Abstract
Study objective:
The National Heart, Lung, and Blood Institute evidence-based guidelines for timeliness of opioid administration for sickle cell disease (SCD) pain crises recommend an initial opioid within 1 hour of arrival, with subsequent dosing every 30 minutes until pain is controlled. No multisite studies have evaluated guideline adherence, to our knowledge. Our objective was to determine guideline adherence across a multicenter network.
Methods:
We conducted a multiyear cross-sectional analysis of children with SCD who presented between January 1, 2016, and December 31, 2018, to 7 emergency departments (EDs) within the Pediatric Emergency Care Applied Research Network. Visits for uncomplicated pain crisis were included, defined with an International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 code for SCD crisis and receipt of an opioid, excluding visits with other SCD complications or temperature exceeding 38.5°C (101.3°F). Times were extracted from the electronic record. Guideline adherence was assessed across sites and calendar years.
Results:
A total of 4,578 visits were included. The median time to first opioid receipt was 62 minutes (interquartile range 42 to 93 minutes); between the first and second opioid receipt, 60 minutes (interquartile range 39 to 93 minutes). Overall, 48% of visits (95% confidence interval 47% to 50%) were guideline adherent for first opioid. Of 3,538 visits with a second opioid, 15% (95% confidence interval 14% to 16%) were guideline adherent. Site variation in adherence existed for time to first opioid (range 22% to 70%) and time between first and second opioid (range 2% to 36%; both P<.001). There was no change in timeliness to first dose or time between doses across years (P>.05 for both).
Conclusion:
Guideline adherence for timeliness of SCD treatment is poor, with half of visits adherent for time to first opioid and one seventh adherent for second dose. Dissemination and implementation research/quality improvement efforts are critical to improve care across EDs.
INTRODUCTION
Background
Sickle cell disease (SCD) is an inherited blood disorder affecting approximately 90,000 people in the United States, of whom 36,000 are children.1,2 The sickling of the abnormal hemoglobin in RBCs results in high rates of emergency department (ED) visits and hospitalizations.3,4 The most common reason for these acute visits is painful vaso-occlusive crises. The Health and Medicine Division of the National Academies described pain management as a “moral imperative,” stating that pain is undertreated, especially in disadvantaged populations.5 An excellent example of the need for improved pain management in a disadvantaged population is pain caused by SCD. Acute care use rates in SCD are among the highest of any disease, are commonly due to painful vaso-occlusive crises, and are associated with high readmission rates.3,4 It is estimated that the average cost of care for a patient with SCD during a lifetime is $460,151.6 The painful episodes are unpredictable and severe, and result in significantly impaired health-related quality of life.7
Released in 2014, evidence-based guidelines from the National Heart, Lung, and Blood Institute (NHLBI) recommend timely evaluation and treatment of patients with painful vaso-occlusive crises. The NHLBI endorsed evidence-based SCD-specific treatment guidelines to ensure that the highest quality of care is received for treatment of painful vaso-occlusive crisis episodes. Evidence-based guidelines for the treatment of vaso-occlusive crises in children with SCD recommend that the first dose of pain medication be given within 30 minutes of ED triage or 60 minutes of registration. In addition, reassessment of pain with subsequent dosing is recommended every 15 to 30 minutes until pain is under control.8 Unfortunately, data suggest that the implementation and dissemination of evidence-based guidelines is not universal.9–11 Single-site studies conducted before the release of the NHLBI guideline also suggested that the ED treatment for sickle cell vaso-occlusive crises would not have met the guideline.12–15
The Pediatric Emergency Care Applied Research Network (PECARN) Registry, a multicenter clinical repository of electronic health record data from 7 pediatric ED sites, is composed of more than 2.4 million overall pediatric ED visits annually.16 The registry data include time of arrival and names, as well as routes and times of all medication administrations, including opioids. All registry sites are children’s hospitals with established sickle cell expertise and EDs that are staffed primarily by pediatric emergency medicine–trained physicians and use established sickle cell treatment guidelines. Our primary objective was to determine guideline adherence since the release of the NHLBI guideline for time to initial opioid dose and time between first and second opioid for sickle cell pain crisis within registry sites. Our secondary objectives were to measure differences by site and changes during the 3 years of the study.
MATERIALS AND METHODS
Study Design
We conducted a cross-sectional study by year of all visits in the PECARN Registry between January 1, 2016, and December 31, 2018, by children aged 18 years and younger who presented to the ED with an uncomplicated pain crisis (defined later). All eligible visits, whether they resulted in hospitalization or treat and release from the ED, were included. The institutional review board at each site approved this study as part of an existing registry approval for research. The 7 clinical sites participating in the study, listed in author order, were Children’s Hospital of Wisconsin, Ann & Robert H. Lurie Children’s Hospital, Children’s National Hospital, Children’s Hospital of Philadelphia, Children’s Hospital Colorado, Nationwide Children’s Hospital, and Cincinnati Children’s Hospital Medical Center.
We developed a computable algorithm to accurately identify children with uncomplicated pain crises to ensure that we were analyzing children who met criteria for the national guideline for acute pain. To accomplish this, we validated by manual electronic health record review a cohort of children presenting to the Children’s Hospital of Wisconsin ED between January 1, 2014, and April 30, 2018, with a primary billing diagnosis for SCD (International Classification of Diseases, Ninth Revision [ICD-9] code 282.4* or 282.6*; ICD-10 code D57.*) or a chief complaint of sickle cell pain crisis or sickle cell fever and who received at least one opioid pain medication. This cohort was identified by the electronic health record data warehouse, using the above ICD-9 and -10 codes. A definition of uncomplicated pain crisis was defined as a visit in which the primary complaint was pain and there were no known complications of SCD (eg, acute chest syndrome) identified during the visit. A single reviewer extracted data from 1,198 visits by 350 patients and categorized each visit as an uncomplicated pain crisis or not. A second reviewer categorized 10% of those visits as uncomplicated or not, using the same definition, with greater than 90% concordance. Of the 1,198 visits, 902 (75.3%) were categorized as uncomplicated pain crises. After exclusion of children from the cohort who had a secondary diagnosis of acute chest syndrome or priapism or a temperature in the ED greater than 38.5°C, 92% of the sample remained with an uncomplicated pain crisis. This algorithm was then applied to all sites for identification of the eligible population and analysis of guideline adherence, using data from each site within the PECARN Registry.
The PECARN Registry was developed through an Agency for Healthcare Research and Quality (AHRQ)-supported R01 grant (HS020270) and captures electronic health record data on all ED visits to the sites.16 The registry includes demographics, clinical event time stamps, vital signs, medications administered in the ED, and coded diagnoses. All registry data are submitted monthly through a secure Web system hosted by a central data coordinating center. For this study, we used registry data from 2016, 2017, and 2018.
The primary outcomes were the timeliness of ED opioid treatment and the percentage of guideline adherence for the first and second doses of opioids. Timeliness of first opioid was measured in minutes from arrival (the time recorded for initial registration) to the ED to receipt of first opioid; second opioid dosing was measured as the time between the receipt of the first and second opioid dose. In addition to median (and interquartile ranges) for timeliness, the percentage of guideline adherence for first dose within 60 minutes of arrival and time between first and second doses of 30 minutes were calculated. Each visit was treated as an independent event. We included all visits in the analysis regardless of pain scores because they are only one marker of severity, because opioids are first line if children have failed other medications at home, and to be consistent with other studies that have assessed adherence using pain scores other than severe.14 Of the visits, 75% of initial pain scores were severe, 18% moderate, and 7% mild. Medians and interquartile ranges were used to summarize time to first dose and time between first and second opioid doses. Differences in the percentage of visits meeting the guideline for first and second opioid administrations by site were compared with χ2 tests. A Cochran Armitage test for trend was used to test for a change in the percentage of adherent visits and the percentage of visits for patients who received intranasal fentanyl across years. We present data with sites identified only by number.
RESULTS
Characteristics of Study Subjects
During the 3 study years, there were 4,578 eligible visits by 1,374 individual patients. The average age at the visit was 13 years (SD 4.8 years), and 52% of the visits were by female patients.
Main Results
The analysis of timeliness of treatment revealed that the median time to first dose was 62 minutes (interquartile range 42 to 93 minutes) (Table 1). Variation between the sites for median time to first dose ranged from 45 to 88 minutes. The median time between the first and second dose was 60 minutes (interquartile range 39 to 93 minutes), with variation by site for median time between the first and second dose ranging from 48 to 106 minutes (Table 2).
Table 1.
ED arrival to receipt of first opioid for children with SCD who presented with an uncomplicated pain crisis.
| Site | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall | |
| Overall N | 352 | 1,252 | 326 | 1,458 | 147 | 543 | 500 | 4,578 |
| Visits | ||||||||
| Median | 68 | 66 | 68 | 57 | 59 | 45 | 88 | 62 |
| q1, q3 | 47, 98 | 42, 103 | 45, 102 | 42, 78 | 41, 98 | 29, 66 | 64, 122 | 42, 93 |
| 2016 | ||||||||
| Median | 69 | 65 | 72 | 58 | 69 | 56 | 79 | 62 |
| q1, q3 | 48, 94 | 41, 99 | 48, 102 | 42, 77 | 46, 83 | 41, 77 | 59, 113 | 44, 90 |
| 2017 | ||||||||
| Median | 65 | 67 | 68 | 60 | 61 | 39 | 90 | 63 |
| q1, q3 | 43, 101 | 41, 100 | 48, 104 | 43, 80 | 43, 90 | 24, 57 | 69, 137 | 41, 92 |
| 2018 | ||||||||
| Median | 70 | 69 | 63 | 55 | 55 | 37 | 90 | 61 |
| q1, q3 | 48, 100 | 44, 114 | 42, 100 | 41, 74 | 38, 101 | 24, 60 | 66, 123 | 42, 96 |
Data are presented as median and interquartile range (in minutes). Guideline-adherent care is an initial dose of opioid within 60 minutes of arrival.
Table 2.
Receipt of first and second opioid for children who received at least 2 opioids in the ED for an uncomplicated sickle cell pain crisis.
| Site | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall | |
| Overall N | 278 | 971 | 259 | 1,180 | 93 | 424 | 333 | 3,538 |
| Visits | ||||||||
| Median | 70 | 59 | 48 | 53 | 106 | 48 | 78 | 60 |
| q1, q3 | 51, 100 | 38, 102 | 19, 86 | 38, 83 | 74, 149 | 34, 75 | 58, 111 | 39, 93 |
| 2016 | ||||||||
| Median | 76 | 57 | 44 | 50 | 109 | 57 | 76 | 58 |
| q1, q3 | 51, 106 | 38, 100 | 20, 80 | 35, 77 | 77, 139 | 42, 86 | 55, 118 | 39, 92 |
| 2017 | ||||||||
| Median | 68 | 62 | 49 | 53 | 109 | 42 | 78 | 58 |
| q1, q3 | 50, 97 | 38, 96 | 22, 89 | 39, 82 | 76, 140 | 30, 66 | 56, 101 | 38, 88 |
| 2018 | ||||||||
| Median | 68 | 60 | 58 | 59 | 102 | 45 | 83 | 62 |
| q1, q3 | 52, 101 | 39, 108 | 18, 89 | 39, 95 | 70, 154 | 29, 65 | 64, 112 | 39, 99 |
Data are presented as median and interquartile range (in minutes). Guideline-adherent care is time between first and second dose less than or equal to 30 minutes. Sites are deidentified but consistent across tables and figures.
Overall analysis of guideline adherence showed that 48% of the 4,578 visits (95% confidence interval 47% to 50%) were guideline adherent for receipt of first opioid within 1 hour of arrival (Figure 1). Of the 3,538 visits in which patients received at least 2 opioid doses, only 15% (95% confidence interval 14% to 16%) were guideline adherent for receipt of the second dose within 30 minutes of the first dose (Figure 2). When only individuals with an initial pain score classified as severe were evaluated, 50% were guideline adherent for time to first dose and 15% were guideline adherent for time between first and second dose. For individuals with an initial pain score that was moderate or severe, 49% were guideline adherent for time to first dose and 15% were guideline adherent for time between first and second dose.
Figure 1.
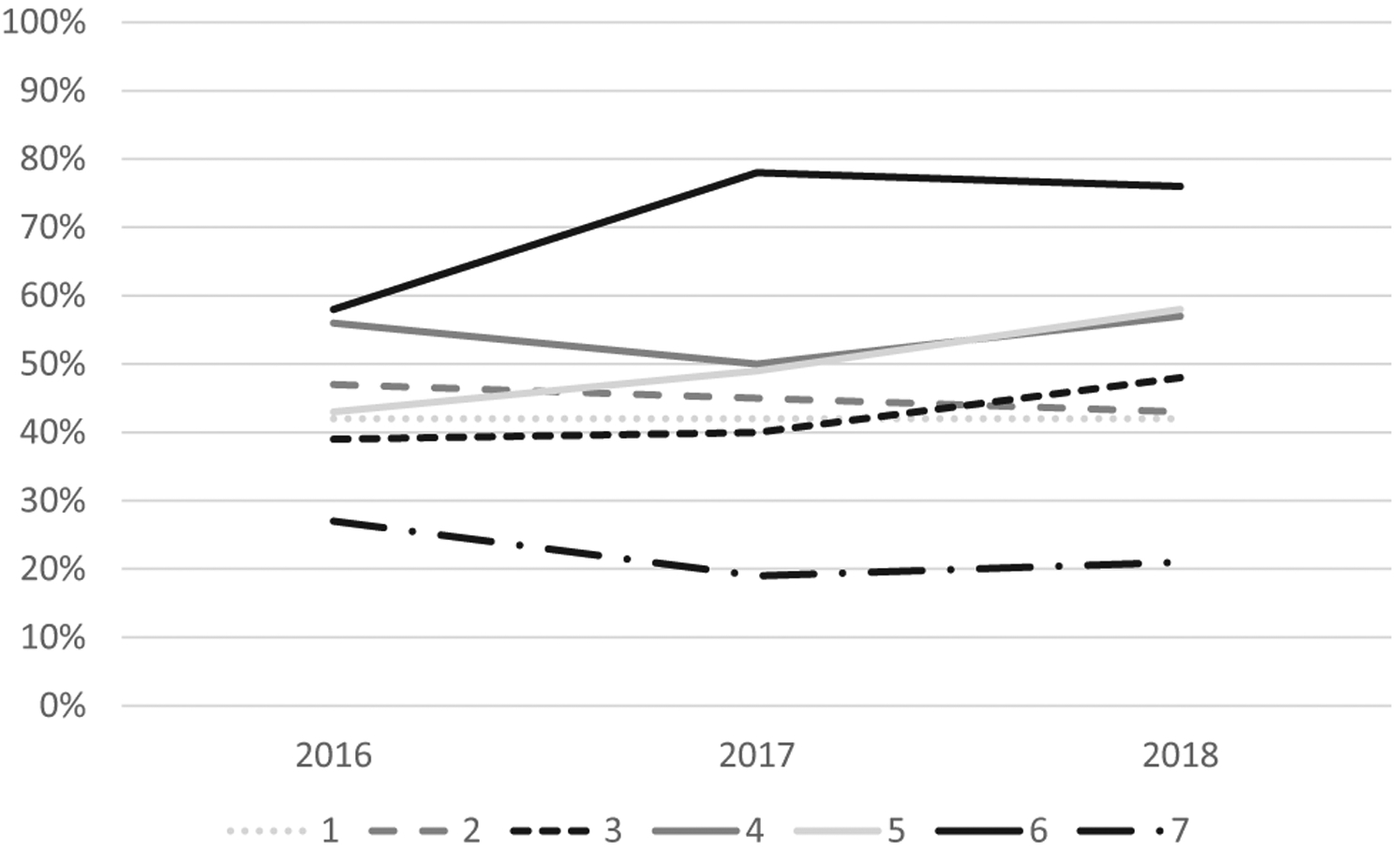
Percentage of children with SCD who presented to the ED with a pain crisis and received their first opioid analgesic within 60 minutes of arrival. Sites are deidentified but consistent across tables and figures.
Figure 2.
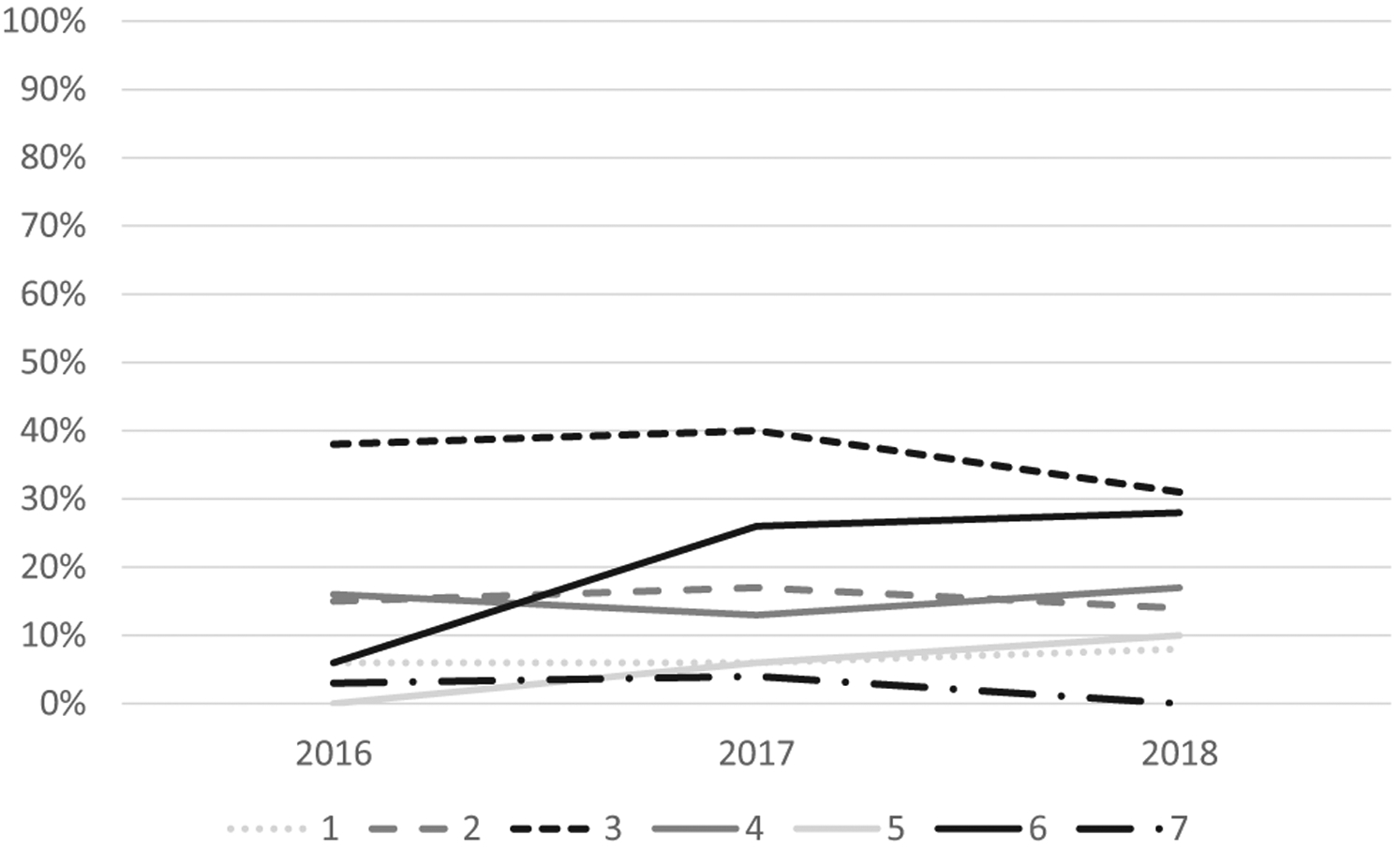
Percentage of children with SCD who presented to the ED, received at least 2 doses of opioids, and received their second opioid analgesic within 30 minutes of their first one. Sites are deidentified but consistent across tables and figures.
Significant site variation existed for guideline adherence for time to first opioid (range 22% to 70%) and time between first and second opioid (range 2% to 36%; both P<.001). In analyses of changes over time, there was no improvement in the percentage of visits with an opioid administered in the first 60 minutes (P=.78) or the percentage of visits meeting the second opioid administration guideline (P=.06).
Analysis of intranasal fentanyl use revealed a significant increasing trend in use across the 3 study years, with 228 of 1,559 visits (15%) in 2016 receiving at least 1 dose of intranasal fentanyl compared with 326 of 1,499 (22%) in 2017 and 395 of 1,520 (26%) in 2018 (P<.001). All sites used intranasal fentanyl for an eligible patient during each year of the study.
LIMITATIONS
Our study has several limitations. The use of a registry data set, although providing access to large numbers of children across multiple sites, relies on documentation in the medical record for the measurement of the timeliness of medication administration and to determine which visits included uncomplicated pain crises. Nevertheless, these are the official events as recorded in the health record and they were validated by manual chart review. Each study site independently validated the timing of opioid administrations in the ED and they were consistent with the registry data. We also performed our initial validation study that demonstrated that greater than 90% of our identified visits would be for uncomplicated sickle cell crises. Another limitation of the registry is that we did not have accurate data on treatments children may have received before ED arrival, including whether they received treatment at another facility before being transferred to one of the included EDs. Additionally, PECARN sites are academic tertiary and quaternary centers that have large pediatric hematology centers; therefore, these results may not be generalizable to other hospitals in the United States, such as general and community hospitals that care for many children and most adults with SCD. Because these other centers often do not have as many resources, these results may exceed the level of care many children receive in the United States. Finally, the study design did not allow us to identify site barriers and facilitators to achieving adherence with the guideline. Because barriers and facilitators to guideline-based care vary by site, future work will evaluate differences between sites in processes of care to help explain site differences and improve guideline-adherent care.
DISCUSSION
This analysis of a multicenter registry of large children’s hospitals demonstrates that guideline adherence for timeliness of opioid treatment for children with SCD presenting with a painful vaso-occlusive crisis is poor. Less than half of patients received a first opioid within 60 minutes of arrival, and only 1 in 7 who received a second opioid received it within 30 minutes of the first dose. These poor rates of guideline adherence occurred despite the presence of dedicated sickle cell care teams and pediatric EDs with established sickle cell pain guidelines at each site.
Recent work to improve timeliness of ED care for children with SCD and painful vaso-occlusive crises suggests improvement in timeliness with the use of intranasal fentanyl and standardized clinical protocols.14,17 Historically, only 20% of hospitals have reported having SCD care protocols, although this proportion has increased with electronic health record use.15 A single-site study within a pediatric ED, conducted predominantly with patients older than 18 years, used a standard algorithm for those with SCD and vaso-occlusive crises that included a first dose of intranasal fentanyl and 2 subsequent doses of intravenous opioids that could be given every 20 to 30 minutes.14 The authors reduced the mean time to first dose of parenteral opioid from 56 to 23 minutes and the mean time to second intravenous opioid dose from 106 to 83 minutes. Although the time to first-dose opioid met the guideline for the majority of patients, data on guideline adherence were not presented for subsequent dosing, but the results suggested improvement. Despite the combined release of the NHLBI guideline and the increasing use of intranasal fentanyl in our study, our data suggest that widespread improvement has not occurred, with significant delays in receipt of first opioid pain medication still present for more than half of children with uncomplicated crises. It is unclear why the expected improvement has not occurred, but simply developing guidelines is not sufficient; rather, the guidelines require dedicated efforts at dissemination and implementation during numerous years to allow broad, consistent use of recommended therapies. That guidelines often contain recommendations that are difficult to implement in a busy clinical setting and are perceived to promote “cookbook medicine” are reasons cited for slow uptake.18
The opioid abuse epidemic and concern about opioid overuse has potentially also had an influence on the timeliness of opioid administration. ED physicians rate opioid misuse higher in the sickle cell population than do the hematologists who provide most of their sickle cell–related care.19 With the recent increasing concern about opioid misuse, patients with SCD are reporting that their opioid prescriptions have become more difficult to fill and their opioid use has come under more intense scrutiny.20 With SCD predominantly affecting blacks, the extent to which implicit bias may also play a role is unclear.21,22 Together, these factors might present barriers to implementation of a rapid treatment guideline for opioid treatment in sickle cell patients. The extent to which these factors explain our findings in a population of children with SCD presenting to children’s hospital EDs is unknown but warrants further evaluation.
In conclusion, the timeliness of opioid treatment for children with SCD who present with a painful vaso-occlusive crisis is poor. Dissemination and implementation research/quality improvement efforts are critical to improve care across EDs.
Funding and support:
By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award 1U01HL143477-01. This project is also supported in part by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS), the Emergency Medical Services for Children program through the following grants: HOMERUN-Cincinnati Children’s Hospital Medical Center (U03MC22684), DCC-University of Utah (U03MC00008), GLEMSCRN-Nationwide Children’s Hospital (U03MC28844), PEMNEWS-Columbia University Medical Center (U03MC00007), PRIME-University of California at Davis Medical Center (U03MC00001), and WBCARN-Children’s National Medical Center (U03MC00006). Publication of this supplement was supported by the Office of Minority Health of the US Department of Health and Human Services.
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and should not be construed as the official position or policy of, nor should any endorsements be inferred by, HRSA, HHS, or the US government. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
The authors acknowledge Mark Nimmer and Cagla Muslu for their work in defining the cohort; and acknowledge other members of the PECARN Registry working group for their assistance in integrating this project into the registry infrastructure: Joe Zorc, MD, MSCE, Robert Grundmeier, MD, Diego Campos, MS, Sara Deakyne, MPH, Norma Jean Simon, MPH, MPA, Lynn Babcock, MD, MS, and Bashar Shihabuddin, MD, MS.
REFERENCES
- 1.Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol. 2010;85:77–78. [DOI] [PubMed] [Google Scholar]
- 2.Hassell KL. Population estimates of sickle cell disease in the US. Am J Prev Med. 2010;38(4 suppl):S512–521. [DOI] [PubMed] [Google Scholar]
- 3.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. [DOI] [PubMed] [Google Scholar]
- 4.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine. Pain in America: a blueprint for transforming prevention, care, education, and research. Available at: http://nationalacademies.org/hmd/Reports/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research.aspx. Published 2011. Accessed January 20, 2020.
- 6.Kauf TL, Coates TD, Huazhi L, et al. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84:323–327. [DOI] [PubMed] [Google Scholar]
- 7.Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: past, present, and future. Pediatr Blood Cancer. 2012;59:377–385. [DOI] [PubMed] [Google Scholar]
- 8.Evidence-Based Buchanan G, Yawn BP. Management of Sickle Cell Disease: Expert Panel Report, 2014. Available at: http://www.nhlbi.nih.gov/sites/www.nhlbi.nih.gov/files/sickle-cell-disease-report.pdf. Accessed August 24, 2020.
- 9.Erasmus V, Kuperus MN, Richardus JH, et al. Improving hand hygiene behaviour of nurses using action planning: a pilot study in the intensive care unit and surgical ward. J Hosp Infect. 2010;76:161–164. [DOI] [PubMed] [Google Scholar]
- 10.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? a framework for improvement. JAMA. 1999;282:1458–1465. [DOI] [PubMed] [Google Scholar]
- 11.Rello J, Lorente C, Bodi M, et al. Why do physicians not follow evidence-based guidelines for preventing ventilator-associated pneumonia? a survey based on the opinions of an international panel of intensivists. Chest. 2002;122:656–661. [DOI] [PubMed] [Google Scholar]
- 12.Mathias MD, McCavit TL. Timing of opioid administration as a quality indicator for pain crises in sickle cell disease. Pediatrics. 2015;135:475–482. [DOI] [PubMed] [Google Scholar]
- 13.Lin SM, Strouse JJ, Whiteman LN, et al. Improving quality of care for sickle cell patients in the pediatric emergency department. Pediatr Emerg Care. 2016;32:14–16. [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh PL, Sprinz PG, Wolfgang TL, et al. Improving the management of vaso-occlusive episodes in the pediatric emergency department. Pediatrics. 2015;136:e1016–1025. [DOI] [PubMed] [Google Scholar]
- 15.Silbergleit R, Jancis MO, McNamara RM. Management of sickle cell pain crisis in the emergency department at teaching hospitals. J Emerg Med. 1999;17:625–630. [DOI] [PubMed] [Google Scholar]
- 16.Deakyne Davies SJ, Grundmeier RW, Campos DA, et al. The Pediatric Emergency Care Applied Research Network Registry: a multicenter electronic health record registry of pediatric emergency care. Appl Clin Inform. 2018;9:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fein DM, Avner JR, Scharbach K, et al. Intranasal fentanyl for initial treatment of vaso-occlusive crisis in sickle cell disease. Pediatr Blood Cancer. 2017;64:e26332. [DOI] [PubMed] [Google Scholar]
- 18.Vander Schaaf EB, Seashore CJ, Randolph GD. Translating clinical guidelines into practice: challenges and opportunities in a dynamic health care environment. N C Med J. 2015;76:230–234. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro BS, Benjamin LJ, Payne R, et al. Sickle cell–related pain: perceptions of medical practitioners. J Pain Symptom Manage. 1997;14:168–174. [DOI] [PubMed] [Google Scholar]
- 20.Sinha CB, Bakshi N, Ross D, et al. Management of chronic pain in adults living with sickle cell disease in the era of the opioid epidemic: a qualitative study. JAMA Netw Open. 2019;2:e194410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson SC, Hackman HW. Race matters: perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451–454. [DOI] [PubMed] [Google Scholar]
- 22.Haywood C Jr, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]


