Learning points for clinicians
One of the coronavirus disease 2019 (COVID-19) vaccination-associated miserable side effects is Guillain–Barré syndrome (GBS). We report a rare case of sensory ataxic immunoglobulin G anti-GM1 antibody-positive GBS following first mRNA COVID-19 vaccine BNT162b2. Although rare, we need to be careful as GBS has been reported even after this vaccination.
Introduction
Coronavirus disease 2019 (COVID-19) vaccines have been used since December 2020 to stop the pandemic progression. Although the vaccines are extremely effective, their side effects are largely unknown and require further research.1
Guillain–Barré syndrome (GBS) is an immune-mediated acute polyradiculoneuritis.2 Approximately one-third of patients develop respiratory failure. The effects of infectious diseases and vaccination on immunity may be related to pathological conditions. However, the definitive causal relationship between GBS and vaccination remains unknown.
We report a case of sensory ataxic GBS with immunoglobulin G (IgG) anti-GM1 antibodies following the first dose of mRNA COVID-19 vaccine BNT162b2 (Pfizer).
Case presentation
A 55-year-old woman with no medical history visited the hospital because of difficulty in walking for about 4 days. She had received the first dose of mRNA COVID-19 vaccine BNT162b2 13 days before her onset. No infectious symptoms, including diarrhea or coughing, preceded the difficulty walking. Deep tendon reflexes were absent generally. She had pseudoathetosis (Figure 1). A decrease in vibration sensation and warm pain sensation was reported in the limbs and trunk. Muscle weakness and autonomic or cranial nerve symptoms were absent.
Figure 1.

Pseudoathetosis. A state in which the fingers are separated from each other and cannot be aligned straight.
Blood samples tested positive for IgG anti-GM1 and anti-SS-A antibodies but negative for other autoantibodies and infectious diseases. Cerebrospinal fluid findings showed protein 44.5 mg/dl, cell number 1/μl and sugar 70 mg/dl. Head magnetic resonance imaging (MRI), spinal cord MRI and neck-to-pelvis contrast-enhanced computed tomography findings showed no obvious abnormalities.
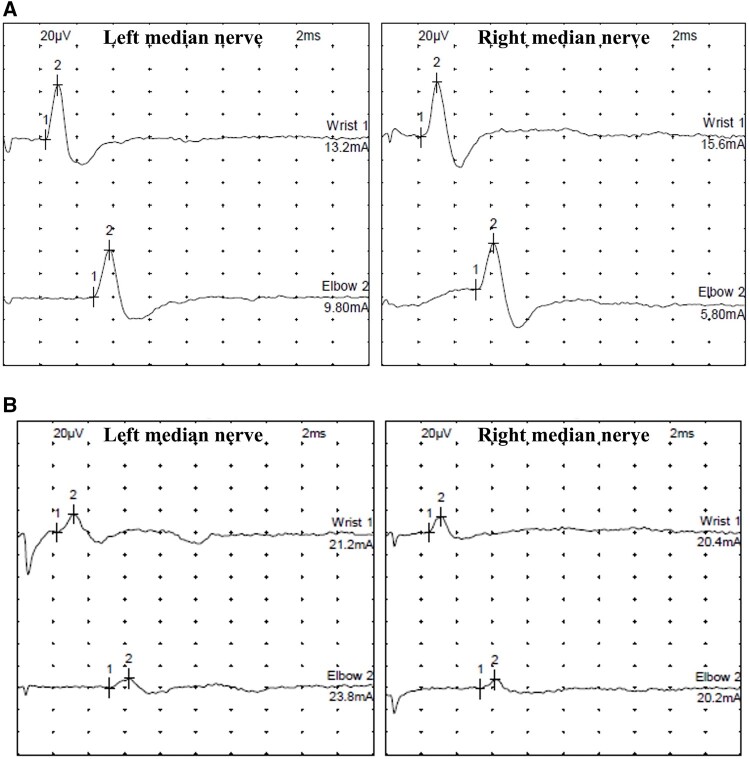
A nerve conduction study (NCS) performed on Day 1 of admission was normal. However, NCS on Day 6 demonstrated reduced amplitude of sensory nerve action potentials (SNAPs) in the median (Figure 2A and B), ulnar and peroneal nerves. Motor conduction study and F-wave persistence results were otherwise normal. Schirmer’s test and salivary gland biopsy results were negative.
Figure 2.
NCS; left and right median nerve. (A) NCS performed on Day 1 of admission were normal. (B) NCS on Day 6 demonstrated reduced amplitude of SNAP in the median, ulnar and peroneal nerve.
Although the patient had no weakness, sensory GBS was diagnosed based on clinical presentation, supportive investigations and the exclusion of alternative diagnoses.
Intravenous Ig 0.4 gm/kg/day was administered for 5 days. Her symptoms improved rapidly after treatment initiation, and she was able to walk using a walker 3 days later and walk independently 10 days later. The extent of decreased temperature and pain sensation was localized to the forearm and parts of the trunk and thigh. She was transferred to a rehabilitation hospital 20 days after admission.
Discussion
We encountered a case of GBS that developed after the first dose of mRNA COVID-19 vaccine BNT162b2. The symptoms improved after high-dose intravenous Ig therapy and rehabilitation.
COVID-19 vaccines used worldwide are greatly beneficial. However, side effects are also being frequently reported, including minor side effects of fever, malaise and headache, severe side effects of anaphylaxis and thrombosis and the grave side effect of GBS.1 Several studies have reported GBS after ChAdOx1-S (CovishieldTM/Vaxzevria, Astra-Zeneca)3 and Ad26.COV2.S (Janssen) vaccinations,4 with a tendency of bilateral facial paralysis, muscle weakness in the limbs and negative anti-ganglioside antibodies. However, only a few GBS cases after BNT162b2 vaccination have been reported, with a similar tendency of muscle weakness in the limbs and negative anti-ganglioside antibodies.5,6 In this case, GBS post-BNT162b2 vaccination showed both sensory ataxia symptoms and GM1 ganglioside antibody positivity.
In conclusion, we report a patient with GBS who presented with GM1 ganglioside antibody positivity and only sensory ataxia symptoms without muscle weakness after BNT162b2 vaccination, which serves as an important case for clarifying the pathophysiology of BNT162b2 vaccination-induced GBS and disseminating vaccines in the future.
Patient consent
I have a consent form from the patient.
Conflict of interest. None declared.
References
- 1. Hadj Hassine I. Covid-19 vaccines and variants of concern: a review. Rev Med Virol 2021; e2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shahrizaila N, Lehmann HC, Kuwabara S.. Guillain-Barré syndrome. Lancet 2021; 397:1214–28. [DOI] [PubMed] [Google Scholar]
- 3. Maramattom BV, Krishnan P, Paul R, Padmanabhan S, Cherukudal Vishnu Nampoothiri S, Syed AA, et al. Guillain-Barré syndrome following ChAdOx1-S/nCoV-19 vaccine. Ann Neurol 2021; 90:312–4. [DOI] [PubMed] [Google Scholar]
- 4. Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N.. Association of receipt of the Ad26.COV2.S COVID-19 vaccine with presumptive Guillain-Barré syndrome, February-July 2021. JAMA 2021; 326:1606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trimboli M, Zoleo P, Arabia G, Gambardella A.. Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Neurol Sci 2021; 42:4401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS.. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus 2021; 13:e13426. [DOI] [PMC free article] [PubMed] [Google Scholar]