Abstract
We compared neutralizing antibody titers of convalescent samples collected before and after the emergence of novel strains of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), against the wild-type virus and Alpha (B.1.1.7) and Beta (B.1.351) variants. Plasma samples collected in 2020 before emergence of variants showed reduced titers against the Alpha variants, and both sets of samples demonstrated significantly reduced titers against Beta. Comparison of microneutralization titers with those obtained with pseudotype and hemagglutination tests showed a good correlation between their titers and effects of strain variation, supporting the use of these simpler assays for assessing the potency of convalescent plasma against currently circulating and emerging strains of SARS-CoV-2.
Keywords: SARS-CoV-2, variants, neutralizing antibodies, convalescent plasma, pseudotype, hemagglutination test, live virus neutralizing antibody assay
Convalescent plasma collected during the first pandemic wave showed significantly reduced neutralization capacity against the Alpha variant when compared with wild-type virus, highlighting the importance of assessing its potency against currently circulating strains before clinical use.
Strategies for mitigating the impact of coronavirus disease 2019 include immunization and targeted therapies to inhibit replication of the causative virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These are challenged by the rapid emergence of new SARS-CoV-2 variants with greater transmissibility and propensity to cause severe disease [1, 2]. Antigenic changes may also impair the effectiveness of vaccines based on the original strain of SARS-CoV-2 [1–3]. Although the results from immunotherapy with anti–SARS-CoV-2 immunoglobulins in convalescent plasma have been generally disappointing [4], such treatment may be beneficial earlier in infection [5] and supplement humoral immunity in those vulnerable to severe disease, including the immunocompromised [6].
Variability in neutralizing antibody (nAb) titers and antigenic specificity in previously SARS-CoV-2–infected donors of source plasma complicate the provision of therapeutically effective convalescent plasma [6]. It is also affected by the plethora of different methods to quantify nAb titers and the lack of information on what constitutes an effective titer to neutralize SARS-CoV-2 in vivo. However, the recent development of an international standard for SARS-CoV-2 nAbs to calibrate in vitro assays promises greater comparability of data generated by different laboratories [7]. nAbs provide the best metric of protection against SARS-CoV-2 [1]. However, the emergence of strains of SARS-CoV-2 with substantially reduced susceptibility to neutralization, such as the Beta (B.1.351) variant that emerged in South Africa [1], emphasizes the importance of performing these assessments with currently circulating virus strains.
In the current study, we investigated the impact of antigenic variation of SARS-CoV-2 on neutralization by convalescent plasma collected in the United Kingdom during the first year of pandemic. The study provides evidence for a substantial component of strain-specific antibody to the Alpha and Beta variants and suggests a possible future strategy of donor antibody and patient virus strain matching to improve the therapeutic efficacy of convalescent plasma.
MATERIALS AND METHODS
Study Participants
Convalescent plasma samples were collected from 75 individuals with previous SARS-CoV-2 infection, as described [8]. Samples from the donors infected with SARS-CoV-2 during the first pandemic wave (n=20) originated from 25 April to 4 December 2020, and samples from the second wave (n=55) originated from 24 January to 20 February 2021. Those included in the latter group donated in London and were positive for SARS-CoV-2 between 15 December 2020 and 4 January 2021. All samples were tested for SARS-CoV-2 immunoglobulin G antibodies with the Euroimmun assay (Perkin Elmer); a sample-to-cutoff ratio ≥6.0 was considered to demonstrate sufficient levels of antibodies in a sample to enable its clinical use [8].
Ethical Statement
Signed consent was obtained from each donor at the time of donation. It included the use of data for the purpose of clinical audit to assess and improve the service provided by NHS Blood and Transplant as well as for research to improve our knowledge of the donor population. The study was approved by our National Blood Supply Committee for Audit and Research Ethics.
nAb Testing
SARS-CoV-2 nAb titers in plasma samples were determined using a live virus microneutralization assay with wild-type (WT; England-2), Alpha (B.1.1.7), and Beta (B.1.351) strains [9] and a pseudotype assay [10]. D614G and several recently reported amino acid substitutions (L335V, E484K, S477N, N501Y, 69/70del, 69/70del + N501Y) were introduced into the spike protein of the pseudovirus. Samples were also assayed with the hemagglutination test (HAT), which uses red blood cell agglutination to detect antibodies to the receptor-binding domain of SARS-CoV-2 [11]. Statistical analysis was performed using SPSS software, version 26.
RESULTS
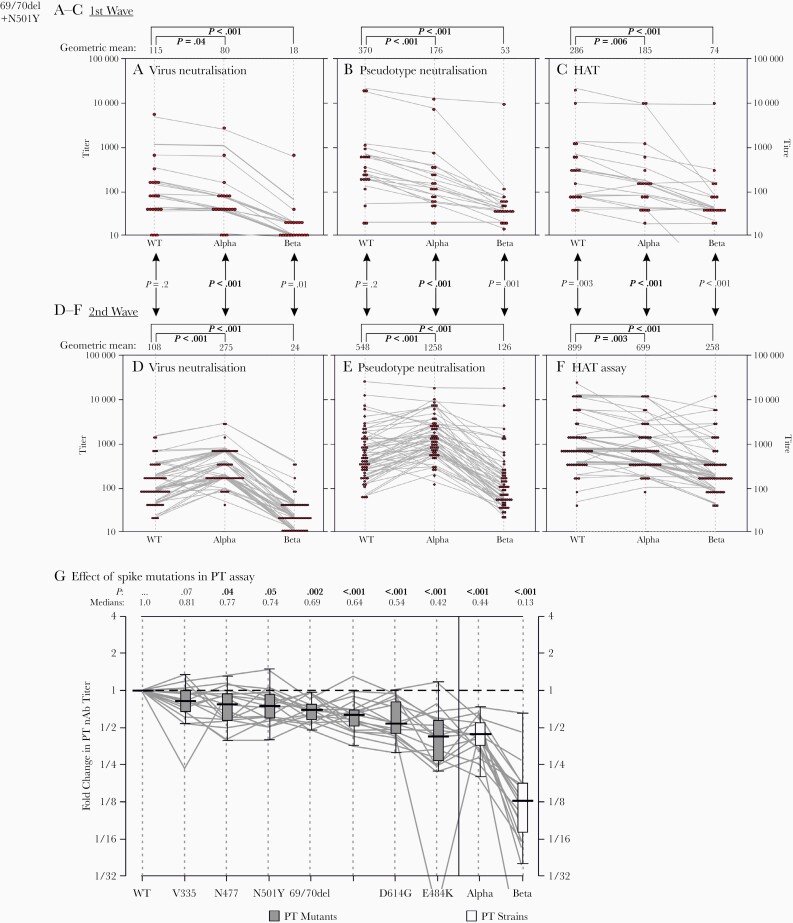
We compared nAb titers of convalescent samples collected before and after the emergence of the Alpha and Beta variants. All samples were first tested for nAbs against the WT virus and Alpha and Beta variants in a live virus microneutralization assay. Three convalescent samples of the 20 collected during the first wave did not have detectable antibodies, while the others showed a mean 1.4-fold reduction in nAb titers against the Alpha variant, compared with WT virus (P=.007) (Figure 1A). Greater reductions in nAb titers were observed for the Beta variant compared with WT virus (6-fold reduction; P<.001) (Figure 1A).
Figure 1.
Effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) strain variation and individual spike protein mutations on neutralization. A–F, Comparison of neutralizing antibody (nAb) titers to virus (A, D) and pseudotype (B, E) neutralization and the hemagglutination test (HAT) results (C, F) for virus isolates and constructs derived from wild-type (WT), Alpha, and Beta strains of SARS-CoV-2. Titers were further compared between samples collected in mid-2020 when WT viruses predominated, before the emergence of SARS-CoV-2 variants (A–C), with those collected when the Alpha variant was primarily circulating in the United Kingdom (D–F). Graphs depict individual reactivities of samples as dot and parallel plots; for the latter, nAb and HAT titers have been jittered for display purposes to avoid superimposition of lines. Titers were compared between viruses by Spearman rank correlation test for paired samples; P values are shown above each graph. For comparison of unpaired samples between time points, titers were compared using Mann-Whitney U test; P values are shown between graphs. P values showing significant associations (P < .05) are highlighted in bold. G, Pseudotype nAb titers of first-wave samples against 6 constructs with individual mutations (x-axis) incorporated into modified spike gene constructs. Titers were normalized to reactivity to the WT construct (y-axis), and ratios compared with those against Alpha and Beta strains of SARS-CoV-2 that incorporate these mutations. Tukey box plots depict medians (black bar), interquartile ranges (boxes), and minimal and maximal values (excluding outliers) (whiskers) of ratios. Distributions were compared using Spearman rank correlation test for paired samples; medians and P values are shown above the graph, with P values <.05 in bold. Abbreviation: PT, Pseudotype.
Parallel analysis of 55 convalescent samples collected during the second wave showed comparable titers of nAbs against WT to those of the first wave (median titers, 108 and 115, respectively; P = 0.2) (Figure 1A and 1D). However, the second-wave samples had significantly higher titers of nAbs against the Alpha strain (median titers, 108 and 275, respectively; P<.001). This reflected an increased neutralizing potency against the homologous Alpha strain in second-wave compared with first-wave samples (median titers, 275 vs 80, respectively; P<.001). Neutralization potencies against the Beta strain were substantially lower than those against the WT strain (4.5-fold reductions in geometric mean value; P<.001).
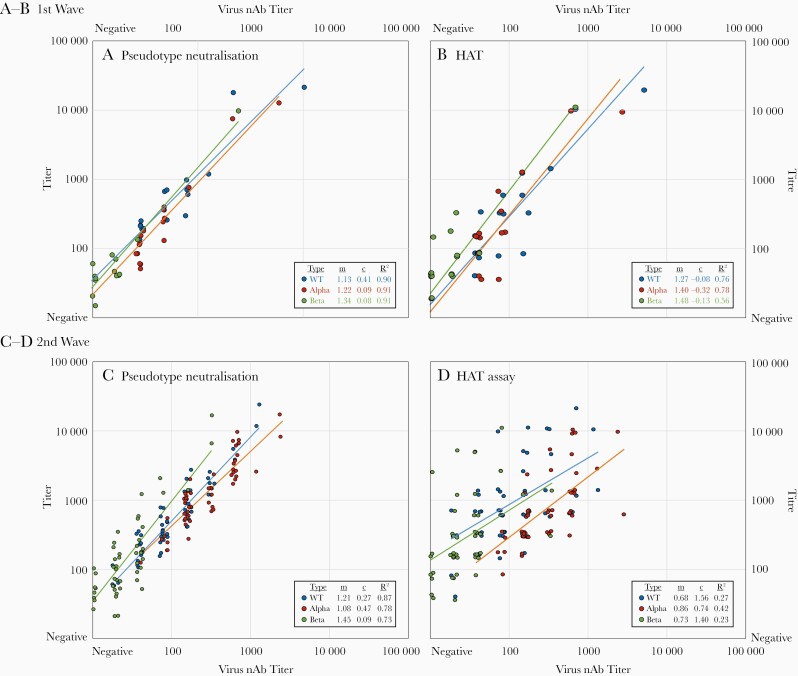
Although live virus neutralization assay is the reference standard for nAb testing, it is laborious and challenging to standardize. For these reasons, we also determined the nAb titers in these samples using pseudotype assay and receptor-binding domain binding antibodies in the HAT, using WT, Alpha, and Beta constructs for both. Absolute titers with both the pseudotype assay and the HAT were uniformly higher than in the virus neutralization assay. In the pseudotype assay, there was high correlation between live virus neutralization and pseudotype titers in both first- and second-wave sample sets for WT (R2=0.90 and 0.87, respectively), Alpha (R2=0.91 and 0.78), and Beta (R2=0.91 and 0.73) virus (Figure 2A and 2B). However, correlations between HAT titers and virus neutralization were better in first-wave than in second-wave samples for WT (R2=0.76 and 0.27, respectively), Alpha (R2=0.78 and 0.42), and Beta (R2=0.56 and 0.23) virus (Figure 2C and 2D). Particularly for the Beta variant HAT results, some samples showed a higher HAT titer but low nAb titer (Figure 2D).
Figure 2.
Correlations between titers in virus neutralization assay (x-axis) with those in pseudotype (A, C) and hemagglutination test (HAT) (B, D) (y-axis) results for samples collected in first (A, B) and second (C, D) waves of severe acute respiratory syndrome coronavirus 2 circulation. Titers for virus neutralization and HATs have been jittered to avoid overlap. Correlations were measured by linear regression of log-transformed values that approximate to normal distributions. Regression line metrics (multiplier [m], offset [c], and R2 correlation coefficients) are tabulated for each assay, sample set, and virus strain. All interassay correlations were significant at the P=.001 level. Abbreviations: nAb, neutralizing antibody; WT, wild type.
The apparent strain specificity of virus neutralization was closely reproduced in the pseudotype assay and HAT results (Figure 1B, 1C, 1E, and 1F). Pseudotype data of most first-wave samples showed reduction in nAb titers against Alpha and Beta pseudovariants compared with WT (2.4- and 9-fold reductions, respectively; P<.001) (Figure 1B). In contrast, 84% of samples collected in the second wave neutralized Alpha better than WT pseudovirus (3.1-fold increase; P<.001) (Figure 1E). Loss of neutralization potency against the Beta variant was reproduced in the pseudotyped assay, with values 10- and 4.4-fold lower than for Alpha and WT, respectively (P<.001) (Figure 1E). Similar trends were observed with the HAT for samples collected in the first wave; 1.5- and 3.9-fold reductions in nAb titers against Alpha and Beta variants were demonstrated, compared with WT (P=.006 and P < .001, respectively) (Figure 1C). Only 3 samples collected in the second wave had better neutralization with Alpha than with WT in the HAT results, and 38% of samples actually had better neutralization with WT than with Alpha (1.3-fold increase in titer) (Figure 1F). In general, HAT titers against WT were 3.5-fold higher for second-wave than for first-wave samples (P=.003) (Figure 1C and 1F). HAT titers against Beta were also lower overall than titers against Alpha and WT (2.7- and 3.4-fold decreases, respectively; P<.001) (Figure 1F).
To investigate which specific mutations accounted for the differences in neutralization between strains, mutations at several sites were introduced into the pseudotype spike gene, and nAb titers were compared with those of the WT construct (Figure 1G). All mutations introduced into the pseudotype construct led to reductions in neutralization susceptibility using samples collected from the first wave, the most influential being the E484K mutation, with >2-fold titer reduction compared with WT.
DISCUSSION
Our first 2 convalescent plasma trials coincided with the emergence of the Alpha variant, first reported at the end of September 2020 and within the following 4 months, became the major circulating strain in the United Kingdom [12]. As convalescent plasma samples are collected a minimum of 28 days after recovery from SARS-CoV-2 infection, most plasma donations supplied for these clinical trials would have been collected from individuals infected with the WT virus, and hence it was important to assess the cross-neutralization characteristics of plasma used in the trial.
Using a live virus assay, we showed that the neutralization capacity of convalescent plasma collected during the first pandemic wave in the United Kingdom was significantly reduced for the Alpha variant compared with WT virus. This finding is consistent with those of previous studies reporting similar reductions in neutralization against the Alpha variant [2]. Although this variant is unlikely to fully escape from protection induced by vaccination using the WT strain, strain-associated differences in neutralization titers have potentially relevant implications for the convalescent plasma trials. From 20 convalescent plasma samples collected in the first wave analyzed in the current study, 13 would have been supplied for clinical trials based on Euroimmun testing [8]. Whereas 7 of these donations had >1:100 titers against WT and another 4 had 1:80 titers, and were hence considered therapeutically effective [8], only 2 of 11 (18%) had corresponding titers against the Alpha variant. Because about 30% of patients in the REMAP-CAP convalescent plasma trial were infected with that Alpha variant [12], a substantial proportion would have received convalescent plasma with suboptimal titers.
In contrast, titers of nAbs against the Alpha variant were increased compared with titers against WT virus in the samples collected during the second pandemic wave. Although Alpha infections were not confirmed in these second-wave samples, we can make a reasonable inference of their identity based on the wider SARS-CoV-2 population analysis [12]. Samples collected during the first wave in the United Kingdom were most likely obtained from individuals infected primarily with SARS-CoV-2 WT strains, whereas infections acquired during the second wave—toward the end of 2020 and early 2021—were derived from a period when predominantly Alpha (lineage Alpha) strains were circulating. The observation of reciprocal reactivity argues for future careful consideration of the match between infecting strains of SARS-CoV-2 and the variants that previously infected the convalescent plasma donors in treatment selection. This would be even more relevant for persons infected with the Delta variant of SARS-CoV-2 (B1.617.2), which is antigenically more distinct from WT (and the Alpha variant) [13].
Samples collected during the second wave showed relatively higher titers with the HAT compared with virus neutralization (Figure 2D and 2F). All 55 samples with Alpha variant HAT titers ≥40 had nAb titers ≥20, and all 32 with HAT titers >480 had nAb titers ≥100, consistent with previous observations for first-wave samples [10]. However, for the Beta variant in the second wave, where neutralization was reduced but HAT titers, maintained, the predictive value of the HAT was reduced; 40 of 55 samples with HAT titers ≥40 had nAb titers ≥20, but only 2 of 12 with HAT titers >480 had nAb titers ≥100. (Supplementary Figure 1; Supplementary Materials). This difference is likely to result from antibodies that bind conserved epitopes on the receptor-binding domain, particularly class 4 [14], which do not neutralize virus efficiently in vitro. In terms of protection from disease, however, nonneutralizing antibodies may still be protective through Fc-mediated mechanisms.
Effects of individual mutations on susceptibility to neutralization were further investigated, concentrating on sites in the spike protein previously associated with the emergence of Alpha strain; the E484K mutation was associated with highest (2-fold) reduction in neutralization titers. Interestingly, none of the investigated mutations approached the >8-fold reduction in neutralization susceptibility of the Beta strain. Beta variants possess a combination of mutations (including K417N, E484K, and N501Y) that may lead to additive or potentially synergistic effects on antigenicity and neutralization susceptibility. This variant has indeed been previously associated with antigenic escape, and these mutations are known to affect antibody binding by several different mechanisms [15].
The ever-increasing emergence of novel SARS-CoV-2 variants necessitates effective and timely public health surveillance programs to monitor the appearance and properties of these new strains that may potentially escape from vaccine-induced immunity. As shown in the current study, both pseudotype assays and HATs can be used to determine the magnitude of nAb titer against different variants by simply cloning the variant spike sequence in question while a virus isolate is obtained for confirmatory microneutralization work. The HAT has the advantages of not requiring any special equipment and being low cost, but it may overestimate nAb titers when the prevailing variant changes. Judicious use of these low-containment tests could support further convalescent plasma trials and large-scale prospective surveillance programs.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank everybody involved in the NHS Blood and Transplant convalescent plasma program and specifically Hatice Baklan within the Microbiology Services Surveillance team.
Financial support. This study was supported by the European Commission (SUPPORT-E; grant 101015756). We are grateful for generous donations to the Townsend-Jeantet Prize Charitable Trust (registered charity no. 1011770), which enable free distribution of the hemagglutination test for detection of antibodies to the variants of concern (enquiries to alain.townsend@imm.ox.ac.uk).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021; 184:2348–61.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Faulkner N, Ng KW, Wu MY, et al. Reduced antibody cross-reactivity following infection with B.1.1.7 than with parental SARS-CoV-2 strains. Elife 2021; 10:e69317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021; 593:130–5. [DOI] [PubMed] [Google Scholar]
- 4. Piechotta V, Iannizzi C, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev 2021; 5:Cd013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Libster R, Pérez Marc G, Wappner D, et al. ; Fundación INFANT–COVID-19 Group. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 2021; 384:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood 2020; 136:2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen D, Simmonds P, Steenhuis M, et al. SARS-CoV-2 neutralising antibody testing in Europe: towards harmonisation of neutralising antibody titres for better use of convalescent plasma and comparability of trial data. Euro Surveillance 2021; 26:2100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harvala H, Mehew J, Robb ML, et al. Convalescent plasma treatment for SARS-CoV-2 infection: analysis of the first 436 donors in England, 22 April to 12 May 2020. Eurosurveillance 2020; 25:2001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harvala H, Robb ML, Watkins N, et al. Convalescent plasma therapy for the treatment of patients with COVID-19: assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfus Med 2021; 31:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamikanra A, Nguyen D, Simmonds P, et al. Comparability of six different immunoassays measuring SARS-CoV-2 antibodies with neutralizing antibody levels in convalescent plasma: from utility to prediction. Transfusion 2021; 61:2837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Townsend A, Rijal P, Xiao J, et al. A haemagglutination test for rapid detection of antibodies to SARS-CoV-2. Nat Commun 2021; 12:1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ratcliff J, Nguyen D, Fish M, et al. ; REMAP-CAP Immunoglobulin Domain UK Investigators. Virological characterization of critically ill patients with COVID-19 in the United Kingdom: interactions of viral load, antibody status, and B.1.1.7 infection. J Infect Dis 2021; 224:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 2021; 397:2331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barnes CO, Jette CA, Abernathy ME, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020; 588:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greaney AJ, Starr TN, Barnes CO, et al. Mapping mutations to the SARS-CoV-2 RBD that escape binding by different classes of antibodies. Nat Commun 2021; 12:4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.