Abstract
Background
Multisystem inflammatory syndrome in adults (MIS-A) was reported in association with the coronavirus disease 2019 (COVID-19) pandemic. MIS-A was included in the list of adverse events to be monitored as part of the emergency use authorizations issued for COVID-19 vaccines.
Methods
Reports of MIS-A patients received by the Centers for Disease Control and Prevention (CDC) after COVID-19 vaccines became available were assessed. Data collected on the patients included clinical and demographic characteristics and their vaccine status. The Vaccine Adverse Events Reporting System (VAERS) was also reviewed for possible cases of MIS-A.
Results
From 14 December 2020 to 30 April 2021, 20 patients who met the case definition for MIS-A were reported to CDC. Their median age was 35 years (range, 21–66 years), and 13 (65%) were male. Overall, 16 (80%) patients had a preceding COVID-19-like illness a median of 26 days (range 11–78 days) before MIS-A onset. All 20 patients had laboratory evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Seven MIS-A patients (35%) received COVID-19 vaccine a median of 10 days (range, 6–45 days) before MIS-A onset; 3 patients received a second dose of COVID-19 vaccine 4, 17, and 22 days before MIS-A onset. Patients with MIS-A predominantly had gastrointestinal and cardiac manifestations and hypotension or shock.
Conclusions
Although 7 patients were reported to have received COVID-19 vaccine, all had evidence of prior SARS-CoV-2 infection. Given the widespread use of COVID-19 vaccines, the lack of reporting of MIS-A associated with vaccination alone, without evidence of underlying SARS-CoV-2 infection, is reassuring.
Keywords: coronavirus, COVID-19, MIS-A, MIS-C, multisystem inflammatory syndrome in adults
Seven of 20 MIS-A patients received COVID-19 vaccination before illness onset. All patients had evidence of prior SARS-CoV-2 infection. Given widespread COVID-19 vaccinations in the United States, the lack of reporting of MIS-A associated with vaccination alone is reassuring.
A novel multisystem inflammatory syndrome in children (MIS-C) temporally associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was first described in April 2020, initially in the United Kingdom and subsequently in other countries [1–5]. Almost all patients reported in the United States had evidence of SARS-CoV-2 infection, with a delayed occurrence in most patients of 2–6 weeks after acute infection [5, 6]. A similar multisystem inflammatory syndrome was identified in adults (MIS-A) beginning in June 2020 [7]. The clinical course of both MIS-C and MIS-A predominantly includes fever, elevated inflammatory markers, gastrointestinal symptoms such as vomiting and diarrhea, shock or hypotension, cardiac dysfunction, and in some cases rash and bilateral conjunctivitis [5, 7]. Similar to MIS-C, patients with MIS-A typically have evidence of prior SARS-CoV-2 infection by serologic testing [5, 7].
As the coronavirus disease 2019 (COVID-19) pandemic spread in multiple waves throughout the United States, the occurrence of MIS-C peaks followed COVID-19 peaks by 2–5 weeks [5]. As of June 2021, 4018 MIS-C cases have been reported to the Centers for Disease Control and Prevention’s (CDC’s) national surveillance system [8]. In contrast, during the same period, fewer than 150 MIS-A cases have been reported to CDC or published in the scientific literature [7]. The temporal association of both MIS-C and MIS-A with the COVID-19 pandemic, their delayed occurrence by 2–6 weeks after acute SARS-CoV-2 infection, evidence of generalized inflammation, and multi-organ involvement suggest that both syndromes result from a dysregulated immune response to SARS-CoV-2 infection [9, 10]. However, the mechanisms driving this immune dysregulation and any potential predisposing environmental or host factors are unknown.
Data on patients with MIS-A published in the scientific literature and spontaneously reported to CDC by health departments and clinicians across the United States were previously analyzed to learn more about the clinical phenotype of the illness [7]. Due to the theoretical risk of MIS-A after exposure to any SARS-CoV-2-derived antigen, MIS-A was included in the list of potential adverse events following COVID-19 vaccination to be monitored as part of the Food and Drug Administration (FDA) emergency use authorization (EUA) [11]. In the present analysis, we assessed the clinical characteristics of MIS-A patients reported to CDC after COVID-19 vaccines were authorized for use among adults in the United States and we compared the demographic and clinical features of patients with and without a vaccination history. For patients who received a COVID-19 vaccine, we evaluated timing of vaccine receipt in relation to the onset of MIS-A and presence or absence of evidence of natural SARS-CoV-2 infection.
METHODS
The analysis included clinical data from patients with suspected MIS-A identified from different sources, including spontaneous reporting by treating clinicians and state health departments, during the period 14 December 2020 to 30 April 2021. Clinical information was evaluated using the criteria in the CDC case definition for MIS-A (Table 1) [12]. The case definition included hospitalized patients with fever, primary and secondary clinical criteria, and laboratory evidence of inflammation and SARS-CoV-2 infection. Only patients who met the CDC case definition for MIS-A were included in the analysis. In addition to the clinical data, information about the COVID-19 vaccine type, timing, and the number of doses administered was reviewed. Available laboratory evidence of natural SARS-CoV-2 infection by molecular testing and serology for anti-nucleocapsid antibody, which is not induced by spike-based vaccines, was also evaluated. All COVID-19 vaccines currently available in the United States are based on SARS-CoV-2 spike protein. Some cases following COVID-19 vaccine were reviewed by infectious disease specialists and other medical experts in CDC’s Clinical Immunization Safety Assessment Project which is established to review adverse events for vaccines used in the United States [13]. Information from MIS-A patients was obtained using a case report form developed to collect demographic, clinical, laboratory, imaging and treatment data or by using information from the Vaccine Adverse Event Reporting System (VAERS) and available medical records. VAERS is the national passive surveillance system for adverse events after immunization, which is jointly operated by CDC and FDA [14].
Table 1.
Case Definition for Multisystem Inflammatory Syndrome in Adults
| A patient aged ≥21 years hospitalized for ≥24 hours, or with an illness resulting in death, who meets the following clinical and laboratory criteria.The patient should not have a more likely alternative diagnosis for theillness(eg, bacterial sepsis, exacerbation of achronicmedicalcondition). |
|---|
| I.\tClinical criteria
\tSubjective fever or documented fever (≥38.0C) for ≥24 hours prior to hospitalization or within the first 3 days of hospitalizationa and atleast3ofthe followingclinical criteriaoccurringprior to hospitalization or withinthe first 3 daysof hospitalization.aAt least 1 must be a primary clinical criterion. |
| A.\tPrimary clinical criteria
\t\t1.\tSevere cardiac illness \t\t\tIncludes myocarditis, pericarditis, coronary artery dilatation/aneurysm, ornew-onset right or left ventricular dysfunction (LVEF < 50%), 2nd/3rd degree A-V block, or ventriculartachycardia. (Note: cardiac arrest alone does not meet this criterion) \t\t2.\tRash AND nonpurulent conjunctivitis \tB.\tSecondary clinical criteria \t\t1.\tNew-onset neurologic signs and symptoms \t\t\tIncludes encephalopathy in a patient without prior cognitive impairment, seizures, meningeal signs, or peripheral neuropathy (including Guillain-Barré syndrome) \t\t2.\tShock or hypotension not attributable to medical therapy (eg, sedation, renal replacement therapy) \t\t3.\tAbdominal pain, vomiting, or diarrhea \t\t4.\tThrombocytopenia (platelet count < 150 000/ microliter) |
| II.\tLaboratory evidence
\tThe presence of laboratory evidence of inflammation AND SARS-CoV-2 infection. |
| \tA.\tElevated levels of at least 2 of the following: C-reactive protein, ferritin, IL-6, erythrocyte sedimentation rate, procalcitonin
\tB.\tA positive SARS-CoV-2 testfor current or recent infectionby RT-PCR, serology, or antigen detection |
Abbreviations: IL-6, interleukin 6; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, sever acute respiratory syndrome coronavirus 2.
These criteria must be met by the end of hospital day 3, where the date of hospital admission is hospital day 0. The case definition is available at https://www.cdc.gov/mis-c/mis-a/hcp.html.
Cases of MIS-A among vaccine recipients are required to be reported to VAERS as part of the COVID-19 vaccine EUAs [11]. A VAERS search was completed for reports received from 14 December 2020 to 30 April 2021, using text and automated coding searches for key terms from the MIS-A case definition. Cases were identified if one of the following three search strategies were fulfilled: (1) search for “systemic inflammatory response syndrome” which is the designated term by medical dictionary for regulatory activities (MedDRA) for the suspected diagnosis of multisystem inflammatory syndrome in adults; (2) text string search of “multi-system inflammatory syndrome,” “MIS-A,” “MISA,” or “MIS-C” found in symptoms text, laboratory data, illness at time of vaccination, preexisting illness, and allergies on the initial VAERS report form; or (3) combinations of the following preferred terms: (a) “pyrexia” and (b) “myocarditis,” or “conjunctivitis” or “rash”; and (c) “abdominal pain upper” or “thrombocytopenia” or “shock” or “encephalopathy”; and (d) “ESR increased” or “C-reactive protein increased” or “serum ferritin increased” or “procalcitonin increased.”
Demographic and clinical data of MIS-A patients who received the COVID-19 vaccine were compared with those of MIS-A patients who did not receive the vaccine. Fisher exact tests were used to assess differences in categorical variables, and Mann-Whitney U tests were used to assess differences in continuous variables. When available, the timing of preceding COVID-19 illness onset, SARS-CoV-2 laboratory testing, and/or COVID-19 vaccination dates were assessed and described in relation to MIS-A onset. The activity was determined to meet criteria for public health surveillance as defined in 45 CFR 46.102(l) [2] at the CDC.
RESULTS
From 14 December 2020 to 30 April 2021, 26 patients with suspected MIS-A were reported to CDC. Of these, 20 (77%) patients met the case definition for MIS-A and are included in the present analysis. The demographic and clinical characteristics of these MIS-A patients are summarized in Tables 2 and 3. The median age of the patients was 35 years (range, 21–66 years), and 13 (65%) patients were male (Table 2). Eight (40%) patients were non-Hispanic White, 7 (35%) were non-Hispanic Black, 2 (10%) were Hispanic, 2 (10%) were non-Hispanic Asian, and 1 (5%) was listed as multiple races. Overall, 16 (80%) of the 20 patients had a preceding COVID-19-like illness, 3 of the 4 patients without preceding COVID-19-like illness had a history of COVID-19 exposure, and previous exposure or illness status was unknown for 1 patient. All 20 patients had a positive viral or antibody test for SARS-CoV-2 confirming past or current infection: 15 (80%) patients tested positive by serology, 15 (80%) patients by reverse transcription polymerase chain reaction (RT-PCR), and 10 (50%) by both serology and RT-PCR. Previous COVID-19 illness was reported a median of 26 days (range 11–78 days) before MIS-A onset.
Table 2.
Characteristics of Patients With Multisystem Inflammatory Syndrome in Adults by COVID-19 Vaccination Status, December 2020 to April 2021, United States
| Total (n = 20) | Vaccinated
(n = 7) |
Unvaccinated(n = 13) | P-value | |
|---|---|---|---|---|
| Age in years, median (range) | 35 (21–66) | 37 (22–49) | 35 (21–66) | .843 |
| Sex | .022 | |||
| Female | 7 (35%) | 5 (71%) | 2 (15%) | |
| Male | 13 (65%) | 2 (29%) | 11 (85%) | |
| Race/Ethnicity | ||||
| Non-Hispanic Asian | 2 (10%) | 0 (0%) | 2 (15%) | .521 |
| Hispanic | 2 (10%) | 0 (0%) | 2 (15%) | .521 |
| Multiple | 1 (5%) | 1 (14%) | 0 (0%) | .350 |
| Non-Hispanic Black | 7 (35%) | 2 (29%) | 5 (38%) | 1.000 |
| Non-Hispanic White | 8 (40%) | 4 (57%) | 4 (31%) | .356 |
| Signs and symptoms | ||||
| Diarrhea | 16 (80%) | 7 (100%) | 9 (69%) | .249 |
| Abdominal pain | 12 (60%) | 6 (86%) | 6 (46%) | .158 |
| Vomiting | 12 (60%) | 3 (43%) | 9 (69%) | .356 |
| Rash | 11 (55%) | 3 (43%) | 8 (62%) | .642 |
| Headache | 11 (55%) | 3 (43%) | 8 (62%) | .642 |
| Shortness of breath | 9 (45%) | 5 (71%) | 4 (31%) | .160 |
| Chest pain or tightness | 7 (35%) | 2 (29%) | 5 (38%) | 1.000 |
| Cough | 6 (30%) | 4 (57%) | 2 (15%) | .122 |
| Conjunctival injection | 5 (25%) | 2 (29%) | 3 (23%) | 1.000 |
| Clinical findings | ||||
| Hypotension | 16 (80%) | 6 (86%) | 10 (77%) | 1.000 |
| Shock | 15 (75%) | 6 (86%) | 9 (69%) | .613 |
| Cardiac dysfunction | 15 (75%) | 6 (86%) | 9 (69%) | .613 |
| Myocarditis | 14 (70%) | 4 (57%) | 10 (77%) | .613 |
| Acute kidney injury | 12 (60%) | 4 (57%) | 8 (62%) | 1.000 |
| Pleural effusion | 10 (50%) | 4 (57%) | 6 (46%) | .350 |
| Pericardial effusion | 9 (45%) | 5 (71%) | 4 (31%) | .160 |
| Pneumonia | 8 (40%) | 3 (43%) | 5 (38%) | 1.000 |
| Mitral regurgitation | 7 (35%) | 2 (29%) | 5 (38%) | 1.000 |
| ARDS | 2 (10%) | 0 (0%) | 2 (15%) | .521 |
| Coronary artery dilatation or aneurysm | 1 (5%) | 0 (0%) | 1 (8%) | 1.000 |
| Laboratory tests | ||||
| Elevated troponin | 17 (85%) | 6 (86%) | 11 (85%) | 1.000 |
| Elevated BNP or NT-proBNP | 16 (80%) | 6 (86%) | 10 (77%) | 1.000 |
| Thrombocytopenia | 9 (45%) | 4 (57%) | 7 (54%) | .642 |
| Lymphopenia | 13 (65%) | 4 (57%) | 9 (69%) | .651 |
| Treatment | ||||
| Steroids | 19 (95%) | 7 (100%) | 12 (92%) | 1.000 |
| Respiratory support, any | 14 (70%) | 6 (86%) | 8 (62%) | .354 |
| IVIG | 13 (65%) | 6 (86%) | 7 (54%) | .329 |
| Anticoagulation medication | 13 (65%) | 2 (29%) | 11 (85%) | .022 |
| Vasoactive medications | 12 (60%) | 4 (57%) | 8 (62%) | 1.000 |
| Antiplatelet medication | 9 (45%) | 3 (43%) | 6 (46%) | 1.000 |
| Intubation and mechanical ventilation | 6 (30%) | 2 (29%) | 4 (31%) | 1.000 |
| Immune modulatorsa | 4 (20%) | 2 (29%) | 2 (15%) | .587 |
| Outcome | ||||
| Days in hospital, median (range) | 6 (2–41) | 6 (3–10) | 6 (2–41) | .685 |
| ICU admission | 14( 70%) | 5 (71%) | 9 (69%) | 1.000 |
| Died | 2 (10%) | 1 (14%) | 1 (8%) | 1.000 |
| SARS-CoV-2 testing | ||||
| Any positive laboratory test | 20 (100%) | 7 (100%) | 13 (100%) | 1.000 |
| PCR positive/Serology negative, not done, or missing | 5 (25%) | 2 (29%) | 3 (23%) | 1.000 |
| Serology positive/PCR negative | 5 (25%) | 1 (14%) | 4 (31%) | .613 |
| PCR positive/Serology positive | 10 (50%) | 4 (57%) | 6 (46%) | 1.000 |
| Vaccine doses | ||||
| 0 dose | 13 (65%) | 0 (0%) | 13 (100%) | … |
| 1 dose | 3 (15%) | 3 (43%) | 0 (0%) | … |
| 2 dosesb | 4 (20%) | 4 (57%) | 0 (0%) | … |
| Preceding COVID-like illness | 16 (80%) | 6 (86%) | 10( 77%) | 1.000 |
| Days from COVID-19 onset to MIS-A onset, median (range) | 26 (11–78) | 28 (25–43) | 26 (11–78) | .913 |
| Days from vaccination (1st dose) to MIS-A onset, median (range) | 10 (6–45) | 10 (6–45) | -
- |
… |
Abbreviations: ARDS, acute respiratory distress syndrome; BNP, B-natriuretic peptide; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MIS-A, multisystem inflammatory syndrome in adults; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Immunomodulators were used in 4 patients: 3 received Anakinra and the 4th patient received tocilizumab.
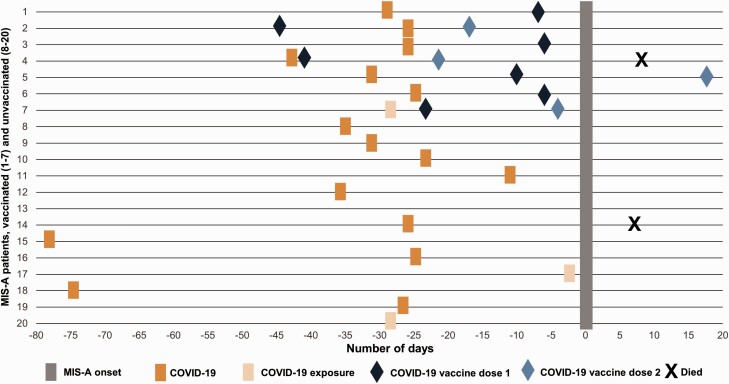
The 2nd dose in 1 patient was administered after recovery from MIS-A illness (Figure 1).
Table 3.
Demographic and Clinical Descriptions of Patients with Multisystem Inflammatory Syndrome in Adults Who Received a COVID-19 Vaccine, December 2020 to April 2021, United States
| Patient | Age Group (years), sex | Race, Ethnicity | Onset Year | Serology | PCR | Clinical Features/ Imaging/ Complications | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 20–24, Female | White, non-Hispanic | 2021 | Nucleocapsid positive | Positive | Shock, shortness of breath, abdominal pain, diarrhea, neck pain, myalgia, cervical lymphadenopathy > 1.5cm
Echocardiography: LV dysfunction, EF 48%, pericardial effusion, ECG (T wave inversion), congestive heart failure, myopericarditis |
Corticosteroids, IVIG ×1 |
| 2 | 20–24, Female | White, non-Hispanic | 2021 | Nucleocapsid positive | Positive | Shock, cough, shortness of breath, abdominal pain, diarrhea, elevated bilirubin/liver function tests
Echocardiography: LV dysfunction, pericardial effusion Abdominal CT: mesenteric lymphadenopathy Chest CT: pneumonia, pleural effusion congestive heart failure, myocarditis |
HFNC, vasoactive medication, corticosteroids, antiplatelet medication, IVIG ×2 |
| 3 | 30–34, Female | White, non-Hispanic | 2020 | Nucleocapsid positive | Positive | Shock, cough, abdominal pain, vomiting, diarrhea, rash, myalgia, elevated LFTs
Echocardiography: LV dysfunction, EF 44%, mild LV hypertrophy, mild pulmonary hypertension, mild pericardial effusion Chest CT/X-ray: pneumonia ECG (non-specific ST seg change, abnormal T-wave), congestive heart failure |
HFNC, corticosteroids |
| 4 | 35–39, Male | White, non-Hispanic | 2021 | Nucleocapsid positive | Negative | Shock, abdominalpain, vomiting, diarrhea, rash,headache, myalgia, conjunctival injection, cervical lymphadenopathy, altered mental status
Elevatedbilirubin/liver function tests Echocardiography: LV/RV dysfunction, pericardial/pleural effusion, mitral regurgitation Chest X-ray: patchy airspacedisease congestive heart failure, myocarditis, AKI |
Mechanical ventilation, ECMO, vasoactive medication, corticosteroids, antiplatelet medication, IVIG ×2 |
| 5 | 45–49, Female | Multiple races, non-Hispanic | 2021 | Nucleocapsid positive | Positive | Shortness of breath, diarrhea, rash, mucocutaneous lesions, encephalopathy, myalgia
Elevated liver function tests Echocardiography: normal Chest CT: atelectasis Encephalitis or aseptic meningitis, AKI |
Non-invasive ventilation, corticosteroids, antiplatelet medication, IVIG ×1 |
| 6 | 45–49, Male | Black, non-Hispanic | 2021 | Not done | Positive | Shock, cough, shortness of breath, chest pain, abdominal pain, diarrhea, headache, encephalopathy
Elevated bilirubin/liver function tests, Echocardiography: cardiac dysfunction, reduced EF Abd CT: enlarged liver Chest CT: ground glass infiltrates, pneumonia, pleural effusion Arrhythmia (supraventricular), congestive heart failure, encephalitis or aseptic meningitis, AKI |
Noninvasive ventilation, vasoactive medication, corticosteroids, tocilizumab, anticoagulation medication, IVIG ×1 |
| 7 | 45–49, Female | Black, non-Hispanic | 2021 | Nucleocapsid positive | Positive | Shock, cough, chest pain, abdominal pain, vomiting, diarrhea, headache, syncope or near syncope, encephalopathy, cervical lymphadenopathy > 1.5
elevated bilirubin/liver function tests Echocardiography: LV/RV dysfunction, EF 25%, pericardial effusion, severe global LV hypokinesis Abdominal ultrasound: gallbladder thickening and trace pericholecystic fluid Chest X-ray: pleural effusion, opacity Congestive heart failure, myopericarditis, AKI, renal failure, |
Mechanical ventilation, vasoactive medication, corticosteroids, anakinra, IVIG ×2 |
Abbreviations: AKI, acute kidney injury; CT, computed tomography; ECG, electrocardiogram; ECMO, extracorporeal membrane oxygenation; EF, ejection fraction; HFNC, high-flow nasal canula; IVIG, intravenous immune globulin; LFT, liver function test; LV, left ventricle; RV, right ventricle.
Seven MIS-A patients (35%) received COVID-19 vaccine before MIS-A onset. Demographic and clinical characteristics of these 7 patients were generally similar to the 13 MIS-A patients who had not received a COVID-19 vaccine (Table 2), although a greater proportion of the vaccinated MIS-A patients (71%) were female. All 7 were reported to CDC and were found on the retrospective VAERS search. No additional MIS-A patient was identified by VAERS search alone. Four patients received the Pfizer-BioNTech vaccine, and 3 patients received the Moderna vaccine. Six of the patients had a history of prior COVID-19 illness and 1 patient had a history of exposure to patients with COVID-19 before MIS-A onset. Six of the 7 patients tested positive for SARS-CoV-2 by RT-PCR; the seventh patient that was negative by RT-PCR tested positive by anti-nucleocapsid serology (Table 3). Among the 7 patients who received a COVID-19 vaccine, the first dose was given a median of 10 days (range, 6–45 days) before MIS-A onset (Figure 1). Three of those 7 patients received the second dose of COVID-19 vaccine prior to MIS-A onset (4, 17, and 22 days before MIS-A onset). One patient received the second dose 18 days after MIS-A onset or 4 days after hospital discharge and had no reported complications following the second dose.
Figure 1.
Patients with multisystem inflammatory syndrome in adults by illness onset and timing of prior COVID-19 illness and vaccine receipt. For patient 13, timing of previous COVID-19 illness or exposure was unknown. Abbreviations: COVID-19, coronavirus disease 2019; MIS-A, multisystem inflammatory syndrome in adults.
Almost all MIS-A patients developed gastrointestinal and cardiovascular manifestations. The predominant signs and symptoms included diarrhea (80%), abdominal pain (60%), vomiting (60%), rash (55%), headache (55%), and shortness of breath (45%) (Table 2). Cough and conjunctival injection were reported in less than one-third of patients. The most common clinical findings reported in at least half of the patients included hypotension (80%), shock (75%), cardiac dysfunction (75%), myocarditis (70%), and acute kidney injury (60%). Almost all patients (95%) were treated with corticosteroids, and 65% of patients received at least 1 dose of intravenous immunoglobulin (IVIG). Six (46%) of the 13 patients treated with IVIG were given a second dose. The median number of days of hospitalization among MIS-A patients was 6 days (range, 2–41 days); 14 (70%) of the patients were admitted to the intensive care unit (ICU). Overall, 2 (10%) of the MIS-A patients died, of whom 1 had received COVID-19 vaccine.
DISCUSSION
MIS-A is a rare, poorly understood complication of SARS-CoV-2 infection believed to result from a delayed dysregulated immune response to the virus [9, 10]. The patients summarized in the present article have evidence of hyperinflammation, including fever, elevated laboratory markers of inflammation, and multisystem organ involvement. Onset of MIS-A occurred a median of about 1 month after initial SARS-CoV-2 infection, consistent with findings from other reports describing patients with MIS-A and MIS-C [5, 7]. The clinical manifestations of MIS-A are generally similar to those of MIS-C with both involving fever, gastrointestinal and cardiovascular involvement, and elevated laboratory markers of inflammation [5, 7]. As described in Table 1, before diagnosing MIS-A other more likely alternative diagnosis such as bacterial sepsis should be excluded. Based on patients reported to CDC and published in the literature, MIS-A appears to occur much less frequently than MIS-C. As of May 2021, the total number of MIS-A patients reported to CDC and in the published literature are below 150, whereas over 3700 MIS-C cases have been reported to CDC as of the same month [7, 8]. Patients with MIS-A reported to date typically have a more severe illness course with higher rates of shock and cardiac involvement. Compared to patients with MIS-C from previously published surveillance data, a higher proportion of the patients with MIS-A in the present analysis had shock (75% vs. 37%), cardiac dysfunction (75% vs 31%), myocarditis (70% vs 17%), and pericardial effusion (45% vs 23%) [5]. The higher rate of cardiac complications in MIS-A may be due to increased recognition, diagnosis, and reporting of patients with a more severe illness course.
Seven of the MIS-A patients in the present report received COVID-19 vaccine within 45 days (median 10 days) before MIS-A onset. However, all 7 patients also had evidence of prior SARS-CoV-2 infection. To date, no patient with MIS-A following COVID-19 vaccination without evidence of prior SARS-CoV-2 infection has been reported to CDC. Nune et al reported a 44-year-old woman who was hospitalized in the United Kingdom 2 days after receiving her first COVID-19 vaccination with arm pain. which progressed to fever, diarrhea, abdominal pain, rash, and hypotension [15]. The patient was reported to have tested negative for SARS-CoV-2 by serology and nasopharyngeal swabs. Two other publications reported similar patients who developed multisystem inflammatory syndrome after prior SARS-CoV-2 infection and receiving COVID-19 vaccine. Uwaydah et al reported a 22-year-old man from United Arab Emirates who was diagnosed with MIS-A 6 weeks after a mild COVID-19 illness [16]. The patient received the first dose of an inactivated SARS-CoV-2 vaccine about 30 days and second dose within hours before symptom onset of an illness that led him to visit the emergency department 4 days after vaccination; he was later diagnosed with MIS-A. Salzman et al reported 3 patients in the United States who were diagnosed with multisystem inflammatory syndrome after receiving COVID-19 vaccine [17]. These patients had illness onset within 20 days of receiving COVID-19 vaccine; 2 of the patients had acute COVID-19 about 6 weeks prior to illness onset and all three patients were positive by serology for nucleocapsid antigen. Reports of MIS-A following COVID-19 vaccination should be interpreted in the context of the large number of vaccinations administered in the United States. Through 30 April 2021, 256759689 COVID-19 vaccine doses were administered to persons aged ≥21 years in the United States, including 111842926 doses of Moderna and 135421590 doses of Pfizer messenger RNA (mRNA) COVID-19 vaccines [18]. Given the widespread use of COVID-19 vaccines in the United States, the lack of reporting of MIS-A associated with vaccination alone, without evidence of underlying SARS-CoV-2 infection, is reassuring.
Although patients with MIS-A commonly reported prior COVID-19 illness, in some patients the only evidence of SARS-CoV-2 infection was a positive serology. All current COVID-19 vaccines elicit an immune response to the spike protein, and vaccinated patients are expected to be seropositive for anti-spike protein antibodies but not for anti-nucleocapsid antibodies. In contrast, natural infection with SARS-CoV-2 results in humoral responses to both the spike and nucleocapsid proteins, providing a plausible laboratory method to distinguish the immune response to vaccination from that resulting from natural infection [19]. However, anti-nucleocapsid antibody is not uniformly detectable following COVID-19 illness, so its absence does not necessarily rule out natural infection with SARS-CoV-2. In particular, younger patients with mild illnesses during acute infection with SARS-CoV-2 may be less likely to mount a humoral immune response to nucleocapsid protein [19].
The present analysis has several limitations. The MIS-A patients were spontaneously reported by health departments, but comprehensive surveillance for MIS-A does not currently exist, leading to potential underreporting of patients with MIS-A. Because VAERS is a passive surveillance system, underreporting of patients with MIS-A among vaccine recipients is also likely. Underreporting may particularly be true for MIS-A patients who have milder illness. Complications such as myocarditis were diagnosed by clinicians managing the patients and the methods used in the diagnosis were not standardized. Absence of imaging details may have limited our assessment of some findings such as pneumonia diagnosed on computed tomography.
The treatment for MIS-A is generally adopted from treatment guidelines developed for MIS-C and primarily consists of administration of corticosteroid and IVIG either alone or in combination. These interventions, with possible addition of other adjunctive immunomodulatory treatment for patients not responding to IVIG and/or corticosteroids, were developed by expert opinion based on currently available evidence [20–22]. Additional treatment with vasopressors and antiplatelet and anticoagulation therapy may be required in some patients. Clinicians who identify patients with an illness similar to MIS-A in temporal association with the COVID-19 vaccine are encouraged to report such cases through VAERS; COVID-19 vaccine providers are required to report suspected MIS-A to VAERS as part of the EUAs. A case definition for MIS-A developed by CDC summarized in Table 1 can be used to assess patients suspected of having the illness [12].
CONCLUSION
MIS-A is a rare complication of SARS-CoV-2 infection. Patients developing MIS-A in temporal association with COVID-19 vaccination have been reported, although all patients reported to date also had evidence of prior SARS-CoV-2 infection. The potential contribution of vaccination to the pathogenesis of MIS-A in these patients is unclear. It is reassuring that, to date, no cases of MIS-A have been reported among vaccine recipients in the United States without evidence of prior SARS-CoV-2 infection despite the administration of COVID-19 vaccines to millions of recipients. Clinicians should maintain a high index of suspicion for the occurrence of similar patients and report suspected cases of MIS-A and other serious adverse events following vaccination to VAERS.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC) Clinical Immunization Safety Assessment (CISA) Project contracts: contract number 200-2012-53661 to Cincinnati Children’s Hospital Medical Center; contract number 200-2012-53664 to Johns Hopkins University; contract number 200-2012-50430 to Vanderbilt University Medical Center, contract number 200-2012-53709 to Boston Medical Center.
Potential conflicts of interest . B. W.’s institution has received partial salary support from a grant from the US Agency for Healthcare Research and Quality (AHRQ); the institution, Intermountain Healthcare has participated in COVID-19 trials sponsored by: AbbVie, Genentech, Gilead, Regeneron, Roche, the US National Institutes of Health (NIH) ACTIV, ACTT, and PETAL clinical trials networks, and the Department of Defense and was a site investigator on these trials but received no direct or indirect remuneration for my effort. Institutions for E. S., K. T., and E. B. have received grants for Pfizer COVID-9 vaccine studies; in addition, E.S. has received honoraria for serving as a consultant for Sanofi Pasteur. K. E. has participated on Data Safety Monitoring Boards or Advisory Boards for Sanofi Pasteur, Pfizer, Merck, X-4 Pharma, Seqirus, and Moderna; received grant funding outside of the submitted work from NIH; received consulting fees from Bionet and IBM; and is Associate Editor Clinical Infectious Diseases. S. B. M. reports participating on a Data Safety Monitoring Board or Advisory Board from PATs-COVID Study Guinea. E. B. reports grants or contracts from RSV and PCV20 vaccine studies paid to the institution outside of the submitted work; consulting fees from Goddard systems for consultation to day care centers about COVID response; honoraria paid to self as Associate Editor, American Academy of Pediatrics Red Book; unpaid position as ex officio member of Committee on Infectious Diseases, American Academy of Pediatrics. K.T. reports being Hopkins Site PI for the Pfizer COVID-19 vaccine study in adults and children; serving on a number of Data and Safety Monitoring boards for non-COVID related studies for Merck, Takeda, Intralytix, PATH, and NIH and serving on 2 NIH Safety Monitoring committees related to vaccines of therapeutics for COVID-19. E. S. reports payments made to the institution for research studies on which they are investigator from the NIH outside of the submitted work and serving on the Data Safety Monitoring Board for an NIH-funding study (NIH Comparison of High vs Standard Dose Flu Vaccine in Pediatric Stem Cell Transplant Recipients). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr 2020; 226:45–54.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belay ED, Abrams J, Oster ME, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr 2021:e210630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Health department-reported cases of multisystem inflammatory syndrome in children (MIS-C) in the United States. Available at: https://www.cdc.gov/mis-c/cases/index.html. Accessed 4 June 2021.
- 9. Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020; 183:968–81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 2020; 20:453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Food and Drug Administration. COVID-19 vaccines. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines.
- 12. Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in adults (MIS-A): case definition. Available at: https://www.cdc.gov/mis-c/mis-a/hcp.html. Accessed 11 May 2021.
- 13. Centers for Disease Control and Prevention. Clinical immunization safety assessment (CISA) project. Available at: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html. Accessed 14 December 2020.
- 14. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015; 33:4398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nune A, Iyengar KP, Goddard C, Ahmed AE. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V). BMJ Case Rep 2021;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uwaydah AK, Hassan NMM, Abu Ghoush MS, Shahin KMM. Adult multisystem inflammatory syndrome in a patient who recovered from COVID-19 postvaccination. BMJ Case Rep 2021; 14:e242060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salzman MB, Huang CW, O’Brien CM, Castillo RD. Multisystem inflammatory syndrome after SARS-CoV-2 infection and COVID-19 vaccination. Emerg Infect Dis 2021; 27:1944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. Available at: https://covid.cdc.gov/covid-data-tracker/#vaccinations. Accessed 22 June 2021.
- 19. Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol 2021; 22:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson LA, Canna SW, Friedman KG, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: Version 2. Arthritis Rheumatol 2021; 3:e13–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med 2021:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory syndrome in children - initial therapy and outcomes. N Engl J Med 2021:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]