Abstract
Objective
To identify which factors influence humoral response to coronavirus disease 2019 (COVID-19) vaccination in rituximab (RTX)-treated patients.
Methods
This was an observational, prospective, usual care study including consecutive patients with inflammatory rheumatic diseases in maintenance therapy with RTX. All patients received a two-dose regimen COVID-19 vaccination. Serum IgG antibody levels against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike proteins were measured at the time of the new RTX infusion.
Results
From the recruited patients, 16/45 (36%) produced antibodies reaching the assay cut-off value of 15 AU/ml and 29/45 (64%) had a negative serology. Within RTX-treated patients, 25 (56%) had undetectable B cells. Negative serology was associated with undetectable B cells (24/25 vs 5/20, P < 0.001). Moreover, SARS-CoV-2 spike antibodies correlated with CD19 counts (r = 0.86, P < 0.001). The effect of RTX and MTX was additive in terms of seroconversion rates (23% vs 50% in patients receiving RTX in monotherapy, P = 0.12) and SARS-CoV-2 spike antibody levels [3.80 (95% CI 3.80, 7.50) vs 75 (95% CI 3.8, 353) AU/ml in patients receiving RTX in monotherapy; P = 0.025]. Multivariate analyses including demographics, disease characteristics, gammaglobulin levels, RTX and other therapies used, CD19 counts, and the time between the last RTX infusion and vaccination identified detectable B cells as the only variable independently associated with seropositivity [odds ratio 35.2 (95% CI 3.59, 344.20)].
Conclusions
B cell depletion is the main independent contributing factor of antibody response to SARS-CoV-2 vaccination in RTX-treated patients. Monitoring CD19 may be of interest to identify the most appropriate period to perform vaccination.
Keywords: COVID-19, vaccination, rituximab, chronic inflammatory rheumatic disorder
Rheumatology key messages
Treatment with rituximab significantly reduce vaccine-induced humoral response.
B cell counts are the main contributing factor of antibody response to SARS-CoV-2 vaccination in rituximab-treated patients.
Monitoring CD19 may be of interest to identify the most appropriate period to perform SARS-CoV-2 vaccination.
Introduction
Rituximab (RTX) is a recognized therapeutic option to treat inflammatory rheumatic disorders. Recent studies have reported the risk of more severe coronavirus disease 2019 (COVID-19) infections in patients receiving RTX [1, 2], highlighting that B cells are critically implicated in viral response. These results have led the French health authorities to consider patients receiving RTX at extremely high priority for anti-severe acute respiratory syndrome coronavirus 2 (anti-SARS-CoV-2) vaccination. However, a major issue relates to the risk of reduced vaccination efficacy in RTX-treated patients. Preliminary studies have shown that RTX treatment might affect the antibody response to SARS-CoV-2 vaccination [3–6]. However, these data were mostly retrospective and were obtained on a limited number of patients, and several important parameters were not or only partially considered when analysing the factors influencing antibody response, including CD19 counts, gammaglobulin levels, cumulative RTX dose, concomitant CS and conventional synthetic DMARDs intake, as well as the underlying diseases. Thus, our aim was to assess antibody response to COVID-19 vaccines in patients undergoing RTX infusion at the end of the treatment interval.
Methods
Study design and patients
This was a prospective, observational, usual care study including consecutive patients hospitalized in the rheumatology department of Cochin Hospital to receive a new RTX infusion between April and June 2021. All patients were in maintenance therapy with RTX for their chronic inflammatory rheumatic disorder and all received at least two doses of a COVID-19 vaccination with BNT162b2 Pfizer/BioNTech or AZD1222 AstraZeneca prior to the new RTX infusion. The protocol and the informed consent document have received Institutional Review Board/Independent Ethics Committee (IRB/IEC) approval before initiation of the study (‘Comité de Protection des Personnes’ Paris Ile de France I, no. CPPIDF-DAP13). The study was declared to the Commission Nationale de l’Informatique et des Libertés (reference 2222937). All patients for our institution (AP-HP) are informed that their clinical data can be used for research and give their consent for the use of their data unless they provide an opposition to it. All patients agreed to participate in this study after written informed consent, which was recorded in the medical source file.
Data collection
We systematically collected patients’ demographic characteristics, underlying disease, disease duration and current medications including cumulative RTX doses. Routine blood tests were performed up to 1 week before the new RTX infusion and included complete blood cell counts and gammaglobulin levels. T, B and NK cell immunophenotyping was performed the day of RTX infusion (Aquios, Beckman Coulter, Pasadena, Ca, USA). The limit of detection of B cells was defined by CD19 <18/µL.
Anti-SARS-CoV-2 testing
COVID-19 serology was performed the day of the new RTX infusion. The LIAISON® SARS-CoV-2 S1/S2 IgG immunoassay (Diasorin, Saluggia, Italy) was used for the quantitative determination of antibodies to the receptor-binding domain of the viral spike protein [6]. Seropositivity was defined by SARS-CoV-2 spike antibodies >15 UA/ml. Previous SARS-CoV-2 infection was ruled out by measuring nucleocapsid-specific antibodies with the AdviseDx SARS-CoV-2 IgG II assay (Abbott microparticulate chemiluminescent immunoassay) [7].
Statistical analysis
All data are expressed as median values with 95% CI or number and percentage (%) for continuous and categorical variables, respectively, unless stated otherwise. Statistical analysis was performed using GraphPad Prism (v9.1.2) and Medcalc (v18.9.1).
Correlations between numeric variables were assessed using Spearman’s rank correlation test. For a two-group comparison, Mann–Whitney test was used (continuous variables). The χ2 test was used to seek differences in frequency (binary variables). A multivariate analysis by logistic regression was also performed to determine the factors independently associated with seropositivity. All relevant identified covariates were entered in the model in one single step. Odds ratios (OR) and 95% CI were then calculated. In this model, a P-value <0.05 was considered statistically significant.
Results
Study population
We included 45 patients (39 females) on maintenance therapy with RTX, with a median age of 66 (95% CI 57, 69) years and a median disease duration of 18 (95% CI 15, 23) years (Table 1). Most patients had RA (34 patients, 76%). Other diseases included SSc (n = 5), SLE (n = 2), MCTD (n = 2) and SS (n = 2) (Table 1).
Table 1.
Study population
| Total (n = 45) | Patients with positive serology (n = 16) | Patients with negative serology (n = 29) | |
|---|---|---|---|
| Age (years), median (95% CI) | 66 (57, 69) | 67 (57, 71) | 63 (55, 69) |
| Females, n (%) | 39 (87) | 16 (100) | 23 (79) |
| BMI (kg/m2), median (95% CI) | 25.6 (23.3, 29.0) | 27.2 (21.6, 31.3) | 25.6 (23.0, 28) |
| BMI >30 kg/m2, n (%) | 10 (34) | 5 (31) | 5 (17) |
| Underlying disease, n | |||
| RA | 34 | 14 | 20 |
| SSc | 5 | 0 | 5 |
| SLE | 2 | 1 | 1 |
| SS | 2 | 0 | 2 |
| MCTD | 2 | 0 | 2 |
| Disease duration (years), median (95% CI) | 18 (15, 23) | 21 (16, 28) | 16 (13, 23) |
| Associated csDMARDs, n (%) | 35 (78) | 11 (69) | 24 (83) |
| MTX, n (%) | 26 (58) | 6 (37.5) | 20 (69) |
| LEF, n (%) | 6 (13) | 2 (12.5) | 4 (14) |
| Other, n (%) | 3 (7) | 3 (19) | 0 0 |
| Current treatment with CS, n (%) | 16 (36) | 4 (26) | 12 (41) |
| CS dose >10 mg/day, n (%) | 0 0 | 0 0 | 0 0 |
| Cumulative RTX dose, median (95% CI) | 5 (3.5, 8) | 6 (2, 10) | 4 (3.5, 7) |
| Cumulative RTX dose <5 g, n (%) | 22 (49) | 6 (38) | 16 (57) |
| Cumulative RTX dose 5–10 g, n (%) | 11 (24) | 5 (31) | 6 (21) |
| Cumulative RTX dose >10 g, n (%) | 12 (27) | 5 (31) | 7 (24) |
| Gammaglobulins, median (95% CI) | 9.1 (8.5, 10.2) | 9.5 (7.8, 11.7) | 9.1 (8.4, 10.3) |
| Gammaglobulin levels <8 g/L, n (%) | 11 (24) | 4 (25) | 7 (24) |
| CD19+ (/µL) (100–600), median (95% CI) | 18 (18, 39) | 100 (50, 150) | 18 (18, 18) |
| CD19 <18/µL, n (%) | 25 (56) | 1 (6.3) | 24 (83) |
| CD3+ (/µL) (850–2500), median (95% CI) | 1239 (110, 1393) | 1363 (1121, 1729) | 1189 (994, 1366) |
| CD3+CD4+ (/µL) (500–1600), median (95% CI) | 897 (757, 974) | 912 (799, 1193) | 774 (596, 972) |
| CD3+CD8+ (/µL) (250–1000), median (95% CI) | 362 (290, 411) | 386 (236, 623) | 354 (271, 417) |
| CD3+CD16+CD56+ (/µL) (100–600), median (95% CI) | 180 (164, 221) | 201 (149, 256) | 178 (151, 227) |
RTX: rituximab; csDMARDs: conventional synthetic DMARDs.
The median cumulative dose of RTX was 5 (95% CI 3.5, 8) g, with 12 patients receiving >10 g. B cells were undetectable in 25 patients (56%). A total of 35 patients were treated with concomitant conventional synthetic DMARDs and 16 with CS (all with a dose <10 mg/day). No change of immunomodulatory drugs or CS after the first or second vaccine doses were reported.
Effect of RTX on the immunogenicity of COVID-19 vaccination
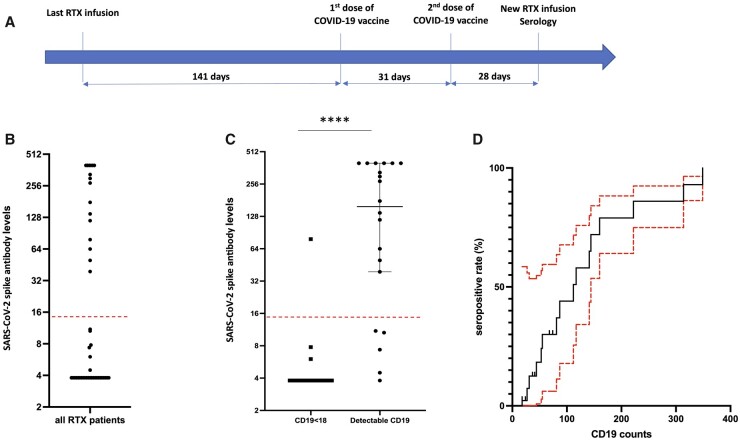
Forty-five patients received a two-dose regimen of COVID-19 vaccination either with BNT162b2 Pfizer/BioNTech (N = 42) or with AZD1222 AstraZeneca (n = 3). The median interval between the last RTX infusion and the first dose of vaccine was 141 (95% CI 126, 177) days, and the median interval between the infusion and the second dose was 170 (95% CI 157, 198) days (Fig. 1A).
Fig. 1.
Antibody response to SARS-CoV-2 vaccination according to B cell depletion
(A) Schematic diagram representing the median time between the last RTX infusion, the first and second doses of vaccine, and the sampling for seropositivity testing the day of the new RTX infusion. (B) Distribution of SARS-CoV-2 spike antibody levels in the whole cohort. (C) S1/S2 antibody levels according to RTX-induced B cell depletion. Results are expressed as medians and 95% CI. ****P < 0.0001 by Mann–Whitney test. Dotted red line corresponds to the seropositivity threshold. (D) Cumulative seropositive rate (with 95% CI in red) according to CD19 counts. COVID-19: coronavirus disease 2019; RTX: rituximab; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
The median interval between the first dose of vaccine and the patient visit aiming at new RTX infusion was 61.5 (95% CI 54, 76) days (Fig. 1A). The seropositivity rate was 36% (16/45 patients) in RTX-treated patients, with SARS-CoV-2 spike antibodies ranging from 3.80 to >400 UA/ml (Fig. 1B). In the subset of patients with RA (supplementary Table S1, available at Rheumatology online), the seropositivity rate was 41% (14/34 patients). No antibodies against the nucleocapsid were detected.
Factors associated with the lack humoral response to vaccination in RTX-treated patients
Negative serology was more likely to occur in patients with undetectable B cells (24/25 vs 5/20, P < 0.001) and SARS-CoV-2 spike antibodies were strikingly lower in patients with undetectable B cells compared with patients with detectable CD19+ cells [3.80 (95% CI 3.80, 3.80) vs 158 (95% CI 39, 400) AU/ml; P < 0.001] (Fig. 1C). Interestingly, B cells were significantly lower in the five patients with detectable B cells and negative serology [41 (95% CI 24, 74) AU/ml] compared with the 15 patients with detectable B cells and positive serology [112 (95% CI 53, 160) AU/ml; P = 0.042]. In addition, SARS-CoV-2 spike antibodies positively correlated with CD19 counts (r = 0.86, P < 0.001) and cumulative seropositive rate gradually increased according to CD19 counts (Fig. 1D). These results were similar in the RA subgroup: a negative serology was more frequently observed in patients with undetectable B cells (17/18 vs 3/16, P < 0.001) and SARS-CoV-2 spike antibodies were strikingly lower in patients with undetectable B cells [3.80 (95% CI 3.80, 3.80) vs 225 (95% CI 46, 400) AU/ml; P < 0.001].
The time interval between the last administration of RTX and COVID-19 vaccination had a significant impact on the vaccine’s immunogenicity (Supplementary Fig. S1A, available at Rheumatology online). Seropositivity rate in patients vaccinated within <6 months after RTX treatment was 23% (7/30 patients) but increased to 60% (9/15 patients) in patients vaccinated at least 6 months after RTX treatment (P = 0.017), and SARS-CoV-2 spike antibodies were significantly increased in patients vaccinated at least 6 months after RTX treatment (Supplementary Fig. S1B, available at Rheumatology online).
RTX and MTX had additive effects in terms of seroconversion and antibody levels: the seropositivity rate was 23% (6/26 patients) in patients receiving both RTX and MTX compared with 50% (5/10 patients) in patients treated with RTX in monotherapy (P = 0.12), and SARS-CoV-2 spike antibody levels were significantly lower in patients receiving both RTX and MTX [3.80 (95% CI 3.80, 3.75) vs 75 (95% CI 3.8, 353) AU/ml; P = 0.025].
Age, gender, the underlying disease, BMI, gammaglobulin levels, cumulative RTX dose and concomitant treatment with CS did not impact seropositivity rates and SARS-CoV-2 spike antibody levels (Table 1 and supplementary Table S2, available at Rheumatology online). Multivariate logistic regression analysis including as covariates age, gender, a BMI >30 kg/m2, the underlying disease, cumulative RTX dose >10 g, detectable B cells (CD19 >18/µL), a time between the last RTX infusion and COVID-19 vaccination >6 months, gammaglobulin levels <8 g/l, current MTX intake and CS identified detectable B cells as the only variable independently associated with seropositivity [OR 35.2 (95% CI 3.59, 344.20)]. A second analysis was performed including only clinical variables: age, gender, a BMI >30 kg/m2, the underlying disease, cumulative RTX dose >10 g, a time between the last RTX infusion and COVID-19 vaccination >6 months, current MTX intake and CS. In this model, a time between the last RTX infusion and COVID-19 vaccination >6 months was the only variable associated with seropositivity [OR 0.13 (95% CI 0.02, 0.85)].
Discussion
Our results show that treatment with RTX significantly reduced vaccine-induced humoral response, with seropositivity of 36%, which is consistent with previous reported data from observational studies (between 33% and 41%) [5, 6, 8]. B cell depletion has a critical role in influencing the response to the vaccine—indeed, it must be underlined that only a single patient from the 25 patients who were RTX-treated and exhibiting undetectable circulating B-cells had a positive serology. Several studies have reported that B cell depletion at the time of vaccination was associated with failure to seroconvert [4, 5, 9, 10]. A preliminary study including five RTX-treated patients showed no antibody response in three patients with complete B cell depletion [4]. In a second study involving 30 RTX-treated patients and for which B cell reconstitution was available for 11 patients, no serological response was observed in the 4 patients with undetectable B cells [5]. In a third study including 74 patients under RTX, the 36 patients without detectable CD19+ peripheral B cells did not develop specific antibodies, except for 1 patient [8]. Interestingly, seropositivity was observed in patients with detectable CD19+ B cells, and the degree of B cell recovery correlated with the extent of SARS-CoV-2 spike antibody levels, suggesting the development of humoral immune response once peripheral B cells are repopulated. Other potential determinants for antibody response were the interval between the administration of RTX and the vaccination, with a longer time allowing an increased chance of B cell repopulation and response to vaccination and concomitant treatment with MTX, which further decreased seroconversion rates and antibody levels, as previously reported [5]. Interestingly, gammaglobulin levels, and cumulative RTX dose and CS concomitant treatment did not influence antibody response, highlighting that these parameters should not be considered in vaccination decision making.
The absence of antibody response does not necessarily mean absence of response to vaccination and other facets of immunity may be developed by vaccination [11]. Indeed, several studies have revealed a T-cell-mediated immune response even in B-cell-depleted patients [4, 8–10]. Thus, it will be critical to assess the involvement of T cell immunity, which was not tested in this study, to protect patients against infection with the virus on vaccination, considering the trend for decreased CD3+CD4+ and CD3+CD8+ T cells in patients with no antibody response to COVID-19 vaccination in our cohort.
Our findings show that B cell counts are the main contributing factor of antibody response in RTX-treated patients. Postponing treatment with RTX in patients with stable quiescent disease might be considered to improve immunogenicity but still needs to be demonstrated. Another option would be to monitor CD19 to detect a window of opportunity for vaccination at the time of B cell repopulation. Further studies evaluating the potential interest of additional vaccine doses in patients treated with RTX and an impaired humoral response might also shed some light on the optimal management of these patients.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work on this manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
All relevant anonymized patient-level data are available upon reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Avouac J, Drumez E, Hachulla E et al. ; FAIR/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 2021;3:e419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strangfeld A, Schäfer M, Gianfrancesco MA et al. ; COVID-19 Global Rheumatology Alliance. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyarsky BJ, Ruddy JA, Connolly CM et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1098–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonelli MM, Mrak D, Perkmann T, Haslacher H, Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis 2021;80:1355–6. [DOI] [PubMed] [Google Scholar]
- 5. Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis 2021;80:1357–9. [DOI] [PubMed] [Google Scholar]
- 6. Furer V, Eviatar T, Zisman D et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 7. Bryan A, Pepper G, Wener MH et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol 2020;58:e00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mrak D, Tobudic S, Koblischke M et al. SARS-CoV-2 vaccination in rituximab-treated patients: b cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021;80:1345–50. [DOI] [PubMed] [Google Scholar]
- 9. Prendecki M, Clarke C, Edwards H et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021;80:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simon D, Tascilar K, Schmidt K et al. Brief report: humoral and cellular immune responses to SARS-CoV-2 infection and vaccination in B cell depleted autoimmune patients. Arthritis Rheumatol 2021. (in press); doi: 10.1002/art.41914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Assen S, Holvast A, Benne CA et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 2010;62:75–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant anonymized patient-level data are available upon reasonable request to the corresponding author.