Abstract
STUDY QUESTION
Can severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA be detected in the reproductive tract of asymptomatic patients undergoing ART?
SUMMARY ANSWER
SARS-CoV-2 mRNA is not detectable in semen, follicular fluid, vaginal secretions or residual medulla from ovarian tissue cryopreservation procedures in asymptomatic patients who undergo ART, irrespective of the results of a triage questionnaire and a nasopharyngeal SARS-CoV-2 RNA detection test.
WHAT IS KNOWN ALREADY
The SARS-CoV-2 pandemic had a huge impact on the activities of fertility clinics. Although some studies reported the presence of SARS-CoV-2 mRNA in the reproductive system during or after acute COVID-19 symptomatic infections, uncertainties remain regarding the presence of viral mRNA in the reproductive material and follicular fluid of asymptomatic patients undergoing ART.
STUDY DESIGN, SIZE, DURATION
An observational cohort trial of residual material samples including semen, follicular fluid, vaginal secretions and ovarian medulla was conducted during the second pandemic wave in Brussels from September 2020 to April 2021.
PARTICIPANTS/MATERIALS, SETTING, METHODS
All patients who underwent ART (IUI, IVF/ICSI, oocyte and ovarian tissue cryopreservation) responded to a triage questionnaire at the beginning and end of the cycle and underwent nasopharyngeal swab collection for SARS-CoV-2 RNA detection by RT-PCR before the procedure according to standard recommendations. For semen analysis, only the questionnaire was requested the day before the sample collection. The ART cycles of patients with positive nasopharyngeal SARS-CoV-2 RNA detection tests and/or questionnaires were cancelled except for those that could not be postponed. After providing informed consent, swabs on residual materials were collected the day of the oocyte, ovarian tissue or semen collection and were processed for RT-qPCR.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 394 samples from 291 patients were analysed. Amongst them, 20 samples were obtained from patients with a positive questionnaire but negative nasopharyngeal SARS-CoV-2 test and 20 others were from patients with a positive nasopharyngeal SARS-CoV-2 test. The remaining samples were collected from patients with a negative or unknown nasopharyngeal SARS-CoV-2 test and/or a negative or unknown triage questionnaire. Viral RNA for SARS-CoV-2 was undetectable in all of the samples.
LIMITATIONS, REASONS FOR CAUTION
Considering the cancellation policy, only a limited number of samples from patients with positive triage questionnaires or nasopharyngeal SARS-CoV-2 tests were included in the analysis.
WIDER IMPLICATIONS OF THE FINDINGS
The study suggested that there was no risk of reproductive tract contamination by SARS-CoV-2 in asymptomatic patients, irrespective of the results from a triage questionnaire or nasopharyngeal SARS-CoV-2 test. The results suggested that no additional measures to prevent staff or cross-patient contamination need to be implemented in the IVF and andrology laboratories.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by the Université Libre de Bruxelles and by a grant from Ferring. A.D. and I.D. received a grant from Ferring for the study. The authors have no other conflict of interest to declare related to this study.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: COVID-19, follicular fluid, semen, vaginal secretions, ovarian tissue, cryopreservation, IVF/ICSI, SARS-CoV-2
Introduction
A new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China in December 2019. Since then, the coronavirus 2019 disease (COVID-19) has infected more than 103 million people worldwide, with more than 2 million deaths confirmed as of January 2021 (WHO COVID Group, 2020). The pandemic had a huge impact on most medical fields, including fertility clinics. Like most fertility centres, all ART activities were interrupted in Belgium (except for oncofertility) in March 2020, including at our centre, and a slow restart was initiated in June 2020 in accordance with local and international recommendations regarding the implementation of specific sanitary measures (ESHRE COVID-19 Working Group, 2020). However, one major concern remains the possible impact of COVID-19 infection on both male and female reproductive health in terms of sexual transmission, vertical transmission and ART outcomes.
To enter the human cell, the SARS CoV-2 virus expresses a membrane Spike-glycoprotein, which binds to its receptor, angiotensin-converting enzyme 2 (ACE2) and uses TransMembrane Serine PRotease 2 (TMPRSS2) as an entry activator in the host cell (Ou et al., 2020; Yan et al., 2020). Viral RNA is released, and then viral replication and transcription occur in the host cell, leading to viral infection. In addition to lung cells, receptors are expressed in other types of cells, including the testis where both Leydig and Sertoli cells highly express ACE2 receptor (Wang and Xu, 2020). Although co-expression of ACE2 and TMPRSS2 was found only in spermatogonia stem cells and spermatids, this observation suggested that viral infection could potentially harm the testes and compromise fertility status (Massarotti et al., 2021; Morelli et al., 2021).
The risks of testis infection and sexual transmission of the virus remain uncertain, although a small study has reported the presence of SARS-CoV-2 in 6 out of 38 semen samples analysed (Li et al., 2020). The presence of SARS-CoV-2 was also reported in the testicular tissue of one patient out of 10 evaluated at autopsy (Yang et al., 2020). Other studies have failed to detect SARS-CoV-2 RNA in the testes from autopsies of men who had previously tested positive for SARS-CoV-2 but the swelling of Sertoli cells and elongation of spermatids suggested an acute testicular injury (Flaifel et al., 2021).
A few studies have shown that SARS-CoV-2 can also indirectly affect the male reproductive system and that spermatogenesis can be altered during and after COVID-19 infection (PaoLi et al., 2020). Postmortem studies have suggested that coronavirus infection can lead to orchitis (Xu et al., 2006). Moreover, fever and inflammation following COVID-19 infection can lead to lower sperm counts and higher DNA fragmentation in symptomatic patients (Holtmann et al., 2020; Li et al., 2020). In infertile patients with altered sperm parameters, fever could have an even more deleterious effect (Hamdi et al., 2020).
Based on the available transcriptomic data, co-expression of ACE2 and TMPRSS2 is also observed in oocytes, but the possible impact of SARS-CoV-2 on reproduction is unknown. Indeed, there is no evidence at present that contamination of female reproductive cells can occur in vivo or in vitro in an ART setting (Rajput et al., 2021). One study evaluated viral mRNA in 16 oocytes from two SARS-CoV-2-positive women and all samples were negative (Barragan et al., 2021). Two other groups did not find either SARS-CoV-2 in vaginal fluid and/or cervical exfoliated cells of symptomatic patients (Cui et al., 2020; Qiu et al., 2020). Nevertheless, data are scarce and the risk of the presence of SARS-CoV-2 in the seminal, follicular and vaginal fluids of asymptomatic and symptomatic patients during ART treatment remains uncertain (Morelli et al., 2021). Such data are therefore urgently needed for the safety of ART laboratory procedures (Tur-Kaspa et al., 2021).
We have conducted a prospective study on residual material during ART treatment in men and women to assess the risk of SARS-CoV-2 virus contamination of seminal, follicular and vaginal fluids as well as ovarian medulla.
Materials and methods
The COVid-ART (COVART) study was a prospective cohort trial on residual material conducted at Erasme Hospital University Medical Center in Brussels, Belgium during the second wave of COVID-19 infection, from September 2020 to April 2021.
The study was approved by the CUB-Erasme Ethical Review Committee and all participants provided informed consent to use the residual material for SARS-CoV-2 RNA detection tests.
Study participants
All men and women undergoing ART treatment or fertility evaluation including sperm analysis, IUI, ovarian stimulation cycle for IVF/ICSI, oocytes and ovarian tissue cryopreservation were invited to participate and sign an informed consent form for using the residual material (semen, follicular fluid, vaginal secretions and ovarian medulla) collected during the procedure for SARS-CoV-2 RNA detection tests.
All patients included in the COVART trial followed the standard procedure to evaluate their primary risk of current infection with SARS-CoV-2. Patients had to complete a triage questionnaire for symptoms of COVID-19 infection at the beginning of the cycle and/or the day before the procedure (Supplementary Table SI). For patients undergoing IVF or ICSI cycles, a nasopharyngeal swab for SARS-CoV-2 test by reverse transcription PCR (RT-PCR) was systematically collected between Day 5 and Day 10 of stimulation. In addition, a nasopharyngeal swab for SARS-CoV-2 testing was also required for patients with a positive questionnaire before undergoing sperm analysis and IUI.
Sample collection and RT-qPCR
The quantitative RT-qPCR methodology used in this study was validated by the national authorities (FAMPH—Federal Agency for Medicine and Health Products) for the detection of SARS-CoV2 in nasopharyngeal swabs in academic Belgian Institutions (Coupeau et al., 2020). Preliminary tests were performed to confirm both the efficacy of the method and the stability of the virus in other biological samples by detecting a defined quantity of SARS-CoV2 virus spiked in the viral universal transport medium (UTM) containing follicular fluids, vaginal secretion and semen swabs (FLOQSwabs, COPAN Diagnostics; Supplementary Fig. S1). A total of 13 samples were spiked with 1 µl of the SARS-CoV-2 viral suspension (kindly provided by Dr Laurent Busson from the CHU Saint-Pierre, Brussels), diluted at 1/10 000 based on previous experiments. All samples tested the day of collection or after 3 days of storage at 4°C (maximum timing before processing) showed positivity with appropriated cycle threshold (CT) values (Supplementary Fig. S2).
For the study, swabs were performed the day of the procedure (IUI, oocyte collection, semen analysis) on semen, follicular fluid or vaginal secretion, and directly immersed in 1 ml of viral UTM (solution mini, COPAN Diagnostics), previously validated for RT-qPC detection of SARS-CoV-2 virus. The vials were stored at 4°C in a secured location until inactivation (maximum 72 h). Medulla was frozen at −80°C before homogenization using MagNA Lyser (Roche Life Science). All vials were transported to the laboratory and disinfected under flow before inactivation in a Biosafety level 2 room. Each UTM sample (100 µl) was inactivated using 1 ml of TRIzol solution (LifeTechnologies) and mixed with an internal control (IC) (RNA sequence of the Schmallenberg virus (SBV) produced by invitro transcription from a plasmid encoding the cDNA sequence of SBV L segment, kindly provided by University of Namur, Belgium). Briefly, RNA was extracted using a chloroform protocol according to the validated protocol (Coupeau et al., 2020). Specific primers and probes (Supplementary Table SII) for SARS-CoV-2 and IC and master mix (Takyon™ One-Step Kit Converter, Eurogentec) were used for quantitative PCR according to manufacturer’s instructions. The Sars-Cov-2 sequence (product length: 113 pb) amplified by the primers corresponds to the virus gene coding for envelope protein (E). A positive SARS-CoV-2 control (SARS-CoV-2 virus amplified in Vero cell culture) was added to each plate as was a negative control (transport media; Supplementary Fig. S1). PCR reactions were performed in duplicates using 4 µl out of the 30 µl of extracted RNA in a Roche Light Cycler 480 (LC480) using the following program: (10 min at 48°C then 3 min at 95°C, 45 cycles 15 s at 95°C, 30 s at 58°C and 30 s at 40°C). Each plate was validated based on the results of the three controls (IC, SARS-CoV2 positive and negative controls). Results of each sample were validated based on the IC control (Supplementary Fig. S1). All samples with discordant results in the duplicate (CT > 1) were re-processed (REDO). The test was considered positive when CT < 40.
Statistics
The study aimed to evaluate the frequency of positive RT-qPCR test for SARS-CoV-2 in the semen, follicular fluid and vaginal secretions according to the results of the questionnaire and the nasopharyngeal SARS-CoV-2 test. As no ART samples were positive, frequencies were not compared between groups. Results of the CT values of the IC were compared using Student’s t-test.
Results
A total of 315 asymptomatic adult patients of reproductive age, including 181 female patients and 134 male patients were enrolled between September 2020 and April 2021 during the second wave of COVID-19 infections in Belgium. A total of 24 patients were excluded: IVF cycle cancelled (n = 4), no genital tract material swab performed (n = 14) and RT-qPCR not validated due to poor RNA quality (n = 6). A total of 291 patients were, therefore, included in this observational study. The majority of the patients participated once, only nine were tested during a second or a third cycle.
A total of 106 and 163 samples from follicular fluid and vaginal fluid, respectively, and 122 semen samples were processed (Table I). Three samples of residual medulla collected during ovarian tissue cryopreservation were also processed.
Table I.
Number of patients included in the study and samples analysed.
| Number of patients |
Number of samples |
|||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Total | FF | Vag | Semen | Medulla | Total | |
| Group 1 | 59 | 93 | 152 | 85 | 89 | 59 | 2 | 235 |
| Group 2 | 6 | 7 | 13 | 7 | 7 | 6 | 0 | 20 |
| Group 3 | 5 | 8 | 13 | 8 | 7 | 5 | 0 | 20 |
| Group 4 | 52 | 61 | 113 | 6 | 60 | 52 | 1 | 119 |
| Total | 122 | 169 | 291 | 106 | 163 | 122 | 3 | 394 |
Four groups were analysed according to the results of the triage questionnaire and/or SARS-CoV-2 testing: Group 1: negative or unknown triage questionnaire and negative nasopharyngeal SARS-CoV-2 test, Group 2: positive triage questionnaire and negative nasopharyngeal SARS-CoV-2 test, Group 3: positive nasopharyngeal SARS-CoV-2 test, Group 4: unknown status.
FF, follicular fluid; Vag, vaginal fluid.
The samples were then divided into four groups: samples from patients with negative or unknown triage questionnaire and negative nasopharyngeal SARS-CoV-2 test (Group 1, n = 235), samples from patients with positive triage questionnaire and negative nasopharyngeal SARS-CoV-2 test (Group 2, n = 20), samples from patients with positive nasopharyngeal SARS-CoV-2 test (Group 3, n = 20) and samples from patients with unknown status (no nasopharyngeal SARS-CoV-2 test) who underwent sperm analysis or IUI (Group 4, n = 119; Table I). Results from the triage questionnaire or nasopharyngeal SARS-CoV-2 tests performed more than 14 days before the collection were not considered for the analysis. The median times between questionnaires, nasopharyngeal tests and study samples collection are reported in Table II.
Table II.
Median timing between study samples collection and triage questionnaires or nasopharyngeal SARS-CoV-2 test in each group defined according to the results of SARS-CoV-2 risk evaluation.
| Results of SARS- CoV-2 risk evaluation | Median timing (days) (min–max) | |
|---|---|---|
| Group 1 | Questionnaire/−/or UK | 1 (0–13) |
| SARS-CoV-2 test/−/ | 5 (0–13) | |
| Group 2 | Questionnaire/+/ | 1 (0–7) |
| SARS-CoV-2 test/−/ | 7 (2–12) | |
| Group 3 | Questionnaire/+/or/−/ | 0 (0–11) |
| (1 questionnaire UK) | ||
| SARS-CoV-2 test/+/ | 4 (0–7) | |
| Group 4 | Questionnaire/+/or/−/ | 1 (0–10) |
| (5 questionnaires UK) | ||
| SARS-CoV-2 test UK | NA |
NA, not applicable; UK, unknown.
No positive RT-qPCR tests for SARS-CoV-2 were obtained in the samples of ovarian medulla, semen, follicular fluid or vaginal fluid in the four study groups. Interestingly, a significant difference in CT values for the IC was observed between samples, with the highest CT values for sperm samples, suggesting that the PCR sensitivity is lower in semen (Fig. 1).
Figure 1.
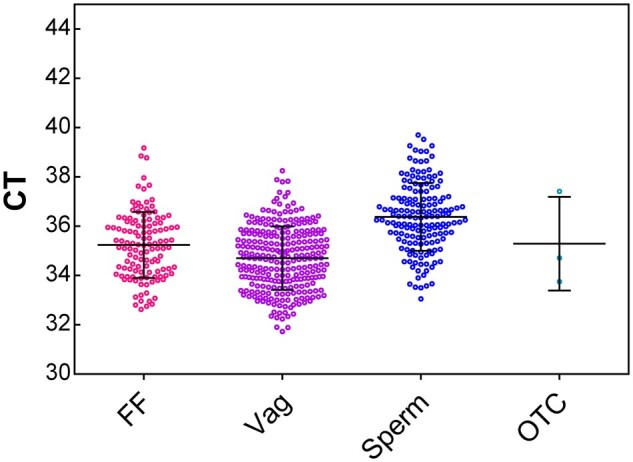
RT-qPCR results of positive internal control used during SARS-CoV-2 test according to the reproductive materials. Data are expressed as CT values (mean ± SD). CT, cycle threshold; FF, follicular fluid; OTC, ovarian tissue cryopreservation; Vag, vaginal secretions.
Pregnancy rates were evaluated in the four study groups for patients who had a fresh embryo transfer (IVF and ICSI cycles) and IUI (Table III), and no difference was observed. Patients who underwent oocyte or ovarian tissue cryopreservation and ‘freeze-all’ cycles were excluded from this analysis.
Table III.
Outcomes of the ART procedure in the different study groups for patients who had fresh embryo transfer.
| IUI |
IVF/ICSI |
||||
|---|---|---|---|---|---|
| N cycles | Pregnancy, n (%) | N cycles with ET | Pregnancy, n (%) | Unknown, n (%) | |
| Group 1 | 0 | 0 | 61 | 21 (34.4%) | 2 (3.3%) |
| Group 2 | 0 | 0 | 7 | 2 (28.6%) | 0 |
| Group 3 | 0 | 0 | 1 | 0 | 0 |
| Group 4 | 57 | 12 (21.0%) | 5 | 3 (60.0%) | 0 |
Group 1: Negative triage questionnaire or unknown + SARS-CoV-2 test negative; Group 2: Positive triage questionnaire + SARS-CoV-2 test negative; Group 3: SARS-CoV-2 test positive; Group 4: No SARS-CoV-2 test.
ET, embryo transfer.
Discussion
In this large prospective cohort study of adult patients undergoing ART treatment or semen analysis from September 2020 to April 2021, there were no positive SARS-CoV-2 RT-qPCR results among vaginal fluid, ovarian tissue, follicular fluid or sperm samples collected for analysis. With the 14-day cumulative number of positive COVID-19 cases per 100 000 reaching 637 for the time period (https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea), Belgium was considered to be in a critical situation during its second wave, based on ESHRE definitions (https://www.eshre.eu/Home/COVID19WG, 14 October 2020). Although this second wave affected all age groups, younger patients of reproductive age were more often infected during this wave compared to the first COVID-19 wave. In Belgium, around 7000 COVID-19-positive cases were diagnosed daily during the study period with a majority being asymptomatic patients. This was associated with a dramatic increase in hospital admissions (>200 patients/day) and the effective reproductive number (Rt) was 1.516 indicating that the pandemic was progressing quickly (https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea). Although Belgian fertility centres interrupted their activities during the first wave (March to June 2020), continuity of care was provided during the second wave under strict sanitary conditions. However, uncertainties remained regarding the risk of sexual transmission of the virus through intercourse and how to ensure safe practices. Moreover, as the ACE2 and TMPRSS2 virus receptors were identified in reproductive organs (Wang and Xu 2020), it became crucial to assess the risk of semen, ovarian tissue, follicular fluid and vaginal secretions for contamination with SARS-CoV-2 in patients undergoing ART cycles. This risk evaluation has important implications for lab procedures such as staff protection, sperm sampling rooms cleaning/disinfection, aeration of the rooms between consecutive patients and use of face masks during sperm retrieval to avoid semen contamination (Hamdi et al., 2020).
In a recent study, one out of every four patients who had recovered from COVID-19 had altered semen quality, suggesting that previous infection could affect spermatogenesis but viral RNA was not detected in any of the semen samples (Gacci et al., 2021). However, a limited number of patients were included and the previous status of semen parameters was unknown. One study reported the presence of viral RNA particles in the semen samples of 6 out of 38 men (Li et al., 2020). This study raised questions about the need for additional safety measures for the fertility clinic lab personnel and regarding the possibility of viral sexual transmission. However, the authors stated that this positive finding should be confirmed in future studies before making conclusions about the possibility of sexual transmission (Li et al., 2020). No additional positive viral RNA tests were reported among 18 semen samples from patients who had recovered after COVID-19 infection and two semen samples from acute COVID-19-infected patients (Holtmann et al., 2020). The presence of SARS CoV-2 RNA was also not detected in 61 prostatic secretions from recovered COVID-19 patients (Ruan et al., 2021). Another study reported one case of positive viral RNA in testicular tissue but the sample contained mainly fibrovascular tissue, suggesting that the virus may have been detected in the blood instead of the testicles (Yang et al., 2020). Electron microscopy confirmed the absence of viral particles (Yang et al., 2020).
Our study confirms the absence of SARS-CoV-2 in a large series of 122 semen samples from asymptomatic men during the second pandemic wave, irrespective of the results of a triage questionnaire or nasopharyngeal SARS-CoV-2 test. We observed no evidence of viral contamination or sexual transmission through the male reproductive tract in the absence of symptomatic COVID-19 infection. Interestingly, our study also suggested that the sensitivity of the testing could be reduced in semen. However, the results of the positive control (IC) remain were within the detection limits in all samples and the TRIzol method was previously described for Sars-CoV-2 detection in semen (Li et al., 2020; Best et al., 2021). RT-qPCR has also been used as a highly sensitive standard technique for detection of other viruses such as hepatitis B or HCV in sperm (Cassuto et al., 2002; Englert et al., 2004; Lesage et al., 2006; Pasquier et al., 2006).
In women, SARS-CoV-2 mRNA was not detected in a report that evaluated viral RNA levels in the follicular fluid from one COVID-19 infected woman (Demirel et al., 2021). In addition, another study reported that no viral RNA was detected in the oocytes of two SARS-CoV-2-positive patients (Barragan et al., 2021). Furthermore, another study confirmed the absence of SARS-CoV-2 in the cervical smears of 35 women (Cui et al., 2020) while another found that SARS-CoV-2 was not detectable in the vaginal fluid of 10 women with severe COVID-19 infection (Qiu et al., 2020). However, one study reported viral contamination in vaginal fluid in 2 out of 35 women hospitalized for acute SARS-CoV-2 infection (Schwartz et al., 2021).
We showed that SARS-CoV-2 RNA was undetectable in a cohort of 163 vaginal fluid samples, 106 follicular fluid samples and three ovarian medulla samples analysed. The median time interval from nasopharyngeal swab to genital tract material swab was 5 days. In our study, ART procedures were not cancelled in a few asymptomatic patients despite positive nasopharyngeal SARS-CoV-2 tests. Moreover, one patient underwent embryo transfer after thorough medical counselling as the infection was acquired more than 40 days prior to the date of the beginning of the ART cycle but the nasopharyngeal swab remained positive.
None of the women included in the study had positive SARS-CoV-2 results in follicular fluid or vaginal secretions. These results are reassuring regarding the possible risk of contamination in the ART laboratory. A recently published study confirmed that, in patients who tested negative for COVID-19 by nasopharyngeal swab before oocytes collection, SARS-CoV-2 was not identified in follicular fluid, vitrification solution and culture media (Rajput et al., 2021). We did not detect additional risk in asymptomatic, positive patients or when status was unknown.
In previously known sexually transmitted diseases (HBV, HCV, HIV), samples of follicular fluid, culture media or liquid nitrogen used for storage tested negative for viral RNA or DNA among seropositive patients with no evidence of cross-contamination (Cobo et al., 2012). Similarly, cross-contamination was considered to be a minimal risk during cryopreservation of reproductive tissues (Pomeroy et al., 2010). Our study further confirms the absence of viral RNA in 106 follicular fluid samples including those of eight patients who tested positive by nasopharyngeal swab.
Although a large number of samples were analysed, this study has some limitations. The number of patients who had a positive SARS-CoV-2 nasopharyngeal test was limited (Group 3, 20 samples) due to the systematic cancellation of the procedure for all symptomatic patients at the beginning or during their ART cycle. A large number of asymptomatic patients had unknown status (Group 4, 119 samples). Another limitation of this study is the accuracy of the questionnaire data as the timing differed between the study participants as it was based on patient compliance.
The strength of our prospective cohort study is that it is the largest study published to date assessing the risk of contamination of reproductive tract material with SARS-CoV-2 during ART and of sexual transmission. All patients were consecutively enrolled during the inclusion period, giving accurate information about the risk of contamination in the daily fertility clinic environment during the second wave. All samples were negative in patients undergoing IUI and sperm analysis despite the facts that triage questionnaire was the only COVID-19 risk assessment and that some patients had unknown questionnaire status. It is likely that some of these patients were infected with COVID-19 at the time of the procedure. These data suggest that no further sanitary measures need to be implemented in the fertility laboratory to avoid the risk of staff or cross-patient contamination for asymptomatic patients. In addition, this study is the first to include ovarian medulla samples collected during ovarian tissue during cryopreservation.
This observational cohort provides compelling evidence for the safety of handling reproductive tract material from asymptomatic patients, and additional safety measures do not need to be implemented by IVF laboratory staff to avoid cross-contamination.
Nevertheless, IVF/ICSI cycle postponement is always advised if patients develop symptoms or test positive during ovarian stimulation. In case postponement is not possible, a freeze-all strategy could be applied.
With the majority of fertility clinics staff being vaccinated for COVID-19, greater access to fertility treatments for the low-risk patient population can be offered, especially for vaccinated patients or patients with positive IgG levels for COVID-19.
According to the data reported in the COVART study, and to maintain ART clinic activities during pandemic waves, routine triage questionnaire at beginning of the cycle and RT-PCR testing of all patients during ART cycles has been shown to be effective.
Overall, this study suggests that SARS-CoV-2 contamination of reproductive tract material is unlikely or even nonexistent in asymptomatic patients. Further studies will be required to determine the possibility of applying further relaxation measures in ART clinics knowing that more people of reproductive age are being vaccinated.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
Anonymized data presented in this article can be shared upon request to the corresponding authors.
Supplementary Material
Acknowledgements
The authors wish to thank the staff of Erasme Fertility Clinic and the Research Laboratory on human reproduction for their support. We thank also the Université de Namur and the CHU Saint-Pierre for providing the positive controls. We acknowledge the contribution of a medical writer, Sandy Field, PhD, for English language editing of the manuscript.
Authors’ roles
I.D., A.O.d.B. and A.B. designed the study. K.K., D.P., O.G. and A.D. recruited the patients. K.K., D.P., O.G., S.J., E.V.d.A. and A.D. collected the samples. K.K., D.P. and J.D. analysed the data and K.K., D.P. wrote the manuscript. P.D.V. and M.D. performed the experiments. All authors contributed to the interpretation of the data, the critical revision of intellectual content and approved of the manuscript.
Funding
The COVART study was funded by the Université Libre de Bruxelles and by a grant from Ferring Pharmaceuticals.
Conflict of interest
A.D. and I.D. received a grant from Ferring for the study. The authors have no other conflict of interest to declare related to this study.
References
- Barragan M, Guillen JJ, Martin-Palomino N, Rodriguez A, Vassena R.. Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum Reprod 2021;36:390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JC, Kuchakulla M, Khodamoradi K, Lima TFN, Frech FS, Achua J, Rosete O, Mora B, Arora H, Ibrahim E. et al. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J Mens Health 2021;39:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto NG, Sifer C, Feldmann G, Bouret D, Moret F, Benifla JL, Porcher R, Naouri M, Neuraz A, Alvarez S. et al. A modified RT-PCR technique to screen for viral RNA in the semen of hepatitis C virus-positive men. Hum Reprod 2002;17:3153–3156. [DOI] [PubMed] [Google Scholar]
- Cobo A, Bellver J, de los Santos MJ, Remohi J.. Viral screening of spent culture media and liquid nitrogen samples of oocytes and embryos from hepatitis B, hepatitis C, and human immunodeficiency virus chronically infected women undergoing in vitro fertilization cycles. Fertil Steril 2012;97:74–78. [DOI] [PubMed] [Google Scholar]
- Coupeau D, Burton N, Lejeune N, Loret S, Petit A, Pejakovic S, Poulain F, Bonil L, Trozzi G, Wiggers L. et al. SARS-CoV-2 detection for diagnosis purposes in the setting of a Molecular Biology Research Lab. Methods Protoc 2020;3: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui P, Chen Z, Wang T, Dai J, Zhang J, Ding T, Jiang J, Liu J, Zhang C, Shan W. et al. Severe acute respiratory syndrome coronavirus 2 detection in the female lower genital tract. Am J Obstet Gynecol 2020;223:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirel C, Tulek F, Celik HG, Donmez E, Tuysuz G, Gokcan B.. Failure to detect viral RNA in follicular fluid aspirates from a SARS-CoV-2-positive woman. Reprod Sci 2021;28:2144–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert Y, Lesage B, Van Vooren JP, Liesnard C, Place I, Vannin AS, Emiliani S, Delbaere A.. Medically assisted reproduction in the presence of chronic viral diseases. Hum Reprod Update 2004;10:149–162. [DOI] [PubMed] [Google Scholar]
- ESHRE COVID-19 Working Group. Safe ART services during the third phase of the COVID-19 pandemic. ESHRE Releases a new guidance on ART and COVID-19; 2020. https://www.eshre.eu/Home/COVID19WG (14 October 2020, date last accessed).
- Flaifel A, Guzzetta M, Occidental M, Najari BB, Melamed J, Thomas KM, Deng FM.. Testicular changes associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Arch Pathol Lab Med 2021;145:8–9. [DOI] [PubMed] [Google Scholar]
- Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, Morselli S, Pecoraro A, Manera A, Nicoletti R, Liaci A. et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod 2021;36:1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi S, Bendayan M, Huyghe E, Soufir JC, Amar E, El Osta R, Plotton I, Delalande C, Perrin J, Leroy C. et al. COVID-19 and andrology: recommendations of the French-speaking society of andrology (Societe d'Andrologie de langue Francaise SALF). Basic Clin Androl 2020;30:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, Kruessel JS, Bielfeld AP.. Assessment of SARS-CoV-2 in human semen—a cohort study. Fertil Steril 2020;114:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage B, Vannin AS, Emiliani S, Debaisieux L, Englert Y, Liesnard C.. Development and evaluation of a qualitative reverse-transcriptase nested polymerase chain reaction protocol for same-day viral validation of human immunodeficiency virus type 1 ribonucleic acid in processed semen. Fertil Steril 2006;86:121–128. [DOI] [PubMed] [Google Scholar]
- Li D, Jin M, Bao P, Zhao W, Zhang S.. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 2020;3:e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, Lu W, Pan F, Meng T, Zhao K. et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 2020;28:100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarotti C, Garolla A, Maccarini E, Scaruffi P, Stigliani S, Anserini P, Foresta C.. SARS-CoV-2 in the semen: where does it come from? Andrology 2021;9:39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli F, Meirelles LEF, de Souza MVF, Mari NL, Mesquita CSS, Dartibale Cb Damke G, Damke E, da Silva VRS, Souza RP. et al. COVID-19 infection in the human reproductive tract of men and nonpregnant women. Am J Trop Med Hyg 2021;104:814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J. et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli D, Pallotti F, Colangelo S, Basilico F, Mazzuti L, Turriziani O, Antonelli G, Lenzi A, Lombardo F.. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest 2020;43:1819–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier C, Anderson D, Andreutti-Zaugg C, Baume-Berkenbosch R, Damond F, Devaux A, Englert Y, Galimand J, Gilling-Smith C, Guist'hau O. et al. ; CREAThE Network. Multicenter quality control of the detection of HIV-1 genome in semen before medically assisted procreation. J Med Virol 2006;78:877–882. [DOI] [PubMed] [Google Scholar]
- Pomeroy KO, Harris S, Conaghan J, Papadakis M, Centola G, Basuray R, Battaglia D.. Storage of cryopreserved reproductive tissues: evidence that cross-contamination of infectious agents is a negligible risk. Fertil Steril 2010;94:1181–1188. [DOI] [PubMed] [Google Scholar]
- Qiu L, Liu X, Xiao M, Xie J, Cao W, Liu Z, Morse A, Xie Y, Li T, Zhu L.. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis 2020;71:813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput SK, Logsdon DM, Kile B, Engelhorn HJ, Goheen B, Khan S, Swain J, McCormick S, Schoolcraft WB, Yuan Y. et al. Human eggs, zygotes, and embryos express the receptor angiotensin 1-converting enzyme 2 and transmembrane serine protease 2 protein necessary for severe acute respiratory syndrome coronavirus 2 infection. F S Sci 2021;2:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, Li R, Luan Y, Liu X, Yu G. et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology 2021;9:99–106. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Yogev Y, Zilberman A, Alpern S, Many A, Yousovich R, Gamzu R.. Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vaginal swabs of women with acute SARS-CoV-2 infection: a prospective study. BJOG 2021;128:97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tur-Kaspa I, Tur-Kaspa T, Hildebrand G, Cohen D.. COVID-19 may affect male fertility but is not sexually transmitted: a systematic review. F S Rev 2021;2:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xu X.. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells 2020;9: 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO COVID Group. European Centre for Disease Prevention and Control: an agency of the European Union. 2020. https://covid19.who.int/ (January 2021, date last accessed).
- Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, Peh S, Gu J.. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod 2006;74:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q.. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367:1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chen S, Huang B, Zhong JM, Su H, Chen YJ, Cao Q, Ma L, He J, Li XF. et al. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus 2020;6:1124–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data presented in this article can be shared upon request to the corresponding authors.


