Abstract
Individuals on immunosuppressive (IS) therapy have increased mortality from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and delayed viral clearance may lead to new viral variants. IS therapy reduces antibody responses following coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccination; however, a comprehensive assessment of vaccine immunogenicity is lacking. Here we show that IS therapy reduced neutralizing, binding, and nonneutralizing antibody functions in addition to CD4 and CD8 T-cell interferon-γ responses following COVID-19 mRNA vaccination compared to immunocompetent individuals. Moreover, IS therapy reduced cross-reactivity against SARS-CoV-2 variants. These data suggest that the standard COVID-19 mRNA vaccine regimens will likely not provide optimal protection in immunocompromised individuals.
Keywords: immunocompromised, COVID, 19 vaccine, SARS, CoV, 2, vaccine immunogenicity, T cells
Individuals on immunosuppression for various indications have impaired neutralizing, binding, and nonneutralizing antibody responses and reduced T-cell activity in response to COVID-19 mRNA vaccination compared to immunocompetent controls. This suggests that standard vaccination may not result in protective immunity in this population.
Individuals on immunosuppressive (IS) therapy for autoimmune disease or transplantation face greater risks of mortality and morbidity from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The observation of prolonged SARS-CoV-2 replication in immunocompromised individuals has raised the question of immunologic control in such populations [1], and recent studies have shown that only 38%–54% of kidney and liver transplant recipients developed detectable SARS-CoV-2 antibodies following the second dose of a coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccine [2–5]. Specific medications including antimetabolite therapy (mycophenolate, azathioprine), high-dose corticosteroids, or maintenance on a 3-medication (triple IS) regimen have been associated with lower odds of antibody response in transplant recipients [2–5]. Prior studies have not reported comprehensive immune profiling or responses to SARS-CoV-2 variants and have only studied limited types of immunosuppression. In this study, we assessed comprehensive antibody and T-cell profiles following COVID-19 mRNA vaccination in individuals on IS therapy for several medical indications.
METHODS
We conducted a cohort study of individuals ≥18 years of age who received the BNT162b2 (Pfizer/BioNTech) or mRNA-1273 (Moderna) COVID-19 vaccine from 19 December 2020 through 8 April 2021. The Beth Israel Deaconess Medical Center institutional review board approved this study (number 2021P000344) and the parent biorepository study (number 2020P000361); participants provided written informed consent. Immune responses were assessed at each vaccine dose and 2–8 weeks after the second dose using comprehensive humoral and cellular immune assays as previously described and detailed in the Supplementary Methods [6].
RESULTS
Ninety participants were enrolled for this study, including 69 participants who were receiving IS therapy for solid organ transplant, bone marrow transplant, and/or chronic inflammatory disease. Eight were excluded from the primary analysis due to known or suspected prior SARS-CoV-2 infection. Twenty-one participants not on IS therapy were enrolled as healthy controls. The 61 participants on IS therapy and without prior SARS-CoV-2 infection were stratified into 2 groups: 47 on single or double IS medications and 14 on triple IS medications. Sampling occurred a median of 24 days (interquartile range, 18–35 days) following the second mRNA vaccine, and most received the BNT162b2 vaccine. Fever following the second dose of vaccination was reported by 37% in the control group, 3% in the single/double IS group, and 0% in the triple IS group (Supplementary Table 1). No participants were diagnosed with new SARS-CoV-2 infection during the study.
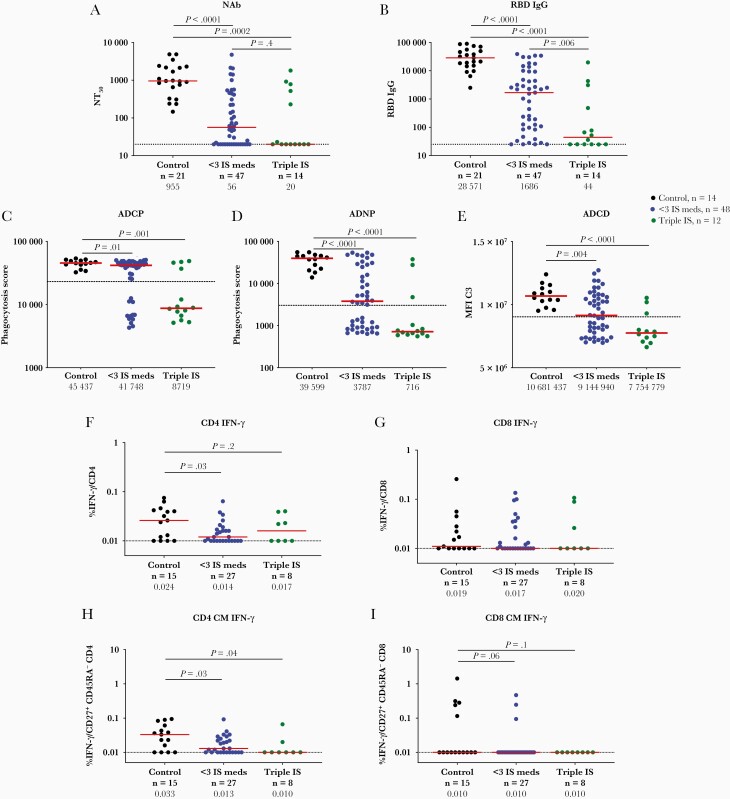
Antibody responses were assessed by pseudovirus neutralization assays, receptor binding domain (RBD)–specific enzyme-linked immunosorbent assays, and Fc functional antibody assays. The median neutralizing antibody titer (NT50) was 955 in healthy controls, 56 in the single/double IS group, and <20 in the triple IS group (P<.001) (Figure 1A). NT50 titers were detected in 100% of healthy controls, in 66% of the single/double IS group, and in 36% of the triple IS group (Supplementary Table 2). Median RBD binding titers (Figure 1B) and functional nonneutralizing antibody responses (Figure 1C–E), including antibody-dependent cellular phagocytosis, antibody-dependent neutrophil phagocytosis, and antibody-dependent complement deposition, were also reduced in the single/double IS group and were markedly reduced in the triple IS group.
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody and T-cell activity following coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccination in individuals on immunosuppressive (IS) therapy. Pseudovirus neutralizing antibody titers (NT50) (A) and SARS-CoV-2 spike receptor-binding domain (RBD) immunoglobulin G titer (B) 2–8 weeks following second dose of COVID-19 mRNA vaccination in individuals on single/double IS therapy (blue), on triple IS therapy (green), and controls (black). Systems serology was used to quantify RBD-specific antibody-dependent monocyte cellular phagocytosis (C), antibody-dependent neutrophil phagocytosis expressed as a phagocytosis score (D), and mean fluorescence index of complement component 3 for antibody-dependent complement deposition (E). Peripheral blood mononuclear cells at 2–8 weeks following second dose of COVID-19 mRNA vaccination in all 3 groups. T-cell responses were measured using multiparameter intracellular cytokine staining to measure percentage interferon-γ production in CD4 T cells (F), CD8 T cells (G), and by CD45RA–CD27+ central memory (CM) CD4 T cells (H) and CD8 CM T cells (I). Medians (red bar) are displayed below each time point on the x-axis. Dotted line indicates limit of detection, and the dashed line in C–E indicates line of positivity. Abbreviations: ADCD, antibody-dependent complement deposition; ADCP, antibody-dependent monocyte cellular phagocytosis; ADNP, antibody-dependent neutrophil phagocytosis; CM, central memory; IFN-γ, interferon gamma; IgG, immunoglobulin G; IS, immunosuppressive; MFI C3, mean fluorescence index of complement component 3; NAb, neutralizing antibody; NT50, median neutralizing antibody titer; RBD, receptor-binding domain.
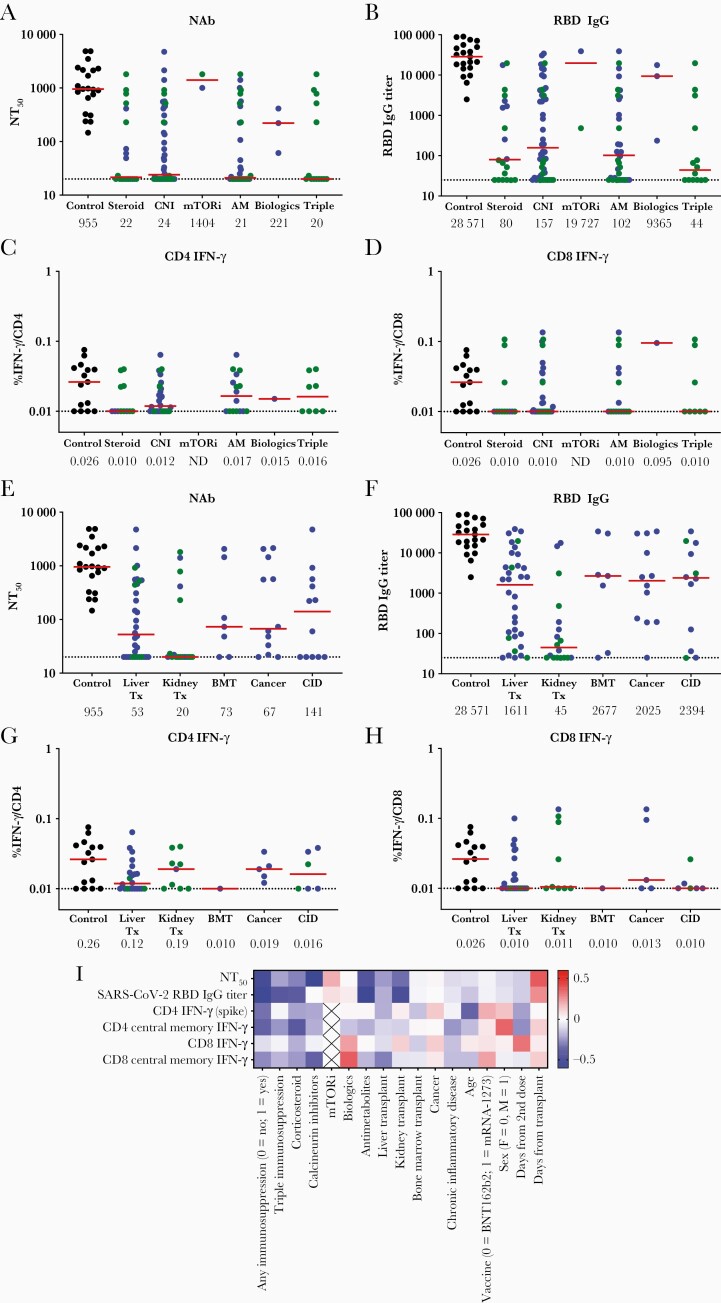
Corticosteroids, calcineurin inhibitors, antimetabolites, triple IS therapy, and kidney transplant were associated with particularly low or undetectable NT50 titers of 49, 28, <20, and <20, respectively (Figure 2A–H), and were all strongly correlated with reduced immune responses (Figure 2I). The 8 participants with prior SARS-CoV-2 infection displayed a high median NT50 titer of 3083 and a high median RBD-specific binding antibody titer of 38058 following vaccination (Supplementary Figure 1).
Figure 2.
Humoral and cellular immune responses in individuals on immunosuppressive (IS) therapy stratified by clinical characteristics (medications and indication for therapy). Pseudovirus neutralizing antibody titers (NT50) (A and E) and receptor-binding domain immunoglobulin G binding antibody titers (B and F) were measured in serum at 2–8 weeks following second dose of coronavirus disease 2019 messenger RNA vaccination in individuals on single/double IS therapy (blue), those on triple IS therapy (green), and controls (black). T-cell responses were measured using intracellular cytokine staining to measure percentage interferon gamma (IFN-γ) production in CD4 T cells (C and G) and CD8 T cells (D and H). Median antibody titers or percentage IFN-γ production (red bar, and listed below x-axis) and the limit of detection (dotted line) are shown. Results are stratified by non–mutually exclusive categories of medication use (A–D) and by medical indication for IS therapy (E–H). “Biologic therapy” included rituximab and infliximab. Heatmap of Spearman correlation coefficient values for correlation between clinical variables on the x-axis and 6 vaccine-induced immune parameters on the y-axis. Red indicates positive and blue indicates negative correlation (I). Abbreviations: AM, antimetabolite therapy (eg, mycophenolate, azathioprine, or leflunomide); BMT, bone marrow transplant; CID, chronic inflammatory disease; CNI, calcineurin inhibitor; F, female; IFN-γ, interferon gamma; IgG, immunoglobulin G; M, male; mTORi, mammalian target of rapamycin inhibitor; NAb, neutralizing antibody; NT50, median neutralizing antibody titer; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Tx, transplantation.
Median spike-specific interferon gamma (IFN-γ) CD4 T-cell responses were 0.024% in healthy controls, as compared with 0.014% and 0.017% in the single/double IS and triple IS groups, respectively (Figure 1F, Supplementary Table 2). A similar pattern was observed for central memory CD4 T cells (Figure 1H). IFN-γ spike-specific CD8 T-cell responses were detectable but low in all groups (Figure 1G and 1I).
Humoral and cellular responses also were reduced against viral variants in individuals on IS therapy (Supplementary Figure 2). Neutralizing and binding antibody responses were typically observed in immunocompetent individuals against the SARS-CoV-2 B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and other variants. Responses to all variants were reduced in the single/double IS group but were nearly ablated in the triple IS group.
DISCUSSION
This study demonstrates markedly reduced humoral and cellular immunogenicity of mRNA COVID-19 vaccines in individuals on IS therapy compared with healthy controls. Multiple immune parameters were decreased, including neutralizing, binding, and functional nonneutralizing antibodies, as well as T-cell responses against both the original vaccine strain and against multiple viral variants. Suppression of vaccine immunogenicity was particularly striking in individuals on triple IS therapy.
Our findings extend prior studies that have recently reported reduced spike-specific antibodies [3, 4, 7] and T-helper responses [8] in transplant recipients following COVID-19 mRNA vaccination. In this study, we show that multiple humoral and cellular immune parameters as well as coverage of viral variants were simultaneously diminished. These observations suggest that there will likely be reduced efficacy of mRNA COVID-19 vaccines for individuals on IS medications. Prior studies have estimated efficacy of 59% compared to 91% in immunocompetent individuals [9], and another study in Israel demonstrated that those on IS therapy represented 40% of breakthrough infections [10]. In our study, suppression of vaccine immunogenicity was particularly striking in individuals on antimetabolites, calcineurin inhibitors, or corticosteroids, or with kidney transplant. Moreover, we observed substantially more suppression of vaccine immunogenicity in individuals on triple IS therapy, suggesting that these populations may be at highest risk of COVID-19 vaccine breakthrough infections.
Recent data support a third dose of mRNA vaccines to boost immunity in transplant recipients [11], and the FDA recently approved use of a third mRNA vaccine dose for immunocompromised individuals [12]. It is currently not known if a third mRNA dose will be immunogenic in individuals on triple IS therapy and will improve protective efficacy.
This study has several limitations. First, the small study size limits definitive conclusions about vaccine tolerability or clinical efficacy and limits the ability to indication for immunosuppression. Second, immune responses were evaluated at a short interval following vaccination; thus, we cannot make conclusions about the durability of immune responses. Third, this was a clinical cohort study rather than a randomized controlled trial.
In conclusion, although analysis of the mRNA-1273 phase 3 trial participants suggests that antibody levels after vaccination correlate to protection against COVID-19 [13] in humans, it is unclear whether or not this would apply to other vaccine platforms or in a population of individuals on medical immunosuppression. The marked suppression of both humoral and cellular immune responses in certain subsets of immunosuppressed individuals suggests that these populations will likely not be protected by the 2-dose regimens of mRNA COVID-19 vaccines.
Supplementary Material
Notes
Author contributions. A. Y. C.: conceptualization, formal analysis, resources, investigation, data curation, writing–original draft, visualization. D. H. B.: conceptualization, formal analysis, writing–review and editing, funding acquisition, supervision. J. Y., K. M., and J. L.: investigation, methodology, data curation. C. A.: methodology, data curation. J. L. A., M. R., and R. A.: project administration, resources, data curation. Z. P. F. and M. P.: investigation, data curation, writing–review and editing. M. P. C.: investigation, resources, writing–review and editing. C. J.-D., J. B., and K. A.: resources, methodology. H. P.: investigation, data curation. D. S.: resources, methodology, investigation. A. G. and G. A.: data curation, methodology, resources. A. C.: project administration, data curation. M. R. H.: validation, resources, formal analysis, writing–review and editing, supervision.
Acknowledgments. The authors thank the participants and the Center for Virology and Vaccine Research Clinical Trials Unit members Caitlin Guiney, Diane Kanjilal, Kate Jaegle, Connor Bradshaw, Lorraine Bermudez Rivera, Rachel Hemond, and Daniel Massillon for assistance with recruitment, enrollment, and sample collection. We thank Barouch laboratory members Shivani Patel, Tochi Anioke, Huahua Wan, Aiquan Chang, Owen Sanborn, and Esther Apraku Bondzie for processing samples and performing assays. We thank Jim Wilbur and Jacob Wohlstadter from MesoScale Discovery for providing the kits for the electrochemiluminescence assay multiplexing kits used in this study.
Data sharing. A. Y. C. and D. H. B. had full access to all study data and take responsibility for the integrity of the data and the accuracy of the data analysis. All data are available in the manuscript or the Supplementary Materials.
Financial support. The authors acknowledge support from the National Institutes of Health (grant number CA260476); the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard; the Massachusetts Consortium for Pathogen Readiness (MassCPR); and the Musk Foundation (to D. H. B.). The authors also acknowledge the Reproductive Scientist Development Program from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and Burroughs Wellcome Fund (HD000849 to A. Y. C.), the Harvard Clinical and Translational Science Center (TR002541 to M. R. H.), and the American Association for the Study of Liver Diseases Foundation (to Z. P. F.).
Potential conflicts of interest. G. A. reported cofounding, serving as a consultant to, and having a patent pending through SeromYx Systems, Inc. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020; 383:2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325:2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-Cov-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 2021; 21:2719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol 2021; 75:435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deepak P, Kim W, Paley MA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv [Preprint]. Posted online 9 April 2021. doi: 10.1101/2021.04.05.21254656. [DOI] [Google Scholar]
- 6. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA 2021; 325:2370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rincon-Arevalo H, Choi M, Stefanski A-L, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol 2021; 6:eabj1031. [DOI] [PubMed] [Google Scholar]
- 8. Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest 2021; 131:e150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 hospitalizations in the United States [manuscript published online ahead of print 6 August 2021]. Clin Infect Dis 2021. doi: 10.1093/cid/ciab687. [DOI] [Google Scholar]
- 10. Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect 2021; 27:1652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021; 385:1244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised. Accessed 13 August 2021.
- 13. Gilbert PB, Montefiori DC, McDermott A, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv [Preprint]. Posted online 15 August 2021. doi: 10.1101/2021.08.09.21261290. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.