Abstract
Aims
Since its emergence in early 2020, the novel severe acute respiratory syndrome coronavirus 2 causing coronavirus disease 2019 (COVID-19) has reached pandemic levels, and there have been repeated outbreaks across the globe. The aim of this two-part series is to provide practical knowledge and guidance to aid clinicians in the diagnosis and management of cardiovascular disease (CVD) in association with COVID-19.
Methods and results
A narrative literature review of the available evidence has been performed, and the resulting information has been organized into two parts. The first, reported here, focuses on the epidemiology, pathophysiology, and diagnosis of cardiovascular (CV) conditions that may be manifest in patients with COVID-19. The second part, which will follow in a later edition of the journal, addresses the topics of care pathways, treatment, and follow-up of CV conditions in patients with COVID-19.
Conclusion
This comprehensive review is not a formal guideline but rather a document that provides a summary of current knowledge and guidance to practicing clinicians managing patients with CVD and COVID-19. The recommendations are mainly the result of observations and personal experience from healthcare providers. Therefore, the information provided here may be subject to change with increasing knowledge, evidence from prospective studies, and changes in the pandemic. Likewise, the guidance provided in the document should not interfere with recommendations provided by local and national healthcare authorities.
Keywords: ACE2, Arrhythmias, Biomarkers, Cardiogenic shock, COVID-19, Myocardial injury, Myocarditis, Non-invasive imaging
Graphical Abstract
Graphical Abstract.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) reached pandemic levels in March 2020 and has caused repeated waves of outbreaks across the globe. Coronavirus disease 2019 shares many manifestations of a systemic disease and has major implications for the cardiovascular (CV) system, which are summarized in a two-part review entitled European Society of Cardiology (ESC) guidance for the diagnosis and management of cardiovascular disease (CVD) during the COVID-19 pandemic.
The first part of the document addresses the topics of impact of CVD comorbidities on the epidemiology of COVID-19 together with the CV manifestations of COVID-19, the pathophysiology of COVID-19 as it concerns the CV system, strategies for diagnosing SARS-CoV-2, and approaches to diagnosing CVD among patients with COVID-19.
Approximately 1 year after SARS-CoV-2 first emerged, we are now better informed about the way in which the infection interacts with the CV system. This review updates our knowledge on the specific types of CVD that appear to be a consequence of severe infection, which include myocardial injury, arrhythmias, heart failure (HF), vascular dysfunction, and thromboembolic disease. Possible mechanisms of CV injury are described. The document provides an update on how SARS-CoV-2 infection is diagnosed, before providing a detailed account of how CV conditions should be diagnosed in patients with COVID-19, covering the clinical presentation, the electrocardiogram (ECG), the effect of disease on cardiac biomarkers, and imaging.
While the document is comprehensive, it is ‘not a guideline’ but rather ‘a guidance’ document. The recommendations are the result of observations and personal experience from healthcare providers. The present publication provides a summary of the guidance until February 2021. Therefore, the information provided here may be subject to change with increasing knowledge, evidence from prospective studies, and changes in the pandemic. Likewise, the guidance provided in the document should in no way interfere with recommendations provided by local and national health care authorities.
Epidemiology
Impact of cardiovascular comorbidities on COVID-19 outcomes
Key points.
CV comorbidities are common in patients with COVID-19.
Presence of CVD is associated with severe COVID-19 and higher mortality.
CV risk factors are linked with severe COVID-19 and higher mortality.
SARS-CoV-2 was first reported in Wuhan, China, on 31 December 2019. It is a new coronavirus strain that had not previously been identified in humans and causes the illness COVID-19. By 7 May 2020, 3.67 million had tested positive and >250 000 had died.1 By 22 March 2021, ∼124 million cases and 2.7 million deaths had been reported globally.2
After the start of the COVID-19 pandemic in Wuhan, China, the epicentre of the pandemic started to shift in March 2020 to Europe, Latin America, and the USA. Numerous countries took different policy measures in an attempt to reduce the spread and the pressure on the national healthcare systems such as lockdowns, curfews and closure of non-essential stores. These measures led to a flattening of the curve and ultimately to a decrease of detected cases between May and August.2 Many countries eased their policy measures over the summer. At the end of the summer, detected cases started to gradually increase in many countries, potentially due to the reopening of many activities that have been known to contribute to higher SARS-CoV-2 diffusion.2 This led to the so-called second wave in which many countries had to enforce similar or even stricter policy measures to reduce the burden of the COVID-19 pandemic.3 Currently, the peak of the second wave seems to be over in many European countries; however, policy measures remain strict and there are concerns that a third wave is commencing in some European countries, leading to new restrictions. Vaccination programmes are now under way across Europe, but rollout has been challenging owing to delays in accessing vaccine supplies. Situation reports of the COVID-19 pandemic are disseminated by the World Health Organization on their website.2
Multiple studies have demonstrated that comorbid CVD is linked to a more severe course and higher mortality of COVID-19.4–8 The meta-analysis by Figliozzi et al.5 showed that a history of CVD tripled the odds [odds ratio (OR) 3.15, 95% confidence interval (CI) 2.26–4.41] of the occurrence of a severe course of COVID-19, which was defined as death, severe COVID‐19 infection, hospitalization in intensive care unit (ICU), and/or use of mechanical ventilation or progression of the disease. Congestive HF was identified both as a risk factor for a more severe course and increased mortality and as a possible consequence of a COVID-19.6,8–10 Tomasoni et al.10 demonstrated that patients with a history of HF have significantly poorer outcomes with higher mortality and in-hospital complications. A meta-analysis of Ssentongo et al.7 concluded that the presence of HF was associated with a doubling of the odds of COVID-19 mortality (OR 2.03, 95% CI 1.28–3.21).
Next to CVD, CV risk factors are associated with higher risk for a more severe course and higher mortality.
Diabetes mellitus and COVID-19 are linked in numerous ways. COVID-19 seems to exacerbate the underlying pathophysiology of hyperglycaemia in people with diabetes.11 A whole-population study in England revealed that the ORs for in-hospital COVID-19-related death were 3.51 (95% CI 3.16–3.90) in people with type 1 diabetes and 2.03 (95% CI 1.97–2.09) in people with type 2 diabetes.11 Increased severity and higher mortality rates of COVID-19 are also observed in obese patients.5,7,12,13 A pooled analysis including 399 461 diagnosed patients concluded that obese individuals were more at risk of COVID-19 positivity (OR 1.46, 95% CI 1.30–1.65), for hospitalization (OR 2.13, 95% CI 1.74–2.60), ICU admission (OR 1.74, 95% CI 1.46–2.08) and for mortality (OR 1.48, 95%CI 1.22–1.80).13 Chronic kidney disease is another comorbidity that is associated with a more severe course of COVID-19.5,7,14,15 Lower estimated glomerular filtration rates were associated with greater hazard ratios for mortality from COVID-19.14,15 Hypertension is one of the most common comorbidities among COVID-19 patients.16–18 Multiple meta-analyses concluded that the presence of hypertension significantly increased the odds for a severe course of COVID-19 or mortality,4,5,7 though relations with obesity, substantially more prevalent in the presence of hypertension, should be further clarified.19,20
Ethnicity seems to be linked to susceptibility for, and outcomes from, COVID-19.21–25 Data from the UK show that one-third of patients admitted to an ICU due to COVID-19 were from an ethnic minority background such as South-Asians and blacks.21,24 Reports from the USA reveal the same message that ethnic minority groups such as African Americans have also been disproportionately affected by COVID-19.22
Case fatality is highest in older age groups. A meta-analysis including 611 583 patients highlighted the determinant effect of age on mortality.26 The highest mortality occurs in patients aged ≥80 years in whom mortality was 6 times higher than in younger patients.26 This underlines the fact that increasing age is the dominant risk factor for a severe course of COVID-19. Multinational cohort analyses will give more insights into the prevalence and risk of CV comorbidities in COVID-19. There are several potential mechanisms explaining why the course of the disease is more severe in patients with underlying CV risk factors and CVD. These are described in Section Pathophysiology—mechanism of disease in relation to the CV system of this manuscript and the Guidance Part 2.
Cardiovascular manifestations and clinical course of COVID-19
Key points.
COVID-19 has comparable cardiac manifestations to previous outbreaks of other coronaviruses.
Cardiac manifestations are associated with worse outcomes of COVID-19.
Long-term manifestations of COVID-19 are unclear, so extensive follow-up is needed.
Previous coronavirus outbreaks such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) were associated with a significant burden of CV morbidity.27,28 Common CV complications in SARS were hypotension, myocarditis, arrhythmias, and sudden cardiac death.29,30 Diagnostic workup during SARS infection revealed electrocardiographic changes, subclinical left ventricular (LV) diastolic impairment, and troponin elevation.31,32
COVID-19 seems to have comparable cardiac manifestations to previous outbreaks with other coronaviruses. Evidence of myocardial injury is frequent at the time of admission in hospitalized COVID-19 patients.17,33,34 Typically, patients with evidence of injury are older and have more CV comorbidities and risk factors. Elevated troponin levels are associated with a greater need for mechanical ventilatory support and higher in-hospital mortality.29,35 Numerous potential links exist between systemic viral infection and acute coronary ischaemic syndromes.36 There is some evidence that active COVID-19 increases the risk of acute myocardial infarction (MI) and ischaemic stroke.37 Plaque-destabilizing and supply–demand imbalance are mechanisms through which COVID-19 may precipitate acute coronary syndromes (ACSs).38 Multiple studies have shown that many patients with an ACS did not receive medical care and hospital admission during the first wave of the pandemic, probably due to the fear of acquiring COVID-19.39–43 Therefore, a proportion of the sudden and unexplained deaths at home in COVID 19-suspected patients may be explained by a type 3 MI.38 Multiple case reports showed the presence of acute myocarditis at the time of symptomatic COVID-19, but the precise incidence is still unclear.44–46 A prospective study in Germany performed cardiac magnetic resonance (CMR) imaging studies in 100 patients recovered from COVID-19. The studies demonstrated that 60% of patients had ongoing myocardial inflammation.47
Arrhythmias are a common manifestation of CVD in patients with COVID-19.38 Bradyarrhythmias specific to COVID-19 have not been described. Data on the frequency of malignant arrhythmias such as ventricular tachycardias (VTs) and atrial fibrillation (AF) in COVID-19 patients are still limited. Small clinical studies estimate the incidence of new-onset AF between 3.6% and 6.7% in patients with COVID-19.35,48,49 A Danish nationwide registry study published that the diagnosis of new-onset AF was 47% lower during the first 3 weeks of the national lockdown compared with the same period of previous year, perhaps for the same reasons that led to a reduction in hospital admissions for ACSs.50
Early studies from China demonstrated that HF decompensation was one of the most common complications of COVID-19.51 Heart failure and COVID-19 may be linked via direct viral infiltration, inflammation, or cardiac fibrosis.52 The increased metabolic demands of COVID-19 could also unmask subclinical HF or exacerbate pre-existing HF.52 Increased levels of serum B-type natriuretic peptide (BNP) were linked with significantly increased odds of mortality.53 A meta-analysis showed that HF was a complication in 11.5% of COVID-19 patients.54
Venous thromboembolic disease is increasingly recognized as a key contributor to the rapid deterioration of hospitalized patients with severe COVID-19.35,38,55,56 Multiple smaller studies reported an incidence of thromboembolic events ranging between 25% and 31%.57,58 A propensity-matched comparison between 150 patients with COVID-19 acute respiratory distress syndrome (ARDS) and 145 non-COVID-19 ARDS patients concluded that venous thromboembolic events, especially pulmonary thromboembolism, were significantly more prevalent in patients with COVID-19 ARDS.59 Those reports also demonstrated an association between inflammatory and prothrombotic markers and with venous thromboembolic disease and mortality.38,57–59
The presence of acute cardiac injury, vascular dysfunction, and thrombosis in patients with COVID-19 raises important questions about potential long-term CV effects. At this moment, it remains unclear if COVID-19 leads to persistent myocardial injury and/or if it is associated with increased long-term risk for developing coronary artery disease and HF.52 Extensive (cardiac) follow-up of COVID-19 patients is needed to mitigate the potential long-term adverse physical and mental health effects.60 Multi-ethnic long-ranging longitudinal observational studies will be critical to elucidate the duration and severity of health consequences attributable to COVID-19.60
Pathophysiology—mechanism of disease in relation to the cardiovascular system
Key points.
The pathobiology of coronavirus infection involves SARS-CoV-2 binding to the host angiotensin-converting enzyme 2 (ACE2) receptor to mediate entry into cells. ACE2 is expressed in the lungs, heart and vessels.
CVD associated with COVID-19 likely involves dysregulation of the ACE/ACE2 system due to SARS-CoV-2 infection and due to comorbidities, such as hypertension.
SARS-CoV-2 directly infects human cardiomyocytes (native and induced pluripotent stem cell-derived) in an ACE2- and cathepsin-dependent manner. These effects can be inhibited by the antiviral drug remdesivir.
CVD comorbidity in COVID-19 may be either primary or secondary due to acute lung injury, leading to increased cardiac workload (particularly relevant in HF).
Other molecules such as neuropilin-1 can facilitate SARS-CoV-2 cell entry and infectivity, although significance of this process for CVD is unclear.
A cytokine storm, originating from an imbalance of T-cell activation with dysregulated release of interleukin (IL)-6, IL-17, and other cytokines, may contribute to CVD in COVID-19. IL-6 targeting is being tested therapeutically.
Immune system activation along with immunometabolism alterations may result in plaque instability, contributing to the development of acute coronary events.
SARS-CoV-2 is a unique strain of novel enveloped, single-stranded ribonucleic acid (RNA) viruses. It has surface projections that correspond to surface spike proteins.61 The natural reservoir of SARS-CoV-2 seems to be the chrysanthemum bat,62 but the intermediate host remains unclear. SARS-CoV-2 is highly virulent and the transmission capacity is greater than the previous SARS virus (outbreak in 2003), with high abundance in infected people (up to a billion RNA copies/mL of sputum) and long-term stability on contaminated surfaces.63 While the infectivity of SARS-CoV-2 is greater than that of influenza or SARS-coronavirus, more data are needed for accurate assessment.64 Transmission occurs primarily by a combination of spread by droplet, and direct and indirect contact, and may possibly be airborne as well. The viral incubation period is 2–14 days (mostly 3–7 days).65 It is contagious during the latency period. SARS-CoV-2 can initially be detected 1–2 days prior to the onset of upper respiratory tract symptoms. Mild cases were found to have an early viral clearance, with 90% of these patients repeatedly testing negative on reverse transcriptase polymerase chain reaction (RT-PCR) by day 10 post-onset.66 By contrast, in severe cases, the median duration of viral shedding was 20 days (interquartile range: 17–24) in survivors.8 The longest observed duration of viral shedding in survivors was 83 days in the upper respiratory tract.67
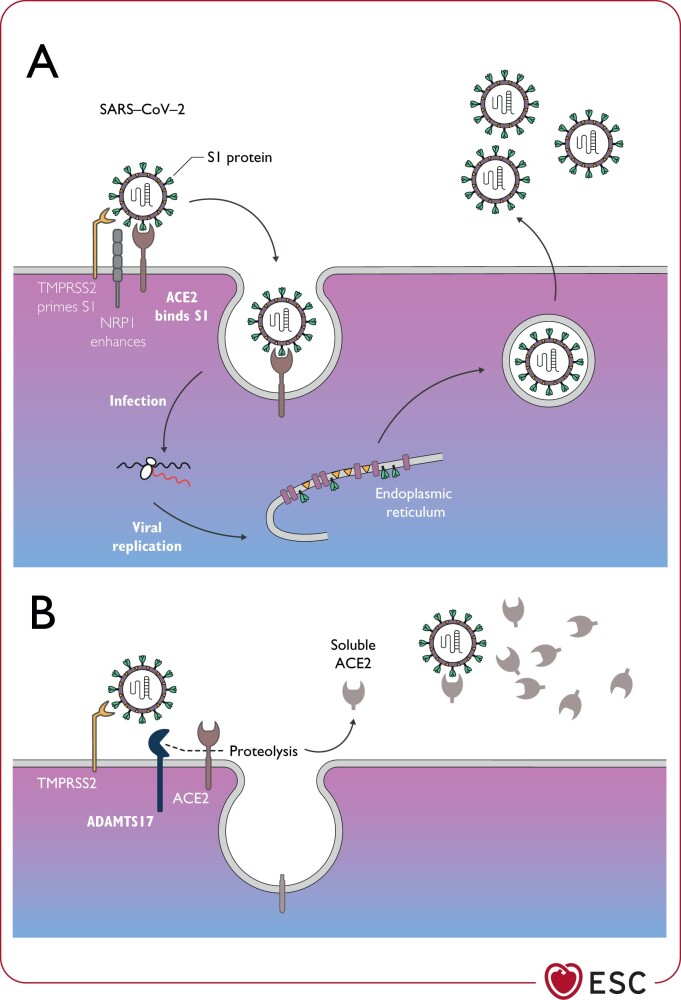
The host receptor through which SARS-CoV-2 enters cells to trigger infection is ACE2 (Figure 1).68,69 ACE2 is a multifunctional protein. Its primary physiological role is the enzymatic conversion of angiotensin (Ang) II to Ang-(1–7), and Ang I to Ang-(1–9), which are CV protective peptides.70 In the context of COVID-19, however, ACE2 is also involved in SARS through its function as the coronavirus receptor.71 Virus entry into lung alveolar epithelial cells is facilitated by high levels of expression of ACE2 in these cells, which lead to enhanced binding of the SARS-CoV-2 spike protein through processes involving cell surface associated transmembrane protein serine 2 (TMPRSS2)72 or proprotein convertase furin (Figure 1). Interestingly, the recently identified neuropilin 1 (NRP1) can greatly enhance infectivity when co-expressed with ACE2 and TMPRSS2. The role of NRP1 appears to be SARS-CoV-2 specific and has not been seen with SARS-CoV.73–75 Within the host cell cytoplasm, the viral genome RNA is released and replicates leading to newly formed genomic RNA, which is processed into virion-containing vesicles that fuse with the cell membrane to release the virus. SARS-CoV-2 is spread mainly through the respiratory tract by droplets, respiratory secretions and direct contact. The ACE/ACE2 seems to be disrupted by SARS-CoV-2 infection, which likely plays a pathogenic role in severe lung injury and respiratory failure in COVID-19.76 In addition to the lungs, ACE2 is highly expressed in the heart, blood vessels and gastrointestinal tract.77,78 Other receptors can also facilitate the entry of SARS-CoV-2.
Figure 1.
Critical role of ACE2 in the regulation of SARS-CoV-2 infection in ACE2-expressing cellsa (A) and ACE2 reduced surface expression by ADAMTS17 (B). (A) SARS-CoV-2 spike protein (S1) is primed by the serine protease transmembrane protein serine 2, which enables its interaction with the membrane bound form of ACE2. This is required for virus internalization and subsequent replication. Other receptors can also facilitate the entry of SARS-CoV-2, e.g. neuropilin 1. (B) Membrane bound ACE2 may be shed from the cell membrane by ADAMTS17 producing soluble ACE2. This mechanism may limit viral invasion. ACE2, angiotensin-converting enzyme 2; ADAMTS17, a disintegrin and metalloproteinase with thrombospondin motifs 17; NRP1, neuropilin-1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; S1, spike protein 1; TMPRSS2, transmembrane protease serine 2. aThis includes type 2 pneumocytes, cardiomyocytes, pericytes, endothelium, and possibly other cell types.
COVID-19 is primarily a respiratory disease, but—as noted in Sections Impact of cardiovascular comorbidities on COVID-19 outcomes and Cardiovascular manifestations and clinical course of COVID-19—many patients also have CVD, such as hypertension and obesity, acute myocardial injury and myocarditis (Figure 2).28,79,80 This may be secondary to the lung disease, since acute lung injury itself leads to increased cardiac workload and can be problematic especially in patients with pre-existing HF. CVD may also be a primary phenomenon considering the important (patho)physiological role of the renin–angiotensin system (RAS)/ACE2 in the CV system and the fact that ACE2 is expressed in human heart, vascular cells, and pericytes.81,82 In vitro studies have demonstrated that SARS-CoV-2 directly infects human-induced pluripotent stem cell-derived cardiomyocytes in an ACE2- and cathepsin-dependent manner. These effects can be inhibited by the antiviral drug remdesivir.83 It is important to note that while biologically able to inhibit cardiomyocyte effects in vitro, remdesivir does not improve overall mortality, initiation of ventilation, or duration of hospital stay.84 SARS-CoV-2-induced pathways within cardiomyocytes are related to viral response and interferon inflammatory signalling, apoptosis, and oxidative stress.
Figure 2.
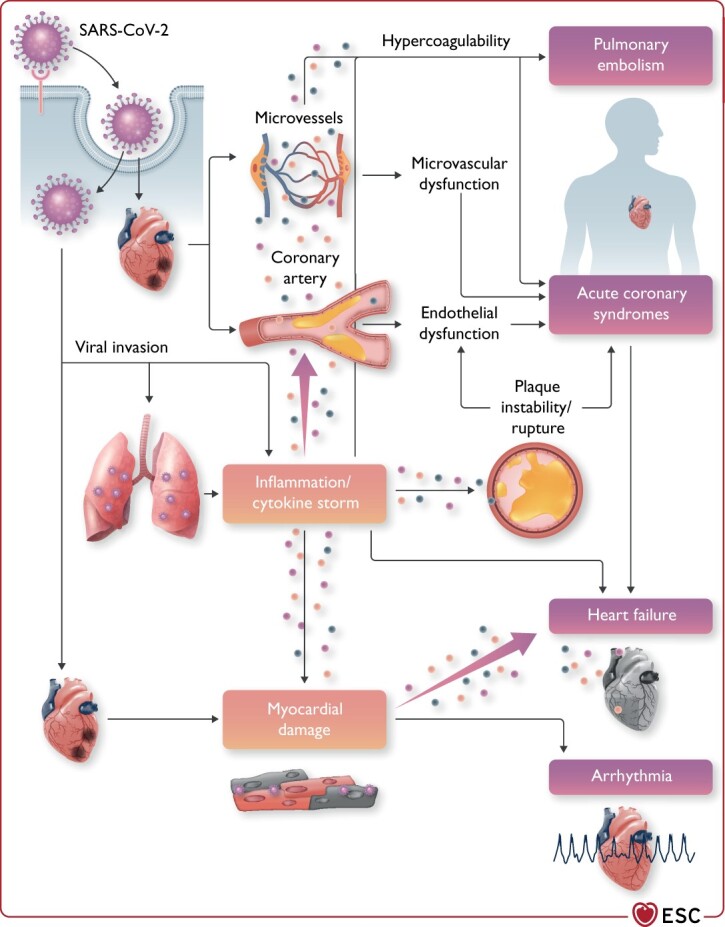
Cardiovascular involvement in COVID-19—key manifestations and hypothetical mechanisms. SARS-CoV-2 anchors on transmembrane angiotensin-converting enzyme 2 to enter the host cells including type 2 pneumocytes, macrophages, endothelial cells, pericytes, and cardiac myocytes, leading to inflammation and multiorgan failure. In particular, the infection of endothelial cells or pericytes could lead to severe microvascular and macrovascular dysfunction. Furthermore, in conjunction with the immune over-reactivity, it can potentially destabilize atherosclerotic plaques and explain the development of the acute coronary syndrome. Infection of the respiratory tract, particularly of type 2 pneumocytes, by severe acute respiratory syndrome coronavirus 2 is manifested by the progression of systemic inflammation and immune cell overactivation, leading to a ‘cytokine storm’, which results in an elevated level of cytokines such as IL-6, IL-7, IL-22, and CXCL10. Subsequently, it is possible that activated T cells and macrophages may infiltrate infected myocardium, resulting in the development of fulminant myocarditis and severe cardiac damage. This process could be further intensified by the cytokine storm. Similarly, the viral invasion could cause cardiac myocyte damage directly leading to myocardial dysfunction and contribute to the development of arrhythmia. CXCL10, C-X-C motif chemokine ligand 10; IL-6, interleukin 6; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Relationships between hypertension, ACE2, and COVID-19
As noted above, the prevalence of pre-existing hypertension seems to be higher in COVID-19 patients who develop severe disease vs. those who do not.8,85 This seems to also be true for ARDS or death. The mechanisms underlying potential relationships between hypertension and COVID-19 are thought most likely to relate to confounding due to age and associated comorbidities, namely obesity.86,87 The earlier studies were not age adjusted, but when age was accounted for in more recent studies, hypertension was associated with a higher risk up to the age of 70 and a lower risk in patients older than 70 years.14 The reasons for the inverse relationship in older patients are unclear but might be related to the decline of prevalence of obesity in the older age-stratum and reverse causality, i.e. that weight loss or underweight in older people is often associated with significant underlying disease.88 Previous speculation suggested that treatment of hypertension with RAS inhibitors may influence SARS-CoV-2 binding to ACE2, promoting disease.89 The extremely frequent cluster of hypertension with obesity suggests that obesity, namely central obesity, may provide a reservoir for viral replication, immune activation, and cytokine amplification, leading to more severe COVID-19 disease.20,90,91 This is based on some experimental findings that RAS inhibitors cause a compensatory increase in tissue levels of ACE292–94 and that angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blockers (ARBs) may be detrimental in patients exposed to SARS-CoV-2.95 It is, however, important to emphasize that there is no clear evidence that using ACEIs or ARBs leads to the up-regulation of ACE2 in human tissues. The available data from blood samples suggest that there is no association between circulating levels of ACE2 and use of renin–angiotensin–aldosterone system antagonists.96 Similar findings have been identified in human kidneys, where increased ACE2 expression was associated with age but not with hypertension, or antihypertensive therapies.97 It also appears that in experimental models ARBs may have a potentially protective influence.98,99 In a recent study performed on a large number of human kidneys, neither hypertension nor antihypertensive treatment altered the expression of the key entry receptor for SARS-CoV-2 showing moreover that ACE2 is most likely nephro-protective but exhibits an age-related increase.97 A recent population-based case–control study in the Lombardy region of Italy of 6272 SARS-CoV-2-infected patients showed no evidence that ACEI or ARBs affected the risk of COVID-19,100 while a Wuhan study demonstrated that in 1128 hospitalized patients use of ACEI/ARB was also not associated with a higher risk of COVID-19 or serious complication or deaths from COVID-19.85,100–105 Very recently, a retrospective analysis conducted in 1584 hospitalized patients with COVID-19 found that discontinuation of ACEI, ARBs, or β-blocker was associated with an increased risk of dying, whereas discontinuation of calcium channel blockers and diuretics was not.91 This supports prior guidance from major CV Societies that stated that patients on ACEIs or ARBs should not stop their treatment.45,96
Acute cardiac injury and myocarditis in COVID-19
Evidence of acute cardiac injury with raised troponin levels appears in COVID-19 patients several days after initiation of fever indicating myocardial damage associated with viral infection. Mechanisms of SARS-CoV-2-induced myocardial injury remain elusive106 but may relate, in part, to direct SARS-CoV-2 effects on cardiac myocytes,83 or through up-regulation of ACE2 in the heart and coronary vessels.45,81 Respiratory failure and hypoxia in COVID-19 may also cause damage to the myocardium and immune mechanisms of myocardial inflammation may be especially important.45,79,107 For example, cardiac injury leads to activation of the innate immune response with the release of proinflammatory cytokines, as well as to the activation of adaptive auto-immune type mechanisms through molecular mimicry.
While initial studies suggested that myocarditis may occur early during COVID-19, more recent studies have been less convincing in showing an association between myocarditis and SARS-CoV-2 infection. A definitive diagnosis of myocarditis should be based on endomyocardial biopsies or autopsy using established histologic and immunohistochemical criteria.108 While the presence of virus has been demonstrated in the heart from patients who died from COVID-19,109 the endomyocardial biopsy criteria for myocarditis, with the classic type of acute lymphocytic myocarditis or lymphocytic inflammatory cardiomyopathy, have yet to be convincingly demonstrated. Thus, myocarditis seems to be an uncommon complication in the course of SARS-CoV-2 infection.110
Immune system dysregulation and cardiovascular disease in COVID-19
Inflammatory mechanisms and activation of immune responses underlie a large range of CVDs including atherosclerosis, HF, and hypertension.111,112 Inflammatory responses in COVID-19 may mediate at least some of the key aspects of CV comorbidity.113,114 First, another receptor through which SARS-CoV-2 may enter cells is cluster of differentiation 209.115 CD209 is expressed in macrophages promoting virus invasion into immune cells in cardiac and vascular tissues. More importantly, in severe cases of COVID-19, systemic increases of numerous cytokines including IL-6 IL-2, IL-7, granulocyte colony-stimulating factor, C-X-C motif chemokine ligand 10, chemokine (C-C motif) ligand 2, and tumour necrosis factor alpha have all been observed in subjects with COVID-19,116 which corresponds to the characteristics of a cytokine release syndrome (CRS).
Altered vascular permeability can result in non-cardiogenic pulmonary oedema and promotes ARDS as well as multiorgan dysfunction. High serum IL-6 levels are a common feature in CRS. IL-6 is a clinical predictor of mortality in COVID-19.117 Thus, IL-6 targeting may be permissive for use in COVID-19 to tackle the CRS. Finally, it has been shown that hypertension is associated with circulating lymphocytes in patients118 and CD8 T-cell dysfunction with development of CVD.119 Obesity is thought to play a key role in the amplification of the inflammatory response and in the immune dysregulation.120 CD8 T cells are a pillar of antiviral immunity; thus, their dysfunction can make the organism inefficiently target virally infected cells.
Strategies for diagnosing SARS-CoV-2
Key points.
Diagnosis of COVID-19 relies on a combination of epidemiological criteria (contact within incubation period), presence of clinical symptoms as well as laboratory testing [nucleic acid amplification tests (NAATs)] and clinical imaging-based tests.
Nucleic acid amplification tests are key diagnostic tests used worldwide.
Quality of sample collection (deep nasal swab) and transport (time) to laboratories are essential to avoid false-negative outcomes of nucleic acid testing.
Widespread testing proved efficient in the containment phase of the epidemic.
Testing should be performed as soon as possible in all symptomatic individuals and contacts of people testing positive to enable efficient isolation.
Anti-SARS-CoV-2 IgM and IgG antibody and SARS-CoV-2 antigen-based enzyme-linked immunosorbent assay tests are now widely used but require further development.
Rapid antigen tests can contribute to overall COVID-19 testing capacity but their sensitivity for is generally lower than for RT-PCR and can be performed best in cases with high viral load.
Lung computed tomography (CT) imaging may be used as a diagnostic test in COVID-19.
As evidenced by previous epidemics, including SARS and MERS, highly sensitive and specific laboratory diagnostics are essential for case identification, contact tracing, animal source finding, and efficient and rational containment measures.121 Precise case identification is essential to isolate vulnerable individuals. Based on the current epidemiological analysis, a history of CVD conveys risk of a more severe outcome of COVID-19;28,79 therefore, testing should be particularly considered in CVD patients. Moreover, similar to influenza, efficient testing of carers and people in contact with high-risk patients may allow the protection of subjects with multiple comorbidities. The decision to test should be based on clinical and epidemiological factors and linked to an assessment of the likelihood of infection, in particular when availability of tests is limited. All subjects with COVID-19 symptoms should be tested immediately after symptom onset to identify and isolate people testing positive. Efficient testing also allows for timely contact tracing. In immediate contacts, testing should be considered irrespective of symptoms.122
European Centre for Disease Prevention and Control also recommends that all healthcare staff and patients are comprehensively and periodically tested and patients admitted for routine CV procedures and hospitalizations should be tested prior to (or during) hospital admission.122 Available testing strategies are outlined below (Table 1).
Table 1.
Types of diagnostic approaches in COVID-19
| Test | Mechanism of detection | Testing material | Availability for POC | Positive test indicates | Use of tests |
|---|---|---|---|---|---|
| Nucleic acid amplification tests | RT-PCR and NGS detection of genetic sequences of conserved regions for regions of the virus, e.g. N, E, S, and RdRP genes. Two independent sequences need to be detected | Ambulatory: nasopharyngeal swabs, sputum In hospital: sputum, endotracheal aspirate, BAL blood, faeces | Under development—SAMBA II SARS-CoV-2 rapid test | Confirms current SARS-CoV-2 infection | Individual testing |
| Antibody based immunoassaya | ELISA detecting IgM or IgG anti-SARS-CoV-2 antibodies | Serum | Yes (depending on test design) | IgM+: 3–5 days post-onset IgG: past infection | Overall infection/immunity rates in a community |
| Antigen-based immunoassaya | ELISA detecting viral proteins, e.g. S (spike protein) or N protein (nucleocapsid) | Nasopharyngeal swabs, sputum and other lower respiratory tract secretions, BAL blood, faeces | Yes (depending on test design) | Confirms current SARS-CoV-2 infection | Individual testing |
| Clinical tests | Clinical symptoms (fever/cough) Epidemiological history Imaging (CT) | CT—detection of radiological features | Yes | Infection possible | Triage to identify candidates for further testing |
BAL, bronchoalveolar lavage; CT, computed tomography; ELISA, antigen-based enzyme-linked immunosorbent assay; NGS, next-generation sequencing; POC, point of care; RT-PCR, reverse transcriptase polymerase chain reaction; SAMBA, simple amplification-based assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Still in experimental phase, now available for research.
While isolation of the virus itself using electron microscopy would be the most specific diagnostic, it requires biosafety level-3 facilities, which are not available in most healthcare institutions. Serum antibody and antigen detection tests would be the easiest and fastest but have not yet been validated, and there may be cross-reactivity with other coronaviruses, especially SARS coronavirus. Furthermore, antibodies are not measurable in the initial phase of the infection. Therefore, real-time PCR remains the most useful laboratory diagnostic test for COVID-19 worldwide.123,124
Comparative specificity and sensitivity of these tests needs to be carefully assessed, when more data are available. It is important to note that negative results of molecular testing (RT-PCR) do not preclude SARS-CoV-2 infection (1–30% false-negative results were reported)125,126 and should not be used as the sole basis for patient management decisions but must be combined with clinical observations, patient history, and epidemiological information. There are a number of factors that may lead to a negative result in an infected individual. These include poor quality of the specimen (small material), collection late or very early in the infection, poor handling/shipping, as well as technical reasons inherent in the test such as virus mutation or PCR inhibition. Therefore, retesting is recommended after 48 h in clinically suspected cases that test negative.
It is essential that adequate standard operating procedures are in use and that staff are trained for appropriate specimen collection, storage, packaging, and transport. This must be observed in order for testing to be reliable and safe for staff and patients.
The optimal testing material includes nasal swabs rather than pharyngeal. To obtain a sufficiently deep swab, the sample must be obtained by experienced and trained staff. According to a comparative study using lung CT as comparator, the sensitivity of nasopharyngeal swab may be limited to 60–70%.127 It has also been concluded that the test does not seem to change clinical decisions and diagnostic considerations in subjects with pre-test probability exceeding 60–70% (e.g. subjects with positive epidemiological and clinical criteria fulfilled). This, however, does not indicate that such tests should not be performed to confirm infection, but it is important that the test is repeated if there is clinical suspicion of SARS-CoV-2 infection. Lung CT has a high sensitivity for diagnosis of COVID-19 in hospitalized patients who are RT-PCR positive. In a study undertaken between 6 January and 6 February 2020 in Tongji Hospital, Wuhan, China, in a population of 1014 patients, when using RT-PCR as a reference, the sensitivity of lung CT imaging for COVID-19 was 97%.127 Importantly, 60–93% of patients had initial positive lung CT consistent with COVID-19 before the initial positive RT-PCR results.
Nucleic acid shedding is also an important tool to verify patient improvement, although 42% of patients showed improvement of follow-up lung CT scans before the RT-PCR results turning negative.127 It is important, however, that nucleic acid shedding does not always indicate presence of live virus.
Data on antigen tests for SARS-CoV-2 are dynamically developing, and they are typically less sensitive than NAATs. However, due to their rapid point-of-care nature they can contribute to overall COVID-19 testing capacity.128
Detection of IgM or IgG antibodies to SARS-CoV-2 in the blood may be useful to identify people who previously had SARS-CoV-2 infection as well as patients with current infection who have had symptoms for 3–4 weeks. Their use in acute settings is limited by the latency of antibody development during infection.
Widespread testing strategies included drive-through testing. However, testing capacity may be insufficient. Thus, testing priorities have been suggested by individual health systems such as one proposed by Centers for Disease Control for the USA.129 Sample pooling strategy has been proposed in relation to sample collection as the most cost-efficient tool for population-wide screening, for example, at airports.
SARS-CoV-2 testing during influenza season:
European Centre for Disease Prevention and Control recommends that during the influenza season, all patients with acute respiratory symptoms in hospitals and within other healthcare settings, and all specimens from sentinel primary care surveillance, should be tested for SARS-CoV-2 and seasonal influenza in parallel, to monitor incidence and trends over time.122
Diagnosis of cardiovascular conditions in COVID-19 patients
Clinical presentation
Chest pain
Key points.
Chest pain and breathlessness are frequent symptoms in COVID-19.
Chronic and ACS presentations can be associated with respiratory symptoms.
Chest pain or tightness is common in patients with active COVID-19. It is usually poorly localized and associated with breathlessness due to the underlying pneumonia. Associated profound hypoxaemia together with tachycardia may result in chest pain and electrocardiographic changes suggestive of myocardial ischaemia. When biomarkers are elevated in conjunction with ECG changes, type 2 MI may be suggested. Patients with ACS do, however, experience typical symptoms related to ischaemia when they have COVID-19. The presence of a COVID-19 can make the differential diagnosis more difficult, as shortness of breath and respiratory symptoms may be present and may precede or precipitate cardiac signs and symptoms.
Dyspnoea, cough, and respiratory distress
Key points.
COVID-19 patients may present with cough, dyspnoea, and ARDS.
Dyspnoea
Dyspnoea (shortness of breath) is one of the typical symptoms in COVID-19. Of 1099 adult inpatients and outpatients in China, 19% presented with dyspnoea.130 With increasing disease severity, the proportion of patients presenting with dyspnoea significantly increases (31–55% in hospitalized patients and up to 92% of patients admitted to ICUs).35,116,131,132
Cough
Cough is present in 59.4–81.1% of patients with COVID-19, irrespective of disease severity.8,18 Unproductive (dry) cough is more frequent, whereas sputum production is present in 23.0–33.7%.8,116,130,132
Acute respiratory distress syndrome
Acute respiratory distress syndrome is an acute diffuse inflammatory lung injury, leading to increased pulmonary vascular permeability, increased lung weight, and loss of aerated lung tissue. It is characterized by bilateral opacifications on chest imaging (e.g. bilateral ground glass opacifications on CT) and hypoxaemia that cannot be explained by other causes.133 Among 1099 adult inpatients and outpatients in China, ARDS occurred in 3.4%,130 but in hospitalized patients, the rates are significantly higher (19.6–41.8%).8,18,132 The median time from disease onset to ARDS is 8–12.5 days.116 The risk of ARDS increases with older age (≥65 years old), presence of comorbidities (hypertension, diabetes, obesity), neutrophilia, lymphocytopenia, elevated laboratory markers of organ dysfunction [e.g. lactate dehydrogenase, inflammation (C reactive protein), and D-dimer].18 Mortality of patients treated for ARDS in COVID-19 is high (i.e. ∼50%).8,18,116,117,130,132,133
Cardiogenic shock
Key points.
In COVID-19 patients with impaired end-organ perfusion at the risk of cardiogenic shock (CS) [e.g. large acute myocardial infarction (AMI)], sepsis or mixed shock should also be considered as a possible aetiology.
Myocarditis and hyperinflammatory syndrome should be considered as precipitating causes of CS.
An early, accurate, and rapid diagnosis of CS in patients with confirmed or suspected COVID-19 is essential.134 The exact incidence of CS in these patients is unknown, but observations of cardiogenic and mixed shock in patients with COVID are accumulating in patients both with and without AMI.135–138 The median duration between onset of symptoms and admission to ICU in critically ill COVID-19 patients has been 9–10 days, suggesting a gradual respiratory deterioration in most patients.139 A simple, actionable classification scheme for CS diagnosis has recently been proposed.140
In critically ill COVID-19 patients at risk for CS (such as those with large AMI, acute decompensated HF; Society for Cardiovascular Angiography and Interventions stage A)103 and sepsis, a mixed aetiology of CS and septic shock should be considered in addition to the sole cardiogenic component. Parameters allowing for a differential diagnosis between CS and septic shock, such as the presence of vasodilatation and central venous oxygen saturation values, may be assessed. In addition, echocardiography may be helpful for differentiation between CS and septic shock as well in the evaluation of CS cause. In selected cases, such as in patients with unclear reasons for haemodynamic deterioration, invasive haemodynamic monitoring via a pulmonary artery catheter may provide useful information.141
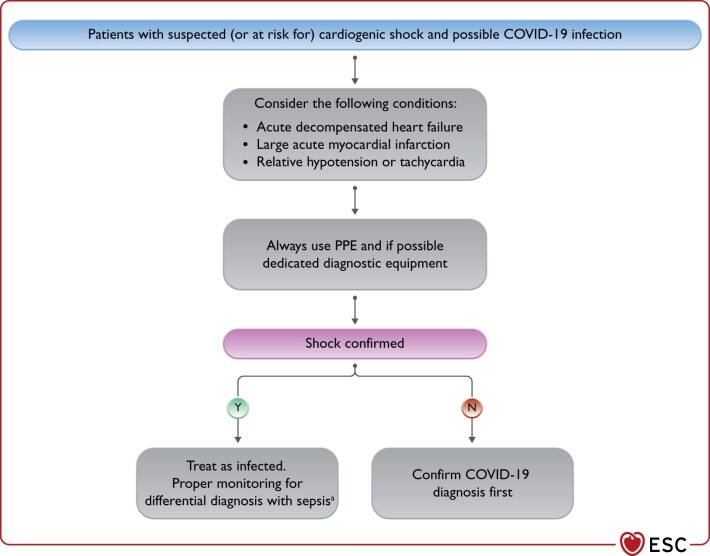
The diagnostic workup of critically ill patients with confirmed or suspected COVID-19 requires specific considerations (Figure 3):
Figure 3.
Considerations in patients with suspected (or at risk for) cardiogenic shock and possible COVID-19 infection. COVID-19, coronavirus disease 2019; HF, heart failure; MI, myocardial infarction; PPE, personal protective equipment. aConsider also myocarditis as potential cause.
The proper level and type of monitoring, in addition to the haemodynamic status of the patient, depend upon available local resources. Importantly, key diagnostic testing in patients with suspected CS, including ECG, bedside echocardiography, and urgent/emergent coronary angiography, should be integrated into local diagnostic protocols (with dedicated and/or protected equipment whenever possible) to ensure both the best deliverable care and a minimal risk of viral transmission to other patients and healthcare providers
Accumulating clinical experience79,142,143 and experimental evidence showing that >7.5% myocardial cells have positive ACE2 receptor expression,78 the target through which SARS-CoV-2 invades human cells indicates that myocarditis complicates COVID-19. This diagnosis should be routinely considered as a potential cause of CS. Importantly, hyperinflammatory syndrome has been associated with CS in patients with biventricular failure.144
Out-of-hospital cardiac arrest, pulseless electric activity, sudden cardiac death, tachyarrhythmias, and bradyarrhythmias
Key points.
Growing evidence worldwide shows a major decrease in the diagnosis and management of cardiac arrhythmias during the current pandemic.
Symptoms of brady- and tachyarrhythmias do not differ from the usual clinical presentation; however, given the overlap with some of the COVID-19 clinical manifestations, both the general public and healthcare professional (HCP) should remain alert for signs and symptoms of cardiac arrhythmias.
There has been an increase in out-of-hospital cardiac arrest (OHCA) in correlation to the COVID-19 pandemic and a worsening in its short-term outcome.
In-hospital cardiac arrest in COVID-19 patients is mainly secondary to pulseless electrical activity (PEA) and/or asystole. Shockable rhythms are only present in a minority of cases.
The occurrence of arrhythmias in stable COVID-19 patients appears to be low. Conversely, arrhythmia incidence appears to be higher in critically ill patients and in patients with elevated markers of myocardial injury.
Multiple reports now support the concern of a drastic reduction in the diagnosis and management of cardiac arrhythmias in relation to the first wave of the COVID-19 pandemic.50,145–150 The true prevalence and nature of arrhythmias in patients with COVID-19 remains unknown, as the available publications report variable numbers and few distinguish between the different types.35 The main observations of the published cohorts include:
A significant increase in OHCA has been described in Italy and France, together with a deterioration of the short-term outcome151–153
Among clinically stable patients, the prevalence appears to be low (9%), according to a single-day assessment in 132 hospitalized patients in Italy, mainly AF, with no patient presenting ventricular arrhythmias.154 On the contrary, the prevalence of cardiac arrhythmias appears to be significantly higher in critically ill patients and in patients with increased markers of myocardial injury.29,132,155–158
In a cohort of 700 hospitalized patients in Pennsylvania (11% in the ICU), 9 experienced cardiac arrest (6 PEA, 2 asystole and 1 torsade de pointes), 25 incident AF and 9 clinically significant bradyarrhythmias.49
In another cohort of 1053 hospitalized patients in New York City, arrhythmias were reported in 25.6% of patients: AF in 15.8% (9.6% incident AF), frequent premature ventricular contractions in 13%, ventricular arrhythmias in 2.6% [1.2% sustained VT, 0.9% polymorphic VT and 0.8% ventricular fibrillation (VF)] and advanced atrioventricular (AV) block in 0.4%.157 The presence of atrial arrhythmias was associated with higher levels of inflammation markers and higher 30-day mortality.159
In Italy, a multicentre retrospective analysis of 414 hospitalized patients revealed that the most frequent arrhythmia was incident AF (17.1%; 12.1% was new onset). Other supraventricular arrhythmias occurred in 1.2%. Ventricular tachycardia (no clear definition) was present in 3.4%.
In an early study, VT/VF was reported as a complication of COVID-19 in 11 of 187 patients (5.9%), with a significantly higher incidence in patients with elevated troponin T.29 However, more recent and larger publications report a lower incidence of sustained ventricular arrhythmias in hospitalized patients.49,157,160,161
Studies assessing the rhythms of critically ill COVID-19 patients with in-hospital cardiac arrest describe a majority of PEA or asystole (74–96%) and an incidence of ventricular arrhythmias between 4% and 13%.162–165 Outcomes of in-hospital cardiac arrest appear to be poor, particularly among older patients.162
Syncope and presyncope have been reported in 3.7% of patients admitted for COVID-19.166 Overall, bradyarrhythmia seems infrequent, with isolated cases of advanced AV block, particularly in patients with more advanced forms of the disease.167–170
The clinical presentation of brady- or tachyarrhythmias in the context of COVID-19 does not differ from those previously described (i.e. palpitations, dyspnoea, dizziness, chest pain, syncope). Most arrhythmias in COVID-19 can be diagnosed by a combination of clinical symptoms and signs (particularly, heart rate measurement) and the review of the ECG in case of symptoms. However, paroxysmal tachy- or bradyarrhythmias may be elusive. In these cases, longer-duration or continuous ECG monitoring may be considered.
If resources are available, a baseline ECG is recommended in any patient admitted with COVID-19, particularly those with severe manifestations or in whom QTc-prolonging drugs will be used. Preferably, this should be done using a 12-lead recording to allow adequate QTc measurements and identification of wavelet morphology,171 but single- or multi-lead ECG from telemetry or hand-held devices may suffice to minimize the HCP exposure.
Hospitalization for pneumonia and time course of increased subsequent risk of cardiovascular death
Key points.
Pneumonia, influenza, and SARS are associated with a markedly increased short-term risk for subsequent CV events.
Alertness for CV events, such as ACS, stroke, and venous thromboembolic events, in the short term after pneumonia and a careful risk management approach in individuals with pre-existing CVD is needed.
Pneumonia and severe influenza infections have been associated with a markedly increased short-term risk of MI and subsequent mortality that is more common among patients at older age, nursing home residents, and patients with histories of HF, coronary disease, hypertension, or obesity.20,172–175 Moreover, an increased rate of venous thromboembolic events has been observed in the context of COVID-19176 and several randomized trials evaluating earlier therapeutic anticoagulation treatment and prolonged thromboprophylaxis in the context of COVID-19 are currently underway.
Furthermore, for influenza epidemics, it has been demonstrated that there is a consistent rise in autopsy-confirmed coronary deaths.177 Fatal AMIs and pulmonary embolism (PE) have been observed in the short term after coronavirus-associated SARS, and a high alertness is required.178
Notably, recent data suggest that myocardial injury during COVID-19—as indicated by elevated troponin levels—represents one predictor of a higher risk of CV complications and an adverse clinical outcome.29,35,179
Electrocardiogram
Key points.
The same ECG diagnostic criteria for cardiac conditions apply in patients affected by SARS-CoV-2 infection and in the general population.
So far, no specific ECG changes have been described in patients with SARS-CoV-2 infection and non-specific ST-T abnormalities are observed in up to 30% of patients with SARS-CoV-2 pneumonia.48 Although we assume that the overall minimal level of myocardial injury associated with the infection (see the following section on biomarkers) does not translate into characteristic ECG manifestations in the majority of patients, a systematic review on COVID-19 myocarditis described ST-segment abnormalities (mostly specific) in 71% of patients.180 For considerations of arrhythmia and corrected QT interval (QTc) prolongation of COVID-19 therapies, see Guidance Part 2.
Key points.
Cardiomyocyte injury, as quantified by cardiac troponin T/I concentrations, and haemodynamic stress, as quantified by BNP and N-terminal B-type natriuretic peptide (NT-proBNP) concentrations, may occur in COVID-19 as in other pneumonias. The level of those biomarkers correlates with disease severity and mortality.
Cardiac troponin T/I and BNP/NT-proBNP concentrations should be interpreted as quantitative variables.
In patients hospitalized with COVID-19, mild elevations in cardiac troponin T/I and/or BNP/NT-proBNP concentrations are in general the result of pre-existing cardiac disease and/or the acute injury/stress related to COVID-19.
In the absence of typical angina chest pain and/or ischaemic ECG changes, patients with mild elevations [e.g. <2–3 times the upper limit of normal (ULN)] do not require workup and/or treatment for type 1 myocardial infarction (T1MI).
In patients with COVID-19, as in patients with other pneumonias, it is suggested to measure cardiac troponin T/I concentrations if the diagnosis of T1MI is being considered on clinical grounds, or in new-onset LV dysfunction. Independently from diagnosis, monitoring of cardiac troponin T/I may potentially help for the purpose of prognostication and risk stratification.
D-dimer quantifies activated coagulation, a prominent feature in COVID-19. Due to the role of endotheliitis and venous thromboembolism (VTE) in COVID-19, serial measurements of D-dimer may possibly help physicians in the selection of patients for VTE imaging and/or the possible use of higher than prophylactic doses of anticoagulation. The same ECG diagnostic criteria for cardiac conditions apply in patients affected by the SARS-CoV-2 infection and in the general population.
Biomarkers
Biomarker elevation suggesting cardiovascular conditions in patients with COVID-19
Cardiac troponin T/I
COVID-19 is a viral pneumonia that may result in severe systemic inflammation and ARDS, and both conditions have profound effects on the heart.8,179,181 As a quantitative marker of cardiomyocyte injury, the concentrations of cardiac troponin T/I in a patient with COVID-19 should be seen as the combination of the presence/extent of pre-existing cardiac disease and/or the acute injury related to COVID-19.8,117,181–186
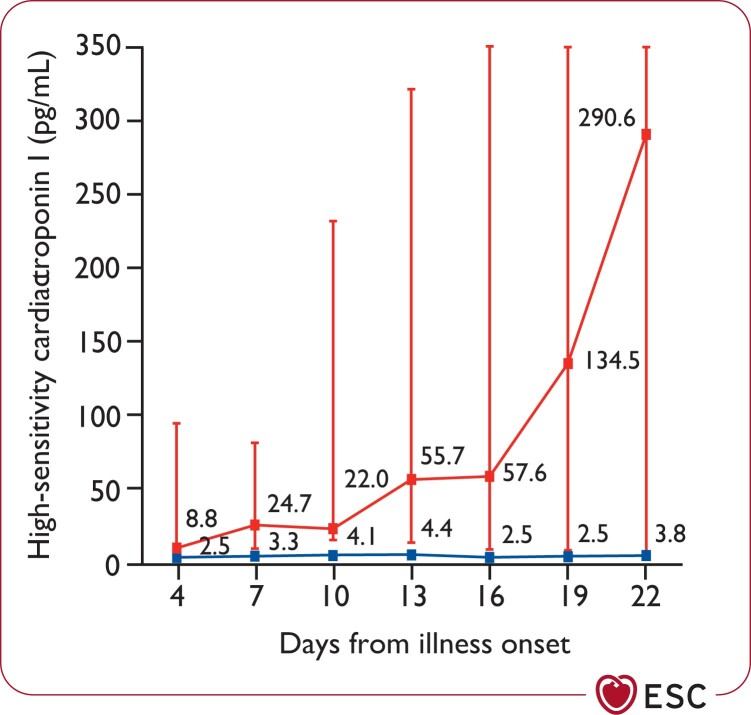
Cohort studies from patients hospitalized with COVID-19 have shown that 5–40% of patients had elevations >ULN in cardiac troponin T/I, and this finding was more common in patients admitted to the ICU and among those who died.34,117,179,181,187,188 Concentrations remained in the normal range in the majority of survivors. In non-survivors, troponin levels progressively increased in parallel with the severity of COVID-19 and the development of ARDS (Figure 4).8,117,179,181,188
Figure 4.
Temporal changes in high-sensitivity cardiac troponin I concentrations from illness onset in patients hospitalized with COVID-19. Differences between survivors and non-survivors were significant for all time points shown. Reprinted from Zhou et al.,8 Copyright (2020), with permission from Elsevier.
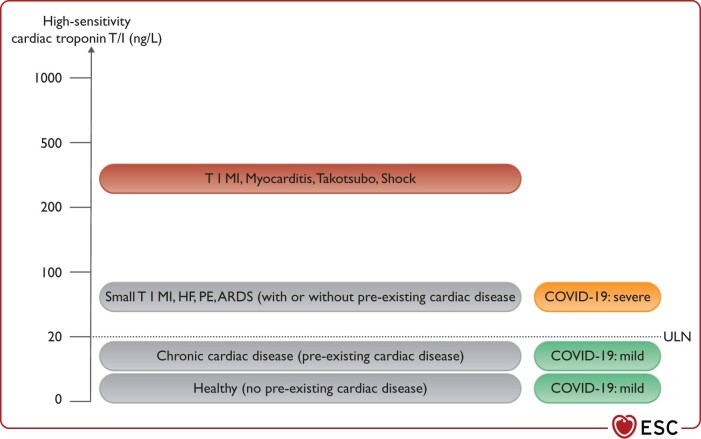
Mild elevations in cardiac troponin T/I concentrations (e.g. <2–3 times the ULN), particularly in older patients with pre-existing cardiac disease, do not require workup or treatment for T1MI, unless the latter is strongly suggested by angina chest pain and/or ECG changes (Figure 5). Such mild elevations are in general well explained by the combination of possible pre-existing cardiac disease and/or the acute injury related to COVID-19.
Figure 5.
High-sensitivity cardiac troponin T/I concentrations should be interpreted as quantitative variables. ARDS, acquired respiratory distress syndrome; HF, heart failure; PE, pulmonary embolism; ULN, upper limit of normal (assay-specific).
In non-critically ill patients with COVID-19, mild elevations (i.e. up to three times the ULN) are in general well explained by the combination of possible prior cardiac disease and the acute cardiomyocyte injury related to COVID-19. Even higher concentrations indicate the presence of specific acute cardiac disease such as T1MI, myocarditis, or Takotsubo syndrome.
Marked elevations in cardiac troponin T/I concentrations (i.e. >5 times the ULN) may indicate the presence of shock as part of COVID-19, severe respiratory failure, tachycardia, systemic hypoxaemia, myocarditis, Takotsubo syndrome, or T1MI triggered by COVID-19.8,179,181,182 In the absence of symptoms or ECG changes suggestive of T1MI, echocardiography should be considered to assess global and regional LV function. Patients with symptoms and ECG changes suggestive of T1MI should be treated according to ESC guidelines irrespective of COVID-19 status.117,183,188,189
B-type natriuretic peptide/N-terminal pro B-type natriuretic peptide
B-type natriuretic peptide/NT-proBNP as quantitative biomarkers of haemodynamic myocardial stress and HF are frequently elevated among patients with severe inflammatory and/or respiratory illnesses.179,190–192 Experience in patients with COVID-19 is limited. Very likely, the experience from other pneumonias can be extrapolated to COVID-19.179,190–192
As quantitative markers of haemodynamic stress and HF, the concentrations of BNP/NT-proBNP in a patient with COVID-19 should be seen as the combination of the presence/extent of pre-existing cardiac disease and/or the acute haemodynamic stress related to COVID-19.179,190–192 At least to some extent, the release of BNP/NT-proBNP seems to be associated with the extent of right ventricular (RV) haemodynamic stress (Figure 6).
Figure 6.
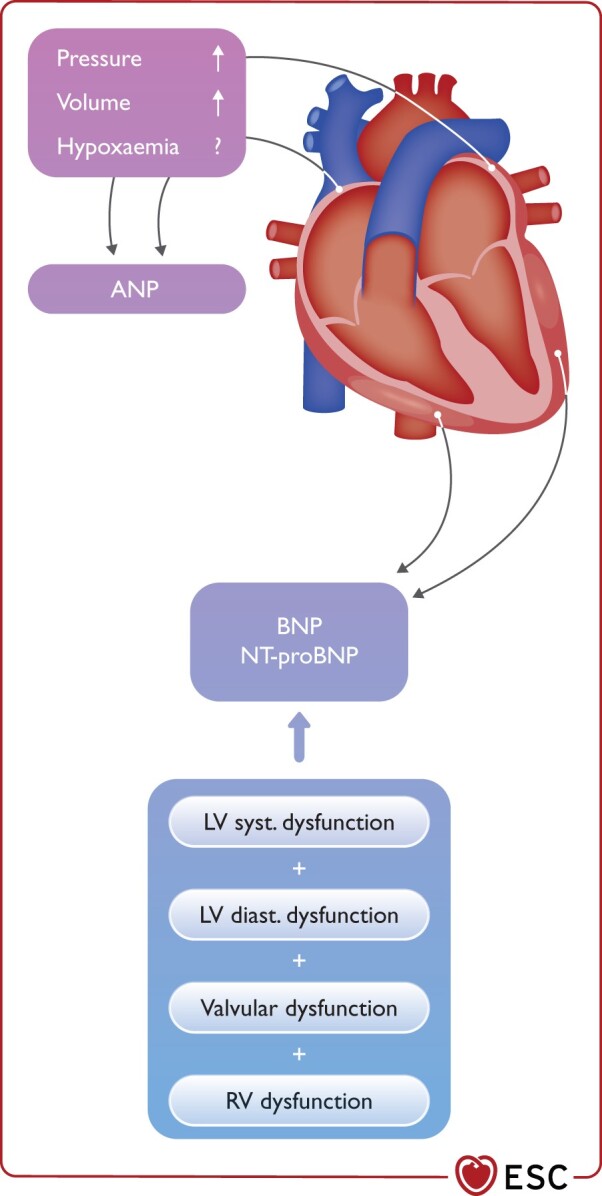
Haemodynamic determinants of natriuretic peptides. ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; LV, left ventricular; NT-proBNP, N-terminal proBNP; RV, right ventricular.
D-dimer
D-dimer is generated by the cleavage of fibrin monomers by prothrombin and indicates the presence of thrombin formation or reflects a non-specific acute-phase response from infection or inflammation. D-dimer may also indicate the presence of disseminated intravascular coagulation associated with shock.193 It is tempting to speculate that markers of activated coagulation or impaired fibrinolysis might contribute to acute myocardial injury, eventually also affecting coronary capillaries. Therefore, markers of haemostasis including activated partial thromboplastin time, prothrombin time, fibrin degradation products and D-dimer may be monitored routinely. In particular, elevations of D-dimer have been associated with poor outcome.194 Although the D-dimer has a lower specificity for the diagnosis of acute PE, 32–53% of patients still have a normal D-dimer and the vast majority has D-dimer below 1000 ng/mL.8,130,132 Therefore, recommended diagnostic algorithms combining pre-test probability assessments and D-dimer tests can be used in case of suspected acute PE.195 In particular, algorithms applying a pre-test probability-dependent D-dimer threshold may yield a decent specificity.196–198
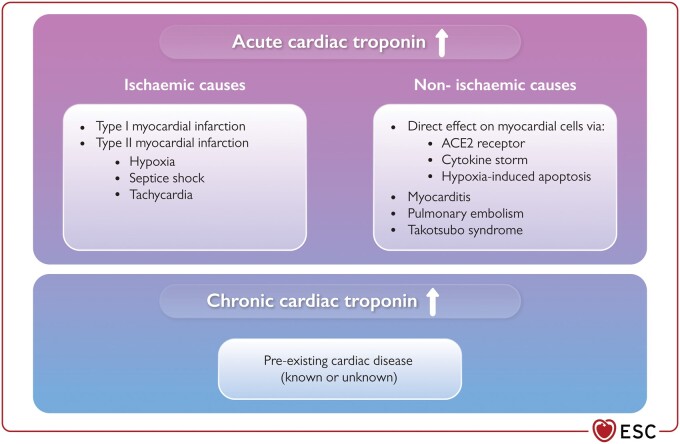
Potential mechanisms underlying biomarker elevation
The potential mechanisms underlying myocardial injury in those with COVID-19 are not fully understood (Figure 7). However, in keeping with other severe inflammatory and/or respiratory illnesses, direct (‘non-coronary’) myocardial injury is most likely the cause. Myocarditis, septic shock, tachycardia, severe respiratory failure, systemic hypoxaemia, Takotsubo syndrome, or T1MI triggered by COVID-19 are alternative causes. Direct myocardial involvement mediated via ACE2, cytokine storm, or hypoxia-induced excessive intracellular calcium leading to cardiac myocyte apoptosis has been suggested as alternative mechanisms.89,199,200
Figure 7.
Potential mechanisms underlying elevations in cardiac troponin and myocardial injury in patients with COVID-19. ACE2, angiotensin-converting enzyme 2; ↑, elevation.
As quantitative biomarkers of haemodynamic myocardial stress and HF, intracardiac filling pressures and end-diastolic wall stress seem to be the predominant triggers of the release of BNP/NT-proBNP.190–192
Which biomarkers should be measured and when?
As in patients without COVID-19, cardiac troponin T/I concentrations should be measured whenever, on clinical grounds, T1MI is suspected.183 In patients with COVID-19, diagnostic algorithms for rapid rule-out and/or rule-in of MI in patients with acute chest discomfort such as the ESC high-sensitivity cardiac troponin (hs-cTn) T/I 0/1-h algorithm can be expected to provide comparable performance characteristics as in other challenging subgroups with higher baseline concentrations such as the elderly and patients with renal dysfunction: very high safety for rule-out and high accuracy for rule-in but reduced efficacy with a higher percentage of patients remaining in the observe zone.183,201–203 Detailed clinical assessment including chest pain characteristics, assessment of COVID-19 severity, hs-cTn T/I measurement at 3 h, and cardiac imaging including echocardiography are the key elements for the identification of MI in this heterogeneous subgroup.183,201–203
Similarly, BNP/NT-proBNP should be measured whenever, on clinical grounds, HF is suspected.179,190–192 In patients who are not critically ill, rule-in cut-offs for HF maintain high positive predictive value even in patients with pneumonia.179,190–192 In contrast, currently recommended cut-offs should not be applied in critically ill patients, as most critically ill patients have substantial elevations in BNP/NT-proBNP, most likely due to the near-universal presence of haemodynamic stress and HF in these patients.179,190–192
There is increasing evidence suggesting that cardiac troponin T/I should possibly also be measured as a prognostic marker in patients with COVID-19. The strong and consistent association with mortality observed in the currently available reports of patients hospitalized with COVID-19, with evidence suggesting cardiac troponin T/I even as an independent predictor of mortality, should be seen in favour of this approach.8,29,34,179,181,187 Furthermore, a structured use of cardiac troponins to facilitate stage categorization and initial triage as well as in-hospital risk stratification, has been proposed.187 On the other hand, a more conservative approach might also be appropriate.8,117,179,181–184 First, beyond cardiac troponin T/I, other routinely available clinical and laboratory variables have also emerged as strong predictors of death in COVID-19 including older age, higher Sequential Organ Failure Assessment (SOFA) score, D-dimer, IL-6, and lymphocyte count. It is unclear whether and to what extent cardiac troponin T/I provide incremental value to a full model. Second, there is a small risk of inappropriate diagnostic and therapeutic interventions triggered based on cardiac troponin T/I concentrations measured for prognostic purposes. Third, in patients with COVID-19 as well as with other pneumonias or patients with ARDS, at this point in time, no specific therapeutic intervention can be justified based on the use of cardiac troponin T/I as a prognostic marker.8,117,179,181–184
Non-invasive imaging
Key points.
Do not perform routine cardiac imaging in patients with suspected or confirmed COVID-19.
COVID-19-positive and -negative patients should not cross in waiting area/scanner area, etc.
Prevent contamination from patients to other patients, to imagers and imaging equipment.
Perform imaging studies in patients with suspected or confirmed COVID-19 only if the management is likely to be impacted by the results.
Re-evaluate which imaging technique is best for your patients both in terms of diagnostic yield and infectious risk for the environment.
The imaging protocols should be kept as short as possible.
Non-urgent or elective cardiac imaging should not be performed routinely in patients with suspected or confirmed COVID-19. Accordingly, non-urgent or elective examinations should be postponed until the patient is known to be free of SARS-CoV-2 infection.204,205
Transthoracic and transoesophageal echocardiography
Key points.
Avoid performing transthoracic, transoesophageal and stress echocardiograms in patients in which test results are unlikely to change the management strategy.
Transoesophageal echocardiography (TOE) carries increased risks of spread of COVID-19 due to exposure of HCP to aerosolization of large viral load and should not be performed if an alternative imaging modality is available.
In COVID-19-infected patients, the echocardiogram should be performed focusing solely on the acquisition of images needed to answer the clinical question to reduce patient contact with the machine and the HCP performing the test.
Point of care focused ultrasound (POCUS), focused cardiac ultrasound study (FoCUS), and critical care echocardiography performed at bedside are effective options to screen for CV complications of COVID-19.
In COVID-19-infected patients, echocardiography should focus solely on the acquisition of images needed to answer the clinical question to reduce patient contact with the machine and HCP. POCUS, FoCUS, and critical care echocardiography are probably the preferred modalities to image patients with COVID-19. Limited evidence exists for the use of lung ultrasound to differentiate ARDS (single and/or confluent vertical artefacts, small white lung regions) from HF.206 Several recent studies have shown cardiac abnormalities in a majority of patients with ongoing COVID-19.207,208 However, it should be acknowledged that the majority of the series included patients in whom the reported abnormalities could be present before infection (particularly LV diastolic dysfunction or valve abnormalities) and, therefore, the role of echocardiography to diagnose new abnormalities is uncertain. The most frequent abnormality is RV dilation. The presence of a dilated right ventricle and pulmonary hypertension indicates the need to perform contrast CT to rule out PE and to differentiate the condition from ARDS. Echocardiography may change the management in 33% of patients with COVID-19.208
It should not be forgotten that the risk of infection remains in the reading rooms and therefore the material used should also be frequently sanitized.
Computed tomography
Key points.
Cardiovascular CT should be performed in hospitalized patients only with indications in which imaging results will likely impact management.
Coronary computed tomography angiography (CCTA) may be the preferred non-invasive imaging modality to diagnose CAD since it reduces the time of exposure of patients and personnel.
Cardiac CT may be preferred to TOE to rule out left atrial appendage and intracardiac thrombus prior to cardioversion.
In patients with respiratory distress, chest CT is recommended to evaluate imaging features typical of COVID-19.
Check renal function when contrast is indicated.
Cardiac CT should be performed when there is a potential impact on clinical management, including evaluation of symptomatic suspected CAD, acute symptomatic heart valve dysfunction, LV assist device dysfunction, PE, and urgent structural intervention.209 Cardiac CT is preferred to TOE to rule out the presence of intracardiac thrombus. In patients with acute chest pain and suspected obstructive CAD, CCTA is the preferred non-invasive imaging modality since it is accurate and fast and minimizes the exposure of patients. In patients with respiratory distress, lung CT is recommended to evaluate imaging features typical of COVID-19 and differentiate from other causes (HF, PE).210 However, it should not be used to screen for or as a first-line test to diagnose COVID 19 and should be reserved for hospitalized patients.211 A dedicated CT scanner for patients with suspected or confirmed COVID-19 is preferred. As in other imaging modalities, local standards for prevention of virus spread and protection of personnel should be followed.
Nuclear cardiology
Key points.
Nuclear cardiology should only be performed in specific indications and when no other imaging modalities can be performed.
The shortest duration of scan time and exposure should be used.
Standard dose imaging with rapid protocols of data acquisition is recommended.
Attenuation-corrected imaging should be considered.
Positron emission tomography (PET) minimizes the acquisition times.
Many of the diagnoses can be evaluated with other imaging modalities that limit the risk of virus spread. Nuclear cardiology tests require long acquisition times and exposure of patients and personnel.212 Guidance and best practices for nuclear cardiology laboratories during the COVID-19 pandemic have been published.212 The use of PET–CT can be limited to patients with suspected endocarditis of prosthetic valves or intracardiac devices when other imaging modalities are inconclusive or to avoid the performance of a TOE which is associated with larger risk of spreading. Single photon emission computed tomography or PET may also be used for diagnosing ischaemia in patients with suspected obstructive CAD when CCTA is not appropriate or available.
Cardiac magnetic resonance
Key points.
Use shortened CMR protocols focused to address the clinical problem.
Check renal function when contrast is indicated.
CMR is preferred in clinically suspected acute myocarditis.
The risks of contamination during a CMR scan are probably similar to a CT scan, but lower than during an echocardiographic study. Only clinically urgent CMR scans should be accepted.213
Longer time exposure in the scanner will probably increase the chances of contamination of equipment and staff. To minimize the examination time, shortened CMR protocols focused to address the clinical problem should be used.214 A dedicated mitral regurgitation scanner for patients with suspected or confirmed COVID-19 is a clear advantage. Allow time for a deep cleaning after each patient with suspected or confirmed COVID-19.
The role of CMR in patients with COVID-19 is mainly the diagnosis of myocarditis186,215 and, in cases of MI with non-obstructive coronary arteries, to evaluate the underlying diagnosis.183 The diagnosis of myocarditis should be suspected when there are elevated troponins, ventricular dysfunction, and/or severe arrhythmias that cannot be explained by other diagnostics and imaging methods.27
A few recent studies have found a very high prevalence of myocarditis by CMR in asymptomatic patients with recovered COVID-19.186,216 However, the clinical significance of these CMR findings is currently unclear. Accepted diagnostic indications for CMR should be considered as appropriate in COVID-19 patients, and CMR should not be performed unless clinically necessary and after a reconsideration of best-suited imaging technique.205,213
Another important attention is the use of late-gadolinium enhancement in patients with COVID-19. Renal function might be decreased in patients with COVID-19 and, therefore, it should follow the same considerations as in patients without COVID-19.217
Exercise testing
Key points.
During physical exercise the risk of virus spread is increased due to greater amount of aerosol and droplets production.
Exercise testing should be avoided in COVID-19-suspect or -positive patients.
Exercise testing should be deferred whenever possible in every patient in COVID-19 epidemic areas and alternative diagnostic methods should be preferred. If exercise testing is necessary (i.e. cardiopulmonary exercise test in advanced HF), it may be considered to rule out SARS-CoV-2 infection by nasopharyngeal swab prior to exercise testing and additional precautions are needed.
Performance of exercise testing (either conventional, echocardiography, or nuclear imaging) has major limitations in the COVID-19 era. Wearing a mask may strongly affect patient exercise capacity. Moreover, during physical exercise, both breath rate and the amount of aerosol/droplets production are increased, even when wearing a surgical mask. This problem is further amplified since rooms of outpatient clinics are rarely large and well aerated. Thus, performance of exercise testing in COVID-19 suspect or positive patients should be avoided to prevent virus spread and protect personnel. In general, exercise tests should be deferred in every patient in COVID-19 epidemic areas and alternative diagnostic methods should be preferred whenever possible. In patients with suspected CAD and stable angina symptoms, a CCTA should be preferred with exercise testing when patients’ characteristics do not limit image quality. When functional imaging for myocardial ischaemia is necessary, pharmacological stress testing (e.g. vasodilatation using adenosine or regadenoson) should be preferred.218 If CAD is highly likely and patients present with typical angina or severe symptoms refractory to medical therapy, invasive coronary, angiography may be the first choice.
However, there are conditions where exercise testing is necessary, such as for the functional evaluation of patients with HF. Namely, cardiopulmonary exercise testing is of primary importance in the staging of patients with advanced HF. Peak exercise oxygen uptake (peak VO2) and minute ventilation/carbon dioxide relationship slope (VE/VCO2 slope) are prognostic and can guide the decision of advanced therapies, including heart transplantation.219 In such patients, the exercise test cannot be delayed. In addition, exercise testing is proposed as the method of choice for the diagnosis of heart failure with preserved ejection fraction (HFpEF) in patients with breathlessness and intermediate scores for HFpEF diagnosis. A low-level exercise may be sufficient in these cases.220
When exercise testing is necessary, prior screening for the symptoms of active SARS-CoV-2 infection (e.g. cough, sore throat, fever, loss of sense of smell and taste) is recommended. Furthermore, SARS-CoV-2 infection should be ruled out by nasopharyngeal swab prior to exercise testing.221 Single-patient use equipment should be preferred, including face masks and disposable parts such as mouth pieces, and nose clips. Filtration devices within mask/mouthpiece apparatus may be considered. Since the risk of virus transmissions increases with aerosolizing tests, the personal protective equipment requirements must be appropriate. Negative-pressure rooms should be preferred when available.222 A sufficient time should be allowed for air changes before cleaning the room and between different patients.223
Differential diagnosis
Key points.
The presence of COVID-19 should not preclude a systematic search for CV events, including ACS.
COVID-19-related injury should be kept in mind as differential diagnosis.
Other manifestations and complications of COVID-19 mimicking heart disease should also have been ruled out.
In COVID-19 patients with clinical presentation compatible with CVD, three main entities should be considered:
Patients with COVID-19 can present with cardiac events that can be favoured by the infection or unrelated. Those include ACS, acute HF, arrhythmias, venous thromboembolic events, CS, and cardiac arrest. Those syndromes require a quick diagnosis and management, and should not be overlooked due to the presence of COVID-19.
Infection-related cardiac injury can also lead to a clinical presentation suggestive of cardiac event, and should also be considered as a differential diagnosis.
Patients with COVID-19 can present with symptoms mimicking CV events, including chest pain, dyspnoea, and shock, even in the absence of cardiac injury.
Acknowledgements
The following people reviewed the document: Victor Aboyans (France), Stefan D. Anker (Germany), Robert A. Byrne (Ireland), A. John Camm (Italy), Andrew J.S. Coats (Italy), Rudolf A. de Boer (The Netherlands), Stefanie Dimmeler (Germany), Donna Fitzimons (UK), Christoph Gräni (Switzerland), Christian Hamm (Germany), Richard Haynes (UK), Bernard Iung (France), Adnan Kastrati (Germany), Patrizio Lancellotti (Belgium), Julinda Mehilli (Germany), Béla Merkely (Hungary), Lis Neubeck (UK), Katja E. Odening (Switzerland), Raffaele Piccolo (Italy), Lorenz Räber (Switzerland), Tobias Reichlin (Switzerland), Manel Sabate (Spain), P. Christian Schulze (Germany), Iain A. Simpson (UK), Lars Sondergaard (Denmark), Miguel Sousa-Uva (Portugal), Stefan Stortecky (Switzerland), Didier Tchétché (France), and Katja Zeppenfeld (The Netherlands). Support for title page creation and format was provided by AuthorArranger, a tool developed at the National Cancer Institute. Matthieu Depuydt (European Society of Cardiology) coordinated the development of the manuscript.
Conflict of interest: C.B. reports grants or contracts as follow: Medical Research Council: Population Health Research Unit (Director) 2019–24; Medical Research Council: PHRU capital award 2019–20; Medical Research Council: Therapy Acceleration Laboratory Award 2021; BHF: Project Grant no. PG/18/16/33570 Cholesterol Treatment Trialists’ (CTT) Collaboration: Meta-analyses of individual participant adverse event data from randomized controlled trials of statin therapy (co-applicant) 2018–20; Boehringer Ingelheim: EMPA-KIDNEY trial (study co-chair) 2018–22; NIHR HTA 17/140/02: Cost-effectiveness of statin therapies evaluated using individual participant data from large randomized clinical trials (co-applicant) 2018–22. C.B. reports unpaid roles as chair of the European Society of Cardiology Clinical Practice Guidelines Committee and as vice-chair of the British Heart Foundation Clinical Studies Committee. S.W. reports research and educational grants via his institution from Abbott, Amgen, Astra Zeneca, BMS, Bayer, Biotronik, Boston Scientific, Cardinal Health, CardioValve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Johnson & Johnson, Medicure, Novartis, Polares, OrPha Suisse, Pfizer, Regeneron, Sanofi Aventis, Sinomed, Terumo, and V-Wave. S.W. is an unpaid member of the Pfizer Research Award selection committee in Switzerland and of the Women as One Awards Committee. He is a member of the Clinical Study Group of the Deutsches Zentrum für Herz Kreislauf-Forschung and of the Advisory Board of the Australian Victorian Heart Institute. He is a chairperson of the ESC Congress Program Committee and a former chairperson of the ESC Clinical Practice Guidelines Committee. S.W. serves as unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave, and Xeltis but has not received personal payments by pharmaceutical companies or device manufacturers. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. E.A. reports honoraria for lectures from Biosense Webster. D.C. reports personal consulting fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, and Daiichi Sankyo. J-P.C. reports grants or contracts from Medtronic, BMS-Pfizer for the ATLANTIS trial; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from BMS-Pfizer, Webmed, AstraZeneca, and Sanofi. T.C. reports consulting fees from Boston Scientific, Medtronic, and Edwards; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Boston Scientific, Medtronic, and Edwards; and participation on a Data Safety Monitoring Board or Advisory Board for CERC. J.R.G.-J. payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Ferrer International, Menarini, MSD, Novartis, Novo Nordisk, Pfizer, Sanofi Aventis, and Servier, outside the submitted work. T.J.G. is the editor-in-chief from the Cardiovascular Research journal. T.J.G. reports speaker fees from Merck and participation to the steering committee of the COMPASS trial from the Public Health Research Institute. H.H. reports unconditional Research Grants for the University of Antwerp and/or University of Hasselt from Bracco Imaging Europe; Daiichi Sankyo, Boehringer Ingelheim, Abbott, Medtronic, Biotronik, St. Jude Medical, and Fibricheck/Qompium. As EHRA president 2018–2020, H.H. reports no personal honorarium for any industry-related speaker or advisory role between March 2017 and September 2020. After September 2020, H.H. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events. B.I. reports other financial or non-financial interests as leader for a small single-centre phase 2 RCT testing the benefits of i.v. metoprolol in critically ill COVID19 patients. Funds from his laboratory were used and there was no relationship with any third party. Metoprolol is not approved for ARDS (COVID related or non-COVID related) and thus this trial was an initial step for its repurposing. Metoprolol is out of patent and has no commercial interest. F.A.K. reports research grants via his institution from Bayer, Bristol Myers Squibb, Boehringer Ingelheim, MSD, Daiichi Sankyo, Actelion, the Dutch thrombosis association, The Netherlands Organisation for Health Research and Development, and the Dutch Heart foundation, all outside the current work. F.K. is a member of the Dutch guideline committee on antithrombotic therapy, and the ASH guideline on thromboprophylaxis in COVID-19 patients. F.K. is a member of the nucleus of the ESC Working Group on RV function and pulmonary circulation. S.V.K. reports grants or contracts via his institution from Bayer AG, Boston Scientific, Servier, Actelion-Janssen, and Daiichi Sankyo; consulting fees from Bayer AG, Pfizer–Bristol Myers Squibb, Daiichi Sankyo, and Boston Scientific; payment or honoraria for lectures, presentations from Bayer AG, Pfizer–Bristol Myers Squibb, Daiichi Sankyo Boston Scientific, and MSD Novartis. U.L. reports grants or contracts from Bayer, Novartis, and Amgen; consulting fees from Bayer, Novartis, Amgen, Sanofi, and Pfizer; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bayer, Novartis, Amgen, Sanofi, and Pfizer. C.L. reports grants or contracts from Biotronik, Medtronic, Boston Scientific, Microport, and Abbott and support for attending meetings and/or travel from Biotronik, Medtronic, Boston Scientific, Microport, and Abbott. M.L. reports honoraria for the role of faculty member of two webinars on COVID-19 from a private society committed to educational initiatives and the Italian Association of Hospital Cardiologists. N.M. reports a research grant from Getinge Global US and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bristol Myers Squibb. C.M. reports research support payments via his institution from Research support from several diagnostic companies, the Swiss National Science Foundation, and the Swiss Heart Foundation; consulting fees from Idorsia; and payment via his institution for his participation on a Data Safety Monitoring Board or Advisory Board from Roche and from Osler. A.S.P reports consulting and proctoring payment via her institution from Medtronic; consulting and proctoring payment from Boston Scientific; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Medtronic, Abbott, and Boston Scientific; payment for expert testimony via her institution from Medtronic, Boston Scientific, and Abbott; and participation to the steering committee for the ongoing SMART trial. F.P. reports travel expenses from Abbott Vascular, Edwards Lifesciences, and Polares Medical. P.P. reports direct consulting fees from Boehringer Ingelheim, Pfizer, and Bayer AG; direct honoraria for lectures from Bayer AG, Bristol Myers Squibb, Boehringer Ingelheim, Pfizer, Sanofi, Roche, and Boston Scientific. M.R. reports research grant via his institution from Medtronic, Boston Scientific, Terumo, Biotronik, and GE Healthcare. S.R. reports research grant via his institution from Actelion, AstraZeneca, Bayer, Janssen, and Novartis; remunerations for lectures from Abbott, Acceleron, Actelion, Arena, Bayer, Ferrer, Janssen, MSD, Novartis, Pfizer, United Therapeutics, and Vifor. G.S. reports research grant via his institution from Boston Scientific; speaker fees from Abbott Vascular and Boston Scientific. H.T. is the president-elect of the German Cardiac Society and is chair of the 2020 ESC NSTE-ACS Guideline. B.W. reports lecture fees for symposia on hypertension, unrelated to the content of those manuscripts from Pfizer, Boehringer, Servier, and Daiichi Sankyo; travel/accommodation fees to conference from Pfizer, Boehringer, Servier, and Daiichi Sankyo. B.W. is the chair of the most recent 2018 ESC-ESH Hypertension Guideline. All other authors declared no conflict of interest.
Data availability
No new data were generated or analysed in support of this research.
Appendix
The Task Force for the management of COVID-19 of the European Society of Cardiology (ESC)
Writing Committee: Colin Baigent* (MRC Population Health Research Unit, Nuffield Department of Population Health, Oxford, UK); Stephan Windecker* (Department of Cardiology, Inselspital, Bern University Hospital, Bern, Switzerland); Daniele Andreini (Centro Cardiologico Monzino, IRCCS, Milan, Italy and Department of Clinical Sciences and Community Health, Cardiovascular Section, University of Milan, Milan, Italy); Elena Arbelo (Arrhythmia Section, Cardiology Department, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain and Institut d’Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain and Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain and ECGen, the Cardiogenetics Focus Group of EHRA); Emanuele Barbato (Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy and Cardiovascular Center Aalst, OLV Hospital, Aalst, Belgium); Antonio L. Bartorelli (Centro Cardiologico Monzino, IRCCS, Milan, Italy and Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy and Department of Biomedical and Clinical Sciences “Luigi Sacco”, University of Milan, Milan, Italy); Andreas Baumbach (Centre for Cardiovascular Medicine and Devices, William Harvey Research Institute, Queen Mary University of London and Barts Heart Centre, London, UK and Yale University School of Medicine, New Haven, CT, USA); Elijah R. Behr (ECGen, the Cardiogenetics Focus Group of EHRA, Cardiology Clinical Academic Group, Institute of Molecular and Clinical Sciences, St George’s, University of London, London, UK and St George’s University Hospitals NHS Foundation Trust, London, UK and European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN GUARDHEART; http://guardheart.ern-net.eu); Sergio Berti (U.O.C. Cardiologia Diagnostica e Interventistica, Dipartimento Cardiotoracico, Fondazione Toscana G. Monasterio – Ospedale del Cuore G. Pasquinucci, Massa, Italy); Héctor Bueno (Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain and Cardiology Department, Hospital Universitario 12 de Octubre and Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain and Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain); Davide Capodanno (Division of Cardiology, A.O.U. Policlinico “G. Rodolico-San Marco” University of Catania, Catania, Italy); Riccardo Cappato (Arrhythmia & Electrophysiology Center, IRCCS Gruppo MultiMedica, Sesto San Giovanni, Milan, Italy); Alaide Chieffo (San Raffaele Scientific Institute, Milan, Italy); Jean-Philippe Collet (Sorbonne Université, ACTION study group, Institut de Cardiologie, Pitié Salpêtrière Hospital (AP-HP), Paris, France); Thomas Cuisset (Département de Cardiologie, CHU Timone, Marseille, France and INSERM, UMR1062, Nutrition, Obesity and Risk of Thrombosis, Marseille, France and Faculté de Médecine, Aix-Marseille Université, Marseille, France); Giovanni de Simone (Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy and Hypertension Research Center, Federico II University Hospital, Naples, Italy); Victoria Delgado (Heart Lung Centrum, Leiden University Medical Center, Leiden, The Netherlands); Paul Dendale (Heart Centre Hasselt, Jessa Hospital, Hasselt, Belgium and Faculty of Medicine and Life Sciences, Uhasselt, Diepenbeek, Belgium); Dariusz Dudek (Institute of Cardiology, Jagiellonian University Medical College, Kraków, Poland and Maria Cecilia Hospital, GVM Care&Research, Cotignola (RA), Ravenna, Italy); Thor Edvardsen (Department of Cardiology Oslo University Hospital, Rikshospitalet, Oslo, Norway); Arif Elvan (Isala Heart Center, Zwolle, The Netherlands); José R. González-Juanatey (Cardiology Department, University Hospital, IDIS, CIBERCV, University of Santiago de Compostela, Santiago de Compostela, Spain); Mauro Gori (Cardiovascular Department & Cardiology Unit, Papa Giovanni XXIII Hospital-Bergamo, Bergamo, Italy); Diederick Grobbee (Julius Global Health, the Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands); Tomasz J. Guzik (Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK and Department of Medicine, Jagiellonian University College of Medicine, Kraków, Poland); Sigrun Halvorsen (Department of Cardiology, Oslo University Hospital Ulleval, Oslo, Norway and University of Oslo, Oslo, Norway); Michael Haude (Medical Clinic I, Städtische Kliniken Neuss, Lukaskrankenhaus GmbH, Neuss, Germany); Hein Heidbuchel (Department of Cardiology, University Hospital Antwerp and University of Antwerp, Antwerp, Belgium); Gerhard Hindricks (Department of Internal Medicine/Cardiology/Electrophysiology, Heart Center Leipzig, University Hospital Leipzig, Leipzig, Germany and Leipzig Heart Institute (LHI), Leipzig, Germany); Borja Ibanez (Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain and Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain and IIS-Fundación Jiménez Díaz Hospital, Madrid, Spain); Nicole Karam (Université de Paris, PARCC, INSERM, Paris, France and European Hospital Georges Pompidou, Paris, France); Hugo Katus (Department of Internal Medicine, University Hospital of Heidelberg, Heidelberg, Germany); Fredrikus A. Klok (Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, The Netherlands); Stavros V. Konstantinides (Center for Thrombosis and Hemostasis, Johannes Gutenberg University Mainz, Mainz, Germany and Department of Cardiology, Democritus University of Thrace, Alexandroupolis, Greece); Ulf Landmesser (Department of Cardiology, Charite University Medicine Berlin, Berlin, Germany and Berlin Institute of Health (BIH), German Center of Cardiovascular Research (DZHK), Partner Site Berlin, Berlin, Germany); Christophe Leclercq (University of Rennes, CHU Rennes, INSERM, LTSI – UMR 1099, Rennes, France); Sergio Leonardi (University of Pavia, Pavia, Italy and Fondazione IRCCS Policlinico S.Matteo, Pavia, Italy); Maddalena Lettino (Cardio-Thoracic and Vascular Department, San Gerardo Hospital, ASST-Monza, Monza, Italy); Giancarlo Marenzi (Centro Cardiologico Monzino, IRCCS, Milan, Italy); Josepa Mauri (Institut del Cor, Hospital Universitari Germans Trias i Pujol, Badalona, Spain and Health Department of the Government of Catalonia, Barcelona, Spain); Marco Metra (Institute of Cardiology, ASST Spedali Civili di Brescia; Department of Medical and Surgical Specialities, Radiological Sciences and Public Health, University of Brescia, Brescia, Italy); Nuccia Morici (Unità di Cure Intensive Cardiologiche e De Gasperis Cardio Center, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy and Dipartimento di Scienze Cliniche e di Comunità, Università degli Studi, Milan, Italy); Christian Mueller (Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, Basel, Switzerland and University of Basel, Basel, Switzerland); Anna Sonia Petronio (Cardiothoracic and Vascular Department, University of Pisa, Ospedale cisanello, Pisa, Italy); Marija M. Polovina (Faculty of Medicine, Belgrade University, Belgrade, Serbia and Department of Cardiology, Clinical Centre of Serbia, Belgrade, Serbia); Tatjana Potpara (School of Medicine, University of Belgrade, Belgrade, Serbia and Department for Intensive Arrhythmia Care, Cardiology Clinic, Clinical Centre of Serbia, Belgrade, Serbia); Fabien Praz (Department of Cardiology, University Hospital Bern, Bern, Switzerland); Bernard Prendergast (St Thomas' Hospital and Cleveland Clinic London, London, UK); Eva Prescott (Department of Cardiology, Bispebjerg University Hospital, Copenhagen, Denmark); Susanna Price (Royal Brompton Hospital, London, UK and National Heart & Lung Institute, Imperial College, London, UK); Piotr Pruszczyk (Department of Internal Medicine & Cardiology, Medical University of Warsaw, Warsaw, Poland); Oriol Rodríguez-Leor (Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain and Institut del Cor, Hospital Universitari Germans Trias i Pujol, Badalona, Spain); Marco Roffi (Department of Cardiology, Geneva University Hospitals, Geneva, Switzerland); Rafael Romaguera (Servicio de Cardiología, Hospital Universitario de Bellvitge-IDIBELL, L'Hospitalet de Llobregat, Barcelona, Spain); Stephan Rosenkranz (Clinic III for Internal Medicine (Cardiology) and Cologne Cardiovascular Research Center (CCRC), Heart Center at the University of Cologne, Cologne, Germany and Center for Molecular Medicine Cologne (CMMC), University of Cologne, Cologne, Germany); Andrea Sarkozy (Department of Cardiology, University Hospital Antwerp and University of Antwerp, Antwerp, Belgium); Martijn Scherrenberg (Heart Centre Hasselt, Jessa Hospital, Hasselt, Belgium and Faculty of Medicine and Life Sciences, Uhasselt, Diepenbeek, Belgium); Petar Seferovic (Faculty of Medicine, Belgrade University, Belgrade, Serbia and Serbian Academy of Sciences and Arts, Belgrade, Serbia); Michele Senni (Cardiovascular Department & Cardiology Unit, Papa Giovanni XXIII Hospital-Bergamo, Bergamo, Italy); Francesco R. Spera (Department of Cardiology, University Hospital Antwerp and University of Antwerp, Antwerp, Belgium); Giulio Stefanini (Department of Biomedical Sciences, Humanitas University, Pieve Emanuele – Milan, Italy and Humanitas Research Hospital IRCCS, Rozzano – Milan, Italy); Holger Thiele (Leipzig Heart Institute (LHI), Leipzig, Germany and Department of Internal Medicine/Cardiology, Heart Center Leipzig at University of Leipzig, Leipzig, Germany); Daniela Tomasoni (Institute of Cardiology, ASST Spedali Civili di Brescia; Department of Medical and Surgical Specialities, Radiological Sciences and Public Health, University of Brescia, Brescia, Italy); Lucia Torracca (Department of Biomedical Sciences, Humanitas University, Pieve Emanuele – Milan, Italy and Humanitas Research Hospital IRCCS, Rozzano – Milan, Italy); Rhian M. Touyz (Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK); Arthur A. Wilde (ECGen, the Cardiogenetics Focus Group of EHRA and European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN GUARDHEART; http://guardheart.ern-net.eu) and Amsterdam UMC, University of Amsterdam, Heart Center; department of Clinical Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam, The Netherlands); Bryan Williams (Institute of Cardiovascular Sciences, University College London, London, UK).
*Joint corresponding authors. Email: colin.baigent@ndph.ox.ac.uk (C.B.); stephan.windecker@insel.ch (S.W.)
Contributor Information
The Task Force for the management of COVID-19 of the European Society of Cardiology:
Colin Baigent, Stephan Windecker, Daniele Andreini, Elena Arbelo, Emanuele Barbato, Antonio L Bartorelli, Andreas Baumbach, Elijah R Behr, Sergio Berti, Héctor Bueno, Davide Capodanno, Riccardo Cappato, Alaide Chieffo, Jean-Philippe Collet, Thomas Cuisset, Giovanni de Simone, Victoria Delgado, Paul Dendale, Dariusz Dudek, Thor Edvardsen, Arif Elvan, José R González-Juanatey, Mauro Gori, Diederick Grobbee, Tomasz J Guzik, Sigrun Halvorsen, Michael Haude, Hein Heidbuchel, Gerhard Hindricks, Borja Ibanez, Nicole Karam, Hugo Katus, Fredrikus A Klok, Stavros V Konstantinides, Ulf Landmesser, Christophe Leclercq, Sergio Leonardi, Maddalena Lettino, Giancarlo Marenzi, Josepa Mauri, Marco Metra, Nuccia Morici, Christian Mueller, Anna Sonia Petronio, Marija M Polovina, Tatjana Potpara, Fabien Praz, Bernard Prendergast, Eva Prescott, Susanna Price, Piotr Pruszczyk, Oriol Rodríguez-Leor, Marco Roffi, Rafael Romaguera, Stephan Rosenkranz, Andrea Sarkozy, Martijn Scherrenberg, Petar Seferovic, Michele Senni, Francesco R Spera, Giulio Stefanini, Holger Thiele, Daniela Tomasoni, Luccia Torracca, Rhian M Touyz, Arthur A Wilde, and Bryan Williams
Corresponding authors. Tel: +44 1865 743743, Fax: +44 1865 743985, Email: colin.baigent@ndph.ox.ac.uk (C.B.); Tel: +41 31 632 21 11, Fax: +41 31 632 47 70, Email: stephan.windecker@insel.ch (S.W.)
References
- 1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (29 October 2020).
- 3. Looi MK. COVID-19: is a second wave hitting Europe? BMJ 2020;371:m4113. [DOI] [PubMed] [Google Scholar]
- 4. Del Sole F, Farcomeni A, Loffredo L et al. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest 2020;50:e13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Figliozzi S, Masci PG, Ahmadi N et al. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Invest 2020;50:e13362. [DOI] [PubMed] [Google Scholar]
- 6. Inciardi RM, Adamo M, Lupi L et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J 2020;41:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One 2020;15:e0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsushita K, Marchandot B, Carmona A et al. Increased susceptibility to SARS-CoV-2 infection in patients with reduced left ventricular ejection fraction. ESC Heart Fail 2021;8:380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tomasoni D, Inciardi RM, Lombardi CM et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the Cardio-COVID-Italy multicentre study. Eur J Heart Fail 2020;22:2238–2247. [DOI] [PubMed] [Google Scholar]
- 11. Barron E, Bakhai C, Kar P et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 2020;8:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caussy C, Pattou F, Wallet F et al. ; COVID Outcomes HCL Consortium and Lille COVID–Obesity Study Group. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol 2020;8:562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Popkin BM, Du S, Green WD et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev 2020;21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Y, Ren Q, Chen G et al. Chronic kidney diseases and acute kidney injury in patients with COVID-19: evidence from a meta-analysis. Front Med (Lausanne) 2020;7:588301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grasselli G, Zangrillo A, Zanella A et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richardson S, Hirsch JS, Narasimhan M et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu C, Chen X, Cai Y et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Hearn M, Liu J, Cudhea F, Micha R, Mozaffarian D. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc 2021;10:e019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah H, Khan MSH, Dhurandhar NV, Hegde V. The triumvirate: why hypertension, obesity, and diabetes are risk factors for adverse effects in patients with COVID-19. Acta Diabetol 2021;58:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Intensive Care National Audit and Research Centre. ICNARC Report on COVID-19 in Critical Care. 2020. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports (6 May 2020).
- 22. Martineau AR, Jolliffe DA, Hooper RL. et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv 2020. doi: 10.1101/2020.03.24.20042937 [DOI]
- 24. Resnick A, Galea S, Sivashanker K. COVID-19: The Painful Price of Ignoring Health Inequities. https://blogs.bmj.com/bmj/2020/03/18/covid-19-the-painful-price-of-ignoring-health-inequities/ (22 September 2021)
- 25. Simpson CR, Steiner MF, Cezard G; et al. Shels Researchers. Ethnic variations in morbidity and mortality from lower respiratory tract infections: a retrospective cohort study. J R Soc Med 2015;108:406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonanad C, García-Blas S, Tarazona-Santabalbina F et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J Am Med Dir Assoc 2020;21:915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Driggin E, Madhavan MV, Bikdeli B et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol 2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guo T, Fan Y, Chen M et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu CM, Wong RS, Wu EB et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J 2006;82:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anupama BK, Chaudhuri D. A review of acute myocardial injury in coronavirus disease 2019. Cureus 2020;12:e8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li SS, Cheng CW, Fu CL et al. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation 2003;108:1798–1803. [DOI] [PubMed] [Google Scholar]
- 33. Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Special article—Acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis 2020;63:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lala A, Johnson KW, Januzzi JL; et al. Mount Sinai Covid Informatics Center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pareek M, Singh A, Vadlamani L et al. Relation of cardiovascular risk factors to mortality and cardiovascular events in hospitalized patients with coronavirus disease 2019 (from the Yale COVID-19 Cardiovascular Registry). Am J Cardiol 2021;146:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Libby P, Loscalzo J, Ridker PM et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol 2018;72:2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Modin D, Claggett B, Sindet-Pedersen C et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation 2020;142:2080–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giustino G, Pinney SP, Lala A et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J Am Coll Cardiol 2020;76:2011–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhatt AS, Moscone A, McElrath EE et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol 2020;76:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Rosa S, Spaccarotella C, Basso C; et al. Società Italiana di Cardiologia and the CCU Academy Investigators Group. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;41:2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: the COVID-19 paradox. J Am Coll Cardiol 2020;76:289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J 2020;41:1852–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pessoa-Amorim G, Camm CF, Gajendragadkar P et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes 2020;6:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fried JA, Ramasubbu K, Bhatt R The variety of cardiovascular presentations of COVID-19. Circulation 2020;141:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Inciardi RM, Lupi L, Zaccone G et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kesici S, Aykan HH, Orhan D, Bayrakci B. Fulminant COVID-19-related myocarditis in an infant. Eur Heart J 2020;41:3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luetkens JA, Isaak A, Zimmer S et al. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ Cardiovasc Imaging 2020;13:e010897. [DOI] [PubMed] [Google Scholar]
- 48. Angeli F, Spanevello A, De Ponti R et al. Electrocardiographic features of patients with COVID-19 pneumonia. Eur J Intern Med 2020;78:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhatla A, Mayer MM, Adusumalli S et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 2020;17:1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holt A, Gislason GH, Schou M et al. New-onset atrial fibrillation: incidence, characteristics, and related events following a national COVID-19 lockdown of 5.6 million people. Eur Heart J 2020;41:3072–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen T, Wu D, Chen H et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Freaney PM, Shah SJ, Khan SS. COVID-19 and heart failure with preserved ejection fraction. JAMA 2020;324:1499. [DOI] [PubMed] [Google Scholar]
- 53. Shoar S, Hosseini F, Naderan M, Mehta JL. Meta-analysis of cardiovascular events and related biomarkers comparing survivors versus non-survivors in patients with COVID-19. Am J Cardiol 2020;135:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vakili K, Fathi M, Pezeshgi A et al. Critical complications of COVID-19: a descriptive meta-analysis study. Rev Cardiovasc Med 2020;21:433–442. [DOI] [PubMed] [Google Scholar]
- 55. Bikdeli B, Madhavan MV, Jimenez D; et al. Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kollias A, Kyriakoulis KG, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol 2020;189:846–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020;18:1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Klok FA, Kruip M, van der Meer NJM et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Helms J, Tacquard C, Severac F et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA 2020;324:1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 2019;17:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou P, Yang XL, Wang XG et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van Doremalen N, Bushmaker T, Morris DH et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020;382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhao S, Lin Q, Ran J et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis 2020;92:214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Guo YR, Cao QD, Hong ZS et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res 2020;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Y, Yang Y, Zhang C et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2021;2:e13–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020;181:281–292.e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367:1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Santos RAS, Sampaio WO, Alzamora AC et al. The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev 2018;98:505–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li W, Moore MJ, Vasilieva N et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hoffmann M, Kleine-Weber H, Schroeder S SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cantuti-Castelvetri L, Ojha R, Pedro LD et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020;370:856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Daly JL, Simonetti B, Klein K et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020;370:861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kielian M. Enhancing host cell infection by SARS-CoV-2. Science 2020;370:765–766. [DOI] [PubMed] [Google Scholar]
- 76. Wu Y. Compensation of ACE2 function for possible clinical management of 2019-nCoV-induced acute lung injury. Virol Sin 2020;35:256–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020;14:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 2020;45:230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Guzik TJ, Mohiddin SA, Dimarco A et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020;116:1666–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020;116:1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nicin L, Abplanalp WT, Mellentin H et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J 2020;41:1804–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bojkova D, Wagner JUG, Shumliakivska M et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res 2020;116:2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Solidarity Trial Consortium WHO, Pan H, Peto R, Henao-Restrepo AM; et al. WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19—interim WHO solidarity trial results. N Engl J Med 2021;384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Williams B, Zhang Y. Hypertension, renin-angiotensin-aldosterone system inhibition, and COVID-19. Lancet 2020;395:1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Iannelli A, Favre G, Frey S et al. Obesity and COVID-19: ACE 2, the missing tile. Obes Surg 2020;30:4615–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kuster GM, Pfister O, Burkard T et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J 2020;41:1801–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring) 2020;28:1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singh S, Offringa-Hup AK, Logtenberg SJJ et al. Discontinuation of antihypertensive medications on the outcome of hospitalized patients with severe acute respiratory syndrome-coronavirus 2. Hypertension 2021;78:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ferrario CM, Jessup J, Chappell MC et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 93. Lopes RD, Macedo AVS, de Barros E; et al. BRACE CORONA Investigators. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA 2021;325:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fosbøl EL, Butt JH, Østergaard L et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 2020;324:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 2014;64:1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jiang X, Eales JM, Scannali D et al. Hypertension and renin-angiotensin system blockers are not associated with expression of angiotensin-converting enzyme 2 (ACE2) in the kidney. Eur Heart J 2020;41:4580–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension 2020;75:1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sun ML, Yang JM, Sun YP, Su GH. [Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:219–222. [DOI] [PubMed] [Google Scholar]
- 100. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bean DM, Kraljevic Z, Searle T et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail 2020;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. de Abajo FJ, Rodríguez-Martín S, Lerma V et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 2020;395:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol 2020;5:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Reynolds HR, Adhikari S, Pulgarin C et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang P, Zhu L, Cai J et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ferrante G, Fazzari F, Cozzi O et al. Risk factors for myocardial injury and death in patients with COVID-19: insights from a cohort study with chest computed tomography. Cardiovasc Res 2020;116:2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;5:831–840. [DOI] [PubMed] [Google Scholar]
- 108. Caforio AL, Pankuweit S, Arbustini E et al. European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 109. Wenzel P, Kopp S, Gobel S et al. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res 2020;116:1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tschope C, Ammirati E, Bozkurt B et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 2021;18:169–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol 2019;19:517–532. [DOI] [PubMed] [Google Scholar]
- 112. Maffia P, Guzik TJ. When, where, and how to target vascular inflammation in the post-CANTOS era? Eur Heart J 2019;40:2492–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bartoloni E, Perricone C, Cafaro G, Gerli R. Hypertension and SARS-CoV-2 infection: is inflammation the missing link? Cardiovasc Res 2020;116:e193–e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Smeda M, Chlopicki S. Endothelial barrier integrity in COVID-19-dependent hyperinflammation: does the protective facet of platelet function matter? Cardiovasc Res 2020;116:e118–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li Z, Guo X, Hao W et al. The relationship between serum interleukins and T-lymphocyte subsets in patients with severe acute respiratory syndrome. Chin Med J 2003;116:981–984. [PubMed] [Google Scholar]
- 116. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Siedlinski M, Jozefczuk E, Xu X et al. White blood cells and blood pressure: a Mendelian randomization study. Circulation 2020;141:1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Youn JC, Yu HT, Lim BJ et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 2013;62:126–133. [DOI] [PubMed] [Google Scholar]
- 120. Mohammad S, Aziz R, Al Mahri S et al. Obesity and COVID-19: what makes obese host so vulnerable? Immun Ageing 2021;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chan JF, Yip CC, To KK et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol 2020;58:e00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. European Centre for Disease Prevention and Control. Testing Strategies for SARS-CoV-2. https://www.ecdc.europa.eu/en/covid-19/surveillance/testing-strategies (12 November 2020).
- 123. China National Health Commission. National Health Commission of the People’s Republic of China. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment, 7th ed. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html (16 April 2020).
- 124. World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance. 2020. https://apps.who.int/iris/handle/10665/331329 (16 April 2020).
- 125. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One 2020;15:e0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Long DR, Gombar S, Hogan CA et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis 2021;72:323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ai T, Yang Z, Hou H et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020;296:E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. European Centre for Disease Prevention and Control. Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. https://www.ecdc.europa.eu/sites/default/files/documents/Options-use-of-rapid-antigen-tests-for-COVID-19.pdf (26 March 2021).
- 129. Center for Disease Control and Prevention. Guidance for Healthcare Workers about COVID-19 (SARS-CoV-2) Testing. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing.html (12 November 2020).
- 130. Guan WJ, Ni ZY, Hu Y; et al. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gao C, Cai Y, Zhang K et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J 2020;41:2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ferguson ND, Fan E, Camporota L et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012;38:1573–1582. [DOI] [PubMed] [Google Scholar]
- 134. Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J 2019;40:2671–2683. [DOI] [PubMed] [Google Scholar]
- 135. Gomila-Grange A, Espasa M, Moglia E. Cardiogenic shock caused by SARS-CoV-2 in a patient with serial negative nucleic acid amplification tests. Case report. SN Compr Clin Med 2020;1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Harari R, Bangalore S, Chang E, Shah B. COVID-19 complicated by acute myocardial infarction with extensive thrombus burden and cardiogenic shock. Catheter Cardiovasc Interv 2021;97:E661–E666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kim HN, Lee JH, Park HS et al. A case of COVID-19 with acute myocardial infarction and cardiogenic shock. J Korean Med Sci 2020;35:e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sánchez-Recalde Á, Solano-López J, Miguelena-Hycka J, Martín-Pinacho JJ, Sanmartín M, Zamorano JL. [COVID-19 and cardiogenic shock. Different cardiovascular presentations with high mortality]. Rev Esp Cardiol 2020;73:669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yang X, Yu Y, Xu J et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Baran DA, Grines CL, Bailey S et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29–37. [DOI] [PubMed] [Google Scholar]
- 141. van Diepen S, Katz JN, Albert NM; et al. American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 142. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J 2021;42:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tavazzi G, Pellegrini C, Maurelli M et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Chau VQ, Giustino G, Mahmood K et al. Cardiogenic shock and hyperinflammatory syndrome in young males with COVID-19. Circ Heart Fail 2020;13:e007485. [DOI] [PubMed] [Google Scholar]
- 145. Bollmann A, Hohenstein S, Meier-Hellmann A, Kuhlen R, Hindricks G. Emergency hospital admissions and interventional treatments for heart failure and cardiac arrhythmias in Germany during the COVID-19 outbreak: insights from the German-wide Helios hospital network. Eur Heart J Qual Care Clin Outcomes 2020;6:221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Boriani G, Palmisano P, Guerra F; et al. AIAC Ricerca Network Investigators. Impact of COVID-19 pandemic on the clinical activities related to arrhythmias and electrophysiology in Italy: results of a survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). Intern Emerg Med 2020;15:1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Compagnucci P, Volpato G, Pascucci R et al. Impact of the COVID-19 pandemic on a tertiary-level electrophysiology laboratory in Italy. Circ Arrhythm Electrophysiol 2020;13:e008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Li J, Mazzone P, Leung LWM et al. Electrophysiology in the time of coronavirus: coping with the great wave. Europace 2020;22:1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Oikonomou E, Aznaouridis K, Barbetseas J et al. Hospital attendance and admission trends for cardiac diseases during the COVID-19 outbreak and lockdown in Greece. Public Health 2020;187:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Toniolo M, Negri F, Antonutti M, Mase M, Facchin D. Unpredictable fall of severe emergent cardiovascular diseases hospital admissions during the COVID-19 pandemic: experience of a single large center in Northern Italy. J Am Heart Assoc 2020;9:e017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Baldi E, Sechi GM, Mare C; et al. Lombardia CARe Researchers. Out-of-hospital cardiac arrest during the COVID-19 outbreak in Italy. N Engl J Med 2020;383:496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Baldi E, Sechi GM, Mare C; et al. the Lombardia CARe Researchers. COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J 2020;41:3045–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Marijon E, Karam N, Jost D et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health 2020;5:e437–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sala S, Peretto G, De Luca G et al. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin Electrophysiol 2020;43:891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Colon CM, Barrios JG, Chiles JW et al. Atrial arrhythmias in COVID-19 patients. JACC Clin Electrophysiol 2020;6:1189–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hou W, Zhang W, Jin R, Liang L, Xu B, Hu Z. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect Dis (Lond) 2020;52:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Peltzer B, Manocha KK, Ying X et al. Arrhythmic complications of patients hospitalized with COVID-19: incidence, risk factors, and outcomes. Circ Arrhythm Electrophysiol 2020;13:e009121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang G, Hu C, Luo L et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Peltzer B, Manocha KK, Ying X et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol 2020;31:3077–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Russo V, Di Maio M, Mottola FF et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: a multicenter observational study. Eur J Clin Invest 2020;50:e13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sultanian P, Lundgren P, Stromsoe A et al. Cardiac arrest in COVID-19: characteristics and outcomes of in- and out-of-hospital cardiac arrest. A report from the Swedish Registry for Cardiopulmonary Resuscitation. Eur Heart J 2021;42:1094–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hayek SS, Brenner SK, Azam TU; et al. STOP-COVID Investigators. In-hospital cardiac arrest in critically ill patients with COVID-19: multicenter cohort study. BMJ 2020;371:m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Shao F, Xu S, Ma X et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation 2020;151:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sheth V, Chishti I, Rothman A et al. Outcomes of in-hospital cardiac arrest in patients with COVID-19 in New York City. Resuscitation 2020;155:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Thapa SB, Kakar TS, Mayer C, Khanal D. Clinical outcomes of in-hospital cardiac arrest in COVID-19. JAMA Intern Med 2021;181:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Oates CP, Turagam MK, Musikantow D et al. Syncope and presyncope in patients with COVID-19. Pacing Clin Electrophysiol 2020;43:1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Azarkish M, Laleh Far V, Eslami M, Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J 2020;41:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Babapoor-Farrokhran S, Batnyam U, Wiener PC et al. Atrioventricular and sinus node dysfunction in stable COVID-19 patients. SN Compr Clin Med 2020;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kir D, Mohan C, Sancassani R. Heart brake: an unusual cardiac manifestation of COVID-19. JACC Case Rep 2020;2:1252–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Turagam MK, Musikantow D, Goldman ME et al. Malignant arrhythmias in patients with COVID-19: incidence, mechanisms, and outcomes. Circ Arrhythm Electrophysiol 2020;13:e008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cheung CC, Davies B, Gibbs K, Laksman ZW, Krahn AD. Multilead QT screening is necessary for QT measurement: implications for management of patients in the COVID-19 era. JACC Clin Electrophysiol 2020;6:878–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Corrales-Medina VF, Alvarez KN, Weissfeld LA et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015;313:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation 2012;125:773–781. [DOI] [PubMed] [Google Scholar]
- 174. Kwong JC, Li P, Redelmeier DA. Influenza morbidity and mortality in elderly patients receiving statins: a cohort study. PLoS One 2009;4:e8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. N Engl J Med 2019;380:171–176. [DOI] [PubMed] [Google Scholar]
- 176. Wichmann D, Sperhake J-P, Lütgehetmann M et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020;173:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Madjid M, Miller CC, Zarubaev VV. et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J 2007;28:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Peiris JS, Chu CM, Cheng VC et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Shi S, Qin M, Shen B et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kariyanna PT, Sutarjono B, Grewal E et al. A systematic review of COVID-19 and myocarditis. Am J Med Case Rep 2020;8:299–305.32747875 [Google Scholar]
- 181. Gao C, Wang Y, Gu X; et al. Community-Acquired Pneumonia–China Network. Association between cardiac injury and mortality in hospitalized patients infected with avian influenza A (H7N9) virus. Crit Care Med 2020;48:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Arentz M, Yim E, Klaff L et al. Characteristics and Outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020;323:1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Collet JP, Thiele H, Barbato E; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 184. Flores F, Walter J, Wussler D et al. Direct comparison of high-sensitivity cardiac troponin t and i for prediction of mortality in patients with pneumonia. J Clin Chem Lab Med 2019;2:1000131. [Google Scholar]
- 185. Knight DS, Kotecha T, Razvi Y et al. COVID-19: myocardial injury in survivors. Circulation 2020;142:1120–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Puntmann VO, Carerj ML, Wieters I et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol 2020;76:1244–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Xu Z, Shi L, Wang Y et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ibanez B, James S, Agewall S; et al. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 190. Christ-Crain M, Breidthardt T, Stolz D et al. Use of B-type natriuretic peptide in the risk stratification of community-acquired pneumonia. J Intern Med 2008;264:166–176. [DOI] [PubMed] [Google Scholar]
- 191. Mueller C, Laule-Kilian K, Frana B et al. Use of B-type natriuretic peptide in the management of acute dyspnea in patients with pulmonary disease. Am Heart J 2006;151:471–477. [DOI] [PubMed] [Google Scholar]
- 192. Mueller C, McDonald K, de Boer RA; Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019;21:715–731. [DOI] [PubMed] [Google Scholar]
- 193. Giannitsis E, Mair J, Christersson C et al. ; Biomarker Study Group of the European Society of Cardiology (ESC) Acute Cardiovascular Care Association (ACCA). How to use D-dimer in acute cardiovascular care. Eur Heart J Acute Cardiovasc Care 2017;6:69–80. [DOI] [PubMed] [Google Scholar]
- 194. Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Konstantinides SV, Meyer G, Becattini C; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 196. Kearon C, de Wit K, Parpia S et al. Diagnosis of pulmonary embolism with d-dimer adjusted to clinical probability. N Engl J Med 2019;381:2125–2134. [DOI] [PubMed] [Google Scholar]
- 197. van der Hulle T, Cheung WY, Kooij S; et al. YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet 2017;390:289–297. [DOI] [PubMed] [Google Scholar]
- 198. van der Pol LM, Tromeur C, Bistervels IM; et al. Artemis Study Investigators. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med 2019;380:1139–1149. [DOI] [PubMed] [Google Scholar]
- 199. Clerkin KJ, Fried JA, Raikhelkar J et al. COVID-19 and cardiovascular disease. Circulation 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 200. Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA 2020;323:1769–1770. [DOI] [PubMed] [Google Scholar]
- 201. Boeddinghaus J, Nestelberger T, Twerenbold R et al. ; APACE, BACC, and TRAPID-AMI Investigators. Impact of age on the performance of the ESC 0/1h-algorithms for early diagnosis of myocardial infarction. Eur Heart J 2018;39:3780–3794. [DOI] [PubMed] [Google Scholar]
- 202. Nestelberger T, Wildi K, Boeddinghaus J et al. Characterization of the observe zone of the ESC 2015 high-sensitivity cardiac troponin 0h/1h-algorithm for the early diagnosis of acute myocardial infarction. Int J Cardiol 2016;207:238–245. [DOI] [PubMed] [Google Scholar]
- 203. Twerenbold R, Badertscher P, Boeddinghaus J et al. 0/1-Hour triage algorithm for myocardial infarction in patients with renal dysfunction. Circulation 2018;137:436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Gluckman TJ. General Guidance on Deferring Non-Urgent CV Testing and Procedures During the COVID-19 Pandemic. https://www.acc.org/latest-in-cardiology/articles/2020/03/24/09/42/general-guidance-on-deferring-non-urgent-cv-testing-and-procedures-during-the-covid-19-pandemic (27 November 2020).
- 205. Skulstad H, Cosyns B, Popescu BA et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging 2020;21:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Soldati G, Smargiassi A, Inchingolo R et al. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med 2020;39:1459–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Szekely Y, Lichter Y, Taieb P et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation 2020;142:342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Dweck MR, Bularga A, Hahn RT et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging 2020;21:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Choi AD, Abbara S, Branch KR et al. Society of Cardiovascular Computed Tomography guidance for use of cardiac computed tomography amidst the COVID-19 pandemic. Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2020;14:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Han Y, Zeng H, Jiang H et al. CSC expert consensus on principles of clinical management of patients with severe emergent cardiovascular diseases during the COVID-19 epidemic. Circulation 2020;141:e810–e816. [DOI] [PubMed] [Google Scholar]
- 211. American College of Cardiology. ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (30 March 2020).
- 212. Skali H, Murthy VL, Al-Mallah MH et al. Guidance and best practices for nuclear cardiology laboratories during the coronavirus disease 2019 (COVID-19) pandemic: an information statement from ASNC and SNMMI. J Nucl Cardiol 2020;27:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kelle S, Bucciarelli-Ducci C, Judd RM et al. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J Cardiovasc Magn Reson 2020;22:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Society for Cardiovascular Magnetic Resonance. SCMR’s COVID-19 Preparedness Toolkit. https://scmr.org/page/COVID19 (25 March 2020).
- 215. Ferreira VM, Schulz-Menger J, Holmvang G et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 216. Huang L, Zhao P, Tang D et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging 2020;13:2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020;22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Wood DA, Mahmud E, Thourani VH et al. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: from the North American Society Leadership. J Am Coll Cardiol 2020;75:3177–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Crespo-Leiro MG, Metra M, Lund LH et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:1505–1535. [DOI] [PubMed] [Google Scholar]
- 220. Pieske B, Tschope C, de Boer RA et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 221. Dengel DR, Evanoff NG. Re-opening exercise science laboratories and testing during the COVID-19 endemic phase. Int J Sports Med 2021;42:789–793. [DOI] [PubMed] [Google Scholar]
- 222. Zoghbi WA, DiCarli MF, Blankstein R; et al. ACC Imaging Council. Multimodality cardiovascular imaging in the midst of the COVID-19 pandemic: ramping up safely to a new normal. JACC Cardiovasc Imaging 2020;13:1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kucharski AJ, Russell TW, Diamond C; et al. Centre for Mathematical Modelling of Infectious Diseases COVID-19 Working Group. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis 2020;20:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.