Abstract
Aims
Since its emergence in early 2020, the novel severe acute respiratory syndrome coronavirus 2 causing coronavirus disease 2019 (COVID-19) has reached pandemic levels, and there have been repeated outbreaks across the globe. The aim of this two part series is to provide practical knowledge and guidance to aid clinicians in the diagnosis and management of cardiovascular (CV) disease in association with COVID-19.
Methods and results
A narrative literature review of the available evidence has been performed, and the resulting information has been organized into two parts. The first, which was reported previously, focused on the epidemiology, pathophysiology, and diagnosis of CV conditions that may be manifest in patients with COVID-19. This second part addresses the topics of: care pathways and triage systems and management and treatment pathways, both of the most commonly encountered CV conditions and of COVID-19; and information that may be considered useful to help patients with CV disease (CVD) to avoid exposure to COVID-19.
Conclusion
This comprehensive review is not a formal guideline but rather a document that provides a summary of current knowledge and guidance to practicing clinicians managing patients with CVD and COVID-19. The recommendations are mainly the result of observations and personal experience from healthcare providers. Therefore, the information provided here may be subject to change with increasing knowledge, evidence from prospective studies, and changes in the pandemic. Likewise, the guidance provided in the document should not interfere with recommendations provided by local and national healthcare authorities.
Keywords: ACE2, Acute coronary syndromes, Arrhythmias, Biomarkers, Cardiogenic shock, COVID-19, Heart failure, Myocarditis, Venous thromboembolism, Pulmonary embolism, Thrombosis
Graphical Abstract
Graphical Abstract.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) reached pandemic levels in March 2020 and has caused repeated waves of outbreaks across the globe. COVID-19 shares many manifestations of a systemic disease and has major implications for the cardiovascular (CV) system, which are summarized in a two part review entitled European Society of Cardiology (ESC) Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic.
The second part of the document addresses the topics of protection measures, triage systems, risk categorization of procedures, management and treatment pathways, therapeutic strategies for SARS-CoV-2 infections, and patient information. Owing to the highly contagious nature of the SARS-CoV-2 virus, appropriate protection of healthcare professionals (HCP) and patients in different encounters, such as ambulatory care setting, hospital wards, emergency room visits, and intermediate and intensive care units, is of pivotal importance. Depending on the extent of pandemic involvement in various regions, prioritization of specialized procedures according to degree of urgency gains prominence, and this document provides guidance on how invasive procedures for coronary artery disease (CAD), valvular heart disease (VHD), acute and chronic heart failure (HF), and arrhythmic heart disease may be categorized. Management and treatment pathways for the most important CV disease (CVD) manifestations that may affect COVID-19 patients are summarized in detail in Section Management/treatment pathways, including acute coronary syndrome (ACS) and chronic coronary syndrome (CCS), acute and chronic HF, VHD, hypertension, pulmonary embolism, and arrhythmias. This is followed by an overview of various therapeutic agents under evaluation to treat SARS-CoV-2 infections highlighting the important issue of drug–drug interactions, particularly as it relates to proarrhythmic properties, such as QTc (corrected QT interval) prolongation. Useful information for patients and updates on vaccinations are summarized in the final chapter.
While the document is comprehensive, it is not a guideline but rather a guidance document. The recommendations are the result of observations and personal experience from healthcare providers. The present publication provides a summary of the guidance until March 2021. Therefore, the information provided here may be subject to change with increasing knowledge, evidence from prospective studies, and changes in the pandemic. Likewise, the guidance provided in the document should in no way interfere with recommendations provided by local and national healthcare authorities.
Management/treatment pathways
This section provides guidance on the specialist management of patients with CV conditions, while general guidance on protective measures and care pathways for healthcare personnel and patients in cardiology is provided as Supplementary material online.
Cardiogenic shock
Key points.
Management of cardiogenic shock (CS) and out-of-hospital cardiac arrest (OHCA) is critically time-dependent, requiring a dedicated network and multidisciplinary expertise.
Resource allocation should still try to deliver a standardized team-based approach including availability and feasibility of mechanical circulatory support (MCS).
Invasive coronary angiography (ICA) remains the mainstay of treatment. However, special considerations need to be taken into account to minimize the risk of widespread nosocomial infections.
In patients with concomitant COVID-19, escalation to MCS should be carefully weighed against the development of coagulopathy associated with COVID-19 and the need for specific treatment (prone position) required for acute lung injury.
In case of requirement for MCS, extracorporeal membrane oxygenation should be the preferred temporary MCS because of the oxygenation capabilities.
In case of acute renal failure, continuous renal replacement should be used restrictively according to established criteria.
Daily sequential organ failure assessment and therapeutic intervention scoring system scores should be assessed for most critical patients, to improve decision-making.
The safety of HCP is of predominant importance to avoid any HCP infections
SARS-Cov-2 infection should be excluded throughout two negative tests performed using a reverse transcriptase-polymerase chain reaction (RT-PCR). For intubated patients, a tracheal aspirate would additionally be required (see Supplementary material online, Section 1 and Supplementary material online, Section 2).
When the patient cannot be placed in the supine position, it may be reasonable to provide cardiopulmonary resuscitation with the patient in the prone position, particularly in patients with advanced airway and circulatory support.1,2
CS and OHCA are time-dependent diseases in need of relevant resources, trained systems, and dedicated networks for optimal outcome. In general, treatment of CS and OHCA should follow current guidelines and current evidence.3–9 However, it should be considered that in an overwhelmed critical care system stressed by the pandemic COVID-19, it will not be possible for all patients to receive intensive care unit (ICU) treatment due to limited resources. This leads to difficult situations based also on the four widely recognized principles of medical ethics (beneficence, non-maleficence, respect for autonomy, and equity), which are also crucial under conditions of resource scarcity. If resources available are insufficient to enable all patients to receive the ideally required treatment, then fundamental principles should be applied in accordance with the following rules of precedence:
Equity: Available resources are to be allocated without discrimination (i.e. without unequal treatment on grounds of age, sex, residence, nationality, religious affiliation, social or insurance status, or chronic disability). The allocation procedure must be fair, objectively justified, and transparent. With a fair allocation procedure, arbitrary decisions, in particular, can be avoided.
Preserving as many lives as possible: Under conditions of acute scarcity, all measures are guided by the aim of minimizing the number of deaths. Decisions should be made in such a way as to ensure that as few people as possible become severely ill or die.
Protection of the professionals involved: Therefore, triage protocols are needed to maximize benefits and relieve HCP from improvising decisions about whom to treat or making those decisions in isolation.
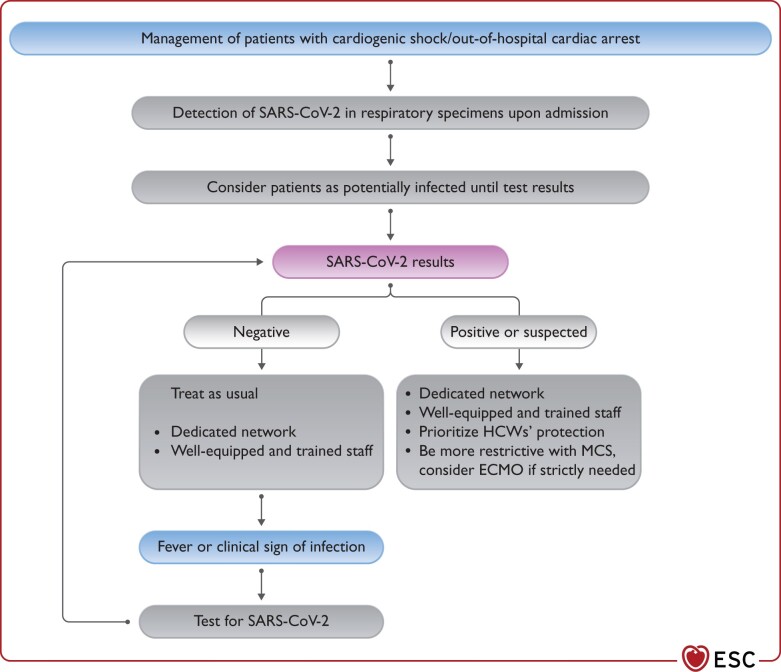
Triage strategies, based on current evidence and a previously established critical care triage protocol developed by working groups for use during a worldwide influenza pandemic,10 are summarized in Table 1. Specific recommendations are provided for patients with and without concomitant infection in Figure 1. Two scenarios will be considered:
Table 1.
Detailed inclusion and exclusion criteria for triage in intensive care unit upon admission
Inclusion criteria:
|
| If at least one criterion is fulfilled, check for exclusion criteria. |
Exclusion criteria:
|
| If not even one criterion is met and ICU beds are not available, check for additional exclusion criteria. |
Additional exclusion criteria to be checked if no ICU beds are available:
|
| If neither of these criteria is fulfilled, consider to withdraw ICU support from patients who arrived earlier to save those with better prognoses. |
Criteria for little or no likelihood of benefit with ICU treatment (occurrence of at least one criterion):
|
COPD, chronic obstructive pulmonary disease; FEV, forced expiratory volume in 1 s; FIO, fraction of inspired oxygen; GOLD, global Initiative for chronic obstructive lung disease; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes; NYHA, New York Heart Association; PaO2, partial pressure of arterial oxygen; SOFA, Sequential Organ Failure Assessment; SpO2, oxygen saturation measured by pulse oximetry; TISS, therapeutic intervention scoring system; TLC, total lung capacity; VC, vital capacity.
Figure 1.
Management of patients with cardiogenic shock/out-of-hospital cardiac arrest during COVID-19 pandemic. COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; HCW, healthcare worker; MCS, mechanical circulatory support; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Non-infected patients and
Possibly infected/COVID-19-positive patients.
The infection should be suspected according to recently defined epidemiological and clinical criteria.11
ST-segment elevation myocardial infarction
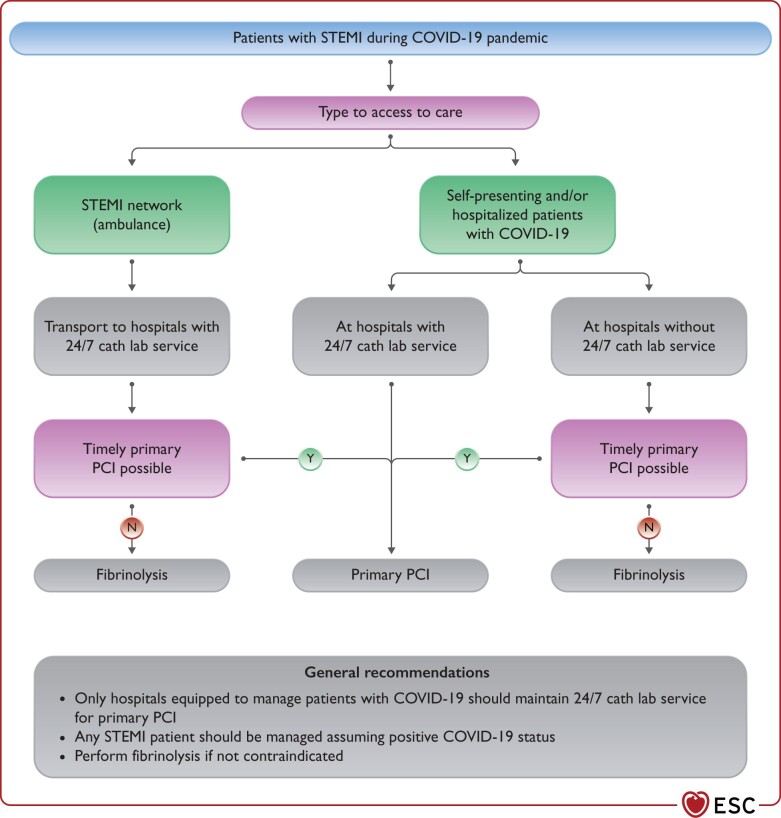
The COVID-19 pandemic should not compromise timely reperfusion of ST-segment elevation MI (STEMI) patients.12–14 In line with current guidelines, reperfusion therapy remains indicated in patients with symptoms of ischaemia of <12 h duration and persistent ST-segment elevation in at least two contiguous electrocardiogram (ECG) leads.5 Concurrently, the safety of HCP should be ensured.15 To that purpose, and in the absence of previous SARS-CoV-2 testing, all STEMI patients should be managed as if they are COVID-19 positive. The main principles of STEMI management in the COVID-19 pandemic are the following (Figure 2):
Figure 2.
Management of patients with STEMI during COVID-19 pandemic. COVID-19, coronavirus disease 2019; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation MI.
-
The maximum delay from STEMI diagnosis to the reperfusion of 120 min should remain the goal for reperfusion therapy under the following considerations:
Primary percutaneous coronary intervention (PCI) remains the reperfusion therapy of choice, if feasible within this time frame and performed in facilities approved for the treatment of COVID-19 patients in a safe manner for healthcare providers and other patients.
Primary PCI pathways may be delayed during the pandemic (up to 60 min in some networks experience) due to delays in the delivery of care and the implementation of protective measures.
If the target time cannot be met and it is not contraindicated, fibrinolysis should be performed in accordance with ESC guidelines recommendations.5
As SARS-CoV-2 test results are not immediately available in STEMI patients, any STEMI patient should be considered potentially infected.
All STEMI patients should undergo testing for SARS-CoV-2 as soon as possible following first medical contact irrespective of reperfusion strategy, at the latest upon admission to the ICU post primary PCI. Until the result of the test is known, all precautionary measures should be taken to avoid potential infection of other patients and HCP.
Consider immediate complete revascularization if indicated and appropriate to avoid staged procedures and reduce hospital stay.
All physicians involved in the management of patients with STEMI should be familiar with indications, contraindications, and dosage of fibrinolysis and adhere to established administration protocols.5
Left ventriculography should be considered during catheterization if echocardiography has not been performed before catheterization laboratory admission or is not feasible soon after the procedure.
The treatment of the non-culprit lesions should be managed according to patients’ clinical stability as well as angiographic features of those lesions. In the presence of persistent symptomatic evidence of ischaemia, subocclusive stenoses, and/or angiographically unstable non-culprit lesions, PCI during the same hospitalization should be considered. Treatment of other lesions should be delayed, planning a new hospitalization after the peak of the outbreak.5
Non-ST-segment elevation acute coronary syndromes
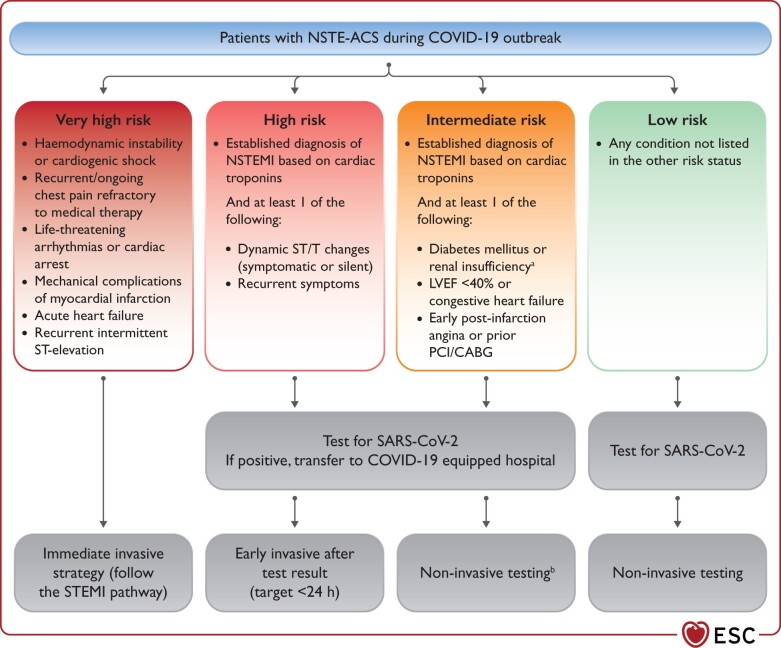
The management of patients with non-ST-segment elevation ACS should be guided by the risk stratification and intensity of involvement in the epidemics.16 In geographic territories with significant pandemic involvement, testing for SARS-CoV-2 should be performed as soon as possible following first medical contact, irrespective of treatment strategy, to allow HCP to implement adequate protective measures and management pathways (see Supplementary material online, Section 1). Patients should be categorized into four risk groups (i.e. very high risk, high risk, intermediate risk, and low risk) and managed accordingly (Figure 3).
Figure 3.
Recommendations for the management of patients with NSTE-ACS in the context of COVID-19 outbreak. CABG, coronary artery bypass graft; COVID-19, coronavirus disease 2019; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST-segment-elevation MI; PCI, percutaneous coronary intervention; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. aEstimated glomerular filtration rate <60 mL/min/1.73 m2. bCoronary computed tomography angiography should be favoured, if equipment and expertise are available. In low-risk patients, other non-invasive testing might be favoured in order to shorten hospital stay. It is suggested to perform left ventriculography during catheterization if echocardiography not performed before catheterization laboratory admission.
For patients at high risk, medical strategy aims at stabilization while planning an early (<24 h) invasive strategy. The time of the invasive strategy may, however, be longer than 24 h according to the timing of testing results.
Patients at intermediate risk should be carefully evaluated taking into consideration alternative diagnoses to Type I myocardial infarction (MI), such as Type II MI, myocarditis, or myocardial injury due to respiratory distress or multiorgan failure or Takotsubo. In the event any of the differential diagnoses seem plausible, a non-invasive strategy should be considered and coronary computed tomography angiography (CCTA) should be favoured, if equipment and expertise are available.
When there is a positive SARS-CoV-2 test, patients should be transferred for invasive management to a COVID-19 hospital equipped to manage COVID-19-positive patients. At times of high demand on the infrastructure and reduced availability of catheterization laboratories or operators, non-invasive conservative management might be considered with early discharge from the hospital and planned clinical follow-up.
Patients with troponin rise and no acute clinical signs of instability (ECG changes, recurrence of pain) might be managed with a primarily conservative approach.17,18 Non-invasive imaging using CCTA may speed up the risk stratification and avoid an invasive approach allowing for early discharge.19,20
Chronic coronary syndromes
HCP managing patients with CCS in geographical areas heavily affected by the COVID-19 pandemic should consider the following main points:
CCS patients are generally at low risk for CV events allowing the deferment of diagnostic and/or interventional procedures, in most cases.
Medical therapy should be optimized and/or intensified depending on the clinical status.
Remote clinical follow-up should be warranted to reassure patients and capture possible changes in clinical status that might require hospital admission in selected high-risk profile patients.
Practical considerations of medical therapy
Nonsteroidal anti-inflammatory drugs have been identified as a potential risk factor for serious clinical presentation of SARS-CoV-2 infection.21 Potential impact of chronic aspirin therapy has been questioned. However, at the low dose administered in CCS, aspirin has very limited anti-inflammatory effect. Therefore, CCS patients should not withdraw aspirin for secondary prevention.
Statin therapy has been variably associated with favourable outcomes in patients admitted with influenza or pneumonia.22,23 On the other hand, patients with COVID-19 have been reported to develop severe rhabdomyolysis or increased liver enzymes.24 In these latter cases, it may be prudent to temporarily withhold statin therapy.
For CCS patients treated with antihypertensive drugs please refer to Section Hypertension.
Non-invasive testing
Non-invasive testing in patients with CCS is tailored upon different clinical presentations.25 In regions with a high rate of SARS-CoV-2 infection, evaluation of asymptomatic CCS patients with non-invasive testing should be postponed in order not to expose these patients to an unnecessary risk of infection or overload the healthcare systems.
For symptomatic patients with suspected CAD and a pre-test probability of 5–15%, functional imaging for the detection of myocardial ischaemia or CCTA is normally recommended as initial tests to diagnose CAD. In regions experiencing a critical situation and medical systems overloaded by the COVID-19 pandemic, CAD screening, even in symptomatic patients, should probably be postponed in the majority of patients. Yet, if necessary, depending upon local availability and expertise, CTA should be preferred (see Guidance Part 1).
However, the increased workload of computed tomography (CT) departments should be acknowledged; they have been heavily disrupted by the high request of pulmonary CT for patients with COVID-19. In addition, feasibility/accuracy of CCTA might be hampered in patients with COVID-19 for the common occurrence of tachycardia and, at times, severe renal dysfunction. In case CCTA is not suitable (e.g. inability of heart rate control) or available, non-invasive testing should be postponed. Alternative imaging modalities should be discouraged during the acute pandemic phase unless severe ischaemia is suspected, to minimize the access of the patients to healthcare system (single photon emission computed tomography/Positron emission tomography) or to prevent close contact between patients and personnel (stress echocardiography).
For known CCS patients, clinical follow-up should be done mostly via tele-health (a dedicated telephone line should be made available to patients). Physicians could therefore address most of the patients’ concerns related to continuation or changes in medical therapy. Possible onset/recurrence of unstable symptoms should be estimated within the clinical history of the patient to weigh the need for hospitalization and diagnostic testing.
Invasive assessment and revascularization
Symptomatic patients with very high clinical likelihood of obstructive CAD are generally referred to ICA without prior non-invasive diagnostic testing.25 However, medical treatment should be attempted first to reserve ICA with possible ad hoc revascularization only in case of clinical instability, especially in regions where healthcare systems are heavily overloaded by patients with COVID-19.26 Revascularization, either by PCI or by coronary artery bypass graft (CABG), can be postponed in most CCS patients. Healthcare systems might identify COVID-19-free hospitals serving as hubs for selected CCS patients in whom invasive and surgical procedures cannot be postponed. In selected patients, hybrid revascularization CABG/PCI or even full-PCI can be considered by the heart team based on the patient’s clinical condition and local situation (see Table 2).
Table 2.
Management of chronic coronary syndromes during COVID-19 pandemic
| Continuation of medications in CCS patients is recommended during COVID-19 pandemic |
| Follow-up of CCS patients via tele-health is recommended |
| Revascularization of CCS patients must be postponed in low- to intermediate-risk patients |
| Postponing of non-invasive testing of CCS patients should be considered during COVID-19 pandemic |
| CT angiography should be preferred to non-invasive functional testing during COVID-19 pandemic |
| Screening for SARS-CoV-2 infection should be considered before cardiac surgery with nasopharyngeal swab and CT scan |
| Revascularization of high-riska CCS patients may be considered during COVID-19 pandemic |
| PCI may be considered over CABG in selected patients during COVID-19 pandemicb |
| Identification of COVID-19-free hospitals may be considered as ‘Hub’ for cardiac surgery |
| Invasive management of CCS in SARS-CoV-2-positive patients should be deferred until the patient has recovered, whenever possible |
CABG, coronary artery bypass graft; CCS, chronic coronary syndrome; COVID-19, coronavirus disease 2019; CT, computed tomography; ICU, intensive care unit; PCI, percutaneous coronary intervention; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Patients with high-risk symptoms and/or coronary anatomy and/or large ischaemia as assessed by Heart team.
To shorten hospital stay and keep ICU beds available for patients with COVID-19.
Heart failure: acute, myocarditis, chronic, left ventricular assist device, and transplantation
Patients with CV comorbidities, namely HF, are at increased risk of the more severe presentation and complications of COVID-19. Chronic HF is associated with a greater risk of hospitalization, requirement of mechanical ventilation, and mortality. Acute HF may occur as a major complication in patients hospitalized for COVID-19.
Acute heart failure
Key points.
Acute HF may complicate the clinical course of COVID-19, particularly in severe cases.
Underlying mechanisms of acute HF in COVID-19 may include the following: acute myocardial injury due to ischaemia, infarction or inflammation (myocarditis), acute respiratory distress syndrome (ARDS), acute kidney injury and hypervolemia, stress-induced cardiomyopathy, and tachyarrhythmia. Acute myocarditis with direct demonstration of SARS-CoV-2, inflammatory infiltrate, and myocardial necrosis is, however, rare.27,28
COVID-19 pneumonia may lead to the worsening haemodynamic status due to hypoxaemia, dehydration, and hypoperfusion.
Since symptoms of COVID-19 and acute/worsening chronic HF can be similar, distinguishing these two entities is challenging. In addition, the two conditions may coexist. Clinical presentation, pre-existing CV comorbidities, and chest imaging findings suggestive of HF (e.g. cardiomegaly and/or bilateral pleural effusion) are of utmost importance. Significantly elevated B-type natriuretic peptide (BNP)/N-terminal B-type natriuretic peptide (NT-proBNP) levels also suggest acute HF, although increased levels of natriuretic peptides may also be found in COVID-19 patients in the absence of HF or left ventricular (LV) systolic dysfunction. Prudent use of bedside point-of-care or transthoracic echocardiography should be considered, keeping in mind the prevention of contamination of personnel and/or equipment.29
The treatment of acute HF in patients with SARS-CoV-2 infection should be equivalent to those without COVID-19, and attention should be given to early detection and treatment of complications, including the need for non-invasive or invasive ventilation, bleeding events, and cardiac arrhythmias.29,30
Data on acute HF in COVID-19 are still scarce. In an earlier report from China, 23% of all hospitalized patients developed HF, while HF prevalence was significantly higher in fatal cases compared with survivors (52% vs. 12%, P < 0.0001).31 In a cohort of 21 patients from the USA admitted to an intensive care unit, 7 (33.3%) patients developed dilated cardiomyopathy, characterized by globally decreased LV systolic function, clinical signs of CS, elevated creatine kinase, or troponin I levels, or hypoxaemia, without a history of systolic dysfunction.32 An analysis of mortality causes in COVID-19 patients (150 hospitalized/68 dead) revealed that myocardial damage/HF and combined respiratory failure/myocardial damage/HF were responsible for 7% and 33% of fatal cases, respectively.33 These early reports require cautious interpretation, because small sample size and inclusion of the more severe cases may have resulted in an overestimation.
Recently, a meta-analysis of 30 studies (6389 patients) published between February and April 2020 including a broader spectrum of COVID-19 patients demonstrated that acute myocardial injury and overt HF occur in 15.7% and 11.5% of patients, respectively.34 In a cohort of 3080 confirmed cases in Spain, acute HF developed more frequently in those with a history of chronic HF; however, 2.5% developed incident HF during SARS-CoV-2 infection.30 Similar results were reported in an Italian multicentre study.35 In addition to chronic HF, advancing age, atrial tachyarrhythmias, and chronic obstructive pulmonary disease (COPD) were identified as independent predictors of acute HF. Patients developing HF have significantly higher 30-day mortality rates compared to those without HF (46.8% vs. 19.7%, P < 0.001), and withdrawal of standard HF medications increased in-hospital mortality.30 Recent studies show that COVID-19 also confers greater risk of right ventricular dysfunction and dilation, which are predictors of poorer outcome.36 In a cohort of 510 COVID-19 in-patients undergoing echocardiographic examinations, right ventricular remodelling was associated with a more than two-fold increase in mortality risk after adjustment for clinical variables and biomarkers.37,38
There are several, not mutually exclusive, mechanisms of acute HF in COVID-19, such as:
acute myocardial injury (defined as serum high-sensitivity troponin I elevation >99th percentile of the upper normal limit or new abnormalities in electrocardiography or echocardiography) occurs in 8–15% of COVID-19 patients.39 It may be caused by ischaemia, infarction, or inflammation (myocarditis). In patients with severe infection, evidence of acute myocardial injury is present in 22.2–31%.31,40,41 A meta-analysis of four studies (n = 341) suggested that in patients with severe infection, high-sensitivity troponin I was significantly higher at admission (mean standardized difference 25.6 ng/L) compared to those with non-severe course.42 In addition, troponin levels remained high in non-survivors throughout the clinical course and increased with illness deterioration.31 A history of HF was more frequently noted in patients with, compared to those without, acute myocardial injury (14.6% vs. 1.5%).43 Acute myocardial injury was also more frequently associated with significantly elevated NT-proBNP levels (median 1689 pg/mL).43 In a Spanish registry of 245 patients hospitalized for COVID-19, elevated troponin I levels were observed in 17.1%.44 On multivariate analysis, elevated troponin I was associated with higher mortality [odds ratio (OR), 4.93; 95% confidence interval (CI), 1.24–19.52; P = 0.023], HF (OR, 4.28; 95% CI, 1.30–14.07; P = 0.017) and the combined outcome of mortality or HF in patients without a history of heart disease (OR, 7.09; 95% CI, 2.28–22.03; P = 0.001), but not in patients with previous heart disease (P = 0.561, P = 0.337 and P = 0.992, respectively).44
ARDS, hypoxaemia, acute kidney injury, hypervolemia, increased adrenergic drive, stress-induced cardiomyopathy, fever, and a profound systemic inflammatory activation (‘cytokine storm’), characteristic of severe infection and multiorgan dysfunction, could also contribute to acute HF or exacerbation of chronic HF in COVID-19.45
Sustained/repetitive cardiac arrhythmia may also lead to deterioration in cardiac function. Apparently, cardiac arrhythmias have been described in 16.7% of all hospitalized COVID-19 patients and in 44.4% of those requiring intensive care admission,41 and atrial tachyarrhythmias have been identified as a predictor of acute HF development.30 An ECG on admission should always be performed and serial ECGs are required in patients with myocardial injury and those receiving pro-arrhythmic drugs.
Management of heart failure in individuals without COVID-19 during the COVID-19 outbreak
Internationally, several reports have suggested a decline in hospitalization rates for acute HF in individuals without SARS-CoV-2 infection during the peak of the COVID-19 pandemic compared with 2019.46–48 Despite similar disposition and management, patients admitted for acute HF in 2020 had more severe symptoms (e.g. New York Heart Association class III–IV in 96% vs. 77%, P = 0.03)49 and higher in-hospital mortality (7.3% vs. 6.1%, P = 0.03) compared with 2019.50 Also, a decline in the emergency department (ED) visits and an increase in out-of-hospital CV mortality have been reported.51,52 These findings call for further research into the causes and long-term prognostic implications to inform strategic plans for the management of chronic CV disorders during the COVID-19 crisis.
Key points.
Acute myocarditis as traditionally defined by viral presence, inflammatory infiltrates, and myocardial injury is seldom demonstrated in COVID-19 patients with increased interstitial myocardial macrophages shown in most of the cases.53
Accumulating clinical experience indicates that myocarditis can occur in SARS-CoV-2-infected individuals, even without pulmonary involvement, with various clinical presentations, including fulminant myocarditis.53
COVID-19 myocarditis should be suspected in patients with acute-onset chest pain, ST-segment changes and/or T wave inversion, cardiac arrhythmias, acute HF, and haemodynamic instability. Mild/moderate LV dilatation, global/multi-segmental LV hypocontractility, increased LV wall thickness (suggestive of oedema), moderately elevated cardiac troponin, and increased NT-proBNP, without significant coronary artery disease, are also suggestive of myocarditis. In particular, suspicion of COVID-19 myocarditis should be raised in patients with rapidly worsening acute HF/CS, without pre-existing CV disorders and acute coronary syndrome.
Cardiac magnetic resonance, if available, is the preferred method for the diagnosis of acute myocarditis.
Endomyocardial biopsy is not recommended for the routine assessment of patients suspected of having COVID-19 myocarditis and should be limited to cases of severe or refractory HF where histological findings may guide therapeutic choices.
No clear recommendation could be given regarding the treatment of patients with COVID-19 myocarditis. MCS, inotropes and/or vasopressors, and mechanical ventilation may be needed in severe cases. There is no compelling evidence to support the use of immunomodulatory therapy, including corticosteroids and intravenous immunoglobulins. However, corticosteroids are indicated when there is respiratory involvement and have been administered to patients who then had favourable clinical outcomes.53–56 Tocilizumab and favipiravir are currently being tested in a randomized trial.57
Myocarditis
Incidence, underlying mechanisms and risk factors of COVID-19 myocarditis are currently unclear. Endomyocardial biopsies have shown cardiotropism, including direct cardiomyocyte infection by SARS-CoV-2, a high degree of interstitial macrophages in a majority of the cases, and multifocal lymphocytic myocarditis in a minority.27,58 However, the mechanisms responsible for myocardial injury and dysfunction remain insufficiently understood. The clinical features vary. Some patients present with fever, dyspnoea, and acute-onset chest pain but without haemodynamic instability. Deterioration to acute HF, hypotension, and CS may also occur.53 In the most severe cases, fulminant myocarditis with CS has been described.59,60
Key points.
The risk of COVID-19 may be higher in chronic HF patients due to the advanced age and presence of several comorbidities.
Chronic HF patients with COVID-19 have a significantly higher risk of adverse outcomes.
In HF patients suspected of COVID-19, routine clinical assessment, temperature measurement, ECG (arrhythmias, ST-T wave changes), chest X-ray (cardiomegaly, COVID-19 pneumonia), and laboratory findings (elevated sedimentation rate, fibrinogen and C-reactive protein, and lymphocytopenia) can provide a diagnostic clue. Transthoracic echocardiography and chest CT scan can be used for further assessment, as indicated. In all instances, attention should be given to the prevention of viral transmission to healthcare providers and contamination of the equipment.
Patients with chronic HF should closely follow protective measures to prevent infection.
Ambulatory HF patients (with no cardiac emergencies) should refrain from hospital visits.
Guideline-directed medical therapy [including angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB) or sacubitril/valsartan, beta-blockers, mineralocorticoid receptor antagonists, and other guideline-directed medications) should be continued in chronic HF patients, irrespective of COVID-19.
Telemedicine should be considered whenever possible to provide medical advice and follow-up of ambulatory HF patients.
Chronic heart failure
Prevention of SARS-CoV-2 infection
During the COVID-19 outbreak, patients with chronic HF should be advised to closely follow protective measures aimed at preventing disease transmission (e.g. self-isolation, social distancing, frequent hand washing, use of hand sanitisers, and wearing a face mask in public spaces). HF outpatients should avoid routine, non-urgent hospital visits. Implementing remote monitoring may be an alternative.
Diagnostic hints
Routine clinical methods, ECG (arrhythmias, myocardial injury, myocarditis), and chest X-ray (cardiomegaly, COVID-19 pneumonia) can provide a diagnostic clue. Due to the relatively low sensitivity of chest X-ray to detect COVID-19 pneumonia, patients with a high degree of clinical suspicion (tachypnoea, hypoxaemia), but with ambiguous chest X-ray findings, should be referred to chest CT,61 which has high sensitivity and specificity to diagnose COVID-19-related pulmonary disease. Laboratory findings, such as increased erythrocyte sedimentation rate, fibrinogen and C-reactive protein, and lymphocytopenia, may suggest COVID-19 pneumonia. Transthoracic echocardiography is useful, not only to evaluate the pre-existing LV dysfunction in HF but also to assess patients suspected of having SARS-CoV-2-associated worsening cardiac function and/or myocarditis.62 Prudent use of bedside point-of-care or transthoracic echocardiography should be considered when the result of an examination is expected to provide a diagnosis and modify therapy.
Chronic heart failure treatment
SARS-CoV-2 utilizes the angiotensin-converting enzyme-2 (ACE2) receptors for cell entry and some data indicate that ACEIs and ARBs may up-regulate ACE2,63 thus hypothetically increasing susceptibility to the infection.
However, there is no clinical evidence of an association between ACEI/ARB treatment and the susceptibility to infection, or the clinical course. A recent study of 111 hospitalized patients with COVID-19 in France treated with ACEI/ARB for CV disorders (9% with HF) has demonstrated no association between exposure to ACEI/ARB and the rate of complications or mortality in a propensity score-adjusted analysis.64 Similarly, in a cohort of 965 patients with COVID-19 from Spain (21.8% on ACEI/ARB), treatment with ACEI/ARB had a neutral effect on mortality (OR, 0.62; 95% CI, 0.17–2.26; P = 0.486), HF (OR, 1.37; 95% CI, 0.39–4.77; P = 0.622), and other complications.44 Furthermore, available data do not support discontinuation of ACEI/ARB in HF patients with COVID-19, as this may increase the risk of death.30 Hence, it could be recommended that HF patients continue all prescribed guideline-directed medications (including ACEI, ARB, or sacubitril/valsartan), irrespective of COVID-19.65
COVID-19 patients may become hypotensive due to dehydration, septic shock, and haemodynamic deterioration; hence adjustment of HF medication doses should be considered.
Chronic heart failure and outcomes in COVID-19
Worse clinical outcomes have been reported among COVID-19 patients with a history of chronic HF. Along with an older age, arrhythmias, dementia, ischaemic heart disease, diabetes, obesity, and hypertension, chronic HF has been associated with a higher risk of hospitalization [hazard ratio (HR), 1.6, 95% CI, 1.2–2.1] and mortality (HR, 2.3, 95% CI, 1.6–3.2) among 2653 COVID-19 patients in Italy.66 Similarly, in 9148 COVID-19 patients from South Korea, a history of chronic HF conferred a 3.17 higher odds ratio (95% CI, 1.88–5.34) for mortality.67 Among 692 patients admitted for COVID-19 in 13 Italian cardiology centres, a history of HF has been associated with an increased risk of death (adjusted HR, 2.25, 95% CI, 1.26–4.02), and in-hospital complications, including acute HF (33.3% vs. 5.1%, P < 0.001), acute renal failure (28.1% vs. 12.9%, P < 0.001), sepsis (18.4% vs. 8.9%, P = 0.006), and multiorgan failure (15.9% vs. 5.8%, P = 0.004). 35 In a cohort of 6439 hospitalized COVID-19 patients from the USA, a history of HF conferred a 3.66 higher odds ratio (95% CI, 2.56–5.16, P < 0.001) for mechanical ventilation and a 1.88 higher odds ratio (95% CI, 1.27–2.78, P = 0.02) for death, regardless of LV ejection fraction or the use of ACEI.68
Telemedicine and home drug delivery
Given the restraints to the usual care and high morbidity and mortality among HF patients contracting COVID-19, the more widespread use of telemedicine should be encouraged to minimize the risk of infection, and to ensure continuity of care and timely optimisation of medical treatment. Successful use of this technology has been reported in providing medical advice, treatment adjustment, and follow-up of ambulatory HF patients during the COVID-19 outbreak.69,70 If feasible (and necessary), home delivery and mailing of standard HF drugs may be a viable option, if permitted by local regulations/laws.
Key points.
Due to the nature of the device, left ventricular assist device (LVAD) patients have greater susceptibility to the infection, and strict preventive measure should be applied to avoid it.
Owing to the state of iatrogenic immunosuppression, heart transplant recipients may be at a higher risk of severe COVID-19 disease or prolonged viral shedding; hence, tight adherence to preventive measures should be advised to avoid infection.
Limited data suggest that heart transplant recipients may have a similar presentation as immunocompetent individuals and a favourable clinical course of COVID-19. However, variable clinical outcomes in solid organ recipients in earlier coronavirus outbreaks [SARS and Middle East respiratory syndrome (MERS)]71,72 suggest that hospitalization, close monitoring, and appropriate treatment of COVID-19 heart transplant patients should be recommended.
Left ventricular assist device and heart transplantation
LVAD patients are fragile, and every measure should be used to prevent viral transmission. Cautious monitoring and management of anticoagulation therapy is advised because both COVID-19 and antiviral medications can affect anticoagulant dosing. If technically feasible, assessment of LVAD function by telemonitoring is preferable. General recommendations for all LVAD patients should also be applied, regardless of COVID-19.
Data on the susceptibility to infection and the clinical course of COVID-19 in heart transplant recipients are sparse. According to a systematic review of four studies (one from China73 and three from the USA74–76) on COVID-19-positive heart transplant recipients (n = 33), the presenting symptoms were similar to those of immunocompetent individuals, including fever (81.8%), cough (94.8%), dyspnoea (75.8%), and gastrointestinal complaints (48.5%).77 The majority of patients (81.8%) were hospitalized, while 24.2% required mechanical ventilation. The treatment included modification of maintenance immunosuppressive therapy (75.8%) and variable approaches with high-dose glucocorticoids, immunoglobulins, fluroquinolone antibiotics, tocilizumab, hydroxychloroquine, and antiviral medications. Of note, the overall mortality rate was 24.2%, while the recovered patients remained rejection free.77 Yet another report of 87 heart transplant recipients from China indicated that high-degree adherence to preventive measures (see above) resulted in a low rate of infection and transition to manifest illness.78
Valvular heart disease
Key points.
Patients with VHD (particularly those with associated left or right ventricular impairment, or pulmonary hypertension) may be at particular risk during the COVID-19 pandemic
Coordinated allocation of resources at hospital and regional level is essential to sustain ICU capacity
Maintained function of the Heart Team is paramount, even if face-to-face meetings are not feasible.
VHD mainly affects the elderly and the symptoms of disease progression (mainly dyspnoea) may mimic those of lung infection or infiltration. In addition, VHD may aggravate the course of COVID-19 and complicate haemodynamic management of the systemic inflammatory response (cytokine storm),79 ARDS, and any superimposed bacterial septicaemia (observed in up to one third of ICU patients).40 In early COVID-19 case series, up to 40% of patients admitted to the ICU had pre-existing congestive HF.32 Excess mortality was reported in patients with VHD who were contaminated with COVID-19. Among 136 elderly patients (mean age 80 years) with severe VHD [54% with aortic stenosis (AS)], 84.6% were treated conservatively and mortality at 30 days was as high as 41.8%.80
Elective surgical and transcatheter interventions for VHD consume significant healthcare resources and many, or all, depending on circumstances, may be inappropriate during the pandemic given the immense pressure on acute and intensive care facilities. During the first pandemic peak in England, a drastic reduction in valve surgery was observed, ranging from 73–76% for surgical aortic valve replacement (SAVR) to 84–85% for surgical mitral valve replacement. Transcatheter aortic valve implantation (TAVI) was less affected with a reduction of 35% and 18% during the months of April and May 2020, respectively.81
Patients with severe VHD must remain under close telephone surveillance and be encouraged to report progressive symptoms. Concentration of resources on the treatment of pandemic victims guides decisions with the overall aim of avoiding shortages of ICU beds and ventilators. Prioritization of valve interventions should therefore balance the immediate and short-term prognosis of individual patients against available resources and the risk to patients and HCP of acquiring in-hospital infection. In this respect, use of less-invasive procedures (particularly TAVI via transfemoral approach performed under conscious sedation and/or local anaesthesia), may present an opportunity to minimize the need for healthcare resources, including ICU and hospital stays. The need for clinical decision-making by Heart Teams remains of paramount importance and the use of telemedicine or other means of virtual communication is essential if face-to-face meetings are difficult, or impossible, during the acute phase of the pandemic.
Management of aortic stenosis
Key points.
Priority should be given to patients with syncope and HF, and those with high (or very high) transvalvular gradients and/or impaired LV function.
Non-urgent procedures should be deferred based on objective criteria assessed by the Heart Team.
Greater use of transfemoral TAVI (as judged appropriate by the Heart Team) may allow optimal utilization of healthcare resources.
The prognosis of patients with severe AS depends on several factors, including age, symptomatic status, peak aortic jet velocity/mean transvalvular gradient,82,83 left ventricular ejection fraction, pulmonary hypertension,84 and elevated biomarkers (natriuretic peptides or troponin).85–87 Mortality of patients with severe symptomatic AS who are treated conservatively is high, reaching 50% at 1 year and 70–80% at 2 years.88 Deferring SAVR or TAVI was associated with an increased risk of hospitalization for valve-related symptoms or worsening HF (19.6% within the first month).89 In another study, 10% of patients awaiting an intervention died or required urgent TAVI.90
In the context of the COVID-19 pandemic, the Heart Team should undertake systematic individual risk assessment based on objective criteria that determine disease progression. Priority should be given to patients with syncope or HF [New York Heart Association (NYHA) Class III/IV], high or very high transvalvular gradients, and those with reduced LV function (See Guidance Part 1), whereas a watchful waiting strategy is more appropriate in those with minimal or no symptoms, provided close follow-up is organized using telemedicine and face-to-face consultation in case of worsening symptoms. TAVI (or balloon aortic valvuloplasty91) may be considered in haemodynamically unstable patients (COVID-19 positive/negative).92 However, the potential benefits of valve intervention in a critically ill COVID-19-positive patient should be carefully weighed against the likelihood of futility given the >60% mortality of COVID-19-positive patients admitted to ICU.93
All cases should be discussed by the Heart Team and indications for TAVI extended to intermediate94,95 and selected low-risk patients.96,97 Increased use of transfemoral TAVI, when feasible, may allow optimal utilization of resources by avoiding general anaesthesia and intubation, shortening or preventing an ICU stay, and accelerating hospital discharge and recovery.98
Management of mitral regurgitation
Key points.
The majority of patients with mitral regurgitation (MR) is stable and surgical or transcatheter intervention can be deferred.
Priority should be given to the treatment of patients with acute MR complicating, e.g. acute myocardial infarction (AMI) or infective endocarditis (IE), and those with severe symptomatic primary MR or secondary MR (SMR) that is not responsive to guideline-directed medical and device treatment and seems likely to require hospital admission. The choice of intervention should be guided by the Heart Team.
The management of MR differs according to its aetiology and presentation. Chronic primary MR (e.g. flail leaflet and Barlow disease) is usually stable and well tolerated. In contrast, SMR is a more variable entity and while many patients remain stable under guideline-directed medical and device treatment (including sacubitril/valsartan and cardiac resynchronization therapy when indicated),99 others may develop unstable HF syndromes that are refractory to medical treatment, particularly in the context of acute infection.100
In the context of the COVID-19 pandemic, priority should be given to the treatment of patients with acute primary MR complicating, e.g. AMI or IE, and those with severe primary or SMR who remain symptomatic despite guideline-directed medical and device treatment and seem likely to require hospital admission. All other patients should be managed conservatively.99–102
Transcatheter mitral edge-to-edge repair may be considered in anatomically suitable high-risk or inoperable patients with acute MR (excluding those with IE) or highly selected patients with highly symptomatic (NYHA III–IV or congestive HF) primary MR or SMR refractory to guideline-directed medical and device treatment. Despite a low risk of complications requiring ICU admission,103 the procedure requires general anaesthesia (in distinction to transfemoral TAVI) and prolonged transoesophageal echocardiographic guidance, thereby exposing echocardiographers and anaesthetists to the risk of COVID-19 transmission. Use of temporary circulatory support (intra-aortic balloon pump or Impella) should be restricted to patients with a good prospect of recovery in the context of available ICU resources.
Hypertension
Key points.
The early reports of an association between hypertension and risk of severe complications or death from COVID-19 were confounded by the lack of adjustment for age and high-risk comorbidities such as obesity and diabetes that commonly co-segregate with hypertension. There is currently no evidence to suggest that hypertension, per se, is an independent risk factor for severe complications or death from COVID-19.
Despite much early speculation of a link between use of ACEIs or ARBs and increased risk from COVID-19, evidence from a series of observational cohort studies from across the world published in major journals has shown that prior or current treatment with ACEIs or ARBs does not increase the risk of COVID-19, or the risk of developing severe complications or death from COVID-19, when compared to the risk in patients taking other antihypertensive drugs.
Two randomized controlled trials have been published (REPLACE COVID) (BRACE-CORONA), both addressing whether ACEIs or ARBS should be continued or withdrawn in patients admitted to hospital with COVID-19. In both studies, there was no difference in major outcomes from COVID-19 whether or not the patients were randomized to continue or discontinue their treatment with ACEIs or ARBs.
Treatment of hypertension should follow existing recommendations in the ESC-European Society of Hypertension (ESH) Guidelines. No change to these treatment recommendations is necessary during the COVID-19 pandemic.
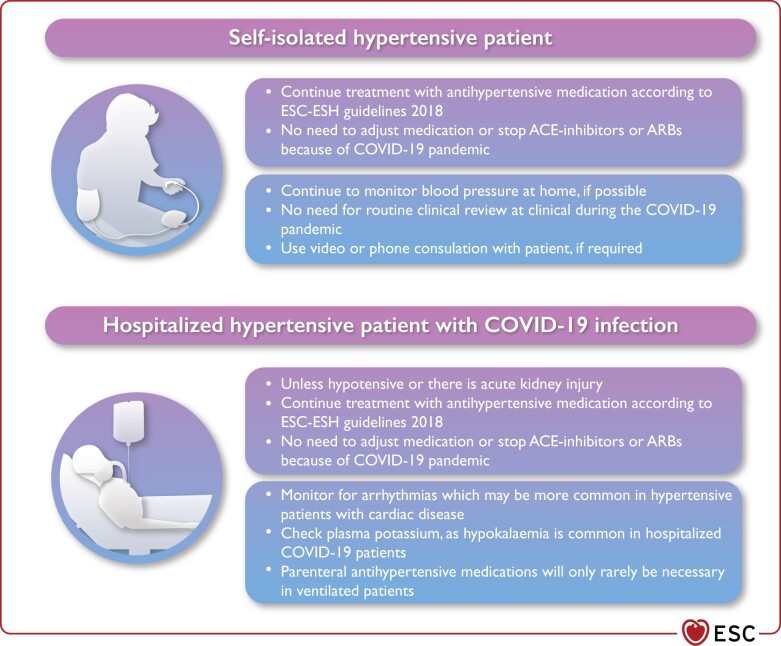
Self-isolated patients with treated hypertension should not need to attend hospital for routine review visits during this pandemic. Patients could make use of periodic home blood pressure (BP) monitoring, with videoconference or phone consultations only if needed (Figure 4).
Hypertensive patients may be at increased risk of cardiac arrhythmias due to underlying cardiac disease, or the reported higher frequency of hypokalaemia in patients with severe COVID-19.
Antihypertensive therapy may need to be temporarily withdrawn in acutely ill patients in hospital who develop hypotension or acute kidney injury secondary to severe COVID-19.
In patients previously treated for hypertension who require invasive ventilation, parenteral antihypertensive medication is only indicated for those developing persistent severe hypertension.
Figure 4.
Hypertension management in the COVID-19 context. ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; COVID-19, coronavirus disease 2019.
Hypertension and COVID-19
Initial reports from China noted that hypertension was one of the most common co-morbidities (20–30% of cases) associated with the need for ventilatory support due to severe respiratory complications of COVID-19.40,41,104–106 These analyses did not adjust for age, which is important because hypertension is very common in older people (∼50% in people over 60 are hypertensive) and hypertension prevalence increases sharply in the very old. Older age is by far the most important risk factor for severe complications and death due to COVID-19; thus, a high frequency of hypertension would be expected in older patients with severe infection. Moreover, obesity and diabetes are significant risk factors for poorer outcomes in patients with COVID-19 and hypertension commonly co-segregates with these comorbidities. New evidence from a very large study involving over 20 million people and 10 000 COVID-19 deaths showed no independent association between hypertension and risk of death from COVID-19.107
It now seems likely that the reported association between hypertension and risk of severe complications or death from COVID-19 is substantially confounded by the lack of adjustment for age and other unmeasured confounders.108 There is currently no evidence to suggest that hypertension, per se, is an independent risk factor for severe complications or death from COVID-19.
Antihypertensive treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers
Renin–angiotensin system (RAS) blockade with ACEIs or ARBs is the foundation of antihypertensive therapy in the current ESC–ESH Guidelines for the management of arterial hypertension (2018).109 The recommended treatment of hypertension for most patients is a combination of an ACEI or ARB with a calcium channel blocker (CCB) or thiazide/thiazide-like diuretic.109
Early in the pandemic, concern had been expressed that treatment with ACEIs or ARBs might increase the risk of infection, or of developing the severe consequences of infection with COVID-19.110–112 This concern originated from a hypothesis linking the observations that COVID-19 invades cells by binding to the enzyme ACE2, which is ubiquitous and expressed on the surface of alveolar cells in the lung.113–115 In some animal studies, but not all, ACEIs or ARBs have been shown to increase ACE2 levels, mainly in cardiac tissue.116–118
There are no studies showing that RAS-blocking drugs increase ACE2 levels in human tissues and no studies in animals or humans showing that RAS-blocking drugs increase ACE2 levels in the lung, or that the level of ACE2 expression in the lung is rate limiting for COVID-19. A recent study of human tissues indicates that neither hypertension nor antihypertensive treatment (including ACEI or ARBs) altered the expression of ACE2 in the human kidney but did show that ACE2 expression was increased in both lungs and kidneys with ageing, which may be relevant to the striking increased risk of Covid-19 with ageing in SARS-CoV-2 infection.119
Series of observational cohort studies have been published in major journals which consistently show that treatment with RAS blockers does not increase the risk of COVID-19 or increase the risk of severe complications or death from COVID-19.120–125 In one study, there was even a substantial reduction in the risk of severe complications or death from COVID-19 in patients with diabetes mellitus.121
Two randomized controlled trials addressing concerns about ACEI and ARBs in patients hospitalized with COVID-19 have now been published. The first study (BRACE CORONA) showed that in 659 patients from 29 sites in Brazil admitted to hospital with COVID-19 and currently treated with ACEIs or ARBs, there was no difference in outcomes (days alive and out of hospital at 30 days), whether or not the patients were randomized to continue or discontinue their treatment with ACEIs or ARBs.126 In the second randomized controlled trial (REPLACE COVID), 152 participants were randomly assigned to either continue or discontinue renin–angiotensin system inhibitor therapy and, irrespective of randomized group, there was no difference in a global rank score of major outcomes.127
This series of large-scale observational studies and the first randomized controlled trials provide a consistent message and reassurance to patients and their doctors that the prior speculation about the safety of RAS blockers in the context of COVID-19 has not been proven.128
Indeed, studies in animal models of infection with influenza or coronaviruses have suggested that ACE2 is important in protecting the lung against severe injury and that RAS-blocking drugs are also protective against severe lung injury due to these viruses.129–131 Human studies of RAS blockade or recombinant ACE2 to prevent respiratory decompensation in COVID-19-infected patients have been suggested, planned, or are ongoing.132,133
In summary, there is currently no evidence to suggest that ACEIs or ARBs increase the risk associated with COVID-19 and there is no reason why these drugs should be discontinued due to concern about COVID-19. Treatment of hypertension, when indicated, should continue to follow the existing ESC–ESH guideline recommendations.134
Remote management of hypertension in the patient isolated at home
Most patients with hypertension require only infrequent visits to the clinic to manage their hypertension. Many patients with treated hypertension will be in self isolation to reduce the risk of COVID-19 and unable to attend their usual routine clinical review. When possible, patients should monitor their own BP as frequently as they usually would, using a validated home BP monitor.109
Videoconference or telephone consultation with patients, when required, may facilitate urgent physician follow-up until normal clinic attendance resumes.
Hypertension and the hospitalized patient with COVID-19
Most patients who are hospitalized will have more severe infection and be hospitalized for respiratory support. They are likely to be older with comorbidities, such as hypertension, diabetes, and chronic kidney disease. Patients with severe disease may also develop multi-organ complications in severe disease.
Hypertensive patients may also have LV hypertrophy or heart disease and be at increased risk of developing arrhythmias, particularly when hypoxic.135 Plasma potassium levels should be monitored because arrhythmias may be exacerbated by the frequent occurrence of low plasma potassium levels, which appears to be more prominent in hospitalized COVID-19-infected patients with more severe disease.136 This is thought to be due to increased urinary loss of potassium, which may be exacerbated by diuretic therapy.
If patients are acutely unwell and become hypotensive or develop acute kidney injury due to their severe disease, antihypertensive therapy may need to be withdrawn. Conversely, parenteral antihypertensive drugs are rarely needed for hypertensive patients who are ventilated and have sustained any significant increases in BP after withdrawal of their usual treatment (i.e. grade 2 hypertension, BP >160/100 mmHg), but the objective in these acute situations is to maintain BP below these levels and not aim for optimal BP control.
Acute pulmonary embolism—prevention and diagnosis
Key points.
Prescribe anticoagulation at standard prophylactic doses in all patients admitted with COVID-19.
Consider the presence of acute pulmonary embolism (PE) in patients with COVID-19 in the setting of unexpected respiratory worsening, new/unexplained tachycardia, a fall in BP not attributable to tachyarrhythmia, hypovolaemia or sepsis, (new-onset) ECG changes suggestive of PE, and signs of deep vein thrombosis of the extremities.
When acute PE is confirmed, treatment should be guided by risk stratification in accordance with the current ESC guidelines.
Observational studies in China, Europe, and the USA have reported a high incidence of thrombotic and thromboembolic complications in patients with COVID-19 pneumonia.137–143 The wide range of described incidence rates is mostly caused by detection bias with variable thresholds for diagnostic testing and, occasionally, limited availability of radiological tests. Most of the studies have demonstrated that acute PE is the most frequent thrombotic complication.137–143 It is debateable whether the contrast-filling defects seen on computed tomography pulmonary angiography represent ‘conventional’ venous thromboembolism (VTE), or if they are induced by in situ immunothrombosis involving, among others, neutrophil extracellular traps.144–146 Likely, VTE and immunothrombosis both contribute to the high incidence of PE in severe COVID-19 pneumonia. Therefore, in view of COVID-19-associated local and systemic inflammation, coagulation activation, hypoxaemia, and immobilization, anticoagulation at standard prophylactic doses should be considered for all patients admitted to the hospital with COVID-19. It has been argued that more intensive anticoagulation [such as low molecular weight heparin (LMWH) at intermediate dose or even full therapeutic-dose anticoagulation] may be indicated in critically ill patients with COVID-19 pneumonia, but such a practice is not supported by current evidence. In fact, it remains unknown whether bleeding rates on more intensive anticoagulation can be acceptably low, or if they outweigh the potential prevention of more thrombotic complications. Of note, patients with COVID-19 pneumonia have been shown to develop acute PE even when they were on full-dose anticoagulation.137–143
Patients with COVID-19 often present with respiratory symptoms and may also report chest pain and haemoptysis.104 These symptoms largely overlap with the presentation of acute PE, and this fact may result in underdiagnosis of this relevant complication.147 Unexpected respiratory worsening, new/unexplained tachycardia, a fall in BP not attributable to tachyarrhythmia, hypovolaemia, or sepsis (new-onset), ECG changes suggestive of PE, and signs of deep vein thrombosis of the extremities should trigger a suspicion of PE. It is recommended to order diagnostic tests for PE only when it is clinically suspected, although the threshold of suspicion should be kept low. The specificity of D-dimer tests may be lower in patients with COVID-19 compared to other clinical settings. Even so, it is still advised to follow diagnostic algorithms starting with pre-test probability and D-dimer testing, especially when pre-test probability-dependent D-dimer thresholds are being used.148–150 This may help to rationalize the deployment of resources and personnel for transporting a patient to the radiology department with all the associated isolation precautions. In the clinical scenario of a patient with COVID who has just undergone computed tomography (CT) of the lungs but the findings cannot explain the severity of respiratory failure, CT pulmonary angiography should be considered before leaving the radiology department.
When acute PE is confirmed, treatment should be guided by risk stratification in accordance with the current ESC guidelines.151 Patients in shock should receive immediate reperfusion therapy. Haemodynamically stable patients should be treated with unfractionated heparin (UFH), LMWH, or a non-vitamin K antagonist oral anticoagulant (NOAC), depending on the feasibility of oral treatment, renal function, and other circumstances. When choosing the appropriate drug and regimen (parenteral vs. oral) for initial, in-hospital anticoagulation, the possibility of rapid cardiorespiratory or renal deterioration due to COVID-19 should be taken into account. Acute renal deterioration or failure precludes continuation of (the same dose of) NOACs and should therefore be closely monitored. Because of the need for anticoagulation monitoring, which may contribute to spreading of the infection, vitamin K antagonists should only be considered in special clinical settings, such as the presence of mechanical prosthetic valves or the antiphospholipid syndrome.151 Of note, several studies have described a high prevalence of antiphospholipid antibodies in patients with COVID-19.152–154 The clinical relevance and implications of this finding are, at present, unknown. Antiphospholipid antibodies are common during infections. Whether the type and titre of the antiphospholipid antibodies described in COVID-19 patients, i.e. IgA isotype alone and low titres, may provoke thrombotic complications remains controversial. Based on current evidence, routine screening for antiphospholipid antibodies in patients with COVID-19 cannot be recommended. However, if triple positivity for antiphospholipid antibodies is demonstrated, i.e. lupus anticoagulant, positive anti-beta-2-glycoprotein antibodies, and positive anti-cardiolipin antibodies, in patients with proven venous or arterial thrombosis, NOACs should be avoided.
Arrhythmias
Key points.
For monitoring and follow-up of patients with cardiac implantable devices, remote monitoring should be utilized as much as possible.
When healthcare resources are scarce, elective ablation and cardiac device implantation procedures should be postponed and urgent procedures should only be performed after careful consideration of all pharmacological treatment options.
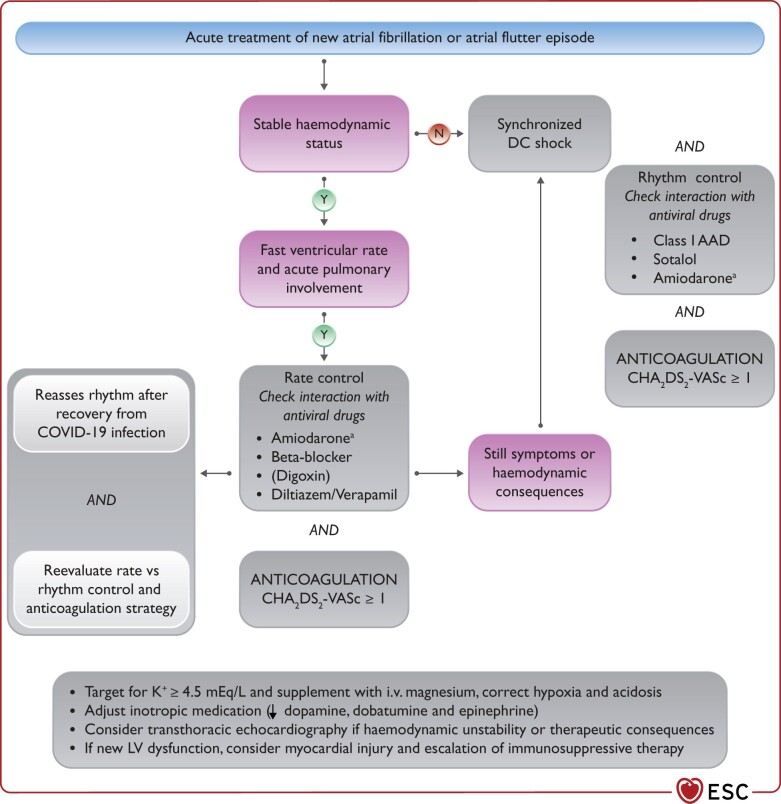
In hospitalized patients with COVID-19, arrhythmias, especially new-onset or recurrent atrial fibrillation (AF) and atrial flutter (AFL), occur frequently. Occurrence of significant arrhythmias is a marker of COVID-19 severity and is associated with higher mortality.
When treating arrhythmias, drug–drug interactions, including antiviral, antiarrhythmic, and anticoagulation therapies, should be considered before co-administration.
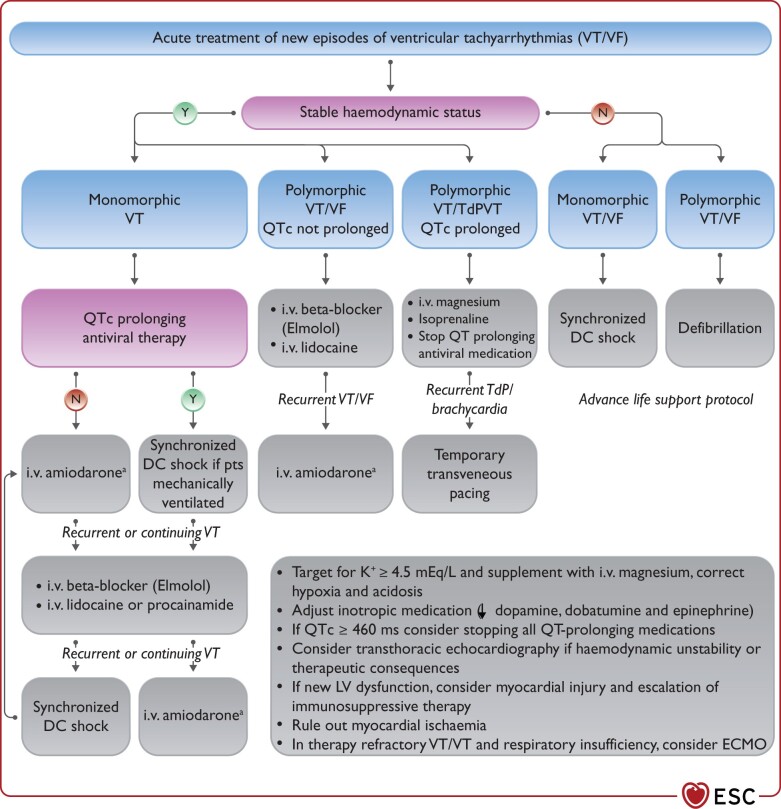
In critically ill patients with hemodynamic instability due to recurrent ventricular tachycardia (VT)/ventricular fibrillation (VF) or AF/AFL, intravenous (i.v.) amiodarone is the choice for antiarrhythmic medication.
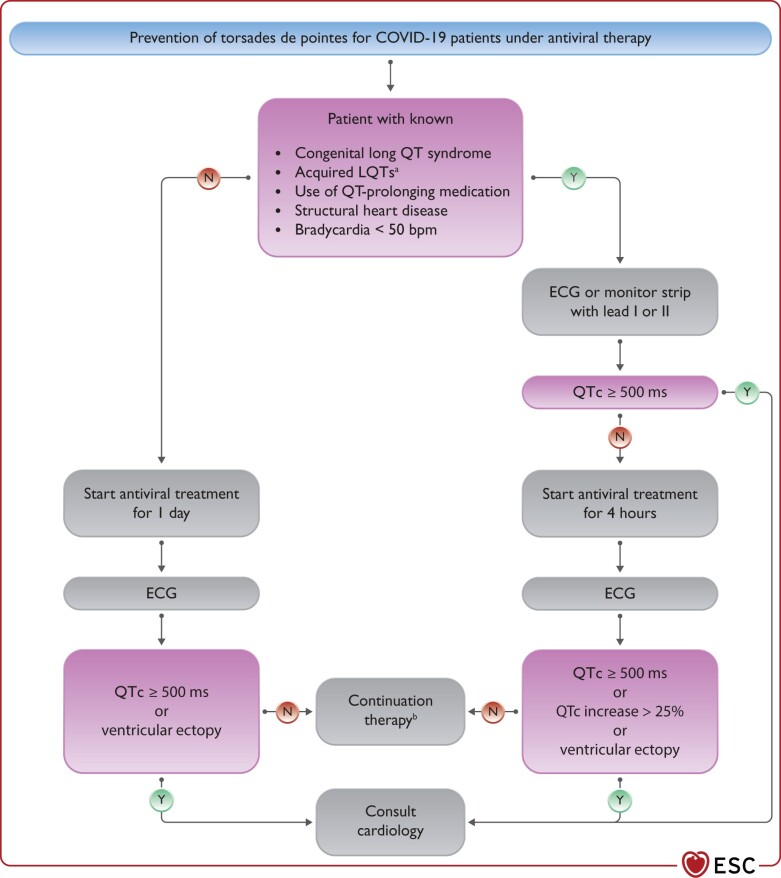
Therapy of TdP VT consists of withdrawal of all QT prolonging drugs, targeting K+ ≥ 4.5 mEq/L, i.v. magnesium supplementation and increasing heart rate (by withdrawing bradycardic agents and if needed by i.v. isoproterenol or temporary pacing); i.v. lidocaine or oral mexiletine may be considered for the treatment of refractory cases based on limited clinical data.
New-onset primary malignant ventricular arrhythmia and sudden arrhythmic death seem to be relatively rare in COVID-19. In critically ill patients, malignant ventricular arrhythmias are a marker of disease severity and occur more frequently, especially in the terminal phase of the disease.
New-onset malignant ventricular tachyarrhythmia or severe bradyarrhythmia not explained by end-stage respiratory failure may be a marker of acute myocardial injury and should trigger diagnostic cardiac evaluation. Ischaemia and hypoxaemia should be excluded, and inflammation and cardiac biomarkers should be followed. Echocardiography should be considered to assess ventricular function and myocardial involvement. In case myocarditis is suspected, magnetic resonance imaging (MRI) may be considered (see Guidance Part 1), as the diagnosis may warrant more aggressive immunosuppressive and antiviral treatment.
After recovery from the COVID-19, in AF/AFL the therapeutic choices of rate and rhythm control should be re-assessed, and long-term anticoagulation should be continued based on the CHA2DS2-VASc score. The need for permanent pacing in bradycardia and for catheter ablation, secondary prophylactic implantable cardiac defibrillator (ICD) or wearable defibrillator in ventricular tachyarrhythmia needs to be re-evaluated.
The general principles of management of patients with cardiac arrhythmias and cardiac implantable devices during the COVID-19 pandemic are based on:
Continuing to provide emergency high-quality care safely to all patients with life-threatening cardiac arrhythmias and implantable devices.
Preserving healthcare resources to allow the appropriate treatment of all patients with COVID-19.
Minimizing the risk of nosocomial infection of non-infected patients and healthcare workers.
Several national and international societies and health services including the Heart Rhythm Society, National Health Service (UK) and the Cardiac Society of Australia and New Zealand have issued similar local recommendations to achieve these goals and guide the management of patients with cardiac arrhythmias and cardiac implantable devices during the COVID-19 pandemic.155–158
Monitoring and follow-up of patients with cardiac implantable devices
Transition to remote interrogation (patient-initiated or automatic prescheduled transmissions) or remote monitoring (i.e. automatic daily or alert-triggered transmissions) of cardiac implantable electronic devices (CIEDs) during the COVID-19 pandemic was proven feasible in a small single-centre Italian study159 and has been reviewed in detail in a recent worldwide document.158
Remote interrogation and monitoring should be utilized as much as possible to replace routine device interrogation visits to hospitals, clinics and practices. In-person office visits should be replaced by remote contact by telephone or internet by the treating physician, using the device information obtained through remote interrogation or monitoring.
For patients who are followed already through remote interrogation/monitoring, deferring in-office evaluation is usually possible. This may have psychological implications, as patients may feel that a delay of their regular check-up may prejudice the integrity of their device. Reassurance on these issues therefore is important when patients are called to postpone their visit.
For patients not already followed via remote interrogation/monitoring, activation requires registering the transmitter, obtaining consent from the patient, and activating the feature in some cases. Initiating remote interrogation/monitoring without the patient coming to the office or hospital may be an option for Boston Scientific and Abbott devices [pacemaker (PM) and ICD] and for newer Medtronic devices using BlueSync, since remote monitoring is programmed ON as default on these CIEDs. Legacy Medtronic devices can be initiated at home by the patient for remote interrogation, but alert-based monitoring of non-BlueSync Medtronic ICDs requires an in-office programming ON. Also, for Biotronik CIEDs, remote monitoring needs an in-office programming ON of the CIED, unless that has been done at the time of implant, as is customary in some countries and centres. When the CIED is ready, for all manufacturers the patient only needs to plug in the transmitter device at home, which then activates automatically (Biotronik; Abbott) after a single push of a button (Boston Scientific or BlueSync Medtronic), or after a series of actions with a removable wand (legacy Medtronic) that can be guided over the phone. Manufacturers point to the restrictions by privacy regulation (like General Data Protection Regulation) to directly send transmitters to the patient’s home and should provide devices to the hospital from where they may be shipped to the patient.
Remote interrogation/monitoring may require hospital re-organization, which can preclude large-scale transitioning from an outpatient setting to a telemetry-based model during hectic COVID-19 times when hospital operations are already stretched.
Device patients for whom a scheduled in-office visit needs to be postponed can also be reassured that major alterations of device integrity will be signalled by an auditory alarm. Patients should be instructed to contact their centre if they notice such an alarm.
Patients without new symptoms or alarms should be rescheduled for device follow-up after the pandemic.
Urgent in-hospital or ambulatory device interrogations may be needed for patients with suspected new and severe lead dysfunction; battery depletion, especially in PM-dependent patients; malignant arrhythmia detection; appropriate or inappropriate ICD therapy delivery if this cannot be sufficiently managed by remote interrogation/monitoring.
-
All patients should be screened for symptoms or exposure to confirmed COVID-19 prior to admission:
-
In patients without suspected or confirmed COVID-19
Preferably, interrogation should use wireless communication to minimize direct contact while maintaining a safe distance and using appropriate personal protective equipment (PPE).
Interrogation should be performed in separate designated non-infected areas (see Supplementary material online, Section 1).
In patients with suspected or confirmed COVID-19: Local hospital protocols for the use of a dedicated single set of programmers with appropriate storage in designated areas, cleaning before and after use, single use wand protection and the use of appropriate PPE (see Supplementary material online, Section 1) are recommended. Preferably, interrogation should use wireless communication, obviating direct contact.
-
Considerations for electrophysiological and implantable device procedures
The categorization of electrophysiology procedures in the context of COVID-19 is depicted in Table 3.
Table 3.
Categorization of electrophysiological procedures in the context of COVID-19
| Urgent (perform within days) | Lower priority (perform within <3 months) | Elective (may be postponed ≥3 months) | Personal protection level | |
|---|---|---|---|---|
| Catheter ablation |
|
|
|
II/III |
| Cardiac implantable electronic device |
|
|
|
II/III |
| Cardioversion/other EP procedures |
|
|
|
II/III |
A, atrial; AF, atrial fibrillation; AV, atrioventricular; CIED, cardiac implantable electronic device; CRT, cardiac resynchronization therapy; EOL, end of life; EP, electrophysiology; ER, emergency room; ERI, elective replacement indicator; ICD, implantable cardioverter–defibrillator; ILR, implantable loop recorder; LAA, left atrial appendage; PM, pacemaker; PSVT, paroxysmal supraventricular tachycardia; PVC, premature ventricular contraction; SVT, supraventricular tachycardia; VF, ventricular fibrillation; VT, ventricular tachycardia; WPW, Wolff–Parkinson–White syndrome.
Management of cardiac arrhythmias in patients with COVID-19
The incidence and type of cardiac arrhythmias in patients with COVID-19 depends on the patient population studied, the intensity of monitoring, the definition of arrhythmias, and the length of follow-up. In an initial single-centre retrospective study including 138 hospitalized patients in Wuhan, China, cardiac arrhythmias occurred in 16.7% of patients. Arrhythmias occurred more frequently in patients who were transferred to the ICU (44% vs. 6.9%, P < 0.001, respectively).41 However, the type and duration of arrhythmias were not specified in this report. In a more recent large study of 1053 hospitalized patients followed for a median of 7 days on telemetry, arrhythmia was reported in 25.6% of patients.160 AF was the most frequent arrhythmia occurring in 15.8% of patients, with 9.6% being newly diagnosed, followed by frequent premature ventricular contractions in 13%, VT or VF in 2.6% (1.9% sustained VT or VF), and atrioventricular (AV) block in 0.4% of the patients. Age, male sex, and hypoxia on presentation were independently associated with occurrence of arrhythmias. The presence of arrhythmias correlated with disease severity, elevated markers of myocardial injury, inflammation, and fibrinolysis and was independently associated with 30-day mortality. Very similar results were recently reported in a large multicentre Italian study with 21.7% incidence of sustained tachyarrhythmias in 414 hospitalized patients.161 Based on these studies, it seems that tachyarrhythmias are a marker of COVID-19 severity occurring more frequently in patients with more severe disease and are associated with higher mortality.
In general, the acute treatment of arrhythmias should not be significantly different from their management in non-COVID-19 patients and should be in line with the current ESC, European Heart Rhythm Association and related guidelines.162–168
Tachyarrhythmias
Supraventricular tachycardia
In an Italian multicentre study of 414 hospitalized patients, the incidence of non-AF/AFL type of supraventricular tachycardia (SVT) was 1.2%.161 In theory, exacerbation of known SVT or new-onset SVT may occur in patients with COVID-19. Special considerations during the COVID-19 pandemic are necessary in a resource-constrained environment considering the transient unavailability of catheter ablation procedures for definitive treatment, the risk of nosocomial infection during repeated ED visits, and the possibility of therapy interactions with antiarrhythmic drugs (AADs) (see Section Treatment of severe acute respiratory syndrome coronavirus 2 infection).
Intravenous adenosine can probably be used safely for acute termination, but confirmatory data are lacking
Maintenance therapy with beta-blockers (or CCBs if beta-blockers are contraindicated) should be initiated with a low threshold. Drug interaction with antiviral drugs should be evaluated, including the avoidance of bradycardia to avoid excessive QT prolongation (see the Treatment of severe acute respiratory syndrome coronavirus 2 infection)
After the COVID-19 pandemic, the indication for catheter ablation should be reassessed.
Atrial fibrillation and flutter
AF/AFL occur in ∼15–20% of patients hospitalized with COVID-19.160,161,169–173 New-onset AF occurs in around 10% of the patients, accounting for up to 60% of COVID-19 patients with AF.169,171,172 The incidence of AF is higher, reaching up to 40% in critically ill COVID-19 patients.169,171–173 Specific precipitating factors in this setting are hypokalaemia and hypomagnesemia (induced by nausea, anorexia, diarrhoea, and medications), metabolic acidosis, the use of inotropic agents (especially dobutamine and dopamine), ventilator dyssynchrony, volume overload, increased sympathetic tone, inflammation, hypoxia, ischaemia, bacterial superinfection, and acute myocardial injury.162 Age, male sex, prior AF, renal disease, and hypoxia on presentation have been independently associated with the occurrence of AF.172 The incidence of AF in COVID-19 is similar to other aetiologies of severe pneumonia, ARDS, and sepsis. Reportedly, 23–33% of critically ill patients with sepsis or ARDS have AF recurrence and 10% develop new-onset AF.162,174–176 New-onset AF in sepsis and ARDS has been associated with higher short- and long-term mortality, very high long-term recurrence rate, and increased risk of HF and stroke.162,174–176 Similarly, in COVID-19, AF has been independently associated in one large US study with significantly higher 30-day mortality (39.2% compared to 13.4% of patients without AF, P < 0.001).172 In this study, 6% of patients with AF/AFL experienced stroke or TIA during their hospitalization, 20% of them while under therapeutic anticoagulation.172 Long-term AF recurrence rate, HF, and mortality risks following recovery from COVID-19 and AF are unknown but are expected to be significant.
As in all patients with AF, treatment goals have to consider ventricular rate control, rhythm control, and thromboembolic prophylaxis. Specifically, in the context of COVID-19, the following considerations should be made (Figure 5):
Figure 5.
Atrial tachyarrhythmias. CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category (female); COVID-19, coronavirus disease 2019; DC, direct current. aThe benefit of intravenous amiodarone treatment should be balanced against the proarrhythmic risk in patients taking QT-prolonging antiviral therapy.
In patients with haemodynamic instability due to new-onset AF and AFL, electrical cardioversion should be considered. This, however, needs to be balanced vs. the need for more equipment and personnel at the side of the patient, and the possible need for intubation (with the risk of increased viral aerosol creation).
In critically ill patients with haemodynamic instability due to new-onset AF/AFL, IV amiodarone is the choice for antiarrhythmic medication for rate and rhythm control. Its combination with hydroxychloroquine and/or azithromycin should be avoided, preferably (see Section Treatment of SARS-CoV-2 infection). Amiodarone may also interfere with cellular SARS-CoV-2 entry and amplification and is being investigated in a study as a candidate antiviral drug in the early stage of the disease.177
In patients with severe acute respiratory insufficiency, cardioversion is unlikely to provide sustained benefit without concomitant intensified treatment of the underlying hypoxaemia, inflammation, and other reversible triggers, such as hypokalaemia and hypomagnesaemia, metabolic acidosis, catecholamine infusion, volume overload, increased sympathetic tone, and bacterial superinfection. In these patients, calcium antagonists may be preferred to beta-blockers for rate control to avoid further worsening of the pulmonary status.
In hospitalized patients with new-onset AFL, rate control may be more challenging than AF. If the patient remains symptomatic or there are haemodynamic consequences, electrical cardioversion may be considered.
Anticoagulation for the prevention of AF-related stroke or systemic embolism should be guided by the CHA2DS2-VASc score. In spite of the thrombophilic environment in COVID-19, there is currently insufficient evidence to recommend a different anticoagulation scheme for patients with or without AF. Therapeutic anticoagulation should be considered in male and female patients with CHA2DS2-VASc score ≥1 and ≥2, respectively, and is indicated in male and female patients with CHA2DS2-VASc score ≥2 and ≥3, respectively.
The need for an echocardiogram should be balanced against the need for close contact between HCP and patient, and contamination of equipment. Only when considered mandatory for immediate therapeutic management, it can be used to assess LV function and pericardial and myocardial involvement. Transthoracic echocardiogram/echocardiography (TTE) is in general preferred to transoesophageal echocardiography (TOE) to avoid aerosol generation. If possible, TTE should be deferred until after convalescence.
Similarly, TOE should be obviated by early start of anticoagulation in new-onset AF and in patients with a low CHA2DS2-VASc score to allow safe electrical cardioversion, also ≥48 h.
Drug–drug interactions including antiviral, antiarrhythmic, and anticoagulation drugs should be considered before administration (see Section Treatment of SARS-CoV-2 infection).
After recovery from the COVID-19, the therapeutic choices of rate and rhythm control should be re-assessed, and long-term anticoagulation should be continued based on the CHA2DS2-VASc score.
Ventricular arrhythmias
An initial single-centre retrospective study from Wuhan analysed the occurrence and significance of malignant ventricular arrythmias in 187 hospitalized patients with COVID-19. Among the 187 patients, 28% of patients had elevated troponin T levels and 23% died. During hospitalization, malignant ventricular arrhythmias (defined as sustained VT or VF) occurred in 5.9% of patients. VT/VF occurred more frequently in patients with elevated troponin levels (17.3% vs. 1.5%, P < 0.001).178 In two more recent larger studies, the reported incidence of sustained ventricular arrhythmias in hospitalized patients was lower at 1.9% and 3.4%, respectively.160,161 An anecdotal case series described critically ill patients with ARDS in the setting of severe COVID-19 dying of refractory ventricular arrhythmias despite normal baseline cardiac function.179 In a recent study of 140 hospitalized patients reaching final disposition of discharge or death in New York, acute malignant cardiac arrhythmia defined as VT/VF or AV block with hemodynamic instability or cardiac arrest occurred in 9% of the study population; 5% had malignant VT/VF; and 3.5% AV block. Patients who died had higher troponin levels and, more frequently, acute malignant arrhythmia with a difference driven by ventricular tachyarrhythmias (17% as compared to 4% of patients who were discharged, P = 0.01). Fatal ventricular tachyarrhythmias invariably occurred in the presence of severe metabolic imbalance and hypoxia. Only 12% of all deaths were classified as CV death, and most (67%) of these deaths occurred in the setting of ST-elevation myocardial infarction.180 In a similar study, also from New York, the last documented rhythm and circumstances of death were analysed in 133 patients who died during the index hospitalizations with COVID-19. Suspected or confirmed arrhythmic death occurred in only 8.3% of the study population and was associated with younger age, ventricular ectopy, mechanical ventilation, vasopressor use, longer QTc and LBBB on admission.181 It should be noted that in all the above-mentioned studies, between 11% and 100% of the patients received hydroxychloroquine and in up to 100% of the patients in combination with azithromycin.160,179–181
In summary, recent studies suggest that sudden cardiac death (SCD) due to primary ventricular arrhythmia is infrequent in hospitalized patients with COVID-19. The incidence of primary malignant ventricular arrhythmias in asymptomatic or mildly symptomatic non-hospitalized patients with COVID-19 is currently unknown but is likely low. In these rare cases, malignant ventricular arrhythmia may occur in the setting of underlying myocardial infarction, pulmonary embolism, stress cardiomyopathy, or acute myocarditis. In contrast, in critically ill patients, malignant ventricular arrhythmias are a marker of disease severity and occur more frequently in the terminal phase of the disease, similar to the high incidence of ventricular arrhythmias in other aetiology ARDS and critical illnesses.182 In patients with a history of CVD and ventricular arrhythmias, exacerbation of the known VT/VF may occur due to COVID-19 as the trigger. Although reports are not yet available for COVID-19, a correlation between influenza epidemic and increased appropriate ICD therapies has been shown.183
Special considerations for the treatment of ventricular arrhythmias during the COVID-19 pandemic are depicted in Figure 6 and summarized below:
Figure 6.
Ventricular tachyarrhythmias. DC, direct current; i.v., intravenous; QT, QT interval; QTc, corrected QT interval; TdP, torsade de pointes; VF, ventricular fibrillation; VT, ventricular tachycardia. aThe benefit of i.v. amiodarone treatment should be balanced against the proarrhythmic risk in patients taking QT-prolonging antiviral therapy.
In unresponsive, unbreathing patients, the local Basic and Advanced Life Support protocol should be followed. During basic life support, ventilation is not performed, only cardiac compressions, to avoid the risk of ingestion of aerosols. For Advanced Life Support, only HCP with full PPE are eligible to perform intubation
In patients with VF, asynchronous defibrillation, and in patients with haemodynamically unstable VT, synchronized electrical cardioversion should be performed;
-
In patients with sustained monomorphic VT:
Electrical cardioversion should be considered, especially if the patient is already ventilated.
Intravenous procainamide (if available and with follow-up of QT interval changes) or lidocaine could be considered in patients taking QT prolonging combination antiviral drugs and if the haemodynamic status permits.
Intravenous amiodarone should be considered in patients with known structural heart disease and impaired LV function. Its action is slow for conversion of VT. Its combination with antiviral drugs should be checked (see Section Treatment of severe acute respiratory syndrome coronavirus 2 infection).
In critically ill patients with COVID-19 and recurrent sustained VT and recurrent VF (‘VT storm’), i.v. amiodarone is the antiarrhythmic medication of choice, though, its combination with antiviral drugs should be checked (see Section Treatment of SARS-CoV-2 infection).
-
Intravenous lidocaine may be considered as a safer but less effective alternative to amiodarone, especially if underlying myocardial ischaemia is suspected:
Addition of sympathetic blockade (e.g. esmolol) should be considered.
Intubation, sedation and ventilation may be considered to abort VT storm.
Temporary PM implantation for overdrive termination may be considered, balancing the possible therapeutic benefit against the invasiveness of the lead placement with risk for personnel. In the absence of a functional cardiac catheterization laboratory, floatation-guided temporary wire insertion may be considered in case of emergency.
In patients with severe acute respiratory insufficiency, correction of underlying reversible triggers should be considered, such as hypoxia, hypovolaemia, electrolyte abnormalities as hypokalaemia and hypomagnesaemia, metabolic acidosis, catecholamine infusions, volume overload, increased sympathetic tone, tamponade, pneumothorax, ischaemia, bacterial superinfection, and proarrhythmic drugs.
Special attention should be paid to the prevention of TdP VT in the setting of COVID-19.
TdP is a polymorphic VT associated with QT prolongation and may be triggered by QT prolonging antiviral drugs, especially in combination with AADs (mainly sotalol), electrolyte disturbances (in particular K+ and Mg2+), renal dysfunction, and/or bradycardia, especially in females and in patients with LV hypertrophy or diminished LV function.
-
Therapy of TdP VT consists of:
TdP withdrawal of all QT prolonging drugs;
Normalizing potassium level (target ≥4.5 mEq/L);
Intravenous magnesium supplementation;
Increasing heart rate by withdrawing bradycardic agents and, if needed, by i.v. isoproterenol or temporary pacing (balancing benefit against the invasiveness of the lead placement with risk for personnel). Isoproterenol is contraindicated in the setting of congenital long QT syndrome (LQTS); and
In therapy refractory cases, i.v. lidocaine184 or oral mexiletine185 may be considered, based on limited clinical data.
New-onset malignant ventricular arrhythmias may be a marker of acute myocardial injury and should trigger diagnostic cardiac evaluation. Polymorphic VT without QT prolongation is not TdP but usually signals ischaemia or acute myocardial injury. Inflammation and cardiac biomarkers should be followed. Echocardiography should be considered in all patients with new malignant ventricular arrhythmia, to assess ventricular function and myocardial involvement. In case myocarditis is suspected, MRI could be considered (see Guidance Part 1), as the diagnosis may warrant more aggressive immunosuppressive and antiviral treatment.
After recovery from COVID-19, the need for secondary prophylactic ICD, catheter ablation, or wearable defibrillator (in case of suspected transient cardiomyopathy due to myocarditis) needs to be evaluated.
Channelopathies
COVID-19 may occur in patients with known congenital LQTS, Brugada syndrome (BrS), catecholaminergic polymorphic ventricular tachycardia (CPVT) and short QT syndrome, with a risk of pro-arrhythmia. The specific interactions of these channelopathies and COVID-19 has recently been reviewed.186
In the initial phase of the pandemic, a combination of antiviral drugs including (hydroxy-) chloroquine and azithromycin was used extensively, with documented prolongation of the QTc and occurrence of related TdP.187,188 Recent randomized drug trials have demonstrated that (hydroxy-)chloroquine is not beneficial, so its use is largely abandoned.189 Similarly, azithromycin has been shown not to confer benefit as compared to usual care.190 However, when used in isolation as an antimicrobial among patients with COVID-19 azithromycin may present a risk of QT prolongation.191 Azithromycin in isolation may still be used and is under ongoing evaluation, though it may still present a risk of further QT prolongation.191
A special consideration in congenital LQTS with COVID-19 is the observation that in COVID-19 patients the QTc is consistently prolonged.192 A combination of factors, including hypoxia, electrolyte disorders, high interleukin (IL)-1, and IL-6 levels, is probably responsible.192 LQTS patients may, therefore, be at increased risk for ventricular arrhythmias. The QTc should be monitored as closely as is safe and practicable. All unnecessary QT prolonging drugs should be stopped, and if QTc is >500 ms or if QTc increases by ≥60 ms from baseline, then the safety of QT prolonging antiviral drugs, if still used, should be reviewed and serum potassium levels should be kept at >4.5 mEq/L (Section Treatment of SARS-CoV-2 infection).
In BrS with COVID-19, the main concern is fever-triggered malignant ventricular arrhythmia. As shown in recently published case reports, COVID-19-induced fever may uncover the type 1 Brugada pattern193 and lead to symptomatic BrS in previously unsuspected cases.194,195 It has also been reported to cause electrical storm in a known BrS patient with an ICD implant.196 Therefore, in all COVID-19 patients with BrS, fever should be aggressively treated with paracetamol. ECG monitoring should be considered if antipyretic therapy is ineffective, and the temperature remains >38.5ºC in higher-risk BrS patients (Figure 7).
Figure 7.
Channelopathies. BrS, Brugada syndrome; COVID-19, coronavirus disease 2019; CPVT, catecholaminergic polymorphic ventricular tachycardia; ECG, electrocardiogram; ICD, implantable cardiac defibrillator. aIdeally ECG recordings with V1 and V2 in the fourth, third, and second intercostal spaces.
In patients with CPVT and COVID-19, beta-blockers and flecainide should be continued with monitoring of drug interactions with antiviral drugs (see Section Treatment of SARS-CoV-2 infection) and in critically ill patients, catecholamine infusions should be administered with great caution, as they require continuous monitoring.
Bradyarrhythmias
In a recent US study of 107 hospitalized patients, first degree AV block was reported in 18.7% of the patients and 0.9% developed transient Mobitz II AV block. PR interval (regardless of medication use or troponin elevation), QRS duration, and QTc interval significantly prolonged in all patients during admission.197 In a study of 135 hospitalized patients in Wuhan, 8.1% were reported to have sinus bradycardia on the ECG, 3.7% first-degree AV block, 0.7% type I second-degree AV block, and 1.5% third-degree AV bock.198 In another study from Wuhan of 319 hospitalized patients, 6% were reported to have sinus bradycardia on the ECG, 3.4% first-degree AV block, and 0.6% second-degree AV block.199 In a large US study of 1053 hospitalized patients followed on telemetry, second-degree or higher AV block was reported in 0.4% of the patients.160 In another recent study of 140 hospitalized patients reaching final disposition of discharge or death in New York, acute malignant AV block defined as AV block with hemodynamic instability or cardiac arrest occurred in 3.5% (five patients) of the study population.180 In two of the five patients, the AV block was associated with AMI, two other patients were critically ill and one patient had non-ST-segment elevation MI and newly depressed LV systolic function.180 Anecdotal reports have described additional cases of in the majority transient type II second degree or third-degree AV block in most cases associated with troponin rise and myocarditis.200–203 In one of these cases, MRI was performed and revealed oedema of the interventricular septum indicative of myocarditis.204 Interestingly, this patient was asymptomatic with COVID-19, had no troponin rise and, as the AV block did not resolve, he underwent permanent PM implantation. Another anecdotal report described two patients with severe COVID-19 and moderate new-onset sinus node dysfunction not resolving during 2 weeks of follow-up but not requiring PM implantation at last follow-up.205
In summary, exacerbation of known conduction system or sinus node disease or severe new-onset AV conduction or sinus node dysfunction may occur in approximately up to 3% of patients with COVID-19. Mild-to-moderate AV conduction or sinus node dysfunction may occur in up to 10–20% of patients with COVID-19 and may be transient. In critically ill patients in the ICU, transient bradycardia and asystole may occur due to patient turning for prone respiration, intubation, or trachea suction and is probably due to transient increase in vagal tone.162 Severe new-onset bradyarrhythmia may be a marker of acute myocardial injury due to ischaemia, hypoxia or myocarditis and, if unexplained by the respiratory status, it should trigger diagnostic cardiac evaluation. Long-term outcomes of new-onset bradyarrhythmia are unknown.
Special considerations for permanent PM implantation in patients with COVID-19 include the poor prognosis of patients requiring mechanical ventilation, increased risk of bacterial superinfection and device infection in the critically ill patients, risk of nosocomial infection during device implantation in COVID-19 negative patients, the possibly transient character of the bradyarrhythmia in myocarditis, and transient bradyarrhythmic side effects of antiviral therapy.
Some treatments used for COVID-19 might increase the likelihood for conduction disturbances (see Section Treatment of SARS-CoV-2 infection). Some of these effects might become apparent only after several weeks.
Recovered COVID-19 patients with mild-to-moderate conduction disturbances or bradyarrhythmic side effects from antiviral medications should be alerted to symptoms of dizziness, presyncope or syncope, and be instructed to contact medical care if these occur.
To avoid bradycardia as the result of drug–drug interactions, monitoring drug levels and dose adjustment may be required (see Section Treatment of SARS-CoV-2 infection).
-
In case of persistent severe symptomatic bradycardia due to AV block or recurrent sinus node dysfunction with pauses:
All medication causing bradycardia should be stopped.
Isoprenaline and atropine should be administered.
Temporary PM implantation should be considered.
New-onset severe symptomatic AV conduction or sinus node dysfunction not explained by respiratory status should trigger diagnostic cardiac evaluation. Ischaemia and hypoxaemia should be excluded. Echocardiography should be considered to assess ventricular function and myocardial involvement. In case myocarditis is suspected, MRI could be considered (see Guidance Part 1) as the diagnosis may warrant more aggressive immunosuppressive and antiviral treatment.
After recovery from the COVID-19, the need for permanent PM implantation should be reassessed.
Treatment of SARS-CoV-2 infection
Key points.
The evidence regarding the efficacy and risk of different treatment strategies in patients with COVID-19 is extensive and continuously evolving; the current and regularly updated version of the World Health Organization (WHO) ‘living guidelines’ is online available.206
Recent randomized clinical trials suggest that, with the exception of glucocorticoids (especially dexamethasone) in hospitalized patients with severe and critical COVID-19, the majority of the initially used antiviral, anti-inflammatory, or immunomodulatory experimental drugs have no or limited effect on the natural history of COVID-19.
In all patients undergoing antiviral treatment, it is of major importance to correct modifiable predisposing factors to QTc prolongation: electrolyte imbalances, concomitant drugs, and bradycardia.
Baseline ECG may not be needed in all before starting treatment, especially if recent prior ECGs are available and there are no clinical signs suggesting CVD (e.g. unexplained syncope).
Resource allocation will need to be adjusted locally depending on availability and demand. According to the context, it is worth exploring alternative ECG monitoring methods (e.g. single lead and telemonitoring, smartphone-enabled mobile ECG, handheld devices).
In COVID-19 patients with an indication for oral anticoagulant therapy, renal and liver function and drug–drug interactions between oral anticoagulant and COVID-19 therapies should be considered to minimize the risk of bleeding or thromboembolic complications.
In NOAC-eligible patients (i.e. those without mechanical prosthetic heart valves, moderate to severe mitral stenosis or antiphospholipid syndrome), NOACs are preferred over vitamin K antagonists (VKAs), owing to their better safety and fixed dosing without the need for laboratory monitoring of anticoagulant effect, notwithstanding the importance of proper NOAC dosing and adherence to treatment.
Whereas apixaban, rivaroxaban, or edoxaban can be given as oral solutions or crushed tablets (via enteral tubes), severely ill COVID-19 patients may be switched to parenteral anticoagulation, which has no clinically relevant drug–drug interactions with COVID-19 therapies (with the exception of azithromycin, which should not be co-administered with UFH).
Acute renal deterioriation or failure precludes continuation of (the same dose of) NOACs and should therefore be closely surveilled.
Medical treatment of COVID-19
Despite the lack of definitive evidence on their efficacy, several drugs with antiviral, anti-inflammatory or immunomodulatory properties have been used ‘off-label’ to treat SARS-CoV-2 infection. Large randomized trials have now identified several therapies, which in combination can approximately halve mortality for patients hospitalized with COVID-19.
Anti-viral therapies
Several agents have been tested as repurposed antiviral agents.
Chloroquine, and its analogue hydroxychloroquine, has been widely used as an antimalarial drug and in the treatment of rheumatological diseases like systemic lupus erythematosus and rheumatoid arthritis. Following the observations of in vitro suppression of SARS-CoV-2 growth,207–209 and in preliminary studies with reduced SARS-CoV-2 positivity in nasopharyngeal secretions,207 these drugs were initially used to treat COVID-19. However, randomized trials have not confirmed that hydroxycholoroquine is beneficial for the treatment of patients hospitalized with COVID-19.189,210–214 Hence, chloroquine and hydroxychloroquine have no indication anymore in the treatment of COVID-19, although trials are ongoing of their role in prophylaxis.215
The protease inhibitor lopinavir–ritonavir was shown to be effective against SARS coronavirus and MERS coronavirus in vitro and in animal models,216–218 but these findings have not been confirmed in randomized controlled trials of hospitalized patients with severe COVID-19.214,219,220
In vitro and animal studies suggest that remdesivir (GS-5734) is effective against zoonotic and epidemic SARS coronavirus and MERS coronavirus.221–224 In vitro studies suggest that remdesivir compared to lopinavir–ritonavir.224 Preliminary studies suggested that remdesivir shortened the recovery time in hospitalized patients with COVID-19.225 However, larger randomized trials have reported little or no effect on key outcomes such as or mortality, initiation of ventilation, and duration of hospital stay among hospitalized patients.214
Antibody-based therapies
Convalescent plasma obtained from people who have recovered from COVID-19 is also being used. Preliminary results from a large expanded-access program in the USA suggested that higher titres of antibody had a larger impact on mortality as well as better outcomes when administered within the first 3 days after diagnosis.226 Importantly, this study did not have an untreated control group, so findings should be considered with caution. The RECOVERY (Randomized Evaluation of COVID-19 Therapy) trial assessed high-titre convalescent plasma among 11 558 patients hospitalized with COVID-19 and found no benefit on major clinical outcomes.227 It remains possible that convalescent plasma may have a role in earlier disease, but this hypothesis needs to be tested.
More recently, synthetic monoclonal antibodies directed against the SARS-CoV-2 spike protein have been assessed in randomized trials. In the USA, Emergency Use Authorization has been given for the use of bamlanivimab with etesevimab, REGEN-COV, and sotrovimab in non-hospitalized patients with mild-to-moderate COVID-19, based on their ability to reduce viral load more quickly and prevent need for hospitalization.228–230 Although small studies of these agents among hospitalized patients were terminated early for futility,231,232 REGEN-COV was assessed among 9785 participants hospitalized with COVID-19 in the RECOVERY trial.233 Among seronegative participants (i.e. those without a detectable humoral response to SARS-CoV-2), allocation to REGEN-COV reduced mortality at 28 days by 20% (24% vs. 30%: rate ratio 0.80, 95% CI 0.70–0.91).
Immunomodulatory therapies
In the RECOVERY trial, dexamethasone reduced mortality in hospitalized COVID-19 patients receiving oxygen, with the largest effect among patients receiving mechanical ventilation.54 This benefit was confirmed in a meta-analysis by the WHO REACT working group of seven randomized clinical trials of critically ill patients with COVID-19. The administration of systemic corticosteroids (dexamethasone, hydrocortisone, or methylpredinisolone), compared with usual care or placebo, was associated with lower 28-day all-cause mortality.56 Benefits were also observed on progression to invasive mechanical ventilation and need for renal replacement therapy.
In vitro, azithromycin has shown to be active against the SARS-CoV-2 virus.234 However, the COALITION I and II and RECOVERY randomized trials did not show any benefit of adding azithromycin therapy in either mild-to-moderate or severe COVID-19.211,235
In COVID-19 patients, IL-6 level is associated with viral load, disease severity, and prognosis.236 Tocilizumab, an IL-6 receptor monoclonal antibody, has been proposed to treat severe COVID-19. The largest trial to date is RECOVERY, which demonstrated that among patients with hypoxia and inflammation (CRP ≥75 mg/L), tocilizumab reduced the risk of death by 15% (rate ratio 0.85, 95% CI 0.76–0.94).237 A WHO-led meta-analysis of all trials of IL-6 antagonists confirms this benefit.238
Colchicine has been proposed as an oral anti-inflammatory medication for the treatment of COVID-19 and several small studies, including the GRECCO-19 trial in 105 hospitalized patients,239 yielded promising findings. In the COLCORONA randomized placebo-controlled trial in community-treated patients including those with suspected but with diagnostic test not confirmed COVID-19, the effect of colchicine on COVID-19-related clinical events was not statistically significant, although an analysis just among patients with PCR-confirmed COVID-19 did suggest that there may be a reduction in the composite of death or hospital admission in such patients.240 However, a much larger randomized trial among patients with more severe illness who had been hospitalized with COVID-19 found no benefit of colchicine.241
Antithrombotic therapies
Antiplatelet agents had been proposed as a potential therapy, in part because of the high rate of venous and arterial thrombosis observed in severe COVID-19. Among 14 892 patients in the RECOVERY trial, aspirin did not improve clinical outcomes.
Trials of heparin-based anticoagulation have shown different results by severity of disease. Among critically ill patients, no benefit of therapeutic anticoagulation compared to usual care was seen in three trials. By contrast, these trials have separately reported that among non-critically ill hospitalized patients therapeutic anticoagulation increased organ support-free days.
In summary, the current version (as of 31 March 2021) of the WHO living guidelines recommends not to use ivermectin in patients with COVID-19 except in the context of a clinical trial; strongly recommends against the use of hydroxychloroquine and lopinavir/ritonavir in patients with COVID-19 of any severity; conditionally recommends against the use of remdesivir in hospitalized patients and systemic corticosteroids in patients with non-severe COVID-19; and strongly recommends for systemic corticosteroids use in patients with severe and critical COVID-19.206
Arrhythmologic consideration of COVID-19 therapies
One major concern with drugs used in COVID-19 is the very rare risk of QTc prolongation and TdP/sudden death or the potential occurrence of conduction disturbances. A recent meta-analysis on arrhythmogenic cardiotoxicity of the quinolines and structurally related antimalarial drugs suggested that this risk is minimal (no events of SCD or documented VF of TdP in 35 448 individuals, 1207 of whom were taking chloroquine).222 During COVID-19, the QT-related risk may be amplified by concomitant use of other QTc-prolonging drugs and/or electrolyte imbalances (hypokalaemia, hypomagnesaemia, and/or hypocalcaemia). Furthermore, important drug–drug interactions have been described [mainly because these potent CYP3A4 inhibitors interfere with (hydroxy)chloroquine metabolism] that should be taken into consideration. In some combinations, dose adjustments or changes may be needed (Table 4).
Table 4.
Pro-arrhythmic considerations of novel experimental pharmacological therapies in COVID-19
| Heart rate | AV conduction | QRS interval | QTc interval | TdP risk | AAD drugs interactions 242 | Comments | |
|---|---|---|---|---|---|---|---|
| Chloroquine | Mild ↓ |
|
|
Very low risk of TdP (2 VT cases with high dosage and 1 case report of TdP in COVID patients)188,250,251 |
|
|
|
| Hydroxychloroquine |
|
No changes in COVID patients256 | See chloroquine |
|
|||
| Azithromycin | Mild ↓266 | Mild ↑266 | Mild ↑266 |
|
In a study during treatment days 1–5, patients receiving azithromycin had significantly increased risk of serious arrhythmia (hazard ratio = 1.77; 95% CI, 1.20–2.62) compared with patients receiving amoxicillin271,272 | ||
| Lopinavir/ritonavir | Moderate ↓273 |
|
Low risk of TdP (1 case of TdP reported in COVID patients)219,273,275 |
|
5 cases of bradycardia and one bundle branch block regressed upon drug discontinuation273 | ||
| Tocilizumab | No ECG changes described276 | No ECG changes described276 | No ECG changes described276 | No ECG changes described276 | Clinical data showed safety277–279 |
Mildc
|
|
| Fingolimod |
|
Mild–moderate ↑ | Unknown | Mild ↑ | Unknown |
Moderateb
|
Reported risk of rare, transient and benign bradycardia and AV conduction abnormalities:281
|
| Remdesivir | No ECG changes described284 | Clinical data showed safety285,286 | Unknown | ||||
| Corticosteroids | No ECG changes described287,288 | Clinical data showed safety286,287 | NR | ||||
| Interferon alfacon-1 | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Limited data: cases of hypotension, arrhythmia, and cardiomyopathy reported |
| Ribavirin | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | No cardiac side effect |
AF, atrial fibrillation; AV, atrio-ventricular; AVB, AV block; AAD, antiarrhythmic drugs; CI, confidence interval; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; HR, heart rate; LAFB, left-anterior fascicle block; LQTS, long QT syndrome; MS, multiple sclerosis; NR, not reported; OR, odd Ratio; QTc, corrected QC interval; RBBB, right-bundle branch block; ROR, reporting odd ratio; RR, risk rate; SCD, sudden cardiac death; SLE, systemic lupus erythematosus; TdP, torsade de pointes; VT, ventricular tachycardia.
These drugs should not be co-administered.
Potential interaction (need dose adjustments/close monitoring).
Weak intensity interaction (need dose adjustments/close monitoring unlikely to be required).
For a detailed overview of all known direct or indirect (through drug–drug interactions) pro-arrhythmic effects of experimental pharmacological therapies in COVID-19 patients, see Table 4.
Corrected QT interval evaluation to prevent drug-induced arrhythmia
QTc prolongation by some drugs can theoretically lead to polymorphic VT (TdP). This is, however, a very rare complication, and its risk has to be balanced against the anticipated benefit of therapy for the COVID-19 patient. Figure 8 provides a practical flow chart for the management of patients to prevent TdP, guidance on the timing and repetition of ECG recordings, and QTc measurements that would alter therapy. Other guidance flowcharts have been published.186,291 Briefly, the following steps are required to reduce the risk of drug-induced TdP:
Figure 8.
QTc management. COVID-19, coronavirus disease 2019; ECG, electrocardiogram; LQTS, long QT syndrome; QTc, corrected QC interval. aAs long as the patient is clinically stable (e.g. no pronounced vomiting, diarrhoea, signs/symptoms of heart failure or deterioration of respiratory, or other organ function).
-
Identify risk factors associated with QTc prolongation:
Non-modifiable risk factors: congenital LQTS, QT prolongation on known QT prolonging drugs, female sex, age >65 years, structural heart disease (ACS, uncompensated HF, hypertrophic cardiomyopathy), renal impairment, and liver impairment.
Modifiable risk factors: hypocalcaemia, hypokalaemia, hypomagnesaemia, concomitant use of QTc-prolonging medications, and bradycardia.
Identify and correct modifiable risk factors in all patients. Serum potassium should be kept in the higher range (≥ 4.5 mEq/L).292
Perform a baseline ECG (12-lead or single strip, depending on resource availability). Patients with a baseline QTc ≥500 ms are at risk of developing TdP or sudden death. The risk-benefit ratio of treatment in this group should be carefully assessed. In some patients with a recent ECG showing normal QTc and no evidence of major CV alterations due to COVID-19, one may consider not taking a baseline ECG to avoid exposure to HCP and contamination of equipment.
Perform ECG once on treatment. If the patient has a QTc ≥500 ms or shows a ΔQTc ≥60 ms, switching to a drug with lower risk of QTc prolongation, reduction of the administered dose, or continuing treatment plan are the options to consider. Close surveillance of QTc interval (preferably including telemetry for arrhythmia monitoring) and electrolyte balance are mandatory.
Bradycardia prolongs QT and facilitates TdP. While some COVID-19 drugs have a weak bradycardic effect, the concomitant use of beta-blockers, CCBs, ivabradine and digoxin should also be evaluated. If digoxin is considered mandatory for the patient, plasma level monitoring should be considered (with ensuing dose reduction if needed).
Technical aspects of QT measurements
For patients with wide QRS complex (≥120 ms) due to bundle branch block or ventricular pacing, QTc adjustment is needed. Formulae are available, but a simpler approach may be to use a QTc cut-off of 550 ms instead of 500 ms. Others propose to calculate adjusted QT interval by subtracting QRS width and adding 100 ms.
A standard 12-lead ECG may not always be feasible to obtain, especially in times of sudden outbreak and scarce healthcare resources. As an alternative, enhanced use of handheld ECG devices should be encouraged to reduce traditional ECG recording to preserve resources and limit virus spread. In a recent study, the QTc in lead‐I and lead‐II derived from a standard 12-lead ECG was compared with the QTc measured from a rhythm strip from a handheld ECG device in 99 healthy volunteers and 20 hospitalized patients in sinus rhythm treated with dofetilide or sotalol.293 QT on the handheld device had an excellent agreement with standard 12‐lead ECG both in the normal range and in patients with QT prolongation.293 This handheld ECG device (KardiaMobile 6L Alivecor) had a high specificity for detecting a QTc >450 ms and should thus be considered as an effective outpatient tool for monitoring patients with prolonged QTc. Recently, KardiaMobile 6L received expedited approval from the FDA for QT monitoring and can thus be used in COVID-19 patients treated with QT prolonging drugs.
Considerations on the use of anticoagulants in COVID-19 patients
Recent studies confirm that COVID-19 is associated with increased risk of venous, arterial, and microvascular thrombotic and thromboembolic disease, including disseminated intravascular coagulation (see Guidance Part 1 and Section Acute pulmonary embolism—prevention and diagnosis).294–297 In general, the risk of thrombotic complications and bleeding should be assessed in all patients with COVID-19, and current guidelines for the prevention and treatment of thrombotic and thromboembolic diseases should be followed.151,298 Specific in COVID-19, in two recent studies, the use of reduced and therapeutic-dose anticoagulation has been associated with improved outcomes and mortality in hospitalized patients.295,296 The indications and details of venous and pulmonary embolism prophylaxis and treatment of thrombotic complications of COVID-19 are discussed in Section Acute pulmonary embolism—prevention and diagnosis and have been reviewed in several recent consensus documents.294,297
Many patients with CV history have an indication for anticoagulation and are already under anticoagulation therapy when affected by COVID-19. Table 5 lists the possible interactions of COVID-19 therapies with VKAs, NOACs, LMWHs, and UFH. The table includes information that was derived from several drug interaction sites, which have been referenced. Drug summary of product characteristics often do not contain information for older drugs and/or drugs with a narrow spectrum of indications (like chloroquine). Antimalarial drugs have a P-glycoprotein inhibiting effect, which may affect NOAC plasma levels. COVID-19 patients on oral anticoagulation may be switched over to parenteral anticoagulation with LMWH and UFH when admitted to an ICU with a severe clinical presentation.
Table 5.
Interactions of anticoagulant drugs with experimental COVID-19 therapies
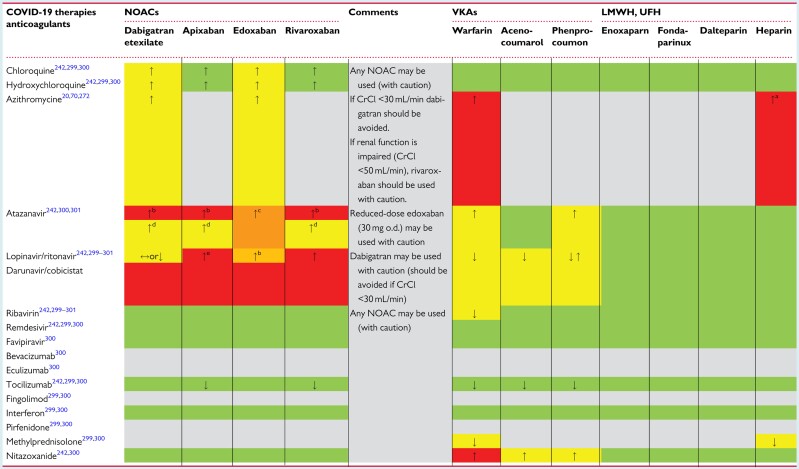
|
Light grey colour: no information found. Green colour: no clinically significant interaction is expected, or potential interaction is likely to be of weak intensity, not requiring additional action/monitoring or dose adjustment. Yellow colour: potential interaction which may require additional monitoring (e.g. more frequent INR monitoring if on VKAs). Orange colour: potential interaction which may require a dose adjustment. Red colour: the drugs should not be co-administered. ↑, potential increased exposure to the anticoagulant drug; ↓, potential decreased exposure to the anticoagulant drug; ↔, no significant effect on the exposure to the drug.
COVID-19, coronavirus disease 2019; CrCl, creatinine clearance; LMWH, low molecular weight heparin; NOACs, non-vitamin K antagonist oral anticoagulants; o.d., once daily; UFH, unfractionated heparin; VKAs, vitamin K antagonists.
Azithromycin increases the effect of heparin by decreasing its metabolism.300
There is an overall agreement that the use of NOACs is not recommended when atazanavir is given in combination with its enhancers, ritonavir or cobicistat.
The EMA product label for edoxaban advises the consideration of dose reduction from 60 mg once daily to 30 mg once daily with concomitant use of strong P-glycoprotein inhibitors.
No data on the safety/efficacy of use of NOACs when co-administered with atazanavir are known; if their use is deemed indicated, one should consider monitoring plasma level of the NOACs in this unknown condition, in line with the recommendation that was made in the last EHRA Practical Guide.298
The US product label for apixaban proposes the use of apixaban at reduced dose (2.5 mg twice daily) if needed.
We would like to reiterate here also that the conventional dose reduction criteria for NOACs for AF patients on oral treatment for stroke prevention can be continued. For more details, including the assessment of renal and liver function and other considerations in patients taking an NOAC, please see the 2021 EHRA Practical Guide on the use of NOACs in patients with AF.302 Of note, none of the NOACs is recommended in patients with a creatinine clearance (CrCl) <15 mL/min according to the EU label.
Apixaban: the standard dose (2 x 5 mg) should be reduced to 2 x 2.5 mg if two out of three criteria are met [body weight ≤60 kg, age ≥80 years, serum creatinine ≥133 µmol/L (1.5 mg/dL)], or if the CrCl is 15–29 mL/min.
Dabigatran: the standard doses 2 x 150 and 2 x 110 mg. No pre-specified dose reduction criteria but, per the drug label, 2 x 110 mg should be used if age >80 years, concomitant verapamil, increased risk of gastrointestinal bleeding.
Edoxaban: the standard dose (1 x 60 mg) should be reduced to 1 x 30 mg if weight <60 kg, CrCl <50 mL/min, concomitant therapy with a strong P-gp inhibitor.
Rivaroxaban: the standard dose (1 x 20 mg) should be reduced to 1 x 15 mg if CrCl <50 mL/min.
For patients with impaired swallowing, NOACs can be administered in the following ways:
Administration in a crushed form (e.g. via a nasogastric tube) does not alter the bioavailability of apixaban, edoxaban and rivaroxaban.303–305
Apixaban can be given as oral solution or via nasogastric or gastric tube on an empty stomach (food impairs bioavailability of the crushed tablets).306
Rivaroxaban tablet can either be crushed and mixed in water or apple puree and taken orally, or suspended in water and given via nasogastric tube (enteral tubes must not be distal to the stomach) followed by food.304
Dabigatran capsules must not be opened, as it would result in a 75% increase in the drug bioavailability.306
Patient information
While there remain unknown features of COVID-19,307 it is clear that transparent patient-centred information is an essential component to support patients to reduce risk of transmission, maintain a healthy lifestyle and manage their CVD (Figure 9). What is the full spectrum of disease severity? What is the transmissibility? What is the role of asymptomatic/pre-symptomatic infected persons? How long is the virus present? What are the risk factors for severe illness? Knowledge is being accumulated very fast and our task is to deliver key information for patients with CVD.
Figure 9.
Advice for patients from the European Society of Cardiology patient forum.
Key points.
Patient-centred information is of paramount importance during the COVID-19 pandemic when the allocation of medical resources is a matter of debate.308
Pre-existing CVD has a direct impact on the risk of SARS-CoV-2, severity of COVID-19 disease, and survival.112
The occurrence of SARS-CoV-2 may lead to CV complications as well as treatments used to cure the COVID-19 disease.
Unambiguous information to the population and patients is key for better control of the disease and the rapid development of specific treatment strategies, including vaccines.
Who is at risk for severe SARS-CoV-2?
There are several clinical features associated with a worse short-term outcome of SARS-CoV-2 manifestations (see Guidance Part 1). These include: age >65-year old with a least one comorbidity, or age >70-year old, with the risk being highest in age >80-year old; COPD, asthma, chronic HF, certain cardiac arrythmias, recent unstable coronary artery disease or coronary revascularization (<3 months), BMI >35 kg/m2 or BMI >30 kg/m2 plus one or more comorbidities, sickle cell anaemia, transplant <6 months, hypertrophic cardiomyopathy with obstruction, chronic kidney disease (eGFR <15 mL/min), and dysregulated diabetes.309 The effect of social background and ethnicity on survival remains controversial, but it appears that long-standing disparities in nutrition and obesity play a crucial role in the health inequities unfolding during the pandemic (see Guidance Part 1).310–313 A cause-and-effect relationship between drug therapy and survival should not be inferred given the lack of ongoing randomized trials. Patients should be informed and take appropriate precautions with emphasis on measures for social distancing when the potential risk is high and medical resources are scarce.
My treatment during the COVID-19 pandemic
COVID-19 disease may trigger destabilization of chronic CVD. This may also be favoured by chronic oral treatment interruption, and patients should be informed to seek medical guidance prior to any treatment modifications.
Aspirin dosage given for the secondary prevention of atherothrombosis has no anti-inflammatory potential and should not be interrupted in COVID-19 patients without any other relevant reasons, such as ongoing bleeding complication or the need for an unplanned invasive procedure.
Many patients at potential risk for SARS-CoV-2 are treated with inhibitors of the RAS, including ACEIs. ACE2 facilitates coronavirus entry into cells, but it is not inhibited by ACEIs or Ang II type 1 receptor blockers or upregulated by these treatments. For these reasons, patients should not discontinue their treatments without medical guidance.134,314 Two randomized controlled trials have shown that there was no difference in major outcomes from COVID-19 whether or not the patients were randomized to continue or discontinue their treatment with ACEIs or ARBs (see Section Hypertension).126,127
There are some treatments that may need to be adjusted when concomitant specific therapy for the COVID-19 disease is initiated. These treatments are initiated during hospital admission and potential drug–drug interactions are summarized in Tables 6 and 7.
Table 6.
Concomitant conditions that may be associated with a more severe course of SARS-CoV-2 infectiona
| Chronic pulmonary disease |
| History of heart failure |
| Waiting list for cardiac surgery |
| Immunodeficiency or prior organ transplantation |
| Hypertension |
| Coronary artery disease |
| Cerebrovascular disease |
| Diabetes |
| Severe overweight (BMI >40 kg/m2) |
| Arrhythmias |
BMI, body mass index.
Many of these features are confounded by age.
Table 7.
Potential interactions of drugs to treat COVID-19a
| Drugs used to treat COVID-19 | Interactions | Action |
|---|---|---|
| Dexamethasone | Warfarin | Monitor INR |
| Methylprednisolone | Warfarin | Monitor INR |
| Antiretroviral drugs | Antiarrhythmics | Use QT prolonging or low-dose digoxin with caution |
| NOACs | Avoid apixaban and rivaroxaban | |
| Statins | Start with low-dose rosuvastatin or atorvastatin | |
| Warfarin | Monitor INR | |
| Colchicine | Statins | Consider reducing dose of statin therapy |
| CYP3A4 inhibitor | Consider reducing dose of colchicine | |
| Chloroquine or hydroxychloroquine | Beta-blockers and QT prolonging drugs | Monitor ECG |
COVID-19, coronavirus disease 2019; ECG, electrocardiogram; INR, international normalized ratio; NOACs, non-vitamin K antagonist oral anticoagulants.
These medications will be administered during hospital admission.
Interactions with others, healthy lifestyle, and medical advice during COVID-19 pandemic
The following information is important for individuals with CVD (Figures 9 and 10):
Figure 10.
Telehealth during COVID-19 for people with cardiovascular disease.
-
Interaction with others:
Avoid people who are sick.
Keep a two-metre distance from other individuals whenever possible.
Wash hands thoroughly with soap and warm water for at least 20 s.
Cover the mouth or nose with a tissue or use the inside of the elbow when you cough or sneeze.
Avoid touching the eyes, nose and mouth when you are with other people.
To remove the virus, clean surfaces like doorknobs or handles often with a disinfectant.
Self-isolate in case of symptoms of fever, cough or a chest infection and look for medical assistance.
Limit/avoid situations with high risk of becoming infected.
Stay at home as much as possible.
Maintain physical activity to avoid VTE and maintain well-being.
In addition, individuals should be encouraged to follow the instruction of the Department of Health and local authorities in the resident countries, as these may differ.
-
Healthy lifestyle:
Maintain a healthy lifestyle (e.g. eat healthy, quit smoking, restrict alcohol intake, get adequate sleep and keep physically active).315
Isolation and physical restrictions may lead to inactivity, increased risk of VTE, and loss of functional autonomy, especially among elderly with co-morbidities.
Physical activity should be strongly encouraged, either in a home setting or outdoor areas with social space, and will also improve well-being.
Attending cardiac rehabilitation (in person or virtual) should be encouraged for those with an indication.
Maintaining a social network (virtually if required) should be encouraged.
Stay mentally active. Undertake enjoyable activities, which require concentration (e.g. read books, listen to music, paint) and take breaks from watching news on COVID-19.
Physical activity trackers significantly increase physical activity and may be a useful adjunct to promote a healthy lifestyle remotely.316
-
Medical advice:
Continue with prescribed medications for CVD.
Seek medical help immediately if experiencing symptoms such as chest pain. Do not neglect symptoms.
Do not interrupt cardiac follow-up. Seek advice of a cardiologist promptly in case of deterioration of the CV condition.
SARS-CoV-2 vaccines
Patients with prior CVD should be informed that:
Vaccines are very effective therapies to prevent severe SARS-CoV-2 infection and have been tested in large-scale randomized trials.
There are very few contraindications to vaccines and CVD are not a contraindication per se.
A time delay is needed prior to vaccine therapy after a recent SARS-CoV-2 infection.
The ones deemed at the highest risk should be treated first, according to local policies.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The following people reviewed the document: Victor Aboyans (France), Stefan D. Anker (Germany), Robert A. Byrne (Ireland), A. John Camm (Italy), Andrew J.S. Coats (Italy), Rudolf A. de Boer (The Netherlands), Stefanie Dimmeler (Germany), Donna Fitzimons (UK), Christoph Gräni (Switzerland), Christian Hamm (Germany), Richard Haynes (UK), Bernard Iung (France), Adnan Kastrati (Germany), Patrizio Lancellotti (Belgium), Julinda Mehilli (Germany), Béla Merkely (Hungary), Lis Neubeck (UK), Katja E. Odening (Switzerland), Raffaele Piccolo (Italy), Lorenz Räber (Switzerland), Tobias Reichlin (Switzerland), Manel Sabate (Spain), P. Christian Schulze (Germany), Iain A. Simpson (UK), Lars Sondergaard (Denmark), Miguel Sousa-Uva (Portugal), Stefan Stortecky (Switzerland), Didier Tchétché (France), and Katja Zeppenfeld (The Netherlands). Support for title page creation and format was provided by AuthorArranger, a tool developed at the National Cancer Institute. Matthieu Depuydt (European Society of Cardiology) coordinated the development of the article.
Conflict of interest: C.B. reports grants or contracts as follow: Medical Research Council: Population Health Research Unit (Director) 2019–24, Medical Research Council: PHRU capital award 2019–20, Medical Research Council: Therapy Acceleration Laboratory Award 2021; BHF: Project Grant no. PG/18/16/33570 Cholesterol Treatment Trialists' (CTT) Collaboration: Meta-analyses of individual participant adverse event data from randomized controlled trials of statin therapy (co-applicant) 2018–20; Boehringer-Ingelheim: EMPA-KIDNEY trial (study co-chair) 2018–22; NIHR HTA 17/140/02: Cost-effectiveness of statin therapies evaluated using individual participant data from large randomized clinical trials (co-applicant) 2018–22. C.B. reports unpaid roles as chair of the European Society of Cardiology Clinical Practice Guidelines Committee and as vice-chair of the British Heart Foundation Clinical Studies Committee. S.W. reports research and educational grants via his institution from Abbott, Amgen, Astra Zeneca, BMS, Bayer, Biotronik, Boston Scientific, Cardinal Health, CardioValve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Johnson&Johnson, Medicure, Novartis, Polares, OrPha Suisse, Pfizer, Regeneron, Sanofi Aventis, Sinomed, Terumo, and V-Wave. S.W. is an unpaid member of the Pfizer Research Award selection committee in Switzerland and of the Women as One Awards Committee. He is a member of the Clinical Study Group of the Deutsches Zentrum für Herz Kreislauf-Forschung and of the Advisory Board of the Australian Victorian Heart Institute. He is a chairperson of the ESC Congress Program Committee and a former chairperson of the ESC Clinical Practice Guidelines Committee. S.W. serves as unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave, and Xeltis but has not received personal payments by pharmaceutical companies or device manufacturers. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. E.A. reports honoraria for lectures Biosense Webster. D.C. reports personal consulting fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, and Daiichi Sankyo. J.-P.C. reports grants or contracts from Medtronic, BMS-Pfizer for the ATLANTIS trial; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from BMS-Pfizer, Webmed, AstraZeneca, and Sanofi. T.C. reports consulting fees from Boston Scientific, Medtronic, Edwards; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Boston Scientific, Medtronic, Edwards; and participation on a Data Safety Monitoring Board or Advisory Board for CERC. J.R.G.-J. receives payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Ferrer International, Menarini, MSD, Novartis, Novo Nordisk, Pfizer, Sanofi Aventis, and Servier outside the submitted work. T.J.G. is the editor-in-chief from the Cardiovascular Research journal. T.J.G. reports speaker fees from Merck and participation to the steering committee of the COMPASS trial from the Public Health Research Institute. H.H. reports unconditional Research Grants for University of Antwerp and/or University of Hasselt from Bracco Imaging Europe, Daiichi-Sankyo, Boehringer-Ingelheim, Abbott, Medtronic, Biotronik, St. Jude Medical, and Fibricheck/Qompium. As EHRA president 2018–2020, H.H. reports no personal honorarium for any industry-related speaker or advisory role between March 2017 and September 2020. After September 2020, H.H. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events. B.I. reports other financial or non-financial interests as leader for a small single-centre phase 2 RCT testing the benefits of i.v. metoprolol in critically ill COVID19 patients. Funds from his laboratory were used, and there was no relationship with any third party. Metoprolol is not approved for ARDS (COVID or non-COVID related) and thus this trial was an initial step for its repurposing. Metoprolol is out of patent and has no commercial interest. F.A.K. reports research grants via his institution from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, Actelion, the Dutch thrombosis association, The Netherlands Organisation for Health Research and Development, and the Dutch Heart foundation, all outside the current work. F.A.K. is a member of the Dutch guideline committee on antithrombotic therapy, and the ASH guideline on thromboprophylaxis in COVID-19 patients. F.A.K. is a member of the nucleus of the ESC Working Group on RV function and pulmonary circulation. S.V.K. reports grants or contracts via his institution from Bayer AG, Boston Scientific, Servier, Actelion-Janssen, Daiichi-Sankyo; consulting fees from Bayer AG, Pfizer–Bristol-Myers Squibb, Daiichi-Sankyo, and Boston Scientific; payment or honoraria for lectures, presentations from Bayer AG, Pfizer–Bristol-Myers Squibb, Daiichi-Sankyo Boston Scientific, and MSD Novartis. U.L. reports grants or contracts from Bayer and Novartis, Amgen; consulting fees from Bayer, Novartis, Amgen, Sanofi, and Pfizer; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Bayer, Novartis, Amgen, Sanofi, and Pfizer. C.L. reports grants or contracts from Biotronik, Medtronic, Boston Scientific, Microport, and Abbott; support for attending meetings and/or travel from Biotronik, Medtronic, Boston Scientific, Microport, and Abbott. M.L. reports honoraria for the role of faculty member of two webinars on COVID-19 from a private society committed to educational initiatives and the Italian Association of Hospital Cardiologists. N.M. reports a research grant from Getinge Global US; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bristol Myers Squibb. C.M. reports research support payments via his institution from Research support from several diagnostic companies, the Swiss National Science Foundation, the Swiss Heart Foundation; consulting fees from Idorsia; payment via his institution for his participation on a Data Safety Monitoring Board or Advisory Board from Roche and from Osler. A.S.P. reports consulting and proctoring payment via her institution from Medtronic; consulting and proctoring payment from Boston Scientific; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Medtronic, Abbott, and Boston Scientific; payment for expert testimony via her institution from Medtronic, Boston Scientific, and Abbott; and participation to the steering committee for the ongoing SMART trial. F.P. reports travel expenses from Abbott Vascular, Edwards Lifesciences, and Polares Medical. P.P. reports direct consulting fees from Boehringer Ingelheim, Pfizer, and Bayer AG and direct honoraria for lectures from Bayer AG, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Sanofi, Roche, and Boston Scientific. M.R. reports research grant via his institution from Medtronic, Boston Scientific, Terumo, Biotronik, and GE Healthcare. S.R. reports research grant via his institution from Actelion, AstraZeneca, Bayer, Janssen, and Novartis and remunerations for lectures from Abbott, Acceleron, Actelion, Arena, Bayer, Ferrer, Janssen, MSD, Novartis, Pfizer, United Therapeutics, and Vifor. G.S. reports research grant via his institution from Boston Scientific and speaker fees from Abbott Vascular and Boston Scientific. H.T. is the president-elect of the German Cardiac Society and is a chair of the 2020 ESC NSTE ACS Guideline. B.W. reports lecture fees for symposia on hypertension, unrelated to the content of those manuscripts from Pfizer, Boehringer, Servier, and Daichi Sankyo; travel/accommodation fees to conference from Pfizer, Boehringer, Servier, and Daichi Sankyo. B.W. is the chair of the most recent 2018 ESC–ESH Hypertension Guideline. The other authors declare that there is no conflict of interest.
Data availability
No new data were generated or analysed in support of this research.
Appendix
The Task Force for the management of COVID-19 of the European Society of Cardiology (ESC)
Writing Committee: Colin Baigent* (MRC Population Health Research Unit, Nuffield Department of Population Health, Oxford, UK); Stephan Windecker* (Department of Cardiology, Inselspital, Bern University Hospital, Bern, Switzerland); Daniele Andreini (Centro Cardiologico Monzino, IRCCS, Milan, Italy and Department of Clinical Sciences and Community Health, Cardiovascular Section, University of Milan, Milan, Italy); Elena Arbelo (Arrhythmia Section, Cardiology Department, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain and Institut d’Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain and Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain and ECGen, the Cardiogenetics Focus Group of EHRA); Emanuele Barbato (Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy and Cardiovascular Center Aalst, OLV Hospital, Aalst, Belgium); Antonio L. Bartorelli (Centro Cardiologico Monzino, IRCCS, Milan, Italy and Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy and Department of Biomedical and Clinical Sciences “Luigi Sacco”, University of Milan, Milan, Italy); Andreas Baumbach (Centre for Cardiovascular Medicine and Devices, William Harvey Research Institute, Queen Mary University of London and Barts Heart Centre, London, UK and Yale University School of Medicine, New Haven, CT, USA); Elijah R. Behr (ECGen, the Cardiogenetics Focus Group of EHRA, Cardiology Clinical Academic Group, Institute of Molecular and Clinical Sciences, St George’s, University of London, London, UK and St George’s University Hospitals NHS Foundation Trust, London, UK and European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN GUARDHEART; http://guardheart.ern-net.eu); Sergio Berti (U.O.C. Cardiologia Diagnostica e Interventistica, Dipartimento Cardiotoracico, Fondazione Toscana G. Monasterio – Ospedale del Cuore G. Pasquinucci, Massa, Italy); Héctor Bueno (Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain and Cardiology Department, Hospital Universitario 12 de Octubre and Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain and Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain); Davide Capodanno (Division of Cardiology, A.O.U. Policlinico “G. Rodolico-San Marco” University of Catania, Catania, Italy); Riccardo Cappato (Arrhythmia & Electrophysiology Center, IRCCS Gruppo MultiMedica, Sesto San Giovanni, Milan, Italy); Alaide Chieffo (San Raffaele Scientific Institute, Milan, Italy); Jean-Philippe Collet (Sorbonne Université, ACTION study group, Institut de Cardiologie, Pitié Salpêtrière Hospital (AP-HP), Paris, France); Thomas Cuisset (Département de Cardiologie, CHU Timone, Marseille, France and INSERM, UMR1062, Nutrition, Obesity and Risk of Thrombosis, Marseille, France and Faculté de Médecine, Aix-Marseille Université, Marseille, France); Giovanni de Simone (Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy and Hypertension Research Center, Federico II University Hospital, Naples, Italy); Victoria Delgado (Heart Lung Centrum, Leiden University Medical Center, Leiden, The Netherlands); Paul Dendale (Heart Centre Hasselt, Jessa Hospital, Hasselt, Belgium and Faculty of Medicine and Life Sciences, Uhasselt, Diepenbeek, Belgium); Dariusz Dudek (Institute of Cardiology, Jagiellonian University Medical College, Kraków, Poland and Maria Cecilia Hospital, GVM Care&Research, Cotignola (RA), Ravenna, Italy); Thor Edvardsen (Department of Cardiology Oslo University Hospital, Rikshospitalet, Oslo, Norway); Arif Elvan (Isala Heart Center, Zwolle, The Netherlands); José R. González-Juanatey (Cardiology Department, University Hospital, IDIS, CIBERCV, University of Santiago de Compostela, Santiago de Compostela, Spain); Mauro Gori (Cardiovascular Department & Cardiology Unit, Papa Giovanni XXIII Hospital-Bergamo, Bergamo, Italy); Diederick Grobbee (Julius Global Health, the Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands); Tomasz J. Guzik (Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK and Department of Medicine, Jagiellonian University College of Medicine, Kraków, Poland); Sigrun Halvorsen (Department of Cardiology, Oslo University Hospital Ulleval, Oslo, Norway and University of Oslo, Oslo, Norway); Michael Haude (Medical Clinic I, Städtische Kliniken Neuss, Lukaskrankenhaus GmbH, Neuss, Germany); Hein Heidbuchel (Department of Cardiology, University Hospital Antwerp and University of Antwerp, Antwerp, Belgium); Gerhard Hindricks (Department of Internal Medicine/Cardiology/Electrophysiology, Heart Center Leipzig, University Hospital Leipzig, Leipzig, Germany and Leipzig Heart Institute (LHI), Leipzig, Germany); Borja Ibanez (Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain and Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain and IIS-Fundación Jiménez Díaz Hospital, Madrid, Spain); Nicole Karam (Université de Paris, PARCC, INSERM, Paris, France and European Hospital Georges Pompidou, Paris, France); Hugo Katus (Department of Internal Medicine, University Hospital of Heidelberg, Heidelberg, Germany); Fredrikus A. Klok (Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, The Netherlands); Stavros V. Konstantinides (Center for Thrombosis and Hemostasis, Johannes Gutenberg University Mainz, Mainz, Germany and Department of Cardiology, Democritus University of Thrace, Alexandroupolis, Greece); Ulf Landmesser (Department of Cardiology, Charite University Medicine Berlin, Berlin, Germany and Berlin Institute of Health (BIH), German Center of Cardiovascular Research (DZHK), Partner Site Berlin, Berlin, Germany); Christophe Leclercq (University of Rennes, CHU Rennes, INSERM, LTSI – UMR 1099, Rennes, France); Sergio Leonardi (University of Pavia, Pavia, Italy and Fondazione IRCCS Policlinico S.Matteo, Pavia, Italy); Maddalena Lettino (Cardio-Thoracic and Vascular Department, San Gerardo Hospital, ASST-Monza, Monza, Italy); Giancarlo Marenzi (Centro Cardiologico Monzino, IRCCS, Milan, Italy); Josepa Mauri (Institut del Cor, Hospital Universitari Germans Trias i Pujol, Badalona, Spain and Health Department of the Government of Catalonia, Barcelona, Spain); Marco Metra (Institute of Cardiology, ASST Spedali Civili di Brescia; Department of Medical and Surgical Specialities, Radiological Sciences and Public Health, University of Brescia, Brescia, Italy); Nuccia Morici (Unità di Cure Intensive Cardiologiche e De Gasperis Cardio Center, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy and Dipartimento di Scienze Cliniche e di Comunità, Università degli Studi, Milan, Italy); Christian Mueller (Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, Basel, Switzerland and University of Basel, Basel, Switzerland); Anna Sonia Petronio (Cardiothoracic and Vascular Department, University of Pisa, Ospedale cisanello, Pisa, Italy); Marija M. Polovina (Faculty of Medicine, Belgrade University, Belgrade, Serbia and Department of Cardiology, Clinical Centre of Serbia, Belgrade, Serbia); Tatjana Potpara (School of Medicine, University of Belgrade, Belgrade, Serbia and Department for Intensive Arrhythmia Care, Cardiology Clinic, Clinical Centre of Serbia, Belgrade, Serbia); Fabien Praz (Department of Cardiology, University Hospital Bern, Bern, Switzerland); Bernard Prendergast (St Thomas' Hospital and Cleveland Clinic London, London, UK); Eva Prescott (Department of Cardiology, Bispebjerg University Hospital, Copenhagen, Denmark); Susanna Price (Royal Brompton Hospital, London, UK and National Heart & Lung Institute, Imperial College, London, UK); Piotr Pruszczyk (Department of Internal Medicine & Cardiology, Medical University of Warsaw, Warsaw, Poland); Oriol Rodríguez-Leor (Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain and Institut del Cor, Hospital Universitari Germans Trias i Pujol, Badalona, Spain); Marco Roffi (Department of Cardiology, Geneva University Hospitals, Geneva, Switzerland); Rafael Romaguera (Servicio de Cardiología, Hospital Universitario de Bellvitge-IDIBELL, L'Hospitalet de Llobregat, Barcelona, Spain); Stephan Rosenkranz (Clinic III for Internal Medicine (Cardiology) and Cologne Cardiovascular Research Center (CCRC), Heart Center at the University of Cologne, Cologne, Germany and Center for Molecular Medicine Cologne (CMMC), University of Cologne, Cologne, Germany); Andrea Sarkozy (Department of Cardiology, University Hospital Antwerp and University of Antwerp, Antwerp, Belgium); Martijn Scherrenberg (Heart Centre Hasselt, Jessa Hospital, Hasselt, Belgium and Faculty of Medicine and Life Sciences, Uhasselt, Diepenbeek, Belgium); Petar Seferovic (Faculty of Medicine, Belgrade University, Belgrade, Serbia and Serbian Academy of Sciences and Arts, Belgrade, Serbia); Michele Senni (Cardiovascular Department & Cardiology Unit, Papa Giovanni XXIII Hospital-Bergamo, Bergamo, Italy); Francesco R. Spera (Department of Cardiology, University Hospital Antwerp and University of Antwerp, Antwerp, Belgium); Giulio Stefanini (Department of Biomedical Sciences, Humanitas University, Pieve Emanuele – Milan, Italy and Humanitas Research Hospital IRCCS, Rozzano – Milan, Italy); Holger Thiele (Leipzig Heart Institute (LHI), Leipzig, Germany and Department of Internal Medicine/Cardiology, Heart Center Leipzig at University of Leipzig, Leipzig, Germany); Daniela Tomasoni (Institute of Cardiology, ASST Spedali Civili di Brescia; Department of Medical and Surgical Specialities, Radiological Sciences and Public Health, University of Brescia, Brescia, Italy); Lucia Torracca (Department of Biomedical Sciences, Humanitas University, Pieve Emanuele – Milan, Italy and Humanitas Research Hospital IRCCS, Rozzano – Milan, Italy); Rhian M. Touyz (Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK); Arthur A. Wilde (ECGen, the Cardiogenetics Focus Group of EHRA and European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN GUARDHEART; http://guardheart.ern-net.eu) and Amsterdam UMC, University of Amsterdam, Heart Center; department of Clinical Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam, The Netherlands); Bryan Williams (Institute of Cardiovascular Sciences, University College London, London, UK).
*Joint corresponding authors. Email: colin.baigent@ndph.ox.ac.uk (C.B.); stephan.windecker@insel.ch (S.W.)
Contributor Information
The Task Force for the management of COVID-19 of the European Society of Cardiology:
Colin Baigent, Stephan Windecker, Daniele Andreini, Elena Arbelo, Emanuele Barbato, Antonio L Bartorelli, Andreas Baumbach, Elijah R Behr, Sergio Berti, Héctor Bueno, Davide Capodanno, Riccardo Cappato, Alaide Chieffo, Jean-Philippe Collet, Thomas Cuisset, Giovanni de Simone, Victoria Delgado, Paul Dendale, Dariusz Dudek, Thor Edvardsen, Arif Elvan, José R González-Juanatey, Mauro Gori, Diederick Grobbee, Tomasz J Guzik, Sigrun Halvorsen, Michael Haude, Hein Heidbuchel, Gerhard Hindricks, Borja Ibanez, Nicole Karam, Hugo Katus, Fredrikus A Klok, Stavros V Konstantinides, Ulf Landmesser, Christophe Leclercq, Sergio Leonardi, Maddalena Lettino, Giancarlo Marenzi, Josepa Mauri, Marco Metra, Nuccia Morici, Christian Mueller, Anna Sonia Petronio, Marija M Polovina, Tatjana Potpara, Fabien Praz, Bernard Prendergast, Eva Prescott, Susanna Price, Piotr Pruszczyk, Oriol Rodríguez-Leor, Marco Roffi, Rafael Romaguera, Stephan Rosenkranz, Andrea Sarkozy, Martijn Scherrenberg, Petar Seferovic, Michele Senni, Francesco R Spera, Giulio Stefanini, Holger Thiele, Daniela Tomasoni, Lucia Torracca, Rhian M Touyz, Arthur A Wilde, and Bryan Williams
Corresponding authors. Tel: +44 1865 743743, Fax: +44 1865 743985, Email: colin.baigent@ndph.ox.ac.uk (C.B.); Tel: +41 31 632 21 11, Fax: +41 31 632 47 70, Email: stephan.windecker@insel.ch (S.W.)
References
- 1. Cave DM, Gazmuri RJ, Otto CW et al. Part 7: CPR techniques and devices: 2010 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010;122:S720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazer SP, Weisfeldt M, Bai D et al. Reverse CPR: a pilot study of CPR in the prone position. Resuscitation 2003;57:279–285. [DOI] [PubMed] [Google Scholar]
- 3. Chioncel O, Parissis J, Mebazaa A et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:1315–1341. [DOI] [PubMed] [Google Scholar]
- 4. Collet JP, Thiele H, Barbato E et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 5. Ibanez B, James S, Agewall S et al. ; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 6. Mebazaa A, Combes A, van Diepen S et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med 2018;44:760–773. [DOI] [PubMed] [Google Scholar]
- 7. Neumann FJ, Sousa-Uva M, Ahlsson A et al. ; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
- 8. Perkins GD, Olasveengen TM, Maconochie I et al. ; European Resuscitation Coucil. European Resuscitation Council Guidelines for Resuscitation: 2017 update. Resuscitation 2018;123:43–50. [DOI] [PubMed] [Google Scholar]
- 9. Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J 2019;40:2671–2683. [DOI] [PubMed] [Google Scholar]
- 10. Christian MD, Hawryluck L, Wax RS et al. Development of a triage protocol for critical care during an influenza pandemic. CMAJ 2006;175:1377–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng SQ, Peng HJ. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med 2020;9: 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choudry FA, Hamshere SM, Rathod KS et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2020;76:1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Rosa S, Spaccarotella C, Basso C et al. ; Società Italiana di Cardiologia and the CCU Academy investigators group. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;41:2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez-Leor O, Cid Alvarez AB, de Prado AP et al. In-hospital outcomes of patients with ST-segment elevation myocardial infarction and COVID-19. EuroIntervention 2021;16:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stefanini GG, Azzolini E, Condorelli G. Critical organizational issues for cardiologists in the COVID-19 outbreak: a frontline experience from Milan, Italy. Circulation 2020;141:1597–1599. [DOI] [PubMed] [Google Scholar]
- 16. Roffi M, Patrono C, Collet JP et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 17. Imazio M, Klingel K, Kindermann I et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart 2020;106:1127–1131. [DOI] [PubMed] [Google Scholar]
- 18. Stefanini GG, Chiarito M, Ferrante G et al. ; Humanitas COVID-19 Task Force. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart 2020;106:1512–1518. [DOI] [PubMed] [Google Scholar]
- 19. Kucharski AJ, Russell TW, Diamond C et al. ; Centre for Mathematical Modelling of Infectious Diseases C-wg. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis 2020;20:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pontone G, Baggiano A, Conte E et al. "Quadruple rule-out" with computed tomography in a COVID-19 patient with equivocal acute coronary syndrome presentation. JACC Cardiovasc Imaging 2020;13:1854–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basille D, Plouvier N, Trouve C, Duhaut P, Andrejak C, Jounieaux V. Non-steroidal anti-inflammatory drugs may worsen the course of community-acquired pneumonia: a cohort study. Lung 2017;195:201–208. [DOI] [PubMed] [Google Scholar]
- 22. Douglas I, Evans S, Smeeth L. Effect of statin treatment on short term mortality after pneumonia episode: cohort study. BMJ 2011;342:d1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleming DM, Verlander NQ, Elliot AJ et al. An assessment of the effect of statin use on the incidence of acute respiratory infections in England during winters 1998-1999 to 2005-2006. Epidemiol Infect 2010;138:1281–1288. [DOI] [PubMed] [Google Scholar]
- 24. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knuuti J, Wijns W, Saraste A et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 26. Maron DJ, Hochman JS, Reynolds HR et al. ; ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Basso C, Leone O, Rizzo S et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020;41:3827–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozieranski K, Tyminska A, Jonik S et al. Clinically suspected myocarditis in the course of severe acute respiratory syndrome novel coronavirus-2 infection: fact or fiction? J Card Fail 2021;27:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y, Coats AJS, Zheng Z et al. Management of heart failure patients with COVID-19: a joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:941–956. [DOI] [PubMed] [Google Scholar]
- 30. Rey JR, Caro-Codon J, Rosillo SO, Iniesta AM et al. ; Card-Covid Investigators. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail 2020;22:2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arentz M, Yim E, Klaff L et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA 2020;323:1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vakili K, Fathi M, Pezeshgi A et al. Critical complications of COVID-19: a descriptive meta-analysis study. Rev Cardiovasc Med 2020;21:433–442. [DOI] [PubMed] [Google Scholar]
- 35. Tomasoni D, Inciardi RM, Lombardi CM et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the Cardio-COVID-Italy multicentre study. Eur J Heart Fail 2020;22:2238–2247. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Li H, Zhu S et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging 2020;13:2287–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Argulian E, Sud K, Vogel B et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging 2020;13:2459–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim J, Volodarskiy A, Sultana R et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol 2020;76:1965–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li B, Yang J, Zhao F et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020;109:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis 2020;63:390–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi S, Qin M, Shen B et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lopez-Otero D, Lopez-Pais J, Antunez-Muinos PJ, Cacho-Antonio C, Gonzalez-Ferrero T, Gonzalez-Juanatey JR. Association between myocardial injury and prognosis of COVID-19 hospitalized patients, with or without heart disease. CARDIOVID registry. Rev Esp Cardiol (Engl Ed) 2021;74:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tomasoni D, Italia L, Adamo M et al. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail 2020;22:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andersson C, Gerds T, Fosbol E et al. Incidence of new-onset and worsening heart failure before and after the COVID-19 epidemic lockdown in Denmark: a nationwide cohort study. Circ Heart Fail 2020;13:e007274. [DOI] [PubMed] [Google Scholar]
- 47. Cannata A, Bromage DI, Rind IA et al. Temporal trends in decompensated heart failure and outcomes during COVID-19: a multisite report from heart failure referral centres in London. Eur J Heart Fail 2020;22:2219–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Frankfurter C, Buchan TA, Kobulnik J et al. Reduced rate of hospital presentations for heart failure during the COVID-19 pandemic in Toronto, Canada. Can J Cardiol 2020;36:1680–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bromage DI, Cannata A, Rind IA et al. The impact of COVID-19 on heart failure hospitalization and management: report from a Heart Failure Unit in London during the peak of the pandemic. Eur J Heart Fail 2020;22:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Konig S, Hohenstein S, Meier-Hellmann A, Kuhlen R, Hindricks G, Bollmann A; Helios Hospitals Germany. In-hospital care in acute heart failure during the COVID-19 pandemic: insights from the German-wide Helios hospital network. Eur J Heart Fail 2020;22:2190–2201. [DOI] [PubMed] [Google Scholar]
- 51. Oikonomou E, Aznaouridis K, Barbetseas J et al. Hospital attendance and admission trends for cardiac diseases during the COVID-19 outbreak and lockdown in Greece. Public Health 2020;187:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu J, Mamas MA, Mohamed MO et al. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart 2021;107:113–119. [DOI] [PubMed] [Google Scholar]
- 53. Inciardi RM, Lupi L, Zaccone G et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Group RC, Horby P, Lim WS et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tomazini BM, Maia IS, Cavalcanti AB et al. ; COALITION COVID-19 Brazil III Investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020;324:1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sterne JAC, Murthy S, Diaz JV et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. ClinicalTrials.gov. Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019. Bethesda (MD): National Library of Medicine (US). Identifier NCT04310228. https://clinicaltrials.gov/ct2/show/NCT04310228 (27 November 2020).
- 58. Wenzel P, Kopp S, Gobel S et al. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res 2020;116:1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Albert CL, Carmona-Rubio AE, Weiss AJ, Procop GG, Starling RC, Rodriguez ER. The enemy within: sudden-onset reversible cardiogenic shock with biopsy-proven cardiac myocyte infection by severe acute respiratory syndrome coronavirus 2. Circulation 2020;142:1865–1870. [DOI] [PubMed] [Google Scholar]
- 60. Sinan U, Erturk M, Yıldırım E et al. The predictors of long-term hospitalization in Turkish heart failure population: a subgroup analysis of journey heart failure-TR study: on behalf of journey heart failure-TR investigators. Int J Cardiovasc Acad 2018;4:82–85. [Google Scholar]
- 61. Yang W, Cao Q, Qin L et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 2020;80:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Čelutkienė J, Lainscak M, Anderson L et al. Imaging in patients with suspected acute heart failure: timeline approach position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:181–195. [DOI] [PubMed] [Google Scholar]
- 63. Furuhashi M, Moniwa N, Mita T et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens 2015;28:15–21. [DOI] [PubMed] [Google Scholar]
- 64. Lafaurie M, Martin-Blondel G, Delobel P, Charpentier S, Sommet A, Moulis G. Outcome of patients hospitalized for COVID-19 and exposure to angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers in France: results of the ACE-CoV study. Fundam Clin Pharmacol 2021;35:194–203. [DOI] [PubMed] [Google Scholar]
- 65. Seferovic PM, Ponikowski P, Anker SD et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:1169–1186. [DOI] [PubMed] [Google Scholar]
- 66. Giorgi Rossi P, Marino M, Formisano D et al. ; the Reggio Emilia COVID-19 Working Group. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS One 2020;15:e0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim DW, Byeon KH, Kim J, Cho KD, Lee N. The correlation of comorbidities on the mortality in patients with COVID-19: an observational study based on the Korean National Health Insurance Big Data. J Korean Med Sci 2020;35:e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alvarez-Garcia J, Lee S, Gupta A et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol 2020;76:2334–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kerr B, Pharithi RB, Barrett M et al. Changing to remote management of a community heart failure population during COVID-19—clinician and patient perspectives'. Int J Cardiol Heart Vasc 2020;31: 100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Salzano A, D'Assante R, Stagnaro FM et al. Heart failure management during the COVID-19 outbreak in Italy: a telemedicine experience from a heart failure university tertiary referral centre. Eur J Heart Fail 2020;22:1048–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant 2015;15:1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe acute respiratory syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant 2003;3:977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant 2020;39:496–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Holzhauser L, Lourenco L, Sarswat N, Kim G, Chung B, Nguyen AB. Early experience of COVID-19 in 2 heart transplant recipients: case reports and review of treatment options. Am J Transplant 2020;20:2916–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Latif F, Farr MA, Clerkin KJ et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease. JAMA Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Russell MR, Halnon NJ, Alejos JC, Salem MM, Reardon LC. COVID-19 in a pediatric heart transplant recipient: emergence of donor-specific antibodies. J Heart Lung Transplant 2020;39:732–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aziz H, Lashkari N, Yoon YC et al. Effects of coronavirus disease 2019 on solid organ transplantation. Transplant Proc 2020;52:2642–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ren ZL, Hu R, Wang ZW et al. Epidemiologic and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in Wuhan, China: a descriptive survey report. J Heart Lung Transplant 2020;39:412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dvir D. Severe valvular heart disease and COVID-19: results from the multicenter international valve disease registry. https://www.tctmd.com/news/valve-disease-plus-covid-19-often-lethal-combination-registry-shows (16 December 2020). [DOI] [PMC free article] [PubMed]
- 81. Mohamed MO, Banerjee A, Clarke S et al. Impact of COVID-19 on cardiac procedure activity in England and associated 30-day mortality. Eur Heart J Qual Care Clin Outcomes 2021;7:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rosenhek R, Binder T, Porenta G et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611–617. [DOI] [PubMed] [Google Scholar]
- 83. Rosenhek R, Zilberszac R, Schemper M et al. Natural history of very severe aortic stenosis. Circulation 2010;121:151–156. [DOI] [PubMed] [Google Scholar]
- 84. Zlotnick DM, Ouellette ML, Malenka DJ, DeSimone JP et al. ; Northern New England Cardiovascular Disease Study Group. Effect of preoperative pulmonary hypertension on outcomes in patients with severe aortic stenosis following surgical aortic valve replacement. Am J Cardiol 2013;112:1635–1640. [DOI] [PubMed] [Google Scholar]
- 85. Bergler-Klein J, Klaar U, Heger M et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004;109:2302–2308. [DOI] [PubMed] [Google Scholar]
- 86. Chin CW, Shah AS, McAllister DA et al. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J 2014;35:2312–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Clavel MA, Malouf J, Michelena HI et al. type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol 2014;63:2016–2025. [DOI] [PubMed] [Google Scholar]
- 88. Otto CM, Prendergast B. Aortic-valve stenosis—from patients at risk to severe valve obstruction. N Engl J Med 2014;371:744–756. [DOI] [PubMed] [Google Scholar]
- 89. Ryffel C, Lanz J, Corpataux N et al. Mortality, stroke, and hospitalization associated with deferred vs expedited aortic valve replacement in patients referred for symptomatic severe aortic stenosis during the COVID-19 pandemic. JAMA Netw Open 2020;3:e2020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ro R, Khera S, Tang GHL et al. Characteristics and outcomes of patients deferred for transcatheter aortic valve replacement because of COVID-19. JAMA Netw Open 2020;3:e2019801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Attisano T, Silverio A, Bellino M et al. Balloon aortic valvuloplasty for urgent treatment of severe aortic stenosis during coronavirus disease. Pandemic: a case report. ESC Heart Fail 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bauernschmitt R, Gabriel P, Gottardi R, Sodian R. Valve-in-valve transcatheter aortic valve replacement in a young patient with a suspected COVID-19 infection: a surgical dilemma in the era of the COVID-19 pandemic. Eur J Cardiothorac Surg 2020;58:188–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yang J, Zheng Y, Gou X et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Leon MB, Smith CR, Mack MJ et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 95. Makkar RR, Thourani VH, Mack MJ, Kodali SK et al. ; PARTNER 2 Investigators. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med 2020;382:799–809. [DOI] [PubMed] [Google Scholar]
- 96. Mack MJ, Leon MB, Thourani VH et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 97. Popma JJ, Deeb GM, Yakubov SJ et al. ; Evolut Low Risk Trial I. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 98. Arora S, Strassle PD, Kolte D et al. Length of stay and discharge disposition after transcatheter versus surgical aortic valve replacement in the United States. Circ Cardiovasc Interv 2018;11:e006929. [DOI] [PubMed] [Google Scholar]
- 99. Ponikowski P, Voors AA, Anker SD, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 100. Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231–1248. [DOI] [PubMed] [Google Scholar]
- 101. Kang DH, Park SJ, Shin SH et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation 2019;139:1354–1365. [DOI] [PubMed] [Google Scholar]
- 102. Zilberszac R, Heinze G, Binder T, Laufer G, Gabriel H, Rosenhek R. Long-term outcome of active surveillance in severe but asymptomatic primary mitral regurgitation. JACC Cardiovasc Imaging 2018;11:1213–1221. [DOI] [PubMed] [Google Scholar]
- 103. Sorajja P, Vemulapalli S, Feldman T et al. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT registry report. J Am Coll Cardiol 2017;70:2315–2327. [DOI] [PubMed] [Google Scholar]
- 104. Guan WJ, Ni ZY, Hu Y et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wu C, Chen X, Cai Y et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhang JJ, Dong X, Cao YY, Yuan YD et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–1741. [DOI] [PubMed] [Google Scholar]
- 107. Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Williams B, Zhang Y. Hypertension, renin-angiotensin-aldosterone system inhibition, and COVID-19. Lancet 2020;395:1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Williams B, Mancia G, Spiering W et al. ; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 110. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sommerstein R, Grani C. Rapid response: re: preventing a covid-19 pandemic: ACE inhibitors as a potential risk factor for fatal Covid-19. BMJ 2020;368:m810.32111649 [Google Scholar]
- 112. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun 2020;525:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280, e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Burrell LM, Risvanis J, Kubota E et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 2005;26:369–375, discussion 322–364. [DOI] [PubMed] [Google Scholar]
- 117. Ferrario CM, Jessup J, Chappell MC et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 118. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004;43:970–976. [DOI] [PubMed] [Google Scholar]
- 119. Jiang X, Eales JM, Scannali D et al. Hypertension and renin-angiotensin system blockers are not associated with expression of angiotensin-converting enzyme 2 (ACE2) in the kidney. Eur Heart J 2020;41:4580–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bean DM, Kraljevic Z, Searle T et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail 2020;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. de Abajo FJ, Rodríguez-Martín S, Lerma V et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 2020;395:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol 2020;5:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Reynolds HR, Adhikari S, Pulgarin C et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang P, Zhu L, Cai J et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lopes RD, Macedo AVS, de Barros E et al. ; BRACE CORONA Investigators. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA 2021;325:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cohen JB, Hanff TC, William P et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med 2021;9:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Williams B. Renin-angiotensin system inhibitors in hospitalised patients with COVID-19. Lancet Respir Med 2021;9:221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Imai Y, Kuba K, Rao S et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kuba K, Imai Y, Rao S et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rodrigues Prestes TR, Rocha NP, Miranda AS, Teixeira AL, Simoes ESAC. The anti-inflammatory potential of ACE2/angiotensin-(1-7)/Mas receptor axis: evidence from basic and clinical research. Curr Drug Targets 2017;18:1301–1313. [DOI] [PubMed] [Google Scholar]
- 132. ClinicalTrials.gov. Recombinant human angiotensin-converting enzyme 2 (rhACE2) as a treatment for patients with COVID-19. Bethesda, MD: National Library of Medicine (US). Identifier NCT04287686. https://clinicaltrials.gov/ct2/show/NCT04287686 (2 April 2020).
- 133. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res 2020;81:537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. de Simone G; ESC Council on Hypertension, On behalf of the Nucleus Members. Position statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang (26 March 2021).
- 135. Lip GYH, Coca A, Kahan T et al. ; Reviewers. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace 2017;19:891–911. [DOI] [PubMed] [Google Scholar]
- 136. Chen D, Li X, Song Q et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open 2020;3:e2011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Klok FA, Kruip M, van der Meer NJM et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Helms J, Tacquard C, Severac F et al. ; CRICS TRIGGERSEP Group. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Llitjos JF, Leclerc M, Chochois C et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 2020;18:1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lodigiani C, Iapichino G, Carenzo L et al. ; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Klok FA, Kruip M, van der Meer NJM et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020;191:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fauvel C, Weizman O, Trimaille A et al. ; for the Critical Covid-19 France Investigators. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020;41:3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost 2020;4:1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. van Dam LF, Kroft LJM, van der Wal LI et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb Res 2020;193:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Middleton EA, He XY, Denorme F et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020;136:1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Nicolai L, Leunig A, Brambs S et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 2020;142:1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Huisman MV, Barco S, Cannegieter SC et al. Pulmonary embolism. Nat Rev Dis Primers 2018;4:18028. [DOI] [PubMed] [Google Scholar]
- 148. Kearon C, de Wit K, Parpia S et al. Diagnosis of pulmonary embolism with d-dimer adjusted to clinical probability. N Engl J Med 2019;381:2125–2134. [DOI] [PubMed] [Google Scholar]
- 149. van der Hulle T, Cheung WY, Kooij S et al. group Ys. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet 2017;390:289–297. [DOI] [PubMed] [Google Scholar]
- 150. van der Pol LM, Tromeur C, Bistervels IM et al. ; Artemis Study Investigators. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med 2019;380:1139–1149. [DOI] [PubMed] [Google Scholar]
- 151. Konstantinides SV, Meyer G, Becattini C et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 152. Hasan Ali O, Bomze D, Risch L et al. Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Reyes Gil M, Barouqa M, Szymanski J, Gonzalez-Lugo JD, Rahman S, Billett HH. Assessment of lupus anticoagulant positivity in patients with coronavirus disease 2019 (COVID-19). JAMA Netw Open 2020;3:e2017539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Devreese KMJ, Linskens EA, Benoit D, Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: a relevant observation? J Thromb Haemost 2020;18:2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cardiac Society of Australia and New Zealand. COVID-19 resources. https://www.csanz.edu.au/resources/ (1 April 2020).
- 156. Lakkireddy DR, Chung MK, Gopinathannair R et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm 2020;17:e233–e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. National Health Society. NHS Clinical guide for the management of cardiology patients during the coronavirus pandemic. https://www.nice.org.uk/Media/Default/About/COVID-19/Specialty-guides/specialty-guide-cardiolgy-coronavirus.pdf (26 March 2021).
- 158. Varma N, Marrouche NF, Aguinaga L et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. Europace 2021;23:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Piro A, Magnocavallo M, D Rocca DG et al. Management of cardiac implantable electronic device follow-up in COVID-19 pandemic: lessons learned during Italian lockdown. J Cardiovasc Electrophysiol 2020;31:2814–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Peltzer B, Manocha KK, Ying X et al. Arrhythmic complications of patients hospitalized with COVID-19: incidence, risk factors, and outcomes. Circ Arrhythm Electrophysiol 2020;13:e009121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Russo V, Di Maio M, Mottola FF et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: a multicenter observational study. Eur J Clin Invest 2020;50:e13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Boriani G, Fauchier L, Aguinaga L et al. ; Group ESCSD. European Heart Rhythm Association (EHRA) consensus document on management of arrhythmias and cardiac electronic devices in the critically ill and post-surgery patient, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin American Heart Rhythm Society (LAHRS). Europace 2019;21:7–8. [DOI] [PubMed] [Google Scholar]
- 163. Brugada J, Katritsis DG, Arbelo E et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia: The Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2020;41:655–720. [DOI] [PubMed] [Google Scholar]
- 164. Brignole M, Auricchio A, Baron-Esquivias G, et al. ; ESC Committee for Practice Guidelines. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 165. Kirchhof P, Benussi S, Kotecha D et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 166. Monsieurs KG, Nolan JP, Bossaert LL et al. ; ERC Guidelines 2015 Writing Group. European Resuscitation Council Guidelines for resuscitation 2015: section 1. Executive summary. Resuscitation 2015;95:1–80. [DOI] [PubMed] [Google Scholar]
- 167. Priori SG, Blomstrom-Lundqvist C, Mazzanti A et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 168. Priori SG, Wilde AA, Horie M et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013;15:1389–1406. [DOI] [PubMed] [Google Scholar]
- 169. Colon CM, Barrios JG, Chiles JW et al. Atrial arrhythmias in COVID-19 patients. JACC Clin Electrophysiol 2020;6:1189–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Goyal P, Choi JJ, Pinheiro LC et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020;382:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Iacopino S, Placentino F, Colella J et al. New-onset cardiac arrhythmias during COVID-19 hospitalization. Circ Arrhythm Electrophysiol 2020;13:e009040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Peltzer B, Manocha KK, Ying X et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol 2020;31:3077–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Sala S, Peretto G, De Luca G et al. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin Electrophysiol 2020;43:891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ambrus DB, Benjamin EJ, Bajwa EK, Hibbert KA, Walkey AJ. Risk factors and outcomes associated with new-onset atrial fibrillation during acute respiratory distress syndrome. J Crit Care 2015;30:994–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Klein Klouwenberg PM, Frencken JF, Kuipers S et al. ; MARS Consortium. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis. A cohort study. Am J Respir Crit Care Med 2017;195:205–211. [DOI] [PubMed] [Google Scholar]
- 176. Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest 2014;146:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sanchis-Gomar F, Lavie CJ, Morin DP, Perez-Quilis C, Laukkanen JA, Perez MV. Amiodarone in the COVID-19 era: treatment for symptomatic patients only, or drug to prevent infection? Am J Cardiovasc Drugs 2020;20:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Guo T, Fan Y, Chen M et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Abrams MP, Coromilas EJ, Wan EY, Rubin GA, Garan H, Dizon JM. Malignant ventricular arrhythmias in patients with severe acute respiratory distress syndrome due to COVID-19 without significant structural heart disease. HeartRhythm Case Rep 2020;6:858–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Turagam MK, Musikantow D, Goldman ME et al. Malignant arrhythmias in patients with COVID-19: incidence, mechanisms, and outcomes. Circ Arrhythm Electrophysiol 2020;13:e008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Abrams MP, Wan EY, Waase MP et al. Clinical and cardiac characteristics of COVID-19 mortalities in a diverse New York City Cohort. J Cardiovasc Electrophysiol 2020;31:3086–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Annane D, Sebille V, Duboc D et al. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am J Respir Crit Care Med 2008;178:20–25. [DOI] [PubMed] [Google Scholar]
- 183. Madjid M, Connolly AT, Nabutovsky Y, Safavi-Naeini P, Razavi M, Miller CC. Effect of high influenza activity on risk of ventricular arrhythmias requiring therapy in patients with implantable cardiac defibrillators and cardiac resynchronization therapy defibrillators. Am J Cardiol 2019;124:44–50. [DOI] [PubMed] [Google Scholar]
- 184. Mitra RL, Greenstein SA, Epstein LM. An algorithm for managing QT prolongation in coronavirus disease 2019 (COVID-19) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: possible benefits of intravenous lidocaine. HeartRhythm Case Rep 2020;6:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Badri M, Patel A, Patel C et al. Mexiletine prevents recurrent torsades de pointes in acquired long QT syndrome refractory to conventional measures. JACC Clin Electrophysiol 2015;1:315–322. [DOI] [PubMed] [Google Scholar]
- 186. Wu CI, Postema PG, Arbelo E et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm 2020;17:1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chorin E, Wadhwani L, Magnani S et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm 2020;17:1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Szekely Y, Lichter Y, Shrkihe BA, Bruck H, Oster HS, Viskin S. Chloroquine-induced torsades de pointes in a patient with coronavirus disease 2019. Heart Rhythm 2020;17:1452–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Group RC, Horby P, Mafham M et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020;383:2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Group RC. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021;397:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Nguyen LS, Dolladille C, Drici MD et al. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the World Health Organization Pharmacovigilance Database. Circulation 2020;142:303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Offerhaus JA, Wilde AAM, Remme CA. Prophylactic (hydroxy)chloroquine in COVID-19: potential relevance for cardiac arrhythmia risk. Heart Rhythm 2020;17:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Vidovich MI. Transient Brugada-like electrocardiographic pattern in a patient with COVID-19. JACC Case Rep 2020;2:1245–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Chang D, Saleh M, Garcia-Bengo Y, Choi E, Epstein L, Willner J. COVID-19 infection unmasking brugada syndrome. HeartRhythm Case Rep 2020;6:237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. van de Poll SWE, van der Werf C. Two patients with COVID-19 and a fever-induced Brugada-like electrocardiographic pattern. Neth Heart J 2020;28:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Maglione TJ, Aboyme A, Ghosh BD, Bhatti S, Kostis WJ. Electrical storm in a febrile patient with Brugada syndrome and COVID-19 infection. HeartRhythm Case Rep 2020;6:676–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Moey MYY, Sengodan PM, Shah N et al. Electrocardiographic changes and arrhythmias in hospitalized patients with COVID-19. Circ Arrhythm Electrophysiol 2020;13:e009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Li Y, Liu T, Tse G et al. Electrocardiograhic characteristics in patients with coronavirus infection: a single-center observational study. Ann Noninvasive Electrocardiol 2020;25:e12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Wang Y, Chen L, Wang J et al. Electrocardiogram analysis of patients with different types of COVID-19. Ann Noninvasive Electrocardiol 2020;25:e12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Azarkish M, Far VL, Eslami M, Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J 2020;41:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. El-Assaad I, Hood-Pishchany MI, Kheir J et al. Complete heart block, severe ventricular dysfunction, and myocardial inflammation in a child with COVID-19 infection. JACC Case Rep 2020;2:1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Eneizat Mahdawi T, Wang H, Haddadin FI, Al-Qaysi D, Wylie JV. Heart block in patients with coronavirus disease 2019: a case series of 3 patients infected with SARS-CoV-2. HeartRhythm Case Rep 2020;6:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Kir D, Mohan C, Sancassani R. Heart brake: an unusual cardiac manifestation of COVID-19. JACC Case Rep 2020;2:1252–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Al-Assaf O, Mirza M, Musa A. Atypical presentation of COVID-19 as subclinical myocarditis with persistent high-degree atrioventricular block treated with pacemaker implant. HeartRhythm Case Rep 2020;6:884–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Peigh G, Leya MV, Baman JR, Cantey EP, Knight BP, Flaherty JD. Novel coronavirus 19 (COVID-19) associated sinus node dysfunction: a case series. Eur Heart J Case Rep 2020;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. World Health Organization. Therapeutics and COVID-19: living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1 (21 June 2021). [PubMed]
- 207. Gautret P, Lagier JC, Parola P et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 208. Wang M, Cao R, Zhang L et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Yao X, Ye F, Zhang M et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;71:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Boulware DR, Pullen MF, Bangdiwala AS et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020;383:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Cavalcanti AB, Zampieri FG, Rosa RG et al. ; Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med 2020;383:2041–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Mitja O, Corbacho-Monne M, Ubals M et al. Bcn Pep-CoV-2 RESEARCH GROUP. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Skipper CP, Pastick KA, Engen NW et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19 : a randomized trial. Ann Intern Med 2020;173:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Pan H, Peto R, Henao-Restrepo AM et al. ; WHO SOLIDARITY Trial Consortium. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med 2021;384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. ClinicalTrials.gov. Chloroquine/hydroxychloroquine prevention of coronavirus disease (COVID-19) in the healthcare setting (COPCOV). Bethesda, MD: National Library of Medicine (US). Identifier NCT0403507. https://clinicaltrials.gov/ct2/show/NCT04303507 (5 July 2021)
- 216. Arabi YM, Asiri AY, Assiri AM et al. ; the Saudi Critical Care Trials group. Treatment of middle east respiratory syndrome with a combination of lopinavir/ritonavir and interferon-beta1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials 2020;21:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Chan JF, Yao Y, Yeung ML et al. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis 2015;212:1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. de Wilde AH, Jochmans D, Posthuma CC et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 2014;58:4875–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Cao B, Wang Y, Wen D et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Agostini ML, Andres EL, Sims AC et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 2018;9:e00221–00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. de Wit E, Feldmann F, Cronin J et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 2020;117:6771–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sheahan TP, Sims AC, Graham RL et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017;9:eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Sheahan TP, Sims AC, Leist SR, Schafer A et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Beigel JH, Tomashek KM, Dodd LE et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 2020;383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Joyner MJ, Senefeld JW, Klassen SA et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv 2020. 2020.2008.2012.20169359.
- 227. Liu STH, Aberg JA. Convalescent plasma in patients hospitalised with COVID-19. Lancet 2021;397:2024–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Gottlieb RL, Nirula A, Chen P et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Chen P, Nirula A, Heller B et al. ; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021;384:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Weinreich DM, Sivapalasingam S, Norton T et al. ; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021;384:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. ACTIV-3/TICO LY-CoV555 Study Group, Lundgren JD, Grund B et al. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med 2021;384:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. National Institutes of Health. NIH-sponsored ACTIV-3 clinical trial closes enrollment into two sub-studies. https://www.nih.gov/news-events/news-releases/nih-sponsored-activ-3-clinical-trial-closes-enrollment-into-two-sub-studies (5 July 2021).
- 233. Horby PW, Mafham M, Peto L et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021:2021.2006.2015.21258542.
- 234. Touret F, Gilles M, Barral K et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci Rep 2020;10: 13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Furtado RHM, Berwanger O, Fonseca HA et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet 2020;396:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Chen X, Zhao B, Qu Y et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis 2020;71:1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021;397:1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021;326:499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Deftereos SG, Giannopoulos G, Vrachatis DA et al. ; GRECCO-19 investigators. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open 2020;3:e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Tardif JC, Bouabdallaoui N, L'Allier PL et al. ; COLCORONA Investigators. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med 2021;9:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Horby PW, Campbell M, Spata E et al. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021:2021.2005.2018.21257267.
- 242. University of Liverpool. COVID-19 drug interactions—prescribing resources. https://www.covid19-druginteractions.org/ (2 May 2020).
- 243. Vicente J, Zusterzeel R, Johannesen L et al. Assessment of multi-ion channel block in a phase I randomized study design: results of the CiPA phase I ECG biomarker validation study. Clin Pharmacol Ther 2019;105:943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Hsia BC, Greige N, Quiroz JA et al. QT prolongation in a diverse, urban population of COVID-19 patients treated with hydroxychloroquine, chloroquine, or azithromycin. J Interv Card Electrophysiol 2020;59:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Mzayek F, Deng H, Mather FJ et al. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials 2007;2:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Sinkeler FS, Berger FA, Muntinga HJ, Jansen M. The risk of QTc-interval prolongation in COVID-19 patients treated with chloroquine. Neth Heart J 2020;28:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Teixeira RA, Borba EF, Pedrosa A et al. Evidence for cardiac safety and antiarrhythmic potential of chloroquine in systemic lupus erythematosus. Europace 2014;16:887–892. [DOI] [PubMed] [Google Scholar]
- 248. van den Broek MPH, Möhlmann JE, Abeln BGS, Liebregts M, van Dijk VF, van de Garde EMW. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth Heart J 2020;28:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Wozniacka A, Cygankiewicz I, Chudzik M, Sysa-Jedrzejowska A, Wranicz JK. The cardiac safety of chloroquine phosphate treatment in patients with systemic lupus erythematosus: the influence on arrhythmia, heart rate variability and repolarization parameters. Lupus 2006;15:521–525. [DOI] [PubMed] [Google Scholar]
- 250. Borba MGS, Val FFA, Sampaio VS et al. ; CloroCovid-19 Team. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020;3:e208857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Saleh M, Gabriels J, Chang D et al. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol 2020;13:e008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Teixeira RA, Martinelli Filho M, Benvenuti LA, Costa R, Pedrosa AA, Nishióka SAD. Cardiac damage from chronic use of chloroquine: a case report and review of the literature. Arq Bras Cardiol 2002;79:85–88. [DOI] [PubMed] [Google Scholar]
- 253. White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis 2007;7:549–558. [DOI] [PubMed] [Google Scholar]
- 254. Yogasundaram H, Putko BN, Tien J et al. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol 2014;30:1706–1715. [DOI] [PubMed] [Google Scholar]
- 255. Capel RA, Herring N, Kalla M et al. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current If: novel electrophysiological insights and therapeutic potential. Heart Rhythm 2015;12:2186–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Gasperetti A, Biffi M, Duru F et al. Arrhythmic safety of hydroxychloroquine in COVID-19 patients from different clinical settings. Europace 2020;22:1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lee JH, Chung WB, Kang JH et al. A case of chloroquine-induced cardiomyopathy that presented as sick sinus syndrome. Korean Circ J 2010;40:604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. McGhie TK, Harvey P, Su J, Anderson N, Tomlinson G, Touma Z. Electrocardiogram abnormalities related to anti-malarials in systemic lupus erythematosus. Clin Exp Rheumatol 2018;36:545–551. [PubMed] [Google Scholar]
- 259. Costedoat-Chalumeau N, Hulot JS, Amoura Z et al. Heart conduction disorders related to antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology (Oxford) 2007;46:808–810. [DOI] [PubMed] [Google Scholar]
- 260. Bessiere F, Roccia H, Deliniere A et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol 2020;5:1067–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Mercuro NJ, Yen CF, Shim DJ et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Ramireddy A, Chugh H, Reinier K et al. Experience with hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc 2020;9:e017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Rosenberg ES, Dufort EM, Udo T et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 2020;323:2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Sridhar AR, Chatterjee NA, Saour B et al. QT interval and arrhythmic safety of hydroxychloroquine monotherapy in coronavirus disease 2019. Heart Rhythm O2 2020;1:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Saleh M, Gabriels J, Chang D et al. ; Northwell COVID-19 Research Consortium. Safely administering potential QTc prolonging therapy across a large health care system in the COVID-19 era. Circ Arrhythm Electrophysiol 2020;13:e008937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Zhang M, Xie M, Li S et al. Electrophysiologic studies on the risks and potential mechanism underlying the proarrhythmic nature of azithromycin. Cardiovasc Toxicol 2017;17:434–440. [DOI] [PubMed] [Google Scholar]
- 267. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012;366:1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Poluzzi E, Raschi E, Motola D, Moretti U, De Ponti F. Antimicrobials and the risk of torsades de pointes: the contribution from data mining of the US FDA Adverse Event Reporting System. Drug Saf 2010;33:303–314. [DOI] [PubMed] [Google Scholar]
- 269. Cheng YJ, Nie XY, Chen XM et al. The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol 2015;66:2173–2184. [DOI] [PubMed] [Google Scholar]
- 270. Maisch NM, Kochupurackal JG, Sin J. Azithromycin and the risk of cardiovascular complications. J Pharm Pract 2014;27:496–500. [DOI] [PubMed] [Google Scholar]
- 271. Lu ZK, Yuan J, Li M et al. Cardiac risks associated with antibiotics: azithromycin and levofloxacin. Expert Opin Drug Saf 2015;14:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Rao GA, Mann JR, Shoaibi A et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med 2014;12:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Fresse A, Viard D, Romani S et al. ; French Network of Pharmacovigilance Centers. Spontaneous reported cardiotoxicity induced by lopinavir/ritonavir in COVID-19. An alleged past-resolved problem. Int J Cardiol 2021;324:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Rathbun CR, Liedtke MD, Blevins SM et al. Electrocardiogram abnormalities with atazanavir and lopinavir/ritonavir. HIV Clin Trials 2009;10:328–336. [DOI] [PubMed] [Google Scholar]
- 275. Moschini L, Loffi M, Regazzoni V, Di Tano G, Gherbesi E, Danzi GB. Effects on QT interval of hydroxychloroquine associated with ritonavir/darunavir or azithromycin in patients with SARS-CoV-2 infection. Heart Vessels 2021;36:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Grange S, Schmitt C, Banken L, Kuhn B, Zhang X. Thorough QT/QTc study of tocilizumab after single-dose administration at therapeutic and supratherapeutic doses in healthy subjects. Int J Clin Pharmacol Ther 2011;49:648–655. [DOI] [PubMed] [Google Scholar]
- 277. Gupta S, Wang W, Hayek SS et al. ; Stop-Covid Investigators. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med 2021;181:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Hermine O, Mariette X, Tharaux PL et al. ; CORIMUNO-19 Collaborative Group. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Salvarani C, Dolci G, Massari M et al. ; Rct-Tcz-Covid-Study Group. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Akbulak RO, Rosenkranz SC, Schaeffer BN et al. Acute and long-term effects of fingolimod on heart rhythm and heart rate variability in patients with multiple sclerosis. Mult Scler Relat Disord 2018;19:44–49. [DOI] [PubMed] [Google Scholar]
- 281. Gold R, Comi G, Palace J et al. ; FIRST Study Investigators. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: a phase 3b, open-label study. J Neurol 2014;261:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Limmroth V, Ziemssen T, Lang M et al. Electrocardiographic assessments and cardiac events after fingolimod first dose—a comprehensive monitoring study. BMC Neurol 2017;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Brown B, Weiss JL, Kolodny S, Meng X, Williams IM, Osborne JA. Analysis of cardiac monitoring and safety data in patients initiating fingolimod treatment in the home or in clinic. BMC Neurol 2019;19:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Humeniuk R, Mathias A, Cao H et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci 2020;13:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Spinner CD, Gottlieb RL, Criner GJ et al. ; GS-US-540-5774 Investigators. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020;324:1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Wang Y, Zhang D, Du G et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395:1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Ramiro S, Mostard RLM, Magro-Checa C et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis 2020;79:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Rubio-Rivas M, Ronda M, Padulles A et al. Beneficial effect of corticosteroids in preventing mortality in patients receiving tocilizumab to treat severe COVID-19 illness. Int J Infect Dis 2020;101:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Al Shibli A, Al Attrach I, Hamdan MA. Bradycardia following oral corticosteroid use: case report and literature review. Arab J Nephrol Transplant 2012;5:47–49. [PubMed] [Google Scholar]
- 290. Sodero A, Squitieri M, Mazzeo S et al. Acute symptomatic sinus bradycardia in high-dose methylprednisolone therapy in a woman with inflammatory myelitis: a case report and review of the literature. Clin Med Insights Case Rep 2019;12:117954761983102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19). Mayo Clin Proc 2020;95:1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Yang T, Roden DM. Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation 1996;93:407–411. [DOI] [PubMed] [Google Scholar]
- 293. Garabelli P, Stavrakis S, Albert M et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016;27:827–832. [DOI] [PubMed] [Google Scholar]
- 294. Bikdeli B, Madhavan MV, Jimenez D et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH NATF EVSM and the IUA, Supported by the ESC Working Group on Pulmonary Circulation Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Nadkarni GN, Lala A, Bagiella E et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol 2020;76:1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Paranjpe I, Fuster V, Lala A et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 2020;76:122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Thachil J, Tang N, Gando S et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020;18:1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Kearon C, Akl EA, Ornelas J et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–352. [DOI] [PubMed] [Google Scholar]
- 299. Driggin E, Madhavan MV, Bikdeli B et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol 2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Medscape. Drug interaction checker. https://reference.medscape.com/drug-interactionchecker (26 March 2021).
- 301. Faragon JJ, Budak JZ. National HIV curriculum. Section 3. Antiretroviral therapy/Topic 3. Drug Interactions with Antiretroviral Medications. https://www.hiv.uw.edu/go/antiretroviral-therapy/drug-drug-interactions/core-concept/all (26 March 2021).
- 302. Steffel J, Collins R, Antz M et al. 2021 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Duchin K, Duggal A, Atiee GJ et al. An open-label crossover study of the pharmacokinetics of the 60-mg edoxaban tablet crushed and administered either by a nasogastric tube or in apple puree in healthy adults. Clin Pharmacokinet 2018;57:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Moore KT, Krook MA, Vaidyanathan S, Sarich TC, Damaraju CV, Fields LE. Rivaroxaban crushed tablet suspension characteristics and relative bioavailability in healthy adults when administered orally or via nasogastric tube. Clin Pharmacol Drug Dev 2014;3:321–327. [DOI] [PubMed] [Google Scholar]
- 305. Song Y, Chang M, Suzuki A, Frost RJ, Kelly A, LaCreta F, Frost C. Evaluation of crushed tablet for oral administration and the effect of food on apixaban pharmacokinetics in healthy adults. Clin Ther 2016;38:1674–1685 e1671. [DOI] [PubMed] [Google Scholar]
- 306. Song Y, Wang X, Perlstein I et al. Relative bioavailability of apixaban solution or crushed tablet formulations administered by mouth or nasogastric tube in healthy subjects. Clin Ther 2015;37:1703–1712. [DOI] [PubMed] [Google Scholar]
- 307. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19—studies needed. N Engl J Med 2020;382:1194–1196. [DOI] [PubMed] [Google Scholar]
- 308. Emanuel EJ, Persad G, Upshur R, Thome B et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020;382:2049–2055. [DOI] [PubMed] [Google Scholar]
- 309. Ellinghaus D, Degenhardt F, Bujanda L et al. ; Severe Covid-19 GWAS Group. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med 2020;383:1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities 2020;7:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Belanger MJ, Hill MA, Angelidi AM, Dalamaga M, Sowers JR, Mantzoros CS. Covid-19 and disparities in nutrition and obesity. N Engl J Med 2020;383:e69. [DOI] [PubMed] [Google Scholar]
- 312. Egede LE, Walker RJ. Structural racism, social risk factors, and Covid-19—a dangerous convergence for Black Americans. N Engl J Med 2020;383:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med 2020;382:2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension 2020;75:1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Piepoli MF, Hoes AW, Agewall S et al. ; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. S Oliveira J, Sherrington C, R Y Zheng E, Franco MR, Tiedemann A. Effect of interventions using physical activity trackers on physical activity in people aged 60 years and over: a systematic review and meta-analysis. Br J Sports Med 2020;54:1188–1194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.