Abstract
Background
Alcohol is one of the most common causes of liver disease in the Western World. Randomised clinical trials have examined the effects of anabolic‐androgenic steroids for alcoholic liver disease.
Objectives
To assess the beneficial and harmful effects of anabolic‐androgenic steroids for patients with alcoholic liver disease based on the results of randomised clinical trials.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, The Cochrane Controlled Trials Register in The Cochrane Library, MEDLINE, EMBASE, LILACS, and Science Citation Index Expanded until June 2006. Electronic searches were combined with full text searches. Manufacturers and researchers in the field were also contacted.
Selection criteria
Randomised clinical trials studying patients with alcoholic steatosis, alcoholic fibrosis, alcoholic hepatitis, and/or alcoholic cirrhosis were included. Interventions encompassed anabolic‐androgenic steroids at any dose or duration versus placebo or no intervention. The trials could be double blind, single blind, or unblinded. The trials could be unpublished or published, and no language limitations were applied.
Data collection and analysis
Outcomes are assessed at maximal follow‐up. All analyses were performed according to the intention‐to‐treat method. The statistical package RevMan Analyses was used. The methodological quality of the randomised clinical trials was assessed.
Main results
Combining the results of five randomised clinical trials randomising 499 patients with alcoholic hepatitis and/or cirrhosis demonstrated no significant effects of anabolic‐androgenic steroids on mortality (relative risk (RR) 1.01, 95% confidence interval (CI) 0.79 to 1.29), liver‐related mortality (RR 0.83, 95% CI 0.60 to 1.15), complications of liver disease (RR 1.25, 95% CI 0.74 to 2.10), and liver histology. Anabolic‐androgenic steroids did not significantly affect a number of other outcome measures, including sexual function and liver biochemistry. Anabolic‐androgenic steroids were not associated with a significantly increased risk of non‐serious adverse events (RR 1.14, 95% CI 0.50 to 2.59) or with serious adverse events (RR 4.54, 95% CI 0.57 to 36.30).
Authors' conclusions
This systematic review could not demonstrate any significant beneficial effects of anabolic‐androgenic steroids on any clinically important outcomes (mortality, liver‐related mortality, liver complications, and histology) of patients with alcoholic liver disease.
Keywords: Humans; Anabolic Agents; Anabolic Agents/therapeutic use; Androgens; Androgens/therapeutic use; Liver Diseases, Alcoholic; Liver Diseases, Alcoholic/drug therapy; Randomized Controlled Trials as Topic; Treatment Outcome
Plain language summary
No evidence to support anabolic‐androgenic steroids for alcoholic liver disease
Alcohol causes a major part of the liver diseases in the Western World. Several trials have addressed the effects of anabolic‐androgenic steroids for alcoholic liver disease. This systematic review could not demonstrate any significant effects of anabolic‐androgenic steroids on mortality, liver‐related mortality, liver complications, and histology of patients with alcoholic liver disease. Anabolic‐androgenic steroid intervention is not associated with a significant increase in non‐serious adverse events, but with the seldom occurrence of serious adverse events. Accordingly, there is no evidence supporting the use of anabolic‐androgenic steroids for alcoholic liver disease, but further randomised clinical trials may be needed to settle the question.
Background
Alcohol is one of the most common causes of liver diseases in the Western World. Alcoholic liver disease has also become an important cause of liver injury in a number of low‐income countries as economics improve and both cultural and religious indictments against drinking are decreasing. In many countries the incidence of alcoholic liver disease is increasing at a time when the incidence of other liver disorders remains steady or is falling (Morgan 1999).
Alcohol is a major hepatotoxin (Morgan 1999). Alcohol leads to fatty liver (Rubin 1968), alcoholic hepatitis, alcoholic fibrosis (Purhoit 2006), and alcoholic cirrhosis (Morgan 1999). Data from long‐term studies in which patients with alcoholic fatty change and alcoholic hepatitis were followed for up to 13 years demonstrate that alcoholic hepatitis is a predictor of later development of liver fibrosis and cirrhosis (Sørensen 1984; Marbet 1987). Acetaldehyde, the first metabolite of ethanol, may increase transcription of collagen I. Alcohol induced necrosis and inflammation may trigger the scarring and the development of fibrosis and later on the development of cirrhosis.
About 70% of patients with clinical alcoholic hepatitis also have alcoholic cirrhosis at the time of diagnosis (Mendenhall 1984a). Survival is adversely affected by continued alcohol abuse. Five‐years survival in patients with alcoholic cirrhosis who stop drinking is 50% to 75%. Five‐years survival in patients continuing to drink rarely exceed 40% (Powell 1968). The progression of liver fibrosis and cirrhosis in alcoholics is enhanced by the presence of hepatitis B and hepatitis C virus markers (Chang 1994; Corrao 1998; Purhoit 2006).
Men with alcoholic cirrhosis have symptoms such as testicular atrophy, azoospermia, reduced body hair, reduced beard growth, reduced prostatic size as well as gynaecomastia, arterial spiders, female escutcheon, female body habitus, and sexual dysfunction (Gluud 1988a; Van Steenbergen 1993). These findings all suggest that there is an endocrine imbalance of sexual hormones. In accordance, plasma concentrations of non‐protein bound testosterone and non‐sex hormone binding globulin bound testosterone ‐ the biologically active fractions ‐ are generally low or decreased in patients with alcoholic cirrhosis (Gluud 1983; Gluud 1987; Gluud 1988a) and levels of oestrogens are often increased (Gluud 1983; Gluud 1988a).
Few studies have addressed sex hormone disturbances in women with chronic alcoholic liver disease (Becker 1993). In female alcoholics ethanol consumption increases the frequency of menstrual disturbances, abortions, and earlier occurrence of menopause. In postmenopausal women with alcoholic liver disease, the main disturbances of sex hormone metabolism consist of elevated oestrone and non‐sex hormone binding globulin concentrations, while serum concentrations of steroid sulphates and 5‐alfa‐dihydrotestosterone are reduced (Becker 1993).
A questionnaire survey among European hospital‐based specialists in gastroenterology/hepatology demonstrated that 5% to 43% of the specialists, depending on the region, considered using anabolic‐androgenic steroids for alcoholic liver disease (Gluud 1993). Several controlled and uncontrolled clinical studies have been undertaken in order to estimate the beneficial and harmful effects of anabolic‐androgenic steroids for alcoholic liver disease (Gluud 1984; Gluud 1988a). The studies have yielded varying results and the power of the controlled studies has been low. Moreover, previous meta‐analyses on the topic have reached conflicting results due to the accumulation of more trial results and differences regarding inclusion criteria (Gluud 1984; Gluud 1988a).
This Review represents an update of our previous Cochrane systematic review on the topic (Rambaldi 2002, Rambaldi 2003), see other published versions of this review.
Objectives
To assess the beneficial and harmful effects of anabolic‐androgenic steroids versus placebo or no intervention in patients with alcoholic steatosis, alcoholic hepatitis, alcoholic fibrosis, and/or alcoholic cirrhosis.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised clinical trials. The trials should have used a proper method of randomisation, ie, central randomisation; serially numbered opaque, sealed envelopes; or other description that contains elements convincing of adequate allocation concealment. Trials with quasi‐randomisation were excluded. Randomised clinical trials could be double blind, single blind, or unblinded. The randomised clinical trials could be unpublished or published as an article, an abstract, or a letter. No language limitations were applied.
Types of participants
Patients with alcoholic liver disease according to the diagnostic work‐up used in the individual randomised clinical trial were included. The analyses examined the efficacy of anabolic‐androgenic steroids in the different subgroups of patients with alcoholic liver disease (fatty liver; alcoholic hepatitis; alcoholic fibrosis; alcoholic cirrhosis) as well as combined.
Types of interventions
Peroral or parenteral administration of anabolic‐androgenic steroids at any dose versus placebo or no intervention. Additional interventions were allowed as long as both intervention groups in the individual trial received the additional intervention(s) equally.
Types of outcome measures
All outcomes were assessed at maximal follow‐up.
Primary outcomes (1) Number of patients dying (total and liver‐related death).
Secondary outcomes (2) Development of clinical symptoms and complications (ie, ascites, variceal bleeding, hepatic encephalopathy, hepato‐renal syndrome, hepatocellular carcinoma). (3) Liver function. (4) Liver biopsy findings. (5) Liver biochemistry. (6) Alcohol consumption during follow‐up. (7) Adverse events. Adverse events were defined as any untoward medical occurrence that did not have a causal relationship with the treatment, but did result in a dose reduction or discontinuation of treatment. Severe adverse events were defined according to the ICH guidelines (ICH‐GCP 1997) as any event that would increase mortality; were life‐threatening; required inpatient hospitalisation; resulted in a persistent or significant disability; or any important medical event, which may have jeopardised the patient or required intervention to prevent it.
In addition, any data on quality of life and health economics (eg, length of hospitalisation) were compared.
Search methods for identification of studies
See: Hepato‐Biliary Group methods used in reviews.
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (June 2006), The Cochrane Central Register of Controlled Trials in The Cochrane Library (Issue 2, 2006), MEDLINE (1950 to June 2006), EMBASE (1980 to June 2006), LILACS (1982 to June 2006), and Science Citation Index EXPANDED (from 1945 to June 2006). See 'Appendix 1' for the search strategies applied to the individual electronic databases.
Further trials were sought by reading the reference lists of the identified studies and through full text searches.
No restrictions of year of publication, language, or publication status were applied.
The principal authors of the identified randomised clinical trials were approached and inquired about additional trials they might know of. Pharmaceutical companies involved in the production of anabolic‐androgenic steroids were contacted in order to obtain additional publications on randomised clinical trials and unpublished randomised clinical trials.
Data collection and analysis
The meta‐analysis was updated following the recommendations given by The Cochrane Collaboration (Higgins 2005).
Trial selection and data extraction The authors independently selected the trials to be included in the review according to the prespecified selection criteria. A third opinion plus discussion resolved disagreements. We wrote to the authors of trials when data were missing in the report. Patient characteristics, diagnosis, and treatments The following items were recorded from the individual randomised clinical trials: mean (or median) age, sex ratio, alcohol consumption, form of liver disease, duration and severity of liver disease at entry, a concomitant cause of liver disease, dose of anabolic‐androgenic steroids intervention, type of intervention in the control group and any additional intervention. The diagnostic work‐up before entry was registered. Hepatitis markers were evaluated and type of alcoholic liver disease included in the randomised clinical trial. Development of clinical symptoms and complications, liver function, liver biochemistry, liver biopsy findings, alcohol consumption, quality of life, health economics (ie, length of hospital stay, cost of medication, and cost of additional follow‐up weighted against any gains in health), and adverse events during follow‐up were registered.
Selection and data‐extraction bias All randomised clinical trials considered for inclusion were analysed by the contributors, who conferred about any disagreements.
All randomised clinical trials had the pertinent data extracted by the contributors. All identified trials were listed and trials excluded from the meta‐analysis of the review were identified with the reason for exclusion.
Data on the number of patients with each outcome event, by allocated treatment group, irrespective of compliance of follow‐up, were sought to allow an intention‐to‐treat analysis. If the above data were not available in the trial reports, further information was sought by correspondence with the principal investigator.
Bias Risk The methodological quality of the randomised clinical trials and hence bias risk were assessed using components of methodological quality (generation of the allocation sequence, allocation concealment, blinding, and withdrawals and dropouts) (Schulz 1995; Moher 1998; Kjaergard 2001).
Generation of the allocation sequence The procedure used to create a random sequence ensuring that each participant has a known, unpredictable, and usually equal chance of being assigned to intervention groups. The allocation sequence generation was classified as:
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice may also be considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not.
Inadequate, if a system involving dates, names, or admittance numbers were used for the allocation of patients. Such studies are known as quasi‐randomised studies and were excluded from the review due to the risk of bias.
Allocation concealment The procedure used to conceal the allocation sequence from the investigators who assign participants to the intervention groups. The allocation concealment was classified as:
Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes. Envelopes should be serially numbered, sealed, and opaque. However, this information is rarely provided, indicating an increased risk of bias. In that case, sealed envelopes may constitute an intermediate category between adequate and unclear.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised. Such studies were excluded due to their risk of bias.
Blinding Blinding was classified as:
Adequate, if the trial was described as double blind and the method of blinding involved identical placebo and active drugs (smell, taste and shape).
Unclear, if the trial was described as double blind, but the method of blinding was not described.
Inadequate, not performed, if the trial was not double blind.
Follow‐up The reported follow‐up was classified as:
Adequate, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals.
Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
Inadequate, if the number or reasons for dropouts and withdrawals were not described.
In addition, we had registered whether the randomised clinical trial had reported the use of intention‐to‐treat analysis or not (Gluud 2001). Statistical methods All analyses were performed according to the intention‐to‐treat method including all randomised patients. Patients without the outcome variable were considered failures (worst‐case scenario analysis).
Dichotomous data were analysed by calculating the relative risk (RR) and continuous outcomes as weighed mean difference (WMD) or standardised mean difference (SMD) both with 95% confidence intervals.
We performed all analyses on mortality according to the intention‐to‐treat method, ie, including all randomised patients. We used the statistical package RevMan Analyses (RevMan 2003) provided by the Cochrane Collaboration. We examined intervention effects by using both a random‐effects model (DerSimonian 1986) and a fixed‐effect model (Demets 1987) with the significance level set at P < 0.05. If the results of the two analyses led to the same conclusion, we presented only the results of the fixed‐effect model analysis. In case of discrepancies of the two models we reported the results of both models.
Potential causes for heterogeneity were explored by performing subgroup analyses. The review performed subgroup analyses with regard to the stage of alcoholic liver disease, methodological quality of included randomised clinical trials (analysing separately randomised clinical trials with adequate quality components and inadequate quality components (Moher 1998; Kjaergard 2001), and way of administration of anabolic‐androgenic steroids as well as preparation, dose, and duration of anabolic‐androgenic steroids treatment.
Due to the risk of chance, statistical findings among the secondary outcome measures were interpreted conservatively.
We planned to examine potential publication bias (Vickers 1998) and other sources of bias using the funnel plot method (Egger 1997), but due to the few trials identified we did not explore it.
Results
Description of studies
Search results We identified 336 references through electronic searches (until June, 2006) of The Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 45), The Cochrane Library (n = 87), MEDLINE (n = 82), EMBASE (n = 85), LILACS (n = 0), and Science Citation Index EXPANDED (n = 37) (see Appendix 1). We found 43 publications; 13 were included and 30 were excluded. Reading bibliographies identified one further randomised clinical trial (Fenster 1966), which was not identified by the electronic searches.
Included studies In 14 included publications, four randomised clinical trials were described in full paper articles (Fenster 1966; Mendenhall 1984b; CSL 1986; Bonkovsky 1991) and one randomised clinical trial in an abstract form only (Mendenhall 1977).
The individual randomised clinical trials are described in the table 'Characteristics of included studies'.
The five trials reported random allocation of 499 patients with alcoholic liver disease (n = 499) to anabolic‐androgenic steroids. All the randomised clinical trials compared anabolic‐androgenic steroids versus placebo except the Bonkovsky trial (Bonkovsky 1991), which compared anabolic‐androgenic steroids versus no intervention.
The entry criteria in the randomised clinical trials varied, but the inclusion criteria were generally of good quality making it highly likely that all patients did in fact have alcoholic liver disease. Patients with alcoholic hepatitis were included in three trials (Mendenhall 1977; Mendenhall 1984b; Bonkovsky 1991). Mendenhall et al (Mendenhall 1977) did not give the proportion of patients also having cirrhosis. However, in the two other trials the proportion of patients with cirrhosis was 71.1% (Mendenhall 1984b) and 54.0% (Bonkovsky 1991).
Patients with alcoholic cirrhosis were included in two randomised clinical trials (Fenster 1966; CSL 1986). Fenster et al (Fenster 1966) did not report the proportion of patients also having alcoholic hepatitis. In the other trial (CSL 1986) the proportion of patients also having histological alcoholic hepatitis was 51.1% (Gluud 1987).
A total of 447 (89.6%) patients were males (Mendenhall 1977; Mendenhall 1984b; CSL 1986; Bonkovsky 1991), while 20 (2.0%) patients were females (Bonkovsky 1991). The exact figure of males and females is not given for 32 (6.4%) patients (Fenster 1966). However, the sex ratio was quite balanced between the two groups of this trial.
The experimental treatment consisted of oxandrolone 80 mg per day orally in three randomised clinical trials (Mendenhall 1977; Mendenhall 1984b; Bonkovsky 1991), micronized‐free testosterone 600 mg day (200 mg three times per day) orally in one randomised clinical trial (CSL 1986), and 100 mg of testosterone propionate or methenolone enanthate every other day intramuscularly in the Fenster 1966 trial.
The duration of the treatment varied from 21 days in Mendenhall 1977 and Bonkovsky 1991, over one month in Fenster 1966 and Mendenhall 1984b to 36 months in CSL 1986.
Excluded studies A total of 22 studies, described in 30 publications, were excluded mainly because they were observational studies or case series. The reasons for exclusion are listed in the table 'Characteristics of excluded studies'. Two of these described randomised clinical trials including patients with mixed aetiologies (Wells 1960; Puliyel 1977). All patients had liver cirrhosis in the Wells trial (Wells 1960), but the aetiology of the cirrhosis was not given. Only 18/53 patients had a history of alcoholism. All patients had also liver cirrhosis in the Puliyel trial (Puliyel 1977), but there were no data on aetiology. These two randomised clinical trials are excluded, due to the fact that only a small proportion of included patients were considered to have alcoholic liver disease. We also excluded the Mendenhall 1993a trial because it examined the combination of two interventions (oxandrolone plus nutritional support) versus placebo. However, we have included this trial in a sensitivity analysis in order to examine the robustness of our findings.
Risk of bias in included studies
Only one of the five randomised clinical trials provided a sample size estimation, which was based on mortality (CSL 1986).
The method to generate the allocation sequence was considered adequate in two randomised clinical trials (CSL 1986; Bonkovsky 1991) and three randomised clinical trials described adequate allocation concealment (Mendenhall 1984b; CSL 1986; Bonkovsky 1991).
All randomised clinical trials but one (Bonkovsky 1991) were described as double blind. However, the placebo was described as having an identical appearance only in two randomised clinical trials (Fenster 1966; CSL 1986).
There was a fair description of follow‐up and withdrawals/drop‐outs in three trials (Fenster 1966; Mendenhall 1984b; CSL 1986). None of the trials stated that they used an intention‐to‐treat method to evaluate their data. All the trials but one presumably used intention‐to‐treat analysis (Fenster 1966).
Effects of interventions
Mortality Combining the results of the five randomised clinical trials demonstrated no significant effect of anabolic‐androgenic steroids on mortality (RR 1.01, 95% confidence interval (CI) 0.79 to 1.29). In the anabolic‐androgenic steroids group 87/275 (31.6%) patients died versus 73/224 (32.6%) patients in the control group (Analysis 1.1).
1.1. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 1 Mortality.
A worst‐case scenario analysis (all patients who dropped‐out or were withdrawn were considered dead) did not change the estimate of no significant effect of anabolic‐androgenic steroids on mortality (Analysis 3.1).
3.1. Analysis.

Comparison 3 Sensitivity analyses, Outcome 1 Mortality ‐ worst‐case scenario.
Subgroup analysis stratifying the trials according to the adequacy of the single methodologic quality components (generation of the allocation sequence, allocation concealment, blinding, and follow‐up) did not demonstrate significant differences regarding the effect of anabolic‐androgenic steroids on mortality between trials with and without adequate methodology (Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5). Comparing the estimate of anabolic‐androgenic steroids intervention effect in adequately blinded trials (RR 1.25, 95% CI 0.79 to 1.98) to that in unblinded trials (RR 0.89, 95% CI 0.68 to 1.18) demonstrated no significant difference between the two estimates (z = ‐0.87 P = 0.38). Comparing trials with adequate methodology regarding all four components to trials with one or more inadequate components gave results similar to Analysis 3.2.
3.2. Analysis.

Comparison 3 Sensitivity analyses, Outcome 2 Mortality and generation of the allocation sequence.
3.3. Analysis.

Comparison 3 Sensitivity analyses, Outcome 3 Mortality and allocation concealment.
3.4. Analysis.

Comparison 3 Sensitivity analyses, Outcome 4 Mortality and double blinding.
3.5. Analysis.

Comparison 3 Sensitivity analyses, Outcome 5 Mortality and follow‐up.
The RR of death of the trials with a follow‐up duration within 21 days was 1.00 (95% CI 0.16 to 6.30), while the RR of the randomised clinical trials with a follow‐up duration of at least six months was 1.01 (95% CI 0.79 to 1.29) (Analysis 3.6).
3.6. Analysis.

Comparison 3 Sensitivity analyses, Outcome 6 Mortality ‐ duration of follow‐up.
The RR of death of the trials evaluating oxandrolone was 0.78 (95% CI 0.54 to 1.13); the RR of the trial evaluating testosterone was 1.19 (95% CI 0.72 to 1.98); and the RR of the trial evaluating testosterone propionate or methenolone was 1.57 (95% CI 0.53 to 4.65) (Analysis 3.7).
3.7. Analysis.

Comparison 3 Sensitivity analyses, Outcome 7 Mortality ‐ different treatment.
The RR of death of the randomised clinical trials including patients with alcoholic hepatitis was 0.89 (95% CI 0.68 to 1.18); the RR of the trials including patients with alcoholic cirrhosis was 1.25 (95% CI 0.79 to 1.98) (Analysis 3.8).
3.8. Analysis.

Comparison 3 Sensitivity analyses, Outcome 8 Mortality ‐ stage of alcoholic liver disease.
Sensitivity analysis adding the excluded trial (Mendenhall 1993b) treating patients with oxandrolone plus nutritional supplement versus placebo did not change this estimate significantly; RR 0.99 (95% CI 0.81 to 1.21) (Analysis 3.9). In the treatment group 128/412 (31.1%) patients died versus 116/360 (32.2%) patients in the control group.
3.9. Analysis.

Comparison 3 Sensitivity analyses, Outcome 9 Mortality ‐ according to co‐intervention.
Liver‐related mortality No significant effect of anabolic‐androgenic steroids on liver‐related mortality could be demonstrated (RR 0.83, 95% CI 0.60 to 1.15). In the anabolic‐androgenic steroids group 50/227 (22.0%) patients died versus 53/187 (28.3%) patients in the control group (Analysis 1.2).
1.2. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 2 Liver‐related mortality.
Liver‐related complications None of the trials considered variceal bleeding or hepato‐renal syndrome as outcome measures.
Anabolic‐androgenic steroids did not significantly affect the incidence of patients with ascites, hepatic encephalopathy, hepatocellular carcinoma, pretibial edema, prostatic hypertrophia, sexual dysfunction, weight (Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.7; Analysis 1.9; Analysis 1.10; Analysis 1.11). Anabolic‐androgenic steroids reduced, however, significantly the prevalence of patients with gynaecomastia (RR 0.32, 95% CI 0.15 to 0.72, P < 0.05) (Analysis 1.8). Combining the results of four trials (Fenster 1966; Mendenhall 1984b; CSL 1986; Bonkovsky 1991) demonstrated no significant effect of anabolic‐androgenic steroids on the combined outcome measure consisting of ascites, hepatic encephalopathy, and hepatocellular carcinoma (RR 1.25, 95% CI 0.74 to 2.10) (Analysis 1.6). Liver haemodynamics and function Only one randomised clinical trial (CSL 1986) determined liver haemodynamics and liver function in 34 patients (22 in the anabolic‐androgenic steroids group and 12 in the control group of the trial, which used skewed randomisation) after one year. No significant effect of testosterone could be found on portal pressure (Analysis 1.12), indocyanine green clearance (Analysis 1.13), and galactose elimination capacity (Analysis 1.14). Liver histology There were no significant effects of anabolic‐androgenic steroids on either histological improvement of liver biopsy findings (Fenster 1966) or histological changes in the liver (CSL 1986) (Analysis 1.15; Analysis 1.16; Analysis 1.17; Analysis 1.18; Analysis 1.19; Analysis 1.20):
1.3. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 3 Ascites.
1.4. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 4 Hepatic encephalopathy.
1.5. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 5 Hepatocellular carcinoma.
1.7. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 7 Pretibial edema.
1.9. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 9 Prostatic hypertrophia.
1.10. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 10 Sexual dysfunction.
1.11. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 11 Weight (kg).
1.8. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 8 Gynaecomastia.
1.6. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 6 Total number of complications (ascites, hepatic encephalopathy, hepatocellular carcinoma).
1.12. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 12 Portal pressure (mmHg).
1.13. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 13 Indocyanine green clearance (ml plasma/min).
1.14. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 14 Galactose elimination capacity (mmol/min).
1.15. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 15 Biopsy finding, improvement (fat).
1.16. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 16 Biopsy finding, significant improvement in follow‐up specimens (fatty).
1.17. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 17 Biopsy finding, improvement (inflammatory cells).
1.18. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 18 Biopsy finding, significant improvement in follow‐up specimens (hepatitis).
1.19. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 19 Biopsy finding, improvement (acute necrosis).
1.20. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 20 Biopsy finding, significant improvement in follow‐up specimens (focal necrosis).
Sexual function The CSL 1986 trial evaluated sexual function in men with alcoholic cirrhosis (Gluud 1988b). At entry, 67% (95% confidence limits 61% to 74%) of 221 patients complained of sexual dysfunction (defined as problems with sexual desire, erection, or ejaculation). During follow‐up (median 30 months (range 1 to 40 months) sexual dysfunction in the total patient group improved significantly (P < 0.05) at 6, 12, and 24 months follow‐up. However, there were no significant differences between the testosterone treated and the placebo treated patients regarding the proportion of patients with sexual dysfunction at any time during follow up (1, 6, 12, 24, and 36 months). At 12 months follow‐up the RR of sexual dysfunction was 1.11 (95% CI 0.83 to 1.48) (Analysis 1.34).
1.34. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 34 Sexual dysfunction at 12 months follow‐up.
Liver biochemistry and other biochemical outcome measures Anabolic‐androgenic steroids significantly increased serum (s)‐testosterone and blood (b)‐haemoglobin levels: ‐ s‐testosterone (mmol/l): WMD 31.90 (95% CI 11.99 to 51.81) P < 0.005 (Analysis 1.27); ‐ b‐haemoglobin (mmol/l): WMD 0.60 (95% CI 0.13 to 1.07) P < 0.05 (Analysis 1.26); ‐ s‐IgM (g/l): WMD ‐0.80 (95% CI ‐1.34 to ‐0.26) (Analysis 1.31) and significantly decreased s‐immunoglobulin (Ig) levels.
1.27. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 27 Serum‐testosterone (nmol/l).
1.26. Analysis.
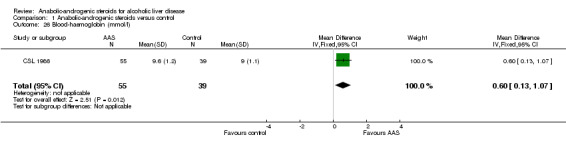
Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 26 Blood‐haemoglobin (mmol/l).
1.31. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 31 Serum‐immunoglobulin M (g/l).
However, anabolic‐androgenic steroids did not significantly affect the majority of the outcome biochemical measures: ‐ s‐bilirubin (mg/dl): WMD 0.01 (95% CI ‐0.25 to 0.27) (Analysis 1.21); ‐ plasma prothrombin time (each study used different units): SMD 0.22 (95% CI ‐0.10 to 0.55) (Analysis 1.22); ‐ s‐albumin (g/dl): WMD 0.16 (95% CI ‐0.04 to 0.36) (Analysis 1.23); ‐ s‐alkaline phosphatases ((U/l): WMD ‐2.51 (95% CI ‐9.86 to 4.85) (Analysis 1.24); ‐ s‐aspartate aminotransferase (U/I): WMD ‐16.69 (95% CI ‐42.24 to 8.86) (Random‐effects model) (Analysis 1.25); ‐ s‐creatinine (umol/l): WMD 3.20 (95% CI ‐10.16 to 16.56) (Analysis 1.28); ‐ s‐Ig G (g/l): WMD ‐1.20 (95% CI ‐3.06 to 0.66) (Analysis 1.29); ‐ s‐IgA (g/l): WMD ‐0.20 (95% CI ‐1.19 to 0.79) (Analysis 1.30).
1.21. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 21 Serum‐bilirubin (mg/dl).
1.22. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 22 Prothrombin time.
1.23. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 23 Serum‐albumin (g/dl).
1.24. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 24 Serum‐alkaline phosphatases (U/l).
1.25. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 25 Serum‐aspartate aminotransferase (U/l).
1.28. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 28 Serum‐creatinine (umol/l).
1.29. Analysis.
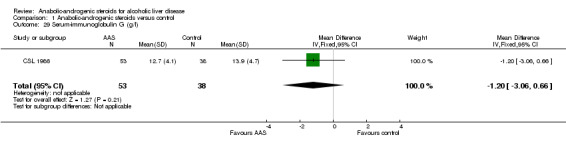
Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 29 Serum‐immunoglobulin G (g/l).
1.30. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 30 Serum‐immunoglobuilin A (g/l).
Adverse events Only two of the five trials reported adverse events (CSL 1986; Bonkovsky 1991). Anabolic‐androgenic steroids had no significant effect on the occurrence of non‐serious adverse events (RR 1.14; 95% CI 0.50 to 2.59). In the anabolic‐androgenic steroids group 11/152 (7.2%) patients had non‐serious adverse events versus 8/108 (7.4%) patients in the control group (Analysis 2.1). The adverse events observed in the anabolic‐androgenic steroids group encompassed mild nausea (n = 4 patients), which was managed by giving oxandrolone with food; and diffuse complaints (n = 5 patients). The adverse events observed in the control group encompassed diffuse complaints (n = 6 patients).
2.1. Analysis.

Comparison 2 Adverse events, Outcome 1 Non‐serious adverse events.
Anabolic‐androgenic steroids had no significant effect on the occurrence of serious adverse events, although there was a trend (RR 4.54; 95% CI 0.57 to 36.30). In the anabolic‐androgenic steroids group 7/152 (4.6%) patients had serious adverse events versus 1/108 (0.9%) patient in the control group (Analysis 2.1). The adverse events observed in the anabolic‐androgenic steroids group encompassed vascular thrombosis in the portal or hepatic veins (n = 3 patients), myocardial infarct (n = 1 patient), and polycythaemia (n = 3 patients). The adverse event observed in the control group encompassed polycythaemia (n = 1 patient) (Analysis 2.2).
2.2. Analysis.

Comparison 2 Adverse events, Outcome 2 Serious adverse events.
Quality of life and health economics Only one trial measured quality of life employing crude measures, ie, numbers of days with normal activity and number of days in hospital within the last 100 days (CSL 1986).
At 12 months, the number of days with normal activity (ie, activity similar to premorbid activity) within the last 100 days was not significantly different between placebo and testosterone treatment: WMD 3.10 (95% CI ‐12.55 to 18.75) (Analysis 1.32).
1.32. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 32 Number of days with normal activity within the last 100 days at 12 months follow‐up.
Anabolic‐androgenic steroids did not significantly affect the number of days in hospital within the last 100 days at 12 months follow up (CSL 1986): WMD 0.50 (‐2.27 to 3.27) (Analysis 1.33). We were unable to identify other data on health economics.
1.33. Analysis.

Comparison 1 Anabolic‐androgenic steroids versus control, Outcome 33 Number of days in hospital within the last 100 days at 12 months follow‐up.
Funnel plot asymmetry Due to the paucity of randomised clinical trials and observed outcome measures reported in the included randomised clinical trials we did not try to analyse for funnel plot asymmetry.
Discussion
We could not demonstrate any significant effects of anabolic‐androgenic steroids on mortality, liver‐related mortality, liver complications, liver histology, and liver biochemistry when tested against placebo or no intervention in patients with alcoholic hepatitis and/or cirrhosis. We were unable to identify any randomised trials in patients with alcoholic steatosis.
Performing subgroup analysis considering methodological quality of the randomised clinical trials, contrasting high‐quality trials versus low‐quality trials did not reveal any significant influence of anabolic‐androgenic steroids on the RR of mortality. This observation may not be in accordance with empirical studies suggesting significant overestimation of intervention effects in unblinded randomised clinical trials (Schulz 1995; Kjaergard 2001), but is likely due to insufficient power. Subgroup analyses taking different treatments (oxandrolone, testosterone, methenolone), stage of liver disease (mainly alcoholic hepatitis or alcoholic cirrhosis), or duration of follow‐up into consideration showed no significant effect of anabolic‐androgenic steroids versus control in any of the subgroups.
The lack of effect of anabolic‐androgenic steroids on mortality was also observed taking into consideration the excluded trial evaluating oxandrolone plus nutritional support versus placebo (Mendenhall 1993b). We excluded this randomised clinical trial because it evaluated the combination of anabolic‐androgenic steroids plus another intervention. On the other hand, nutrition has no verified effect on mortality of patients with alcoholic hepatitis (Cabré 2000; Cabré 2001; Koretz 2001). Accordingly, the present results confirm our previous meta‐analyses on anabolic‐androgenic steroids for alcoholic liver disease (Gluud 1988a, see other published versions of this review).
Some trials have claimed that oxandrolone may provide beneficial effects in patients with alcoholic hepatitis. In a six‐month conditional analysis, removing patients that died within the first two months after randomisation, Mendenhall and associates (Mendenhall 1984a) observed a significant effect of oxandrolone on mortality in patients with moderate disease. Such analyses are difficult to interpret. First, by removing the patients who died early (within one or two months) you are not likely to have a randomised comparison any more. Secondly, this analysis was based on a subgroup analysis of patients. Mendenhall and associates (Mendenhall 1993b) could not demonstrate any significant effect of oxandrolone plus nutritional support versus placebo. In a subgroup of patients with moderate malnutrition and adequate caloric intake, however, oxandrolone plus nutritional support reduced six‐month mortality significantly. Subgroup analyses are often misleading (Yusuf 1991; Oxman 1992; Assmann 2000). Because of such risks researchers need to be cautious about subgroup analyses and the interpretation of the results of the ones that they feel compelled to do.
Anabolic‐androgenic steroids were without any significant effect on liver histology and liver haemodynamics. We were also unable to detect any significant influence of anabolic‐androgenic steroids on liver‐related mortality, ascites, hepatic encephalopathy, hepatocellular carcinoma, pretibial oedema, and weight. Anabolic‐androgenic steroids were also without significant effect on all other liver biochemical and liver function variables. However, anabolic‐androgenic steroids significantly increased the b‐haemoglobin concentration (CSL 1986). These observations may be real, but even then cannot establish any therapeutic indication for anabolic‐androgenic steroids in alcoholic liver disease.
Aromatisable anabolic‐androgenic steroids lead to a significant increase in plasma oestrogen concentrations. This increase is significantly higher in patients with liver cirrhosis than in controls (Gluud 1988a). Due to a higher increase in testosterone than in concentrations of oestrogens, the oestrogen/testosterone‐ratio decreases during testosterone administration (Gluud 1988a). In accordance, a significant decrease was observed in the proportion of patients with gynaecomastia in the testosterone treated group (CSL 1986). This effect may not, however, establish an indication for using testosterone in these patients considering the risk of serious adverse events and the fact that symptomatic gynaecomastia may be treated with surgical ablation.
According to the observations of the CSL trial (CSL 1986), the testosterone‐treated patients did not differ significantly from the placebo‐treated patients regarding changes in sexual function (Gluud 1988a; Gluud 1988b). On the positive side, a number of the male cirrhotic patients showed improvements of their sexual function irrespective of the treatment offered to the patients (Gluud 1988b).
Anabolic‐androgenic steroids were not associated with a significant increase in non‐serious adverse events. There was a trend for the treated group to have more serious adverse events, but that did not achieve statistical significance (P = 0.15). Three patients with portal or hepatic thrombosis, one with myocardial infarction, and three with polycythaemia constituted the serious adverse events reported in the anabolic‐androgenic steroids group versus one with polycythaemia in the control group. We observed that testosterone increased b‐haemoglobin. It has been claimed that oxandrolone increases clotting factors in cirrhotic patients with vitamin K resistant hypoprothrombinaemia (Tamburro 1967). It cannot be excluded that testosterone may increase the incidence of thrombosis, possibly via an increase in estrogens during anabolic‐androgenic steroids administration (Lewis 1983; Gluud 1988b). Acute myocardial infarction and stroke have been reported as serious adverse events in several athletes using androgens (Ferenchick 1991).
Accordingly, we have been unable to identify any convincing evidence supporting anabolic‐androgenic steroids for alcoholic liver disease patients. However, this review does not preclude the possibility of a beneficial effect of anabolic‐androgenic steroids in alcoholic liver disease; or in other form of liver disease (Shahidi 2001). Evidence about how much medical interventions work may change over time. Ioannidis and Lau (Ioannidis 2001) recently applied 'recursive cumulative meta‐analyses' of randomised clinical trials to evaluate the relative change in the pooled treatment effect over time for 60 medical interventions within pregnancy/perinatal medicine and cardiology. With 500 accumulated patients, the pooled relative risk may change by about 0.6 to 1.7 fold in the immediate future. When 2000 patients have been randomised, the pooled relative risk may change by 0.7 to 1.3 fold.
Meta‐analyses and randomised clinical trials have been unable to demonstrate significant effects of colchicine (Rambaldi 2005a), glucocorticosteroids (Christensen 1995; Gluud 2001), insulin/glucagon (Trinchet 1992), milk thistle (Flora 1998; Rambaldi 2005b), propylthiouracil (Rambaldi 2005c), parenteral amino acid supplementation (Mezey 1991), amlodipine (Bird 1998), and polyenylphosphatidylcholine (Lieber 2000; Lieber 2003) for alcoholic liver disease. At present, S‐adenosyl‐L‐methionine (Mato 1999) and pentoxifylline (Akriviadis 2000) may seem promising interventions for alcoholic liver disease (Mato 1999), but more randomised clinical trials are needed before S‐adenosyl‐L‐methionine can be recommended (Rambaldi 2006) and this also applies to pentoxifylline, which has only been evaluated in one published randomised trial including patients with severe alcoholic hepatitis. Abstinence from ethanol remains the best intervention for alcoholic liver disease (Poynard 1994).
Authors' conclusions
Implications for practice.
This systematic review could not demonstrate any significant beneficial effect of anabolic‐androgenic steroids on mortality, liver‐related mortality, liver complications, and liver histology of patients with alcoholic liver disease.
Implications for research.
The absence of evidence for an effect of anabolic‐androgenic steroids on clinically relevant outcome variables, however, does not mean that there is evidence of lack of effect. If researchers wish to conduct new randomised clinical trials they ought to be large and closely monitored for the occurrence of adverse events. We recommend that investigators report their trials according to guidelines described in the CONSORT Statement (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 23 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 21 August 2006 | Amended | Amendment. |
Acknowledgements
We primarily extend our acknowledgements to the patients who took part in the reviewed trials and the researchers who conducted the trials. We are indebted to Nader Salas for the expert technical computer assistance and to Dimitrinka Nikolova and Sarah Klingenberg for expert assistance with the retrieval of publications. We are indebted to Gaetano Iaquinto who revised a previous version of the review. We thank John Villumsen for help with calculation of means and their 95% confidence intervals in the Fenster et al trial into means and standard deviations. Furthermore, we thank Ronald Koretz for helpful comments during the editorial processing of the review. Special thanks to Herbert Bonkovsky and Charles L. Mendenhall for providing us with more information on the trials they were involved in.
Appendices
Appendix 1. Search Strategies
| Database | Time of search | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register, 45 hits | June 2006. | (steroid* OR androgenic OR anabolic OR testosterone OR oxandrolone OR methandrostenolon OR 'methenolone enanthate') AND (alcoholic and ('liver disease*' or steatosis or fibrosis or hepatitis or cirrhosis)) |
| The Cochrane Central Register of Controlled Trials (CENTRAL/CCTR) in The Cochrane Library, 87 hits | Issue 2, 2006. | #1 STEROIDS explode all trees (MeSH) 26594 #2 (steroid* or androgenic or anabolic or testosterone or oxandrolone or methandrostenolon or (methenolone next enanthate)) 10098 #3 (#1 or #2) 31763 #4 LIVER DISEASES ALCOHOLIC explode all trees (MeSH) 330 #5 (alcoholic and ((liver next disease*) or steatosis or fibrosis or hepatitis or cirrhosis)) 680 #6 (#4 or #5) 683 #7 (#3 and #6) 111 |
| MEDLINE (WinSPIRS 5.0), 82 hits | 1950 to June 2006. | #1 555154 explode "Steroids"/ all subheadings #2 243711 steroid* or androgenic or anabolic or testosterone or oxandrolone or methandrostenolon or methenolone enanthate #3 666273 #1 or #2 #4 9371 explode "Liver‐Diseases‐Alcoholic"/ all subheadings #5 13656 alcoholic and (liver disease* or steatosis or fibrosis or hepatitis or cirrhosis) #6 13847 #4 or #5 #7 889 #3 and #6 #8 536466 random* or blind* or placebo* or meta‐analysis #9 82 #7 and #8 |
| EMBASE (WinSPIRS 5.0), 85 hits | 1980 to June 2006. | #1 332319 explode "steroid"/ all subheadings #2 179516 steroid* or androgenic or anabolic or testosterone or oxandrolone or methandrostenolon or methenolone enanthate #3 390950 #1 or #2 #4 6036 explode "alcohol‐liver‐disease"/ all subheadings #5 7201 alcoholic and (liver disease* or steatosis or fibrosis or hepatitis or cirrhosis) #6 9230 #4 or #5 #7 613 #3 and #6 #8 469358 random* or blind* or placebo* or meta‐analysis #9 85 #7 and #8 |
| Science Citation Index Expanded, 37 hits (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | 1945 to June 2006. | http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame #1 >100,000 TS=(steroid* OR androgenic OR anabolic OR testosterone OR oxandrolone OR methandrostenolon OR 'methenolone enanthate') #2 8,661 TS=(alcoholic and ('liver disease*' or steatosis or fibrosis or hepatitis or cirrhosis)) #3 195 #2 AND #1 #4 >100,000 TS=(random* or blind* or placebo* or meta‐analysis) #5 37 #4 AND #3 |
| LILACS, 0 hits (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/) | 1982 to June 2006. | Steroid * and alcoholic and (hepatitis or liver disease) |
Data and analyses
Comparison 1. Anabolic‐androgenic steroids versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 2 Liver‐related mortality | 3 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.60, 1.15] |
| 3 Ascites | 4 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.47, 1.63] |
| 4 Hepatic encephalopathy | 4 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.53, 3.42] |
| 5 Hepatocellular carcinoma | 4 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.13, 2.66] |
| 6 Total number of complications (ascites, hepatic encephalopathy, hepatocellular carcinoma) | 4 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.74, 2.10] |
| 7 Pretibial edema | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.12, 2.17] |
| 8 Gynaecomastia | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.72] |
| 9 Prostatic hypertrophia | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.64, 7.39] |
| 10 Sexual dysfunction | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.58, 1.42] |
| 11 Weight (kg) | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐6.26, 5.46] |
| 12 Portal pressure (mmHg) | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 1.84 [‐2.19, 5.87] |
| 13 Indocyanine green clearance (ml plasma/min) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐33.26 [‐131.01, 64.49] |
| 14 Galactose elimination capacity (mmol/min) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.74, 0.26] |
| 15 Biopsy finding, improvement (fat) | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.71, 1.86] |
| 16 Biopsy finding, significant improvement in follow‐up specimens (fatty) | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| 16.1 Fatty change | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.68, 1.33] |
| 17 Biopsy finding, improvement (inflammatory cells) | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.34, 6.70] |
| 18 Biopsy finding, significant improvement in follow‐up specimens (hepatitis) | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.48, 1.89] |
| 18.1 alcoholic hepatitis | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.48, 1.89] |
| 19 Biopsy finding, improvement (acute necrosis) | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.81, 3.99] |
| 20 Biopsy finding, significant improvement in follow‐up specimens (focal necrosis) | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.10] |
| 20.1 Focal necrosis | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.10] |
| 21 Serum‐bilirubin (mg/dl) | 3 | 165 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.25, 0.27] |
| 22 Prothrombin time | 3 | 163 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.10, 0.55] |
| 23 Serum‐albumin (g/dl) | 2 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.04, 0.36] |
| 24 Serum‐alkaline phosphatases (U/l) | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐2.51 [‐9.86, 4.85] |
| 25 Serum‐aspartate aminotransferase (U/l) | 3 | 164 | Mean Difference (IV, Random, 95% CI) | ‐16.69 [‐42.24, 8.86] |
| 26 Blood‐haemoglobin (mmol/l) | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.13, 1.07] |
| 27 Serum‐testosterone (nmol/l) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 31.90 [11.99, 51.81] |
| 28 Serum‐creatinine (umol/l) | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐10.16, 16.56] |
| 29 Serum‐immunoglobulin G (g/l) | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.06, 0.66] |
| 30 Serum‐immunoglobuilin A (g/l) | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.19, 0.79] |
| 31 Serum‐immunoglobulin M (g/l) | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.34, ‐0.26] |
| 32 Number of days with normal activity within the last 100 days at 12 months follow‐up | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐12.55, 18.75] |
| 33 Number of days in hospital within the last 100 days at 12 months follow‐up | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐2.27, 3.27] |
| 34 Sexual dysfunction at 12 months follow‐up | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.83, 1.48] |
Comparison 2. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐serious adverse events | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.50, 2.59] |
| 2 Serious adverse events | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.54 [0.57, 36.30] |
Comparison 3. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality ‐ worst‐case scenario | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.25] |
| 2 Mortality and generation of the allocation sequence | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 2.1 Adequate generation of the allocation sequence | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.72, 1.98] |
| 2.2 Inadequate generation of the allocation sequence | 3 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.72, 1.24] |
| 3 Mortality and allocation concealment | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 3.1 Adequate allocation concealment | 2 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.26] |
| 3.2 Inadequate allocation concealment | 3 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.54, 3.50] |
| 4 Mortality and double blinding | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 4.1 Adequately blinded | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.79, 1.98] |
| 4.2 Inadequately blinded | 3 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.18] |
| 5 Mortality and follow‐up | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 5.1 Adequate follow‐up | 4 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 5.2 Inadequate follow‐up | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.30] |
| 6 Mortality ‐ duration of follow‐up | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 6.1 Short‐term follow‐up (within 21 days) | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.30] |
| 6.2 Long‐term follow‐up (at least six months) | 3 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 7 Mortality ‐ different treatment | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 7.1 Oxandrolone | 3 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.18] |
| 7.2 Testosterone | 1 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.72, 1.98] |
| 7.3 Testosterone propionate or methenolone | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.53, 4.65] |
| 8 Mortality ‐ stage of alcoholic liver disease | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 8.1 Alcoholic hepatitis | 3 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.18] |
| 8.2 Alcoholic cirrhosis | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.79, 1.98] |
| 9 Mortality ‐ according to co‐intervention | 6 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.21] |
| 9.1 Anabolic‐androgenic steroids | 5 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 9.2 Anabolic‐androgenic steroids plus nutritional therapy | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bonkovsky 1991.
| Methods | Sample size: no justification. Generation of the allocation sequence: adequate, by random number table. Allocation concealment: adequate, by opaque envelope technique. Blinding: inadequate, unblinded. Intention‐to‐treat analysis: not described, but presumably used. Follow‐up: adequate. |
|
| Participants | Multicentre clinical trial including 39 patients (19 men and 20 women) from three hospitals, with severe alcoholic hepatitis. Eight patients (three men and five women) were allocated to the oxandrolone group (Experimental I), mean age (SEM) 39 ± 3 years, range 32 to 46 years. Ten patients (five men and five women) were allocated to the oxandrolone plus nutritional supplementation group (Experimental II), mean age (SEM) 46 ± 4 years, range 36‐51 years. Twelve patients (seven men and five women) were allocated to the control group (Control I), with a mean age (SEM) 41± 2 years, range 35 to 46 years; nine patients (four men and five women) were allocated to the control group treated with nutritional supplementation (Control II), with a mean age (SEM) 44± 2 years, range 37 to 49 years. Inclusion criteria: history of excessive drinking; liver biochemistry: s‐aspartate aminotransferase < 500 U/L, ratio of s‐transaminases > 1.5, s‐albumin< 3.0 g/dl, s‐bilirubin > 5 mg/dl, and prothrombin time < 6 seconds above control; cessation of alcohol intake 5 to 14 days before entry into the trial. Liver biopsies showed florid alcoholic hepatitis in all patients, having a biopsy (n = 31). Exclusion criteria: recent severe gastrointestinal bleeding; severe degree of ascites; severe degree of encephalopathy; renal insufficiency; sepsis; acute pancreatitis; haemodynamic instability; advanced pulmonary disease; diabetes mellitus; active malignancy. |
|
| Interventions | There was a 10‐day baseline period of observation during which dietary and intravenous intakes and weights were recorded daily, and weekly routine and special quantitative tests of liver function were calculated. Experimental I: oxandrolone (20 mg orally four times a day). Experimental II: oxandrolone (20 mg orally four times a day) plus nutritional supplementation consisting of two liters of 3.5% crystalline amino acids in 5% dextrose given by peripheral vein. Control I: no intervention consisting of standard therapy (alcohol abstinence, a balanced nutritionally adequate diet, and multivitamins). Control II: nutritional supplementation consisting of two liters of 3.5% crystalline amino acids in 5% dextrose given by peripheral vein plus no intervention (standard therapy). Duration of treatment and of follow‐up: 21 days. |
|
| Outcomes | Mortality. Biochemistry and function. Liver histology. Adverse events. |
|
| Notes | Sent letter. H. Bonkovsky answered the letter in 2001. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
CSL 1986.
| Methods | Sample size: based on mortality. Generation of the allocation sequence: adequate, by random numbers (skewed randomisation 3:2; testosterone versus placebo). Allocation concealment: adequate, by serially numbered sealed boxes. Blinding: adequate, double blind with placebo of identical appearance, smell, and taste. Intention‐to‐treat analysis: not mentioned, but used. Follow‐up: adequate more than 10% of the patients dropped out or were withdrawn. |
|
| Participants | Multicentre clinical trial including 221 men from five medical departments. Of these 134 patients (53 years, range 24 to 79 years) were allocated to the testosterone group and 87 (53 years, range 29 to 78) to the placebo group. Inclusion criteria: daily ethanol consumption above 50 grams for more than two years; liver cirrhosis diagnosed by liver biopsy for the first time within the last six months; specific etiology of cirrhosis other than ethanol could be excluded. Exclusion criteria: a) unable to cooperate; b) refusal of informed consent; c) hepatitis B surface antigen positive; d) hepatocellular carcinoma; e) other malignancies; f) other reasons. |
|
| Interventions | Experimental:
micronized‐free testosterone tablets 200 mg (two tablets), three times daily. Control: placebo (two tablets three times daily). Patients were advised not to drink alcoholic beverages, and were offered the standard treatment. Duration of treatment and of follow‐up: 36 months. |
|
| Outcomes | Mortality. Complications. Biochemistry and function. Liver histology. Adverse events. Gynaecomastia. Sexual dysfunction. Quality of life. Number of days in hospital. |
|
| Notes | Data on mortality, ascites, hepatic encephalopathy, hepatocellular carcinoma, pretibial edema, prostatic hypertrophia, sexual dysfunction, gynaecomastia were for patients followed‐up and treated for 24 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fenster 1966.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear, no information. Allocation concealment: unclear, no information. Blinding: adequate, double blind with placebo of identical appearance. Intention‐to‐treat analysis: not used. Follow‐up: adequate, more than 10% of the patients dropped out or were withdrawn. |
|
| Participants | Thirty‐two patients were included in the trial. Patients were divided in three treatment groups: 1) nine patients with testosterone; 2) twelve patients with methenolone; 3) eleven patients with placebo. Inclusion criteria: clinical and laboratory evidence of chronic alcoholic liver disease; evidence of active parenchymal dysfunction, as distinct from manifestations of portal hypertension. Exclusion criteria: gastrointestinal bleeding or suspicion of prostatic carcinoma. |
|
| Interventions | Experimental I:
testosterone propionate intramuscularly 100 mg every other day. Experimental II: methenolone enanthate intramuscularly 100 mg every other day. Control: placebo injection. Duration of the treatment: one month. Duration of follow‐up: at least six months. |
|
| Outcomes | Mortality. Biochemistry. Liver histology. Since both testosterone and methenolone are anabolic‐androgenic steroids we combined the results. There was not significant difference in mortality and biochemistry between the testosterone and methenolone groups. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mendenhall 1977.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear, no information. Allocation concealment: unclear, no information. Blinding: unclear, described as double blind, but no information on how blinding was achieved. Intention‐to‐treat analysis: not mentioned, but presumably used. Follow‐up: not described. |
|
| Participants | Thirty‐four patients with alcoholic hepatitis were included in the trial. Inclusion criteria: ethanol ingestion > 100 g/day for at least one year; hepatomegaly ( > 12 cm) and significant jaundice (bilirubin > 5 mg/dl). Liver biopsy was obtained in about 70% of patients to confirm the diagnosis. |
|
| Interventions | Experimental I:
oxandrolone 80 mg/day orally x 14 days then decreased over a period of seven days. Experimental II: prednisolone 60 mg orally per day for four days, then a decreasing dosage per 16 days. Control: placebo. Additional treatment: supportive care. Duration of treatment and of follow‐up: 21 days. |
|
| Outcomes | Mortality. Biochemistry. Liver histology. Rate of improvement. |
|
| Notes | Sent letter. C.L. Mendenhall answered the letter. Only published as abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mendenhall 1984b.
| Methods | Sample size: no justification. Generation of the allocation sequence: unclear ‐ no information. Allocation concealment: adequate, using centralised randomisation. Blinding: unclear, described as double blind, but no information on how blinding was achieved. Intention‐to‐treat analysis: not mentioned but presumably used. Follow‐up: adequate, more than 10% of the patients dropped out or were withdrawn. |
|
| Participants | Multicentre clinical trial including 173 men from six Veteran Administration Medical Centers, 88 (mean age plus standard deviation (SD) 50.4± 9.2 years) in the placebo group and 85 (mean age plus SD 52.2± 8.2 y) in the oxandrolone group. Inclusion criteria: history of alcoholism and liver disease enough to warrant treatment. Exclusion criteria: conditions that precluded the use of prednisolone; clinical or laboratory features atypical of alcoholic hepatitis without biopsy confirmation of the diagnosis; inability or unwillingness to participate; biopsy‐confirmed liver disease other than alcoholic hepatitis; parenteral drug abuse or a positive test for hepatitis B surface antigen; diseases unrelated to the liver disease that would have complicated the interpretation of therapy; unrelated conditions that required corticosteroid therapy either currently or in the recent past; and miscellaneous reasons. |
|
| Interventions | Experimental I: oxandrolone 80 mg orally day per 30 days. Experimental II: prednisolone 60 mg orally per day for four days, then a decreasing dosage per 30 days. Control: placebo. Duration of treatment: 30 days. Patients were then followed and evaluated monthly at outpatient clinics. If alcoholic hepatitis recurred and required re hospitalisation, the patient was reassigned to the same therapy for 30 days with his permission. Duration of follow‐up: one year. |
|
| Outcomes | Mortality. Complications. Biochemistry. Liver histology. Adverse events. Nutritional status. |
|
| Notes | Sent letter. C.L. Mendenhall answered the letter in 2001. The data on patients allocated to the glucocorticosteroid arm were excluded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mendenhall 1993b.
| Methods | We excluded this randomised clinical trial because patients were treated with nutritional support and oxandrolone (two interventions) versus placebo as it was stated in the protocol. Since the etiology of the liver disease is alcoholic hepatitis we consider this randomised clinical trial only for sensitivity analysis. |
|
| Participants | Multicentre clinical trial including 273 men: 137 underwent active treatment; 136 received placebo. | |
| Interventions | Experimental:
The active treatment consisted of oxandrolone 80 mg/die accompanied by high‐calorie, high‐protein food supplement. Control: placebo treatment consisted of placebo tablets accompanied by the low calorie, low‐protein food supplement. |
|
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Eberhardt 1975 | The study is quasi‐randomised. In 66 patients with mostly alcoholic liver disease, the effect of vitamin B versus clostebol (Steranabol) was tested. |
| Fiegel 1959 | The study is observational (case series). The intervention consisted of the administration of high doses of androgenic derivates (durabolin) combined with vitamins and glucocorticosteroids to patients with liver cirrhosis. |
| Figueroa 1973 | The study is observational (case series). Thirty patients with alcoholic and non‐alcoholic liver disease received mesterolone 75 mg per day per three months. No adverse events were observed. |
| Franken 1963 | A non‐randomised study evaluating methandrostenolone versus prednisone in 51 patients with liver cirrhosis of mixed etiologies. |
| Gill 1984 | The randomised clinical trial evaluated depo‐testosterone, prednisolone, and amino acid supplement versus placebo in patients with severe alcoholic hepatitis. |
| Girolami 1958 | The study is observational (case series). Fifty cases of liver cirrhosis were treated with high dosages of testosterone. No serious adverse events were reported. |
| Gluud 1981 | The study is a three‐armed parallel‐group randomised clinical trial comparing testosterone concentrations during 28 days of administration of three testosterone preparations to 24 patients with alcoholic cirrhosis (intramuscular Triolandren® (combination of short‐ and long‐acting testosterone); intramuscular testosterone propionate (short‐acting testosterone); peroral micronized testosterone) (total dose administered during the study 0.7 g, 1.2 g, and 22.4 g, respectively). No adverse events were observed. This trial was excluded due to the lack of a control group. |
| Hirayama 1970 | The trial is not randomised. The effect of short‐term treatment with methenolon and stanozolol for patients with compensated liver cirrhosis was studied. |
| Islam 1973 | The study is quasi‐randomised. Testosterone propionate was given to 32 patients with liver cirrhosis with ascites. Liver complications and survival rates differed significantly from those in the control group of 38 cases. After four years, the survival rate in testosterone group was 65.6%, and in the control group 36.8%. |
| Jabbari 1967 | The study is observational (three cases). Patients with alcoholic fatty liver were treated with either norethandrolone (n = 2 patients) or Anavar (n = 1 patient). |
| Kley 1979 | The study is observational (case series). The effect of testosterone enanthate administration was studied in patients with cirrhosis of the liver by the measurement of testosterone, androstenedione, estrone, and estradiol as well as free testosterone and free estradiol in the plasma. |
| Knobel 1975 | The study is observational (case series). Fifteen patients with histologically proven liver cirrhosis were treated for four weeks with metenolonenanthate. |
| Leevy 1962 | The study is observational (case series). Norethandrolone was given to 270 patients with fatty liver. |
| Lindner 1967 | The study is observational (case series). In 186 patients with alcoholic and non‐alcoholic liver cirrhosis, methenolone acetate was given for a mean time of 94 weeks. |
| Mendenhall 1974 | The study is observational (case series). Nine patients with varying degrees of fatty liver were studied. The authors evaluated hepatic lipoprotein release after norethandrolone administration. |
| Mendenhall 1993a | A randomised clinical trial involving 273 men was performed to evaluate the efficacy of oxandrolone in combination of an enteral food supplement versus no intervention in patients with severe alcoholic hepatitis. There was no significant reduction in the mortality rate in patients treated with oxandrolone and nutritional therapy. |
| Müting 1965 | The study is observational (case series). In 24 patients with compensated liver cirrhosis, the effect on the lipoprotein metabolism of treatment with nandrolondecanoate was evaluated. |
| Puliyel 1977 | The study is randomised, but the etiology of the liver disease was not given. In 23 patients with cirrhosis the effect of testosterone was studied. |
| Rosenak 1950 | The study is observational (case series). In 12 patients with liver cirrhosis the effect of testosterone was evaluated on the protein metabolism. |
| Seifert 1967 | The study is observational (case series) studying methenolone acetate for 33 patients with liver cirrhosis. |
| Tamburro 1967 | The authors investigated clotting factors in 14 patients with liver cirrhosis before and after the therapy with Anavar®. |
| Wells 1960 | The study is randomised, but the etiology of the liver cirrhosis was mixed. In 53 patients the effect of testosterone on mortality and other outcomes was studied. |
Contributions of authors
AR drafted the protocol, coordinated the identification of studies, selected trials for inclusion, performed data extraction, and drafted the review. CG checked and revised all these processes. Both are guarantors for the review.
Sources of support
Internal sources
The Copenhagen Trial Unit, Denmark.
External sources
The Copenhagen Hospital Corporation's Research Council Grant on Getting Research into Practice (GRIP), Denmark.
The 1991 Pharmacy Foundation, Denmark.
The Danish Medical Research Council Grant on Getting Research into Practice (GRIP), Denmark.
Declarations of interest
Christian Gluud has been the principle and coordinating investigator on a randomised trial on testosterone for alcoholic cirrhosis (CSL 1986) as well as the author of two previous meta‐analyses on the topic (Gluud 1984; Gluud 1988a).
Edited (no change to conclusions)
References
References to studies included in this review
Bonkovsky 1991 {published data only}
- Bonkovsky HL, Fiellin DA, Smith GS, Slaker DP, Simon D, Galambos JT. A randomized, controlled trial of treatment of alcoholic hepatitis with parenteral nutrition and oxandrolone. I. Short‐term effects on liver function. The American Journal of Gastroenterology 1991;86(9):1200‐8. [PubMed] [Google Scholar]
- Bonkovsky HL, Fiellin DA, Smith GS, Slaker DP, Simon D, Galambos JT. Treatment of alcoholic hepatitis with parenteral nutrition and oxandrolone: a randomized controlled trial. I. Short‐term effects on liver function. Hepatology 1990;12(4):870. [PubMed] [Google Scholar]
- Bonkovsky HL, Jafri IH, Singh RH, Cotsonis GA, Slaker DP. Treatment of alcoholic hepatitis with parenteral nutrition and oxandrolone: a randomized controlled trial. II. Effects on nitrogen metabolism. Hepatology 1990;12(4):978. [PubMed] [Google Scholar]
- Bonkovsky HL, Singh RH, Jafri IH, Fiellin DA, Smith GS, Simon D, et al. A randomized, controlled trial of treatment of alcoholic hepatitis with parenteral nutrition and oxandrolone. II. Short‐term effects on nitrogen metabolism, metabolic balance, and nutrition. The American Journal of Gastroenterology 1991;86(9):1209‐18. [PubMed] [Google Scholar]
CSL 1986 {published and unpublished data}
- Becker U, Gluud C, Bennett P, the Copenhagen Study Group for Liver Diseases. The effect of oral testosterone on serum TBG levels in alcoholic cirrhotic men. Liver 1988;8:219‐24. [DOI] [PubMed] [Google Scholar]
- Gluud C, Christoffersen P, Eriksen J, Wantzin P, Knudsen BB, the Copenhagen Study Group for Liver Diseases. No effect of long‐term oral testosterone treatment on liver morphology in men with alcoholic cirrhosis. The American Journal of Gastroenterology 1987;82(7):660‐4. [PubMed] [Google Scholar]
- Gluud C, Henriksen JH, the Copenhagen Study Group for Liver Diseases. Liver haemodynamics and function in alcoholic cirrhosis. Relation to testosterone treatment and ethanol consumption. Journal of Hepatology 1987;4:168‐73. [DOI] [PubMed] [Google Scholar]
- Gluud C, Wantzin P, Eriksen J, the Copenhagen Study Group for Liver Diseases. No effect of oral testosterone treatment on sexual dysfunction in alcoholic cirrhotic men. Gastroenterology 1988;95(6):1582‐7. [PMID 3053314] [DOI] [PubMed] [Google Scholar]
- Gluud C, the Copenhagen Study Group for Liver Diseases. Effect of testosterone treatment in men with alcoholic cirrhosis. Journal of Hepatology 1985;Suppl 1:S59. [Google Scholar]
- Gluud C, the Copenhagen Study Group for Liver Diseases. Serum concentrations in men with alcoholic cirrhosis: background for variation. Metabolism 1987;36(4):373‐8. [DOI] [PubMed] [Google Scholar]
- The Copenhagen Study Group for Liver Diseases. Testosterone treatment of men with alcoholic cirrhosis: a double‐blind study. Hepatology 1986;6(5):807‐13. [PubMed] [Google Scholar]
Fenster 1966 {published data only}
- Fenster LF. The nonefficacy of short‐term anabolic steroid therapy in alcoholic liver disease. Annals of Internal Medicine 1966;65(4):738‐44. [Google Scholar]
Mendenhall 1977 {published data only}
- Mendenhall CL, Goldenberg S. Risk factors and therapy in alcoholic hepatitis (AH). Gastroenterology 1977;72(5):1100. [Google Scholar]
Mendenhall 1984b {published data only}
- Mendenhall CL, Anderson S, Garcia‐Pont P, Goldberg S, Kiernan T, Seeff LB, et al. Short‐term and long‐term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. New England Journal of Medicine 1984;311:1464‐70. [DOI] [PubMed] [Google Scholar]
Mendenhall 1993b {published data only}
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veteran Affairs Cooperative Study. Hepatology 1993;17:564‐76. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Eberhardt 1975 {published data only}
- Eberhardt G, Mohr J, Oehlert W, Schmidt E, Schmidt FW, Wondrasek P. Controlled study of the therapeutic effect of B‐vitamins and anabolic steroid in chronic hepatitis [Kontrollierte studie zur therapeutischen wirkung von B vitaminen und einem anabolen steroid bei chronischer hepatitis]. Deutsche Medizinsche Wochenschrift 1975;100:2074‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fiegel 1959 {published data only}
- Fiegel G, Kelling H‐W. The influence of high doses of androgen derivates and of corticosteroids on severe parenchymal diseases [Die beeinflussung des schweren leberparenchymschadens unter zufuhr hochdosierter androgenderivate und von corticosteroiden]. Arztliche Forschung 1959;13:574‐8. [Google Scholar]
Figueroa 1973 {published data only}
- Figueroa RB. Mesterolone in steatosis and cirrhosis of the liver. Acta Hepato Gastroenterologica 1973;20:282‐90. [PubMed] [Google Scholar]
Franken 1963 {published data only}
- Franken FH, Daweke H, Gries EA, Schweinitz HA, Holzgrewe H, Forstmannn U. Long term treatment of chronic liver diseases with prednisone and methandrostenolon [Langzeitbehandlung chronischer lebererkrankungen mit prednison und methandrostenolon]. Deutsche Medizinsche Wochenschrift 1963;88:1979‐85. [DOI] [PubMed] [Google Scholar]
Gill 1984 {published data only}
- Gill R, Zieve L, Logan G. Severe alcoholic hepatitis improved by combined treatment with prednisolone, testosterone and an amino acid supplement. Hepatology 1984;4(5):26. [Google Scholar]
Girolami 1958 {published data only}
- Girolami M. Treatment of ascitic atrophic cirrhosis of the liver with high dosages of testosterone propionate. Journal of the American Geriatrics Society 1958;6:306‐23. [DOI] [PubMed] [Google Scholar]
Gluud 1981 {published data only}
- Gluud C, Bennett P, Dietrichson O, Johnsen SG, Ranek L, Svendsen LB, et al. Short‐term parenteral and peroral testosterone administration in men with alcoholic cirrhosis. Scandinavian Journal of Gastoenterology 1981;16:749‐55. [DOI] [PubMed] [Google Scholar]
Hirayama 1970 {published data only}
- Hirayama C, Kimura N, Masuya T. Anabolic steroid effect on hepatic protein synthesis in patients with liver cirrhosis. Digestion 1970;3:41‐7. [DOI] [PubMed] [Google Scholar]
Islam 1973 {published data only}
- Islam N, Islam A. Testosterone propionate in cirrhosis of the liver. The British Journal of Clinical Practice 1973;27(4):125‐8. [PubMed] [Google Scholar]
Jabbari 1967 {published data only}
- Jabbari M, Leevy CM. Protein anabolism and fatty liver of the alcoholic. Medicine 1967;46(2):131‐9. [DOI] [PubMed] [Google Scholar]
Kley 1979 {published data only}
- Kley HK, Strohmeyer G, Krüskemper HL. Effect of testosterone application on hormone concentrations of androgens and estrogens in male patients with cirrhosis of the liver. Gastroenterology 1979;76(2):235‐41. [PMID 569612] [PubMed] [Google Scholar]
Knobel 1975 {published data only}
- Knobel H, Becker K. Effect of an anabolic steroid (methenolone enanthate) on the intra‐and extravasal albumin pool in liver cirrhosis [Die wirkung eines anabol wirkenden steroids (metenolononanthat) auf den intra‐und extravasalen albuminpool bei leberzirrhose]. Zeitschrift für Gastroenterologie 1975;13(6):583‐7. [PMID 1224752] [PubMed] [Google Scholar]
Leevy 1962 {published data only}
- Leevy CM. Fatty liver: a study of 270 patients with biopsy proven fatty liver and a review of the literature. Medicine 1962;41:249‐76. [DOI] [PubMed] [Google Scholar]
Lindner 1967 {published data only}
- Lindner H. Long‐term therapy of liver cirrhosis with anabolic steroids [Langzeittherapie der leberzirrhose mit anabolen steroiden]. Medizinische Klinik 1967;62:59‐63. [PMID 5592142] [PubMed] [Google Scholar]
Mendenhall 1974 {published data only}
- Mendenhall CL. Anabolic steroid therapy as an adjunct to diet in alcoholic hepatic steatosis. American Journal of Digestive Diseases 1968;13(9):783‐91. [DOI] [PubMed] [Google Scholar]
- Mendenhall CL. Augmented release of hepatic triglycerides with anabolic steroids in patients with fatty liver. Digestive Diseases 1974;19(2):122‐6. [DOI] [PubMed] [Google Scholar]
Mendenhall 1993a {published data only}
- Mendenhall CL, Moritz T, the DVA Coop Study Group 275. Therapy with oxandrolone (OX) and a high calorie supplement improves nutritional status in dempensated alcoholic hepatitis (AH) with moderate proteine calorie malnutrition (PCM). Hepatology 1991;14(4):233A. [Google Scholar]
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veteran Affairs Cooperative Study. Hepatology 1993;17:564‐76. [DOI] [PubMed] [Google Scholar]
- Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, et al. Protein energy malnutrition in severe alcoholic hepatitis: diagnosis and response to treatment. Journal of Parenteral and Enteral Nutrition 1995;19(4):258‐65. [DOI] [PubMed] [Google Scholar]
- Mendenhall CL, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcoholism, Clinical Experimental Research 1995;19(3):635‐41. [DOI] [PubMed] [Google Scholar]
- Mezinskis J, Mendenhall CL, Moritz T, the VA Coop Study Group #275. Improve cognitive function as the result of nutrition therapy for patients with alcoholic hepatitis. Hepatology 1991;14(4):233A. [Google Scholar]
- Morgan TR, Haas R, Moritz T, Mendenhall CL, VA Coop Study #275. Does high protein intake increase the risk of hepatic encephalopathy (HE) in patients with alcoholic hepatitis (AH)?. Hepatology 1991;14(4):233A. [Google Scholar]
- Morgan TR, Mortitz T, Mendenhall CL. Nutritional therapy for alcoholic hepatitis. VA Cooperative 275 Study Group. Gastroenterology 1992;103(1):357‐8. [DOI] [PubMed] [Google Scholar]
Müting 1965 {published data only}
- Müting D. Protein metabolism, excretion, and detoxication functions of the liver after long‐term treatment of liver cirrhosis with an anabolic substance. American Journal of Digestive Diseases 1965;10:790‐5. [DOI] [PubMed] [Google Scholar]
Puliyel 1977 {published data only}
- Puliyel MM, Vyas GP, Mehta GS. Testosterone in the management of cirrhosis of the liver ‐ a controlled study. Australian and New Zealand Journal of Medicine 1977;7:596‐9. [PubMed] [Google Scholar]
Rosenak 1950 {published data only}
- Rosenak BD, Moser RH, Howell JD. The effect of administration of testosterone propionate on excretion of water in patients with cirrhosis of the liver. Gastroenterology 1950;14:382‐5. [Google Scholar]
- Rosenak BD, Moser RH, Kilgore B Jr. Treatment of cirrhosis of the liver with testosterone propionate. Gastroenterology 1947;9:695‐704. [PubMed] [Google Scholar]
Seifert 1967 {published data only}
- Seifert E, Dittrich H. Therapy of chronic liver diseases with dacomid [Therapie chronischer lebererkrankungen mit dacomid]. Medizinische Klinik 1967;62(27):1055‐8. [PMID 4874027] [PubMed] [Google Scholar]
Tamburro 1967 {published data only}
- Tamburro C, Leevy CM. Protein clotting factor synthesis in liver disease. Gastroenterology 1967;52:325. [Google Scholar]
Wells 1960 {published data only}
- Wells R. Prednisolone and testosterone propionate in cirrhosis of the liver. A controlled trial. Lancet 1960;276(7166):1416‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Akriviadis 2000
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short‐term survival in severe acute alcoholic hepatitis: a double‐blind, placebo‐controlled trial. Gastroenterology 2000;119(6):1787‐91. [DOI] [PubMed] [Google Scholar]
Assmann 2000
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other mis(uses) of baseline data in clinical trials. Lancet 2000;355:1064‐9. [DOI] [PubMed] [Google Scholar]
Becker 1993
- Becker U. The influence of ethanol and liver disease on sex hormones and hepatic oestrogen receptors in women. Danish Medical Bulletin 1993;40(4):447‐59. [PMID 8222764] [PubMed] [Google Scholar]
Bird 1998
- Bird GL, Prach AT, McMahon AD, Forrest JA, Mills PR, Danesh BJ. Randomised controlled double‐blind trial of the calcium channel antagonist amlodipine in the treatment of acute alcoholic hepatitis. Journal of Hepatology 1998;28(2):194‐8. [DOI] [PubMed] [Google Scholar]
Cabré 2000
- Cabré E, Rodríguez‐Iglesias P, Caballería J, Quer JC, Sánchez‐Lombrana JL, Parés A, et al. Short‐ and long‐term outcome of severe alcohol‐induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000;32:36‐42. [DOI] [PubMed] [Google Scholar]
Cabré 2001
- Cabré E, Gassull MA. Metabolic and nutritional interventions in the treatment of alcoholic hepatitis. FALK Symposium 121. Steatohepatitis (NASH and ASH). Dordrecht/Boston/London: Kluwer Academic Publishers, 2001:305‐16.
Chang 1994
- Chang TT, Lin CY, Chow NH, Hsu PI, Yang CC, Lin XZ, et al. Hepatitis B and hepatitis C virus infection among chronic alcoholic patients with liver disease in Taiwan. Journal of Formosan Medical Association 1994;93(2):128‐33. [PubMed] [Google Scholar]
Christensen 1995
- Christensen E, Gluud C. Glucocorticoids are inffective in alcoholic hepatitis: a meta‐analysis adjusting for confounding variables. Gut 1995;37(1):113‐8. [PMID: 7672658] [DOI] [PMC free article] [PubMed] [Google Scholar]
CONSORT Statement
- Moher D, Schulz KF, Altman DG for the CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. http://www.consort‐statement.org/Statement/revisedstatement.htm (accessed 29 June 2006). [DOI] [PMC free article] [PubMed]
Corrao 1998
- Corrao G, Zambon A, Torchio P, Arico S, Vecchia C, Iorio F. Attributable risk for symptomatic liver cirrhosis in Italy. Collaborative Groups for the Study of Liver Diseases in Italy. Journal of Hepatology 1998;28(4):608‐14. [DOI] [PubMed] [Google Scholar]
Demets 1987
- Demets DL. Pratical aspects in monitoring: a brief review. Statistics in Medicine 1987;6(7):753‐60. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [PMID: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferenchick 1991
- Ferenchick GS. Anabolic/androgenic steroid abuse and thrombosis: is there a connection?. Medical Hypotheses 1991;35(1):27‐31. [PMID: 1921773] [DOI] [PubMed] [Google Scholar]
Flora 1998
- Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. The American Journal of Gastroenterology 1998;93(2):139‐43. [PMID: 9468229] [DOI] [PubMed] [Google Scholar]
Gluud 1983
- Gluud C, Bahsen M, Bennett P, Brodthagen UA, Dietrichson O, Johnsen SG, et al. Hypothalamic‐pituitary‐gonadal function in relation to liver function in men with alcoholic cirrhosis. Scandinavian Journal of Gastroenterology 1983;18:939‐44. [DOI] [PubMed] [Google Scholar]
Gluud 1984
- Gluud C. Anabolic‐androgenic steroid treatment of liver diseases. Liver 1984;4:159‐69. [DOI] [PubMed] [Google Scholar]
Gluud 1987
- Gluud C, the Copenhagen Study Group for Liver Diseases. Serum testosterone concentrations in men with alcoholic liver cirrhosis: background for variation. Metabolism 1987;36:373‐8. [DOI] [PubMed] [Google Scholar]
Gluud 1988a
- Gluud C. Testosterone and alcoholic cirrhosis. Epidemiologic, pathophysiologic and therapeutic studies in men (Thesis). Danish Medical Bullettin 1988;35(6):564‐75. [PubMed] [Google Scholar]
Gluud 1988b
- Gluud C. Steroid hormones and Budd‐Chiari syndrome. Proceedings of the first international symposium on Budd‐Chiari syndrome. September 10th‐13th, Jinan, China, 1988:107‐14.
Gluud 1993
- Gluud C, Afroudakis A, Caballeria J, Laskus T, Morgan MY, Rueff B, et al. Diagnosis and treatment of alcoholic liver disease in Europe. Gastroenterology International 1993;6(4):221‐30. [Google Scholar]
Gluud 2001
- Gluud C. Alcoholic hepatitis: no glucocorticosteroids?. FALK Symposium 121. Steatohepatitis (NASH and ASH). Dordrecht/Boston/London: Kluwer Academic Publishers, 2001; Vol. 121:323‐42.
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & sons, Ltd. [Google Scholar]
ICH‐GCP 1997
- International conference on harmonisation expert working group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice. 1997 CFR & ICH Guidelines. 1. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Ioannidis 2001
- Ioannidis JPA, Lau J. Evolution of treatment effects over time: empirical insigh from recursive cumulative metaanalyses. Proceedings of the National Academy of Sciences of the United States of America 2001;98:831‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methological quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koretz 2001
- Koretz RL, Lipman TO, Klein S. AGA technical review on parenteral nutrition. Gastroenterology 2001;1214:970‐1001. [DOI] [PubMed] [Google Scholar]
Lewis 1983
- Lewis JH, Tice HL, Zimmerman HJ. Budd‐Chiari syndrome associated with oral contraceptive steroids. Review of treatment of 47 cases. Digestive Disease and Sciences 1983;28:673‐83. [DOI] [PubMed] [Google Scholar]
Lieber 2000
- Liber CS. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. Journal of Hepatology 2000;32(1 Suppl):113‐28. [PMID: 10728799] [DOI] [PubMed] [Google Scholar]
Lieber 2003
- Lieber CS, Weiss DG, Groszmann R, Paronetto F, Schenker S. For the Veterans Affairs Cooperative Study 391 Group. II. Veterans Affairs Cooperative Study of polyenylphosphatidylcholine in alcoholic liver disease. Alcohol Clinical and Experimental Research 2003;27:1765‐72. [DOI] [PubMed] [Google Scholar]
Marbet 1987
- Marbet UA, Bianchi L, Meury U, Stalder GA. Long‐term histological evaluation of the natural history and prognostic factors of alcoholic liver disease. Journal of Hepatology 1987;4(3):364‐72. [DOI] [PubMed] [Google Scholar]
Mato 1999
- Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A, et al. S‐Adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo‐controlled, double‐blind, multicenter clinical trial. Journal of Hepatology 1999;30:1081‐9. [DOI] [PubMed] [Google Scholar]
Mendenhall 1984a
- Mendenhall CL, Anderson S, Garcia‐Pont P, Goldenberg S, Kiernan T, Seeff LB, et al. Short‐term and long‐term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. New England Journal of Medicine 1984;311(23):1464‐70. [DOI] [PubMed] [Google Scholar]
Mezey 1991
- Mezey E, Caballeria J, Mitchell MC, Pares A, Herlong HF, Rodes J. Effect of parenteral amino acid supplementation on short‐term and long‐term outcomes in severe alcoholic hepatitis: a randomised controlled trial. Hepatology 1991;14(6):1090‐6. [PMID: 1959859] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Morgan 1999
- Morgan MY. Alcoholic liver disease: natural history, diagnosis, clinical features, evaluation, managment, prognosis, and prevention. In: Bircher J, Benhamou J‐P, McIntyre N, Rizzetto M, Rodés J editor(s). Oxford Textbook of Clinical Hepatology. Second Edition. Vol. 2, Oxford: Oxford Medical Publications, 1999:1185‐238. [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116:78‐84. [DOI] [PubMed] [Google Scholar]
Powell 1968
- Powell WJ Jr, Klatskin G. Duration of survival in patients with Laennec´s cirrhosis. Influence of alcohol withdrawal, and possible effects of recent changes in general management of the disease. American Journal of Medicine 1968;44(3):406‐20. [DOI] [PubMed] [Google Scholar]
Poynard 1994
- Poynard T, Barhelemy P, Fratte S, Boudjema K, Doffoel M, Vanlemmens C. Evaluation of efficacy of liver transplantation in alcoholic cirrhosis by a case‐control study and simulated control. The Lancet 1994;344(8921):502‐7. [DOI] [PubMed] [Google Scholar]
Purhoit 2006
- Purhoit V, Brenner DA. Mechanisms of alcohol‐induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology 2006;43:872‐8. [DOI] [PubMed] [Google Scholar]
Rambaldi 2005a
- Rambaldi A, Gluud C. Colchicine for alcoholic and non‐alcoholic liver fibrosis and cirrhosis. The Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No.: CD002148. DOI: 10.1002/14651858.CD002148.pub2. [DOI] [PMC free article] [PubMed]
Rambaldi 2005b
Rambaldi 2005c
Rambaldi 2006
RevMan 2003 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Rubin 1968
- Rubin E, Lieber CS. Alcohol‐induced hepatic injury in nonalcoholic volunteers. New England Journal of Medicine 1968;278(16):869‐76. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Haeys RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of American Medical Association 1995;273(5):408‐12. [PMID: 7823387] [DOI] [PubMed] [Google Scholar]
Shahidi 2001
- Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic‐androgenic steroids. Clinical Therapeutics 2001;23:1355‐90. [DOI] [PubMed] [Google Scholar]
Sørensen 1984
- Sørensen TIA, Orholm M, Bentsen KD, Høybye G, Eghøje K, Christoffersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of developement of cirrhosis. Lancet 1984;2(8397):241‐4. [DOI] [PubMed] [Google Scholar]
Tamburro 1967
- Tamburro C, Leevy CM. Protein clotting factor synthesis in liver disease. Gastroenterology 1967;52:325. [Google Scholar]
Trinchet 1992
- Trinchet JC, Balkau B, Poupon RE, Heintzmann F, Callard P, Gotheil C, et al. Treatment of severe alcoholic hepatitis by infusion of insulin and glucagon: a multicenter sequential trial. Hepatology 1992;15(1):76‐81. [PMID: 1727803] [DOI] [PubMed] [Google Scholar]
Van Steenbergen 1993
- Steenbergen W. Alcohol, liver cirrhosis, and disorders in sex hormone metabolism [Alcohol, levercirrhose, en stoornissen in het metabolisme van de geslachtshormonen]. Acta Clinica Belgica 1993;48(4):269‐83. [PMID 8212979] [DOI] [PubMed] [Google Scholar]
Vickers 1998
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Controlled Clinical Trials 1998;19(2):159‐66. [PMID: 9551280] [DOI] [PubMed] [Google Scholar]
Yusuf 1991
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomised clinical trials. Journal of American Medical Association 1991;266:93‐8. [PubMed] [Google Scholar]
References to other published versions of this review
Rambaldi 2002
- Rambaldi A, Iaquinto G, Gluud C. Anabolic‐androgenic steroids for alcoholic liver disease: a Cochrane review. The American Journal of Gastroenterology 2002;97(7):1674‐81. [DOI] [PubMed] [Google Scholar]


