Abstract
A better understanding of serological data and risk factors for coronavirus disease 2019 (COVID-19) infection in healthcare workers (HCWs) is especially important in African countries where human resources and health services are more constrained. We reviewed and appraised the evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seroprevalence and its risk factors in HCWs in Africa to inform response and preparedness strategies during the SARS-CoV-2 pandemic. We followed the Preferred Reporting Items for systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines in this scoping review. Databases including PubMed, Embase and preprint servers were searched accordingly from the start of the COVID-19 pandemic to 19 April 2021. Our search yielded 12 peer-reviewed and four pre-print articles comprising data on 9223 HCWs from 11 countries in Africa. Seroprevalence varied widely and ranged from 0% to 45.1%. Seropositivity was associated with older age, lower education, working as a nurse/non-clinical HCW or in gynaecology, emergency, outpatient or surgery departments. Asymptomatic rates were high and half of the studies recommended routine testing of HCWs. This scoping review found a varying but often high SARS-CoV-2 seroprevalence in HCWs in 11 African countries and identified certain risk factors. COVID-19 public health strategies for policy and planning should consider these risk factors and the potential for high seroprevalence among HCWs when prioritizing infection prevention and control measures and vaccine deployment.
Keywords: Scoping review, seroprevalence, Africa, COVID-19, SARS-CoV-2, health professionals
Key messages.
There are 16 articles on seroprevalence comprising data on 9223 HCWs from 11 countries in Africa.
Seroprevalence varied widely and ranged from 0% to 45.1%.
Seropositivity was associated with older age, lower education, working as a nurse/non-clinical HCW, or in gynaecology, emergency, outpatient, or surgery departments.
COVID-19 policies should consider these risk aspects and the partly high seroprevalence among HCWs when prioritizing IPC measures and vaccine deployment.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has put health systems worldwide to an unprecedented test. As frontline workers, healthcare workers (HCWs) are a critical part of the health system’s pandemic response and are themselves at high risk of infection. Vaccine roll-out, therefor, prioritizes HCWs worldwide (WHO, 2020b), and at the time of writing, 35 African countries had begun vaccine campaigns (Our World in Data, 2021).
Serological studies are investigations using serology testing to look for antibodies in blood (Centers for Disease Control and Prevention, 2021). These studies give insight into the true burden of infection as they have the ability to account for asymptomatic and unreported cases. These studies can provide data on infection trends, effects of interventions, vaccine deployment prioritization guidelines (Maeda and Nkengasong, 2021) and geographical distribution mapping for identification of populations at particularly high risk (Peeling et al., 2020).
A previous systematic review on seroprevalence in HCWs only included three African studies (Galanis et al., 2020). Nevertheless, data on seroprevalence and risk factors are specifically important in the African continent, where countries have predominantly lower access or quality of healthcare (Our-World-in-Data, 2015). This region furthermore experiences constrained human resources in healthcare. Compared to Europe, where there are 80 nurses per 10 000 population, across Africa there are only 10 (WHO, 2021a), emphasizing the high need to protect this scare workforce. Evidence on the implementation of measures that can help detect and mitigate exposures such as triage systems, dedicated COVID-19 wards, as well as surveillance testing and infection prevention and control (IPC) availability is necessary to better understand the risk of becoming overburdened.
This need is further emphasized in a region that does not have ownership of vaccine production and must depend on limited doses from other areas. Serological testing can also provide guidance on vaccine decision-making.
This scoping review aimed to better understand and identify gaps in knowledge on seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in HCWs in the African continent and their risk factors for COVID-19 infection, as well as how these seroprevalence rates compare with the respective general population. The added public health value of this review is that it provides the most up to-date synthesis of all available research and hence can inform public health strategies for policy and planning.
Materials and methods
Given the relative infancy and fast-moving pace of the field, we selected a scoping review method without prior registration in a public domain.
Data sources and search strategy
This scoping review was performed according to the Preferred Reporting Items for systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist (Tricco et al., 2018) and frameworks on scoping reviews (Levac et al., 2010; Arksey and O’malley, 2005; Peters Mdj et al., 2020) including the stages of (1) identifying a research question, (2) identifying relevant studies, (3) study selection, (4) charting the data and (5) summarizing and reporting results. The detailed PRISMA checklist is available in Supplementary Table S1.
We searched PubMed, Embase and preprint servers (ArRvix, BioRvix, ChemRvix, MedRvix, Preprints.org, ResearchSquare and SSRN) for designated terms for COVID-19, seroprevalence, HCWs and African countries. The complete search strategy is detailed in supplement 2. A hand-search was also conducted to add relevant articles not previously identified with the search strategy. Additionally, to put the HCW seroprevalences into context, general population seroprevalences were gathered from the SeroTracker database (Arora et al., 2021) for all countries included on 24 October 2021.
Selection and eligibility criteria
We screened title and abstracts of all publications returned in the search, guided by pre-set inclusion and exclusion criteria detailed as follows. We included articles published or in preprint between the first detection of COVID-19 and 19 April 2021 on HCWs irrespective of prior COVID-19 status or comorbidities in health settings in Africa with data on serological status. We excluded reviews and evidence that focused on non-HCWs or outside of Africa. HCWs were not defined in this scoping review, but we followed the definition of included studies, being mainly ‘clinical and non-clinical care’. We did not restrict our search in terms of publication language, although search terms were all in English. Two researchers independently undertook study selection, and discussion resolved potential disagreement. Selection of studies for general population seroprevalence was restricted to the respective counties and overall study period.
Data extraction and quality assessment
Data from all selected studies were entered independently by two researchers into a form created for this purpose in Microsoft Excel. The following data were charted: author, publication year, title, journal, publication status, study period, city/country, sample size, sampling strategy, response rate, inclusion criteria, exclusion criteria, study design, sex, age, seroprevalence, asymptomatic rate, sample collection, testing strategy, testing rate, type of antibody, type of test, test name, factors investigated, factors associated, level of analysis and additional data such as reported triage system, dedicated COVID-19 wards, routine testing, IPC or personal protective equipment (PPE) availability.
The quality of studies was assessed by two authors independently using Joanna Briggs Institute (JBI) critical appraisal tool for prevalence studies (Munn et al., 2015) adapted with additional specifications (Arora et al., 2021). Bias levels were assessed using a 9-point ranking system with categorization of 8–9 low, 5–7 moderate and ≤4 high risk of bias (Galanis et al., 2020).
Results
Selection of studies
The search identified a total of 102 articles; following the removal of duplicates and critical assessment of title and abstracts, 19 potentially relevant articles were identified for full-text evaluation (Figure 1). Application of the pre-set eligibility criteria resulted in a final inclusion of 16 articles.
Figure 1.
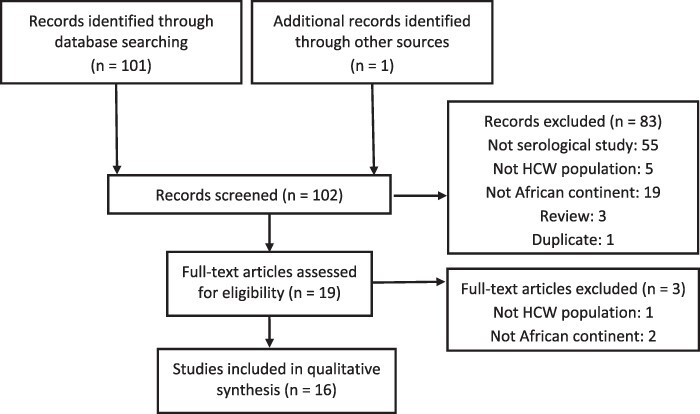
Selection of sources of evidence
Study characteristics
The included 16 studies contain seroprevalence data on 9223 HCWs from 11 countries across Africa: Democratic Republic of Congo (Mukwege et al., 2021), Egypt (Mostafa et al., 2020; 2021; Abdelmoniem et al., 2021; Kassem et al., 2020; Mukhtar et al., 2021), Kenya (Etyang et al., 2021), Libya (Kammon et al., 2020), Malawi (Chibwana et al., 2020), Mauretania (Salem et al., 2021), Nigeria (Majiya et al., 2020; Olayanju et al., 2020), South Africa (Goldblatt et al., 2021), Togo (Halatoko et al., 2020), Zambia (Fwoloshi et al., 2021) and Zimbabwe (Rusakaniko et al., 2021) (Table 1).
Table 1.
Results of included studies
| Reference | City, country | Time | Sample size (n) | Seroprevalence % (95% CI) | Asymptomatic rate % | Factors investigated | Highest level of analysis |
|---|---|---|---|---|---|---|---|
| Abdelmoniem et al. (2021) | Cairo, Egypt | 06/2020 | 203 | 18.2 (13.2–24.2) | NAa | Demographics (age, gender), profession (occupation),
exposure (contact with case), IPC questionnaires (use of PPE, HH), medical condition (comorbidities) |
Univariate |
| Chibwana et al. (2020) | Blantyre, Malawi | 05/2020–06/2020 | 500 | 16.8 (13.6–20.4) | NAa | None | NAa |
| Etyang et al. (2021) | Kilifi, Busia; Nairobi, Kenya | 07/2020–12/2020 | 684 | 20.8 (17.5–24.4) | NRb | Demographics (age, gender), profession (occupation), exposure (site, working in COVID unit), medical condition (chronic illness), symptoms | Multivariate |
| Fwoloshi et al. (2021) | 6 districts, Zambia | 07/2020 | 575 | 2.2 (0.5–3.9) | NRb | Demographics (age, gender), profession (occupation), exposure (district, health facility type, contact, direct patient care, care for confirmed COVID-19, travel, visit of health facility, in-person attendance at work/school, market visit, transportation), medical condition (unknown HIV status, pregnancy) |
Univariate |
| Goldblatt et al. (2021) | Cape Town, South Africa | 06/2020–08/2020 | 222 | 10.4 (6.7–15.1) | 68.9 | None | NAa |
| Halatoko et al. (2020) | Lomé, Togo | 04/2020–05/2020 | 370 | 1.4 (0.4–3.1) | NRb | None | NAa |
| Kammon et al. (2020) | Alzintan, Libya | 04/2020–05/2020 | 77 | 0 (0.0–4.7) | NRb | None | NAa |
| Kassem et al. (2020) | Cairo, Egypt | 06/2020 | 74 | 12.2 (5.7–21.8) | 62.5 | Demographics (age, gender), profession (occupation), exposure (contact with case/suspect), IPC questionnaires (use of PPE, HH), medical condition (comorbidities), symptoms |
Univariate |
| Majiya et al. (2020) | Niger State, Nigeria | 06/2020 | 43 | 37.2 (23.0–53.3) | NRb | None | NAa |
| Mostafa et al. (2020) | Cairo, Egypt | 04/2020–05/2020 | 4040 | 1.3 (1.0–1.7) | 68.2 | Demographics (age, gender, residence, marital status, education), profession (occupation, department), medical condition (immunological disorder, tobacco use), exposure (location of contact with confirmed/suspected case, duration, type of contact), symptoms (severity, fever, cough, change/loss of smell/taste/appetite) | Multivariate |
| Mostafa et al. (2021) | Cairo, Egypt | 05/2020–06/2020 | 2282 | 4.0 (3.6–5.3) | 64.0 | Demographics (age, gender, residence, marital status, education), profession (occupation), exposure (location of contact with confirmed/suspected case, duration, type of contact), medical condition (comorbidities, pregnancy, tobacco use), symptoms (severity, fever, muscle pain, joint pain, sneezing, shortness of breathing, other respiratory symptoms, loss of appetite, change/loss of taste, change/loss of smell, conjunctivitis) | Multivariate |
| Mukhtar et al. (2021) | Cairo, Egypt | 05/2020–06/2020 | 455 | 7.9 (5.8–10.8) | 31.0 | Demographics (age, gender), medical condition (clinical history, medication intake, smoking history), symptoms | Univariate |
| Mukwege et al. (2021) | Bukavu, DRC | 07/2020–08/2020 | 359 | 41.2 (36.1–46.5) | 77.7 | Demographics (gender), profession (occupation), exposure (type of work, use of PPE, contact with confirmed case), medical condition (comorbidities), confirmed case | Multivariate |
| Olayanju et al. (2020) | Ibadan, Nigeria | 04/2020 | 133 | 45.1 (36.5–54.0) | NAa | Demographics (age, gender), profession (occupation, department) | Univariate |
| Rusakaniko et al. (2021) | Bulawayo, Zimbabwe | 06/2020 | 635 | 26.1 (22.8–29.7) | NRb | Demographics (age), profession (occupation, department), medical condition (comorbidities) | Univariate |
| Salem et al. (2021) | Nouakchott, Mauritania | 05/2020 | 853 | 1.7 (0.9–2.7) | NRb | None | NAa |
NA: not applicable as the study excluded symptomatic HCWs.
NR: not reported, bolded factors are those significantly associated with seropositivity.
Twelve articles were published in peer-reviewed journals (Goldblatt et al., 2021; Halatoko et al., 2020; Abdelmoniem et al., 2021; Kassem et al., 2020; Mostafa et al., 2020; Olayanju et al., 2020; Mostafa et al., 2021; Mukwege et al., 2021; Mukhtar et al., 2021; Salem et al., 2021; Fwoloshi et al., 2021; Rusakaniko et al., 2021) and four were published in preprint services (Chibwana et al., 2020; Kammon et al., 2020; Majiya et al., 2020; Etyang et al., 2021). Twelve studies used total population sampling, reflecting that all HCWs of a given setting were included (Chibwana et al., 2020; Goldblatt et al., 2021; Abdelmoniem et al., 2021; Kassem et al., 2020; Mostafa et al., 2020; 2021; Etyang et al., 2021; Mukhtar et al., 2021; Salem et al., 2021; Mukwege et al., 2021; Fwoloshi et al., 2021; Rusakaniko et al., 2021) and four reported to have used a random sampling strategy (Halatoko et al., 2020; Kammon et al., 2020; Majiya et al., 2020; Olayanju et al., 2020). All studies were conducted between April and December of 2020 in hospital settings, with the exception of two studies which did not report details on HCW recruitment (Halatoko et al., 2020; Majiya et al., 2020). Three studies took place in specific departments, such as emergency (Abdelmoniem et al., 2021), paediatrics (Goldblatt et al., 2021) or gastroenterology (Kassem et al., 2020). In six studies, the percentage of males was higher than the percentage of females (Kammon et al., 2020; Abdelmoniem et al., 2021; Majiya et al., 2020; Mukhtar et al., 2021; Mukwege et al., 2021; Salem et al., 2021).
Reported sensitivity ranged from 71.1% (Mukwege et al., 2021) to 100% (Goldblatt et al., 2021) and specificity from 85.0% (Halatoko et al., 2020) to 100% (Fwoloshi et al., 2021; Mostafa et al., 2020; 2021; Mukwege et al., 2021). More than 60% of the studies used tests that met the commonly set minimum performance criteria of sensitivity ≥90% and test specificity >95% (Biocentury, 2020). Please see Supplementary Table S5 for detailed information on all study and antibody test characteristics discussed above.
Six studies assessed rates of asymptomatic infection, five of which reported asymptomatic rates above 60% (Goldblatt et al., 2021; Kassem et al., 2020; Mostafa et al., 2021; 2020).
Quality assessment
Following the JBI critical appraisal tool, studies were rated out of a total of 9 points, and all were found to be of moderate-to-low risk of bias (ranging between 5 and 8) (Supplementary Table S3). Risk of bias primarily arose from aspects such as test characteristics or non-reporting of response rates (Supplementary Table S3).
Seroprevalence and its geographic distribution
Seroprevalence among studies ranged from 0% to 45.1%, with highest prevalence in Nigeria (45.1%) (Olayanju et al., 2020) and DRC 41.2% (Mukwege et al., 2021) and lowest in Libya, Togo and Egypt (0%, 1.4% and 1.3%, respectively) (Kammon et al., 2020; Halatoko et al., 2020; Mostafa et al., 2020). Data collection across countries started in April 2020 and ended in December 2020 (Table 1). Five studies originated from Egypt, four of which were cross-sectional, while one was a consecutive follow-up cohort study for one of the three studies (Mostafa et al., 2021). The follow-up cohort study found a seroconversion rate of 4% at a 3-week interval (Mostafa et al., 2021), being 2.7 percentage points higher than the baseline cross-sectional study finding of 1.3% seroprevalence (Mostafa et al., 2020). The three other cross-sectional studies found a prevalence of 12.2%, 18.2% and 31.0% among HCWs (Kassem et al., 2020; Abdelmoniem et al., 2021; Mukhtar et al., 2021).
Risk factors
Ten studies investigated factors associated with seropositivity, eight of which found association reported by odds ratio (OR) or hazard ratio (HR) (Table 1).
Risk of seropositivity increased with age. This was shown in detail in two studies from Egypt with the exemption of age group 40–49 years [Mostafa et al., 2020: reference age: 18–24 years; 25–29 years, OR = 3.9, confidence interval (CI) 0.96–16.1; 30–39 years, OR = 4.3, CI 1.1–17.3; 40–49 years, OR 2.8, CI 0.6–12.9; ≥50 years, OR = 4.2, CI 0.8–21.2 and Mostafa et al., 2021: reference age 18–29 years; 30–39 years, HR = 2.6, CI 1.3–4.9; 40–49 years, HR = 2.4, CI 1.2–4.7; ≥50 years, HR = 2.7, CI 1.3–5.6]. Sex was not associated with seropositivity. Lower levels of education had higher risks for seropositivity (reference education: university or higher; secondary HR = 2.0, CI 1.2–3.3; primary/preparatory HR = 3.9, CI 1.9–8.0; less than primary HR = 3.3, CI 1.4–7.8; Mostafa et al., 2021). Odds of seropositivity were higher for HCWs in the operating room (OR = 3.2, CI 1.3–8.0; Mostafa et al., 2020), emergency department (OR = 3.2, CI 1.1–9.4; Olayanju et al., 2020), outpatient department (OR = 2.3, CI not reported; Rusakaniko et al., 2021) or obstetrics and gynaecology department (OR = 19.3, CI 2.0–183.4; Olayanju et al., 2020). Two studies reported higher risk of seropositivity for nurses (OR = 4.7, CI 2.0–11.2; OR = 3.0, CI 1.6–5.5; reference group physicians, respectively; Mostafa et al., 2020; 2021), and one study reported higher risks for non-clinical HCWs (OR 7.8, CI 1.7–34.9, reference group nurses; Fwoloshi et al., 2021).
Symptoms like fever, dry cough and change/loss of smell were associated with seropositivity (Kassem et al., 2020; Mostafa et al., 2020; 2021), with change/loss of smell having the highest odds (OR = 26.2, CI 2.1–329.7; Mostafa et al., 2020). Medical conditions like pregnancy and chronic kidney disease were associated with seropositivity (OR = 3.5, CI 1.1–11.9; OR = 4.4, CI 1.0–19.0, respectively; Mostafa et al., 2021). More than 15 minutes of contact with a confirmed case were positively associated (OR = 2.2, CI 1.2–4.1) with seropositivity, whereby exposure at work was negatively associated (OR = 0.5, CI 0.3–0.8; Mostafa et al., 2021). No association between seropositivity and use of IPC measures could be found, but in-person attendance at work/school and frequent market visits (3–5 visits/month) were found to be risk factors (OR = 4.8, CI 1.6–13.9; OR = 3.0, CI 1.3–7.0, respectively; Fwoloshi et al., 2021).
Gap analysis
None of the studies reported facilities having a COVID-19 triage in place. Only one specified existence of dedicated wards (Kassem et al., 2020) or that HCWs were specifically trained in COVID-19 IPC measures (Fwoloshi et al., 2021). Five studies raised concerns about insufficient PPE availability or asked to increase supply (Majiya et al., 2020; Mukwege et al., 2021; Etyang et al., 2021; Fwoloshi et al., 2021; Rusakaniko et al., 2021), whereby one study asked for PPE especially for HCWs with patient contact (Rusakaniko et al., 2021). Fifty percent of included studies stated a lack of routine testing of HCWs or recommended its implementation due to the high seroprevalence and asymptomatic rates.
General population seroprevalence comparison
Seroprevalence in the general population in the included countries ranged between 0% (Elfakhri et al., 2020) and 44.6% (Marion et al., 2021). For four countries, there were no general population data available: Malawi, Mauritania, Togo and Zimbabwe. Seroprevalence in the other included countries ranged from 16.6% (Nkuba et al., 2021) to 40.8% (Philippe et al., 2021) in DRC, from 29.8% (Girgis et al., 2021) to 41.0% (Musa et al., 2021) in Egypt, from 1.3% (Lucinde et al., 2021) to 34.7% (Ngere et al., 2021) in Kenya, from 0% (Elfakhri et al., 2020) to 4.2% (Kammon et al., 2020) in Libya, from 16.1% (Okpala et al., 2021) to 42.0% (Ijeoma et al., 2021) in Nigeria, from 23% (Kleynhans et al., 2021) to 44.6% (Marion et al., 2021) in South Africa and from 2.1% (Mulenga et al., 2021) to 8.2% (Hines et al., 2021) in Zambia.
Discussion
The overall seroprevalence among HCWs across 11 Africa countries varied widely reflecting recent data from a systematic review of seroprevalence of anti-SARS-CoV-2 antibodies in Africa, which combined the general population and specific working groups (Chisale et al., 2021).
Direct comparison with the general population is challenging and was not the primary aim of the current manuscript, but data available from the general population in the 11 included countries suggest highly varying seroprevalences similar to those of HCWs with rates between 0% and 44.6%. The seroprevalence among HCWs could be influenced by variety of factors that may differ between levels (national and local) and countries. National Public Health Institutes vary widely in breadth and depth (Africa CDC, 2019), especially in West Africa, where the strengthening of public health structures (Meda et al., 2016) and surveillance is urgently needed (Massinga Loembé et al., 2020). This need could be reflected in the suspected underreporting of SARS-CoV-2 cases. The Africa Centres for Disease Control and Prevention (CDC) together with Ministries of Health have recognized this challenge and taken action with their joint continental Strategy and the creation of Africa Task Force for Coronavirus (Africa CDC, 2020).
HCWs in Africa are potentially more often in contact with undiagnosed cases due to low surveillance and limited testing capacities (Chitungo et al., 2020). Furthermore, the clinical COVID-19 diagnosis is even more challenging due to other endemic febrile diseases such as malaria or typhoid fever, which also act as competing priorities for frontline HCWs (Maze et al., 2018). These competing priorities make it difficult for hospitals to implement triage and infectious disease treatment centres specifically dedicated for the COVID-19 pandemic (Ayebare et al., 2020). This aspect is reflected by the fact that only one included study reported having a COVID-19 ward and none to have a triage system in place.
Additionally, even where cases are accurately diagnosed, self-protection of HCWs is difficult due to IPC measure limitations in infrastructure and equipment, such as overcrowded settings (van de Ruit et al., 2020), in which proper distancing is more challenging and lacking supply or access to PPE material (Desai et al., 2019). This lack of PPE availability or the need for increased supply was confirmed by five of the included studies. However, in contrast to studies in the UK or USA (Nguyen et al., 2020), no significant association between IPC and seropositivity among HCWs in Africa was shown in our review (Abdelmoniem et al., 2021; Kassem et al., 2020; Mukwege et al., 2021). This lack of association could arise from the assessment procedure through questionnaire only. Observation should be used in conjunction with questionnaires to realistically measure the impact of IPC on infection mitigation (Sax et al., 2009).
The highest HCW prevalence rate was found in Nigeria (45.1%) and supports reports that this country has Africa’s second largest population incidence (WHO, 2021b) This high rate was also reflected in the comparative general population data that showed Nigeria to be second highest after South Africa. Providing care with minimal precautionary measures was a reported factor that is likely influencing high seroprevalence rates among HCWs in particular (Olayanju et al., 2020).
Three of the included studies reporting a HCW seroprevalence <5% were conducted in April 2020, before the first wave peaked in Africa (WHO, 2021b).
Detection and subsequent isolation of SARS-CoV-2 cases is known to be especially challenging when asymptomatic rates are high. In this scoping review, asymptomatic rates were predominately >60% (Goldblatt et al., 2021; Kassem et al., 2020; Mostafa et al., 2021; 2020; Mukwege et al., 2021) and hence much higher than in a current meta-analysis (He et al., 2020). These high asymptomatic rates warrant extra vigilance in case detection strategies since asymptomatic HCWs can not only infect their peers, colleagues and families but also the vulnerable group of patients they are caring for. These high asymptomatic rates are in particular worrisome in the African region where continuous mass screening of HCWs is rarely done, asymptomatic infections are broadly undetected and hence HCWs are more likely to transmit the infections onwards. Consecutively, half of the included studies recommended the implementation of routine screening of HCWs to avoid hidden onward transmission. Routine testing in practice can hence mitigate the possibility of HCWs being a potential source of infection to the vulnerable patients and protect the scarce workforce of HCWs in low-resource settings.
In the African region, significant association was shown between seropositivity and working in operating rooms, obstetrics, outpatient clinic, gynaecology and emergency department (Mostafa et al., 2020; Olayanju et al., 2020; Rusakaniko et al., 2021). These risk areas are in accordance with results from Denmark that found emergency units to be a high-risk area (Iversen et al., 2020). One included study found that nurses and non-clinical HCWs were at higher risk of seropositivity than doctors (Mostafa et al., 2020), supporting our found association between seropositivity and lower level of education or the non-prioritization for IPC trainings. These associations show that non-clinical HCWs should also be included in training regardless of their level of patient contact. Interestingly, a study from Cairo found that exposure to a confirmed case at home had a significantly higher HR than exposure at work (Mostafa et al., 2021), suggesting that HCWs’ non-occupational risk should also be considered. This finding supports the determined general population seroprevalence range being similar to that of HCWs. Seroprevalence was associated with higher age in three included studies (Rusakaniko et al., 2021; Mostafa et al., 2020; 2021), which is concerning since higher age is known to be associated with higher risk for severe disease (ECDC, 2021).
Limitations
There are several limitations in the present scoping review. No grey literature of news and press releases were included. We searched in databases and public preprint servers to assess as much available scientific information as possible. As studies from only 11 countries could be identified, extrapolation of findings to all HCWs in the African continent could not be made. However, all African regions were represented, although data on risk factors mainly stemmed from Egyptian studies. Most studies used total population or random sampling, and none of the studies were rated to be of high risk of bias. No study explicitly included HCWs on sick leave or in quarantine leading to a potential underestimation. A valid and direct comparison between HCW and general population seroprevalence rates was challenging as neither methods nor timing of most studies were aligned.
Serological studies per se have some limitations. Given antibody kinetics, infections can be undetectable for up to 2 weeks from the onset of infection or after 3 months (Isho et al., 2020). In addition to antibody kinetics, infection through immunoglobulin G testing may also be undetected as some individuals may not develop antibodies (Mostafa et al., 2021). Overestimation, on the other hand, could be possible due to cross-reactive antibodies that have been detected in sub-Saharan Africa (Tso et al., 2020). Test characteristics and test validity also affect seropositivity. Ten of the 16 studies used tests that met the commonly set minimum performance criteria of sensitivity ≥90% and test specificity >95% (Biocentury, 2020). These varying tests are leading to potentially divergent results that stress the need for validation in study populations, especially in African countries, where the concern of potential lower specificity of SARS-CoV-2 commercial tests has been raised (Nkuba Ndaye et al., 2021). Confirmation through neutralizing antibody testing and interpretation with caution has therefore been recommended (Nkuba Ndaye et al., 2021).
Public health contribution
To our knowledge, this is the first scoping review including all preprint and published studies estimating seroprevalence and risk factors for SARS-CoV-2 seropositivity among HCWs from 11 African countries with evidence appraisal. The present scoping review has shown that data on seroprevalence in HCWs across the African continent are scarce and hence data on risk factors even scarcer. Even though there is no direct and valid comparison, this review offers the first hint that differences between seroprevalences of HCWs and the general population are not as prominent as in other regions (Nguyen et al., 2020). Little standardization of study protocols, including harmonization of tests, is done, and consecutively, seroprevalence rates and risk factors are only marginally comparable between studies and countries. This scoping review therefor stands as a roadmap to compiling and understanding available data on the COVID-19 burden in HCWs across Africa, a region in need for increased investment in research (Guleid et al., 2021). This roadmap provides the most up-to-date synthesis of all available research and its gaps. It is a beneficial resource for larger projects such as the planned representative national studies by the Africa CDC (Maeda and Nkengasong, 2021) and surveillance unity studies by the World Health Organization (WHO, 2020a). This serological data will help to assess the true burden and risk factors among HCWs across the African continent and hence provide knowledge to more strategically apply limited resources in response aspects such as IPC measures and vaccine programmes. Furthermore, serological data provides the possibility to make vaccination prioritization more efficient with adaptations such as prioritization of HCWs without detectible antibodies or single-dose vaccination of those who are seropositive (Krammer et al., 2021). Such a strategic development of the vaccination programme could better utilize limited vaccination resources given the high seroprevalence rate of HCWs found in some African countries.
Conclusion
This scoping review found a varying, but often high SARS-CoV-2 seroprevalence among HCWs in the African region. HCWs of older age and lower education, as well as nurses and those working in gynaecology, emergency department, outpatient clinic and operating rooms are at even higher risk. These results point to a clear need to target specific risks of HCWs, whether they arise due to their social position or due to increased exposure of certain functions/settings within the clinic. Further research is needed to better understand which factors lead to increased risk of certain cadres of health workers. Asymptomatic rates were high and half of the studies recommended routine testing of HCWs. Public health strategies should take these risk aspects and the partly high seroprevalence among HCWs into account when prioritizing IPC measures and high-risk groups for vaccine deployment.
Supplementary Material
Acknowledgements
We would like to thank Jessica Sturm and the team at RKI library for their support.
Contributor Information
Sophie Alice Müller, Centre for International Health Protection, Robert Koch Institute, Nordufer 20, Berlin 13353, Germany.
Rebekah Ruth Wood, Evidence-Based Public Health, Centre for International Health Protection, Robert Koch Institute, Nordufer 20, Berlin 13353, Germany.
Johanna Hanefeld, Centre for International Health Protection, Robert Koch Institute, Nordufer 20, Berlin 13353, Germany.
Charbel El-Bcheraoui, Evidence-Based Public Health, Centre for International Health Protection, Robert Koch Institute, Nordufer 20, Berlin 13353, Germany.
Abbreviations
CI: Confidence interval
HCW: Healthcare worker
HR: Hazard ratio
IPC: Infection prevention and control
JBI: Joanna Briggs Institute
NA: Not applicable
NR: Not reported
OR: Odds ratio
PPE: Personal protective equipment
PRISMA-ScR: Preferred Reporting Items for systematic reviews and Meta-Analyses extension for Scoping Reviews
SARS-CoV-2: severe acute respiratory syndrome coronavirus type 2
Supplementary data
Supplementary data are available at Health Policy and Planning online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Funding
No funding is received for this work.
Ethical approval.
Ethical approval for this type of research is not required.
Conflict of interest statement.
The authors declare that they have no competing interests.
References
- Abdelmoniem R, Fouad R, Shawky S et al. 2021. SARS-CoV-2 infection among asymptomatic healthcare workers of the emergency department in a tertiary care facility. Journal of Clinical Virology 134: 104710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Africa CDC . 2019. Framework for Development of National Public Health Institutes in Africa. https://africacdc.org/download/framework-for-development-of-national-public-health-institutes-in-africa/, accessed 13 May 2021.
- Africa CDC . 2020. Africa CDC Establishes Continent-Wide Task Force to Respond to Global Coronavirus Epidemic. https://africacdc.org/news-item/africa-cdc-establishes-continent-wide-task-force-to-respond-to-global-coronavirus-epidemic/, accessed 12 May 2021.
- Arksey H, O’malley L. 2005. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology 8: 19–32. [Google Scholar]
- Arora RK, Joseph A, Van WYK et al. 2021. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. The Lancet Infectious Diseases 21: e75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayebare RR, Flick R, Okware S, Bodo B, Lamorde M. 2020. Adoption of COVID-19 triage strategies for low-income settings. The Lancet Respiratory Medicine 8: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biocentury . 2020. Good Test Hunting: FDA Authorizations Point to Benchmarks for COVID-19 Serology. https://www.biocentury.com/article/305095/good-test-hunting-fda-authorizations-point-to-benchmarks-for-covid-19-serology, accessed 29 April 2021.
- Centers for Disease Control and Prevention . 2021. COVID-19 Serology Surveillance Strategy. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/serology-surveillance/index.html, accessed 11 May 2021.
- Chibwana MG, Jere KC, Kamng’ona R et al. 2020. High SARS-CoV-2 seroprevalence in health care workers but relatively low numbers of deaths in urban Malawi. medRxiv, 10.1101/2020.07.30.20164970. [Google Scholar]
- Chisale MRO, Ramazanu S, Mwale SE et al. 2021. Seroprevalence of anti-SARS-CoV-2 antibodies in Africa: a systematic review and meta-analysis. Reviews in Medical Virology e2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitungo I, Dzobo M, Hlongwa M, Dzinamarira T. 2020. COVID-19: unpacking the low number of cases in Africa. Public Health in Practice 1: 100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AN, Ramatowski JW, Lassmann B et al. 2019. Global infection prevention gaps, needs, and utilization of educational resources: a cross-sectional assessment by the International Society for Infectious Diseases. International Journal of Infectious Diseases 82: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . 2021. Risk Factors and Risk Groups. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/risk-factors-risk-groups, accessed 8 June 2021.
- Elfakhri A, Abdelghffar A, Elhassi A et al. 2020. Population-based random survey for detection of COVID-19 infection and seroprevalence in Benghazi-Libya May/2020. Journal of Preventive Medicine 5: 19. [Google Scholar]
- Etyang AO, Lucinde R, Karanja H et al. 2021. Seroprevalence of antibodies to SARS-CoV-2 among health care workers in Kenya. Clinical Infectious Diseases, 10.1093/cid/ciab346. [Google Scholar]
- Fwoloshi S, Hines JZ, Barradas DT et al. 2021. Prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among health care workers-Zambia, July 2020. Clinical Infectious Diseases 73: e1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. 2020. Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: a systematic review and meta-analysis. The Journal of Hospital Infection 108: 120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis SA, Hafez HM, Elarab HE et al. 2021. SARS-CoV-2 PCR positivity rate and seroprevalence of related antibodies among a sample of patients in Cairo: pre-wave 2 results of a screening program in a university hospital. PLoSOne 16: e0254581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt D, Johnson M, Falup-pecurariu O et al. 2021. Cross sectional prevalence of SARS-CoV-2 antibodies in health care workers in paediatric facilities in eight countries. Journal of Hospital Infection 110: 60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleid FH, Oyando R, Kabia E et al. 2021. A bibliometric analysis of COVID-19 research in Africa. BMJ Global Health 6: e005690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatoko WA, Konu YR, Gbeasor-komlanvi FA et al. 2020. Prevalence of SARS-CoV-2 among high-risk populations in Lomé (Togo) in 2020. PLoS One 15: e0242124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Yi GY, Zhu Y. 2020. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: meta-analysis and sensitivity analysis. Journal of Medical Virology 92: 2543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines J, Fwoloshi S, Kampamba D et al. 2021. SARS-CoV-2 prevalence among outpatients during community transmission, Zambia, July 2020. Emerging Infectious Disease Journal 27: 2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijeoma I, Emmanuel N, Uchenna O et al. 2021. Sero-pravelence of SARS CoV-2 IgM and IgG antibodies amongst blood donors in Nigeria. Research Square.
- Isho B, Abe KT, Zuo M et al. 2020. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Science Immunology 5: eabe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen K, Bundgaard H, Hasselbalch RB et al. 2020. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. The Lancet Infectious Diseases 20: 1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammon AM, El-arabi AA, Erhouma EA, Mehemed TM, Mohamed OA 2020. Seroprevalence of antibodies against SARS-CoV-2 among public community and health-care workers in Alzintan City of Libya. medRxiv 2020.05.25.20109470. [Google Scholar]
- Kassem AM, Talaat H, Shawky S et al. 2020. SARS-CoV-2 infection among healthcare workers of a gastroenterological service in a tertiary care facility. ArabJournal of Gastroenterology 21: 151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleynhans J, Tempia S, Wolter N et al. 2021. SARS-CoV-2 Seroprevalence in a rural and urban household cohort during first and second waves of infections, South Africa, July 2020–March 2021. Emerging Infectious Disease Journal 27. doi: 10.3201/eid2712.211465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Srivastava K, Alshammary H et al. 2021. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. New England Journal of Medicine 384: 1372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levac D, Colquhoun H, O’brien KK. 2010. Scoping studies: advancing the methodology. Implementation Science 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucinde R, Mugo D, Bottomley C et al. 2021. Sero-surveillance for IgG to SARS-CoV-2 at antenatal care clinics in two Kenyan referral hospitals. medRxiv 2021.02.05.21250735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda JM, Nkengasong JN. 2021. The puzzle of the COVID-19 pandemic in Africa. Science 371: 27–8. [DOI] [PubMed] [Google Scholar]
- Majiya H, Aliyu-paiko M, Balogu VT et al. 2020. Seroprevalence of COVID-19 in Niger State. medRxiv 2020.08.04.20168112. [Google Scholar]
- Marion V, Laurette M, Wendy S et al. 2021. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in South Africa during the period January-May 2021. Research Square.
- Massinga Loembé M, Tshangela A, Salyer SJ et al. 2020. COVID-19 in Africa: the spread and response. Nature Medicine 26: 999–1003. [DOI] [PubMed] [Google Scholar]
- Maze MJ, Bassat Q, Feasey NA et al. 2018. The epidemiology of febrile illness in sub-Saharan Africa: implications for diagnosis and management. Clinical Microbiology and Infection 24: 808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda N, Dabis F, Desenclos J-C, Crespin X, Delfraissy J-F. 2016. Network for strong, national, public health institutes in west Africa. The Lancet 387: 2196–7. [DOI] [PubMed] [Google Scholar]
- Mostafa A, Kandil S, El-sayed MH et al. 2020. Universal COVID-19 screening of 4040 health care workers in a resource-limited setting: an Egyptian pilot model in a university with 12 public hospitals and medical centers. International Journal of Epidemiology 50: 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa A, Kandil S, El-sayed MH et al. 2021. SARS-CoV-2 seroconversion among 4040 Egyptian healthcare workers in 12 resource-limited healthcare facilities: a prospective cohort study. International Journal of Infectious Diseases 104: 534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar A, Afishawy M, Alkhatib E et al. 2021. Asymptomatic SARS-CoV-2 infection among healthcare workers in a non-COVID-19 Teaching University Hospital. Journal ofPublic HealthResearch 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukwege D, Byabene AK, Akonkwa EM et al. 2021. High SARS-CoV-2 seroprevalence in healthcare workers in Bukavu, Eastern Democratic Republic of Congo. The American Journal of Tropical Medicine and Hygiene 104: 1526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga LB, Hines JZ, Fwoloshi S et al. 2021. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: a cross-sectional cluster sample survey. The Lancet Global Health 9: e773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn Z,MS, Lisy K, Riitano D, Tufanaru C. 2015. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. International Journal of Evidence-Based Healthcare 13: 147–53. [DOI] [PubMed] [Google Scholar]
- Musa S, Abdel Alem S, Amer K et al. 2021. Prevalence of SARS-CoV-2 infection and dynamics of antibodies response among previously undiagnosed healthcare workers in a university hospital: a prospective cohort study. Journal of Infection and Public Health 14: 1466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngere I, Dawa J, Hunsperger E et al. 2021. High seroprevalence of SARS-CoV-2 but low infection fatality ratio eight months after introduction in Nairobi, Kenya. International Journal of Infectious Diseases 112: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Drew DA, Graham MS et al. 2020. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. The Lancet Public Health 5: e475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkuba AN, Makiala SM, Guichet E et al. 2021. High prevalence of anti–severe acute respiratory syndrome coronavirus 2 (Anti–SARS-CoV-2) antibodies after the first wave of coronavirus disease 2019 (COVID-19) in Kinshasa, Democratic Republic of the Congo: results of a cross-sectional household-based survey. Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkuba Ndaye A, Hoxha A, Madinga J et al. 2021. Challenges in interpreting SARS-CoV-2 serological results in African countries. The LancetGlobal Health 9: e588–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okpala OV, Dim CC, Ugwu CI et al. 2021. Population seroprevalence of SARS-CoV-2 antibodies in Anambra State, South-East, Nigeria. International Journal of Infectious Diseases 110: 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayanju O, Bamidele O, Edem F et al. 2020. SARS-CoV-2 seropositivity in asymptomatic frontline health workers in Ibadan, Nigeria. The American Journal of Tropical Medicine and Hygiene 104: 91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Our World in Data . 2021. COVID-19 Vaccination Policy. https://ourworldindata.org/grapher/covid-vaccination-policy?region=Africa&country=∼VUT, accessed 15 May 2021.
- Our-World-in-Data . 2015. Healthcare Access and Quality Index. https://ourworldindata.org/grapher/healthcare-access-and-quality-index, accessed 23 February 2021.
- Peeling RW, Wedderburn CJ, Garcia PJ et al. 2020. Serology testing in the COVID-19 pandemic response. The Lancet Infectious Diseases 20: e245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters Mdj GC, Mcinerney P, Munn Z, Tricco AC, Khalil H 2020. Chapter 11: scoping reviews (2020 version). In: Aromataris E, Munn Z (eds). JBI Manual for Evidence Synthesis, JBI, 2020. https://synthesismanual.jbi.global. 10.46658/JBIMES-20-12. [Google Scholar]
- Philippe BK, Aimé M, Prince A et al. 2021. Séroprévalence des anticorps anti-SARS-CoV-2 parmi les voyageurs et travailleurs dépistés à la clinique Saint Luc de Bukavu, à l´Est de la République Démocratique du Congo, de mai en août 2020. PAMJ 38: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakaniko S, Sibanda EN, Mduluza T et al. 2021. SARS-CoV-2 serological testing in frontline health workers in Zimbabwe. PLOSNeglected Tropical Diseases 15: e0009254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem MLO, Sidiya MAMM, Eibih ABA et al. 2021. Serological tests for SARS-CoV-2 in a health workers population in Nouakchott-Mauritania. Pan African Medical Journal 38: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax H, Allegranzi B, Chraïti MN et al. 2009. The World Health Organization hand hygiene observation method. American Journal of InfectionControl 37: 827–34. [DOI] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W et al. 2018. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Annals of Internal Medicine 169: 467–73. [DOI] [PubMed] [Google Scholar]
- Tso FY, Lidenge SJ, Peña PB et al. 2020. High prevalence of pre-existing serological cross-reactivity against SARS-CoV-2 in sub-Sahara Africa. International Journal of Infectious Diseases 102: 577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ruit C, Lahri SA, Wallis LA 2020. Clinical teams’ experiences of crowding in public emergency centres in Cape Town, South Africa. African Journal of Emergency Medicine 10: 52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020a. Coronavirus Disease (COVID-19) Technical Guidance: The Unity Studies: Early Investigation Protocols. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations, accessed 09 February 2021.
- WHO . 2020b. Health Workers at Risk, Older Adults and Residents of Long-Term Care Facilities to be Prioritized for COVID-19 Vaccination. https://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/news/news/2020/11/health-workers-at-risk,-older-adults-and-residents-of-long-term-care-facilities-to-be-prioritized-for-covid-19-vaccination, accessed 30 April 2021.
- WHO . 2021a. Global Health Workforce Statistics Database. https://www.who.int/data/gho/data/themes/topics/health-workforce, accessed 19 May 2021.
- WHO . 2021b. Weekly Epidemiological Update - 2 February 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update—2-February-2021, accessed 09 February 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


