Lay Summary
A 47-year-old woman developed a de novo occurrence of Crohn’s disease after coronavirus disease 2019. This is an unusual occurrence and suggests that severe acute respiratory syndrome coronavirus 2 could trigger inflammatory bowel disease in predisposed people.
To the Editors:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a significant impact on the management of all diseases, and inflammatory bowel disease (IBD) is no exception.1,2 While the clinical picture of IBD during coronavirus disease 2019 (COVID-19) becomes clearer,1 less is known about the impact of COVID-19 as a potential risk factor for IBD occurrence.
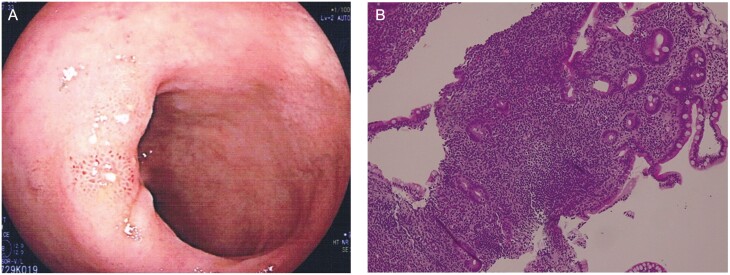
In May 2021, a 47-year-old woman experienced weakness, myalgia, and diarrhea, with up to 5 bowel movements per day, without the presence of a cough or breathlessness. As symptoms persisted for 5 days, a nasopharyngeal swab was performed, resulting in a positive result for SARS-CoV-2. Thoracic radiographs and laboratory tests were unremarkable, except for a raised C-reactive protein (CRP) of 12mg/dL. She received paracetamol and was advised to self-isolate. Weakness and myalgia disappeared within a week, and her diarrhea also improved. However, during the following 4 months, she had recurrent episodes of diarrhea despite monthly treatment with probiotics. At the beginning of September 2021, she developed frequent and large-volume diarrhea episodes of up to 10 bowel movements per day. Laboratory tests showed hemoglobin 10.5g/dL, erythrocyte sedimentation rate 78mm/h, CRP 39mg/dL, and fecal calprotectin 290mg/g. Stool microscopy culture did not reveal any pathogens, and the nasopharyngeal swab was negative. Ileocolonoscopy showed multiple erosions located in the terminal ileum (Figure 1A). Histopathology showed focal architectural distortion and dense lymphoplasmacytic and eosinophils infiltration through the lamina propria (Figure 1B). Computed tomography enterography showed a 7-cm-long segmental wall thickening (8mm) of the terminal ileum, with mucosal hyperenhancement, mesenteric vascularity, and lymphoadenomegaly. The diagnosis of de novo Crohn’s disease (CD) was made.
Figure 1.
A, Endoscopy findings. Evidence of erosions of the terminal ileum. B, Histology findings. Significant lymphoplasmacytic and eosinophils infiltration through the lamina propria, together with glandular destruction. Hematoxylin and eosin, ×10.
Oral budesonide 9mg/kg was prescribed for 8 weeks, and diarrheal episodes improved over the following 3 weeks. The patient is currently in clinical remission, and follow-up laboratory tests showed hemoglobin 12.1g/d, erythrocyte sedimentation rate 22mm/h, CRP 9mg/dL, and fecal calprotectin 112mg/g.
There are established links between enteric infection-related dysbiosis and the future development of IBD.3 After binding to the angiotensin-converting enzyme 2 receptor on the intestinal epithelial cells, SARS-CoV-2 enters the cell, causing enhanced tumor necrosis factor α production.4 A T helper cell 17 cytokine storm has been identified in COVID-19,5 similar to that seen in CD. Therefore, it is hypothesized that this immune dysregulation with related intestinal inflammation due to SARS-CoV-2 might create a favorable milieu that can trigger the onset of CD in susceptible individuals.
Author Contributions
A.T. and R.N. planned and conducted the study; A.T. and R.N. collected data; A.T. interpreted data and drafted the manuscript; A.T. and R.N. approved the final draft submitted.
Funding
No financial support was obtained for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1. Papa A, Gasbarrini A, Tursi A. Epidemiology and the impact of therapies on the outcome of COVID-19 in patients with inflammatory bowel disease. Am J Gastroenterol. 2020;115:1722-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mantovani Cardoso E, Hundal J, Feterman D, et al. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol. 2020;39:2811-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porter CK, Tribble DR, Aliaga PA, et al. Infectious gastroenteritis and risk of developing inflammatory bowel disease. Gastroenterology. 2008;135:781-786. [DOI] [PubMed] [Google Scholar]
- 4. Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105:7809-7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368-370. [DOI] [PMC free article] [PubMed] [Google Scholar]