Abstract
Purpose
To analyze the visual performance in contact lens wearers with keratoconus.
Methods
A retrospective study including contact lens (CL) wearers was performed. The current best-corrected visual acuity with contact lens (BCVA-CL) and with spectacles (BCVA-S) correction, contrast sensitivity (CS) (by Metrovision-MonPack3®), analysis of light scattering in the retina and vision break-up time (HD Analyzer®), and corneal tomography (Oculus Pentacam® HR) were evaluated.
Results
This study included 96 eyes of 59 patients with Keratoconus. Rigid gas permeable contact lenses (RGPCL), hybrid contact lenses (HCL), and silicone hydrogel/hydrogel contact lenses (HGCL) were fitted in 67, 17, and 12 eyes, respectively. Dynamic objective scatter index (OSI) (p = 0.024), minimum OSI (p = 0.037) and maximum OSI (p = 0.040) were significantly better with RGPCL and worse with HGCL. Mean CS in photopic conditions was significantly worse with HGCL and better with HCL (p = 0.006), without differences in mesopic conditions (p = 0.121). RGPCL wearers showed a higher mean K (p = 0.020), and a lower corneal thickness at the thinnest point (p=0.011).
Conclusion
Visual quality varied significantly with different types of CL. Although RGPCL was fitted in patients with worse Pentacam tomographic parameters, RGPCL was associated with a better dynamic visual quality.
Keywords: keratoconus, contact lens, visual performance, optical quality
Introduction
National Organization for Rare Disorders defines keratoconus as an eye (ocular) disorder characterized by progressive thinning and changes in the shape of the cornea.1 It is a noninflammatory corneal ectasia that induces irregular astigmatism, myopia, and protrusion, leading to impairment in the quality of vision.2 Spectacles are useful in the early stages of keratoconus when the astigmatism is mild. With advanced keratoconus, spectacles play a very limited role and contact lenses become necessary for improving the vision, sometimes avoiding surgery that is more invasive.3 Corneal transplant is a therapeutic option for advanced disease, when spectacle correction is insufficient, contact lens wear is intolerable and visual acuity is at an unacceptable level.4 Various options for contact lenses (CLs) are available, which include toric soft, corneal gas-permeable (RGPCL), piggyback, hybrid (HCL), and scleral lenses.4 To fit CLs is a challenge in patients with keratoconus. The choice of CL often depends on the anatomical fit on the cornea/sclera, the comfort provided by the CL, the cost of the lens, and the visual performance.5
Regarding visual performance, the monocular high-contrast distance visual acuity is the most commonly used measurement of vision in clinical practice but it is not representative enough of the full complexity of the patient’s routine visual experience. It can be a poor predictor of some aspects of vision function.6–12 Quality of vision is a subjective entity based on an individual’s unique and multifactorial perception of their vision, influenced by both visual and psychological factors.13 It is difficult to define the quality of vision by a single parameter. The assessment can include contrast sensitivity, disability glare, intraocular stray light, and aberrometry.14 For example, contrast sensitivity has been found to correlate well with various aspects of visual ability, including orientation and mobility,8,11,15 reading speed,16 and driving.15
For keratoconus, the improvement of monocular high-contrast visual acuity with CLs is well-established, but fewer reports are available on the improvement of quality of vision with different CL modalities, all relative to spectacles, and do not necessarily compare different CL designs.5,17–22 Our study aimed to analyze the visual performance in keratoconus patients already fitted with different CL materials and designs.
Materials and Methods
Study Design
A cohort study including keratoconus patients fitted with CLs in the Ophthalmology Department of Centro Hospitalar Universitário do Porto (CHUPorto), between June 2019 and March 2021. This study was conducted following the tenets of the Declaration of Helsinki (1964). The authors ensured that all patients’ anonymity was carefully protected. Informed consent was signed for all procedures. Approval was obtained from the “Departamento de Ensino, Formação e Investigação” (DEFI).
Participants
The inclusion criteria were as follows: adult patients; diagnosis of keratoconus (according to the criteria developed by the Global Consensus on Keratoconus and Ectatic Diseases); and well-fitted CL wearers (comfortable lens for as long as necessary on a case-by-case basis; daily use; none or minimal lens awareness; clear and stable vision; no or occasional need for rewetting drop).23 Patients who missed the complete clinical evaluation (complete functional and structural analysis detailed in parameters section) were included in “loss to follow-up” and they were excluded from outcome analysis. The use of different types of CLs in the past was not an exclusion criterion.
Parameters
The following variables were analyzed: demographic characteristics; functional and structural outcomes.
a) The demographic characteristics:
-age and gender;
-previous ocular surgeries;
-CL specifications (current CL fitted): type, time of wear, and spherical equivalent.
b) The functional analysis:
-current monocular high-contrast distance best-corrected visual acuity with contact lens (BCVA-CL) and with spectacles (BCVA-S) correction evaluated by Snellen Chart;
-contrast sensitivity (CS) in the various spatial frequencies under photopic (PCS) and mesopic conditions (MCS) evaluated by Metrovision-MonPack3® (grating luminance’s of 80 cd/m2 in the photopic exam and 0.08 cd/m2 in the low mesopic exam): mean PCS and MCS were considered the value on the 2–5cpd interval;
-analysis of light scattering in the retina evaluated by HD Analyzer®: the objective scatter index (OSI), the modular transfer function (MTF), the predicted visual acuities (decimal) within the 100% contrast level (PVA100), the 20% contrast level (PVA20) and the 9% contrast level (PVA9);
-the impact of the tear film on dynamic optical quality evaluated by HD Analyzer®: vision break-up time (VBUT), OSI dynamically measured 40 times in a 20-second period as a surrogate of tear film stability (dOSI). Minimum OSI (minOSI), maximum OSI (maxOSI), and OSI amplitude (ampOSI) were considered in that period.
c) The structural analysis:
-corneal tomographic parameters measured by Oculus Pentcam® HR: maximum, minimum and mean K, corneal thickness at the thinnest point (TP), Belin-Ambrósio deviation index (BAD-D), root mean square higher-order aberration (RMS-HOA), and root mean square total (RMS-TOTAL);
-retinal tomographic parameters measured by Heildelberg Spectralis® spectral-domain optical coherence tomography: central macular thickness (CMT), subfoveal choroidal thickness (SCT), and optic disc nerve fiber layer thickness (OD-NFL).
All evaluations considered and detailed above were performed in 2 different timings:
without CL and previously to CL fitting: functional analysis (BCVA-S) and structural analysis (corneal tomographic parameters)
with CL fitted and in this order: functional analysis (BCVA-CL, CS, analysis of light scattering in the retina and the impact of the tear film on dynamic optical quality) and structural analysis (retinal tomographic parameters).
The OCT was performed while patients wearing CLs due to logistic reasons (so that patients did not need to take off CL in the second evaluation time). There are some contradictory reports about the influence of CL during analysis with optical coherence tomography.24–26 This structural evaluation was performed to control other causes of poor quality of vision, which can present in these patients.
Statistical Analysis
Statistical analysis was performed using the SPSS program (SPSS Statistics, version 22.0 for Windows, SPSS Inc., IBM, Somers, NY). The normality of the variables was evaluated by the Kolmogorov–Smirnov test. The comparison between independent continuous variables was performed using the Mann–Whitney test and T-Student test. The Fisher exact test was used for nominal scaled data. Spearman’s bivariate correlation test was applied to study correlations. P values less than 0.05 were considered statistically significant.
Results
Demographic Data
Sixty-eight patients were initially included. Nine patients belong to “loss to follow-up” due to non-attendance for complete clinical evaluation and they were not included in the analysis: 3 patients were emigrant, 3 patients had given up wearing CL and were waiting to corneal transplant, 2 patients were recovering from other health problems and 1 was very satisfied and did not want such an early review. Therefore, we analysed 96 eyes of 59 patients, 64.4% (38/59) male and 35.6% (21/59) female, aged 19 to 70 years, with a mean age of 42.9 ± 12.8 years (y) at the time of complete visual performance evaluation. Concerning ocular history, 29.17% (28/96) of cases had undergone previous ocular surgery: 15.6% (15/96) intrastromal corneal ring implant, 11.5% (11/96) penetrating keratoplasty, and 2.1% (2/96) deep anterior lamellar keratoplasty. There was no case of corneal cross-linking in our sample.
Rigid gas permeable contact lenses (RGPCL), hybrid contact lenses (HCL), and silicone hydrogel/hydrogel contact lenses (HGCL) were fitted in 67, 17, and 12 eyes respectively. The specific CLs fitted were:
RGPCL group: Menicon EX/Z/S9/B4PM/KRC (31/67), Rose K2 (15/67), Hanita BXO K/Wolhk KE (15/67), Ocellus k2 (3/67), Fluorolens 50 Menicon David Thomas (2/67) and OCP (1/67);
HCL group: SynergEyes A/KC (15/17) and Duette (2/17);
HGCL group: Biofinity toric (6/12), Soflex k67 (2/12), Frequency Xcel toric (2/12), Purevision toric (1/12), and AirOptix for astigmatism (1/12).
The age (p = 0.382), gender (p = 0.875), time of CL wear (p = 0.382) and spherical equivalent (p = 0.078) were similar among groups (Table 1).
Table 1.
Demographic Data
| RGPCL (n = 67) | HCL (n = 17) | HGCL (n = 12) | p-value | |
|---|---|---|---|---|
| Age, years (mean±SD) | 42.2±12.7 | 40.7±14.9 | 47.1±9.3 | 0.382 |
| Gender (%) | ||||
| Male | 61.2% (41/67) | 88.2% (15/17) | 50.0% (6/12) | 0.875 |
| Female | 38.8% (26/67) | 11.8% (2/17) | 50.0% (6/12) | |
| Previous ocular surgery (%) | ||||
| ICRS | 14.9% (10/67) | 11.8% (2/17) | 25% (3/12) | 0.002 |
| PK | 7.5% (5/67) | 17.6% (3/17) | 25% (3/12) | |
| DALK | 0% (0/67) | 0% (0/17) | 16.7% (2/12) | |
| Time of CL wear* (%) | ||||
| ≤5 years | 46.3% (31/67) | 41.2% (7/17) | 58.3% (7/12) | 0.382 |
| 5–10 years | 16.4% (11/67) | 11.8% (2/17) | 33.3% (4/12) | |
| ≥10 years | 37.3% (25/67) | 47.1% (8/17) | 8.3% (1/12) | |
| SE, diopters (mean±SD) | −6.4±4.8 | −3.7±3.0 | −5.9±3.4 | 0.078 |
Notes: *Refers to the current CL fitted in this study. Bold number represents a statistically significant p-value.
Abbreviations: CL, contact lens; DALK, deep anterior lamellar keratoplasty; HCL, hybrid contact lens; HGCL, silicone hydrogel/hydrogel contact lens; ICRS, intra-stromal corneal ring segments; PK, penetrating keratoplasty; RGPCL, rigid gas permeable contact lens; SE, spherical equivalent.
Visual Quality Analysis
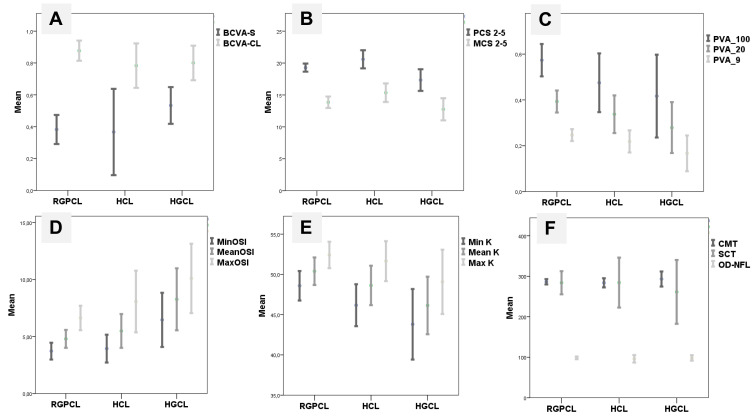
All parameters analysed are detailed in Table 2. Overall, the mean BCVA-CL was better than BCVA-S (p < 0.001) and it was similar among CL groups (p = 0.618) (Figure 1A). Mean PCS was better with RGPCL (p = 0.020) and HCL (p = 0.004) than with HGCL. There were no statistically significant differences (p = 0.071) between PCS with RGPCL and HCL. Mean MCS was similar among groups (p = 0.121) (Figure 1B). Concerning light scattering analysis, mean OSI (p = 0.134), PVA100 (p = 0.131), PVA20 (p = 0.120), PVA9 (p = 0.054) and MTF (p = 0.105) were similar among groups. Mean PVA by groups is represented in Figure 1C. Dynamic optical quality showed statistical differences: mean dOSI (p = 0.024), maxOSI (p = 0.040) and minOSI (p = 0.037) were better in RGPCL group and worse in HGCL group. The mean ampOSI was similar (p = 0.165) among groups (Figure 1D). OSI was significant correlated with age (r = 0.429, p < 0.001), time of CL wear (r = 0.249, p = 0.017), spherical equivalent (r = −0.392, p < 0.001) and SCT (r = −0.470, p < 0.001), but not with other structural parameters (Table 3). Patients with previous ocular surgery had similar visual performance to others in all functional parameters (p > 0.05).
Table 2.
Functional and Structural Outcomes
| RGPCL (n = 67) | HCL (n = 17) | HGCL (n = 12) | p-value | ||
|---|---|---|---|---|---|
| Functional Analysis | BCVA | ||||
| BCVA-S, decimal | 0.4±0.2 | 0.4±0.3 | 0.5±0.2 | 0.199 | |
| BCVA-CL,decimal | 0.9±0.2 | 0.9±0.2 | 0.8±0.1 | 0.618 | |
| Contrast sensitivity | |||||
| Mean PCS, dB | 19.3±2.6 | 20.6±2.8 | 17.3±2.7 | 0.006 | |
| Mean MCS, dB | 13.9±3.7 | 15.4±2.8 | 12.8±2.7 | 0.121 | |
| Analysis of light scattering in the retina | |||||
| OSI | 3.7±2.9 | 3.8±2.7 | 5.8±3.4 | 0.134 | |
| PVA100, decimal | 0.6±0.3 | 0.5±0.2 | 0.4±0.3 | 0.131 | |
| PVA20, decimal | 0.4±0.2 | 0.3±0.2 | 0.3±0.2 | 0.120 | |
| PVA9, decimal | 0.2±0.1 | 0.2±0.1 | 0.2±0.1 | 0.054 | |
| MTF, c/deg | 17.1±8.6 | 14.3±6.8 | 12.0±8.4 | 0.105 | |
| Pupil size, mm | 4.3±2.1 | 3.2±2.3 | 4.4±2.5 | 0.161 | |
| Impact of tear film on dynamic optical quality | |||||
| VBUT, sec | 2.1±2.6 | 2.1±2.5 | 2.9±2.0 | 0.227 | |
| dOSI | 4.8±3.1 | 5.5±2.8 | 8.3±4.3 | 0.024 | |
| maxOSI | 6.6±4.2 | 8.1±5.1 | 10.1±4.8 | 0.040 | |
| minOSI | 3.7±2.9 | 3.9±2.3 | 6.5±3.7 | 0.037 | |
| ampOSI | 2.9±2.7 | 4.1±4.6 | 3.6±2.0 | 0.165 | |
| STRUCTURAL ANALYSIS | Corneal tomographic parameters | ||||
| minK, dioptre | 48.6±6.1 | 46.2±3.9 | 43.8±5.2 | 0.034 | |
| maxK, dioptre | 52.4±5.5 | 51.6±3.7 | 49.1±4.8 | 0.294 | |
| meanK, dioptre | 50.4±5.7 | 48.6±3.6 | 44.0±7.6 | 0.020 | |
| TP, µm | 423.5±74.0 | 489.7±77.1 | 484.4±76.6 | 0.011 | |
| BAD-D | 11.7±5.4 | 7.1±3.9 | 7.7±4.5 | 0.013 | |
| RMS-HOA, µm | 3.7±1.6 | 3.2±1.7 | 3.0±1.7 | 0.372 | |
| RMS-TOTAL, µm | 11.6±4.3 | 11.0±3.8 | 10.0±5.8 | 0.601 | |
| Posterior pole tomographic parameters | |||||
| CMT, µm | 286.4±26.4 | 283.8±22.0 | 293.2±29.3 | 0.617 | |
| SCT, µm | 284.0±115.2 | 284.4±120.0 | 261.4±124.1 | 0.822 | |
| OD-NFL, µm | 98.6±13.2 | 96.2±18.0 | 98.4±10.5 | 0.818 | |
Notes: Bold number represents a statistically significant p-value. Values represent mean ± standard deviation.
Abbreviations: ampOSI, OSI amplitude; BAD-D, Belin-Ambrósio deviation index; BCVA, best-corrected visual acuity, BCVA-CL, best-corrected visual acuity with contact lens, BCVA-S, best-corrected visual acuity with spectacles; CMT, central macular thickness; dOSI, OSI dynamically measured 40 times in a 20 second period as a surrogate of tear film stability; HCL, hybrid contact lens; HGCL, silicone hydrogel/hydrogel contact lens; MCS, contrast sensitivity under mesopic conditions; maxOSI, OSI maximum; minOSI, OSI minimum; MTF, modular transfer function; OD-NFL, optic disc nerve fiber layer thickness; OSI, objective scatter index; PCS, contrast sensitivity under photopic conditions; PVA100, predicted visual acuities (decimal) within the 100% contrast level; PVA20, predicted visual acuities (decimal) within the 20% contrast level; PVA9, predicted visual acuities (decimal) within the 9% contrast level; RGPCL, rigid gas permeable contact lens; RMS-HOA, root mean square higher-order aberration; RMS-TOTAL, root mean square total; SCT, subfoveal choroidal thickness; TP, corneal thickness at the thinnest point; VBUT, vision break-up time.
Figure 1.
The variation of some variables according to different patients groups: rigid gas permeable contact lens (RGPCL), hybrid contact lens (HCL) and silicone hydrogel/hydrogel contact lens (HGCL). (A) Mean best-corrected visual acuity with spectacles (BCVA-S) (p = 0.199) and with contact lens (BCVA-CL) (p = 0.618). (B) Mean contrast sensitivity under photopic conditions (PCS) (p = 0.006) and mesopic conditions (MCS) (p = 0.121). (C) Mean predicted visual acuities within the 100% contrast level (PVA100) (p = 0.131), within the 20% contrast level (PVA20) (p = 0.120) and within the 9% contrast level (PVA9) (p = 0.054). (D) Mean dynamically measured OSI: minimum (MinOSI) (p = 0.037), mean (p = 0.024) and maximum (MaxOSI) (p = 0.040). (E) Mean k minimum (mink) (p = 0.034), mean (p = 0.020) and maximum (maxK) (p = 0.294). (F) Mean central macular thickness (CMT) (p = 0.617), subfoveal choroidal thickness (SCT) (p = 0.822) and optic disc nerve fiber layer thickness (OD-NFL) (p = 0.818).
Table 3.
OSI Correlations
| R value | p value | ||
|---|---|---|---|
| Demographic Parameters | |||
| Age | 0.429 | <0.001 | |
| Time of contact lens wear | 0.249 | 0.017 | |
| Spherical equivalent | −0.392 | <0.001 | |
| Strutural Parameters | |||
| Anterior | Maximum K | 0.237 | 0.063 |
| BAD-D | 0.164 | 0.207 | |
| TP | −0.069 | 0.592 | |
| Posterior | CMT | 0.161 | 0.121 |
| SCT | −0.470 | <0.001 | |
| OD-NFL | −0.185 | 0.074 | |
Note: Bold number represents a statistically significant p-value.
Abbreviations: BAD-D, Belin-Ambrósio deviation index; CMT, central macular thickness; OD-NFL, optic disc nerve fiber layer thickness; SCT, subfoveal choroidal thickness; TP, corneal thickness at the thinnest point.
Structural Analysis
Concerning corneal tomographic parameters, mean minK (p = 0.034), mean K (p = 0.020), TP (p = 0.011) and BAD-D (p = 0.013) were worse in RGPCL and better in HGCL group. Mean K values in each group are represented in Figure 1E. RMS-HOA (p = 0.372) and RMS-TOTAL (p = 0.601) were similar among groups.
Regarding posterior pole structural analysis, the mean CMT (p = 0.617), SCT (p = 0.822), and OD-NFL (p = 0.818) were similar among groups (Figure 1F).
Examples
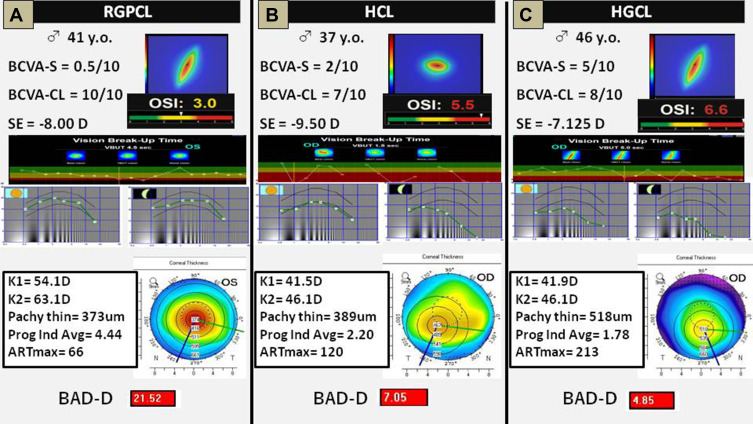
In Figures 2, 3 clinical cases are represented with similar age, gender, and spherical equivalent, fitted with 3 different groups of CLs. In all these cases, there was an increased BCVA with CL, but the other visual quality parameters differed among groups. Along with the cases (Figure 2A–C), OSI, VBUT, and CS worsened, despite better corneal tomographic parameters.
Figure 2.
Three clinical examples. They were chosen because they have a similar age, gender, and spherical equivalent, fitted with 3 different contact lenses. (A) Patient fitted with a rigid gas permeable contact lens (RGPCL). (B) Patient fitted with a hybrid contact lens (HCL). (C) Patient fitted with a silicone hydrogel/hydrogel contact lens (HGCL). In all these cases, it was an increased best-corrected visual acuity with contact lens, but the other visual quality parameters differed among groups. Along with the cases (A–C), objective scatter index, vision break-up time, and contrast sensitivity worsened, despite better corneal tomographic parameters.
Discussion
The results of the present study suggest a significant visual quality variation for different types of CL fitted in patients with keratoconus, despite the similar BCVA-CL. Although this is a real-life study, our CLs groups are significantly homogeneous as to age, gender, time of CL wear, and spherical equivalent, which allows comparisons among them.
Regarding contrast sensitivity, HGCL showed worse performance in photopic conditions, and all CLs were associated with a decreased contrast sensitivity in mesopic conditions. This is a characteristic of keratoconus patients even without CLs27 and may impact on patients’ daily activities and professional performance, like the ability to drive, despite good BCVA. Although analysis of light scattering in the retina revealed no differences, the dynamic optical quality analysis highlighted the impact that tear film can have on visual quality. As to this parameter RGPCL showed a significant advantage in visual performance despite more advanced keratoconus and HGCL had the worst results. Tear lens is the main reason that RGPCL or HCL provides better visual quality by decreasing higher-order aberrations arising from anterior corneal surface irregularity. Although the role of tear exchange is less discussed, it should be clinically paid attention to in those cases with decreased quality of vision compared to spectacle wearing. Tear exchange beneath CLs is lower during soft lenses wear when compared with rigid lenses wear, resulting in more accumulation of tear film debris and metabolic by-products between the cornea and CLs.28 The correlations found between OSI and age29 and spherical equivalent30 are not new. The correlation found between OSI and the time of CL wear may be explained by the fact that patients with more time of CL wear are older. The correlation between OSI and SCT can be explained by the fact that patients with lower SCT are myopic. However, there was no correlation between corneal tomographic parameters and OSI.31 Probably, the fitted CL can contribute to decreasing aberrations and decreasing light scattering on the retina.
Concerning corneal structural analysis, we concluded that the 3 groups were not homogenous as regards the degree of disease. Nevertheless, patients fitted with RGPCL had more advanced degrees of keratoconus and were simultaneously the group with the best visual performance. Lastly, the posterior pole tomographic parameters analysis allowed us to exclude differences in retinal and optic nerve parameters that might influence our visual quality analysis interfering with the results.
Since not all types of contact lens are suitable to all patients with keratoconus, prescription can be a big challenge. Therefore, one of the aims of this work is to highlight that in CL fitting process, professionals must take into account not only visual acuity but also the quality of vision, as this may impact daily-life activities.
Two of the strengths of this study are the inclusion of real-life patients and the multimodal assessment of visual quality, allowing us to understand most of the limitations of these patients. We did not want to exclude patients with previous corneal treatments because these patients are an increasingly frequent reality in our medical appointments. The assessment was performed by only 1 doctor (IB) and 2 technicians (DA and DJ).
The major limitation of this study is that it consists in comparing heterogeneous groups regarding stages of the disease. However, it is almost impossible to find patients well fitted with these 3 types of CLs for all stages of the disease. Additionally, this study is not randomized and has no control group. The randomization is not plausible because this study is an evaluation of patients already well adapted to CLs and not chosen for this study. A control group, especially in cases of more advanced disease, is not suitable, as visual acuity is bad without correction and correction with glasses is not possible and surgical options are not to be considered when achieving good visual performance with contact lenses. Comparing the visual performance of the different CLs in the same patient would be ideal, but it would not be feasible in patients with keratoconus. In most cases, fitted lenses are the only suitable option, and so it would not be possible to assess the quality of vision with poorly fitted CLs, which do not stabilize, might cause corneal trauma, or do not allow acceptable visual acuity, etc. These formats are without doubt very interesting in theoretical terms, but they do not work with CLs fitted for use in everyday life, especially in more advanced keratoconus. Occasionally, especially in less developed keratoconus, CLs could be put on only for the assessment of vision and quality of vision even if they were not well-fitted enough to be prescribed. Besides, CLs often seem well-fitted, but the patient sees poorly with them. For this reason, it is not appropriate to test the quality of vision, because they will not be prescribed either. In our opinion, the ideal methodology would be to compare the quality of vision with different types of CLs, well adapted to the same eye. However, they would always have to be adapted under the same conditions, allowing enough time between evaluations, to regularize the shape of the cornea after CL removal, a situation that we consider completely utopian. Another limitation of this study is that it did not address the issue of quality of life in the CL choice process. Sometimes, patients can prefer lower visual acuity and quality of vision to choose an option that provides them a better quality of life or lower costs. The quality of vision assessed in this study was also not correlated with daily performance. Lastly, this study did not include all CLs options available in the international market for keratoconus (eg, ClearKone) and included some CLs not specifically designed for keratoconus (eg, Duette), which means that the results must be interpreted with caution and cannot be generalized.
Overall, our study demonstrates that contact lenses should not be forgotten as a valid option in keratoconus patients baring in mind that in the CLs fitting process not only visual acuity but also the quality of vision must be taken into account, which is often forgotten, despite the fact that both aspects may impact daily-life activities.
Acknowledgments
The authors want to acknowledge all the support granted by the Head of the Ophthalmology Department of Centro Hospitalar e Universitário do Porto, Prof. Dr. Pedro Menéres. The manuscript has been presented in public as an oral communication at 2 events: World Ophthalmology Congress 2020 Virtual and CIRP 2021 (Reunion of Cornea, cataract and ocular surface specialists), June, Santa Eulália, Portugal.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Keratoconus [Internet]. NORD (National Organization for Rare Disorders). [cited June 7, 2021]. Available from: https://rarediseases.org/rare-diseases/keratoconus/. Accessed December 2, 2021.
- 2.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/S0039-6257(97)00119-7 [DOI] [PubMed] [Google Scholar]
- 3.Rathi VM, Mandathara PS, Dumpati S. Contact lens in keratoconus. Indian J Ophthalmol. 2013;61(8):410–415. doi: 10.4103/0301-4738.116066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandathara PS, Stapleton FJ, Willcox MDP. Outcome of keratoconus management: review of the past 20 years’ contemporary treatment modalities. Eye Contact Lens. 2017;43(3):141–154. doi: 10.1097/ICL.0000000000000270 [DOI] [PubMed] [Google Scholar]
- 5.Kumar P, Bandela PK, Bharadwaj SR. Do visual performance and optical quality vary across different contact lens correction modalities in keratoconus? Contact Lens Anterior Eye. 2020;43(6):568–576. doi: 10.1016/j.clae.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 6.Hess R, Woo G. Vision through cataracts. Invest Ophthalmol Vis Sci. 1978;17(5):428–435. [PubMed] [Google Scholar]
- 7.Paulsson LE, Sjöstrand J. Contrast sensitivity in the presence of a glare light. Theoretical concepts and preliminary clinical studies. Invest Ophthalmol Vis Sci. 1980;19(4):401–406. [PubMed] [Google Scholar]
- 8.Marron JA, Bailey IL. Visual factors and orientation-mobility performance. Am J Optom Physiol Opt. 1982;59(5):413–426. doi: 10.1097/00006324-198205000-00009 [DOI] [PubMed] [Google Scholar]
- 9.Owsley C, Sloane ME. Contrast sensitivity, acuity, and the perception of ‘real-world’ targets. Br J Ophthalmol. 1987;71(10):791–796. doi: 10.1136/bjo.71.10.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott DB, Hurst MA. Simple clinical techniques to evaluate visual function in patients with early cataract. Optom Vis Sci. 1990;67(11):822–825. doi: 10.1097/00006324-199011000-00006 [DOI] [PubMed] [Google Scholar]
- 11.Elliott DB, Hurst MA, Weatherill J. Comparing clinical tests of visual function in cataract with the patient’s perceived visual disability. Eye (Lond). 1990;4(Pt 5):712–717. doi: 10.1038/eye.1990.100 [DOI] [PubMed] [Google Scholar]
- 12.Mangione CM, Phillips RS, Lawrence MG, Seddon JM, Orav EJ, Goldman L. Improved visual function and attenuation of declines in health-related quality of life after cataract extraction. Arch Ophthalmol. 1994;112(11):1419–1425. doi: 10.1001/archopht.1994.01090230033017 [DOI] [PubMed] [Google Scholar]
- 13.McAlinden C, Pesudovs K, Moore JE. The development of an instrument to measure quality of vision: the Quality of Vision (QoV) questionnaire. Invest Ophthalmol Vis Sci. 2010;51(11):5537–5545. doi: 10.1167/iovs.10-5341 [DOI] [PubMed] [Google Scholar]
- 14.Parede TRR, Torricelli AAM, Mukai A, Vieira Netto M, Bechara SJ. Quality of vision in refractive and cataract surgery, indirect measurers: review article. Arq Bras Oftalmol. 2013;76:386–390. doi: 10.1590/S0004-27492013000600016 [DOI] [PubMed] [Google Scholar]
- 15.Rubin GS, Roche KB, Prasada-Rao P, Fried LP. Visual impairment and disability in older adults. Optom Vis Sci. 1994;71(12):750–760. doi: 10.1097/00006324-199412000-00005 [DOI] [PubMed] [Google Scholar]
- 16.Leat SJ, Woodhouse JM. Reading performance with low vision aids: relationship with contrast sensitivity. Ophthalmic Physiol Opt. 1993;13(1):9–16. doi: 10.1111/j.1475-1313.1993.tb00420.x [DOI] [PubMed] [Google Scholar]
- 17.Negishi K, Kumanomido T, Utsumi Y, Tsubota K. Effect of higher-order aberrations on visual function in keratoconic eyes with a rigid gas permeable contact lens. Am J Ophthalmol. 2007;144(6):924–929. doi: 10.1016/j.ajo.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 18.Montalt JC, Porcar E, España-Gregori E, Peris-Martínez C. Visual quality with corneo-scleral contact lenses for keratoconus management. Cont Lens Anterior Eye. 2018;41(4):351–356. doi: 10.1016/j.clae.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 19.Metlapally S, Bharadwaj SR, Roorda Aet al. Binocular cross-correlation analyses of the effects of high-order aberrations on the stereoacuity of eyes with keratoconus. J Vis. 2019;19(6):12. doi: 10.1167/19.6.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilagiri VK, Metlapally S, Kalaiselvan P, Schor CM, Bharadwaj SR. LogMAR and stereoacuity in keratoconus corrected with spectacles and rigid gas-permeable contact lenses. Optom Vis Sci. 2018;95(4):391–398. doi: 10.1097/OPX.0000000000001205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherafat H, White JE, Pullum KW, Adams GG, Sloper JJ. Anomalies of binocular function in patients with longstanding asymmetric keratoconus. Br J Ophthalmol. 2001;85(9):1057–1060. doi: 10.1136/bjo.85.9.1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gumus K, Gire A, Pflugfelder SC. The impact of the Boston ocular surface prosthesis on wavefront higher-order aberrations. Am J Ophthalmol. 2011;151(4):682–690.e2. doi: 10.1016/j.ajo.2010.10.027 [DOI] [PubMed] [Google Scholar]
- 23.Gomes JAP, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369. doi: 10.1097/ICO.0000000000000408 [DOI] [PubMed] [Google Scholar]
- 24.Aviram T, Beeri I, Berkow D, Zayit-Soudry S, Blumenthal EZ, Shapira Y. The effect of contact lens wear on retinal spectral domain optical coherence tomography. Clin Exp Optom. 2020;103(6):792–797. doi: 10.1111/cxo.13064 [DOI] [PubMed] [Google Scholar]
- 25.Berkenstock MK, Parikh RA, Collins MD, et al. Use of contact lenses to optimize OCT scans of the optic nerve in glaucoma suspects or patients with glaucoma with high myopia. Ophthalmol Glaucoma. 2020;3(3):196–201. doi: 10.1016/j.ogla.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 26.Salchow DJ, Hwang AM, Li F-Y, Dziura J. Effect of contact lens power on optical coherence tomography of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2011;52(3):1650–1654. doi: 10.1167/iovs.10-6118 [DOI] [PubMed] [Google Scholar]
- 27.Zarei-Ghanavati S, Khakshour H, Vejdani M, Ghooshkhanei H, Vejdani A. Evaluation of changes in visual acuity, contrast sensitivity and aberrations in patients with keratoconus after corneal collagen cross-linking. J Ophthalmic Vis Res. 2017;12(3):260–264. doi: 10.4103/jovr.jovr_30_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muntz A, Subbaraman LN, Sorbara L, Jones L. Tear exchange and contact lenses: a review. J Optom. 2015;8(1):2–11. doi: 10.1016/j.optom.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artal P, Benito A, Pérez GM, et al. An objective scatter index based on double-pass retinal images of a point source to classify cataracts. PLoS One. 2011;6(2):e16823. doi: 10.1371/journal.pone.0016823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao H, Tian M, He L, Zhao J, Mo X, Zhou X. Objective optical quality and intraocular scattering in myopic adults. Invest Ophthalmol Vis Sci. 2014;55(9):5582–5587. doi: 10.1167/iovs.14-14362 [DOI] [PubMed] [Google Scholar]
- 31.Leonard AP, Gardner SD, Rocha KM, Zeldin ER, Tremblay DM, Waring GO. Double-pass retina point imaging for the evaluation of optical light scatter, retinal image quality, and staging of keratoconus. J Refract Surg. 2016;32(11):760–765. doi: 10.3928/1081597X-20160728-02 [DOI] [PMC free article] [PubMed] [Google Scholar]