Alawam et al. demonstrate in this issue that prolonged damage in thymic medullary epithelial cells causes the failure in self-tolerance in newly generated T cells and provokes post-transplant autoimmunity.
Abstract
Whether autologous hematopoietic stem cell transplantation is free from graft-versus-host disease is controversial. Alawam et al. (2021. J. Exp. Med. https://doi.org/10.1084/jem.20211239) now demonstrate that prolonged damage in thymic medullary epithelial cells causes the failure in self-tolerance in newly generated T cells and provokes post-transplant autoimmunity.
“You don’t know what you’ve got till it’s gone.” —Joni Mitchell, “Big Yellow Taxi”
Hematopoietic stem cell transplantation (HSCT) is an important treatment of cancer and other diseases (Copelan, 2006; Saad et al., 2020). Complications associated with HSCT include graft-versus-host disease (GVHD), which has been detected even when the combination between grafts and hosts is autologous (Kline et al., 2008). However, whether and how autologous HSCT triggers the breakdown of self-tolerance in the immune system is controversial (Otegbeye et al., 2014). The new study by Alawam et al. published in this issue of Journal of Experimental Medicine demonstrates that in a mouse model of syngeneic HSCT, the recovery of medullary thymic epithelial cells (mTECs) in the thymus was noticeably inefficient and slow compared with cortical TECs (cTECs; Alawam et al., 2021). Importantly, as mTECs have an essential role for the establishment of self-tolerance in newly generated T cells (Abramson and Anderson, 2017), Alawam et al. report that the sustained failure in mTEC recovery during syngeneic HSCT is accompanied by reduced number in thymic dendritic cells (DCs), delayed development of Foxp3+ regulatory T (Treg) cells, and defective T cell self-tolerance, which triggers autoimmunity in mice (Alawam et al., 2021). The prolonged damage in mTECs may explain the onset of GVHD-like complications during autologous HSCT.

Insights from Yousuke Takahama.
In the new study, Alawam et al. examined how syngeneic bone marrow cell transplantation in irradiated mice affects TECs (Alawam et al., 2021). They noticed that the number of mTECs was significantly reduced, while the number of cTECs was not altered, during the process of syngeneic bone marrow cell transplantation. The reduced mTECs included the Aire+ MHC IIhigh CD80high subpopulation, which has an essential role in inducing self-tolerance in newly generated T cells, by supporting the promiscuous expression of virtually all genes to display genome-wide self-components in the thymic medullary microenvironment (Kyewski and Klein, 2006). Other mTEC subpopulations, including CCL21+ cells and thymic tuft cells (Kadouri et al., 2020), were also reduced. CCL21+ mTECs have an essential role in inducing self-tolerance in T cells, by supporting the migration of positively selected cortical thymocytes into the thymic medulla (Kozai et al., 2017). Interestingly, the number of Aire+ mTECs, which was significantly reduced during the first 3 wk after transplantation, recovered afterward, suggesting that the post-HSCT damage in mTECs is transient and the recovery of mTECs is slower than other cells. Along with the prolonged decrease in mTECs, the number of thymic DCs was also reduced after the bone marrow cell transplantation and their recovery was inefficient and delayed.
cTECs are responsible for the production and positive selection of T cells in the thymic cortex (Takahama et al., 2017). Alawam et al. showed that no significant reduction in cTECs during HSCT coincided with the rapid progression of thymocyte development after the bone marrow cell transplantation. In contrast, mTECs and DCs in the thymic medulla are cooperatively responsible for the establishment of self-tolerance in T cells by inducing negative selection of self-reactive T cells and by promoting the generation of Treg cells (Abramson and Anderson, 2017). Importantly, Alawam et al. showed the reduction in caspase 3–cleaved apoptotic TCRβhigh CD5high thymocytes and the escape of self-mammary tumor virus superantigen-reactive thymocytes from thymic deletion. They also showed a prolonged reduction and delayed recovery in the number of Foxp3+ Treg cells during the bone marrow cell transplantation. These results indicate that during syngeneic bone marrow cell transplantation, the thymic cortex keeps its functional capability to produce T cells, but thymic medulla function is substantially impaired, resulting in a failure to establish self-tolerance in T cells newly produced in the cortex. Alawam et al. further showed that Treg cell–depleted CD4+CD8− mature thymocytes generated after transplantation readily cause autoimmunity in mice by detecting lymphocyte infiltration in the liver and autoantibodies against various tissues upon the transfer in athymic nude mice. Collectively, Alawam et al. report that during syngeneic bone marrow cell transplantation, the thymus can support production of TCRαβ+ T cells rapidly and efficiently, despite that the thymic function is not fully operational as the thymic medulla remains damaged and insufficient to cause defective self-tolerance in T cells.
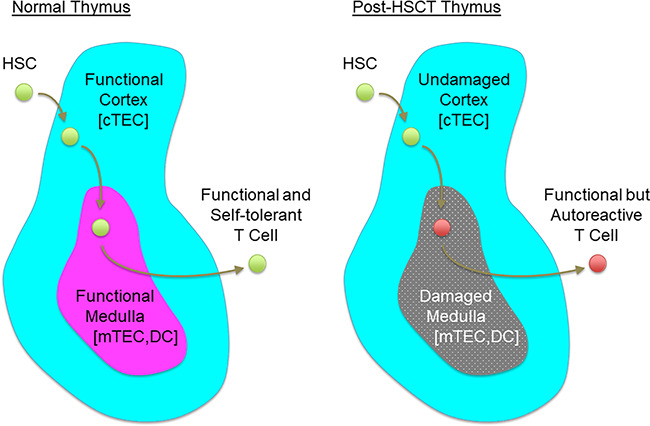
In a normal thymus, hematopoietic stem cell (HSC)–derived precursors enter the thymic cortex to become T-lineage cells and to generate functionally capable repertoire of T cell specificities. Newly generated T-lineage cells migrate to the thymic medulla to acquire self-tolerance with the help of mTECs and DCs. Stepwise supports by thymic cortex and medulla establish a pool of functional and self-tolerant T cells (left). Alawam et al. (2021) demonstrate that after HSCT, the thymic cortex functions undisturbed but the thymic medulla is damaged. Selective damage in the thymic medulla causes the failure in self-tolerance in newly generated T cells and leads to the generation of functional but autoreactive T cell pool (right).
By revealing the uncoupling of T cell–producing function by the thymic cortex and self-tolerance–inducing function by the thymic medulla, the study by Alawam et al. suggests a scenario that prolonged failure in thymic medulla is responsible for the loss of self-tolerance in T cells during autologous HSCT. Such a failure in thymic medulla and subsequent autoimmunity during immune reconstitution may explain GVHD-like tissue damages and associated symptoms detected in patients who received autologous HSCT (Abudayyeh et al., 2015; El-Jurdi et al., 2017; Hammami et al., 2018; Hierlmeier et al., 2018).
Total body irradiation before HSCT is likely responsible for the damage in mTECs. However, mechanisms for the selective and sustained loss in mTECs (when cTECs seem to remain undisturbed) and the prolonged failure in thymic medulla function during HSCT are interesting issues. Further studies would lead toward a better understanding of the mechanism for the generation and regeneration of heterogenous TEC subpopulations, including cTECs, Aire+ mTECs, and CCL21+ mTECs. How long the defects persist is another interesting question. Alawam et al. reports that total mTEC number stayed reduced even after 8 wk after transplantation, although Aire+ mTECs and Foxp3+ Treg cells recovered by then. It is possible that thymic medulla failures in initial stages of immune reconstitution are accompanied by autoimmunity but are later tempered by the availability of Treg cells. Seeking a synchronized regeneration of multiple TEC subpopulations appears an important challenge toward further successful hematopoietic cell reconstitution without complications with the risk of autoimmunity.
References
- Abramson, J., and Anderson G.. 2017. Annu. Rev. Immunol. 10.1146/annurev-immunol-051116-052320 [DOI] [PubMed] [Google Scholar]
- Abudayyeh, A., et al. 2015. Clin. Kidney J. 10.1093/ckj/sfv036 [DOI] [Google Scholar]
- Alawam, A.S., et al. 2021. J. Exp. Med. 10.1084/jem.20211239 [DOI] [Google Scholar]
- Copelan, E.A. 2006. N. Engl. J. Med. 10.1056/NEJMra052638 [DOI] [PubMed] [Google Scholar]
- El-Jurdi, N., et al. 2017. Bone Marrow Transplant. 10.1038/bmt.2017.20 [DOI] [PubMed] [Google Scholar]
- Hammami, M.B., et al. 2018. Gastroenterol. Res. 10.14740/gr925w [DOI] [Google Scholar]
- Hierlmeier, S., et al. 2018. PLoS One. 10.1371/journal.pone.0204914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadouri, N., et al. 2020. Nat. Rev. Immunol. 10.1038/s41577-019-0238-0 [DOI] [PubMed] [Google Scholar]
- Kline, J., et al. 2008. Bone Marrow Transplant. 10.1038/sj.bmt.1705931 [DOI] [Google Scholar]
- Kozai, M., et al. 2017. J. Exp. Med. 10.1084/jem.20161864 [DOI] [Google Scholar]
- Kyewski, B., and Klein L.. 2006. Annu. Rev. Immunol. 10.1146/annurev.immunol.23.021704.115601 [DOI] [PubMed] [Google Scholar]
- Otegbeye, F., et al. 2014. Bone Marrow Transplant. 10.1038/bmt.2014.169 [DOI] [PubMed] [Google Scholar]
- Saad, A., et al. 2020. J. Natl. Compr. Canc. Netw. 10.6004/jnccn.2020.0021 [DOI] [Google Scholar]
- Takahama, Y., et al. 2017. Nat. Rev. Immunol. 10.1038/nri.2017.12 [DOI] [PubMed] [Google Scholar]


