaviruses and research has demonstrated that PVP-I has virucidal effects against SARS-CoV-2.7,12,24 Thus, PVP-I is a reasonable candidate as a novel anti-coronavirus disinfectant. The commercially available PVP-I disinfectant concentration is usually 5%, while the clinical concentration for oral use is usually 1%. A previous study has demonstrated that 1–5% PVP-I disinfectant can completely inactivate SARS-CoV-2 after 60 seconds of action on Vero 76 cells that were infected with SARS-CoV-2. 24 The experimental results of this previous study further demonstrate that PVP-I disinfectant is highly suitable for clinical use. 25 It is hoped that the appropriate concentration obtained in this study can be used to prepare disinfectant sprays specifically for COVID-19, which is very promising for oral and skin disinfection in high-risk groups or in public places. 26
Povidone-iodine is a water-soluble complex composed of iodine and a solubilizing carrier polymer. Iodine is released by PVP-I in aqueous conditions, rapidly contacting with the virus and oxidizing key proteins and/or nucleotides, eventually inactivating the virus or preventing it from proliferating. 26 Previous studies have shown that PVP-I is a broad-spectrum, highly effective and safe disinfectant.27,28 From the existing research on the other antiviral mechanisms of PVP-1, it is known that the free iodine is mainly responsible for its antiviral effects because it can oxidize key structures in the pathogen, such as amino acids, nucleic acids and membrane components. 29 In addition to inactivating the virus directly, PVP-I can also exert its antiviral activity in virus-infected cells, thereby reducing the production of viral progeny. 29 Therefore, it can be inferred that the antiviral effect of PVP-I can be attributed to its inhibition of the binding activity of virus to host cells and the pathway of virus release and transmission. 30 However, there are reports of adverse reactions caused by PVP-I. 11 In this current study, PVP-I showed no significant cytotoxicity at all the concentrations tested (CC50 > 100 µM), which was similar to the results of previous studies.31,32 This current study demonstrated that PVP-I administered at 1 mg/ml for 1 min can achieve an ideal antiviral effect against SARS-CoV-2 (TCID50 = 2.2 log10 mg/ml). This means that under these conditions, viral nucleic acid load tests can be negative. This current research on PVP-1 may help healthcare professionals to optimize their use of PVP-1 for gargling or other oral care, so as to prevent unnecessary COVID-19 infections.
This current study had several limitations. First, compared with the simple single-cell controlled experimental model used in this study, the human oral microenvironment is more complex. For example, saliva, teeth and other substances can act as viral carriers. 22 This limitation has encouraged our research team to more comprehensively study the antiviral effects of PVP-I on SARS-CoV-2 in animal models, against other similar viruses and to conduct clinical trials. In order to make the experimental conditions more consistent with the in vivo situation, the use of respiratory epithelial cells as host cells will be considered. Secondly, disinfectants when used in the oral cavity often only contact cell-free surfaces and do not completely contact the cells. Considering that the delivery formulation and the oral microenvironment are under the influence of many complex factors, an attempt to build a three-dimensional model of the human oral cavity with realistic contact areas might be useful. Changing the delivery formulation of PVP-I to an aerosol for example is another avenue for potential future research.
In conclusion, comprehensive clinical trials of PVP-I will be able to confirm whether the routine clinical use of this oral disinfectant in dentistry is effective in reducing the transmission and controlling the spread of COVID-19.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study was funded by grants from the General project of the Traditional Chinese Medicine Administration of Zhejiang Province (no. 2016ZA134), the Key Technologies R&D Programme of the National Ministry of Science (no. 2018ZX10734-401) and the Zhejiang Provincial Natural Science Foundation of China (no. LQ20H190004).
ORCID iD: Keda Chen https://orcid.org/0000-0002-9469-0991
References
- 1.Coronavirus Resource Center: John Hopkins University, COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU), https://coronavirus.jhu.edu/map.html (2020).
- 2.Gottsauner MJ, Michaelides I, Schmidt B, et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin Oral Investig 2020; 24: 3707–3713. DOI: 10.1007/s00784-020-03549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020; 63: 457–460. DOI: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020; 382: 1199–1207. DOI: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19, 11 March 2020, World Health Organization, https://www.who.int/zh/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 6.Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int J Infect Dis 2020; 92: 214–217. DOI: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DE, Sivalingam V, Kang AEZ, et al. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease. Infect Dis Ther 2020; 9: 669–675. DOI: 10.1007/s40121-020-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habibzadeh P andStoneman EK.. The Novel Coronavirus: A Bird's Eye View. Int J Occup Environ Med 2020; 11: 65–71. DOI: 10.15171/ijoem.2020.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shamsi A, Mohammad T, Anwar S, et al. Glecaprevir and Maraviroc are high-affinity inhibitors of SARS-CoV-2 main protease: possible implication in COVID-19 therapy. Biosci Rep 2020; 40: BSR20201256. DOI: 10.1042/BSR20201256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamel Boulos MN and, Geraghty EM. Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic and associated events around the world: how 21st century GIS technologies are supporting the global fight against outbreaks and epidemics. Int J Health Geogr 2020; 19: 8.DOI: 10.1186/s12942-020-00202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vergara-Buenaventura A andCastro-Ruiz C.. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg 2020; 58: 924–927. DOI: 10.1016/j.bjoms.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bidra AS, Pelletier JS, Westover JB, et al. Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse. J Prosthodont 2020; 29: 529–533. DOI: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morawska L andCao J.. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ Int 2020; 139: 105730. DOI: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrera D, Serrano J, Roldan S, et al. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Investig 2020; 24: 2925–2930. DOI: 10.1007/s00784-020-03413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang GY. Experts recommendation on the diagnosis and treatment of oral mucosal diseases during prevention and control stage of the novel coronavirus infection. Shanghai Kou Qiang Yi Xue 2020; 29: 118–122 [Article in Chinese, English abstract]. [PubMed] [Google Scholar]
- 16.Tsuda S, Soutome S, Hayashida S, et al. Topical povidone iodine inhibits bacterial growth in the oral cavity of patients on mechanical ventilation: a randomized controlled study. BMC Oral Health 2020; 20: 62. DOI: 10.1186/s12903-020-1043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggers M Eickmann M andZorn J.. Rapid and Effective Virucidal Activity of Povidone-Iodine Products Against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA). Infect Dis Ther 2015; 4: 491–501. DOI: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang T, Yim N, Tummala S, et al. Povidone-Iodine versus antibiotic irrigation in breast implant surgery: Revival of the ideal solution. J Plast Reconstr Aesthet Surg 2020; 73: 391–407. DOI: 10.1016/j.bjps.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishnan MA. Determination of 50% endpoint titer using a simple formula. World J Virol 2016; 5: 85–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Committee for Standardization. European Standard EN 14476: chemical disinfectants and antiseptics e quantitative suspension test for the evaluation of virucidal activity in the medical area e test method and requirements (Phase 2/Step 1). 2019. Brussels.
- 21.Hurlburt NK, Seydoux E, Wan YH, et al. Structural basis for potent neutralization of SARS-CoV-2 and role of antibody affinity maturation. Nat Commun 2020; 11: 5413. DOI: 10.1038/s41467-020-19231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect 2020; 81: 357–371. DOI: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggers M Koburger-Janssen T andEickmann M.. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect Dis Ther 2018; 7: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelletier JS, Tessema B, Frank S, et al. Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). Ear Nose Throat J 2021; 100(2_suppl): 192S–196S. DOI: 10.1177/0145561320957237. [DOI] [PubMed]
- 25.Elzein R, Abdel-Sater F, Fakhreddine S, et al. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. J Evid Based Dent Pract 2021; 21: 101584. doi:10.1016/j.jebdp.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepelletier D, Maillard J, Pozzetto B, et al. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob Agents Chemother 2020; 64: e00682–20. DOI: 10.1128/aac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vercammen Y, Dauwe D, De Vlieger G, et al. Povidone Iodine Disinfection Associated with Hypothyroidism and Potentially Contributing to Prolonged Kidney Failure. Case Rep Crit Care 2021; 2021: 5528210. DOI:10.1155/2021/5528210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sani D, Abdu PA, Mamman M, et al. Research Note: Evaluation of acute oral toxicity of povidone-iodine in cockerels using the up-and-down procedure. Poult Sci 2021; 100: 631–634. DOI:10.1016/j.psj.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh S, Sawant OB, Mian SI, et al. Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses. Biomolecules 2021; 11: 753. doi:10.3390/biom11050753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sriwilaijaroen N, Wilairat P, Hiramatsu H, et al. Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: its effects on hemagglutination and sialidase activities. Virol J 2009; 6: 124. doi:10.1186/1743-422X-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barreto R, Barrois B, Lambert J, et al. Addressing the challenges in antisepsis: focus on povidone iodine. Int J Antimicrob Agents 2020; 56: 106064. DOI:10.1016/j.ijantimicag.2020.106064. [DOI] [PubMed] [Google Scholar]
- 32.Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, et al. Povidone iodine in wound healing: A review of current concepts and practices. Int J Surg 2017; 44: 260–268. DOI:10.1016/j.ijsu.2017.06.073. [DOI] [PubMed] [Google Scholar]