Abstract
Background
Azithromycin has been widely used in the management of COVID-19. However, the evidence on its actual effects remains disperse and difficult to apply in clinical settings. This systematic review and meta-analysis summarizes the available evidence to date on the beneficial and adverse effects of azithromycin in patients with COVID-19.
Methods
The PRISMA 2020 statement criteria were followed. Randomized controlled trials (RCTs) and observational studies comparing clinical outcomes of patients treated with and without azithromycin, indexed until 5 July 2021, were searched in PubMed, Embase, The Web of Science, Scopus, The Cochrane Central Register of Controlled Trials and MedRXivs. We used random-effects models to estimate pooled effect size from aggregate data.
Results
The initial search produced 4950 results. Finally, 16 studies, 5 RCTs and 11 with an observational design, with a total of 22 984 patients, were included. The meta-analysis showed no difference in mortality for those treated with or without azithromycin, in observational studies [OR: 0.90 (0.66–1.24)], RCTs [OR: 0.97 (0.87–1.08)] and also when both types of studies were pooled together [with an overall OR: 0.95 (0.79–1.13)]. Different individual studies also reported no significant difference for those treated with or without azithromycin in need for hospital admission or time to admission from ambulatory settings, clinical severity, need for intensive care, or adverse effects.
Conclusions
The results presented in this systematic review do not support the use of azithromycin in the management of COVID-19. Future research on treatment for patients with COVID-19 may need to focus on other drugs.
Introduction
Azithromycin has been widely used in the management of COVID-19.1,2 It is a broad-spectrum antibiotic, which is rapidly absorbed after oral intake, and has a long half-life. Evidence suggests that azithromycin has antiviral activity in bronchial epithelial cells, together with anti-inflammatory and immunoregulatory effects.1,2 The association of azithromycin with improved outcome in patients with other viral pneumonias, and in those with acute lung injury admitted in intensive care, has also been reported.2 The possible beneficial effect of azithromycin in patients with COVID-19 and bacterial superinfection has been considered as well.1 It is an economical drug that can be used in the early stages of COVID-19. However, the possible QT prolongation and cardiotoxicity associated with azithromycin are a concern.1–4
A number of individual studies have investigated the effect of azithromycin on different clinical outcomes among patients with COVID-19. The reviews where these articles are summarized are either narrative, they focus on the effect of azithromycin in combination with hydroxychloroquine, or are restricted to studies with a particular design. Therefore, the evidence on the actual beneficial or harmful effects of azithromycin in patients with COVID-19 remains disperse and difficult to apply in clinical settings.1 This systematic review and meta-analysis summarizes the evidence on the beneficial and adverse effects of azithromycin in patients with COVID-19.
Methods
This systematic review and meta-analysis was registered in the International prospective register of systematic reviews (PROSPERO) with the reference CRD 42021252219, and it was conducted following the PRISMA 2020 statement criteria.5,6 Randomized controlled trials (RCTs) and observational studies comparing clinical outcomes of patients treated with and without azithromycin were searched and considered for inclusion. All the publications indexed up to 5 July 2021 in the following six databases were reviewed: PubMed, Embase, The Web of Science, Scopus, The Cochrane Central Register of Controlled Trials (CENTRAL) and MedRXivs. The following search strategy was used: ((‘Azithromycin’[Mesh]) OR ‘Macrolides’[Mesh]) AND (((‘Coronavirus’[Mesh]) OR ‘COVID-19’[Mesh]) OR ‘SARS-CoV-2’[Mesh]). Studies in the bibliography of all the relevant reviews identified in the initial search were also considered for inclusion. The forward citation tool in The Web of Science was used and all papers that cited those included in the review were also considered for inclusion. There were no restrictions on the basis of language, sample size, or duration of follow-up. Studies were not included in the following cases: (i) they reported outcomes of specific participants (i.e. cancer patients only); (ii) there was no comparison arm; (iii) azithromycin was compared against an intervention different to placebo or standard care; (iv) the effect of azithromycin in combination with another drug was the objective of the study; (v) the exposure was not specifically azithromycin (i.e. antibiotics, macrolides); (vi) the study had an observational design but no multivariate analysis, with adjustment for potential confounders, had been conducted.
Where several articles reported results from the same population, data were taken from the publication with the longest follow-up. The quality of all studies was assessed according to accepted criteria.7 Authors of studies were contacted when it was unclear whether papers met the inclusion criteria, and to verify methods and results that may not have been reported.
We used random-effects models to estimate pooled effect size from aggregate data.8 The majority of the studies provided estimates on the risk of death, ORs, relative risks (RRs), or HRs. We pooled these using ORs, where RRs were transformed to ORs, and HRs were used as proxy measures for ORs as the percentage of the outcome was relatively small and the follow-up period was short.9 When an RCT did not report a summary estimate, but provided numbers of deaths in each exposure group, the OR was calculated and used in the pooled summary estimates. When observational studies did not provide numerical measurements of effect for death, they could not be included in the meta-analysis and their results are presented narratively. Observational studies and RCTs are displayed in one forest plot stratified by study design.
The summaries for outcomes other than death were reported narratively, due to the different methods used across studies and the small number of studies that investigated each outcome.
Between-study heterogeneity was assessed using I2 statistics, which describes the percentage of variation across studies that is due to heterogeneity rather than chance.10 Publication bias was assessed visually using funnel plots and contour-enhanced funnel plots, and the Egger test was used to assess asymmetry and small study effects.11 Statistical analysis was performed using the software STATA V.16.
Results
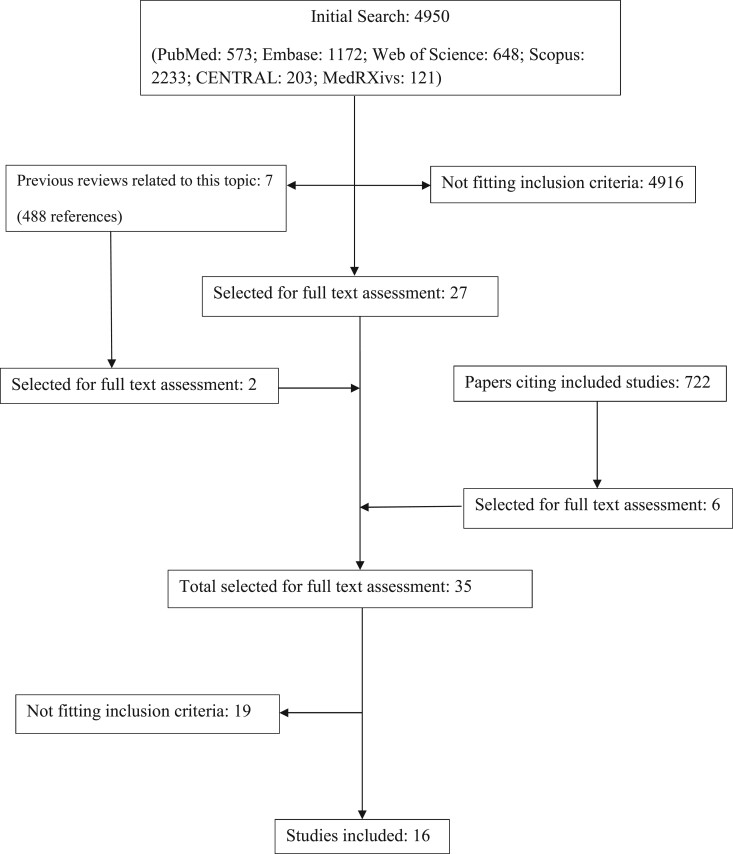
The initial search produced 4950 results, seven of them were reviews relevant to this topic.2,12–17 The full text of 35 articles was assessed. Finally, our systematic review included 16 studies, with a total of 22 984 patients.18–33 The studies assessed at each stage of the search are presented in Figure 1. Five of the studies were RCTs and 11 had an observational design. They had been conducted in Brazil, France, Italy, Iran, Spain, Turkey, the UK and the USA. Four studies had been conducted in ambulatory settings, 11 in hospitals, and one included both hospital and ambulatory patients. The sample size ranged from 111 to 7763 patients.18,19 The characteristics of the studies included in this systematic review are presented in Tables 1 and 2.
Figure 1.
Studies identified at each stage of the search.
Table 1.
RCTs included in the systematic review
| Author/year/ country | Setting | N AZM/standard care | Outcome | Outcome n AZM/comparison | Effect |
|---|---|---|---|---|---|
| ATOMIC2/ 2021/UK | Comm | 147/148 | Death or need for admission | 15/17 | OR: 0.91 (0.43–1.92), P = 0.80 |
| Time to admission | HR: 0.95 (0.46–1.96), P = 0.89 | ||||
| Maximum clinical severity | OR: 0.91 (0.57–1.46), P = 0.69 | ||||
| COALITION II/ 2020/Brazil | Hosp | 214/183 | Higher category of clinical status score | OR: 1.36 (0.94–1.97) | |
| Death | 90/73 | HR: 1.08 (0.79–1.47), P = 0.63 | |||
| Difference median length of admission (days) | 26/18 | 8.00 (0.81–15.19), P = 0.064 | |||
| Serious adverse events | 102/75 | No difference in adverse events P = 0.35 | |||
| QT prolongation | 47/42 | No difference. No difference in ventricular arrythmia or need for resuscitation | |||
| PRINCIPLE/ 2021/UK | Comm | 540/875 | Clinical recovery Day 28 | 402/631 | |
| Time to clinical recovery | HR: 1.08 (0.95–1.23) | ||||
| Need of admission | 16/28 | OR: 0.3 (–1.7 to 2.2) | |||
| RECOVERY/ 2021/UK | Hosp | 2582/5181 | Death | 561/1162 | RR: 0.97 (0.87–1.107), P = 0.50. This result was also not significant in different age or gender categories |
| Median length of admission (days) | 10/11 | ||||
| Discharged alive on Day 28 (%) | 68/69 | RR: 1.04 (0.98–1.10), P = 0.19 | |||
| Need for mechanical ventilation or death (%) | 25/26 | RR: 0.95 (0.87–1.03), P = 0.24 | |||
| Serious adverse events | 1/0 | No difference in arrythmias | |||
| Sekhavati/ Iran/2020 | Hosp | 56/55 | Mean length of admission (days) | 4.61/5.96 | P = 0.02 |
| Need for ICU | 2/7 | P = 0.070 | |||
| Death | 0/1 | P = 0.495 | |||
| No difference in QT prolongation or arrythmia |
AZM, azithromycin; Hosp, hospital; Comm, community.
Table 2.
Observational studies included in the systematic review
| Author/year/country | Setting | N AZM/ standard care | Outcome | Outcome n AZM/ comparison | Effect |
|---|---|---|---|---|---|
| Albani/2020/Italy | Hosp | 421/605 | Death | 69/172 | OR: 0.60 (0.42–0.85) |
| Need for ICU | 20/46 | OR: 1.08 (0.57–2.05) | |||
| Median length of admission (days) | 6/6 | OR: 1.17 (1.10–1.25) | |||
| Arshad/2020/USA | Hosp | 147/409 | Death | 33/108 | HR: 1.050 (0.682–1.616) |
| Ayerbe/2020/Spain | Hosp | 1223/796 | Death | 146/140 | OR: 0.53 (0.19–1.50), P = 0.233 |
| Guérin/2020/France | Comm | 34/34 | Time to clinical recovery (days) | 12.9/25.8 | (P = 0.0149) |
| Need for ICU | 8/1 | ||||
| Serious adverse effects | None reported in the AZM group | ||||
| Cardiovascular events | None reported in the AZM group | ||||
| Ip/2020/USA | Hosp | 256/2256 | Death | HR: 0.89 (0.72–1.10), P = 0.28 | |
| Length of admission | HR: 1.45 (0.88–2.41), P = 0.150 | ||||
| Kokturk/2021/Turkey | Hosp | 738/762 | Death | 34/33 | OR: 1.54 (0.48–4.98), P = 0.472 |
| Pathak/2021/USA | Hosp | Need for ICU. ICU death | No association between azithromycin and the outcomes | ||
| Rodriguez- Molinero/ 2020/Spain | Hosp | 120/63 | Death | 7/6 | P = 0.501 |
| Length of admission | HR: 1.45 (0.88–2.41), P = 0.150 | ||||
| Rosenberg/2020/USA | Hosp | 121/211 | Death | 21/28 | HR: 0.56 (0.26–1.21) |
| Cardiac arrest | 5/7 | HR: 0.64 (0.27–1.56) | |||
| Abnormal ECG | HR: 0.95 (0.47–1.94) | ||||
| Szente-Fonseca/ 2020/Brazil | Comm | 380/337 | Need for admissions (114) | OR: 0.93 (0.60–1.45) | |
| Wang/2020/USA | Hosp and comm | 535/4165 | Death | 124/168 | OR: 1.57 (1.14–2.16), P = 0.006 |
AZM, azithromycin; Hosp, hospital; Comm, community.
The dose of azithromycin was reported in 11 studies. In four of them, patients received 500 mg/day for 5 days.19–22 Patients were given 500 mg on the first day and then 250 mg/day the following 4 days in three studies.23–25 In two studies, patients were treated with 500 mg/day for 10 days.18,26 In one study, patients received 500 mg/day for 14 days27 and in another one 500 mg/day for 3 days.28 All studies compared the outcomes of patients treated with azithromycin against those for the ones that had only received standard care. In two studies, it was specified that patients did not received hydroxychloroquine, neither in the intervention arm, nor in the comparison one.30,33 Two studies reported that patients in both arms had received hydroxychloroquine and lopinavir/ritonavir.19,24 Finally, one study reported that patients in both arms had been treated with hydroxychloroquine and steroids.22
Death was the outcome in 13 studies, length of hospital admission in 6 studies, need for ICU care in 4 studies and need for admission in 2 studies. Other secondary outcomes were also reported in individual studies. Tables 1 and 2 present the outcomes reported in each study. All RCTs were considered of good quality. While one of them had a substantially smaller sample size, its results on mortality were consistent with the ones reported in larger studies.19 All observational studies were also considered to have good quality. In one of them, only available as an abstract, the quality was difficult to assess.29 However, while this study reported results consistent with the ones presented in the other papers, no numerical results were presented; therefore, it was not included in the meta-analysis (Tables S1 and S2, available as Supplementary data at JAC Online).
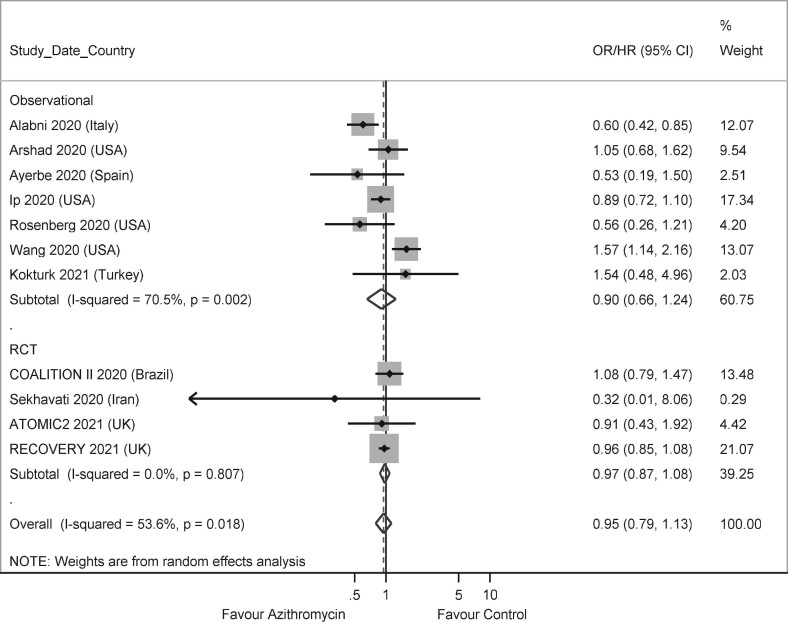
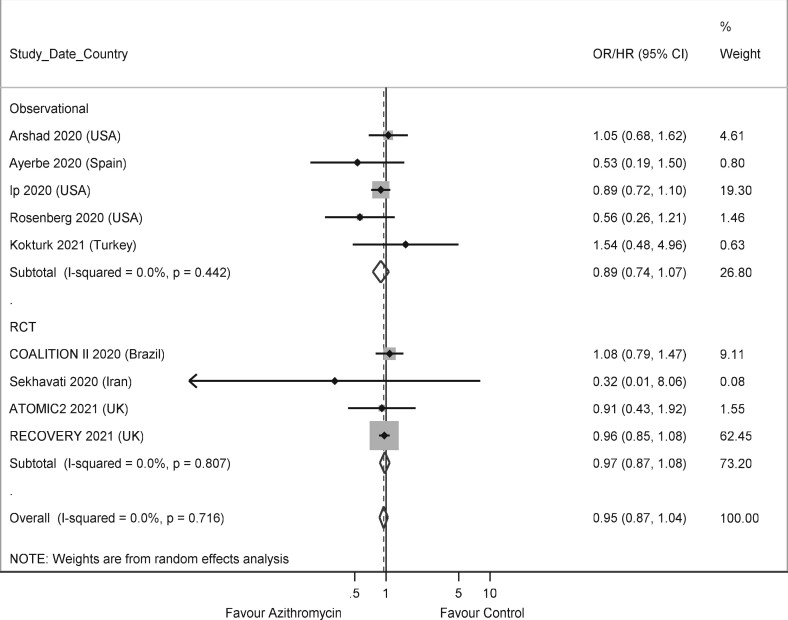
The meta-analysis showed no difference in death for those treated with or without azithromycin, in observational studies [OR: 0.90 (0.66–1.24)], RCTs [OR: 0.97 (0.87–1.08)] and when studies with both designs were pooled together [OR: 0.95 (0.79–1.13)] (Figure 2). In the study by Hinks and colleagues,27 the outcome was death or need for admission, and this was used as a proxy for death in the meta-analysis. Excluding this study had minimal effect on the magnitude and the significance of the results, with an overall OR 0.95 (0.79–1.14). Two further observational studies, which did not present a measure of effect and could not be included in the meta-analysis, reported narratively no evidence of association between treatment with azithromycin and death.24,29 Two observational studies contributed with large weight to the meta-analysis.20,33 Azithromycin was associated with reduced mortality in one of them,20 and with increased mortality in the other.33 When these two studies were removed from the meta-analysis, a lower heterogeneity was observed, but the pooled association between azithromycin and mortality remained not significant [OR: 0.95 (0.87–1.04)] (Figure 3).
Figure 2.
Forest plot of observational studies and RCTs on the association between treatment with azithromycin and death.
Figure 3.
Forest plot of observational studies and RCTs on the association between treatment with azithromycin and death (studies by Albani et al.20 and Wang et al.33 excluded).
Three observational studies and one RCT reported no association between the use of azithromycin and the length of hospital admission,20,24,26,31 while another RCT showed evidence of hospital admission shorter by 1 day for those who received azithromycin.19 The two observational studies and the two RCTs that had need for ICU as an outcome reported it not to be associated with azithromycin.18–20,29 One observational study and one RCT, conducted both in the community, reported no association between treatment with azithromycin and need for admission.22,28 Azithromycin was not associated with serious adverse events,18,26 QT prolongation,19,26,30 arrythmia,18,19,26 or need for resuscitation26,30 (Tables 1 and 2). The reasonably symmetrical funnel plot, and the contour-enhanced funnel plot at significance levels 1%, 5% and 10%, supports the hypothesis that there was no publication bias (Figures S1–S4).
Discussion
This systematic review and meta-analysis presents strong evidence on the lack of association between azithromycin and any clinical benefit in the management of COVID-19.34 This evidence is consistent across 16 studies conducted in Europe, America and Asia with diverse methodology and design. There is also no evidence of azithromycin being associated with any serious adverse events, including cardiovascular disease.
This systematic review has some limitations. Only one person extracted most of the data (L.A.). Even so, all data were checked for accuracy on repeated occasions and all analyses were conducted several times and checked by a senior statistician (S.A.). It is also possible that some publications may have been missed. The use of standard care, provided in addition to azithromycin in the intervention arm, and on its own in the comparison arm, was not described in some studies. It is not clear how differences in standard care across the studies may have affected the results. Even in studies where other drugs are mentioned, clinicians use numerous pharmacological, and not pharmacological, interventions that are not possible to account for, and this limitation affects all reviews of clinical studies. Some RCTs adjusted the treatment effect for confounders, but not all did so. In those adjusted however, there was no considerable difference between the unadjusted and adjusted estimates, likely due to the balanced characteristics of the compared groups, achieved by randomization.
The comprehensive search in six databases and critical assessment of 16 studies, which added together a large number of patients, represent the strengths of this systematic review. The inclusion of both observational and interventional studies, based in different settings and looking at various outcomes, are also positive aspects of this research. Furthermore, the sensitivity analyses that were conducted add consistency to the results. The use of a random-effects model was a conservative choice. The overall estimate remained significant despite the increased width of the confidence intervals, providing support to the significance of the findings.
The results presented in this systematic review do not support the use of azithromycin in the management of COVID-19. They also show no evidence of any harm caused to patients who received it, which would be consistent with the well-established safety profile reported before for azithromycin.4 Future research on repurposed or innovative treatment for patients with COVID-19 may need to consider alternative drugs.
Funding
This study was funded by a grant from the Claire Wand Fund. S.A. was funded by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Transparency declarations
None to declare.
Author contributions
L.A. had the original idea, which then received input from I.F., C.R.-R., M.P.-P. and S.A. L.A. conducted the searches. I.F. and M.P.-P. assessed the quality of the studies. L.A. and S.A. extracted the data. S.A. conducted the statistical analyses. L.A. wrote the first draft, which was later amended with contributions and references provided by I.F., C.R.-R., M.P.-P. and S.A.
Supplementary data
Tables S1 and S2 and Figures S1 to S4 are available as Supplementary data at JAC Online.
Supplementary Material
References
- 1. Sultana J, Cutroneo PM, Crisafulli S. et al. Azithromycin in COVID-19 patients: pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Saf 2020; 43: 691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gyselinck I, Janssens W, Verhamme P. et al. Rationale for azithromycin in COVID-19: an overview of existing evidence. BMJ Open Respir Res 2021; 8: e000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Connell TF, Bradley CJ, Abbas AE. et al. Hydroxychloroquine/azithromycin therapy and QT prolongation in hospitalized patients with COVID-19. JACC Clin Electrophysiol 2021; 7: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ. et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 2014; 143: 225–45. [DOI] [PubMed] [Google Scholar]
- 5. International Prospective Register of Systematic Reviews. (PROSPERO) https://www.crd.york.ac.uk/PROSPERO/.
- 6. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). http://www.prisma-statement.org/.
- 7. Scottish Intercollegiate Guidelines Network (SIGN). Methodology Assessment. https://www.sign.ac.uk/what-we-do/methodology/checklists/.
- 8. DerSimonian R, Kacker R.. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–14. [DOI] [PubMed] [Google Scholar]
- 9. Spruance SL, Reid JE, Grace M. et al. Hazard ratio in clinical trials. Antimicrob Agents Chemother 2004; 48: 2787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 11. Sterne JA, Gavaghan D, Egger M.. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000; 53: 1119–29. [DOI] [PubMed] [Google Scholar]
- 12. Fiolet T, Guihur A, Rebeaud M.. Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients: a systematic review and meta-analysis. Clin Microbiol Infect 2021; 27: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juul S, Nielsen EE, Feinberg J. et al. Interventions for treatment of COVID-19: a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS Med 2020; 17: e1003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim MS, An MH, Kim WJ. et al. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS Med 2020; 17: e1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lepere P, Escarguel B, Yolartiran S. et al. COVID-19: can early home treatment with Azithromycin alone or with Zinc help prevent hospitalisation, death, and long-COVID-19? A review. medRxiv 2021; 10.1101/2020.12.29.20248975. [DOI] [Google Scholar]
- 16. Parra-Lara LG, Martínez-Arboleda JJ, Rosso F.. Azithromycin and SARS-CoV-2 infection: where we are now and where we are going. J Glob Antimicrob Resist 2020; 22: 680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamel AM, Monem MSA, Sharaf NA. et al. Efficacy and safety of azithromycin in Covid-19 patients: a systematic review and meta-analysis of randomized clinical trials. Rev Med Virol 2021; 10.1002/rmv.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Group RC. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397: 605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sekhavati E, Jafari F, SeyedAlinaghi S. et al. Safety and effectiveness of azithromycin in patients with COVID-19: an open-label randomised trial. Int J Antimicrob Agents 2020; 56: 106143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albani F, Fusina F, Giovannini A. et al. Impact of azithromycin and/or hydroxychloroquine on hospital mortality in COVID-19. J Clin Med 2020; 9: 2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayerbe L, Risco C, Ayis S.. The association of treatment with hydroxychloroquine and hospital mortality in COVID-19 patients. Intern Emerg Med 2020; 15: 1501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Szente-Fonseca SN, de Queiroz-Sousa A, Giandoni-Wolkoff A. et al. Risk of hospitalization for COVID-19 outpatients treated with various drug regimens in Brazil: comparative analysis. Travel Med Infect Dis 2020; 38: 101906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arshad S, Kilgore P, Chaudhry ZS. et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis 2020; 97: 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodríguez-Molinero A, Pérez-López C, Gálvez-Barrón C. et al. Observational study of azithromycin in hospitalized patients with COVID-19. PLoS One 2020; 15: e0238681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guérin V, Lévy P, Thomas J-L. et al. Azithromycin and hydroxychloroquine accelerate recovery of outpatients with mild/moderate COVID-19. Asian J Med Health 2020; 18: 45. [Google Scholar]
- 26. Furtado RHM, Berwanger O, Fonseca HA. et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet 2020; 396: 959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hinks T, Cureton L, Knight R. et al. Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): an open-label, randomised trial. Lancet Respir Med 2021; 9: 1130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collaborative Group PT. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 2021; 397: 1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pathak V, Conklin C.. Predictors of ICU admission and mortality in patients with coronavirus disease - 2019 (COVID 19) in community hospitals. Am J Respir Crit Care Med 2021; 203: A2551. [Google Scholar]
- 30. Rosenberg E, Dufort E, Udo T. et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 2020; 23: 2493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ip A, Berry DA, Hansen E. et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-an observational study. PLoS One 2020; 15: e0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kokturk N, Babayigit C, Kul S. et al. The predictors of COVID-19 mortality in a nationwide cohort of Turkish patients. Respir Med 2021; 183: 106433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang AL, Zhong X, Hurd YL.. Comorbidity and sociodemographic determinants in COVID-19 mortality in an US Urban Healthcare System. medRxiv 2020; 10.1101/2020.06.11.20128926. [DOI] [Google Scholar]
- 34. Oxford Centre for Evidence Based Medicine Levels of Evidence Working Group. ‘The Oxford 2011. Levels of Evidence’. Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.