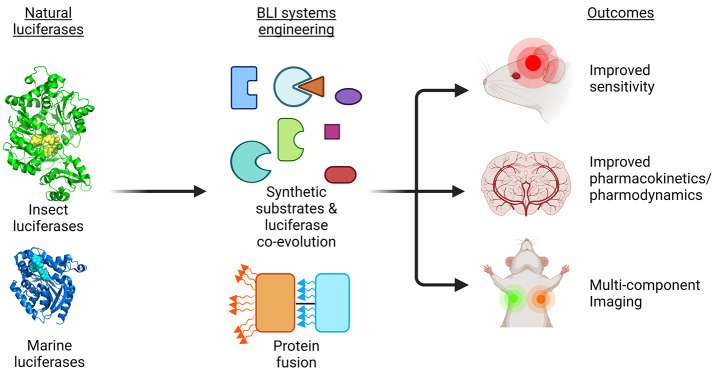
Abstract
Bioluminescence imaging (BLI) using luciferase reporters is an indispensable method for the noninvasive visualization of cell populations and biochemical events in living animals. BLI is widely performed with preclinical rodent models to understand disease processes and evaluate potential cell- or gene-based therapies. However, in vivo BLI remains constrained by low photon production and tissue attenuation, limiting the sensitivity of reporting from small numbers of cells in deep locations and hindering its application to larger animal models. This Review highlights recent advances in the development of luciferase systems that improve the sensitivity of in vivo BLI and discusses the expanding array of biological applications.
Introduction
Bioluminescence imaging (BLI) with luciferase reporters is a highly valuable technique for tracking genetically labeled cell populations and monitoring molecular processes in vivo. In its simplest form, BLI detects the presence of labeled cells at a particular location over time, which can reveal changes in cellular proliferation or survival throughout development, disease, or therapy.1 In a more specific scheme, the expression of luciferase reporters can be made responsive to biochemical signals, allowing BLI to track these signals over time.2 A major advantage of BLI is that it can be performed longitudinally and noninvasively. Other benefits of BLI include the lack of an intrinsic bioluminescence signal in animals allowing for high sensitivity and specificity, its relative simplicity and affordability, and the ability to perform simultaneous whole-body imaging of model animals such as mice.3−5
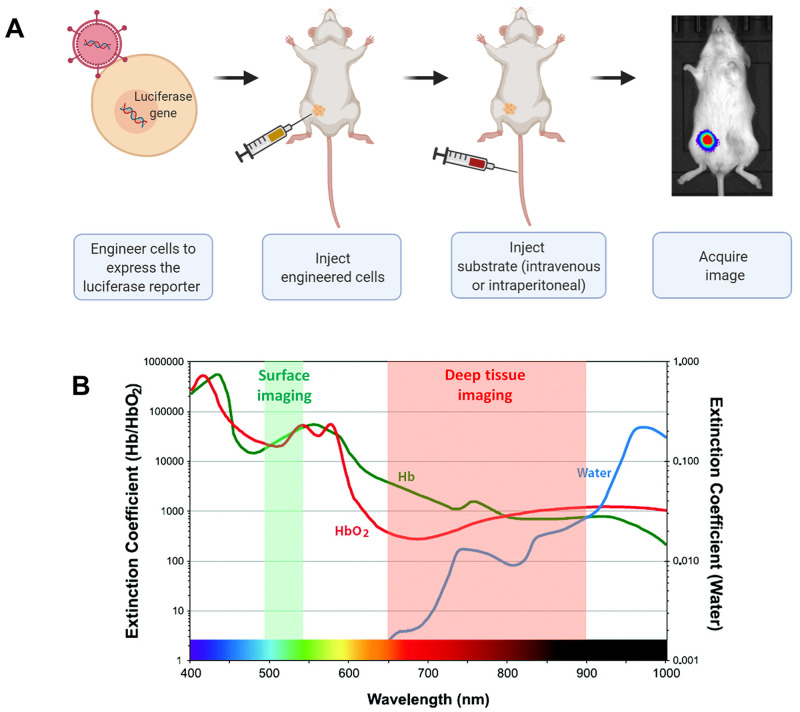
To perform BLI, cells are genetically engineered to express a luciferase enzyme that reacts with an exogenously delivered substrate to produce light, which is then detected by a camera6 (Figure 1A). The most thoroughly studied luciferases are those isolated from insects, with the Photinus pyralis firefly luciferase (FLuc) being the most widely used. In insect luciferases, oxidation of the substrate d-luciferin in the presence of oxygen, ATP, and Mg2+ produces yellow light with an emission peak at 578 nm at 25 °C.7,8 Other insect luciferases that are used for BLI include those from the firefly Luciola italica and the click beetle Pyrophorus plagiophthalamus. A larger number of known luciferases, however, originate from marine organisms and produce blue light (emission peak 450–500 nm) from the oxidation of coelenterazine (CTZ) in the absence of cofactors other than oxygen. Commonly used CTZ-dependent systems include Renilla (RLuc), Gaussia (GLuc), and NanoLuc luciferases.9
Figure 1.
In vivo bioluminescence imaging (BLI) of engineered cells. (A) Cells are first genetically engineered to express the luciferase, then transplanted into the animal. The appropriate substrate is injected, and the emitted light is captured to generate an image. (B) Extinction coefficient value of water, oxyhemoglobin (HbO2), and deoxyhemoglobin (Hb) for wavelengths in the visible to near-infrared region. Adapted with permission from Kobayashi et al.10 Copyright 2010 American Chemical Society.
BLI detection favors superficially located cells due to absorption and scattering of light by tissue. In mammalian tissues, hemoglobin impairs transmission of wavelengths below 600 nm, which partially or completely overlaps with the emission spectra of most natural luciferases discovered to date.8 Therefore, to improve the detection of sparse cell populations at greater tissue depths, it would be advantageous to develop luciferases with red-shifted emission spectra (Figure 1B). Alternatively, luciferases with higher specific activity or higher levels of expression (through more efficient translation or slower degradation) would improve limits of detection for small numbers of cells but alone would not reduce depth-dependent attenuation. Additionally, many current substrates possess uneven biodistribution within mice. For example, intravenously injected 14C–d-luciferin showed early high uptake in the kidney and liver, late predominant uptake in the bladder, and limited access to the brain.11 Substrate solubility and stability are also limiting factors of CTZ and its analogs.12
Due to these limitations, significant efforts have been invested in the development of improved BLI luciferase/luciferin pairs for improved monitoring of cellular processes. Here, we summarize recent advances in luciferase systems aimed to improve in vivo sensitivity in mammalian model systems, and we highlight novel biological applications of the expanding BLI toolbox.
BLI Systems Engineering
With the goal of improving detection sensitivity from deep locations in mammalian subjects, multiple efforts have attempted to mutate luciferases to increase catalytic activity or to red-shift emission (Table 1). One of the earliest examples of wavelength shifting comes from the S284T mutant of FLuc, PpeRS, discovered by random mutagenesis to create a ∼50 nm red-shift in the emission peak.13 However, the brightness of PpeRS in vivo is not significantly increased, suggesting the mutation merely eliminates the production of green photons rather than increasing the output of red photons. A similar observation of selective loss of green emission without an increase in red emission was made with a Y257A mutation in Luciola firefly luciferase, which also produced a ∼50 nm red-shift.14 Efforts on RLuc engineering have been relatively more successful in emission shifting. The RLuc8 variant, consisting of eight mutations, displays a 4-fold increase in brightness,15 while RLuc8.6–535 exhibits a 50 nm peak wavelength shift to 535 nm and 2.6-fold brighter emission than RLuc8 at a tissue depth of 1–2 mm.16 This suggests that the increased brightness of RLuc8.6–535 is indeed due to an increased production of red photons, as desired. However, the emission spectrum of RLuc8.6–535 lies nearly completely below 600 nm, reducing the sensitivity of imaging by RLuc8.6–535 to lower than that of FLuc.
Table 1. Engineered Luciferase/Luciferin Pairs with Demonstrated in Vivo Applications.
| category | luciferase(s) | substrate(s) | emission peak | advantages | disadvantages | refs |
|---|---|---|---|---|---|---|
| firefly luciferase (FLuc) | FLuc | d-luciferin | 578 nm | effective at shallow tissue imaging | poor substrate blood-brain barrier permeability | (7, 8, 54) |
| low background emission | ATP-dependent (as are all FLuc-based systems) | |||||
| FLuc | aminoluciferins (e.g., CycLuc1, cybLuc) | up to 591 nm | superior to d-luciferin at several-fold lower dose | substrate solubility may be lower than d-luciferin | (22, 23, 55) | |
| improved blood–brain barrier permeability | ||||||
| more sustained signal | ||||||
| FLuc | near-infrared (NIR) luciferins | up to 690 nm | longer half-life in blood and higher affinity to FLuc | (32) | ||
| Akaluc | AkaLumine-hydrochloride (AkaLumine-HCl) | 650 nm | capable of single-cell detection in deep tissues | background hepatic signal | (27, 30) | |
| demonstrated video-rate deep-brain imaging in marmosets | potential toxicity due to acidosis | |||||
| Akaluc | seMpai | 675 nm | no background hepatic signal | lower brightness and affinity to Akaluc compared to AkaLumine-HCl | (31) | |
| FLuc mutants (x5, x5 S284T) | infraluciferin | up to 706 nm | produces distinct bioluminescent colors with various mutants | (33, 56) | ||
| click beetle luciferase | click beetle mutants (CBR2, CBG2) | NH2–NpLH2 | up to 730 nm | produces distinct bioluminescent colors with various mutants | (34, 57) | |
| Renilla luciferase (RLuc) | RLuc | CTZ | 482 nm | ATP-independent (as are all CTZ-based systems) | poor tissue penetration due to blue light emission | (36, 58) |
| high substrate autoluminescence | ||||||
| rapid inactivation in serum | ||||||
| RLuc | ViviRen | 482 nm | reduced autoluminescence compared to CTZ | poor tissue penetration due to blue light emission | (39) | |
| RLuc8 | CTZ | 487 nm | 4-fold brighter in vitro and 200-fold more resistant to inactivation in serum than RLuc | poor tissue penetration due to blue light emission | (15) | |
| RLuc8.6–535 | CTZ | 535 nm | 2.6-fold brighter than RLuc8 in vivo | (16) | ||
| NanoLuc | NanoLuc | furimazine, hydrofurimazine (HFz), fluorofurimazine (FFz) | 460 nm | small size and high structural stability | poor tissue penetration due to blue light emission | (44, 59) |
| 9-fold brighter with FFz than furimazine | ||||||
| Antares | furimazine, HFz, FFz | 584 nm | 13-fold brighter than FLuc/d-luciferin at equivalent dose in vivo | (30, 53) | ||
| teLuc | diphenylterazine (DTZ) | 502 nm | 54-fold brighter than FLuc/d-luciferin at equivalent dose in vivo | substrate solubility is lower than d-luciferin | (45) | |
| Antares2 | DTZ | 584 nm | 35–90% signal increase over teLuc/DTZ in vivo | substrate solubility is lower than d-luciferin | (45) | |
| LumiLuc | 8pyDTZ | 525 nm | enhanced substrate solubility compared to DTZ | (46) | ||
| 3-fold brighter than Akaluc/AkaLumine-HCl in vivo | ||||||
| LumiScarlet | 8pyDTZ | 600 nm | 3-fold brighter than LumiLuc/8pyDTZ in vivo | (46) | ||
| Gaussia luciferase (GLuc) | GLuc | CTZ | 480 nm | secreted into circulation | poor tissue penetration due to blue light emission | (60) |
| high substrate autoluminescence | ||||||
| rapidly decaying signal | ||||||
| GLuc variants (e.g., I90L, 8990, Monsta) | CTZ | up to 503 nm | up to 7-fold brighter than native GLuc in vivo | up to only 1.7% of photons >600 nm | (61) | |
| more sustained signal |
Taken together, these early studies suggest that mutagenesis of insect and marine luciferases alone may not be sufficient to dramatically improve in vivo performance of these BLI systems. Thus, additional strategies, such as engineering of luciferase substrates with improved emission characteristics or dramatically red-shifting emission using bioluminescence resonance energy transfer (BRET), may be necessary.
Synthetic Substrate Analogs
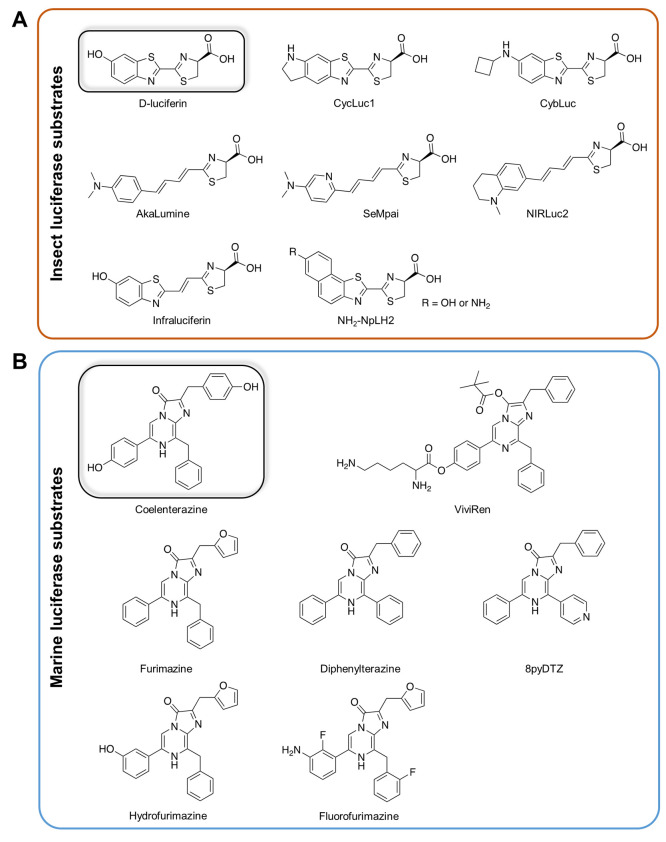
An orthogonal approach to modifying bioluminescence properties, and one that can overcome wavelength limitations inherent to d-luciferin or coelenterazine, is to chemically alter the substrate (Figure 2). Substrate engineering is often then accompanied by enzyme engineering to ensure maximal optimization of the system. For the firefly luciferase system, it has been discovered that emission color is primarily determined by the charge distribution on the oxyluciferin product.17 Various methods to redistribute the electronic charge, such as replacing substituent groups or extending conjugation on the luciferin, have led to the synthesis of novel luciferin analogs capable of red-shifting the emission light.18,19 Efforts have also focused on modifying luciferins to enhance the circulation time, water solubility, and membrane permeability of luciferins for deep tissue imaging.20,21
Figure 2.
Chemical structures of native luciferins and their analogs: (A) Native insect luciferase substrate d-luciferin and its analogs. (B) Native marine luciferase substrate coelenterazine and its analogs.
A major group of d-luciferin analogs is based on aminoluciferins. The first aminoluciferin analog was developed in 1966 by replacing the 6′-hydroxyl group of d-luciferin with an amino group, resulting in a peak emission shift to 590 nm and a ∼10-fold increased affinity for FLuc.22 Research focused on optimizing analogs for in vivo imaging led to the development of the cyclic alkylaminoluciferin CycLuc1. When injected at a dose 20-fold lower than d-luciferin, CycLuc1 can still lead to brighter signal and achieve sensitive brain imaging in FLuc-expressing mice.23,24 While wild-type FLuc is promiscuous, Adams et al. later engineered some FLuc mutants that exhibit 40–50-fold selectivity for aminoluciferins (including CycLuc1) over d-luciferin in mouse brains.25 Notably, when paired with aminoluciferins, the engineered FLuc variants did not exhibit increased brightness, indicating the primary cause of the selectivity is likely a loss of utilization of d-luciferin rather than increased utilization of the synthetic aminoluciferins. Recently, Wu et al. investigated a series of novel N-cycloalkylaminoluciferins called cyaLucs and reported one analog, cybLuc, with improved sensitivity in vivo. At maximum doses (100 mM for d-luciferin and 10 mM for CybLuc), CybLuc provided 7-fold brighter bioluminescence in the mouse brain, which suggests that cybLuc can efficiently traverse the blood–brain barrier and has higher accessibility to deep brain tissues. In addition, the cybLuc signal plateaued for more than 30 min, whereas the d-luciferin signal began to drop after only 5 min, suggesting cybLuc can be used in applications requiring long-time observation in vivo.
Recent efforts have succeeded in further shifting bioluminescence into the near-infrared (NIR) region. Kuchimaru et al. synthesized a novel luciferin analog, AkaLumine-hydrochloride (AkaLumine-HCl), by replacing the benzothiazole structure in d-luciferin with a dimethylaniline moiety and extending the π-conjugated system.26 When paired with native FLuc, AkaLumine-HCl emits NIR light peaking at 677 nm and possesses a more favorable biodistribution. Iwano et al. further refined this system by engineering FLuc through successive rounds of mutagenesis to optimize pairing with AkaLumine-HCl, and their work yielded the novel luciferase Akaluc.27 Imaging with Akaluc/AkaLumine-HCl exhibited up to 1000 times brighter emissions in vivo compared to FLuc/d-luciferin and was capable of single-cell detection in deep lung tissues in mice. Additionally, improved BLI in the brain owing to efficient blood–brain-barrier penetration of AkaLumine-HCl enabled video-rate imaging of striatal neurons in marmosets, a significant milestone in in vivo BLI. Later, the superiority of Akaluc over FLuc was recaptured in the mouse brain.28 However, it was questioned by another study when applied in the imaging fate of cell therapy outside the brain, although in this research d-luciferin was administered at a dose 6-fold higher than common usage.29 Some potential drawbacks of AkaLumine-HCl include the background hepatic signal30,31 and noticeable toxicity to skin and heart,29,31 likely due to the acidity in AkaLumine-HCl solution. A structurally similar luciferin analog, seMpai, does not produce a detectable hepatic signal when paired with Akaluc in vivo and may be more suitable for sensitive imaging of the liver, although at the cost of lower brightness compared to AkaLumine-HCl.31
In 2020, Ikeda et al. further expanded the palette of NIR-emitting substrates with the development of NIR luciferins (NIRLucs).32 Using the structure of AkaLumine as a framework, they synthesized a series of novel luciferins via a ring fusion strategy and reached peak wavelengths up to 690 nm when paired with FLuc. One candidate, NIRLuc2, was tested in vivo. Although NIRLuc2 was dimmer than AkaLumine in vitro, it was comparable to AkaLumine in a subcutaneous tumor model which might be due to its improved pharmacokinetics properties (although the dose was questionably maintained at 5 mM and intravenous injection was adopted instead of intraperitoneal). More sustained bioluminescence was also achieved as a result of longer half-life in blood and higher affinity to FLuc.
Other NIR luciferins include the beetle luciferin analog infraluciferin, with an emission peak at 708 nm.33 Moreover, infraluciferin produces distinct bioluminescent colors with various luciferase mutants and therefore may be useful for imaging multiple cell populations or biochemical processes within the same animal (see the Multicomponent BLI section). Similarly, the naphthyl-based luciferin NH2–NpLH2 can be paired with the mutant click beetle red luciferase (CBR2) to produce NIR emission (730 nm), with potential applications in multicomponent imaging.34
Investigation into novel CTZ analogs that enhance bioluminescence in animals has also been a wide field of study. However, this has been much more challenging due to the blue emission of native CTZ systems.35 It should also be noted that due to its unstable imidazopyridine structure, CTZ can undergo enzyme-independent oxidation, resulting in background autoluminescence.36 Substitution at the C-2, C-5, C-6, and C-8 positions of the imidazopyrazinone core has yielded enhancements in bioluminescence properties, with changes at the C-2 position modifying the reaction rate and the C-6/C-8 positions influencing the emission spectra.37,38 One notable CTZ analog developed by Promega Corporation, ViviRen, exhibited up to a 10-fold higher signal in vivo than CTZ when paired with RLuc, while showing reduced autoluminescence.39 Recent studies and overviews covering the progress of the chemical synthesis and structure–activity studies of CTZ analogs exist for interested readers.37,40−43
In 2012, Promega Corporation synthesized furimazine, a CTZ analog containing a furan group at the C-2 position that could pair with NanoLuc, an engineered luciferase derived from the small luciferase subunit (19 kDa) of the deep sea shrimp Oplophorus gracilirostris.44 The NanoLuc/furimazine pair produced ∼150-fold greater bioluminescence in vitro compared to RLuc/CTZ, with a slightly blue-shifted emission wavelength (460 nm). Because of its brightness, small size, and high stability, NanoLuc was quickly commercialized for widespread use in cell-based assays; however, its blue emission still limited in vivo applications. Yeh et al. were successful in red-shifting its emission by engineering it to pair with the CTZ analog diphenylterazine (DTZ), resulting in the teLuc luciferase with an emission peak at 502 nm.45 Imaging in deep tissues of mice with teLuc/DTZ generated ∼54-fold brighter emission than FLuc/d-luciferin at an equivalent dose. Yeh and colleagues then synthesized 8pyDTZ, an analog with 4- to 14-fold enhanced solubility.46 They then performed directed evolution of teLuc to optimize pairing with 8pyDTZ, resulting in the LumiLuc luciferase which also exhibited a shifted emission peak at 525 nm. LumiLuc/8pyDTZ produced ∼3-fold higher photon flux than Akaluc/AkaLumine-HCl from superficial implants in mice.
BRET-Based Luciferase Systems
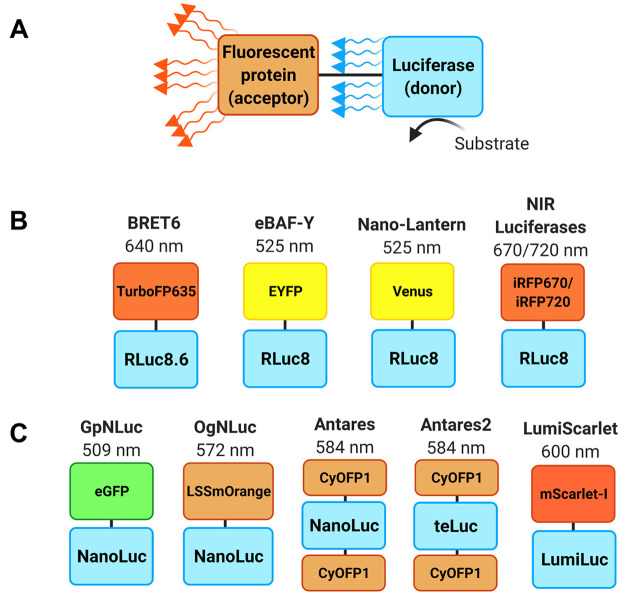
In nature, multiple marine organisms have evolved bright luciferase-fluorescent protein systems that utilize the phenomenon of BRET. In BRET, the excited luciferase-bound substrate transfers its energy to an acceptor chromophore through a nonradiative dipole–dipole coupling mechanism, of which the efficiency is dependent on the spectral overlap and intermolecular distance between the two molecules (Figure 3A). The resulting emission light from the acceptor chromophore exhibits a red-shifted spectrum and overall higher quantum yield. Inspired by nature, multiple engineering efforts on marine luciferases have focused on using BRET to red-shift emission and enhance bioluminescence brightness.
Figure 3.
Bioluminescence resonance energy transfer (BRET)-based luciferase systems: (A) A luciferase donor reacts with its substrate to produce light which is then absorbed by a fused fluorescent protein and re-emitted at a longer wavelength. (B) RLuc-based BRET systems and (C) NanoLuc-based BRET systems and their respective peak emission wavelengths.
Most of the early examples of BRET-based reporters were based on RLuc or its enhanced variants (Figure 3B). BRET6, a TurboFP635-RLuc8.6 fusion protein, enabled direct imaging of drug-mediated protein–protein interaction in deep tissues of living mice.47 eBAF-Y, consisting of enhanced yellow fluorescent protein (EYFP) and RLuc8, displayed 26-fold bioluminescence enhancement over RLuc and 3-fold over RLuc8.48 In a more recent study, by replacing EYFP with Venus and extensively optimizing the unstructured linker, Saito et al. developed an enhanced BRET-based reporter termed Nano-Lantern with a 2.9 times brighter emission than eBAF-Y. Notably, the enhanced signal enabled video rate (30 Hz) imaging of luciferase-expressing tumors in freely moving unshaved mice.49 In another interesting example, RLuc8 was fused with iRFP670 or iRFP720, phytochrome-based NIR fluorescent proteins that have an excitation peak at around 380 nm, to create reporters with NIR emission.50 Compared to NIR fluorescent in vivo imaging, NIR BLI provided 10-fold increased sensitivity, enabling detection of as low as 104 tail vein-injected MTLn3 tumor cells in the lungs of CFW mice. However, fluorescent proteins alone may still be beneficial when substrate delivery is an issue or in hypoxic regions for oxygen-dependent luciferases.
Ever since its development, NanoLuc has become a popular donor for engineering novel BRET-based reporters due to its superior catalytic efficiency over RLuc variants (Figure 3C). By replacing RLuc8 in Nano-Lantern with NanoLuc, a series of enhanced Nano-Lanterns (eNL) were reported.51 Specifically, fluorescent proteins mTurquoise2, mNeonGreen, Venus, mKOκ, and tdTomato were chosen as BRET acceptors to generate cyan (CeNL, 475 nm), green (GeNL, 520 nm), yellow (YeNL, 530 nm), orange (OeNL, 565 nm), and red (ReNL, 585 nm) eNLs. Among them, CeNL and GeNL showed a roughly 2-fold increased total luminescence intensity compared to NanoLuc, while YeNL and OeNL were 4- or 1.8- fold brighter than their Nano-Lantern counterparts YNL and ONL. Meanwhile, in an independent study, two other chimeric fusion reporter proteins termed LumiFluors were developed using a similar design.52 The green LumiFluor, eGFP-NanoLuc (GpNLuc), displayed 45-fold more luminescence than YNL in vitro. In NOD/SCID mice, following a tail vein injection of 500 000 GpNLuc-expressing tumor cells, colonization could easily be detected in the lungs. The orange LumiFluor, LSSmOrange-NanoLuc (OgNLuc), was even several fold brighter than GpNLuc for imaging allografts of mouse lymphoma cells in Albino C57Bl/6 mice. Given their similar signal intensity in vitro, this improvement in vivo supports the strategy of red-shifting luciferases for enhanced tissue penetration.
While most BRET systems exploit one copy of the fluorescent protein as the acceptor, introducing multiple copies may further enhance the BRET efficiency. On the basis of this hypothesis, two copies of CyOFP1, a bright cyan-excitable orange fluorescent protein, were fused to both the N- and C-termini of NanoLuc to create a highly sensitive bioluminescent reporter called Antares.53 When compared to the spectrally similar bioluminescent protein ONL, Antares produced 20-fold more photons across all wavelengths and 28-fold more above 600 nm in vitro.
Other engineered luciferases based on NanoLuc, such as teLuc and LumiLuc mentioned above, were also exploited as donors in BRET systems. Compared to NanoLuc/furimazine, the emission of teLuc/DTZ peaking at 502 nm overlaps better with the absorbance of CyOFP1 in Antares. Thus, NanoLuc in Antares was replaced by teLuc to generate Antares2, which consistently exhibits a 584 nm emission peak. In BALB/c mice, when luciferase-coding plasmids were hydrodynamically delivered to have luciferases expressed in the liver, and equimolar doses of luciferase substrates were administered intraperitoneally, Antares2 exhibited an approximately 35–90% signal increase over teLuc and Antares.45 For the BRET system that utilizes LumiLuc/8pyDTZ, which is further red-shifted from teLuc-DTZ, a recently reported red fluorescent protein mScarlet-I was adopted instead as the BRET acceptor.46 The resulting fusion protein mScarlet-I-LumiLuc, named LumiScarlet, has an emission peak at 600 nm and has 51% of its emitted photons above 600 nm. When applied to image luciferase-expressing HeLa cells trapped in the lungs of NU/J mice with intravenously injected substrates, LumiScarlet-8pyDTZ gave a comparable signal to that from Akaluc with a comparable amount of intravenous AkaLumine-HCl, and 3-fold brighter than that from LumiLuc/8pyDTZ.
However, the brightness of Antares or LumiScarlet with the more convenient intraperitoneal injection of furimazine or 8pyDTZ does not reach the brightness attainable by AkaLuc with intraperitoneal AkaLumine delivery.30,45 Two NanoLuc substrates, hydrofurimazine (HFz) and fluorofurimazine (FFz), with a higher aqueous solubility that can be injected into higher total amounts were recently reported.30 When coupled with Antares, these novel substrates enabled comparable or even brighter bioluminescence in vivo compared to AkaLuc/AkaLumine at limiting doses for each substrate.
BLI Systems Application—Multicomponent BLI
In many biological studies, the need to observe different cellular events within the same animal requires the cells of interest to be tagged with multiple luciferases. Applications include investigations into the endogenous expression patterns of two distinct genes, tracking of the differentiation/activation status of individual cells by regulating the expression of each luciferase with state-dependent promoters and the trafficking of two separate cell populations such as therapeutic cells (e.g., cytotoxic T cells) and diseased cells (e.g., cancer cells). Unlike fluorescence imaging where the cells of interest can be visualized by simply switching a filter, multicomponent BLI requires more complex methods which can pose limitations on the detection sensitivity and accuracy.
One option is to use substrate-resolved luciferases, where the injection of a single substrate is used to activate the respective luciferase. This requires tagging of luciferases with minimal cross-reactivity with the other substrate(s), also known as orthogonal luciferase-luciferin systems. The dual-BLI proof-of-concept was first demonstrated in 2002 by Bhaumik and Gambhir, who subcutaneously implanted C6 rat glioma cells expressing either RLuc or FLuc into single mice, followed by their sequential visualization using CTZ and d-luciferin, respectively.35 Since then, several other groups have used dual-BLI to visualize distinct molecular events or cell populations in vivo, using other luciferase pairs such as FLuc/d-luciferin and GLuc/CTZ,62 and FLuc/d-luciferin and NanoLuc/furimazine.63 A triple-BLI system has also been demonstrated using FLuc/d-luciferin, GLuc/CTZ, and the codon-optimized luciferase from Vargula hilgendorfii (Vluc) with its substrate vargulin.64 Prescher’s group has expanded the toolbox of multicomponent BLI by synthesizing novel orthogonal luciferase–luciferin systems based on FLuc/d-luciferin system,65,66 including a class of π-extended luciferins enabling red-shifted emission.67 The challenge with these systems, however, remains their low sensitivity in vivo, limiting their detection capability in deep tissues.
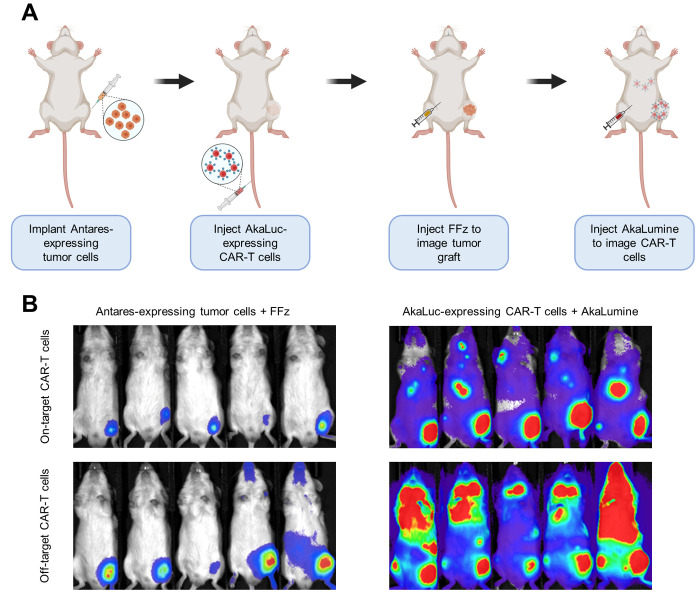
The low sensitivity issue could be resolved with the state-of-the-art bioluminescent reporters described above. The AkaLuc/AkaLumine pair has been used orthogonally with Antares/fluorofurimazine. With intraperitoneally administered FFz or AkaLumine, the expansion or recession of engrafted Antares-expressing MG63.3 osteosarcoma tumor and the circulation and localization of intravenously injected CAR-T cells could be sensitively visualized within the same NSG mice, over a period of 30 days30 (Figure 4). This pair of luciferase and luciferins for dual-BLI of two cell populations is the most sensitive reported to date.
Figure 4.
Example of sensitive multicomponent BLI enabled by state-of-the-art bioluminescent systems: (A) Scheme of experimental design for in vivo tracking of both Antares-expressing tumor cells and AkaLuc-expressing CAR-T cells. (B) Representative BLI images showing the change in tumor size and the localization of expanded CAR-T cells. Adapted with permission from Su et al.30 Copyright 2020 Springer Nature.
Another limitation with substrate-resolved luciferases is the need for the signal from the previous luciferase to clear, which can take hours to days. A solution to this issue was recently developed by Rathbun et al., which relies on substrate unmixing algorithms: substrates are administered in rapid succession, beginning with the dimmest substrate, so that the residual signal from the previous luciferase becomes part of the background noise of the next.68 The set of images can then be used to deconvolute individual substrate signatures. Using this technique, Yao et al. demonstrated for the first time multicomponent imaging of four luciferase-luciferin pairs in vitro.67 In 2021, Moroz et al. demonstrated quadruple-BLI in vivo with a slightly different approach, using light wavelength-blocked filters to separate signals.69 They multiplexed RLuc, CBRLuc, CBGLuc, and a new reporter named membrane-anchored Cypridina luciferase (maCLuc), which is paired with the vargulin substrate, to visualize a metastatic tumor model.
With these developments, interpretation of substrate-resolved multicomponent BLI can still be biased by differences in substrate kinetics and biodistribution. Another way to perform multicomponent BLI is through spectrally resolved luciferase pairs, whereby the injection of a single substrate produces distinct emission spectra with multiple luciferases which can be separated using spectral unmixing algorithms. This often employs green- and red-shifted mutant luciferases to minimize overlap in emission spectra.13 Mezzanotte et al. demonstrated in vivo dual-BLI of human embryonic kidney cells expressing green CBG99 (λmax = 537 nm) and red PpyRE8 (λmax = 618 nm) luciferases.70 They achieved efficient spectral unmixing and sensitive imaging of distinct cell populations injected subcutaneously in mice. However, imaging in deeper tissues can be hampered by greater attenuation of the lower wavelength light which would introduce bias into quantitative comparisons. Recently, Aswendt et al. developed an improved dual-color BLI system with the further mutated x5r/x5g pair showing better separated spectra and higher photon flux with the ability to accurately predict cell population ratios from quantitative unmixing results.71 The x5r/x5g pair can also be coupled with infraluciferin for red-shifted dual-color imaging.56 In 2021, Zambito et al. introduced a further improved NIR dual-BLI system using the substrate NH2–NpLH2 paired with novel click beetle mutants CBG2 (λmax = 660 nm) and CBR2 (λmax = 730 nm).57 They showed sensitive deep tissue imaging in a lung model and were able to spectrally resolve and quantify the emissions. Furthermore, the versatility of the system was demonstrated with in vivo dual-BLI of CBG2/NH2–NpLH2 and Akaluc/Akalumine-HCl.
Technological Advancements
In addition to the engineering of luciferase systems at the chemical level, developments in camera technologies and imaging methods have advanced the capabilities of in vivo BLI (Table 2). In brief, these imaging systems consist of a light-tight chamber in which the subject is placed and a cooled charge-coupled device (CCD) camera which collects emitted photons. The sensitivity of imaging systems has been greatly improved through the development of intensified CCD (ICCD) and electron multiplying CCD (EMCCD) cameras. This increased sensitivity has subsequently decreased acquisition times from several minutes to milliseconds, enabling video-rate imaging of unanesthetized animals.27,49,72 In addition, development of bioluminescence tomography (BLT) that incorporates CT/MRI information has aided anatomical localization in three dimensions.73,74 Traditional CCD cameras are blue sensitive, but newer cameras have been optimized for higher quantum efficiency at wavelengths >600 nm, which is especially useful for imaging NIR-emitting luciferases.75 In summary, technological breakthroughs that complement chemical engineering of bioluminescent systems is expected to improve imaging sensitivity and expand future BLI applications.
Table 2. Summary of Key Advancements in Instrumentation and Imaging Methods for in Vivo BLI.
| year | technological advancement | ref |
|---|---|---|
| 2001 | first commercial system designed for BLI of small animals (IVIS, Xenogen Corporation) | (75) |
| 2003 | first bioluminescence tomography (BLT) prototype developed, enabling integration with CT for quantitative 3D localization of signals | (76) |
| 2006 | development of spectral unmixing algorithms for separating signals from multiple BLI reporters | (77) |
| 2008 | new imaging system developed for video-rate imaging of nonanaesthetized and freely moving mice | (72) |
| 2012 | first time multiple BLI reporter genes were imaged in vivo using BLT | (78) |
| 2013 | development of a 3D BLI method based on multiple rotating cameras for accelerated data acquisition and improved signal quantification | (79) |
| 2018 | video-rate imaging of Akaluc-expressing striatal neurons in nonanaesthetized marmosets using a MIIS system (Molecular Devices Japan) | (27) |
| 2020 | in vivo BLI in the second near-infrared region (1000–1700 nm) achieved using a InGaAs CCD camera (Princeton Instruments, NIRvana-LN) | (80) |
Conclusions
Over the past few decades BLI has emerged as a powerful tool for preclinical visualization of cellular and molecular processes in vivo. The high signal-to-noise ratio, direct relationship of signal to cell viability, and ability to survey processes on a whole-animal scale offers an unparalleled approach to noninvasive imaging. However, the attenuation of light by tissue raises a pressing need for the development of novel red-shifted luciferases and luciferins that can enable sensitive, deep-tissue imaging. Recent advancements in protein engineering and substrate synthesis have pushed the limits of detection, and the expanding toolbox of multicomponent BLI has improved imaging of complex cellular interactions.
The in vivo behavior of BLI systems remains incompletely understood. For substrates, in vivo evaluation should not only characterize bioluminescence output from one location but should also address pharmacodynamics and pharmacokinetics since these substrates act like drugs in vivo.21 An ideal synthetic luciferin should have its organ/tissue biodistribution clearly characterized,11 cause little to no toxicity or behavioral abnormality to the injected animal30 or have its safe dosage established, and be free of background bioluminescence caused by auto-oxidation.30,31 For d-luciferin, distribution has been assessed with radioactive tracers, but this has yet to be performed for other substrates. We expect animal testing of advanced luciferase systems to be an area of growing interest as the field of BLI continues to expand.
Another interesting future development is in the combination of the most sensitive BLI reporters with other imaging methods. For example, a multimodal system consisting of a BLI reporter for ease of use and affordability with either an MRI reporter for high 3D resolution or a PET reporter for high 3D sensitivity may overcome limitations inherent to each modality.
In conclusion, we envision further development of luciferase/luciferin systems, along with in vivo testing and incorporation with other imaging reporters, to be a growing and exciting area as we continue to challenge the limits of in vivo imaging.
Acknowledgments
Figures were created with BioRender.com. Funds were provided by NINDS (grant 1R21NS122055).
Glossary
Keywords
- Bioluminescence imaging (BLI)
an imaging technique that allows the visualization of biochemical events and labeled cells via the detection of light emitted by enzyme-catalyzed reactions
- Luciferase
an enzyme that produces bioluminescence by oxidizing a compatible substrate
- Luciferin
the substrate oxidized by a luciferase to produce bioluminescence
- Near-infrared luciferins (NIRLucs)
a class of luciferins emitting light in the near-infrared range (∼650–900 nm), enabling higher light penetration at deep tissues
- Bioluminescence resonance energy transfer (BRET)
an energy transfer event occurring between an excited luciferase-bound substrate and an acceptor fluorescent protein; the emitted wavelength of a BRET system is higher than that of the donor luciferase
- Dual-BLI
BLI of a pair of orthogonal luciferases and luciferins, allowing the tracking of independent biochemical or cellular events
- Substrate-resolved luciferases
a pair of luciferases that are independently activated following administration of the appropriate substrate
- Spectrally resolved luciferases
a pair of luciferases that utilize the same substrate but produce distinct emission spectra; their activity is separated using spectral unmixing algorithms.
The authors declare no competing financial interest.
References
- Kang J. H.; Chung J. K. Molecular-genetic imaging based on reporter gene expression. J. Nucl. Med. 2008, 49 (Suppl 2), 164S–179S. 10.2967/jnumed.107.045955. [DOI] [PubMed] [Google Scholar]
- Badr C. E.; Tannous B. A. Bioluminescence imaging: progress and applications. Trends Biotechnol. 2011, 29, 624–633. 10.1016/j.tibtech.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger M.; Cao Y. A.; Hornig Y. S.; Jenkins D. E.; Verneris M. R.; Bachmann M. H.; Negrin R. S.; Contag C. H. Advancing animal models of neoplasia through in vivo bioluminescence imaging. Eur. J. Cancer 2002, 38, 2128–2136. 10.1016/S0959-8049(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Mandl S.; Schimmelpfennig C.; Edinger M.; Negrin R. S.; Contag C. H. Understanding immune cell trafficking patterns via in vivo bioluminescence imaging. J. Cell. Biochem. 2002, 87, 239–248. 10.1002/jcb.10454. [DOI] [PubMed] [Google Scholar]
- Paroo Z.; Bollinger R. A.; Braasch D. A.; Richer E.; Corey D. R.; Antich P. P.; Mason R. P. Validating bioluminescence imaging as a high-throughput, quantitative modality for assessing tumor burden. Mol. Imaging 2004, 3, 117–124. 10.1162/1535350041464865. [DOI] [PubMed] [Google Scholar]
- Dothager R. S.; Flentie K.; Moss B.; Pan M. H.; Kesarwala A.; Piwnica-Worms D. Advances in bioluminescence imaging of live animal models. Curr. Opin. Biotechnol. 2009, 20, 45–53. 10.1016/j.copbio.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J.; Pinto da Silva L.; Esteves da Silva J. C. Advances in the knowledge of light emission by firefly luciferin and oxyluciferin. J. Photochem. Photobiol., B 2012, 117, 33–39. 10.1016/j.jphotobiol.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Doyle T. C.; Coquoz O.; Kalish F.; Rice B. W.; Contag C. H. Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. J. Biomed. Opt. 2005, 10, 041210. 10.1117/1.2032388. [DOI] [PubMed] [Google Scholar]
- Fleiss A.; Sarkisyan K. S. A brief review of bioluminescent systems (2019). Curr. Genet. 2019, 65, 877–882. 10.1007/s00294-019-00951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H.; Ogawa M.; Alford R.; Choyke P. L.; Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F.; Paulmurugan R.; Bhaumik S.; Gambhir S. S. Uptake kinetics and biodistribution of 14C-d-luciferin--a radiolabeled substrate for the firefly luciferase catalyzed bioluminescence reaction: impact on bioluminescence based reporter gene imaging. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2275–2285. 10.1007/s00259-008-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. W.; Ai H. W. Development and Applications of Bioluminescent and Chemiluminescent Reporters and Biosensors. Annu. Rev. Anal. Chem. 2019, 12, 129–150. 10.1146/annurev-anchem-061318-115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchini B. R.; Southworth T. L.; Khattak N. F.; Michelini E.; Roda A. Red- and green-emitting firefly luciferase mutants for bioluminescent reporter applications. Anal. Biochem. 2005, 345, 140–148. 10.1016/j.ab.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Akiyama H.; Terakado K.; Nakatsu T. Impact of site-directed mutant luciferase on quantitative green and orange/red emission intensities in firefly bioluminescence. Sci. Rep. 2013, 3, 2490. 10.1038/srep02490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening A. M.; Fenn T. D.; Wu A. M.; Gambhir S. S. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng., Des. Sel. 2006, 19, 391–400. 10.1093/protein/gzl023. [DOI] [PubMed] [Google Scholar]
- Loening A. M.; Wu A. M.; Gambhir S. S. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat. Methods 2007, 4, 641–643. 10.1038/nmeth1070. [DOI] [PubMed] [Google Scholar]
- Cheng Y. Y.; Liu Y. J. Theoretical Development of Near-Infrared Bioluminescent Systems. Chem. - Eur. J. 2018, 24, 9340–9352. 10.1002/chem.201800416. [DOI] [PubMed] [Google Scholar]
- Pirrung M. C.; Biswas G.; De Howitt N.; Liao J. Synthesis and bioluminescence of difluoroluciferin. Bioorg. Med. Chem. Lett. 2014, 24, 4881–4883. 10.1016/j.bmcl.2014.08.048. [DOI] [PubMed] [Google Scholar]
- Reddy G. R.; Thompson W. C.; Miller S. C. Robust light emission from cyclic alkylaminoluciferin substrates for firefly luciferase. J. Am. Chem. Soc. 2010, 132, 13586–13587. 10.1021/ja104525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran S. S.; Williams S. A.; Denmeade S. R. Extended-release PEG-luciferin allows for long-term imaging of firefly luciferase activity in vivo. Luminescence 2009, 24, 35–38. 10.1002/bio.1060. [DOI] [PubMed] [Google Scholar]
- Mofford D. M.; Miller S. C. Luciferins behave like drugs. ACS Chem. Neurosci. 2015, 6, 1273–1275. 10.1021/acschemneuro.5b00195. [DOI] [PubMed] [Google Scholar]
- Shinde R.; Perkins J.; Contag C. H. Luciferin derivatives for enhanced in vitro and in vivo bioluminescence assays. Biochemistry 2006, 45, 11103–11112. 10.1021/bi060475o. [DOI] [PubMed] [Google Scholar]
- Evans M. S.; Chaurette J. P.; Adams S. T.; Reddy G. R.; Paley M. A.; Aronin N.; Prescher J. A.; Miller S. C. A synthetic luciferin improves bioluminescence imaging in live mice. Nat. Methods 2014, 11, 393–395. 10.1038/nmeth.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofford D. M.; Reddy G. R.; Miller S. C. Aminoluciferins extend firefly luciferase bioluminescence into the near-infrared and can be preferred substrates over d-luciferin. J. Am. Chem. Soc. 2014, 136, 13277–13282. 10.1021/ja505795s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams S. T. Jr.; Mofford D. M.; Reddy G. S.; Miller S. C. Firefly Luciferase Mutants Allow Substrate-Selective Bioluminescence Imaging in the Mouse Brain. Angew. Chem., Int. Ed. 2016, 55, 4943–4946. 10.1002/anie.201511350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchimaru T.; Iwano S.; Kiyama M.; Mitsumata S.; Kadonosono T.; Niwa H.; Maki S.; Kizaka-Kondoh S. A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat. Commun. 2016, 7, 11856. 10.1038/ncomms11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano S.; Sugiyama M.; Hama H.; Watakabe A.; Hasegawa N.; Kuchimaru T.; Tanaka K. Z.; Takahashi M.; Ishida Y.; Hata J.; Shimozono S.; Namiki K.; Fukano T.; Kiyama M.; Okano H.; Kizaka-Kondoh S.; McHugh T. J.; Yamamori T.; Hioki H.; Maki S.; Miyawaki A. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. 10.1126/science.aaq1067. [DOI] [PubMed] [Google Scholar]
- Bozec D.; Sattiraju A.; Bouras A.; Jesu Raj J. G.; Rivera D.; Huang Y.; Junqueira Alves C.; Tejero R.; Tsankova N. M.; Zou H.; Hadjipanayis C.; Friedel R. H. Akaluc bioluminescence offers superior sensitivity to track in vivo glioma expansion. Neurooncol Adv. 2020, 2 (2), vdaa134 10.1093/noajnl/vdaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadeo F., Plagge A., Chacko A., Wilm B., Hanson V., Liptrott N., Murray P., Taylor A. (2021) Firefly luciferase offers superior performance to AkaLuc for tracking the fate of administered cell therapies, Eur. J. Nucl. Med. Mol. Imaging, 10.1007/s00259-021-05439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.; Walker J. R.; Park Y.; Smith T. P.; Liu L. X.; Hall M. P.; Labanieh L.; Hurst R.; Wang D. C.; Encell L. P.; Kim N.; Zhang F.; Kay M. A.; Casey K. M.; Majzner R. G.; Cochran J. R.; Mackall C. L.; Kirkland T. A.; Lin M. Z. Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals. Nat. Methods 2020, 17, 852–860. 10.1038/s41592-020-0889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J.; Saito R.; Hayashi Y.; Kitada N.; Tamaki S.; Han Y.; Semba K.; Maki S. A. High Sensitivity In Vivo Imaging of Cancer Metastasis Using a Near-Infrared Luciferin Analogue seMpai. Int. J. Mol. Sci. 2020, 21, 7896. 10.3390/ijms21217896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y.; Nomoto T.; Hiruta Y.; Nishiyama N.; Citterio D. Ring-Fused Firefly Luciferins: Expanded Palette of Near-Infrared Emitting Bioluminescent Substrates. Anal. Chem. 2020, 92, 4235–4243. 10.1021/acs.analchem.9b04562. [DOI] [PubMed] [Google Scholar]
- Jathoul A. P.; Grounds H.; Anderson J. C.; Pule M. A. A dual-color far-red to near-infrared firefly luciferin analogue designed for multiparametric bioluminescence imaging. Angew. Chem., Int. Ed. 2014, 53, 13059–13063. 10.1002/anie.201405955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. P.; Woodroofe C. C.; Wood M. G.; Que I.; Van’t Root M.; Ridwan Y.; Shi C.; Kirkland T. A.; Encell L. P.; Wood K. V.; Lowik C.; Mezzanotte L. Click beetle luciferase mutant and near infrared naphthyl-luciferins for improved bioluminescence imaging. Nat. Commun. 2018, 9, 132. 10.1038/s41467-017-02542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S.; Gambhir S. S. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 377–382. 10.1073/pnas.012611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.; Doyle T. C.; Wong R. J.; Cao Y.; Stevenson D. K.; Piwnica-Worms D.; Contag C. H. Characterization of coelenterazine analogs for measurements of Renilla luciferase activity in live cells and living animals. Mol. Imaging 2004, 3, 43–54. 10.1162/153535004773861714. [DOI] [PubMed] [Google Scholar]
- Jiang T.; Du L.; Li M. Lighting up bioluminescence with coelenterazine: strategies and applications. Photochem. Photobiol. Sci. 2016, 15, 466–480. 10.1039/C5PP00456J. [DOI] [PubMed] [Google Scholar]
- Shakhmin A.; Hall M. P.; Machleidt T.; Walker J. R.; Wood K. V.; Kirkland T. A. Coelenterazine analogues emit red-shifted bioluminescence with NanoLuc. Org. Biomol. Chem. 2017, 15, 8559–8567. 10.1039/C7OB01985H. [DOI] [PubMed] [Google Scholar]
- Otto-Duessel M.; Khankaldyyan V.; Gonzalez-Gomez I.; Jensen M. C.; Laug W. E.; Rosol M. In vivo testing of Renilla luciferase substrate analogs in an orthotopic murine model of human glioblastoma. Mol. Imaging 2006, 5, 57–64. 10.2310/7290.2006.00006. [DOI] [PubMed] [Google Scholar]
- Nishihara R.; Citterio D.; Suzuki K. Synthetic Bioluminescent Coelenterazine Derivatives. Methods Mol. Biol. 2016, 1461, 19–31. 10.1007/978-1-4939-3813-1_2. [DOI] [PubMed] [Google Scholar]
- Jiang T.; Yang X.; Zhou Y.; Yampolsky I.; Du L.; Li M. New bioluminescent coelenterazine derivatives with various C-6 substitutions. Org. Biomol. Chem. 2017, 15, 7008–7018. 10.1039/C7OB01554B. [DOI] [PubMed] [Google Scholar]
- Coutant E. P.; Goyard S.; Hervin V.; Gagnot G.; Baatallah R.; Jacob Y.; Rose T.; Janin Y. L. Gram-scale synthesis of luciferins derived from coelenterazine and original insights into their bioluminescence properties. Org. Biomol. Chem. 2019, 17, 3709–3713. 10.1039/C9OB00459A. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang X.; Dong G.; Yan C.; Cui Y.; Zhang Z.; Du L.; Li M. Novel furimazine derivatives for nanoluciferase bioluminescence with various C-6 and C-8 substituents. Org. Biomol. Chem. 2021, 19, 7930–7936. 10.1039/D1OB01098K. [DOI] [PubMed] [Google Scholar]
- Hall M. P.; Unch J.; Binkowski B. F.; Valley M. P.; Butler B. L.; Wood M. G.; Otto P.; Zimmerman K.; Vidugiris G.; Machleidt T.; Robers M. B.; Benink H. A.; Eggers C. T.; Slater M. R.; Meisenheimer P. L.; Klaubert D. H.; Fan F.; Encell L. P.; Wood K. V. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. W.; Karmach O.; Ji A.; Carter D.; Martins-Green M. M.; Ai H. W. Red-shifted luciferase-luciferin pairs for enhanced bioluminescence imaging. Nat. Methods 2017, 14, 971–974. 10.1038/nmeth.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. W.; Xiong Y.; Wu T.; Chen M.; Ji A.; Li X.; Ai H. W. ATP-Independent Bioluminescent Reporter Variants To Improve in Vivo Imaging. ACS Chem. Biol. 2019, 14, 959–965. 10.1021/acschembio.9b00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragulescu-Andrasi A.; Chan C. T.; De A.; Massoud T. F.; Gambhir S. S. Bioluminescence resonance energy transfer (BRET) imaging of protein-protein interactions within deep tissues of living subjects. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 12060–12065. 10.1073/pnas.1100923108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H.; Nakajima Y.; Ohmiya Y. Luciferase-YFP fusion tag with enhanced emission for single-cell luminescence imaging. Nat. Methods 2007, 4, 637–639. 10.1038/nmeth1069. [DOI] [PubMed] [Google Scholar]
- Saito K.; Chang Y. F.; Horikawa K.; Hatsugai N.; Higuchi Y.; Hashida M.; Yoshida Y.; Matsuda T.; Arai Y.; Nagai T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat. Commun. 2012, 3, 1262. 10.1038/ncomms2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumyantsev K. A.; Turoverov K. K.; Verkhusha V. V. Near-infrared bioluminescent proteins for two-color multimodal imaging. Sci. Rep. 2016, 6, 36588. 10.1038/srep36588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K.; Kimura T.; Shinoda H.; Bai G.; Daniels M. J.; Arai Y.; Nakano M.; Nagai T. Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nat. Commun. 2016, 7, 13718. 10.1038/ncomms13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub F. X.; Reza M. S.; Flaveny C. A.; Li W.; Musicant A. M.; Hoxha S.; Guo M.; Cleveland J. L.; Amelio A. L. Fluorophore-NanoLuc BRET Reporters Enable Sensitive In Vivo Optical Imaging and Flow Cytometry for Monitoring Tumorigenesis. Cancer Res. 2015, 75, 5023–5033. 10.1158/0008-5472.CAN-14-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J.; Oh Y.; Sens A.; Ataie N.; Dana H.; Macklin J. J.; Laviv T.; Welf E. S.; Dean K. M.; Zhang F.; Kim B. B.; Tang C. T.; Hu M.; Baird M. A.; Davidson M. W.; Kay M. A.; Fiolka R.; Yasuda R.; Kim D. S.; Ng H. L.; Lin M. Z. A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nat. Biotechnol. 2016, 34, 760–767. 10.1038/nbt.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H.; Byun S. S.; Paik J. Y.; Lee S. Y.; Song S. H.; Choe Y. S.; Kim B. T. Cell uptake and tissue distribution of radioiodine labelled d-luciferin: implications for luciferase based gene imaging. Nucl. Med. Commun. 2003, 24, 1003–1009. 10.1097/00006231-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Wu W.; Su J.; Tang C.; Bai H.; Ma Z.; Zhang T.; Yuan Z.; Li Z.; Zhou W.; Zhang H.; Liu Z.; Wang Y.; Zhou Y.; Du L.; Gu L.; Li M. cybLuc: An Effective Aminoluciferin Derivative for Deep Bioluminescence Imaging. Anal. Chem. 2017, 89, 4808–4816. 10.1021/acs.analchem.6b03510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe C. L.; Burley T. A.; Allan H.; Vinci M.; Kramer-Marek G.; Ciobota D. M.; Parkinson G. N.; Southworth T. L.; Agliardi G.; Hotblack A.; Lythgoe M. F.; Branchini B. R.; Kalber T. L.; Anderson J. C.; Pule M. A.. Near-infrared dual bioluminescence imaging in mouse models of cancer using infraluciferin, eLife 2019, 8, 10.7554/eLife.45801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambito G.; Hall M. P.; Wood M. G.; Gaspar N.; Ridwan Y.; Stellari F. F.; Shi C.; Kirkland T. A.; Encell L. P.; Löwik C.; Mezzanotte L. Red-shifted click beetle luciferase mutant expands the multicolor bioluminescent palette for deep tissue imaging. iScience 2021, 24, 101986. 10.1016/j.isci.2020.101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz W. W.; Cormier M. J.; O’Kane D. J.; Hua D.; Escher A. A.; Szalay A. A. Expression of the Renilla reniformis luciferase gene in mammalian cells. J. Biolumin. Chemilumin. 1996, 11, 31–37. . [DOI] [PubMed] [Google Scholar]
- Gaspar N.; Walker J. R.; Zambito G.; Marella-Panth K.; Lowik C.; Kirkland T. A.; Mezzanotte L. Evaluation of NanoLuc substrates for bioluminescence imaging of transferred cells in mice. J. Photochem. Photobiol., B 2021, 216, 112128. 10.1016/j.jphotobiol.2021.112128. [DOI] [PubMed] [Google Scholar]
- Tannous B. A.; Kim D. E.; Fernandez J. L.; Weissleder R.; Breakefield X. O. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 2005, 11, 435–443. 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Kim S. B.; Suzuki H.; Sato M.; Tao H. Superluminescent variants of marine luciferases for bioassays. Anal. Chem. 2011, 83, 8732–8740. 10.1021/ac2021882. [DOI] [PubMed] [Google Scholar]
- Serganova I.; Moroz E.; Vider J.; Gogiberidze G.; Moroz M.; Pillarsetty N.; Doubrovin M.; Minn A.; Thaler H. T.; Massague J.; Gelovani J.; Blasberg R. Multimodality imaging of TGFbeta signaling in breast cancer metastases. FASEB J. 2009, 23, 2662–2672. 10.1096/fj.08-126920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacer A. C.; Nyati S.; Moudgil P.; Iyengar R.; Luker K. E.; Rehemtulla A.; Luker G. D. NanoLuc reporter for dual luciferase imaging in living animals. Mol. Imaging 2013, 12, 1–13. 10.2310/7290.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire C. A.; Bovenberg M. S.; Crommentuijn M. H.; Niers J. M.; Kerami M.; Teng J.; Sena-Esteves M.; Badr C. E.; Tannous B. A. Triple bioluminescence imaging for in vivo monitoring of cellular processes. Mol. Ther.--Nucleic Acids 2013, 2, e99 10.1038/mtna.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. J.; Hwang C. S.; Prescher J. A. Orthogonal Bioluminescent Probes from Disubstituted Luciferins. Biochemistry 2021, 60, 563–572. 10.1021/acs.biochem.0c00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C. K.; Ornelas M. Y.; Yao Z. W.; Prescher J. A. Multicomponent Bioluminescence Imaging with Naphthylamino Luciferins. ChemBioChem 2021, 22, 2650. 10.1002/cbic.202100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z.; Zhang B. S.; Steinhardt R. C.; Mills J. H.; Prescher J. A. Multicomponent Bioluminescence Imaging with a π-Extended Luciferin. J. Am. Chem. Soc. 2020, 142, 14080–14089. 10.1021/jacs.0c01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun C. M.; Ionkina A. A.; Yao Z.; Jones K. A.; Porterfield W. B.; Prescher J. A. Rapid Multicomponent Bioluminescence Imaging via Substrate Unmixing. ACS Chem. Biol. 2021, 16, 682–690. 10.1021/acschembio.0c00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz M. A.; Zurita J.; Moroz A.; Nikolov E.; Likar Y.; Dobrenkov K.; Lee J.; Shenker L.; Blasberg R.; Serganova I.; Ponomarev V. Introducing a new reporter gene, membrane-anchored Cypridina luciferase, for multiplex bioluminescence imaging. Mol. Ther Oncolytics 2021, 21, 15–22. 10.1016/j.omto.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte L.; Que I.; Kaijzel E.; Branchini B.; Roda A.; Löwik C. Sensitive dual color in vivo bioluminescence imaging using a new red codon optimized firefly luciferase and a green click beetle luciferase. PLoS One 2011, 6, e19277 10.1371/journal.pone.0019277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswendt M.; Vogel S.; Schäfer C.; Jathoul A.; Pule M.; Hoehn M. Quantitative in vivo dual-color bioluminescence imaging in the mouse brain. Neurophotonics 2019, 6, 025006. 10.1117/1.NPh.6.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncali E.; Savinaud M.; Levrey O.; Rogers K. L.; Maitrejean S.; Tavitian B. New device for real-time bioluminescence imaging in moving rodents. J. Biomed. Opt. 2008, 13, 054035. 10.1117/1.2976426. [DOI] [PubMed] [Google Scholar]
- Wang G.; Cong W.; Shen H.; Qian X.; Henry M.; Wang Y. Overview of bioluminescence tomography--a new molecular imaging modality. Front. Biosci., Landmark Ed. 2008, 13, 1281–1293. 10.2741/2761. [DOI] [PubMed] [Google Scholar]
- Klose A. D.; Beattie B. J. Bioluminescence tomography with CT/MRI co-registration. Annu. Int. Conf IEEE Eng. Med. Biol. Soc. 2009, 2009, 6327–6330. 10.1109/IEMBS.2009.5333170. [DOI] [PubMed] [Google Scholar]
- Rice B. W.; Cable M. D.; Nelson M. B. In vivo imaging of light-emitting probes. J. Biomed. Opt. 2001, 6, 432–440. 10.1117/1.1413210. [DOI] [PubMed] [Google Scholar]
- Wang G.; Hoffman E.; McLennan G.. Development of the first bioluminescent tomography system. Radiology Suppl. (Proceedings of the RSNA); Radiological Society of North America, 2003; p 229.
- Gammon S. T.; Leevy W. M.; Gross S.; Gokel G. W.; Piwnica-Worms D. Spectral unmixing of multicolored bioluminescence emitted from heterogeneous biological sources. Anal. Chem. 2006, 78, 1520–1527. 10.1021/ac051999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin M.; Akin A. R.; Collins S. A.; Meganck J.; Kim J. B.; Baban C. K.; Joyce S. A.; van Dam G. M.; Zhang N.; van Sinderen D.; O’Sullivan G. C.; Kasahara N.; Gahan C. G.; Francis K. P.; Tangney M. High resolution in vivo bioluminescent imaging for the study of bacterial tumour targeting. PLoS One 2012, 7, e30940 10.1371/journal.pone.0030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. A.; Richer E.; Slavine N. V.; Kodibagkar V. D.; Soesbe T. C.; Antich P. P.; Mason R. P. A Multi-Camera System for Bioluminescence Tomography in Preclinical Oncology Research. Diagnostics 2013, 3, 325–343. 10.3390/diagnostics3030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.; Li B.; Ding S.; Fan Y.; Wang S.; Sun C.; Zhao M.; Zhao C. X.; Zhang F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 2020, 11, 4192. 10.1038/s41467-020-18051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]