Abstract
The severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine–induced humoral response and reactogenicity profile are described in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Findings showed that 75.0% (by Simoa assay) or 80.0% (by Roche assay) of the HSCT cohort had a positive antibody response on series completion, compared with 100% in the healthy cohort.
Keywords: SARS-CoV-2, mRNA vaccines, immunocompromised, immunogenicity
Allogeneic hematopoietic stem cell transplant (HSCT) recipients are more likely to experience severe coronavirus disease 2019 (COVID-19), with a high mortality rate [1]. Although 2 nanoparticle-encapsulated messenger RNA (mRNA) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines, mRNA-1273 (Moderna) and BNT162b2 (Pfizer) have demonstrated high efficacy and safety for the general population [2, 3], the immunogenicity and safety profile for these novel vaccines are not known for allogeneic HSCT recipients. The SARS-CoV-2 vaccine–induced humoral response is significantly decreased for solid organ transplant recipients and patients with hematological cancer [3, 4], leading to the hypothesis that HSCT recipients may also have inadequate responses, which has been demonstrated for non–SARS-CoV-2 respiratory viral vaccines, such as for influenza [5]. Further understanding of the immunogenicity and tolerability of mRNA SARS-CoV-2 vaccines in the context of heterogeneous host characteristics is essential to improve protection for these patients who are at higher risk for poor outcomes from COVID-19.
METHODS
Study Design
A prospective cohort study of allogeneic HSCT recipients and healthy participants age ≥18 years was conducted from December 2020 to June 2021 at Brigham and Women’s Hospital and the Dana-Farber Cancer Institute, with institutional review board approval. All participants provided written informed consent. Participants received the 2-dose series of either mRNA-1273 or BNT162b2 under the Food and Drug Administration\'s (FDA’s) emergency use authorization, via their care providers. Allogeneic HSCT recipients who were ≥100 days after transplantation were enrolled and asked to complete a 7-day symptom diary after each dose. Demographic and other information were captured from the electronic medical record.
Serum and Plasma Collection and Assays
Blood samples were obtained at baseline, at the time of dose 2, and approximately 28 days after series completion. A FDA-cleared multiplexed, single molecule array (Simoa) immunoassay measured anti-nucleocapsid (N), anti-spike (S), anti-S1, and anti–receptor-binding domain (RBD) immunoglobulin (Ig) G titers from plasma [6, 7]. The Simoa assay has a positivity threshold of 1.07 and 5.2 normalized average enzymes per bead (AEB) for anti-S IgG and anti-N IgG, respectively. Samples were also evaluated using the FDA-cleared Elecsys anti–SARS-CoV-2 and anti–SARS-CoV-2 S total antigen-capture (IgG, IgA, IgM) immunoassays on the cobas c602 module (Roche Diagnostics), detecting anti-S and anti-N total antibody from serum samples, with positivity thresholds of 0.8 U/mL and a 1.0 cutoff index, respectively [8].
Statistical Analysis
Analyses included descriptive and graphic summaries. The Mann-Whitney test compared the magnitude of the antibody response for the HSCT cohort with that of the healthy cohort, with statistical significance set at a level of α = 0.05. Spearman correlation coefficients were calculated to compare the anti-S levels detected by the Simoa and Roche assays. Analyses were performed using R software (version 4.1.0 www.R-project.org/).
RESULTS
A total of 24 healthy participants and 20 allogeneic HSCT recipients were enrolled. The median age was 24.0 and 66.0 years for the healthy and allogeneic HSCT cohorts; 54.2% and 50.0%, respectively, were female. The vaccine type was more likely to be mRNA-1273 (62.5%) in the healthy cohort and BNT162b2 (71.4%) in the HSCT cohort. In the HSCT cohort, the median duration after transplantation (interquartile range) was 173.0 (111.0–334.0) days, and 9 participants were prescribed corticosteroids and/or tacrolimus at the time of vaccination. Further disease and treatment characteristics for the HSCT recipients are described in Supplementary Table 1. No participants in either cohort had a reported history of COVID-19 before enrollment or during the study, and no participants received intravenous immunoglobulin within 3 months of vaccination.
Eighteen participants (18 of 20) completed the first dose diary, with reports of mild injection site pain (10 of 18 [55.6%]), mild to moderate headache (3 of 18 [16.7%]), chills without fever (1 of 18 [5.6%]), mild to moderate fatigue (5 of 18 [27.8%]), and gastrointestinal symptoms (2 of 18 [11.1%]) (Supplementary Table 3). Eighteen participants (18 of 20) completed the second dose diary, with reports of mild to severe injection site pain (10 of 18 [55.6%]), low-grade fever (2 of 18 [11.1%]), mild to severe headache (4 of 18 [22.2%]), mild to severe fatigue (8 of 18 [44.4%]), gastrointestinal symptoms (3 of 18 [16.7%]), myalgias (5 of 18 [27.8%]), and chills (3 of 18 [16.7%]). Participants who did not complete the entries initially reported no severe events during a follow-up safety call, and reactogenicity was collected retrospectively. No participants were hospitalized or died during the study period.
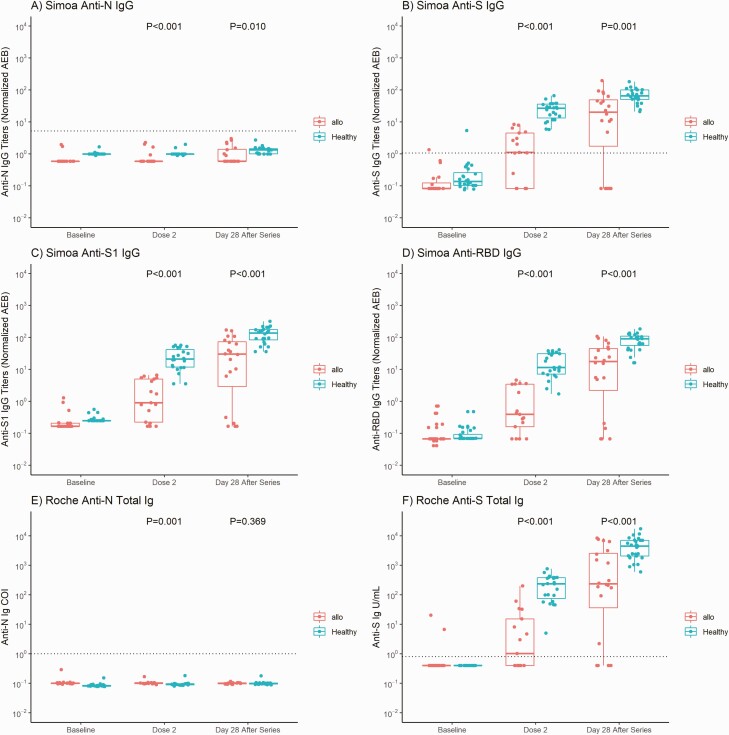
The anti-N titers were negative for both the Simoa and Roche assays in all participants, indicating no SARS-CoV-2 infection before or during the study in either cohort. The median titer magnitudes of anti-S, anti-RBD, and anti-S1 IgG were all significantly higher in the healthy than in the HSCT cohort at the time of dose 2 (anti-S, 26.90 vs 1.11; anti-RBD, 11.53 vs 0.40; anti-S1, 21.22 vs 0.91 normalized average enzymes per bead; P < .001) and 28 days after series completion (anti-S, 65.70 vs 20.27; anti-RBD 90.04 vs 17.63; anti-S1, 136.39 vs 30.04 normalized average enzymes per bead; P < .001) for the Simoa assay, with similar findings for the Roche assay (anti-S, 233.60 vs 1.02 U/mL before dose 2 and 4435.00 vs 205.05 U/mL after series completion; both P < .001) (Figure 1).
Figure 1.
Anti–severe acute respiratory syndrome coronavirus 2 antibody results for allogeneic hematopoietic stem cell transplant (HSCT) recipients and healthy participants. A–D, Simoa assay titers for anti-N immunoglobulin (Ig) G (A), anti-S IgG (B), anti-S1 IgG (C), and anti–receptor-binding domain (RBD) IgG. E, F, Roche assay results for anti-N (E) and anti-S (F) total immunoglobulin (Ig) values at baseline (before vaccination), at dose 2, and 28 days after completion of the vaccine series. Thick horizontal bars and error lines represent medians and interquartile ranges, respectively, and dotted lines represent cutoff thresholds for each assay (Simoa assay: 1.07 normalized average enzymes per bead [AEB] for anti-S IgG and 5.2 normalized AEB for anti-N IgG [no thresholds determined for anti-RBD or anti-S1]; Roche assay: 0.8 U/mL for anti-S and 1.0 cutoff index [COI] for anti-N total immunoglobulin). All participants in the allogeneic HSCT and healthy cohorts had negative anti-N IgG (A) and anti-N total immunoglobulin (E) results throughout the study. P values represent comparisons between the allogeneic HSCT and healthy cohorts at dose 2 and 28 days after series completion. All temporal values within the allogeneic HSCT or healthy cohort for anti-S, anti-S1, and anti-RBD (Simoa) and anti-S (Roche) represent significant differences (P < .001), indicating a significant rise in antibody magnitude from baseline to the time of dose 2, and from dose 2 to 28 days after series completion.
The Spearman correlation between the Simoa and Roche assays for anti-S IgG/Ig was r = 0.89 (95% confidence interval, .81–.94) for the HSCT cohort and r = 0.92 (.87–.96) for the healthy cohort (Supplementary Figure 1). All healthy participants had a positive anti-S antibody response after just 1 dose by both assays. In contrast, only 58.5% (Simoa) or 52.9% (Roche) of HSCT recipients responded after 1 dose. By 28 days after series completion, 75.0% (Simoa) or 80.0% (Roche) of the HSCT recipients responded and had an increased magnitude titer compared with the time of dose 2, suggesting that the second dose is essential to achieve a more robust antibody response. Interestingly, 5 HSCT recipients did not respond by series completion with the Simoa assay, compared with 4 as measured with the Roche assay. There was no clear trend based on the clinical history in terms of donor type, time from transplantation, HLA match, graft-vs-host disease prophylaxis, or steroid use among these nonresponders (Supplementary Table 2).
DISCUSSION
In summary, we described the SARS-CoV-2 mRNA vaccine-induced responses from baseline through approximately 28 days after series completion in a cohort of allogeneic HSCT recipients, using 2 serological assays with high concordance. Both mRNA vaccines were well tolerated with limited reactogenicity. Our data show that not all HSCT recipients mount a humoral response (75.0% with the Simoa and 80.0% with the Roche assay) 1 month after completing the vaccination series, which aligns with other findings described for allogeneic HSCT recipients [9].
Our study is unique in describing significantly lower magnitude of response after both 1 and 2 doses, compared with a healthy control group that had a 100% high-titer response. While there may be a potential benefit of additional vaccine doses for immunocompromised hosts [10, 11], there are likely heterogenous host factors that affect response, as demonstrated by the disparate disease characteristics and treatments in our cohort. Allogeneic HSCT recipients who fail to produce antibodies after vaccination may have specific B-cell pathway defects that limit response, and thus additional doses may not necessarily augment antibody production. Our results also demonstrate the importance of orthogonal testing, especially in populations whose host immune responses may be altered. Without a clear reference standard established for SARS-CoV-2 antibody diagnostics, the discordant assay results (Simoa vs Roche) observed for the individuals in our study could reflect intrinsic differences in assay measurements or differences in host factors. If future policies (eg, additional booster doses or monoclonal antibodies) are based on antibody responses, the assays chosen should best reflect the immune status of the individual, with orthogonal testing perhaps the best approach.
Our study has limitations. We did not evaluate vaccine efficacy, and in the absence of a known correlate of protection, protection against COVID-19 cannot be assessed solely from immunogenicity data. The sample is small and is not powered to detect significant differences in antibody response based on vaccine type, age, or disease or treatment characteristics. Effects of immunosenescence may have affected the results, since the median age was older for the HSCT cohort. However, immunogenicity data from the mRNA phase 1/2 trials demonstrated high binding IgG and neutralization responses in healthy, older individuals that were comparable to titers elicited by young adults [12], and other studies have shown age-dependent decreased immunogenicity that was most significant in individuals >80 years old [13, 14], whereas all of our participants were <80 years old.
Further larger studies should assess the humoral response, neutralization titers, and clinical efficacy based on specific clinical characteristics that are likely to affect the immune system. A more in-depth understanding will aid in optimization of vaccine strategies, which may include specific timing after transplantation, additional vaccinations at targeted intervals between immunosuppression therapies, and/or deferring to other preventive options for individuals who are unable to produce an endogenous humoral response.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study conceptualization: N. C. I. and L. R. B. Study design: A. C. S., M. D., N. C. I., and L. R. B. Enrollment of study participants: A. C. S., M. D., B. B., and N. I. Statistical planning and analysis: A. C. S. and G. Z. Extraction of clinical data: B. B. and N. I. Sample processing: J. K. Laboratory support and performance of assays: C. C. and N. T. Assay expertise: D. R. W. Monitoring of study conduct: N. C. I. and L. R. B. Drafting of manuscript: A. C. S. Editing of manuscript: A. C. S., M. D., B. B., N. I., D. R. W., R. S., V. T. H., N. C. I., and L. R. B. Critical review of manuscript: M. D., C. C., G. Z., J. K., N. T., D. R. W., R. S., V. T. H., N. C. I., and L. R. B.
Acknowledgments. The authors thank Xiaofang Li, Salena Von, John Kupelian, and Yasmeen Senussi for their assistance with processing samples and Maria Fernandez for sample testing. Megan Powell, Julia Klopfer, Noah Abasciano, and Omolola Ometoruwa assisted with participant scheduling, enrollment, and follow-up visits. Stacy Melanson provided helpful insights for the Roche assay and critically reviewed the manuscript. They also thank Tal Gilboa for helpful discussions.
Potential conflicts of interest. A. C. S. reports support for the present work from Chleck Foundation and from Barbara and Amos Hostetter and served as principal investigator for Merck studies on cytomaegalovirus therapeutics. M. D. reports grants or contracts from Centre Hospitalier de l’Université de Montreal (CHUM) and the CHUM Foundation, outside the submitted work. G. Z. reports receiving $5000 from University of British Columbia/British Columbia Children’s Hospital for statistical consulting on multiple projects involving continuous physiological monitoring to improve pediatric care in resource-limited settings, funded by the Bill & Melinda Gates Foundation; the nature of this work is unrelated to the current study. N. T. reports grants or contracts from Abbott Diabetes Care and Biomerieux to the Department of Pathology and from CIC Health to herself; consulting fees from Health Advances; support for attending meetings and/or travel from the American Association for Clinical Chemistry; and leadership or fiduciary roles in other board, society, committee, or advocacy group, paid or unpaid, for the American Association for Clinical Chemistry, the College of American Pathologists, and The Journal of Applied Laboratory Medicine. D. R. W. has a financial interest in Quanterix, a company developing an ultrasensitive digital immunoassay platform; is an inventor of the Simoa technology, a founder of the company, and a member of its board of directors; and received financial support for the present work from Barbara and Amos Hostetter and from the Chleck Foundation. D. R. W.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Mass General Brigham, in accordance with their conflict of interest policies. R. S. reports being a consultant for Cugene (2021), Takeda (2021 to present), Jasper (2021 to present), Jazz Pharmaceuticals (2021 to present), Precision Biosciences (2020 to present), Alexion (2020 to present), and Rheos Therapeutics (2015 to present); and serving on a Data Safety Monitoring Board for Juno Therapeutics (2020 to present), on the board of directors for Kiadis, the Netherlands (2016 to present), on a career development award committee for Gilead (2015 to present), and on the board of directors for the National Marrow Donor Program—Be the Match (2014 to present). N. C. I. received funding to conduct sponsored clinical research from GlaxoSmithKline, Merck, Astellas, and AiCuris. L. R. B. reports research support from the National Institutes of Health (NIH; including National Institute of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences), Wellcome Trust, and the Bill & Melinda Gates Foundation, outside the submitted work; has served on a Data and Safety Monitoring Board, SMC, and advisory committee for NIH and the Food and Drug Administration; and is involved in human immunodeficiency virus (HIV), coronavirus (COVID), and other vaccine clinical trials conducted in collaboration with the NIH, HIV Vaccine Trials Network, COVID Vaccine Prevention Network, International AIDS Vaccine Initiative, Crucell/Janssen, Moderna, Military HIV Research Program, the Bill & Melinda Gates Foundation, and the Ragon Institute. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sharma A, Bhatt NS, Martin AS, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol 2021; 8:e185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325:2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halasa NB, Savani BN, Asokan I, et al. Randomized double-blind study of the safety and immunogenicity of standard-dose trivalent inactivated influenza vaccine versus high-dose trivalent inactivated influenza vaccine in adult hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant 2016; 22:528–35. [DOI] [PubMed] [Google Scholar]
- 6. Ogata AF, Cheng C-A, Desjardins M, et al. Circulating SARS-CoV-2 vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Norman M, Gilboa T, Ogata AF, et al. Ultrasensitive high-resolution profiling of early seroconversion in patients with COVID-19. Nat Biomed Eng 2020; 4:1180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roche Diagnostics. Elecsys Anti-SARS-CoV-2. 2020. [Google Scholar]
- 9. Redjoul R, Bouter AL, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet 2021; 398:298–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. New Engl J Med 2021; 385:661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. New Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021; 596:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis 2021. doi: 10.1093/cid/ciab381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.