Abstract
Background
Beta‐blocker therapy has mortality benefit in patients with hypertension, heart failure and coronary artery disease, as well as during the perioperative period. These drugs have traditionally been considered contraindicated in patients with reversible airway disease.
Objectives
To assess the effect of cardioselective beta‐blockers in patients with asthma or chronic obstructive pulmonary disease (COPD).
Search methods
We performed a search of the Cochrane Airways Group Register of trials up to June 2011. We checked reference lists of trial reports and review articles.
Selection criteria
Randomized, blinded, placebo‐controlled trials of the effects of single‐dose or continued‐treatment cardioselective beta‐blockers in patients with reversible airway disease.
Data collection and analysis
Two independent review authors extracted data from the selected articles, reconciling differences by consensus. We divided beta1‐blockers into those with or without intrinsic sympathomimetic activity (ISA). Interventions were: administration of single‐dose or continued beta1‐blocker, and response to beta2‐agonist given after the study drug.
Main results
Nineteen studies on single‐dose treatment and 10 studies on continued treatment met the inclusion criteria. Single‐dose cardioselective beta‐blocker produced a 7.46% (95% confidence interval (CI) 5.59 to 9.32) reduction in forced expiratory volume in one second (FEV1), but with a 4.63% (95% CI 2.47 to 6.78) increase in FEV1 with beta2‐agonist, compared to placebo. Treatment lasting three to 28 days produced no change in FEV1 (mean difference (MD) ‐0.42% change from baseline; 95% CI ‐3.74 to 2.91), symptoms or inhaler use, whilst maintaining an 8.74% (95% CI 1.96 to 15.52) response to beta2‐agonist. There was no significant change in FEV1 treatment effect for those patients with chronic obstructive pulmonary disease (COPD): single doses (MD ‐5.28%; 95% CI ‐10.03 to ‐0.54); continued treatment (MD 1.07%; 95% CI ‐3.30 to 5.44).
With continued treatment there was no significant difference in FEV1 response for beta1‐blockers without ISA compared to those with ISA: ‐3.22% (95% CI ‐7.79 to 1.36) compared to 2.72% (95% CI ‐2.12 to 7.57). Those without ISA produced a 12.0% increase in FEV1 after beta2‐agonist administration compared to placebo (95% CI 4.12 to 19.87) while beta1‐blockers with ISA produced no change compared to placebo (MD ‐0.60%; 95% CI ‐13.93 to 12.73). These results were obtained in a small number of studies with few patients. The difference was not significant.
Authors' conclusions
Cardioselective beta‐blockers given in mild to moderate reversible airway disease or COPD do not produce adverse respiratory effects. Given their demonstrated benefit in conditions such as heart failure, cardiac arrhythmias and hypertension, these agents should not be withheld from such patients. Long‐term safety still needs to be established.
Keywords: Female; Humans; Male; Adrenergic beta-Antagonists; Adrenergic beta-Antagonists/therapeutic use; Asthma; Asthma/drug therapy; Forced Expiratory Volume; Forced Expiratory Volume/drug effects; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/drug therapy; Randomized Controlled Trials as Topic; Sensitivity and Specificity
Plain language summary
Cardioselective beta‐blockers for reversible airway disease
Beta‐blockers reduce mortality in patients with hypertension, heart failure and coronary arterial disease. Traditionally they have not been given to patients with reversible airway disease (asthma or chronic obstructive pulmonary disease with a reversible obstructive component), for fear of adverse respiratory effects. This review of randomized controlled trials, which evaluated cardioselective beta‐blocker use in patients with reversible airway disease, demonstrated no increase in adverse respiratory effects. From the available evidence, it appears to be safe to prescribe these drugs to people with reversible airways disease.
Background
Beta‐adrenergic blocking agents, or beta‐blockers, are indicated in the management of angina pectoris, myocardial infarction, hypertension, congestive heart failure, cardiac arrhythmia and thyrotoxicosis, as well as to reduce complications in the perioperative period (Jones 1980; Koch‐Weser 1984; IPPPSH 1985; Wadworth 1991; Klein 1994; Kendall 1995; Mangano 1996; Doughty 1997; JNC VI 1997; Lechat 1998; Freemantle 1999; Heidenreich 1999). Despite clear evidence of their effectiveness and mortality benefit, clinicians are often hesitant to administer them in the presence of a variety of common conditions for fear of adverse reactions (Kennedy 1995; Viskin 1996; Gottlieb 1998; Chafin 1999). Review articles and practice guidelines have listed asthma as a contraindication to beta‐blocker use, citing cases of acute bronchospasm occurring during non‐cardioselective beta‐blocker use (Tattersfield 1986; Tattersfield 1990; O'Malley 1991; Belli 1995; Craig 1996; JNC VI 1997; Kendall 1997). Cardioselective beta‐blockers, or beta1‐blockers, have over 20 times more affinity for beta‐1 receptors as for beta‐2 receptors, and theoretically should have significantly less risk for bronchoconstriction (Wellstein 1987).
We evaluated only patients with documented reactive airway disease in this review, as these patients have been thought to be particularly susceptible to the respiratory effects of beta‐blockers. Patients with COPD, in general, are at greater risk for ischemic heart disease than asthmatics, so may benefit more from the use of beta‐blockers. This study evaluates a subgroup of patients with a documented chronic obstructive component, but was not designed to make recommendations about patients with COPD. Another recent Cochrane Review evaluated the use of cardioselective beta‐blockers in patients with COPD, given either as a single dose or for continued treatment (Salpeter 2011). Pooled data from 22 trials demonstrated no adverse effect on forced expiratory volume in one second (FEV1) or respiratory symptoms for beta1‐blockers compared to placebo, even for those with severe chronic airway obstruction.
Objectives
To evaluate the effect of cardioselective beta1‐blockers on respiratory function in patients with reversible airway disease, as assessed by FEV1, the incidence of symptoms and use of inhaled short‐acting beta2‐agonists.
To evaluate the FEV1 response to beta2‐agonists administered after beta1‐blockers in these same participants.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized trials (RCTs) of cardioselective beta‐blockers and included beta‐blockers regardless of whether or not they were considered to possess intrinsic sympathomimetic activity (ISA) (Palmer 1969; Nicolaescu 1972; Skinner 1975a; Singh 1976; Decalmer 1978; Lofdahl 1983b; Falliers 1985; Fitzgerald 1991; McAreavey 1991; Wadworth 1991; Lois 1997; Lois 1999) (Table 1). We included studies of intravenous or oral beta1‐blockers either as a single dose or for continued treatment.
1. Beta‐blocker categories.
| Nonselective (‐ ISA) | Nonselective (+ ISA) | 1: Selective (‐ ISA) | 2: Selective (+ ISA) |
| Propranolol | Oxprenolol | Atenolol | Celiprolol (+ alpha block) |
| Timolol | Pindolol | Metoprolol | Acebutolol |
| Nadolol | Dilevalol | Bisoprolol | Xamoterol |
| Sotalol (antiarrhythmic) | Prenalterol | Esmolol | |
| Ibutmonide (+ alpha block) | Labetalol (+ alpha block) | Practolol | |
| Pafenolol | |||
| Tolamolol | |||
| Bevantolol (+ alpha agonist) |
We included single‐dose trials if they:
reported forced expiratory volume at one second (FEV1) at baseline and follow‐up or provided per cent variance;
withheld beta2‐agonists for at least eight hours prior to initial FEV1 measurements;
did not include or exclude subjects for prior response, positive or negative, to beta‐blockers;
were randomized and single or double‐blinded;
placebo‐controlled; and
included only subjects with documented reversible airway disease, demonstrated by a mean increase of at least 15% in FEV1 in response to beta2‐agonist, response to methacholine challenge, or as asthma defined by the American Thoracic Society (ATS 1993).
We decided, a priori, that the above inclusion criteria (3) through (6) would be applied to trials of continued treatment. We included studies of continued treatment if they did not report FEV1, but instead evaluated the amount of beta2‐agonist use and respiratory symptoms reported compared to placebo. In addition, we included trials even if beta2‐agonists were not withheld during the trial.
We evaluated only cardioselective beta1‐blockers in this study as these are the ones most frequently used in clinical practice. We included studies only if participants had clearly documented reversible airway disease. We chose FEV1 to evaluate respiratory function because it is a standardized and reproducible method of airway function. For single‐dose trials, only studies that withheld beta2‐agonists for at least eight hours prior to measurements were included, to maximize the response seen with beta2‐blockers. We analyzed separately clinical trials that did not meet our inclusion criteria, but gave information on FEV1 responses to beta1‐blockers in patients with reversible airway disease.
Types of participants
We included participants with reversible airway disease, defined as asthma or chronic obstructive pulmonary disease (COPD) with reversible bronchial obstruction. We included definitions of reversible airway disease determined by response to methacholine challenge, an increase in FEV1 of at least 15% to beta2‐agonist administration, or the presence of asthma as defined by the American Thoracic Society (ATS 1993).
Types of interventions
We included two interventions.
Intravenous or oral beta1‐blockers versus placebo, given either as a single dose or during continued treatment.
Administration of a beta2‐agonist, either intravenously or by inhalation, given after the study medication (beta‐blocker) or after placebo.
Types of outcome measures
Two of us independently extracted data on the following outcomes:
change in FEV1 in response to placebo or study drug;
FEV1 response to beta2‐agonist administered after placebo or study drug;
number of patients with reported symptoms during the trial, such as wheezing, dyspnea or asthma exacerbation; and
for trials of continued treatment, the weekly use of inhaled short‐acting beta2‐agonists.
Search methods for identification of studies
Electronic searches
Two investigators jointly developed strategies, with the help of an information service librarian and the Cochrane Airways Group Trial Search Co‐ordinator. We performed a comprehensive search to identify all relevant randomized controlled trials published between 1966 and June 2011 using the Cochrane Airways Group Register (CAGR) (please see Appendix 1). We searched all records in the CAGR concerned with reversible airway disease using the following terms:
(adrenergic* and antagonist*) or (adrenergic* and block*) or (adrenergic* and beta‐receptor*) or (beta‐adrenergic* and block*) or (beta‐blocker* and adrenergic*) or (blockader* or Acebutolol or Alprenolol or Atenolol or Betaxolol or Bisoprolol or Bupranolol or Butoxamine or Carteolol or Celiprolol or Dihydroalprenolol or Iodocyanopindolol or Labetalol or Levobunolol or Metipranolol or Metoprolol or Nadolol or Oxprenolol or Penbutolol or Pindolol or Practolol or Propranolol or Sotalol or Timolol)
There was no restriction on the language of publication.
Searching other resources
We also scanned the reference lists of identified trial reports or review articles, and of abstracts at clinical symposia.
Data collection and analysis
Selection of studies
We performed a search to identify all relevant published clinical trials that addressed the effects of cardioselective beta‐blockers on airway function in patients with reversible airway disease. Two of us independently evaluated studies for inclusion. In choosing articles, investigators were blinded to results but not to journal, author or institution of studies. We calculated the observed percentage agreement between raters for the assessment of inclusion.
Data extraction and management
We classified each beta1‐blocker used into one of two categories. Group 1 included cardioselective beta‐blockers without intrinsic sympathomimetic activity (ISA) and Group 2 included cardioselective beta‐blockers with ISA (Palmer 1969; Nicolaescu 1972; Lofdahl 1983b; McAreavey 1991; Lois 1997; Lois 1999; Fitzgerald 1991) (Table 1).
Two of us extracted data from the selected articles, reconciling differences by consensus. We included only published data from the trials in the analysis. We did not attempt to contact the original authors to verify the data or obtain more information, as all of the trials identified were small and many were published several years ago.
Assessment of risk of bias in included studies
We assessed the methodological quality of each trial, using the following factors.
Was the study randomized? If so, was the randomization procedure adequate and was there allocation concealment?
Were the patients and people administering the treatment blind to the intervention?
Were withdrawals and drop‐outs described and was the analysis by intention‐to‐treat?
Based on these criteria, we broadly subdivided studies into the following three categories: (a) all quality criteria met, (b) one or more of the quality criteria only partially met, or (c) one or more criteria not met.
Measures of treatment effect
We estimated the net treatment effect by measuring the ratio of the lowest FEV1 value seen after study drug to baseline FEV1, for both placebo and active treatment, and recorded the per cent change from baseline. We then compared the treatment response to the placebo response. For studies of the response to beta2‐agonists, we used the baseline of the FEV1 taken after study drug but prior to beta2‐agonist or placebo. We calculated the net treatment effect by calculating the ratio of FEV1 measured after beta2‐agonist to the new baseline for both placebo and active treatment, and then compared the treatment‐agonist response to the placebo‐agonist response.
We measured the results for respiratory symptoms as a risk difference, by subtracting the number of patients with respiratory symptoms after treatment from the number of patients with symptoms after placebo. We then pooled the risk differences using the fixed‐effect model for dichotomous outcomes. For inhaler use during longer duration studies, we averaged the standard deviation (SD) for both placebo and treatment from the available data, and we calculated the weighted mean treatment effects using the fixed‐effect model for continuous outcomes.
Dealing with missing data
We have changed the methods used to obtain SD in the updated version of the review, to get a better assessment of the net treatment effect in these cross‐over trials. Whenever possible, we calculated the SD for the net treatment effect from individual patient data or P values, and then used these figures to derive the SDs for the analysis. Some trials only provided SDs calculated for treatment response and placebo response separately. For those trials that did not report any information on SDs, we obtained the average SD from trials that provided such data and calculated these separately for placebo, treatment and beta2‐agonist responses. We performed sensitivity analyses to evaluate the effect of including these trials, by using the lowest and highest available SD in place of the pooled SD, and also by excluding these trials from the analysis.
Assessment of heterogeneity
In order to test for inter‐study heterogeneity, we calculated the Chi2 for the assumption of homogeneity. In addition, we compared the confidence intervals from the fixed‐effect model to those using the random‐effects model (DerSimonian 1986). We chose the fixed‐effect model to report the results, as minimal heterogeneity was noted in most of the analyses. When heterogeneity was noted, we reported the results from both the random‐effects model and fixed‐effect model. In addition we evaluated the Q‐parameter, as described by Berlin (Berlin 1989) and the values were found to be compatible with homogeneity.
Data synthesis
We then pooled the mean treatment effects to get a weighted average of the study means using a fixed‐effect model for continuous outcomes (Mantel 1959; Yusuf 1985). We obtained confidence intervals (CI) with 95% significance for the pooled study means.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis to compare the treatment effects of cardioselective beta‐blockers with and without ISA. We also performed another analysis to evaluate the response of patients with concomitant chronic airways obstruction, documented by a baseline FEV1 of less than 80% normal predicted value or less than 1.8 liters, or as defined by the American Thoracic Society (ATS 1995). A third subgroup analysis evaluated the treatment response for those participants known to have comorbid cardiovascular conditions such as hypertension.
Sensitivity analysis
Many of the trials were performed 20 or 30 years ago and did not provide adequate information to calculate SDs for the net treatment effect. We performed sensitivity analyses to evaluate the effect of including trials that did not provide any information on SDs.
Results
Description of studies
Results of the search
The database search identified 281 potentially relevant articles. After review of articles and bibliographies, we found 104 trials of beta‐blockers in patients with reversible airway disease. Of these trials 29 met inclusion criteria.
Included studies
Of the 29 included studies, 19 gave information on singe‐dose studies (Johnsson 1975; Skinner 1975b; Benson 1978; Ruffin 1979; Ellis 1981; Lofdahl 1981; van den Bergh 1981; Adam 1982; Lawrence 1982; Greefhorst 1984; Lammers 1984; Lammers 1985a; Chatterjee 1986; Doshan 1986a; Doshan 1986b; Falliers 1986; Lammers 1986a; Chodosh 1988; Lammers 1988; Tantucci 1990) and 10 provided data on treatment of longer duration (Nicolescu 1972; Nicolaescu 1973; Lawrence 1982; Butland 1983; Fenster 1983; Lammers 1985a; Dorow 1986c; van Zyl 1989; Fogari 1990; Bauer 1994). One of the articles (Lawrence 1982) gave data for both. Inter‐rater agreement for study eligibility was 94% (96 of 102 studies). We reached consensus on the remaining six trials.
Excluded studies
We excluded 66 trials for the following reasons: 24 trials evaluated nonselective beta‐blockers only; 27 studies failed to provide baseline and follow‐up FEV1 values; seven studies did not meet definitions for reversible airway disease; three single‐dose studies did not withhold beta2‐agonists for at least eight hours prior to study measurements; one study presented data that were published elsewhere; four studies were not blinded; two were not placebo‐controlled; four included or excluded patients based on a prior response to beta2‐blockers; and one was performed during exercise provocation.
Risk of bias in included studies
To maintain high methodological quality of the studies, we set strict inclusion criteria so that all trials were randomized, blinded and placebo‐controlled. We required all participants to have documented reversible airway disease using a conventional definition, and for single‐dose studies, beta2‐agonists had to be withheld for at least eight hours prior to measurements. We excluded studies that did not meet these methodological criteria, and were evaluated separately for sensitivity analysis.
The dropout rate was low: 2% for single‐dose studies and 1.3% for longer duration trials. All the studies evaluated were small cross‐over trials that received a B quality score on the Jadad scale, due to the fact that the randomization process was not described in detail or the trial was single‐blind instead of double‐blind.
Effects of interventions
Cardioselective blockers without intrinsic sympathomimetic activity (ISA) that were included in the study were atenolol, metoprolol, bisoprolol and practolol. Cardioselective blockers with ISA that were studied were celiprolol, acebutolol and xamoterol (Table 1).
Single‐dose treatment results
Nineteen studies on single‐dose treatment included 240 patients, 79% of whom were men. There was an average of 12.6 patients per study and the total dropout rate was 2.0%. From the available information the age range of participants was 19.5 to 65.1 years and the mean age was 40.1 years. These baseline characteristics were the same for the placebo and treatment groups because all of the trials were cross‐over by design. The baseline forced expiratory volume in one second (FEV1) measured in the treatment group was 2.41 (+/‐ 0.15) L/sec, and in the placebo group was 2.42 (+/‐ 0.2) L/sec.
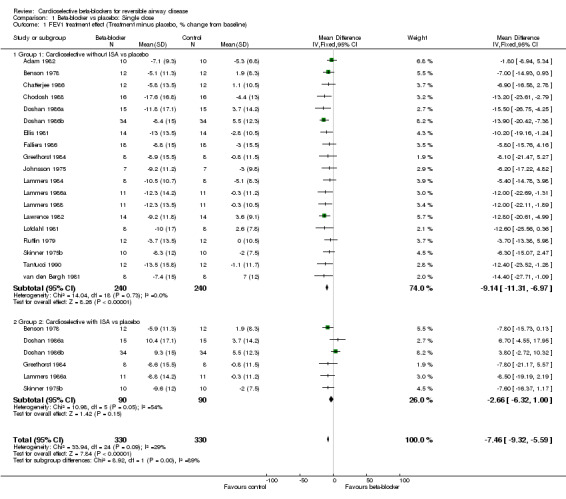
Compared to placebo, single doses of cardioselective beta‐blockers as a group were associated with a reduction in FEV1 (mean difference (MD) ‐7.46; 95% confidence interval (CI) ‐9.32 to ‐5.59), but with an increase in FEV1 after beta2‐agonist was given (MD 4.63; 95% CI 2.47 to 6.78). There was no increase in respiratory symptoms seen in any of the 19 studies (risk difference (RD) 0.01; 95% CI ‐0.02 to 0.03).
Continued treatment results
Data from 10 studies involving 141 participants (77% of whom were men) were evaluated for response to continued treatment ranging from three days to four weeks. There was an average of 15.4 patients in each study, with a 1.3% dropout rate. From the available information the average age of the participants was 51.3 years. The average baseline FEV1 for the treatment group was 1.81 (+/‐ 0.13) liters and for the placebo group was 1.81 (+/‐ 0.15) liters. Five of the studies (54 participants) did not provide data on FEV1 and were used for information on symptoms and inhaler use.
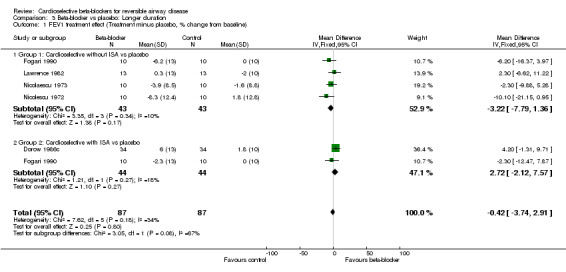
In the continued treatment trials the use of cardioselective beta‐blockers as a group was not significantly different from placebo in terms of FEV1 (MD ‐0.42; 95% CI ‐3.74 to 2.91), symptoms (RD 0.01; 95% CI ‐0.02 to 0.04) or inhaler use (MD ‐0.11; 95% CI ‐6.75 to 6.54), and produced an increase in FEV1 compared to placebo after beta2‐agonist was given (MD 8.74; 95% CI 1.96 to 15.52).
Subgroup analysis
For single‐dose trials, beta1‐blockers without ISA were associated with a 6.5% reduction in FEV1 compared to those with ISA (MD ‐9.14%; 95% CI ‐11.31 to ‐6.97) compared to (MD ‐2.66%; 95% CI ‐6.32 to 1.00). However, treatment with beta1‐blockers without ISA was associated with a 9.7% increase in FEV1 in response to beta2‐agonist compared to those with ISA (MD 6.59%; 95% CI 4.18 to 9.01) compared to (MD ‐3.10%; 95% CI ‐7.89 to 1.69). In the continued treatment trials there was no significant change in FEV1 compared to placebo for beta1‐blockers without ISA (MD ‐3.22%; 95% CI ‐7.79 to 1.36) or for those with ISA (MD 2.72%; 95% CI ‐2.12 to 7.57). However, those without ISA produced a 12% increase in FEV1 after beta2‐agonist administration compared to placebo (95% CI 4.12 to 19.87%) while those with ISA produced no increase in FEV1 (MD ‐0.60%; 95% CI ‐13.93 to 12.73). Whilst this difference appears large its is not statistically significant. In addition, it should be noted that these results were obtained in only a small number of studies with a small number of patients.
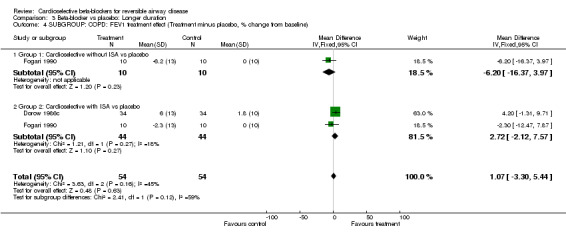
In order to evaluate the treatment effect in patients with concomitant chronic obstructive pulmonary disease (COPD), we analyzed separately 10 trials that included only patients with documented chronic airways obstruction (Johnsson 1975; Lofdahl 1981; Adam 1982; Butland 1983; Fenster 1983; Lammers 1985a; Chatterjee 1986; Dorow 1986c; Fogari 1990; Bauer 1994). When the analysis was limited to those with COPD there was no significant difference in results for FEV1 treatment effect, in single‐dose trials (MD ‐5.28%; 95% CI ‐10.03 to ‐0.54) or continued treatment (MD 1.07%; 95% CI ‐3.30 to 5.44) and there was no increase in symptoms in any of the trials.
In eight of the trials, all participants had a comorbid condition such as hypertension (Adam 1982; Lawrence 1982; Lammers 1985a; Chatterjee 1986; Dorow 1986c; van Zyl 1989; Fogari 1990; Bauer 1994). When only these trials were included in the analysis there was no significant change in results for FEV1 treatment effect in single‐dose trials (MD ‐6.83%; 95% CI ‐11.46 to ‐2.20) or continued treatment (MD 1.31; 95% CI ‐2.62 to 5.24).
Inter‐study variance
There was no significant inter‐study variance found in the measurements of the FEV1 treatment effect, symptoms and long‐term inhaler use. Heterogeneity was detected between studies in the measurements of FEV1 made after beta2‐agonist use, in both the single‐dose and continued‐treatment studies. This heterogeneity was noted only in the subgroup of beta‐blockers without ISA. When we compared the random‐effects model to the fixed‐effect model for the beta2‐agonist response in the subgroup of beta‐blockers without ISA, there was less than 1% difference for single‐dose studies (5.66%; 95% CI 1.81 to 9.51) compared to 6.59% (95% CI 4.18 to 9.01) and a 1.7% difference for continued treatment (10.32%; 95% CI ‐6.38 to 27.01) compared to 12.0% (95% CI 4.12 to 19.89).
Sensitivity analysis
We performed a sensitivity analysis to evaluate the effect of including studies that did not provide any information on standard deviations (SDs) (Ruffin 1979; Ellis 1981; Chatterjee 1986; Lammers 1988). When we excluded the trials that did not provide SD data from the analysis, there was less than 0.5% difference for all parameters measured. When we performed the analysis by replacing the pooled SD with the lowest and highest available SD, the difference in results between the highest and lowest SD was 2% or less.
We also performed sensitivity analysis to evaluate the effect of including trials that did not meet the inclusion criteria, but that provided information on FEV1 and symptoms for cardioselective beta1‐blocker use in patients with reversible airway disease. Data analysis of 10 excluded studies, with 141 participants, showed no significant difference in any parameters compared to studies that met inclusion criteria (Vilsvik 1976; Boye 1977; Beumer 1978; Mue 1979a; Abraham 1981; Larsson 1982; Schindl 1986; Blaive 1988; Pujet 1992; Cazola 2000).
Discussion
This meta‐analysis pooled the data from 29 randomized, placebo‐controlled trials of the use of cardioselective beta‐blockers in patients with reversible airway disease. The results showed that, compared to placebo, the first dose of active treatment produced a small drop in forced expiratory volume in one second (FEV1), which was not associated with adverse respiratory effects. After continued treatment for a few days to weeks there was no difference in FEV1, symptoms or incidence of inhaler use. Cardioselective beta‐blockers, given in a single dose or for continued treatment, were associated with an increase in response to beta2‐agonists, as compared to placebo. No individual trial demonstrated an increase in respiratory symptoms compared to placebo. In fact, a review of all the 80 trials on cardioselective beta‐blockers identified in the database search found no study that demonstrated an increase in respiratory symptoms for beta1‐blockers compared to placebo or baseline.
We performed subgroup analyses to evaluate the effect of cardioselective beta‐blockers on patients with concomitant chronic obstructive pulmonary disease (COPD) or cardiovascular diseases such as hypertension, as these patients are most often targeted for beta‐blocker treatment. There was no significant difference in the FEV1 treatment effect, number of patients with symptoms, or incidence of inhaler use in these patients.
This meta‐analysis has several limitations (Ioannidis 1999). Most of the participants were relatively young with mild to moderate airway obstruction, and those with recent asthma exacerbation were often excluded from study. In addition, many of the studies were of short duration, so it is not possible to comment on the effect of cardioselective beta‐blockers on the frequency or severity of acute asthma exacerbations after several months of treatment. Furthermore, this analysis was based only on published literature and therefore is subject to publication bias. However, funnel plots of effect size versus standard error for the trials in this analysis showed no evidence of bias. We believe that these pooled results provide valuable information on the safety of cardioselective beta‐blockers in patients with reversible airway disease, with or without concomitant COPD or cardiovascular disease.
In the past, the standard of care was to consider reversible airway disease to be a contraindication to the use of all beta‐blockers (Tattersfield 1986; Tattersfield 1990; O'Malley 1991; Belli 1995; Craig 1996; JNC VI 1997; Kendall 1997). Due to the proven mortality benefit of beta‐blockers, many of the other relative or absolute contraindications traditionally listed for beta‐blockers have been questioned and disproved, including impaired left ventricular function, peripheral vascular disease, diabetes mellitus, depression and advanced age (Kjekshus 1990; Opie 1990; Wicklmayr 1990; Radack 1991; Beto 1992; Bright 1992; Rosenson 1993; Jonas 1996; Gottlieb 1998; Lechat 1998; Krumholz 1999). Now, with the accumulated evidence of the safety of cardioselective beta‐blockers in reversible airway disease, the most recent expert panel guidelines from the National Asthma Education and Prevention Program state that these agents may be used in patients with asthma (Expert Panel Report 2007).
The original evidence of a potential adverse effect of beta‐blockers in reversible airway disease was based on case reports of acute bronchospasm precipitated by high doses of non‐cardioselective blockers, presumably due to their blockade of beta‐2 receptors on bronchial smooth muscle (McNeill 1964; Zaid 1966; Anderson 1979; Raine 1981). A search of the literature found 16 trials that met the inclusion criteria for this analysis but that evaluated non‐cardioselective beta‐blockers (Johnsson 1975; Skinner 1975a; Benson 1978; Ruffin 1979; Ellis 1981; Sue 1981; Adam 1982; George 1983; Light 1983; Falliers 1985; Lammers 1985b; Doshan 1986b; Giulekas 1986; Chodosh 1988; Fogari 1990; Devereux 1998). Pooled results showed that regular use of nonselective beta‐blockers, compared to placebo, caused a 13.5% reduction (95% confidence interval (CI) ‐23.0 to ‐4.0) in FEV1, and a 22.5% decrement (95% CI ‐32.5 to ‐12.5) in the FEV1 response after beta2‐agonists were given. No significant increases in symptoms or inhaler use were found. However, one cannot rule out the possibility that the decrement in beta2‐agonist response seen with nonselective beta‐blockers may increase the risk of a clinically significant adverse effect occurring during an asthma exacerbation.
Cardioselective beta‐blockers such as atenolol, bisoprolol and metoprolol are at least 20 times more potent at blocking beta‐1 receptors than beta‐2 receptors, so that at therapeutic doses the beta‐2 blocking effect is negligible (Wellstein 1987). The doses of beta1‐blockers evaluated in this analysis varied from therapeutic to supratherapeutic doses, those that are not generally used for initiation of treatment. For example, in single‐dose studies using 50 to 200 mg of atenolol or metoprolol, no clinically apparent effect on respiratory function was demonstrated. Linear regression analysis could not differentiate a treatment effect between low and high doses, as there were so few low‐dose trials.
The results of this meta‐analysis indicate that for cardioselective beta1‐blockers without intrinsic sympathomimetic activity (ISA), the minimal decrement in FEV1 noted on a single dose is attenuated over a few days to weeks. In addition, there is an increase in beta2‐agonist response that is maintained with continued treatment. These results could be explained by an up‐regulation or sensitization of beta‐2 receptors, accompanied by an increased effect of endogenous or exogenous beta2‐agonist stimulation (Hall 1983; Hall 1989; Motomura 1990). This paradoxical effect of upregulation on beta‐2‐agonist receptors may, in fact, be beneficial for patients with asthma (Dickey 2010; Page 2011). There has been recent interest in studying the use of beta‐blockers in the treatment of asthma (Bond 2007; Lipworth 2009).
There is now accumulating evidence that the use of inhaled beta2‐agonists in patients with reactive airway disease is associated with a tolerance to beta2‐agonist stimulation and a worsening of asthma control (Molinoff 1983; Kraan 1985; Borst 1990; Taylor 1992; Cockcroft 1993; Wahedna 1993; Salpeter 2004). Meta‐analyses of randomized, placebo‐controlled trials have shown that the regular use of beta‐agonists in asthma results in an increase in severe asthma exacerbations and asthma‐related deaths, compared with placebo (Salpeter 2006; Salpeter 2010).
There is also evidence that treatment with beta‐blockers that have intrinsic beta2 ISA is associated with down‐regulation of beta2 receptors (Busso 1985; Sbirrazzuoli 1989; Brodde 1990; Jakubetz 1999). This is consistent with data from this analysis that showed that continued treatment with beta1‐blockers with ISA did not produce the increase in beta2‐agonist response that was seen with beta1‐blockers without ISA.
Only a small fraction of patients with heart disease who would benefit from beta‐blockers are presently given this treatment, mainly due to unfounded fears of their adverse effects (Sial 1994; Soumerai 1997; Krumholz 1998; Wang 1998). A study by Gottlieb 1998 on survivors of myocardial infarction included 46,000 patients with asthma and chronic obstructive lung disease, and showed a significant reduction in total mortality for those treated with beta‐blockers compared to those who were not. Other studies that have investigated the use of beta1‐blockers in patients with cardiac disease and concomitant chronic obstructive lung disease or asthma found that these medicines were well tolerated (Quan 1983; Dorow 1986b; Mooss 1994). Trials that evaluated the use of beta1‐blockers in hypertensive patients, many of whom had reversible airway disease, did not demonstrate a worsening of respiratory symptoms or FEV1 in these patients (Formgren 1976; George 1983; Krauss 1984; Falliers 1985). A recent study by Heller 2000 showed that COPD and asthma were the co‐morbidities most commonly associated with beta‐blockers being withheld in elderly patients after a myocardial infarction.
It is generally considered that patients with COPD are at greater risk than those with reversible airway disease for developing ischemic heart disease and other cardiovascular conditions requiring the use of beta‐blockers. There is, however, significant overlap in the presenting features of COPD and reversible airway disease. Another recent meta‐analysis evaluated the effect of cardioselective beta‐blockers in patients with COPD and found no change in FEV1 or respiratory symptoms for single doses or continued use of these agents compared to placebo (Salpeter 2011). Subgroup analyses revealed no difference in results for those with concomitant reversible airway disease, or with severe chronic airways obstruction as demonstrated by a baseline FEV1 of less than 1.4 liters or less than 50% normal predicted values. Three of the trials from that meta‐analysis are also included in this analysis (Adam 1982; Fenster 1983; Fogari 1990). The cumulative evidence from these two meta‐analyses indicates that cardioselective beta‐blockers should not be withheld in patients with reactive airway disease or COPD.
Authors' conclusions
Implications for practice.
Beta‐blocker treatment reduces mortality in patients with cardiovascular disease. The available data suggest that there is a small reduction in forced expiratory volume in one second (FEV1) following a single dose of a cardioselective beta‐blocker, but the response to inhaled beta2‐agonists is preserved, without any adverse respiratory effects. Continued treatment with relatively high doses of cardioselective beta‐blockers without intrinsic sympathomimetic activity (ISA) produces no reduction in FEV1, while increasing the sensitivity to beta2‐agonist stimulation. It would be reasonable to start treatment with a low daily dose of a cardioselective beta‐blocker without ISA and then titrate the dose up as needed.
Implications for research.
From the accumulated evidence we have now, it is apparent that patients with reversible airway disease should not be excluded from future beta1‐blocker trials so that the treatment effect can be studied in this substantial population. It would also be helpful to conduct trials on the regulation of airway responsiveness in patients with reversible airway disease treated with cardioselective beta‐blockers, to understand the effects of these medications on respiratory function. Due to the paradoxical effect of upregulation of beta‐receptors, studies are now emerging to evaluate the potential beneficial effect of beta‐blockers in the treatment of asthma.
What's new
| Date | Event | Description |
|---|---|---|
| 9 June 2011 | New search has been performed | New literature search run. No new eligible studies identified. We made minor edits for style. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 22 July 2008 | Amended | Converted to new review format. |
| 3 July 2002 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We would like to acknowledge Steve Milan for his guidance with this review, Toby Lasserson for his technical assistance and Karen Blackhall for co‐ordinating the trials search. Thanks also to Kirsty Olsen who has copy edited this review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (T he Cochrane Library) | Quarterly (4 issues per year) |
| PsycINFO (Ovid) | Monthly |
| CINAHL (Ebsco) | Monthly |
| AMED (Ebsco) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Condition search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
17. exp Aspergillosis, Allergic Bronchopulmonary/
18. lung diseases, fungal/
19. aspergillosis/
20. 18 and 19
21. (bronchopulmonar$ adj3 aspergillosis).mp.
22. 17 or 20 or 21
23. 16 or 22
24. Lung Diseases, Obstructive/
25. exp Pulmonary Disease, Chronic Obstructive/
26. emphysema$.mp.
27. (chronic$ adj3 bronchiti$).mp.
28. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
29. COPD.mp.
30. COAD.mp.
31. COBD.mp.
32. AECB.mp.
33. or/24‐32
34. exp Bronchiectasis/
35. bronchiect$.mp.
36. bronchoect$.mp.
37. kartagener$.mp.
38. (ciliary adj3 dyskinesia).mp.
39. (bronchial$ adj3 dilat$).mp.
40. or/34‐39
41. exp Sleep Apnea Syndromes/
42. (sleep$ adj3 (apnea$ or apnoea$)).mp.
43. (hypopnea$ or hypopnoea$).mp.
44. OSA.mp.
45. SHS.mp.
46. OSAHS.mp.
47. or/41‐46
48. Lung Diseases, Interstitial/
49. Pulmonary Fibrosis/
50. Sarcoidosis, Pulmonary/
51. (interstitial$ adj3 (lung$ or disease$ or pneumon$)).mp.
52. ((pulmonary$ or lung$ or alveoli$) adj3 (fibros$ or fibrot$)).mp.
53. ((pulmonary$ or lung$) adj3 (sarcoid$ or granulom$)).mp.
54. or/48‐53
55. 23 or 33 or 40 or 47 or 54
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Data and analyses
Comparison 1. Beta‐blocker vs placebo: Single dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 treatment effect (Treatment minus placebo, % change from baseline) | 19 | 660 | Mean Difference (IV, Fixed, 95% CI) | ‐7.46 [‐9.32, ‐5.59] |
| 1.1 Group 1: Cardioselective without ISA vs placebo | 19 | 480 | Mean Difference (IV, Fixed, 95% CI) | ‐9.14 [‐11.31, ‐6.97] |
| 1.2 Group 2: Cardioselective with ISA vs placebo | 6 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐2.66 [‐6.32, 1.00] |
| 2 Patients with symptoms (Treatment minus placebo) | 19 | 845 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.03] |
| 2.1 Group 1: Cardioselective without ISA vs placebo | 18 | 584 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.03] |
| 2.2 Group 2: Cardioselective with ISA vs placebo | 7 | 261 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.03, 0.06] |
| 3 SUBGROUP: COPD: FEV1 treatment effect (Treatment minus placebo, % change from baseline) | 4 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐5.28 [‐10.03, ‐0.54] |
| 3.1 Group 1: Cardioselective without ISA vs placebo | 4 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐5.28 [‐10.03, ‐0.54] |
| 4 SUBGROUP: Cardiovascular disease: FEV1 treatment effect (Treatment minus placebo, % change from baseline) | 3 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐6.83 [‐11.46, ‐2.20] |
| 4.1 Group 1: Cardioselective without ISA vs placebo | 3 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐6.83 [‐11.46, ‐2.20] |
1.1. Analysis.
Comparison 1 Beta‐blocker vs placebo: Single dose, Outcome 1 FEV1 treatment effect (Treatment minus placebo, % change from baseline).
1.2. Analysis.

Comparison 1 Beta‐blocker vs placebo: Single dose, Outcome 2 Patients with symptoms (Treatment minus placebo).
1.3. Analysis.

Comparison 1 Beta‐blocker vs placebo: Single dose, Outcome 3 SUBGROUP: COPD: FEV1 treatment effect (Treatment minus placebo, % change from baseline).
1.4. Analysis.

Comparison 1 Beta‐blocker vs placebo: Single dose, Outcome 4 SUBGROUP: Cardiovascular disease: FEV1 treatment effect (Treatment minus placebo, % change from baseline).
Comparison 2. Beta‐blocker + agonist vs placebo + agonist: Single dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 treatment effect after Beta‐agonist (Treatment minus placebo, % change) | 15 | 444 | Mean Difference (IV, Fixed, 95% CI) | 4.63 [2.47, 6.78] |
| 1.1 Group 1: Cardioselective without ISA vs placebo | 15 | 332 | Mean Difference (IV, Fixed, 95% CI) | 6.59 [4.18, 9.01] |
| 1.2 Group 2: Cardioselective with ISA vs placebo | 5 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐7.89, 1.69] |
2.1. Analysis.

Comparison 2 Beta‐blocker + agonist vs placebo + agonist: Single dose, Outcome 1 FEV1 treatment effect after Beta‐agonist (Treatment minus placebo, % change).
Comparison 3. Beta‐blocker vs placebo: Longer duration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 treatment effect (Treatment minus placebo, % change from baseline) | 5 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐3.74, 2.91] |
| 1.1 Group 1: Cardioselective without ISA vs placebo | 4 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐3.22 [‐7.79, 1.36] |
| 1.2 Group 2: Cardioselective with ISA vs placebo | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [‐2.12, 7.57] |
| 2 Inhaler use per patient‐week (Treatment minus placebo) | 3 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐6.75, 6.54] |
| 2.1 Group 1: Cardioselective without ISA vs placebo | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐16.97, 16.97] |
| 2.2 Group 2: Cardioselective with ISA vs placebo | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐7.35, 7.10] |
| 3 Patients with symptoms (Treatment minus placebo) | 8 | 504 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 3.1 Group 1: Cardioselective without ISA vs placebo | 8 | 410 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 3.2 Group 2: Cardioselective with ISA vs placebo | 2 | 94 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 4 SUBGROUP: COPD: FEV1 treatment effect (Treatment minus placebo, % change from baseline) | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | 1.07 [‐3.30, 5.44] |
| 4.1 Group 1: Cardioselective without ISA vs placebo | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐6.2 [‐16.37, 3.97] |
| 4.2 Group 2: Cardioselective with ISA vs placebo | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [‐2.12, 7.57] |
| 5 SUBGROUP: Cardiovascular disease: FEV1 treatment effect (Treatment minus placebo, % change from baseline) | 3 | 134 | Mean Difference (IV, Fixed, 95% CI) | 1.31 [‐2.62, 5.24] |
| 5.1 Group 1: Cardioselective without ISA vs placebo | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐8.10, 5.31] |
| 5.2 Group 2: Cardioselective with ISA vs placebo | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [‐2.12, 7.57] |
3.1. Analysis.
Comparison 3 Beta‐blocker vs placebo: Longer duration, Outcome 1 FEV1 treatment effect (Treatment minus placebo, % change from baseline).
3.2. Analysis.

Comparison 3 Beta‐blocker vs placebo: Longer duration, Outcome 2 Inhaler use per patient‐week (Treatment minus placebo).
3.3. Analysis.

Comparison 3 Beta‐blocker vs placebo: Longer duration, Outcome 3 Patients with symptoms (Treatment minus placebo).
3.4. Analysis.
Comparison 3 Beta‐blocker vs placebo: Longer duration, Outcome 4 SUBGROUP: COPD: FEV1 treatment effect (Treatment minus placebo, % change from baseline).
3.5. Analysis.

Comparison 3 Beta‐blocker vs placebo: Longer duration, Outcome 5 SUBGROUP: Cardiovascular disease: FEV1 treatment effect (Treatment minus placebo, % change from baseline).
Comparison 4. Beta‐blocker + agonist vs placebo + agonist: Longer duration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 treatment effect after Beta‐agonist (Treatment minus placebo, % change) | 3 | 80 | Mean Difference (IV, Fixed, 95% CI) | 8.74 [1.96, 15.52] |
| 1.1 Group 1: Cardioselective without ISA vs placebo | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | 12.00 [4.12, 19.87] |
| 1.2 Group 2: Cardioselective with ISA vs placebo | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐13.93, 12.73] |
4.1. Analysis.

Comparison 4 Beta‐blocker + agonist vs placebo + agonist: Longer duration, Outcome 1 FEV1 treatment effect after Beta‐agonist (Treatment minus placebo, % change).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adam 1982.
| Methods | Single‐dose Double‐blind Cross‐over Dropout: 0% | |
| Participants | n = 10 Mean age = 65.1 Inclusion: hypertension and reversible chronic airway obstruction, with > 15% improvement FEV1 and FEV1/FVC < 70% Exclusions: none given | |
| Interventions | #1: Intervention: Metoprolol 100 mg Atenolol 100 mg Placebo #2: Inhaled salbutamol after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Nonselectives studied: labetalol, propranolol | |
Bauer 1994.
| Methods | Longer duration: 1 week treatment and placebo, cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 18 Mean age = 48.6 Inclusion: hypertension and stable asthma with > 15% improvement, FEV1 > 50% predicted Exclusion: angina, heart block, HR < 50, impaired renal or hepatic function, diabetes mellitus, pregnancy, lactation or adverse reaction to beta‐blocker | |
| Interventions | Metoprolol 14/190 mg Atenolol 100 mg Placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Benson 1978.
| Methods | Single‐dose Cross‐over Single‐blind Dropout prior to treatment: 14% Dropout with treatment: 0% | |
| Participants | n = 12 Mean age = 32.2 Inclusion: reversible airway obstruction, with spontaneous variability > 15%. All stable at time of study Exclusion: none given | |
| Interventions | #1: Acebutolol 300 mg Atenolol 100 mg Placebo #2: Inhaled isoprenaline after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Nonselectives studied: propranolol, pindolol | |
Butland 1983.
| Methods | Longer duration: 4 weeks treatment and placebo Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 12 Mean age = 61 Inclusion: reversible COPD: FEV1 < 1 L with > 20% reversal. Could continue taking inhalers, steroids. Exclusion: other lung disease or serious disease of other organs | |
| Interventions | Atenolol 100 mg/d Metoprolol 100 mg/d Placebo | |
| Outcomes | Symptoms Exercise tolerance | |
| Notes | FEV1 reported only as % of predicted normal value | |
Chatterjee 1986.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 12 Mean age = 60 Inclusion: hypertension and bronchial asthma, with FEV1/vital capacity < 30% and > 15% improvement with agonist Exclusion: pregnancy, overt heart failure, renal failure, heart block. Current antihypertensive and inhaler treatment prohibited. | |
| Interventions | #1: Atenolol 100 mg Bisoprolol 10 mg Bisoprolol 20 mg #2: Inhaled salbutamol after Tx and placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Chodosh 1988.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 11% prior to study beginning | |
| Participants | n = 16 Mean age = 39 Inclusion: normotensive patients with stable bronchial asthma, > 15% increase FEV1 and FEV1 60% to 90% predicted Exclusion: taking cromolyn, their steroid or inhaler use changed, recent asthma attack requiring Tx , upper respiratory infection within 2 weeks or recent status asthmaticus | |
| Interventions | #1: Metoprolol 200 mg Placebo #2: Inhaled isoproterenol after Tx and placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Nonselective studied: dilevalol | |
Dorow 1986c.
| Methods | Longer duration: 12 weeks treatment, 4 weeks placebo Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 34 Inclusion: hypertension + reversible airway obstruction, > 15% increase FEV1 Exclusion: severe hypertension | |
| Interventions | Celiprolol 100 to 600 mg/d Placebo | |
| Outcomes | FEV1 Symptoms Inhaler use/wk | |
| Notes | ||
Doshan 1986a.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 6% prior to study | |
| Participants | n = 15 Age: 19 to 55 Inclusion: mild asthma, otherwise good health, with >15% increase FEV1, and FEV1 > 50% predicted Exclusion: those that require asthma medications other than theophylline or inhalers | |
| Interventions | #1: Celiprolol 400 mg Celiprolol 600 mg Atenolol 100 mg Placebo #2: Inhaled albuterol after Tx and placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Doshan 1986b.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 34 Age: 18 to 57 Inclusion: normotensive + asthma, with > 15% increase and FEV1 > 50% predicted Exclusion: those requiring cromolyn or steroids | |
| Interventions | Celiprolol 200 mg Celiprolol 400 mg Atenolol 100 mg Placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Nonselective studied: propranolol | |
Ellis 1981.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 14 Inclusion: reversible airways obstruction, average increase FEV1 23% Exclusion: heart failure, heart block, irreversible airways obstruction | |
| Interventions | #1: Atenolol 50 mg Atenolol 100 mg Atenolol 200 mg Placebo #2: Inhaled isoprenaline after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Nonselective studied: propranolol | |
Falliers 1986.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n =18 Age: 21 to 60 yrs Inclusion: asthma, with < 80 predicted and >15% improvement FEV1 Exclusion: hypertension, hematological dz, CVA within 3 months, cardiac dysrhythmia, heart block, recent asthma attack or upper respiratory infection in past 2 weeks, or status asthmaticus, or if taking cromolyn | |
| Interventions | #1: Intervention: Metoprolol 100 mg Metoprolol 200 mg Placebo #2: Inhaled isoproterenol after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Nonselective studied: labetalol | |
Fenster 1983.
| Methods | Longer duration: 1 wk Tx, 1 wk placebo Cross‐over Single‐blind Dropout: 0% | |
| Participants | n = 6 Mean age = 48.6 Inclusion: reversible COPD: < 60% predicted FEV1 + > 15% reversal Exclusion: angina, MI, hypertension, valvular dz, heart failure, LVH or clinically unstable | |
| Interventions | Metoprolol 200/d Placebo | |
| Outcomes | Increase in symptoms | |
| Notes | FEV1 reported only as % of predicted | |
Fogari 1990.
| Methods | Longer duration: 1 week treatment, 2 weeks placebo Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 10 Mean age = 57 Inclusion: HTN + reversible airway dz, with > 20% increase FEV1 Exclusion: heart failure, A‐V block, valve dz, obstructive arteriopathy, renal insufficiency | |
| Interventions | #1: Intervention: Atenolol 100/d Celiprolol 200/d Placebo #2: Inhaled salbutamol after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Nonselectives studied: propranolol, oxprenolol | |
Greefhorst 1984.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 8 Mean age = 29 Inclusion: asthma with > 20% increase FEV1, all with intrinsic atopic asthma. All in stable respiratory state. Exclusion: cardiovascular dz | |
| Interventions | #1: Metoprolol 100 mg Acebutolol 400 mg Placebo #2: IV terbutaline after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Johnsson 1975.
| Methods | Single‐dose Cross‐over Single‐blind Dropout: 0% | |
| Participants | n = 7 Mean age = 44 Inclusion: endogenous asthma longer than 2 years, >15% reversibility Exclusion: no acute exacerbation at time of study, no history or signs of heart disease | |
| Interventions | Metoprolol 0.12 mg/kg IV Placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | 1) IV isoprenaline given, but baseline FEV1 not reported 2) Nonselective studied: propranolol | |
Lammers 1984.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 8 Mean age = 39 Inclusion: bronchial asthma, defined as paroxysmal reversible generalized airway obstruction, with FEV1 > 15 increase. Some with chronic bronchitis. Exclusion: no respiratory infection or increase in bronchoconstriction within 4 weeks of study | |
| Interventions | #1: Bisoprolol 10 mg Bisoprolol 20 mg Metoprolol 100 mg Placebo #2: Inhaled terbutaline after Tx and placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Lammers 1985a.
| Methods | Longer duration: 4 weeks treatment and placebo Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 8 Mean age = 52.7 Inclusion: COPD + hypertension Average reversal > 15% FEV1. All in stable respiratory state: no recent respiratory infection or event. Exclusion: none listed | |
| Interventions | Metoprolol 100 mg BID Placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Nonselective studied: pindolol | |
Lammers 1986a.
| Methods | Single‐dose Cross‐over Single + double‐blind Dropout: 0% | |
| Participants | n = 11 Mean age = 36.6 Inclusion: bronchial asthma as per ATS criteria, with > 15% increase. None with cardiovascular disease Exclusion: none listed | |
| Interventions | #1: Xamoterol 200 mg Atenolol 100 mg Placebo #2: Inhaled terbutaline after Tx and placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Single‐blind: placebo on first day Double‐blind: Tx drugs | |
Lammers 1988.
| Methods | Single‐dose Cross‐over Single‐blind Dropout: 0% | |
| Participants | n = 11 Age: 22 to 60 yrs Inclusion: asthma as defined by ATS, with > 15% increase FEV1. FEV1 ranged 40% to 74% predicted. All in stable phase without steroids or theophylline. Exclusion: none listed | |
| Interventions | #1: Atenolol 50 mg Placebo #2: Inhaled terbutaline after Tx and placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Lawrence 1982.
| Methods | Single‐dose + longer duration: 3 weeks Tx and placebo Cross‐over Single‐blinded Dropout: 0% | |
| Participants | n = 14 Mean age = +55.7 Inclusion: asthma + hypertension > 15% reversal Exclusion: pregnant, renal failure, heart failure, heart block | |
| Interventions | Acute: #1: Intervention: Atenolol 100 mg Metoprolol 100 mg Placebo #2 Intervention: Inhaled salbutamol after Tx or placebo Longer duration: Atenolol 100 mg/d Metoprolol 100 mg BID | |
| Outcomes | FEV1 Acute: symptoms Longer duration: Asthma attacks Inhaler use/wk | |
| Notes | ||
Lofdahl 1981.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 8 Mean age = 52 Inclusion: intrinsic asthma, constant reversibility > 20% None had an acute exacerbation at time of study Exclusion: none listed | |
| Interventions | #1: Atenolol 100 mg Metoprolol 100 mg Placebo #2: Terbutaline (IV then inhaled) after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Nicolaescu 1973.
| Methods | Longer duration: 3 days treatment and placebo Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 10 Mean age = 46.6 Inclusion: severe bronchial asthma for at least 5 yrs, average increase FEV1 > 15%. Steroids continued. Inhalers held prior to measurements. Exclusions: no asthma attack in past several days | |
| Interventions | #1: Intervention: Practolol 200 mg/d Placebo #2: Inhaled orciprenaline after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Nicolescu 1972.
| Methods | Longer duration: 3 days treatment and placebo Cross‐over Double‐blinded Dropout: 0% | |
| Participants | n = 10 Mean age = 43.7 Inclusion: mild bronchial asthma for at least 5 yrs, defined according to WHO, with average decrease in FEV1 to acetylcholine 26% Exclusion: none listed | |
| Interventions | #1: Practolol 50 mg QID Placebo #2: Inhaled orciprenaline after Tx and placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Ruffin 1979.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 12 Mean age = 30.8 Inclusion: asthma with episodic dyspnea + wheeze, > 20% drop in FEV1 with histamine Exclusion: FEV1 < 70% predicted at start of study or symptoms out of control | |
| Interventions | Metoprolol 100 mg Placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Nonselectives studied: propranolol, timolol | |
Skinner 1975b.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 10 Mean age = 36.8 Inclusion: asthma, with > 15% increase FEV1 Exclusion: none given | |
| Interventions | #1: Acebutolol 300 mg Practolol 300 mg Placebo #2: Inhaled isoprenaline after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
Tantucci 1990.
| Methods | Single‐dose Cross‐over Double‐blind Dropout: 0% | |
| Participants | n = 12 Mean age = 37.3 Inclusion: reversible airway dz, stable, with > 15% improvement FEV1 Exclusion: hx atopy, recent respiratory tract infection, contraindications to beta‐blocker, pregnancy | |
| Interventions | Metoprolol OROS 14/190 mg Atenolol 100 mg Placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | ||
van den Bergh 1981.
| Methods | Single‐dose Cross‐over Single + double‐blind Dropout: 0% | |
| Participants | n = 8 Mean age = 24 Inclusion: bronchial asthma, with > 20% increase FEV1. Mild to moderate in severity. All in stable respiratory state Exclusion: none listed | |
| Interventions | #1: Metoprolol OT 100 mg Metoprolol OT 200 mg Metoprolol SR 200 mg Placebo #2: IV terbutaline after Tx or placebo | |
| Outcomes | FEV1 Symptoms | |
| Notes | Single‐blind: metoprolol 100 + placebo Double‐blind: metoprolol 200 mg x 2 doses | |
van Zyl 1989.
| Methods | Longer duration: 4 weeks treatment, 2 weeks placebo Cross‐over Single/double‐blind Placebo‐controlled Dropout: 16% with placebo, 0% with treatment | |
| Participants | n = 12 Mean age = 45.9 Inclusion: asthma + hypertension Asthma: < 85% predicted FEV1, > 15% reversal Exclusion: none listed | |
| Interventions | Atenolol 100 mg/d Celiprolol 400 mg/d Placebo | |
| Outcomes | FEV1 Symptoms Inhaler use/wk | |
| Notes | Single‐blind: placebo run in and cross‐over Double‐blind: Tx drugs | |
ATS: American Thoracic Society BID: twice a day COPD: chronic obstructive pulmonary disease CVA: cerebrovascular accident d: day dz: disease FEV1: forced expiratory volume in one second FVC: forced vital capacity HR: heart rate HTN: hypertension hx: history IV: intravenous LVH: Left ventricular hypertrophy MI: myocardial infarction QID: four times a day (from the Latin quarter in die) Tx: treatment WHO: World Health Organization wk: week
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraham 1981 | Beta‐agonists not held: given 2 hrs before study Data analyzed separately in results |
| Astrom 1975 | No FEV1 data No asthma definition Not blinded |
| Bernecker 1970 | No FEV1 data No placebo |
| Beumer 1971 | Not blinded No placebo |
| Beumer 1978 | No FEV1 data (just % predicted) Data analyzed separately in results |
| Blaive 1988 | No placebo Data analyzed separately in results |
| Boskabady 2000 | Nonselective Not controlled |
| Boye 1977 | Beta‐agonists not held 8 hours Data analyzed separately in results |
| Bruschi 1988 | No FEV1 data: only in figure form |
| Cannon 1982 | Nonselective Asthma not defined, ? reversal Agonists not held No FEV1 data |
| Carpentiere 1988 | Nonselective Patients excluded for previous response to propranolol |
| Cazola 2000 | Baseline FEV1 data not given Beta‐agonist not held |
| Christensen 1978 | Nonselective Asthma not defined, ? reversal No FEV1 data |
| Clague 1984 | No FEV1 data |
| Coleman 1974 | Bronchial asthma: not defined |
| Connolly 1970 | Nonselective No placebo No FEV1 data |
| Decalmer 1978 | No FEV1 data: only in graph form |
| Devereux 1998 | Nonselective |
| Dorow 1982a | No FEV1 data |
| Dorow 1982b | No FEV1 data |
| Dorow 1986a | No FEV1 data: only graph form |
| Dorow 1986b | No FEV1 data |
| Falliers 1985 | Nonselective Inclusion based on prior response to propranolol Agonists not held 8 hours |
| Fleming 1978 | No FEV1 data: only in graph form |
| Formgren 1976 | No FEV1 data |
| Gayrard 1975 | Nonselective No FEV1 data |
| George 1983 | Nonselective No FEV1 data |
| George 1985 | Nonselective Inclusion based on prior response to propranolol |
| Giulekas 1986 | Nonselective |
| Greefhorst 1981 | Patients excluded on basis of prior bronchoconstriction to propranolol |
| Greefhorst 1983 | Nonselective |
| Groth 1986 | No FEV1 data: only in graph form |
| Hamm 1970 | Nonselective No FEV1 data |
| Hedner 1989 | Nonselective No FEV1 data |
| Hugues 1978 | Not blinded No placebo |
| Krauss 1984 | Not blinded |
| Lammers 1985b | FEV1 measured during exercise provocation |
| Lammers 1986b | Duplicate patients to Lammers 1984 |
| Larsson 1982 | Asthma: < 15% increase in FEV1 (average 11%) Data analyzed separately in the results |
| Leary 1973 | Not blinded |
| Light 1983 | Nonselective For single‐dose study, agonists not held |
| Lofdahl 1982 | Nonselective No FEV1 data |
| Lofdahl 1983b | No FEV1 data: graph form only |
| Lofdahl 1984 | No FEV1 data: only in figure form |
| Lofdahl 1988 | No FEV1 data: only in figure form |
| Macquin‐Mavier 1988 | No FEV1 data: specific airway conductance |
| Matthys 1985 | Patients included on basis of prior response to propranolol |
| Matthys 1986 | Patients included on basis or prior response to propranolol |
| Meier 1966 | Nonselective No FEV1 data |
| Mue 1979a | Asthma not defined ? reversal Data analyzed separately in the results |
| Mue 1979b | No FEV1 data: graph only Asthma with provocation test (? 15%) |
| Nair 1981 | No FEV1 data: graph only |
| Oosterhoff 1995 | Nonselective No FEV1 data |
| Palmer 1969 | Asthma not defined ? agonist held No FEV1 data |
| Patakas 1983 | Nonselective No FEV1 data |
| Philip‐Joet 1986 | No FEV1 data: only in figure form |
| Pujet 1992 | No placebo Data analyzed separately in results |
| Quan 1983 | Beta‐agonists not held Data studied separately in results |
| Ranchod 1982 | Chronic bronchitis: response to acetylcholine but ? > 15% No FEV1 data |
| Richardson 1969 | Nonselective No FEV1 data |
| Ruffin 1982 | No FEV1 data: only in figure form |
| Schindl 1986 | No asthma definition (? reversal) Data analyzed separately in the results |
| Sheppard 1986 | No FEV1 data: specific airway resistance |
| Singh 1976 | No FEV1 data: specific airway resistance |
| Skinner 1975a | Nonselective Asthma criteria not defined |
| Skinner 1976 | No FEV1 data No asthma definition |
| Sue 1981 | Nonselective |
| Sue 1983 | Nonselective No FEV1 data Only > 10% in FEV1 |
| Szmidt 1999 | No FEV1 data: only in figure form |
| Vilsvik 1976 | No placebo Data analyzed separately in results |
| Vilsvik 1977 | No FEV1 data |
| Von Graffenried 1978 | Nonselective No FEV1 data |
| Wilcox 1986 | No FEV1 data: only in figure form |
FEV1: forced expiratory volume in one second
Differences between protocol and review
We changed the methods used to obtain standard deviations in the updated version of the review, to get a better assessment of the net treatment effect in these cross‐over trials(see Dealing with missing data).
Contributions of authors
Shelley Salpeter: developed review protocol, search strategy, trial selection, data extraction, manuscript preparation, management of RevMan (RevMan 2011) protocol.
Thomas Ormistion: search strategy, trial selection, data extraction, manuscript preparation.
Edwin Salpeter: Prof. Salpeter died November 2008, prior to the preparation of the 2011 update. His contributions from 2001 to 2010 were: data analysis, statistical management, manuscript preparation.
Richard Wood‐Baker: editor.
Sources of support
Internal sources
Santa Clara Valley Medical Center, USA.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adam 1982 {published data only}
- Adam WR, Meagher EJ, Barter CE. Labetalol, beta blockers, and acute deterioration of chronic airway obstruction. Clinical & Experimental Hypertension ‐ Part A, Theory & Practice 1982;4(8):1419‐28. [DOI] [PubMed] [Google Scholar]
Bauer 1994 {published data only}
- Bauer K, Kaik G, Kaik B. Osmotic release oral drug delivery system of metoprolol in hypertensive asthmatic patients. Pharmacodynamic effects on beta 2‐adrenergic receptors. Hypertension 1994;24(3):339‐46. [DOI] [PubMed] [Google Scholar]
Benson 1978 {published data only}
- Benson MK, Berrill WT, Cruikshank JM, Sterling GS. A comparison of four beta‐adrenoceptor antagonists in patients with asthma. British Journal of Clinical Pharmacology 1978;5(5):415‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butland 1983 {published data only}
- Butland RJ, Pang JA, Geddes DM. Effect of beta‐adrenergic blockade on hyperventilation and exercise tolerance in emphysema. Journal of Applied Physiology: Respiratory, Environmental & Exercise Physiology 1983;54(5):1368‐73. [DOI] [PubMed] [Google Scholar]
Chatterjee 1986 {published data only}
- Chatterjee SS. The cardioselective and hypotensive effects of bisoprolol in hypertensive asthmatics. Journal of Cardiovascular Pharmacology 1986;8(Suppl 11):S74‐7. [DOI] [PubMed] [Google Scholar]
Chodosh 1988 {published data only}
- Chodosh S, Tuck J, Blasucci DJ. The effects of dilevalol, metoprolol, and placebo on ventilatory function in asthmatics. Journal of Cardiovascular Pharmacology 1988;11(Suppl 2):S18‐24. [DOI] [PubMed] [Google Scholar]
Dorow 1986c {published data only}
- Dorow P, Clauzel AM, Capone P, Mayol R, Mathieu M. A comparison of celiprolol and chlorthalidone in hypertensive patients with reversible bronchial obstruction. Journal of Cardiovascular Pharmacology 1986;8(Suppl 4):S102‐4. [DOI] [PubMed] [Google Scholar]
Doshan 1986a {published data only}
- Doshan HD, Brown R, Applin WJ, Kapoor M, Caruso FS. Effects of high doses of celiprolol in asthmatic patients. Journal of Cardiovascular Pharmacology 1986;8(Suppl 4):S109‐11. [DOI] [PubMed] [Google Scholar]
Doshan 1986b {published data only}
- Doshan HD, Rosenthal RR, Brown R, Slutsky A, Applin WJ, Caruso FS. Celiprolol, atenolol and propranolol: a comparison of pulmonary effects in asthmatic patients. Journal of Cardiovascular Pharmacology 1986;8(Suppl 4):S105‐8. [PubMed] [Google Scholar]
Ellis 1981 {published data only}
- Ellis ME, Sahay JN, Chatterjee SS, Cruickshank JM, Ellis SH. Cardioselectivity of atenolol in asthmatic patients. European Journal of Clinical Pharmacology 1981;21(3):173‐6. [DOI] [PubMed] [Google Scholar]
Falliers 1986 {published data only}
- Falliers CJ, Vincent ME, Medakovic M. Effect of single doses of labetalol, metoprolol, and placebo on ventilatory function in patients with bronchial asthma: interaction with isoproterenol. Journal of Asthma 1986;23(5):251‐60. [DOI] [PubMed] [Google Scholar]
Fenster 1983 {published data only}
- Fenster PE, Hasan FM, Abraham T, Woolfenden J. Effect of metoprolol on cardiac and pulmonary function in chronic obstructive pulmonary disease. Clinical Cardiology 1983;6(3):125‐9. [DOI] [PubMed] [Google Scholar]
Fogari 1990 {published data only}
- Fogari R, Zoppi A, Tettamanti F, Poletti L, Rizzardi G, Fiocchi G. Comparative effects of celiprolol, propranolol, oxprenolol, and atenolol on respiratory function in hypertensive patients with chronic obstructive lung disease. Cardiovascular Drugs and Therapy 1990;4(4):1145‐9. [DOI] [PubMed] [Google Scholar]
Greefhorst 1984 {published data only}
- Greefhorst AP, Herwaarden CL. Ventilatory and haemodynamic effects of beta 1‐selective and beta 2‐ selective stimulation in asthmatic patients. European Journal of Respiratory Diseases Supplement 1984;135:147‐50. [PubMed] [Google Scholar]
Johnsson 1975 {published data only}
- Johnsson G, Svedmyr N, Thiringer G. Effects of intravenous propranolol and metoprolol and their interaction with isoprenaline on pulmonary function, heart rate and blood pressure in asthmatics. European Journal of Clinical Pharmacology 1975;8(3‐4):175‐80. [DOI] [PubMed] [Google Scholar]
Lammers 1984 {published data only}
- Lammers JW, Folgering HT, Herwaarden CL. Ventilatory effects of beta 1‐receptor‐selective blockade with bisoprolol and metoprolol in asthmatic patients. European Journal of Clinical Pharmacology 1984;27(2):141‐5. [DOI] [PubMed] [Google Scholar]
Lammers 1985a {published data only}
- Lammers JW, Folgering HT, Herwaarden CL. Ventilatory effects of atenolol and bevantolol in asthma. Clinical Pharmacology & Therapeutics 1985;38(4):428‐33. [DOI] [PubMed] [Google Scholar]
Lammers 1986a {published data only}
- Lammers JW, Muller ME, Folgering HT, Herwaarden CL. A comparative study on the ventilatory and haemodynamic effects of xamoterol and atenolol in asthmatic patients. British Journal of Clinical Pharmacology 1986;22(5):595‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lammers 1988 {published data only}
- Lammers JW, Muller ME, Folgering HT, Herwaarden CL. Effects of terbutaline and atenolol on large and small airways in asthmatic patients. European Respiratory Journal 1988;1(5):453‐7. [PubMed] [Google Scholar]
Lawrence 1982 {published data only}
- Lawrence DS, Sahay JN, Chatterjee SS, Cruickshank JM. Asthma and beta‐blockers. European Journal of Clinical Pharmacology 1982;22(6):501‐9. [DOI] [PubMed] [Google Scholar]
Lofdahl 1981 {published data only}
- Lofdahl CG, Svedmyr N. Cardioselectivity of atenolol and metoprolol. A study in asthmatic patients. European Journal of Respiratory Diseases 1981;62(6):396‐404. [PubMed] [Google Scholar]
Nicolaescu 1973 {published data only}
- Nicolaescu V, Racoveanu C, Manicatide M. Effects of exercise on practolol‐treated asthmatic patients. European Journal of Clinical Pharmacology 1973;6(1):3‐8. [DOI] [PubMed] [Google Scholar]
Nicolescu 1972 {published data only}
- Nicolaescu V, Manicatide M, Stroescu V. Beta‐adrenergic blockade with practolol in acetylcholine‐sensitive asthma patients. Respiration 1972;29(2):139‐54. [DOI] [PubMed] [Google Scholar]
Ruffin 1979 {published data only}
- Ruffin RE, Frith PA, Anderton RC, Kumana CR, Newhouse MT, Hargreave FE. Selectivity of beta adrenoreceptor antagonist drugs assessed by histamine bronchial provocation. Clinical Pharmacology & Therapeutics 1979;25(5 Pt 1):536‐40. [DOI] [PubMed] [Google Scholar]
Skinner 1975b {published data only}
- Skinner C, Palmer KNV, Kerridge DF. Comparison of the effects of acebutolol (Sectral) and practolol (Eraldin) on airways obstruction in asthmatics. British Journal of Clinical Pharmacology 1975;2(5):417‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tantucci 1990 {published data only}
- Tantucci C, Bruni B, Dottorini ML, Peccini F, Motolese M, Lecaillon JB, et al. Comparative evaluation of cardioselectivity of metoprolol OROS and atenolol: a double‐blind, placebo‐controlled crossover study. American Heart Journal 1990;120(2):467‐72. [DOI] [PubMed] [Google Scholar]
van den Bergh 1981 {published data only}
- Bergh JH, Herwaarden CL. Ventilatory effects of ordinary and slow‐release tablets of metoprolol in asthmatic patients. European Journal of Respiratory Diseases 1981;62(3):168‐72. [PubMed] [Google Scholar]
van Zyl 1989 {published data only}
- Zyl AI, Jennings AA, Bateman ED, Opie LH. Comparison of respiratory effects of two cardioselective beta‐blockers, celiprolol and atenolol, in asthmatics with mild to moderate hypertension. Chest 1989;95(1):209‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abraham 1981 {published data only}
- Abraham TA, Hasan FM, Fenster PE, Marcus FI. Effect of intravenous metoprolol on reversible obstructive airways disease. Clinical Pharmacology & Therapeutics 1981;29(5):582‐7. [DOI] [PubMed] [Google Scholar]
Astrom 1975 {published data only}
- Astrom H. Comparison of the effects on airway conductance of a new selective beta‐adrenergic blocking drug, atenolol, and propranolol in asthmatic subjects. Scandinavian Journal of Respiratory Diseases 1975;56(6):292‐6. [PubMed] [Google Scholar]
Bernecker 1970 {published data only}
- Bernecker C, Roetscher I. The beta‐blocking effect of practolol in asthmatics. Lancet 1970;Sept 26:662. [DOI] [PubMed] [Google Scholar]
Beumer 1971 {published data only}
- Beumer HM, Hardonk HJ. Effect of beta‐adrenergic blockaders in bronchial asthma (in German) [Zur wirkung beta‐adrenerg blockierender substanzen bei asthma bronchiale]. Medizinische Klinik 1971;66(52):1804‐7. [PubMed] [Google Scholar]
Beumer 1978 {published data only}
- Beumer HM, Teirlinck C, Wiseman RA. Comparative investigation of the respiratory and cardiovascular effect of mepindolol, propranolol and pindolol in asthmatic patients. International Journal of Clinical Pharmacology Research and Biopharmacy 1978;16(6):249‐53. [PubMed] [Google Scholar]
Blaive 1988 {published data only}
- Blaive B, Nahon F, Lemoigne F, Lewest G. Les antagonistes beta‐adrenergiques au long cours dans les bronchopathies spastiques. Allergie et Immunologie 1988;20(4):161‐5. [PubMed] [Google Scholar]
Boskabady 2000 {published data only}
- Boskabady MH, Snashall PD. Bronchial responsiveness to beta‐adrenergic stimulation and enhanced beta‐blockade in asthma. Respirology 2000;5(2):111‐8. [DOI] [PubMed] [Google Scholar]
Boye 1977 {published data only}
- Boye NP, Vale JR. Effect in bronchial asthma of a new beta‐adrenergic blocking drug atenolol (ICI 66, 082). European Journal of Clinical Pharmacology 1977;11(1):11‐4. [DOI] [PubMed] [Google Scholar]
Bruschi 1988 {published data only}
- Bruschi C, Casali L, Cerveri I, Peona V, Zoia M. Effects of celiprolol on the bronchial reactivity in asthma. American Journal of Cardiology 1988;61:53C‐4C. [DOI] [PubMed] [Google Scholar]
Cannon 1982 {published data only}
- Cannon RE, Slavin RG, Gonasun LM. The effect on asthma of a new beta blocker, pindolol. American Heart Journal 1982;104:438‐42. [DOI] [PubMed] [Google Scholar]
Carpentiere 1988 {published data only}
- Carpentiere G, Castello F, Marino S. Increased responsiveness to histamine after propranolol in subjects with asthma nonresponsive to the bronchoconstrictive effect of propranolol. Journal of Allergy & Clinical Immunology 1988;82:595‐8. [DOI] [PubMed] [Google Scholar]
Cazola 2000 {published data only}
- Cazola M, Noschese P, D'Amato M, D'Amato G. Comparison of the effects of single oral doses of nebivolol and celiprolol on airways in patients with mild asthma. Chest 2000;118:1322‐6. [DOI] [PubMed] [Google Scholar]
Christensen 1978 {published data only}
- Christensen CC, Boye NP, Erikson H, Hansen G. Influence of pindolol (Visken) on respiratory function in 20 asthmatic patients. European Journal of Clinical Pharmacology 1978;13:9‐12. [DOI] [PubMed] [Google Scholar]
Clague 1984 {published data only}
- Clague HW, Ahmad D, Carruthers SG. Influence of cardioselectivity and respiratory disease on pulmonary responsiveness to beta‐blockade. European Journal of Clinical Pharmacology 1984;27(5):517‐23. [DOI] [PubMed] [Google Scholar]
Coleman 1974 {published data only}
- Coleman AJ, Leary WP, Asmal AC. Isopropterenol blockade in man; comparison between penbutalol and acebutolol. Current Therapeutic Research 1974;16:64‐79. [PubMed] [Google Scholar]
Connolly 1970 {published data only}
- Connolly CK, Batten JC. Comparison of the effect of alprenolol and propranolol on specific airway conductance in asthmatic subjects. British Medical Journal 1970;2(708):515‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Decalmer 1978 {published data only}
- Decalmer P, Chatterjee S, Cruickshank J, Benson M, Sterling G. Beta‐blockers and asthma. British Heart Journal 1978;40:184‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Devereux 1998 {published data only}
- Devereux G, Fishwick K, Aiken TC, Bourke SJ, Hendrick DJ. Adverse effects of a single dose of (+)‐sotalol in patients with mild stable asthma. British Journal of Clinical Pharmacology 1998;46(1):79‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorow 1982a {published data only}
- Dorow P. The role of alpha‐receptors in bronchoconstriction induced by beta‐blockade. Respiration 1982;43:359‐62. [DOI] [PubMed] [Google Scholar]
Dorow 1982b {published data only}
- Dorow P. Influence of intrinsic sympathomimetic activity (ISA) during beta‐adrenoceptor blockade in asthmatics. British Journal of Clinical Pharmacology 1982;13:321S‐2S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorow 1986a {published data only}
- Dorow P, Schiess W. Bopindolol, pindolol, and atenolol in patients with chronic obstructive lung disease. Klinische Wochenschrift 1986;64(8):366‐9. [DOI] [PubMed] [Google Scholar]
- Schiess W, Dorow P. Bopindolol, pindolol, and atenolol in patients with chronic obstructive lung disease. Journal of Cardiovascular Pharmacology 1986;8(Suppl 6):S30‐3. [DOI] [PubMed] [Google Scholar]
Dorow 1986b {published data only}
- Dorow P, Bethge H, Tonnesmann U. Effects of single oral doses of bisoprolol and atenolol on airway function in nonasthmatic chronic obstructive lung disease and angina pectoris. European Journal of Clinical Pharmacology 1986;31(2):143‐7. [DOI] [PubMed] [Google Scholar]
Falliers 1985 {published data only}
- Falliers CJ, Vrchota J, Blasucci DJ, Maloy JW, Medakovic M. The effects of treatments with labetalol and hydrochlorothiazide on ventilatory function of asthmatic hypertensive patients with demonstrated bronchosensitivity to propranolol. Journal of Clinical Hypertension 1985;1(1):70‐9. [PubMed] [Google Scholar]
Fleming 1978 {published data only}
- Fleming GM, Chester EH, Schwartz HJ, Jones PK. Beta‐adrenergic blockade of the lung. Chest 1978;73:807‐12. [DOI] [PubMed] [Google Scholar]
Formgren 1976 {published data only}
- Formgren H. The effect of metoprolol and practolol on lung function and blood pressure in hypertensive asthmatics. British Journal of Clinical Pharmacology 1976;3:1007‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gayrard 1975 {published data only}
- Gayrard P, Orehek J, Grimaud C, Charpin J. Beta‐adrenergic function in airways of health and asthmatic subjects. Thorax 1975;30:657‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
George 1983 {published data only}
- George RB, Manocha K, Burford JG, Conrad SA, Kinasewitz GT. Effects of labetalol in hypertensive patients with chronic obstructive pulmonary disease. Chest 1983;83(3):457‐60. [DOI] [PubMed] [Google Scholar]
George 1985 {published data only}
- George RB, Light RW, Hudson LD, Conrad SA, Chetty K, Manocha K, et al. Comparison of the effects of labetalol and hydrochlorothiazide on the ventilatory function of hypertensive patients with asthma and propranolol sensitivity. Chest 1985;88(6):815‐8. [DOI] [PubMed] [Google Scholar]
Giulekas 1986 {published data only}
- Giulekas D, Georgopoulos D, Papakosta D, Vanvalis C. Influence of pindolol on asthmatics and effects of bronchodilators. Respiration 1986;50(3):158‐66. [DOI] [PubMed] [Google Scholar]
Greefhorst 1981 {published data only}
- Greefhorst AP, Herwaarden CL. Comparative study of the ventilatory effects of three beta 1‐selective blocking agents in asthmatic patients. European Journal of Clinical Pharmacology 1981;20(6):417‐21. [DOI] [PubMed] [Google Scholar]
Greefhorst 1983 {published data only}
- Greefhorst APM, Herwaarden CLA. Ventilatory and haemodynamic effects of prenalterol and terbutaline in asthmatic patients. European Journal of Clinical Pharmacology 1983;24:173‐8. [DOI] [PubMed] [Google Scholar]
Groth 1986 {published data only}
- Groth S, Tonnesen P, Asted M, Dirksen H, Sorensen PG. Assessment of the relative safety of the beta‐blockers ICI 141,292 and atenolol in patients with bronchial asthma [published erratum appears in Eur J Clin Pharmacol 1987; 31(6):744]. European Journal of Clinical Pharmacology 1986;30(6):653‐8. [DOI] [PubMed] [Google Scholar]
Hamm 1970 {published data only}
- Hamm J, Hunekohl E, Schmidt A. The effect of an adrenergic beta receptor blockader (Ko 592) on respiration (in German) [Wirkungen eines adrenergischen Beta‐Receptorenblockers (Ko592) auf die Atmung]. Klinische Wochenschrift 1970;48(8):457‐64. [DOI] [PubMed] [Google Scholar]
Hedner 1989 {published data only}
- Hedner J, Ullman A, Lemne C, Svedmyr N. Effects of dilevalol, a beta‐adrenoceptor antagonist with intrinsic sympathetic activity in asthmatic patients. Pulmonary Pharmacology 1989;2(3):155‐9. [DOI] [PubMed] [Google Scholar]
Hugues 1978 {published data only}
- Hugues FC, Julien D, Munera Y, Marche J. Study of the respiratory tolerance of adrenolytic beta‐blockers in the asthmatic (in French) [Etude de la tolerance respiratoire des adrenolytiques beta‐bloquers chez l'asthmatique]. La Nouvelle Presse Medicale 1978;7(31):2711‐5. [PubMed] [Google Scholar]
Krauss 1984 {published data only}
- Krauss S, Spitz E, Krauss A, Grizotzki B, Clement S. Treatment of hypertension in mild asthmatic patients with atenolol. Angiology 1984;Dec:773‐8. [DOI] [PubMed] [Google Scholar]
Lammers 1985b {published data only}
- Lammers JW, Folgering HT, Herwaarden CL. Ventilatory effects of atenolol and bevantolol in asthma. Clinical Pharmacology & Therapeutics 1985;38(4):428‐33. [DOI] [PubMed] [Google Scholar]
Lammers 1986b {published data only}
- Lammers JW, Folgering HT, Herwaarden CL. Respiratory tolerance of bisoprolol and metoprolol in asthmatic patients. Journal of Cardiovascular Pharmacology 1986;8(Suppl 11):S69‐73. [DOI] [PubMed] [Google Scholar]
Larsson 1982 {published data only}
- Larsson K. Influence of labetalol, propranolol and practolol in patients with asthma. European Journal of Respiratory Diseases 1982;63(3):221‐30. [PubMed] [Google Scholar]
Leary 1973 {published data only}
- Leary WP. Respiratory effects of acebutolol hydrochloride: a new selective beta‐adrenergic blocking agent. South African Medical Journal 1973;July:1245‐8. [PubMed] [Google Scholar]
Light 1983 {published data only}
- Light RW, Chetty KG, Stansbury DW. Comparison of the effects of labetalol and hydrochlorothiazide on the ventilatory function of hypertensive patients with mild chronic obstructive pulmonary disease. American Journal of Medicine 1983;75(4A):109‐14. [DOI] [PubMed] [Google Scholar]
Lofdahl 1982 {published data only}
- Lofdahl CG. Selectivity of beta‐adrenoceptor agonists and antagonists in asthmatics. European Journal of Respiratory Diseases Supplement 1982;120:1‐53. [PubMed] [Google Scholar]
Lofdahl 1983b {published data only}
- Lofdahl CG, Marlin GE, Svedmyr N. Pafenolol, a highly selective beta 1‐adrenoceptor‐antagonist, in asthmatic patients: interaction with terbutaline. Clinical Pharmacology & Therapeutics 1983;33(1):1‐9. [DOI] [PubMed] [Google Scholar]
Lofdahl 1984 {published data only}
- Lofdahl CG, Marlin GE, Svedmyr N. Effects of beta 1‐adrenoceptor stimulation and blockade studied in asthmatic airways. European Journal of Respiratory Diseases Supplement 1984;135:151‐2. [PubMed] [Google Scholar]
Lofdahl 1988 {published data only}
- Lofdahl CG, Dahlof C, Westergren G, Olofsson B, Svedmyr N. Controlled‐release metoprolol compared with atenolol in asthmatic patients: interaction with terbutaline. European Journal of Clinical Pharmacology 1988;33(Suppl):S25‐32. [DOI] [PubMed] [Google Scholar]
Macquin‐Mavier 1988 {published data only}
- Macquin‐Mavier I, Roudot‐Thoraval F, Clerici C, George C, Harf A. Comparative effects of bisoprolol and acebutolol in smokers with airway obstruction. British Journal of Clinical Pharmacology 1988;26(3):279‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthys 1985 {published data only}
- Matthys H, Doshan HD, Ruhle KH, Braig H, Pohl M, Applin WJ, et al. The bronchosparing effect of celiprolol, a new beta1‐alpha2‐receptor antagonist on pulmonary function of propranolol‐sensitive asthmatics. Journal of Clinical Pharmacology 1985;25:354‐9. [DOI] [PubMed] [Google Scholar]
Matthys 1986 {published data only}
- Matthys H, Doshan HD, Ruhle KH, Applin WJ, Braig H, Pohl M. Bronchosparing properties of celiprolol, a new beta1‐alpha2‐blocker, in propranolol‐sensitive asthmatic patients. Journal of Cardiovascular Pharmacology 1986;8 (Suppl 4):S40‐2. [PubMed] [Google Scholar]
Meier 1966 {published data only}
- Meier J, Lydtin H, Zollner N. The action of adrenergic beta‐receptor blockers on ventilatory functions in obstructive lung diseases. German Medical Monthly 1966;11(1):1‐3. [PubMed] [Google Scholar]
Mue 1979a {published data only}
- Mue S, Sasaki T, Shibahara S, Takahashi M, Ohmi T, Yamauchi K, et al. Influence of metoprolol on hemodynamics and respiratory function in asthmatic patients. International Journal of Clinical Pharmacology and Biopharmacy 1979;17(8):346‐50. [PubMed] [Google Scholar]
Mue 1979b {published data only}
- Mue S, Sasaki T, Ohmi T, Hida W, Suzuki S, Takahashi M, et al. Influence of acebutolol on the haemodynamic and respiratory function of asthmatic patients. Journal of Clinical Pharmacology 1979;2:67‐71. [PubMed] [Google Scholar]
Nair 1981 {published data only}
- Nair S, Maguire WC, Laddu AR. The effect of acebutolol, a beta adrenergic blocking agent, and placebo on pulmonary functions in asthmatics. International Journal of Clinical Pharmacology, Therapy, & Toxicology 1981;19(12):519‐26. [Google Scholar]
Oosterhoff 1995 {published data only}
- Oosterhoff Y, Koeter GH, Postma DS. Effects of propranolol inhalation on the diurnal increase in FEV1 and on propranolol airways responsiveness in atopic subjects with asthma. Thorax 1995;50:937‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 1969 {published data only}
- Palmer KN, Hamilton WF, Lge JS, Diament ML. Effect of a selective beta‐adrenergic blocker in preventing falls in arterial oxygen tension following isoprenaline in asthmatic subjects. Lancet 1969;2(7630):1092‐4. [DOI] [PubMed] [Google Scholar]
Patakas 1983 {published data only}
- Patakas D, Argiropoulou V, Louridas G, Tsara V. Beta‐blockers in bronchial asthma: effect of propranolol and pindolol on large and small airways. Thorax 1983;38:108‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philip‐Joet 1986 {published data only}
- Philip‐Joet F, Saadjian A, Bruguerolle B, Arnaud A. Comparative study of the respiratory effects of two beta 1‐selective blocking agents atenolol and bevantolol in asthmatic patients. European Journal of Clinical Pharmacology 1986;30(1):13‐6. [DOI] [PubMed] [Google Scholar]
Pujet 1992 {published data only}
- Pujet JC, Debreuil C, Fleury B, Provendier O, Abelia ML. Effects of celiprolol, a cardioselective beta‐blocker, on respiratory function in asthmatic patients. European Respiratory Journal 1992;5:196‐200. [PubMed] [Google Scholar]
Quan 1983 {published data only}
- Quan SF, Fenster PE, Hanson CD, Coaker LA, Basista MP. Suppression of atrial ectopy with intravenous metoprolol in chronic obstructive pulmonary disease patients. Journal of Clinical Pharmacology 1983;23(8‐9):341‐7. [DOI] [PubMed] [Google Scholar]
Ranchod 1982 {published data only}
- Ranchod A, Keeton GR, Benatar SR. The effect of beta‐blockers on ventilatory function in chronic bronchitis. South African Medical Journal 1982;61(12):423‐4. [PubMed] [Google Scholar]
Richardson 1969 {published data only}
- Richardson PS, Sterling GM. Effects of beta‐adrenergic receptor blockade on airway conductance and lung volume in normal and asthmatic subjects. British Medical Journal 1969;3:143‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruffin 1982 {published data only}
- Ruffin RE, McIntyre EL, Latimer KM, Ward HE, Crockett AJ, Alpers JH. Assessment of beta‐adrenoceptor antagonists in asthmatic patients. British Journal of Clinical Pharmacology 1982;13(Suppl):325S‐35S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schindl 1986 {published data only}
- Schindl R. The effect of the cardio‐selective beta blocker celiprolol on pulmonary function in asthmatic patients. Journal of Cardiovascular Pharmacology 1986;8(Supp 4):S99‐101. [DOI] [PubMed] [Google Scholar]
Sheppard 1986 {published data only}
- Shepard D, DiStefano S, Byrd RC, Eschenbacher WL, Bell V, Steck J, et al. Effects of esmolol on airway function in patients with asthma. Journal of Clinical Pharmacology 1986;26:169‐74. [DOI] [PubMed] [Google Scholar]
Singh 1976 {published data only}
- Singh B, Whitlock R, Comber R, Williams F, Harris E. Effects of cardioselective beta adrenoceptor blockade on specific airways resistance in normal subjects and in patients with bronchial asthma. Clinical Pharmacology & Therapeutics 1976;19(5):493‐501. [DOI] [PubMed] [Google Scholar]
Skinner 1975a {published data only}
- Skinner C, Gaddie J, Palmer KNV. Comparison of intravenous AH 5158 (ibidomide) and propranolol in asthma. British Medical Journal 1975;2(5962):59‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 1976 {published data only}
- Skinner C, Gaddie J, Palmer KNV. Comparison of effects of metoprolol and propranolol on asthmatic airway obstruction. British Medical Journal 1976;6008(1):504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sue 1981 {published data only}
- Sue DY, Hansen J, Wasserman K. Beta‐adrenergic blockade with pindolol (LB‐46) in mild to moderate asthma. Chest 1981;80(5):537‐42. [DOI] [PubMed] [Google Scholar]
Sue 1983 {published data only}
- Sue DY, Meter R, Hansen JE, Wasserman K. Exercise gas exchange in asthmatics after beta‐adrenergic blockade. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 1983;55(2):529‐33. [DOI] [PubMed] [Google Scholar]
Szmidt 1999 {published data only}
- Szmidt M, Minc P, Wasiak W. Comparison of the influence of celiprolol, metoprolol and atenolol on ventilation in asthmatic patients [Polish]. Pneumonolgia i Alergologia Polska 1999;67(9‐10):452‐61. [PubMed] [Google Scholar]
Vilsvik 1976 {published data only}
- Vilsvik JS, Schaanning J. Effect of atenolol on ventilatory and cardiac function in asthma. British Medical Journal 1976;2(6033):453‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vilsvik 1977 {published data only}
- Vilsvik JS, Schaanning J. Effect of atenolol on cardiac and ventilatory function in patients with chronic asthma. Postgraduate Medical Journal 1977;53(Suppl 3):142. [PubMed] [Google Scholar]
Von Graffenried 1978 {published data only}
- Graffenried B. Competitive effects of pindolol and salbutamol on airway resistance in asthmatics (in French) [Effets competitifs du pindolol et du salbutamol sur la resistance des voies aeriennes chez les asthmatiques]. La Nouvelle Presse Medicale 1978;7(31):2717‐20. [PubMed] [Google Scholar]
Wilcox 1986 {published data only}
- Wilcox PG, Ahmad D, Darke AC, Parsons J, Carruthers SG. Respiratory and cardiac effects of metoprolol and bevantolol in patients with asthma. Clinical Pharmacology & Therapeutics 1986;39(1):29‐34. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 1979
- Anderson EG, Calcraft B, Jariwalla AG, Al‐Zaibak M. Persistent asthma after treatment with beta‐blocking agents. British Journal of Diseases of the Chest 1979;73(4):407‐8. [PubMed] [Google Scholar]
ATS 1993
- American Thoracic Society. American Thoracic Society: Guidelines for the evaluation of impairment/disability in patients with asthma. American Review of Respiratory Disease 1993;147:1056‐61. [DOI] [PubMed] [Google Scholar]
ATS 1995
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1995;152(5 Pt 2):S77‐121. [PubMed] [Google Scholar]
Belli 1995
- Belli G, Topol EJ. Adjunctive pharmacologic strategies for acute MI. Contemporary Internal Medicine 1995;7(8):51‐9. [PubMed] [Google Scholar]
Berlin 1989
- Berlin JA, Laird NM, Sacks HS, Chalmers TC. A comparison of statistical methods for combining event rates from clinical trials. Statistics in Medicine 1989;8:141‐51. [DOI] [PubMed] [Google Scholar]
Beto 1992
- Beto JA, Bansal VK. Quality of life in treatment of hypertension: a metaanalysis of clinical trials. American Journal of Hypertension 1992;5(3):125‐33. [DOI] [PubMed] [Google Scholar]
Bond 2007
- Bond RA, Spina D, Parra S, Page CP. Getting to the heart of asthma: can beta‐blockers be useful to treat asthma?. Pharmacology and Therapeutics 2007;115:360‐74. [DOI] [PubMed] [Google Scholar]
Borst 1990
- Borst S, Hui K, Conolly M. Beta‐adrenergic receptors on human lymphocytes: comparison of down‐regulation in vivo and in vitro. Pharmacology 1990;40(6):325‐9. [DOI] [PubMed] [Google Scholar]
Bright 1992
- Bright J, Everitt DE. Beta‐blockers and depression: evidence against an association. JAMA 1992;267(13):1783‐7. [DOI] [PubMed] [Google Scholar]
Brodde 1990
- Brodde O, Daul A, Michel M. Subtype‐selective modulation of human beta‐1‐ and beta‐2‐adrenoceptor agonist and antagonists. Clinical Physiology and Biochemistry 1990;8(Suppl 2):11‐7. [PubMed] [Google Scholar]
Busso 1985
- Busso A, Piantanelli L, Cognini G, Giunta S, Andreoni A, Paciaroni E. Acebutolol‐induced decrease of mononuclear leukocyte beta‐adrenoceptors in hypertension. Pharmacology 1985;31:278‐83. [DOI] [PubMed] [Google Scholar]
Chafin 1999
- Chafin CC, Soberman JE, Demirkan K, Self T. Beta‐blockers after myocardial infarction: do benefits ever outweigh risks in asthma?. Cardiology 1999;92:99‐105. [DOI] [PubMed] [Google Scholar]
Cockcroft 1993
- Cockcroft D, McParland C, Britto S, Swystun V, Rutherford B. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet 1993;342:833‐7. [DOI] [PubMed] [Google Scholar]
Craig 1996
- Craig T, Richerson HB, Moeckli J. Problem drugs for the patient with asthma. Comprehensive Therapy 1996;22(6):339‐44. [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dickey 2010
- Dickey BF, Walker JK, Hanania NA, Bond RA. Beta‐adrenoceptor inverse agonists in asthma. Current Opinion in Pharmacology 2010;10(3):254‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doughty 1997
- Doughty RN, Rodgers A, Sharpe N, MacMahon S. Effects of beta‐blocker therapy on mortality in patients with heart failure. European Heart Journal 1997;18:560‐5. [DOI] [PubMed] [Google Scholar]
Expert Panel Report 2007
- National Heart Lung and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. National Asthma Education and Prevention Program 2007; Vol. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf.
Fitzgerald 1991
- Fitzgerald J. The applied pharmacology of beta‐adrenoceptor antagonist (beta‐blockers) in relation to clinical outcomes. Cardiovascular Drugs and Therapy 1991;5:561‐76. [DOI] [PubMed] [Google Scholar]
Freemantle 1999
- Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottlieb 1998
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta‐blockade on mortality among high‐risk and low‐risk patients after myocardial infarction. New England Journal of Medicine 1998;339(8):489‐97. [DOI] [PubMed] [Google Scholar]
Hall 1983
- Hall J, Kaumann A, Wells F, Brown M. Beta‐2‐adrenoceptor mediated inotropic responses of human atria: receptor subtype regulation by atenolol. British Journal of Pharmacology Proceedings 1983;Suppl 93:116P. [Google Scholar]
Hall 1989
- Hall J, Kaumann A, Brown M. Increased beta‐2 adrenoceptor coupling to inotropic effects in human atrial myocardium due to atenolol treatment. British Journal of Pharmacology Proceedings 1989;Suppl 87:391P. [Google Scholar]
Heidenreich 1999
- Heidenreich PA, McDonald KM, Hastie T, Fadel B, Lee BK, Hlatky MA. Meta‐analysis of trials comparing beta‐blockers, calcium antagonists, and nitrates for stable angina. JAMA 1999;281(20):1927‐36. [DOI] [PubMed] [Google Scholar]
Heller 2000
- Heller DA, Ahern PM, Kozak M. Changes in rates of beta‐blocker use between 1994 and 1997 among elderly survivors of acute myocardial infarction. American Heart Journal 2000;140:663‐71. [DOI] [PubMed] [Google Scholar]
Ioannidis 1999
- Ioannidis JP, Lau J. Pooling research results: benefits and limitations of meta‐analysis. The Joint Commission Journal on Quality Improvement 1999;25(9):462‐9. [DOI] [PubMed] [Google Scholar]
IPPPSH 1985
- The IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta‐blocker oxprenolol: The International Prospective Primary Prevention Study in Hypertension (IPPPSH). Journal of Hypertension 1985;3:379‐92. [DOI] [PubMed] [Google Scholar]
Jakubetz 1999
- Jakubetz J, Schmuck S, Poller U, Fuchs B, Gorf A, Radke J, et al. Cardiac effects of beta‐adrenoceptor antagonist with intrinsic sympathomimetic activity in humans: beta‐1‐ and/or beta‐2‐adrenoceptor mediated?. Journal of Cardiovascular Pharmacology 1999;33:461‐72. [DOI] [PubMed] [Google Scholar]
JNC VI 1997
- JNC VI. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Archives of Internal Medicine 1997;157:2413‐46. [DOI] [PubMed] [Google Scholar]
Jonas 1996
- Jonas M, Reicher‐Reiss H, Boyko V, Shotan A, Mandelzweig L, Goldbourt U, et al. Usefulness of beta‐blocker therapy in patients with non‐insulin‐dependent diabetes mellitus and coronary artery disease. American Journal of Cardiology 1996;77:1273‐7. [DOI] [PubMed] [Google Scholar]
Jones 1980
- Jones M, John R, Jones G. The effect of oxprenolol, acebutolol and propranolol on thyroid hormones in hyperthyroid subjects. Clinical Endocrinology 1980;13:343‐7. [DOI] [PubMed] [Google Scholar]
Kendall 1995
- Kendall MJ, Lynch KP, Hjalmarson A, Kjekshus J. Beta‐blockers and sudden cardiac death. Annals of Internal Medicine 1995;123(5):358‐67. [DOI] [PubMed] [Google Scholar]
Kendall 1997
- Kendall MJ. Clinical relevance of pharmacokinetic differences between beta blockers. American Journal of Cardiology 1997;80(9B):15J‐9J. [DOI] [PubMed] [Google Scholar]
Kennedy 1995
- Kennedy HL, Rosenson RS. Physician use of beta‐adrenergic blocking therapy: a changing perspective. Journal of the American College of Cardiology 1995;26(2):547‐52. [DOI] [PubMed] [Google Scholar]
Kjekshus 1990
- Kjekshus J, Gilpin E, Cali G, Blackey AR, Henning H, Ross J. Diabetic patients and beta‐blockers after acute myocardial infarction. European Heart Journal 1990;11:43‐50. [DOI] [PubMed] [Google Scholar]
Klein 1994
- Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Annals of Internal Medicine 1994;121(3):281‐8. [DOI] [PubMed] [Google Scholar]
Koch‐Weser 1984
- Koch‐Weser J. Beta‐adrenergic blockade for survivors of acute myocardial infarction. New England Journal of Medicine 1984;310:830‐7. [DOI] [PubMed] [Google Scholar]
Kraan 1985
- Kraan J, Koeter G, Mark TW, Sluiter H, Vries K. Changes in bronchial hyperreactivity induced by 4 weeks of treatment with antiasthmatic drugs in patients with allergic asthma: a comparison between budesonide and terbutaline. Journal of Allergy & Clinical Immunology 1985;76:628‐36. [DOI] [PubMed] [Google Scholar]
Krumholz 1998
- Krumholz HM, Radford MJ, Wang Y, Chen J, Heiat A, Marciniak TA. National use and effectiveness of beta‐blockers for the treatment of elderly patients after acute myocardial infarction. JAMA 1998;280(7):623‐9. [DOI] [PubMed] [Google Scholar]
Krumholz 1999
- Krumholz HM, Radford MJ, Wang Y, Chen J, Marciniak TA. Early beta‐blocker therapy for acute myocardial infarction in elderly patients. Annals of Internal Medicine 1999;131(9):648‐54. [DOI] [PubMed] [Google Scholar]
Lechat 1998
- Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel J. Clinical effects of beta‐adrenergic blockade in chronic heart failure: a meta‐analysis of placebo‐controlled, randomized trials. Circulation 1998;98:1184‐91. [DOI] [PubMed] [Google Scholar]
Lipworth 2009
- Lipworth BJ, Williamson PA. Think the impossible: beta‐blockers for treating asthma. Clinical Science (London) 2009;118(2):115‐20. [DOI] [PubMed] [Google Scholar]
Lois 1997
- Lois M, Honig E. Beta‐blockade post‐MI: safe for patients with asthma or COPD?. Journal of Respiratory Diseases 1997;18(6):568‐91. [Google Scholar]
Lois 1999
- Lois M, Roman J, Honig E. Using beta‐blockers for CHF in patients with COPD. Journal of Respiratory Diseases 1999;20(1):31‐43. [Google Scholar]
Mangano 1996
- Mangano DT, Layug EL, Wallace A, Tateo I. Atenolol reduced mortality and cardiovascular events after noncardiac surgery. New England Journal of Medicine 1996;335:1713‐20. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel WH. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22:719‐48. [PubMed] [Google Scholar]
McAreavey 1991
- McAreavey D, Vermeulen R, Robertson J. Newer beta blockers and the treatment of hypertension. Cardiovascular Drugs & Therapy 1991;5:577‐88. [DOI] [PubMed] [Google Scholar]
McNeill 1964
- McNeill RS. Effect of a beta‐adrenergic‐blocking agent, propranolol, on asthmatics. Lancet 1964;2:1101‐2. [DOI] [PubMed] [Google Scholar]
Molinoff 1983
- Molinoff P, Aarons R. Effects of drugs on beta‐adrenergic receptors on human lymphocytes. Journal of Cardiovascular Pharmacology 1983;5:S63‐7. [DOI] [PubMed] [Google Scholar]
Mooss 1994
- Mooss AN, Hillerman DE, Syed MM, Hunter CB. Safety of esmolol in patients with acute myocardial infarction treated with thrombolytic therapy who had relative contraindications to beta‐blocker therapy. Annals of Pharmacotherapy 1994;28:701‐3. [DOI] [PubMed] [Google Scholar]
Motomura 1990
- Motomura S, Deighton N, Zerkowski H, Doetsch N, Michel M, Brodde O. Chronic beta‐1‐adrenergic antagonist treatment sensitizes beta‐2‐adrenoceptors, but desensitizes M2‐muscarinic receptors in the human right atrium. British Journal of Pharmacology 1990;101(2):363‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicolaescu 1972
- Nicolaescu V, Manicatide M, Stroescu V. Beta‐adrenergic blockade with practolol in acetylcholine‐sensitive asthma patients. Respiration 1972;29(2):139‐54. [DOI] [PubMed] [Google Scholar]
O'Malley 1991
- O'Malley K, Cox JP, O'Brien E. Choice of drug treatment for elderly hypertensive patients. American Journal of Medicine 1991;90(Suppl 3A):27S‐33S. [DOI] [PubMed] [Google Scholar]
Opie 1990
- Opie LH. Required beta blocker profile in the elderly. Cardiovascular Drugs & Therapy 1990;4:1273‐80. [DOI] [PubMed] [Google Scholar]
Page 2011
- Page C. Paradoxical pharmacology: turning our pharmacological models upside down. Opinion 2011;32(4):197‐200. [DOI] [PubMed] [Google Scholar]
Radack 1991
- Radack K, Deck C. Beta‐adrenergic blocker therapy does not worsen intermittent claudication in subjects with peripheral arterial disease: a meta‐analysis of randomized controlled trials. Archives of Internal Medicine 1991;151:1769‐76. [PubMed] [Google Scholar]
Raine 1981
- Raine JM, Palazzo MG, Kerr JH, Sleight P. Near fatal bronchospasm after oral nadolol in a young asthmatic and response to ventilation with halothane. British Medical Journal 1981;282:548‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosenson 1993
- Rosenson RS. The truth about beta‐blocker adverse effects ‐ depression, claudication, and lipids. Journal of Ambulatory Monitoring 1993;6(2):163‐71. [Google Scholar]
Salpeter 2004
- Salpeter SR, Ormiston TM, Salpeter EE. Meta‐analysis: respiratory tolerance to regular beta2‐agonist use in patients with asthma. Annals of Internal Medicine 2004;140:802‐13. [DOI] [PubMed] [Google Scholar]
Salpeter 2006
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Annals of Internal Medicine 2006;144:904‐12. [DOI] [PubMed] [Google Scholar]
Salpeter 2010
- Salpeter SR, Wall AJ, Buckley NS. Long‐acting beta‐agonists with and without inhaled corticosteroids and catastrophic asthma events. American Journal of Medicine 2010;123:322‐8. [DOI] [PubMed] [Google Scholar]
Salpeter 2011
- Salpeter S, Ormiston T, Salpeter E. Cardioselective beta‐blocker use in patients with COPD. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD003455.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sbirrazzuoli 1989
- Sbirrazzuoli V, Drici M, Garraffo R, Candito M, Gibelin P, Morand P, et al. Changes in lymphocyte beta‐adrenoceptor density after dilevalol oral treatment. Drugs Under Experimental & Clinical Research 1989;15(5):223‐9. [PubMed] [Google Scholar]
Sial 1994
- Sial SH, Malone M, Freeman JL, Battiola R, Nachodsky J, Goodwin JS. Beta blocker use in the treatment of community hospital patients discharged after myocardial infarction. Journal of General Internal Medicine 1994;9:599‐605. [DOI] [PubMed] [Google Scholar]
Soumerai 1997
- Soumerai SB, McLaughlin TJ, Spiegelman D, Hertzmark E, Thibault G, Goldman L. Adverse outcomes of underuse of beta‐blockers in elderly survivors of acute myocardial infarction. JAMA 1997;277(2):115‐21. [PubMed] [Google Scholar]
Tattersfield 1986
- Tattersfield AE. Beta adrenergic antagonists and respiratory disease. Journal of Cardiovascular Pharmacology 1986;8(Suppl 4):S35‐9. [DOI] [PubMed] [Google Scholar]
Tattersfield 1990
- Tattersfield AE. Respiratory function in the elderly and the effects of beta blockade. Cardiovascular Drugs & Therapy 1990;4:1229‐32. [DOI] [PubMed] [Google Scholar]
Taylor 1992
- Taylor D, Sears M, Herbison G, Flannery E, Print C, Lake D, et al. Regular inhaled beta agonist in asthma: effects on exacerbations and lung function. Thorax 1992;48:134‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viskin 1996
- Viskin S, Barron HV. Beta blockers prevent cardiac death following a myocardial infarction: so why are so many infarct survivors discharged without beta blockers?. American Journal of Cardiology 1996;78:821‐2. [DOI] [PubMed] [Google Scholar]
Wadworth 1991
- Wadworth AN, Murdoch D, Brogden RN. Atenolol: a reappraisal of its pharmacological properties and therapeutic use in cardiovascular disorders. Drugs 1991;42(3):468‐510. [DOI] [PubMed] [Google Scholar]
Wahedna 1993
- Wahedna I, Wong C, Wisniewski A, Pavord I, Tattersfield A. Asthma control during and after cessation of regular beta‐2‐agonist treatment. American Review of Respiratory Disease 1993;148:707‐12. [DOI] [PubMed] [Google Scholar]
Wang 1998
- Wang TJ, Stafford RS. National patterns and predictors of beta‐blocker use in patients with coronary artery disease. Archives of Internal Medicine 1998;158:1901‐6. [DOI] [PubMed] [Google Scholar]
Wellstein 1987
- Wellstein A, Palm D, Belz G, Butzer R, Polsak R, Pett B. Reduction of exercise tachycardia in man after propranolol, atenolol and bisoprolol in comparison to beta‐adrenoceptor occupancy. European Heart Journal 1987;8(Suppl M):3‐8. [DOI] [PubMed] [Google Scholar]
Wicklmayr 1990
- Wicklmayr M, Rett K, Dietze G, Mehnert H. Effects of beta‐blocking agents on insulin secretion and glucose disposal. Hormone and Metabolic Research 1990;22(5):29‐33. [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta‐blockade during and after myocardial infarction: an overview of the randomized trials. Progress in Cardiovascular Diseases 1985;27:335‐71. [DOI] [PubMed] [Google Scholar]
Zaid 1966
- Zaid G, Beall GN. Bronchial response to beta‐adrenergic blockade. New England Journal of Medicine 1966;275:580‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Salpeter 2002a
- Salpeter S, Ormiston T, Salpeter E. Cardioselective [bgr]‐blockers in patients with reactive airway disease: a meta‐analysis. Annals of Internal Medicine 2002;137:715‐25. [DOI] [PubMed] [Google Scholar]


