Abstract
Background
Patients with Crohn’s disease (CD) undergo frequent endoscopic procedures, with visualization of the gastrointestinal mucosa central to treatment decision-making. Subsequently, a noninvasive alternative to optical colonoscopy (OC) would be welcomed. One such technology is capsule endoscopy, including the PillCam COLON 2 (PCC2), though research validating its use in ileocolonic CD is limited. This study aims to compare PCC2 with ileocolonoscopy (OC) in assessing mucosal CD through use of a standardized scoring system.
Methods
At an Australian tertiary hospital, same-day PCC2 and ileocolonoscopy results of 47 CD patients, with known nonstricturing disease, were prospectively collected and analyzed for correlation and agreement. Deidentified recordings were reported by a single expert gastroenterologist. Mucosal disease was quantified using the Simple Endoscopic Score for Crohn’s Disease (SES-CD). The SES-CD results of paired endoscopic modalities were compared in total per bowel segment and per SES-CD variable.
Results
Of 47 PCC2 recordings, 68% were complete, fully assessing terminal ileum to rectum, and OC was complete in 89%. Correlation (r) between total SES-CD scores was strongest in the terminal ileum (r = 0.77, P < .001), with the SES-CD variable of “ulcer detection” showing the strongest agreement. The PCC2 (vs OC) identified additional ulcers in the terminal ileum; ascending, transverse, and descending colon; and rectum; scores were 5 (1), 5 (3), 1 (1), 2 (1), and 2 (2), respectively.
Conclusions
The PCC2 shows promise in assessing ileocolonic mucosa, especially in proximal bowel segments, with greater reach of visualization in the small bowel. Given the resource and safety considerations raised by the Coronavirus disease 2019 pandemic, capsule endoscopy has particular significance.
This article aims to contribute to the limited body of research surrounding the validity of capsule endoscopy technology in assessing ileocolonic mucosa in Crohn’s Disease patients. In doing so, an alternative option for patients enduring frequent endoscopies is given potential.
Keywords: Crohn’s disease, capsule endoscopy, PillCam Colon 2, mucosal healing, colonoscopy
Introduction
Crohn’s disease (CD) is a chronic inflammatory condition affecting the gastrointestinal (GI) tract, anywhere from mouth to anus.1 Under the umbrella of inflammatory bowel disease (IBD), along with ulcerative colitis (UC), it is an idiopathic, relapsing, and remitting condition, with clinical features dependent on location. Crohn’s disease differs from UC in that inflammation can be panenteric and transmural; inflammatory cells invade deep mucosal layers sometimes forming granulomas, leading to patches of ulceration, bowel wall thickening, narrowing, and penetrating disease.2
With many patients diagnosed in early adulthood, quality of life among IBD patients can be significantly impaired.3 As CD progresses, complications, bowel obstruction, and fistulae lead to high rates of surgery, with approximately 50% of postoperative cases relapsing and requiring further surgery.2,4 Associated morbidity combined with increasing prevalence places importance on research surrounding optimal CD surveillance.5
Relevance of Endoscopic Assessment in Crohn’s Disease
Therapeutic goals for IBD have evolved over time, particularly for CD, with the understanding that targeting clinical response does not necessarily alter the natural history of disease.6 The new paradigm of treating to target endoscopic response came with the introduction of biological drug therapies, making mucosal healing (MH) an achievable goal.7,8 Maintenance of mucosal integrity is associated with reduced risk of hospitalization and higher rates of clinical remission.9,10 With MH as an important therapeutic goal, endoscopic visualization is key in management decisions.
The Gold Standard for Ileocolonic Endoscopic Assessment in Crohn’s Disease
Optical colonoscopy (OC), or ileocolonoscopy, remains the principal method for CD diagnosis. Despite the terminal ileum (TI) being the most commonly affected small bowel (SB) segment, one-third of patients can present with isolated, more proximal lesions.11 With difficulty in observing such lesions via conventional endoscopies, these patients are at increased risk of complications.11 Optical colonoscopy carries burden in poor patient tolerance, invasiveness, and surgical risks, but despite the use of new technologies such as bowel-ultrasonography, the need for mucosal visualization remains.12
The Advent of Capsule Endoscopy
In 2001, capsule endoscopy (CE) first emerged as a potential answer to the challenging visualization of the SB mucosa.13 The ingestible capsule has an inbuilt camera that continually captures images that are sent wirelessly to a recorder worn around the patient’s waist. Small bowel capsule endoscopy (SBCE) has been established as a valid investigation tool for iron deficiency anemia or overt gastrointestinal bleeding of unknown origin and can be useful in monitoring CD in the small bowel.14 The second-generation colon CE, PillCam COLON 2 (PCC2) by Medtronic, has extended battery life and 2 cameras, allowing for nearly 360º of observation of the visualized bowel and in particular, the colon (image in Supplementary Data Content 1, which shows examples of PCC2 captured images).
Use of PCC2 has been studied extensively in UC and for cancer detection, but its feasibility as an endoscopic monitoring tool in CD, specifically in the colon, has been largely unavailable.15 Limiting factors noted in published literature include the poorer completion rates of PCC2 in comparison with ileocolonoscopy due to insufficient bowel preparation and the risk of capsule retention due to bowel strictures.16 Hence, the suitability of PCC2 as a viable adjunct or even alternative to optical colonoscopy in monitoring of colonic mucosal disease needs further study to assess feasibility and safety.
Scoring Mucosal Disease Activity in Crohn’s Disease
Assessment of mucosal healing endoscopically is encompassed in the Crohn’s Disease Endoscopic Index of Severity (CDEIS), which is regarded as the best endoscopic severity index with good reproducibility and correlation with the clinical score of the Crohn’s Disease Activity Index.17 However, it is also considered complex, requiring training and experience in estimating size, depth, and surface area of ulcerations. A simplified index, the Simple Endoscopic Score for Crohn’s Disease (SES-CD), was then developed, which has improved interobserver agreement, removing the requirement of a subjective estimate of affected mucosal surface area, and scoring anywhere from 0% to 100% in the CDEIS.18 In the majority of studies using SES-CD cutoffs to define CD severity, a score of 0 to 2 is defined as inactive, 3 to 6 as mild, 7 to 15 as moderate, and >15 as severe.19
With the introduction of CE capable of assessing the small bowel, the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI) was created, which was modified to the panenteric “extended CECDAI” for the development of colon CE.20 The current study assesses the feasibility of PCC2 in detecting ileocolonic disease; hence, a panenteric scoring system was not needed. Therefore, the SES-CD was used, being a quick and reproducible tool and reliably correlating with the widely used CDEIS.19
Aims and Hypothesis
This study aims to assess the feasibility of PCC2 as a modality for endoscopic detection of colon and terminal ileal Crohn’s disease compared with the gold standard, optical colonoscopy, using the SES-CD scoring system. We hypothesize that PCC2 would provide visualization of gastrointestinal mucosa at least comparable with OC. The study to date represents an advanced pilot study addressing this hypothesis and is the largest research experiment of its kind.
Methods
Study Design
This study is a single-center, prospective, and comparative analysis of PillCamCOLON2 video recordings in 47 patients with known Crohn’s disease at the Royal Melbourne Hospital (RMH). In light of the Coronavirus disease 2019 (COVID-19) pandemic, patient recruitment was ceased.
Participants
Recruitment began in 2015; suitable CD patients, 18 years and older requiring surveillance ileocolonoscopy, were identified through inflammatory bowel disease clinics and analysis of ileocolonoscopy waiting lists. Patients were excluded if there was clinical suspicion of stricturing disease or known prior evidence of strictures on imaging (exclusion criteria, Supplementary Data Content 2, for full exclusion criteria). Not all patients underwent cross-sectional imaging for the purpose of the study if there was no clinical suspicion of obstruction. Each potential participant was provided with an information booklet outlining the research purpose and risks, with emphasis on freedom to withdraw at any stage. Patients were consented in clinic and again prior to capsule ingestion. Bowel preparation regimens were provided upon confirmed ileocolonoscopy date (see Supplementary Data Content 3 for the bowel preparation regimen provided to participants). After their bowel preparation, patients ingested the capsule prior to ileocolonoscopy, instructed and supervised by a research nurse, aided by the research student and principal investigator. In a portion of patients, tolerability of capsule ingestion (38 of 47) and impact on treatment recommendations (35 of 47) were assessed via patient and clinician questionnaires (see Supplementary Data Content 4 for the patient and clinician questionnaires). Analysis of PCC2 recordings ensured no retained capsule was unrecognized.
Test Methods
The PCC2 recordings were securely uploaded and deidentified using the PILLCAM SOFTWARE V9. Recordings were read by a single expert gastroenterologist blinded to patients’ ileocolonoscopy outcomes. Each camera view on the capsule was assessed separately to determine any difference in visualization. Points of identified disease and anatomical landmarks were thumbnailed; timing was noted, and estimation of ulcer size was aided by the RAPID-9 software. The Simplified Endoscopic Score for Crohn’s disease (SES-CD) was completed by the colonoscopist at ileocolonoscopy and the reader at PCC2 viewing, both blinded to respective outcomes in each circumstance (Table 1). Outcomes of endoscopies, including bowel cleanliness, were stored securely on a password-protected Excel spreadsheet.
Table 1.
The Simple Endoscopic Score for Crohn’s Disease (SES-CD) adapted from the 2004 study Daperno M et al.
| Simple Endoscopic Score For Crohn’s Disease Values | ||||
|---|---|---|---|---|
| Variable | 0 | 1 | 2 | 3 |
| Size of Ulcers | None | Aphthous ulcers < 0.5cm | Large ulcers 0.5–2cm | Very Large ulcers > 2cm |
| Ulcerated Surface | None | <10% | 10%–30% | >30% |
| Affected Surface | Unaffected segment | <50% | 50%–75% | >75% |
| Presence of Narrowing | None | Single, can be passed | Multiple, can be passed | Cannot be passed |
| Total Score: Sum Of All Bowel Segments | ||||
|
SES-CD Activity
Cutoff |
0–2 | 3–6 | 7–15 | >15 |
| Inactive | Mild | Moderate | Severe |
Analysis
Basic analysis of patient demographics and outcomes were completed through Microsoft Excel. Complete evaluation was defined as capture of images from the terminal ileum to the rectum. STATA software was used to assess correlation and agreement between paired endoscopic SES-CD results determined by OC and PCC2. Using Pearson coefficient, total and per bowel segment SES-CD values were correlated between paired endoscopies; only complete endoscopies (terminal ileum to rectum) had total scores assessed. Limits of agreement were assessed through the Bland-Altman test of average difference. Weighted Cohen’s kappa (κ) coefficient of agreement was calculated for each SES-CD variable per bowel segment, given the categorical nature of the score (0-3). The level of significance was determined under a 95% confidence interval (CI; P < .05). Because this was a pilot feasibility study (interrupted by COVID considerations), no calculation of necessary sample size was made.
Ethical Considerations
The protocol of the current study was approved by the site’s ethical committee, conducted in alignment with the ethical principles set out by the Declaration of Helskinki and Good Clinical Practice. This clinical trial is registered with clinicaltrials.gov (NCTO2624414). Protocol for this study was approved by the local institutional review boards of the participating sites. Ethical considerations, including the risk and management of capsule retention and the interaction between the bowel preparation required for CE and the need for fasting prior to sedation for ileocolonoscopy were discussed extensively with the ethics committee.
Participants were enrolled in clinics and from endoscopy waiting lists from a single-tertiary center in Melbourne, Australia, from February 2015 to July 2020. All patients in the study were enrolled after written and informed consent, with opportunity to withdraw their participation at any stage.
Results
Patient Characteristics and Outcomes
Forty-seven patients were included in this study (26 male and 21 female). The median age was 35, with an age range of 18 to 74 years old. The completion rates of PCC2 and OC were 68% (32) and 89% (42), respectively, meaning that terminal ileum to rectum was fully visualized. Two patients had no PCC2 recording of the colon due to unsuspected stricturing disease, with the capsule either passed after the study or retrieved on subsequent ileocolonoscopy. There were no complications associated with capsule procedures. Of the 5 (11%) patients with incomplete colonoscopies, 3 had stricturing disease of the colon (ascending colon, descending colon, and at the ileocecal valve), 1 had poor bowel preparation allowing only for rectal assessment, and 1 patient was suspected to have aspirated during the OC (not substantiated by subsequent radiology), limiting assessment only to the terminal ileum with the need for rapid colonoscope withdrawal. Rates of endoscopic assessments per bowel segment for each modality are detailed in Table 2.
Table 2.
Rates of complete and near-complete assessments per bowel segment by PillCam Colon 2 (PCC2) and ileocolonoscopy (OC).
| Endoscopic Modality | Terminal Ileum | Ascending Colon | Transverse Colon | Descending Colon | Rectum |
|---|---|---|---|---|---|
| PCC2 (n = 47) | 100% (47) | 96% (45) | 91% (43) | 83% (39) | 68% (32) |
| OC (n = 47) | 93% (44) | 93% (44) | 93% (44) | 96% (45) | 98% (46) |
| PCC2 and OC (n = 47) | 93% (44) | 89% (42) | 85% (40) | 83% (39) | 68% (32) |
Bowel cleanliness was reported at ileocolonoscopy and PCC2 viewing. Of the 47 patients, 36% (17) had excellent bowel preparation, 30% (14) good, 26% (12) fair, and 8% (4) poor bowel preparation. There was a reduction in PCC2 completion rate of capsules as bowel cleanliness worsened, with most completed studies in the context of excellent bowel preparation (14 of 32) in comparison with good,10 fair,7 and poor.1 The average length of transit time for completed PCC2 recording was 11 hours.
Mucosal Assessment in Completed Endoscopies: PillCamCOLON2 vs Colonoscopy
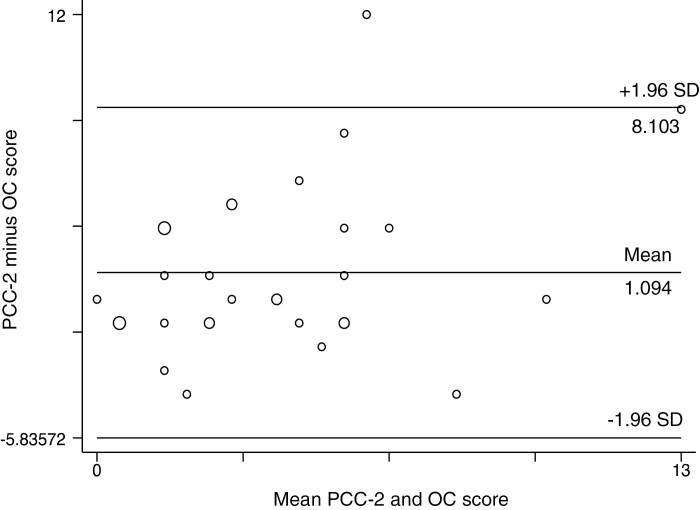
There was moderate correlation between all available complete SES-CD scores and paired PCC2 and OC assessments (n = 32, r = 0.49, P = .001). Figure 1 illustrates this, showing that as SES-CD totals increased, so too did the difference between paired PCC2 and OC scores. To further assess agreement between the endoscopic modalities, total SES-CD scores (n = 32) were decoded into disease activity levels based on the most commonly used cutoffs in current literature (Table 1), where a score of 0 to 2 inferred inactive, 3 to 6 mild, 7 to 15 moderate, and >15 severe disease activity.
Figure 1.
Bland-Altman plot of average difference between the total SES-CD values of paired PCC2 and ileocolonoscopy in 32 CD patients. Larger circles delineate a larger portion of patients, with positive differences inferring PCC2 scored higher. Limits of agreement (within 2 standard deviations [SD]) of the mean showed wide extremes of differences between paired scores (−5.94 to 8.10), with a mean difference of 1.10 (95% CI, −0.13 to 2.38).
Overall agreement between disease activity levels was fair (79%, κ = 0.21, P = .04). Out of the patients deemed to have inactive disease on PCC2 viewing, 4 out of 10 were categorized as mild on OC. A larger portion of patients15 were viewed to have inactive disease on OC, with PCC2 assessing 6 of 15 and 3 of 15 to have mild and moderate activity, respectively. Of those found to have mild disease on CE,15 only 1 patient was rated more severe on OC (moderate activity), in which there was further recognition of ulceration in the ascending colon by OC. This patient was noted to have poor bowel preparation. Findings of moderate activity on PCC2 viewing had little agreement on OC (1 of 6), and all others5 were determined to be of lower severity on OC. Only 1 patient was scored to have severe disease activity on PCC2, with OC scoring this patient moderately; in this case, the capsule detected more ulceration in the terminal ileum. The rates and frequencies of disease activities, as well as the proportion of absolute agreement between paired PCC2 and OC, are tabulated in Table 3.
Table 3.
Disease activity statuses of 32 Crohn’s disease patients as determined by capsule endoscopy (PCC2) and ileocolonoscopy (OC).
| Disease Activity Statusa | Rate and Frequency | ||
|---|---|---|---|
| PCC2 | OC | Agreement | |
| Inactive (SES-CD 0–2) | 31% (10) | 47% (15) | 6/10 |
| Mild (SES-CD 3–6) | 47% (15) | 44% (14) | 8/15 |
| Moderate (SES-CD 7–15) | 19% (6) | 9% (3) | 1/6 |
| Severe (SES-CD > 15) | 3% (1) | 0% (0) | 0/1 |
| Total | 100% (32) | 100% (32) | 15/32 |
aThe Simple Endoscopic Score of Crohn’s Disease (SES-CD) was used to categorize patients into mucosal disease activity states. When PCC2 evaluation agreed with the reference, OC, agreement was noted.
Mucosal Assessment Per Bowel Segment: PillCamCOLON2 vs Colonoscopy
The respective PCC2 and OC SES-CD scores were correlated per bowel segment: terminal ileum (TI), ascending colon (AC), transverse colon (TC), descending colon (DC), and rectum (R). Sample sizes change based on the amount of completed capsule and colonoscopy assessments in each bowel segment. The strongest correlation (r = 0.77, P ≤ .001) was seen in the TI, with correlation weakening in the more distal segments: AC (r = 0.38, P = .01), TC (r = 0.43, P = .01), DC (r = 0.46, P ≤ .001), and insignificant correlation in the rectum (r = 0.16, P = .38). Table 4 outlines these correlations between paired PCC2 and OC scores, with respective limits of difference.
Table 4.
SES-CD correlation and Bland-Altman limits of agreement between PCC2 and OC per bowel segment.
| Bowel Segment | Sample size (n)a | Correlation (R) | P | Limits of Agreement |
|---|---|---|---|---|
| TI | 44 | 0.77 | .001 | −2.91 to 3.45 |
| AC | 42 | 0.38 | .01 | −3.05 to 2.86 |
| TC | 40 | 0.43 | .01 | −2.31 to 2.26 |
| DC | 39 | 0.46 | .001 | −2.30 to 2.76 |
| R | 32 | 0.16 | .38 | −2.30 to 2.92 |
aSample sizes represent the number of complete assessments by both endoscopic modalities by bowel segment: terminal ileum (TI), ascending colon (AC), transverse colon (TC), descending colon (DC) and rectum (R).
Mucosal Assessment per SES-CD Variable: PillCamCOLON2 vs Colonoscopy
The scores per category of the SES-CD scoring system, size of ulcers, ulcerated surface area, and affected surface area were assessed for agreement (κ) between the 2 modalities; 0.01 to 0.2 was considered to be none or slight, 0.21 to 0.4 fair, 0.41 to 0.6 moderate, 0.61 to 0.8 substantial, and 0.81 to 1 almost perfect. Additionally, 95% confidence intervals were used.
Assessment by PCC2 and OC of size of ulcers showed substantial agreement in the TI (89%, κ = 0.63, P < .001), with fair agreement in the AC (κ = 0.23, P = .03), and moderate agreement in the TC and DC (κ = 0.38 and κ = 0.53, respectively; P < .001).
Agreement in assessment of ulcerated surface was again substantial in the TI (93%, κ = 0.72, P < .001), none or slight in the AC, moderate in the TC (κ = 0.47, P < .001), and substantial in the DC (κ = 0.64, P < .001).
Overall estimation of affected surface area, which includes assessment of inflammation, showed the least agreement, where PCC2 tended systematically to score higher. Agreement in the rectum was insignificant in all assessed variables.
The final SES-CD variable, presence of narrowing, was not assessed for agreement, given the inherent difference in the ability of a capsule to pass a stricture against that of a larger colonoscope. In the TI (n = 44), there were 4 strictures noted on PCC2, though all could be passed. Of these, 1 was unable to be negotiated by OC.
Additional Findings on PillCamCOLON2 vs Colonoscopy
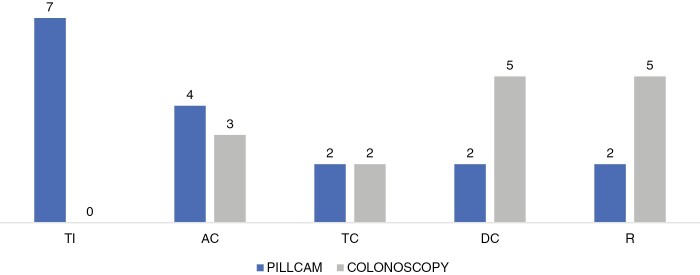
Detection of additional ulcers by either PCC2 or OC was determined through analysis of unassessed segments and segments scored to have no ulcers on either modality. The PCC2 detected more additional ulcers in the more proximal segments of bowel—TI and AC—as opposed to ileocolonoscopy, which had higher additional detections in the DC and rectum. Overall, more additional ulcers were detected on PCC217 compared with OC.15 The differences in additional ulcers detected exclusively by one modality vs the other are illustrated in Figure 2.
Figure 2.
The number of ulcers detected exclusively by either PillCam Colon 2 or ileocolonoscopy in each bowel segment. Detection of additional ulcers by one modality were still included even if the other modality did not assess the bowel segment. Abbreviations: TI, terminal ileum, AC, ascending colon, TC, transverse colon, and R, rectum.
Looking specifically at the sizes of additional ulcers detected on PCC2, 8 of 17 were aphthous (<0.5 cm), 8 were large (0.5-2cm), and 1 was determined to be very large (>2 cm). When examined with OC, 11 of 15 were aphthous, 2 large and 2 very large. If we consider differences in completion rates, of the 15 bowel segments in which OC found additional ulceration, 7 were not assessed by PCC2. The 2 very large ulcers viewed in the DC of 1 patient on OC were not assessed by PCC2 due to a stricture. In the same patient however, PCC2 detected a very large ulcer proximal to the stricture, which was impassable by the OC.
Of the ulcers detected in the terminal ileum on OC,13 PCC2 detected all but 1. The PCC2 missed 3 ulcers in the AC and 1 in the TC detected on ileocolonoscopy, though these were only small (aphthous) ulcers. In the distal segments, DC and rectum, there were 10 ulcers undetected on PCC2, largely due to areas not being assessed (7 of 10). Those missed were in the majority aphthous (70%).
Other additional findings on PCC2 included diverticulae, gastric disease, polyps, and proximal small-bowel inflammation. The latter was not formerly assessed but was noted in 6 of 47 patients. Ileocolonoscopy had advantage of identifying anal disease, and further characterizing polyps through ancillary optical techniques.
Management Outcomes and Tolerability
Follow-up occurred in outpatient clinic following the procedures. Formal questionnaires were provided to clinicians in 35 of 47 patients, assessing treatment recommendation agreement based on PCC2 and OC findings. Post hoc analysis of correspondence for the remaining patients allowed for inference of agreement on treatment recommendations: continue current management or treatment upgrade. This aspect for these patients was, therefore, not prospective.
Forty-four of 47 patients had treatment recommendations recorded. Of the patients requiring treatment upgrades,16 agreement between PCC2 and OC assessments of mucosal disease severity was seen in 14 out of 16. The 2 “disagreeing” assessments had treatment upgrades based on PCC2 findings of TI disease. One other patient was found to have proximal SB involvement on PCC2, further supporting treatment upgrade.
Tolerability of capsule vs colonoscopy was formally assessed via questionnaire in 38 of 47 patients; 71% (27) preferred CE, and 8% (3) had no preference. The remaining 21% (8) preferred colonoscopy describing lack of familiarity and the extra bowel preparation to be factors in their choice.
Discussion
This study, comparing PCC2 assessment of mucosal disease in adult Crohn’s patients against the gold-standard ileocolonoscopy, demonstrated that capsule endoscopy is a useful tool in ulcer detection within the terminal ileum and colon. Considering its noninvasive nature, PCC2 presented advantages unmatched by colonoscopy in detection of ileal disease and patient tolerability.
Assessment of Disease Activity
In comparing totals of paired SES-CD assessments, PCC2 vs colonoscopy, correlation was moderate, with capsule scoring higher on average. As noted in the Bland-Altman plot of average differences between paired scores, the greatest difference was noted at more severe scores. For example in 1 patient, total PCC2 score was 12, whereas OC scored 0. In this case, as in most other cases in which PCC2 categorized patients into more severe statuses, estimation of affected surface was a common point of difference. The PCC2 was seen to systematically determine mucosal area affected by inflammation to be higher than OC across all bowel segments, leading to outliers in data sets. This may be due to limits in optical resolution of the PCC2 captured images, with blurring of the mucosa. Overestimation of the size of the bowel segment itself by capsule could also lead to higher scores.
Adding to the differences noted in disease severity assessments by SES-CD was the additional mucosal disease noted in the terminal ileum on capsule. For example, there was only 1 patient found to have severe disease activity status by PCC2, detected in the terminal ileum ulceration—this was not noted on OC. In our study, these results can be attributed to the capsule’s increased visualization of this segment. Although not marketed or approved for ileal endoscopy, our results indicate there is important gain to be had from this off-label aspect of the PCC2.
As bowel cleanliness worsened, complete PCC2 assessment diminished, emphasizing the importance of adequate bowel preparation in allowing for successful visualization and adequate comparable sample sizes. Other studies with improved colon capsule completion rates have used sodium phosphate boosters; use of sodium phosphate is restricted in Australia due to associated acute kidney injury.21,22
In determining the feasibility of PCC2 against colonoscopy, we postulate that analyzing differences in disease statuses determined by the overall SES-CD is not as useful as analyzing ulcer detection rates. This is due to the inherent differences described previously in scoring the variables: affected surface area and presence of narrowing, in addition to the lack of clinical worth for cutoff scores influenced by these less objective variables.
Ulcer Detection
The capsule showed strength in detection of ulcers: additional ulcers were found more frequently on PCC2 viewing than OC, even in the context of segments being not fully assessed by capsule. This is important clinically, considering the impact of ulceration and further mucosal healing in outcomes for Crohn’s patients.
All colonoscopy assessments of the terminal ileum were assessed by capsule (n = 44), in which agreement levels were the strongest. However, in the context of fewer sufficient PCC2 captured images more distally due to effects of increasing fecal matter in the large bowel, agreement levels worsened.
Incidences of extra ulcers found on capsule were greatest in the terminal ileum and ascending colon. Successful assessment of the terminal ileum is important when considering the distribution of Crohn’s disease, with approximately three-quarters of patients having ileal involvement.23 This is further supported in the analysis of treatment recommendations, where treatment upgrades were based on capsule findings in 4 patients; 3 were found to have terminal ileum ulceration, and 1 had more proximal small-bowel involvement detected on capsule. However in the distal colon, OC had an advantage through stronger completion rates and hence detection of more ulcers in comparison with capsule.
Limitations
The Simple Endoscopic Score of Crohn’s Disease, which provided the basis of mucosal disease assessment in this study, has certain limitations. Despite being the most widely used endoscopic assessment tool for Crohn’s disease, it presents issues in the same allocation of points across different pathologies. For example, a score of 3 will be given for very large ulcers, >30% ulcerated surface area, >75% affected mucosal surface, and for a narrowing that could not be passed. Thus, when SES-CD scores are totaled and converted into activity statuses, comparisons will not reflect agreement amongst different pathologies (eg, ulceration, mucosal inflammation, and stricturing disease). The adoption of the SES-CD scoring system, including the variable presence of narrowing, was used only for the post hoc analysis of modality comparisons, not for any selection purpose, given that selection of patients excluded those suspected to have obstruction or bowel narrowing. Additionally, the number of assessed bowel segments is not taken into account by the SES-CD; meaning for this study to assess total scores, all partially incomplete endoscopies had to be removed from the sample. This was problematic as sample sizes were reduced significantly, from 47 patients (who had ingested PCC2 with subsequent colonoscopy) to 32 patients (who had complete paired endoscopies). The cohort size itself is recognized to limit this study’s comment on safety and utility of PCC2; however, this is a pilot study.
As discussed previously, the reading of CE recordings in assessment of bowel mucosa is heavily dependent on adequate bowel preparation, which presents a limitation to PillCam technology otherwise negated by the insufflation and washing abilities of colonoscopy.
In analyzing ulcer detection rates between modalities, calculations of sensitivity and specificity were not addressed, given that an ulcer detected on colonoscopy was not then exactly matched to the same ulcer detected on capsule. Further, subsequent histological confirmation of disease found on colonoscopy was not reported, and so diagnostic accuracy of capsule findings vs that of pathologically verified findings at colonoscopy could not be assured.
Clinical Impact and Future Direction
Capsule agreement with colonoscopy on management recommendations was not noted to be close, supporting a role for PCC2 findings to guide clinical practice independently of OC findings.
The current study evaluates the feasibility of PillCam Colon 2 in its assessment of mucosal disease in Crohn’s patients; to our knowledge, this is the largest comparative study of its kind reported to date. Its use in monitoring of Crohn’s disease has clear purpose in predicting prognosis, including hospitalization and surgery, with the potential for equal if not better ulcer detection than colonoscopy, given equal chance of completion. In this, we see potential for PCC2 application in patient follow-up over time and measurement of response to therapy, with the use of the SES-CD scoring system supported in previous studies to quantify mucosal response to treatment; a score of 0 to 3 or a 50% reduction in previous score indicates a good response.24,25 Because the small bowel capsule (SBCE) has a recognized advantage in assessing the small bowel, the only missing piece is improved colonic assessment. This may come with further advancements in technology, now with the release of the latest capsule, PillCam Crohn’s, with battery life permitting complete panenteric views and software that allows for more accurate assessment of location and mucosal disease. The figure in Supplementary Data Content 5 depicts the location software of PillCam Crohn’s.
To fully appreciate all that PillCam technology can provide for Crohn’s patients, endoscopic visualization must be given emphasis in management and treatment decisions. In this, an endoscopic index must be validated for panenteric CE to support its use practically in patient management. Relative impact on clinical management needs further study, with histological assessment used as adjunct.
Endoscopic assessments with reduced associated patient and hospital burden and avoidance of sedation are the goal, especially when considering the potential impact of improved tolerability on patient compliance. Additionally, in the setting of the COVID-19 pandemic, reducing contact and hospital admission for immunocompromised patients, such as CD patients, is now highly relevant.
Despite the potential advantages presented in CE, further research assessing its accuracy, aiming for improved completion rates, is needed before it can be used a stand-alone tool, outside the requirements of same-day ileocolonoscopy.
The current study has identified at least a complementary role for PillCam Colon 2 in the assessment of mucosal healing in Crohn’s disease, with visualization approximating those achieved at ileocolonoscopy, although there remain certain caveats relating to completion rates and bowel cleanliness.
Conclusions
PillCam Colon 2 shows promise in its assessment of colonic mucosa in the context of additional small bowel assessment not fully appreciated in this study. In assessed bowel segments, PCC2 adequately detected ulcers, though often overestimated surface area of inflamed mucosa. Because PCC2 is a more tolerable, noninvasive option for patients, its use in monitoring mucosal disease in CD patients, who require frequent endoscopic visualization, would be appreciated. Further study is needed to validate its use in ileocolonic assessment in the setting of improved completion rates not confounded by simultaneous ileocolonoscopy. As technology advances, reliability in panenteric capsule endoscopy will only expand, leaving room for further research into its use in Crohn’s disease.
Supplementary Material
Acknowledgements
Authors would like to acknowledge the contribution of research staff, Zafirah Khan and Virginia Bird, and the Department of Colorectal Medicine and Genetics at The Royal Melbourne Hospital, leading Australian research in colon capsule endoscopy in Crohn’s disease. Authors are most grateful to the participants of this study, in giving their trust and time to the efforts of the research.
This investigator-initiated trial received supportive funding and devices and PillCam capsule endoscopy systems from Medtronic (Award number ISR-2015-10565). This funding did not contribute to the wages of any author and had no part in the study’s design, practice, or analysis. This article aims to contribute to the limited body of research surrounding the validity of capsule endoscopy technology in assessing ileocolonic mucosa in Crohn’s Disease patients. In doing so, an alternative option for patients enduring frequent endoscopies is given potential.
Supplement Sponsorship
This supplement was sponsored by the Crohn’s & Colitis Foundation.
Conflicts of Interest
None declared.
References
- 1. Wilkins T, Jarvis K, Patel J. Diagnosis and management of Crohn’s disease. Am Fam Physician. 2011;84:1365–1375. [PubMed] [Google Scholar]
- 2. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrews JM, Mountifield RE, Van Langenberg DR, et al. . Un-promoted issues in inflammatory bowel disease: opportunities to optimize care. Intern Med J. 2010;40:173–182. [DOI] [PubMed] [Google Scholar]
- 4. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. [DOI] [PubMed] [Google Scholar]
- 5. Wilson J, Hair C, Knight R, et al. . High incidence of inflammatory bowel disease in Australia: a prospective population-based Australian incidence study. Inflamm Bowel Dis. 2010;16:1550–1556. [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ. Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology. 2008;135:1442–1447. [DOI] [PubMed] [Google Scholar]
- 7. van Dullemen HM, van Deventer SJ, Hommes DW, et al. . Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995;109:129–135. [DOI] [PubMed] [Google Scholar]
- 8. Lichtenstein GR, Loftus EV, Isaacs KL, et al. . ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 9. Efthymiou A, Viazis N, Mantzaris G, et al. . Does clinical response correlate with mucosal healing in patients with Crohn’s disease of the small bowel? A prospective, case-series study using wireless capsule endoscopy. Inflamm Bowel Dis. 2008;14:1542–1547. [DOI] [PubMed] [Google Scholar]
- 10. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group . Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 11. Flamant M, Trang C, Maillard O, et al. . The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1390–1396. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Leung WK. Colon capsule endoscopy for inflammatory bowel disease. J Dig Dis. 2018;19:386–394. [DOI] [PubMed] [Google Scholar]
- 13. Adler SN. The history of time for capsule endoscopy. Ann Transl Med. 2017;5:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koulaouzidis A, Rondonotti E, Giannakou A, Plevris JN. Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: a systematic review. Gastrointest Endosc. 2012;76:983–992. [DOI] [PubMed] [Google Scholar]
- 15. Hong SN, Kang SH, Jang HJ, Wallace MB. Recent advance in colon capsule endoscopy: what’s new? Clin Endosc. 2018;51:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boal Carvalho P, Rosa B, Dias de Castro F, et al. . PillCam COLON 2 in Crohn’s disease: a new concept of pan-enteric mucosal healing assessment. World J Gastroenterol. 2015;21:7233–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daperno M, D’Haens G, Van Assche G, et al. . Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 19. Koutroumpakis E, Katsanos KH. Implementation of the simple endoscopic activity score in crohn’s disease. Saudi J Gastroenterol. 2016;22:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niv Y, Gal E, Gabovitz V, et al. . Capsule Endoscopy Crohn’s Disease Activity Index (CECDAIic or Niv Score) for the Small Bowel and Colon. J Clin Gastroenterol. 2018;52:45–49. [DOI] [PubMed] [Google Scholar]
- 21. Oliva S, Cucchiara S, Civitelli F, et al. . Colon capsule endoscopy compared with other modalities in the evaluation of pediatric Crohn’s disease of the small bowel and colon. Gastrointest Endosc. 2016;83:975–983. [DOI] [PubMed] [Google Scholar]
- 22. Russmann S, Lamerato L, Marfatia A, et al. . Risk of impaired renal function after colonoscopy: a cohort study in patients receiving either oral sodium phosphate or polyethylene glycol. Am J Gastroenterol. 2007;102:2655–2663. [DOI] [PubMed] [Google Scholar]
- 23. Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn’s disease. Dis Mon. 2018;64:20–57. [DOI] [PubMed] [Google Scholar]
- 24. Grover Z, Muir R, Lewindon P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J Gastroenterol. 2014;49: 638–645. [DOI] [PubMed] [Google Scholar]
- 25. Ferrante M, Colombel JF, Sandborn WJ, et al. ; International Organization for the Study of Inflammatory Bowel Diseases . Validation of endoscopic activity scores in patients with Crohn’s disease based on a post hoc analysis of data from SONIC. Gastroenterology. 2013;145:978–986.e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.