Abstract
Background and Aims
The health consequences of coronavirus disease 2019 [COVID-19] among patients with ulcerative colitis [UC] and Crohn’s disease [CD] remain largely unknown. We aimed to investigate the outcomes and long-term effects of COVID-19 in patients with UC or CD.
Methods
We conducted a prospective, population-based study covering all Danish patients with CD or UC and confirmed COVID-19 between January 28, 2020 and April 1, 2021, through medical records and questionnaires.
Results
All 319 patients with UC and 197 patients with CD who developed COVID-19 in Denmark were included in this study and compared with the Danish background population with COVID-19 [N = 230 087]. A significantly higher risk of COVID-19-related hospitalization was observed among patients with UC (N = 46 [14.4%], relative risk [RR] = 2.49 [95% confidence interval, CI, 1.91–3.26]) and CD (N = 24 [12.2%], RR = 2.11 [95% CI 1.45–3.07]) as compared with the background population (N = 13 306 [5.8%]). A similar pattern was observed for admission to intensive care (UC: N = 8 [2.51%], RR = 27.88 [95% CI 13.88–56.00]; CD: N = 3 [1.52%], RR = 16.92 [95% CI 5.46–52.46]). After a median of 5.1 months (interquartile range [IQR] 4.5–7.9), 58 [42.3%] and 39 [45.9%] patients with UC and CD, respectively, reported persisting symptoms which were independently associated with discontinuation of immunosuppressive therapies during COVID-19 (odds ratio [OR] = 1.50 [95% CI 1.07–10.22], p = 0.01) and severe COVID-19 (OR = 2.76 [95% CI 1.05–3.90], p = 0.04), but not with age or presence of comorbidities.
Conclusion
In this population-based study of 516 patients with IBD and COVID-19, 13.6% needed hospitalization and 2.1% required intensive care. Furthermore, sequelae were frequent, affecting 43.7% of COVID-19-infected patients. These findings might have implications for planning the healthcare of patients in the post-COVID-19 era.
Keywords: Inflammatory bowel diseases, COVID-19 outcomes and sequelae, population-based
1 Introduction
Coronavirus disease 2019 [COVID-19], caused by the coronavirus SARS-CoV-2 and first reported in December 2019, is a new form of acute infectious respiratory syndrome, with a broad spectrum of multi-organ manifestations and severities.1 The disease has become a pandemic, with over 177 million affected cases and 3.8 million deaths as of June 2021, resulting in global healthcare crises and strained healthcare resources.2 As the vast majority of patients survive COVID-19, it is therefore paramount to investigate the possible long-term effects of COVID-19. To date, only two hospital-based cohort studies are available.3,4 Together, their data indicate a high risk of clinical sequelae following COVID-19, which also seem to be evident among younger and middle-aged patients with no comorbidities.3,4
Inflammatory bowel diseases [IBD], consisting of ulcerative colitis [UC] and Crohn’s disease [CD], are chronic, immune-mediated inflammatory diseases with an increasing incidence.5 Although patients frequently require immunosuppressive therapies, which have been shown to increase the risk of some infections,6 the susceptibility to SARS-CoV-2 infection seems not to differ among patients with and without IBD, regardless of IBD-related medications.7–9 However, only three population-based studies have assessed the outcomes of COVID-19 among patients with IBD,7,10,11 and data on the influence of IBD medication on COVID-19 outcomes are conflicting.7,8,12–14 Furthermore, population-based estimates of the long-term effects of COVID-19 in IBD patients are lacking.
In this study, we aimed to investigate the severity, outcomes and long-term health effects of COVID-19 in a Danish generalizable population-based cohort of patients with IBD.
2 Materials and Methods
2.1 Study design and population
The Danish COVID-IBD cohort is a prospectively maintained population-based cohort.7 Briefly, the cohort was established among all internal medicine and gastrointestinal departments in Denmark at the beginning of April 2020 and prospectively included all patients with an established diagnosis of IBD according to current diagnostic criteria15,16 who developed COVID-19. The inclusion period was from the first test for SARS-CoV-2 in Denmark, which took place on January 28, 2020, until April 1, 2021. In Denmark, all residents could book an appointment for PCR [polymerase chain reaction] testing beginning May 18, 2020 without a prescription; before that, a PCR test required a physician’s referral. All healthcare is free and universal in Denmark.
Four out of five geographically well-defined regions covering 4.43 million residents [equal to 78.6% of the Danish population], and each responsible for maintaining all healthcare within their respective geographical areas, delivered complete population-based data on individual patients for this study. The data included test results from reverse transcription PCR [RT-PCR] analysis for the presence of SARS-CoV-2. These data were linked to IBD-related and COVID-19-related clinical data from the patients’ unique medical records and their answers to validated questionnaires, as described below. The fifth region, the Region of Southern Denmark, with 1.2 million residents, was covered by including all relevant hospital departments and performing a manual assessment of their outpatient lists.
For the assessment of COVID-19 outcomes within the background population, data were provided by all five regions; however, these data were limited to the outcomes of COVID-19 and the patients’ ages.
2.2 Outcomes and definitions
The primary outcomes of this study were the severity of COVID-19 and development of any long-term effects following it, including their characterization and associated factors. Secondary outcomes included health-related quality of life, clinical relapse of IBD and re-infection with COVID-19 during follow-up.
The clinical diagnosis of COVID-19 relied on a positive nucleic acid amplification test [RT-PCR] for SARS-CoV-2,17 and the severity of COVID-19 was categorized as either adverse, defined as a need for hospitalization or death due to COVID-19, severe, defined as a composite of intensive care unit [ICU] admission, mechanical ventilation or death, or mild, defined as an absence of adverse or severe COVID-19. Patients with severe COVID-19 were included in the analysis of adverse COVID-19.
Sequelae following COVID-19 were defined as symptoms that [i] develop during or after an infection consistent with COVID-19, [ii] and are present for more than 12 weeks, [iii] and are not attributable to alternative diagnoses.18 This outcome was primarily patient-reported through secure surveys, which were sent to the whole population irrespective of hospital contact. Survey answers were subsequently verified by physicians when possible.
Similarly, patient-reported health-related quality of life was measured prospectively after COVID-19, using the EuroQol five-dimension five-level [EQ-5D-5L] questionnaire,19 EuroQol Visual Analogue Scale [EQ-VAS],20 Short Inflammatory Bowel Disease Questionnaire [SIBDQ],21 IBD Disability Index [IBD-DI]22 and IBD Fatigue Score, regardless of the severity of a patient’s COVID-19.23
Disease activity of IBD was assessed on an individual patient level using either the Simple Clinical Colitis Activity Index [SCCAI] in UC or unclassified IBD, in which a value of ≤2 indicates clinical remission, or the Harvey–Bradshaw Index [HBI] for CD, in which a score of ≤4 indicates clinical remission. Biochemical remission was defined as having C-reactive protein [CRP] <5 mg/L or faecal calprotectin <250 µg/g. Endoscopic remission was defined as a Mayo Endoscopic Subscore of ≤1 for UC upon sigmoidoscopy or colonoscopy, and a Simple Endoscopic Score Crohn’s Disease [SES-CD] of <2 for CD upon colonoscopy. Clinical relapse was defined as a loss of clinical remission within the first 12 weeks following COVID-19 [short-term relapse] or after 12 weeks [long-term relapse].
Recent guidelines suggest the following criteria for COVID-19 re-infection, which were adapted in this study:24 [i] confirmation of a first episode of COVID-19, [ii] proof of a re-infection with two positive SARS-CoV-2 RT-PCR tests, and [iii] at least one, and ideally two, negative RT-PCR tests, on two different specimens collected between the first and second episodes. A less stringent approach, presented in the same guidelines,24 was adopted in this study for a separate analysis and includes the clinical recurrence of symptoms compatible with COVID-19 accompanied by a positive PCR test for SARS-CoV-2 more than 90 days after the onset of the primary infection and with no evidence of another cause of infection.
2.3 Statistical analysis
Descriptive statistics were used to measure prevalences and incidences. Differences between UC and CD were investigated using the chi-squared test and Fisher’s exact test, as appropriate. In addition, binary logistic regression models were used to investigate possible categorical variables as predictive factors for the disease course of COVID-19 and IBD. The primary predictor variables, which were determined a priori, included IBD medications with an emphasis on immunosuppressive therapies and 5-aminosalicylic acid [5-ASA], IBD activity, and severity of COVID-19. In addition, covariates were determined by backward selection to obtain statistically relevant variables for bivariate analysis. We considered age, gender, comorbidities, and IBD localization and behaviour according to the Montreal classification. Outcomes including associated factors were analysed separately for UC and CD. If an analysis could not be conducted due to a limited sample size, data on CD and UC patients were pooled, and adjusted analysis was conducted; p-values <0.05 were considered to be significant for all analyses. Statistical analyses, including data preparation, were performed using R version 3.6.1, developed by the R Foundation for Statistical Computing.
2.4 Ethical considerations
This study was approved by the Danish Patient Safety Authority [R-20049371] and the Danish Data Protection Agency [P-2020-299]. The findings were reported according to Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] recommendations.25
2.5 Data availability statement
All data are incorporated within the article and its online Supplementary Data.
2.6 Patient and public involvement
No patients were involved in setting the research question or the outcome measures, planning the study design or interpretation, or in writing up the results.
3 Results
3.1 Baseline characteristics of patients with IBD and COVID-19 in Denmark
During the inclusion period, a total of 516 patients with IBD and 230 087 patients without IBD developed COVID-19, and all were included in this study. The baseline demographic and clinical characteristics were available on patients with IBD and are reported in Table 1 and in Supplementary Data p. 5. The cohorts of UC and CD were comparable in terms of age and gender but not in terms of the proportion of patients in remission or patients receiving or not receiving IBD-related medications. All patients who received systemic steroids did so due to IBD-related disease activity.
Table 1.
Baseline characteristics of patients with IBD and COVID-19
| Ulcerative colitis [UC] | Crohn’s disease [CD] | p-value | ||
|---|---|---|---|---|
| Patients | N | 319 | 197 | |
| Age at diagnosis of COVID-19 [years] | Median [IQR] | 48 [35–61] | 44 [30–59] | 0.03 |
| Female gender | N [%] | 154 [48.3] | 116 [58.9] | 0.11 |
| IBD localization and behaviour | ||||
| E1: ulcerative proctitis | N [%] | 70 [21.9] | ||
| E2: left-sided UC | N [%] | 101 [31.7] | ||
| E3: extensive colitis | N [%] | 80 [25.1] | ||
| L1: ileal CD | N [%] | 45 [22.8] | ||
| L2: colonic CD | N [%] | 66 [33.5] | ||
| L3: ileocolonic CD | N [%] | 61 [31.0] | ||
| L4: upper gastrointestinal CD | N [%] | 7 [3.6] | ||
| Unknown localization | N [%] | 68 [21.3] | 18 [9.1] | |
| B1: non-stricturing, non-penetrating CD | N [%] | 119 [60.4] | ||
| B2: stricturing CD | N [%] | 25 [12.7] | ||
| B3: penetrating CD | N [%] | 10 [5.1] | ||
| Perianal disease | N [%] | 14 [7.1] | ||
| Unknown behaviour | N [%] | 43 [21.8] | ||
| Smoking at the time of COVID-19 | N [%] | 97 [30.4] | 76 [38.6] | 0.06 |
| Body mass index [kg/m2] | Median [IQR] | 25.3 [22.2–28.4] | 25.4 [21.4–29.4] | 0.68 |
| Co-occurring IMIDs | N [%] | 49 [15.4] | 33 [16.8] | 0.67 |
| Comorbidities other than IMIDs | N [%] | 95 [29.8] | 56 [28.4] | 0.74 |
| No comorbidities | N [%] | 154 [48.3] | 98 [49.7] | 0.75 |
| IBD disease activity at diagnosis of COVID-19 | ||||
| Clinical remission | N [%] | 200 [83.0] | 110 [72.8] | 0.02 |
| Biochemical remission | N [%] | 169 [55.0] | 104 [79.4] | <0.01 |
| Endoscopic remission | N [%] | 41 [62.1] | 24 [57.1] | 0.61 |
| IBD-related treatment at the time of COVID-19 | ||||
| None | N [%] | 71 [22.3] | 70 [35.5] | <0.01 |
| Topical 5-ASA | N [%] | 88 [27.6] | 3 [1.5] | <0.01 |
| Systemic 5-ASA | N [%] | 136 [42.6] | 6 [3.0] | <0.01 |
| Topical steroids | N [%] | 11 [3.4] | 5 [2.5] | 0.56 |
| Systemic steroids | N [%] | 12 [3.8] | 8 [4.1] | 0.86 |
| Immunomodulators | N [%] | 42 [13.2] | 53 [26.9] | <0.01 |
| Azathioprine | N [%] | 30 [9.4] | 43 [21.8] | <0.01 |
| Mercaptopurine | N [%] | 9 [2.8] | 5 [2.5] | 0.85 |
| Methotrexate | N [%] | 2 [0.6] | 5 [2.5] | 0.11 |
| Biologic therapies | N [%] | 42 [13.2] | 76 [38.6] | <0.01 |
| Infliximab | N [%] | 19 [6.0] | 25 [12.7] | <0.01 |
| Adalimumab | N [%] | 5 [1.6] | 29 [14.7] | <0.01 |
| Vedolizumab | N [%] | 11 [3.4] | 17 [8.6] | 0.01 |
| Golimumab | N [%] | 5 [1.6] | 2 [1.0] | 0.71 |
| Ustekinumab | N [%] | 2 [0.6] | 3 [1.5] | 0.37 |
A total of 15 patients had an unclassified type of IBD and were added to the ulcerative colitis group due to similarities in all characteristics and outcomes. Missing data: clinical remission [UC: 78, CD: 46], biochemical remission [UC: 12: CD: 66], endoscopic remission [UC: 253, CD: 155]. IBD, inflammatory bowel disease; IMID, immune-mediated inflammatory disease; 5-ASA, 5-aminosalicylates. Localization and behaviour of IBD are categorized according to the Montreal Classification. The p-value is based on a chi-squared test, with p < 0.05 [bold] considered significant.
3.2 Severity and outcomes of COVID-19
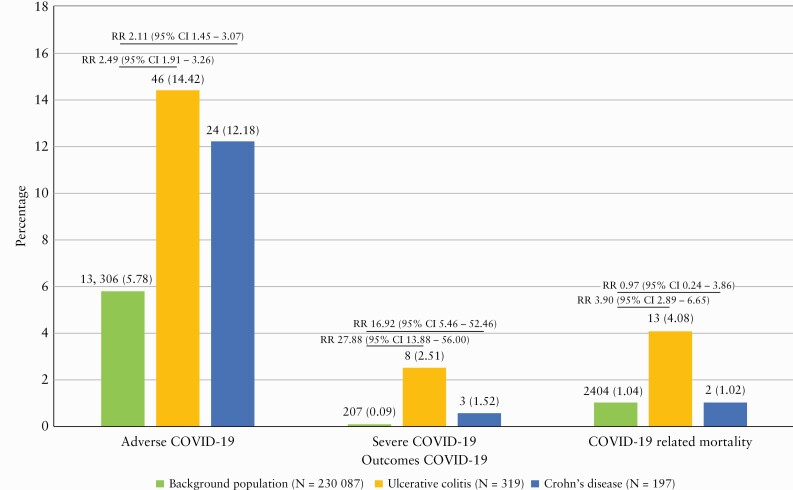
The outcomes of COVID-19 are depicted in Figure 1. A significantly higher risk of adverse COVID-19 was observed among patients with UC (N = 46 [14.4%], relative risk [RR] = 2.49 [95% confidence interval, CI, 1.91–3.26], p < 0.01) and CD (N = 24 [12.2%], RR = 2.11 [95% CI 1.45–3.07], p < 0.01) than in the background population (N = 13 306 [5.8%]). A similar pattern was observed in the risk of severe COVID-19 (UC: N = 8 [2.51%], RR = 27.88 [95% CI 13.88–56.00], p < 0.01; CD: N = 3 [1.52%], RR = 16.92 [95% CI 5.46–52.46], p < 0.01), and risk of COVID-19-related mortality [Figure 1]; however, as shown, very few patients with IBD were available for the analysis of these outcomes. The sub-analysis of outcomes of COVID-19 in relation to age showed that these outcomes merely appeared in elderly patients, which was particularly evident among IBD patients [Supplementary Data pp. 5–6].
Figure 1.
Outcomes of COVID-19.
In patients with UC, adverse COVID-19 was independently associated with being older than 50 years (odds ratio [OR] = 5.10 [95% CI 1.68–17.80], p < 0.01), the presence of comorbidities (OR = 3.26 [95% CI 1.32–8.25], p < 0.01), being female (OR = 0.38 [95% CI 0.14–0.92], p = 0.03), and UC being in clinical remission (OR = 2.45 [95% CI 1.05–6.25], p = 0.04) [Supplementary Data pp. 7–8]. In contrast, only current smoking at the time of COVID-19 was an independent predictor for severe COVID-19 among patients with UC (OR = 6.62 [95% CI 1.08–2.78], p < 0.01) [Supplementary Data pp. 7–8]. Among patients with CD, being older than 50 years (OR = 5.10 [95% CI 1.68–17.80], p < 0.01), colonic disease location (OR = 4.13 [95% CI 1.47–12.32], p < 0.01), use of systemic steroids (OR = 13.62 [95% CI 1.98–17.77], p = 0.01) and the presence of comorbidities (OR = 3.44 [95% CI 1.19–10.36], p = 0.02) were independently associated with adverse COVID-19, while no factors were found to be associated with severe COVID-19 [Supplementary Data pp. 9–10].
IBD-related medications were not associated with the disease course of COVID-19 in patients with either UC or CD [Supplementary Data pp. 7–10]. However, when including 5-ASA into an adjusted and pooled analysis of UC and CD, this medication was associated with an increased risk of COVID-19-related mortality compared with patients who did not receive 5-ASA and those who did not receive any IBD medications [Supplementary Data p. 11]. However, the patients were all high-risk individuals and are described individually in detail [Supplementary Data p. 12].
3.3 Long-term outcomes following COVID-19
The median time to assess long-term outcomes of COVID-19 was 5.1 months (interquartile range [IQR] 4.5–7.9] after infection.
3.3.1 Post-COVID-19 syndrome and other physical COVID-19 sequelae
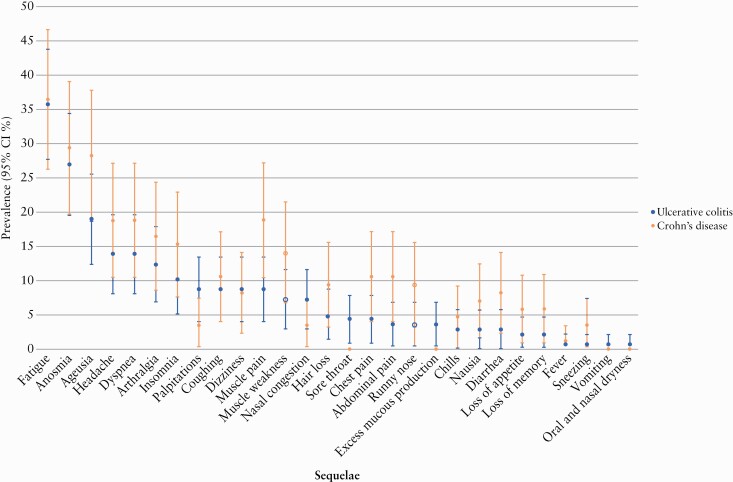
Among UC and CD patients with COVID-19, a total of 137 [42.9%] and 85 [43.1%], respectively, participated in a subsequent investigation of post-COVID-19 sequelae. No difference between patients participating or not participating in this assessment was identified in terms of the characteristics of IBD or COVID-19 [Supplementary Data pp. 13–14]. An equal proportion of patients with UC (58 [42.3%]) and CD (39 [45.9%], p = 0.60) reported persisting symptoms of COVID-19 for at least 12 weeks, consistent with the development of post-COVID-19 syndrome; Figure 2 specifies the symptoms. The most common persisting patient-reported symptoms included fatigue (UC: 49 [35.8%], CD: 31 [36.5%], p = 0.92), anosmia (UC: 37 [27.0%], CD: 25 [29.4%], p = 0.70), ageusia (UC: 26 [19.0%], CD: 24 [28.2%], p = 0.11), headache (UC: 19 [13.9%], CD: 16 [18.8%], p = 0.32), dyspnea (UC: 19 [13.9%], CD: 16 [18.8%], p = 0.32) and arthralgia (UC: 17 [12.4%], CD: 14 [16.5%], p = 0.40) [Figure 2]. Among the 19 [13.9%] patients with UC and 10 [11.8%] patients with CD who had their persisting symptoms assessed by a physician, all symptoms were confirmed as new-onset sequelae of COVID-19.
Figure 2.
Prevalence of clinical sequelae following COVID-19 in patients with IBD.
The persisting symptoms are outlined according to the severity of COVID-19 in the Supplementary Data [pp. 15–16]. Only discontinuation of immunosuppressive therapies for UC during COVID-19 (OR = 1.50 [95% CI 1.07–10.22], p = 0.01) and the severity of COVID-19 among patients with CD were independently associated with the long-term effects of COVID-19 (OR = 2.76 [95% CI 1.05–3.90], p = 0.04) [Table 2].
Table 2.
Factors associated with post-COVID-19 syndrome among patients with inflammatory bowel diseases
| Ulcerative colitis | Crohn’s disease | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| OR | 95% CI | P | OR | 95% CI | p | OR | 95% CI | P | OR | 95% CI | p | ||
| Female gender | 0.92 | 0.46–1.84 | 0.80 | 1.97 | 0.80–4.94 | 0.13 | |||||||
| Age >50 years | 0.97 | 0.48–1.96 | 0.93 | 2.17 | 0.86–5.60 | 0.10 | |||||||
| Age >60 years | 0.99 | 0.43–2.26 | 0.99 | 1.29 | 0.41–4.10 | 0.65 | |||||||
| Age >70 years | 1.09 | 0.30–3.83 | 0.88 | 6.06 | 0.91–11.91 | 0.10 | |||||||
| Body mass index >25 | 0.74 | 0.20–2.45 | 0.63 | 2.10 | 0.67–7.04 | 0.20 | |||||||
| Smoking at the time of COVID-19 | 2.13 | 0.88–5.27 | 0.09 | 1.46 | 0.54–4.05 | 0.45 | |||||||
| Disease activity of IBD at diagnosis of COVID-19 | |||||||||||||
| Clinical remission | 1.74 | 0.86–3.60 | 0.12 | 1.8 | 0.76–4.57 | 0.17 | |||||||
| Biochemical remission | 1.32 | 0.66–2.65 | 2.64 | 2.31 | 0.94–5.84 | 0.06 | |||||||
| Endoscopic remission | 0.83 | 0.26–2.46 | 0.74 | 0.40 | 0.08–1.58 | 0.21 | |||||||
| Phenotype of IBD | |||||||||||||
| E1: ulcerative proctitis | 1.18 | 0.53–2.60 | 0.67 | L1: ileal disease | 1.39 | 0.50–3.94 | 0.51 | ||||||
| E2: left-sided UC | 0.80 | 0.38–1.64 | 0.54 | L2: colonic disease | 0.55 | 0.21–1.39 | 0.21 | ||||||
| E3: extensive colitis | 0.67 | 0.28–1.55 | 0.36 | L3: ileocolonic disease | 0.85 | 0.33–2.19 | 0.74 | ||||||
| L4: upper gastrointestinal disease | 0.33 | 0.01–2.73 | 0.35 | ||||||||||
| B1: non-stricturing, non-penetrating | 0.52 | 0.20–1.33 | 0.18 | ||||||||||
| B2: stricturing | 4.26 | 0.95–29.99 | 0.08 | ||||||||||
| B3: penetrating | NA | ||||||||||||
| Perianal disease | NA | ||||||||||||
| Comorbidities | NA | ||||||||||||
| Comorbidities other than IMIDs | 0.67 | 0.28–1.55 | 0.36 | 1.21 | 0.44–3.34 | 0.69 | |||||||
| IMIDs | 1.19 | 0.46–3.06 | 0.71 | 2.16 | 0.67–7.68 | 0.20 | |||||||
| IBD-related treatment | |||||||||||||
| Topical 5-ASA | 1.21 | 0.56–2.60 | 0.61 | NA | |||||||||
| Systemic 5-ASA | 1.09 | 0.55–2.19 | 0.79 | NA | |||||||||
| Topical steroids | 0.24 | 0.01–1.56 | 0.20 | 1.05 | 0.04–27.25 | 0.97 | |||||||
| Systemic steroids | 1.96 | 0.31–15.29 | 0.46 | 3.33 | 0.40–69.04 | 0.30 | |||||||
| Immunomodulators | 0.54 | 0.17–1.47 | 0.24 | 1.06 | 0.36–3.08 | 0.90 | |||||||
| Azathioprine | 0.39 | 0.08–1.39 | 0.17 | 0.77 | 0.25–2.33 | 0.65 | |||||||
| Methotrexate | 1.28 | 0.04–32.85 | 0.86 | NA | |||||||||
| Biologic therapy | 1.33 | 0.50–3.49 | 0.55 | 1.08 | 0.43–2.69 | 0.86 | |||||||
| Tumour necrosis factor antagonist | 0.83 | 0.26–2.46 | 0.74 | 0.56 | 0.17–1.70 | 0.31 | |||||||
| Infliximab | 1.02 | 0.24–4.04 | 0.97 | 1.06 | 0.27–4.12 | 0.93 | |||||||
| Adalimumab | 1.28 | 0.15–10.98 | 0.80 | 0.5 | 0.06–2.72 | 0.44 | |||||||
| Vedolizumab | NA | 2.77 | 0.70–13.67 | 0.163 | |||||||||
| Ustekinumab | NA | NA | |||||||||||
| No treatment | 1.33 | 0.50–3.49 | 0.55 | 1.86 | 0.42–9.63 | 0.41 | |||||||
| Discontinuation of immunosuppressive therapy | 2.42 | 1.38–16.16 | 0.02 | 1.50 | 1.07–10.22 | 0.01 | 0.88 | 0.37–2.16 | 0.78 | ||||
| Antidrug antibody formation | NA | NA | |||||||||||
| Adverse COVID-19 | 1.89 | 0.57–6.72 | 0.29 | 5.03 | 1.16–34.96 | 0.04 | 2.76 | 1.05–3.90 | 0.04 | ||||
| Severe COVID-19 | NA | NA | |||||||||||
IBD, -nflammatory bowel disease; IMID, immune-mediated inflammatory disease; 5-ASA, 5-aminosalicylates; COVID-19, coronavirus disease 2019; NA, not applicable; OR, odds ratio. Localization of IBD is categorized according to the Montreal Classification. The p-values originate from regression analyses, and p < 0.05 [bold] was considered significant.
3.3.2 Health-related quality of life after COVID-19
The health-related quality of life after COVID-19 was assessed among 137 [42.9%] patients with UC including 125 [45.8%] and 12 [26.1%, p = 0.01] patients with mild and adverse COVID-19, respectively, as well as 85 [43.1%] patients with CD, of whom 74 [42.8%] and 11 [45.8%, p = 0.78] patients experienced mild and adverse COVID-19, respectively. No difference was observed in terms of EQ-5D-5L among patients with UC and CD or patients with mild, adverse or severe COVID-19 [Supplementary Data pp. 17–18]. The SIBDQ scores were similar among patients with mild, adverse or severe COVID-19 and UC (mild: median 59 [IQR 50–65], adverse: 62 [54–65], severe: 62 [54–65], p = 0.89) or CD (mild: 57 [46–65], 58 [49–64], 58 [49–64], p = 0.91) as well, and no difference was observed in the subscores. Similarly, the IBD Disability Index (UC: median 10 [IQR 6–16], CD: 13 [6–20], p = 0.16) scores were not associated with the severity of COVID-19 [details in Supplementary Data p. 19]. Finally, while patients with UC and adverse and mild COVID-19 did not differ in their Fatigue Score (47 [IQR 42–48], 41 [IQR 31–47], p = 0.40), CD patients with adverse COVID-19 experienced more fatigue than patients with mild COVID-19 (26 [IQR 25–35], 41 [IQR 29–46], p = 0.03).
3.3.3 Disease activity of IBD after COVID-19
Following COVID-19, seven patients with UC [2.3%] and eight patients with CD [4.1%] experienced clinical relapse after a median of 7.0 weeks [IQR 3.0–10.0], while three [1.0%] and six [3.1%] patients, respectively, experienced a clinical relapse at long-term follow-up (median 17.5 weeks [IQR 14.8-30.9]). No association was found between changes in IBD-related treatments and the severity of COVID-19 [Supplementary Data pp. 20–21] and no clinical factors were associated with short-term or long-term clinical relapse of UC [Table 3 and Supplementary Data pp. 22–23]. However, short-term relapse in CD was independently associated with adverse COVID-19 (OR = 5.11 [95% CI 1.64–15.12], p < 0.01), upper gastrointestinal CD (OR = 10.57 [95% CI 1.89–54.15], p < 0.01) and treatment with ustekinumab (OR = 18.32 [95% CI 1.44-43.44], p = 0.03), while long-term clinical relapse was associated with CD in clinical remission at the time of infection (OR = 0.21 [95% CI 0.04–0.90], p = 0.04), upper gastrointestinal CD (OR = 1.50 [95% CI 1.20–5.25], p = 0.01) and treatment with infliximab (OR = 5.17 [95% CI 1.14–21.33], p = 0.02) [Table 3, Supplementary Data pp. 22–23].
Table 3.
Factors associated with short-term clinical relapse in ulcerative colitis and Crohn’s disease following COVID-19
| Ulcerative colitis [univariate analysis] | Crohn’s disease [univariate analysis] | Crohn’s disease [multivariate analysis] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||
| Female gender | 0.61 | 0.23–1.50 | 0.29 | 0.64 | 0.26–1.59 | 0.33 | ||||
| Age >50 years | 0.29 | 0.34–2.09 | 0.74 | 1.37 | 0.55–3.35 | 0.48 | ||||
| Age >60 years | 1.18 | 0.41–3.04 | 0.73 | 1.03 | 0.32–2.81 | 0.94 | ||||
| Age >70 years | 0.88 | 0.20–2.74 | 0.84 | 1.27 | 0.28–4.19 | 0.71 | ||||
| Body mass index >25 | 0.54 | 0.08–2.02 | 0.43 | 1.47 | 0.48–4.07 | 0.46 | ||||
| Smoking at the time of COVID-19 | 2.01 | 0.75–5.66 | 0.16 | 0.71 | 0.27–1.80 | 0.48 | ||||
| Disease activity of IBD at diagnosis of COVID-19 | ||||||||||
| Clinical remission | 1.20 | 0.48–3.26 | 0.69 | 0.62 | 0.25–1.52 | 0.30 | ||||
| Biochemical remission | 1.84 | 0.74–4.98 | 0.19 | 0.88 | 0.35–2.16 | 0.78 | ||||
| Endoscopic remission | 0.69 | 0.10–2.53 | 0.63 | 1.15 | 0.25–3.77 | 0.82 | ||||
| Phenotype of IBD | ||||||||||
| E1: ulcerative proctitis | 1.46 | 0.50–3.77 | 0.44 | L1: ileal disease | 0.99 | 0.31–2.69 | 0.98 | |||
| E2: left-sided UC | 0.85 | 0.29–2.18 | 0.76 | L2: colonic disease | 1.43 | 0.56–3.52 | 0.43 | |||
| E3: extensive colitis | 1.21 | 0.42–3.11 | 0.69 | L3: ileocolonic disease | 1.04 | 0.38–2.63 | 0.92 | |||
| L4: upper gastrointestinal disease | 6.75 | 1.25–32.90 | 0.01 | 10.57 | 1.89–54.15 | <0.01 | ||||
| B1: non-stricturing, non-penetrating | 1.16 | 0.47–3.05 | 0.74 | |||||||
| B2: stricturing | 1.62 | 0.43–4.88 | 0.41 | |||||||
| B3: penetrating | 0.87 | 0.04–5.02 | 0.90 | |||||||
| Perianal disease | ||||||||||
| Comorbidities other than IMIDs | 1.85 | 0.73–4.54 | 0.17 | 1.20 | 0.43–3.03 | 0.70 | ||||
| IMIDs | 1.32 | 0.36–3.78 | 0.62 | 1.54 | 0.47–4.27 | 0.42 | ||||
| IBD-related treatment | ||||||||||
| Topical 5-ASA | 1.34 | 0.49–3.35 | 0.53 | NA | ||||||
| Systemic 5-ASA | 1.01 | 0.40–2.47 | 0.97 | NA | ||||||
| Topical steroids | NA | NA | ||||||||
| Systemic steroids | 1.30 | 0.06–7.26 | 0.80 | 2.81 | 0.39–13.20 | 0.22 | ||||
| Immunomodulators | 0.68 | 0.10–2.47 | 0.61 | 1.65 | 0.62–4.12 | 0.29 | ||||
| Azathioprine | 1.01 | 0.15–3.76 | 0.98 | 1.39 | 0.47–3.66 | 0.51 | ||||
| Methotrexate | NA | 2.03 | 0.10–14.58 | 0.53 | ||||||
| Biologic therapy | 1.11 | 0.25–3.47 | 0.87 | 1.37 | 0.55–3.36 | 0.48 | ||||
| Tumour necrosis factor antagonist | 0.48 | 0.02–2.46 | 0.48 | 1.15 | 0.39–2.99 | 0.78 | ||||
| Infliximab | 0.78 | 0.04–4.09 | 0.81 | 1.09 | 0.24–3.56 | 0.88 | ||||
| Adalimumab | NA | 1.09 | 0.24–3.56 | 0.88 | ||||||
| Vedolizumab | 1.44 | 0.07–8.12 | 0.73 | 0.50 | 0.02–2.71 | 0.52 | ||||
| Ustekinumab | 14.90 | 0.57–386.28 | 0.18 | 17.40 | 1.59–384.42 | 0.02 | 18.32 | 1.44–43.44 | 0.03 | |
| No treatment | 0.56 | 0.12–1.73 | 0.37 | 0.65 | 0.22–1.66 | 0.39 | ||||
| Discontinuation of immunosuppressive therapy | 0.94 | 0.05–5.05 | 0.95 | 3.98 | 0.81–15.39 | 0.07 | ||||
| Antidrug antibody formation | NA | NA | ||||||||
| Adverse COVID-19 | 0.99 | 0.22–3.09 | 0.99 | 4.33 | 1.49–11.91 | <0.01 | 5.11 | 1.64–15.12 | <0.01 | |
| Severe COVID-19 | NA | 4.11 | 0.18–44.80 | 0.25 | ||||||
IBD, inflammatory bowel disease; IMID, immune-mediated inflammatory disease; 5-ASA, 5-aminosalicylates; COVID-19, coronavirus disease 2019; NA, not applicable; OR, odds ratio. Localization of IBD is categorized according to the Montreal Classification. The p-values originate from regression analyses, and p < 0.05 [bold] was considered to be significant.
3.3.4 Re-infection with SARS-CoV-2
Before vaccination of the cohort began on December 27, 2020, the diagnosis of COVID-19 was established among 253 patients with UC and 163 patients with CD. Of these, 203 [80.2%] UC and 112 [68.7%] CD patients were subsequently tested for COVID-19; none, however, appeared to be re-infected after 3 months. Broadening the definition of re-infection, as described previously, three patients with UC (1.48% [95% CI 1.20–1.62]), all with a mild first episode of COVID-19, experienced a second and mild COVID-19 infection following two negative RT-PCR tests. Furthermore, one patient with CD, a 78-year-old female with severe chronic obstructive pulmonary disease and asthma who was not receiving immunosuppressive treatment, developed COVID-19 a second time after two negative RT-PCR tests (0.89% [95% CI 0.70–1.00]). This second COVID-19 episode resulted in acute kidney failure and death due to severe COVID-19 pneumonia. This patient’s first COVID-19 infection was also adverse and required hospitalization.
4 Discussion
The burden of COVID-19, including the number of affected individuals, constitutes an unprecedented health challenge. To manage these patients, data from extensive population-based studies are urgently needed to ensure informed decision-making based on generalizable conclusions in the planning and prioritization of healthcare. To our knowledge, this is the first population-based study with data at the level of individual patients to assess the short- and long-term health consequences of COVID-19 and the first study to address the long-term effects of COVID-19 in patients with UC and CD. Within our cohort, we demonstrate a relatively high risk of adverse and severe COVID-19 among patients with IBD. Second, we found that approximately 40% of patients with UC and CD experienced persisting symptoms of COVID-19 during follow-up after a median of 5 months, which was associated with discontinuation of immunosuppressive therapies for UC due to COVID-19 and adverse COVID-19 among patients with CD. Assessing the disease activity of IBD following COVID-19, we found that relapse of CD was independently associated with adverse COVID-19, the L4 phenotype, and use of ustekinumab and infliximab during COVID-19 infection.
To our knowledge, only one study has conducted a direct comparison of outcomes of COVID-19 among patients with IBD and the background population.11 As with that study, we found a high risk of adverse COVID-19 among patients with UC and CD as compared with the background population. We also found a high risk of severe COVID-19, something that was not detected in the Swedish study. An important distinction between our study and the report from Sweden is that the latter included all patients with IBD as a reference; in contrast, we only included those with confirmed COVID-19. Furthermore, the Swedish report could not distinguish between UC and CD or assess the results in relation to IBD disease activity or medications. Nonetheless, our analyses should also be interpreted with caution as the analysis was based on very few patients and therefore needs to be confirmed in larger population-based generalizable cohorts.
In our cohort, a large proportion of patients with UC and CD reported sequelae after a median of 5 months, which most frequently included fatigue, anosmia and ageusia. This is consistent with findings from another large, ambidirectional cohort study with 6 months of follow-up of non-IBD patients discharged from hospitals in China.3 As in that study, we found no association between clinical sequelae and age or the presence of comorbidities, which is clinically relevant as it indicates that sequelae following COVID-19 are to be expected not only among elderly patients with comorbidities. However, emerging data suggest that sequelae might become more prevalent with increasing age and comorbidities.4 Our study also found that discontinuation of immunosuppressive therapies during COVID-19 was independently associated with the development of clinical sequelae following COVID-19 among patients with UC. As far as we know, this topic has not yet been investigated among patients with IBD or other immune-mediated inflammatory diseases and, therefore, might have implications for the management and awareness of this group of patients in the post-COVID-19 era.
The finding that short-term clinical relapse of CD was independently associated with the severity of COVID-19 is intriguing, but should be interpreted with caution until it has been verified in other cohorts, in light of the limited sample size in ours.26,27
The initial concern about treatment with immunosuppressive therapies during the COVID-19 pandemic was related to the balance between the risk of deterioration of COVID-19 and the risk of clinical relapse of UC and CD.7 Reassuringly, we found no association between discontinuation of immunosuppressive therapies during COVID-19 and clinical relapse of IBD, and no association between use of these medications and the severity of COVID-19. However, data regarding the latter are conflicting.7,12,13,28–30 Of particular interest is the consistent finding that 5-ASA is independently associated with severe COVID-19.13,28,29 However, this finding was not verified in the most recent update from the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease [SECURE-IBD] registry.14 In the current study, we did find 5-ASA to be associated with COVID-19-related mortality in an adjusted analysis; however, all patients who died of COVID-19 while receiving 5-ASA were already high-risk individuals, and a similar pattern can be recognized in the most recent description of the SECURE-IBD registry.14 The unclear causal relationship between 5-ASA and COVID-19-related outcomes is further complicated by different control groups, study designs and treatment guidelines for the use of 5-ASA across countries.
To our knowledge, the current study provides the first evidence for the development of clinical sequelae in a population-based setting that focuses on patients with UC and CD. The strengths of this study include its population-based design with individual-level data for the whole spectrum of patients with a confirmed diagnosis of IBD and COVID-19. Linking population-based data with patients’ unique medical records and their responses to validated questionnaires provides novel insights into the clinical course during, and sequelae following, COVID-19, including assessment of associated factors in a real-life setting in one of the largest cohorts to date. This was further reinforced by standardized case ascertainment methods within our relatively homogeneous population treated according to uniform national guidelines. However, we acknowledge several limitations to our study, the first being its observational study design, which hindered a systematic approach, such as using systematic pulmonary CT scans, as reported in a study of non-IBD patients.3 Second, the clinical sequelae following COVID-19, which were investigated among less than every second patient, were not limited to physician-verified outcomes and were limited by sample size and wide confidence intervals. Finally, this study was not designed to investigate specific variants of SARS-CoV-2 or the disease activity of IBD following COVID-19 in comparison with a non-COVID-19 population. These limitations warrant further investigation.
In conclusion, we have found that in this prospective, population-based cohort of unselected patients with either UC or CD who developed COVID-19, the outcomes appeared to be more unfavourable among these patients than among the background population, albeit with small sample sizes in the subanalyses, and that patients with IBD were prone to develop clinical and psychological sequelae. This study identifies clinical factors that might help guide physicians in decision-making and could have implications in the healthcare planning for post-COVID-19 sequelae in patients with IBD.
Supplementary Material
Acknowledgments
The authors are grateful to all Danish COVID-IBD Study Group members who have screened the outpatient lists for COVID-19. The group members are listed in the Supplementary Data, p. 2.
Funding
None.
Conflict of Interest
MA, JFD, AP, MRH, MKVA, SE, APP, NP, LL, TJ, AN, KVH, AM, ABL, HG, AMO, MDJ, KZ, MKK: None. JBS: Research grants from Takeda and the Capital Region Denmark, national coordinator of studies from AbbVie, Arena Pharmaceuticals, Ely Lilly and Boehringer Ingelheim. None of these pertain to the research submitted here. JB: personal fees from AbbVie, Janssen-Cilag, Celgene, Samsung Bioepis and Pfizer; grants and personal fees from Takeda, MSD and Tillots Pharma; grants from Novo Nordisk Foundation, and Bristol Meyers Squibb. None of these pertain to the research submitted here.
Author Contributions
Guarantor of the article: JB. Verification of the underlying data: MA and JB. MA: study concept design, patient inclusion, data extraction, analysis and interpretation of data, and manuscript drafting. JFD, AP, MRH, MKVA, SE, APP, NP, LL, TJ, AN, KVH, AM, ABL, HG, AMO, MDJ, KT, MKK: patient inclusion, data extraction and critical revision of the manuscript. JBS, JB: study concept design, critical revision of the manuscript and supervision. All authors approved the final version of the manuscript, including the authorship list.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO. WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization. https://covid19.who.int/. Accessed May 23, 2021. [Google Scholar]
- 3. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. Epub ahead of print 2021. Doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2021;373:n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. Epub ahead of print 2017. Doi: 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 6. Zabana Y, Rodríguez L, Lobatón T, et al. Relevant infections in inflammatory bowel disease, and their relationship with immunosuppressive therapy and their effects on disease mortality. J Crohns Colitis 2019;13:828–37. [DOI] [PubMed] [Google Scholar]
- 7. Attauabi M, Poulsen A, Theede K, et al. Prevalence and outcomes of COVID-19 among patients with inflammatory bowel disease—a Danish prospective population-based cohort study. J Crohns Colitis. Epub ahead of print 2021. Doi: 10.1093/ecco-jcc/jjaa205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Attauabi M, Seidelin JB, Felding OK, et al. Coronavirus disease 2019, immune-mediated inflammatory diseases and immunosuppressive therapies – a Danish population-based cohort study. J Autoimmun. Epub ahead of print 2021. Doi: 10.1016/j.jaut.2021.102613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan N, Patel D, Xie D, et al. Are patients with inflammatory bowel disease at an increased risk of developing SARS-CoV-2 than patients without inflammatory bowel disease? Results from a nationwide veterans’ affairs cohort study. Am J Gastroenterol. Epub ahead of print 2020. Doi: 10.14309/ajg.0000000000001012 [DOI] [PubMed] [Google Scholar]
- 10. Derikx LAAP, Lantinga MA, de Jong DJ, et al. Clinical outcomes of Covid-19 in patients with inflammatory bowel disease: a nationwide cohort study. J Crohns Colitis 2021;15:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ludvigsson JF, Axelrad J, Halfvarson J, et al. Inflammatory bowel disease and risk of severe COVID-19: a nationwide population-based cohort study in Sweden. United European Gastroenterol J 2021;9:177–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Attauabi M, Seidelin J, Burisch J; Danish COVID-IBD Study Group . Association between 5-aminosalicylates in patients with IBD and risk of severe COVID-19: an artefactual result of research methodology? Gut 2021;70:2020–2. [DOI] [PubMed] [Google Scholar]
- 13. Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut 2021;70:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ungaro R, Brenner E, Agrawal1 M, et al. 5-Aminosalicylates are not associated with adverse outcomes in inflammatory bowel disease patients with COVID-19: analysis from an international registry. ECCO Week 2021;15:S006. [Google Scholar]
- 15. Langholz E. Ulcerative colitis. An epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan Med Bull 1999;46:400–15. [PubMed] [Google Scholar]
- 16. Munkholm P. Crohn’s disease–occurrence, course and prognosis. An epidemiologic cohort-study. Dan Med Bull 1997;44:287–302. [PubMed] [Google Scholar]
- 17. WHO COVID-19: Case Definitions. World Health Organization. 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2. Accessed May 23, 2021.
- 18. Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021;372:n136. [DOI] [PubMed] [Google Scholar]
- 19. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337–43. [DOI] [PubMed] [Google Scholar]
- 21. Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 91:1571–8. [PubMed] [Google Scholar]
- 22. Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. Epub ahead of print 2012. Doi: 10.1136/gutjnl-2011-300049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Czuber-Dochan W, Norton C, Bassett P, et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J Crohns Colitis 2014;8:1398–406. [DOI] [PubMed] [Google Scholar]
- 24. Yahav D, Yelin D, Eckerle I, et al. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect. Epub ahead of print 2021. Doi: 10.1016/j.cmi.2020.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. Epub ahead of print 2007. Doi: 10.1016/S0140-6736(07)61602-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Effenberger M, Grabherr F, Mayr L, et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 2020;69:1543–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reuken PA, Wüst M, Löffler B, Bauer M, Stallmach A. Letter: SARS-CoV-2-induced gastrointestinal inflammation. Aliment Pharmacol Ther 2020;52:1748–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an International Registry. Gastroenterology 2020;159:481–91.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lamb CA, Sebastian S, Kent AJ, et al. ; PREPARE-IBD Study Group. Letter: Risk of severe COVID-19 outcomes associated with inflammatory bowel disease medications-reassuring insights from the United Kingdom PREPARE-IBD multicentre cohort study. Aliment Pharmacol Ther 2021;53:1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyer A, Semenzato L, Zureik M, Weill A, Carbonnel F, Dray-Spira R. Risk of severe COVID-19 in patients treated with IBD medications: a French nationwide study. Aliment Pharmacol Ther 2021;54:160–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.