ABSTRACT
Background
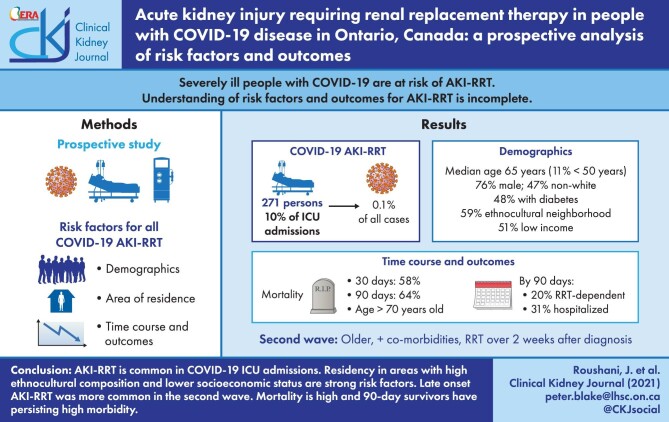
Severely ill people with coronavirus disease 2019 (COVID-19) are at risk of acute kidney injury treated with renal replacement therapy (AKI-RRT). The understanding of the risk factors and outcomes for AKI-RRT is incomplete.
Methods
We prospectively collected data on the incidence, demographics, area of residence, time course, outcomes and associated risk factors for all COVID-19 AKI-RRT cases during the first two waves of the pandemic in Ontario, Canada.
Results
There were 271 people with AKI-RRT, representing 0.1% of all diagnosed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases. These included 10% of SARS-CoV-2 admissions to intensive care units (ICU). Median age was 65 years, with 11% <50 years, 76% were male, 47% non-White and 48% had diabetes. Overall, 59% resided in the quintile of Ontario neighborhoods with the greatest ethnocultural composition and 51% in the two lowest income quintile neighborhoods. Mortality was 58% at 30 days after RRT initiation, and 64% at 90 days. By 90 days, 20% of survivors remained RRT-dependent and 31% were still hospitalized. On multivariable analysis, people aged >70 years had higher mortality (odds ratio 2.4, 95% confidence interval 1.3, 4.6). Cases from the second versus the first COVID-19 wave were older, had more baseline comorbidity and were more likely to initiate RRT >2 weeks after SARS-CoV-2 diagnosis (34% versus 14%; P < 0.001).
Conclusions
AKI-RRT is common in COVID-19 ICU admissions. Residency in areas with high ethnocultural composition and lower socioeconomic status are strong risk factors. Late-onset AKI-RRT was more common in the second wave. Mortality is high and 90-day survivors have persisting high morbidity.
Keywords: acute dialysis, acute kidney injury, COVID-19, renal replacement therapy
Graphical Abstract
Graphical Abstract.
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of novel coronavirus disease 2019 (COVID-19) [1]. As of 31 January 2021, in Ontario, Canada's most populous province, there had been >270 000 diagnosed infections, equivalent to 1.9% of the population, and >6000 deaths, representing a case fatality rate of 2.3% [2].
Acute kidney injury (AKI) is a well-recognized complication of COVID-19, with a significant proportion of those affected receiving renal replacement therapy (RRT) [3–14]. AKI has been associated with direct SARS-CoV-2 infection of renal tubular cells, but the principal cause is now considered to be acute tubular injury caused by volume depletion and multi-organ failure associated with COVID-19 [7].
The literature on people with COVID-19-related AKI-RRT is still quite limited. Gupta et al. reported a 20.6% rate of AKI-RRT in 637 people admitted to an intensive care unit (ICU) in 60 hospitals across the USA [6]. Mortality was 55% and, of those discharged from hospital, 34% still required RRT [6]. Risk factors for mortality included older age and severe oliguria [6]. Other AKI-RRT studies are small and single-center [10–12]. Multiple COVID-19 AKI studies do not focus specifically on patients with AKI-RRT but also report high mortality [3–5, 8, 9, 13].
We have used prospectively collected data since the start of the pandemic in Ontario in March 2020 to look at COVID-19 AKI-RRT both inside and outside the ICU in all the centers in the province. We specifically looked at the demographic characteristics, including neighborhood of residence, of the AKI-RRT population, the type of RRT they received and their outcomes, the risk factors for mortality and the intervals between diagnosis of SARS-CoV-2 infection, initiation of RRT and recovery or mortality. We also examined differences between the pattern of AKI-RRT in the first and second COVID-19 waves.
MATERIALS AND METHODS
Setting
Ontario has a population of ∼14.5 million [15]. The Ontario Renal Network (ORN), a part of Ontario Health, is a provincial government agency that funds and manages kidney disease services working with 27 Renal Programs [16, 17]. All dialysis in Ontario, including RRT for AKI, is funded by a single-payer—the provincial government—operating through the ORN [16, 17]. For the purpose of this study, the first wave of the pandemic in Ontario comprised the period 1 March to 1 September 2020, and the second wave from 1 September 2020 to 31 January 2021.
Data sources
Nine linked data sets were used. The ORN COVID-19 data collection tool captured information each week on people with AKI-RRT after SARS-CoV-2 infection: name, health card number, type of residence, hospitalization status, RRT modality, ICU and ventilator status, and disposition (active, recovered, deceased) [17]. This was a minimal data set but included personal health information that could be linked to other data sets. Data were collected prospectively from the second week of March 2020, when the first AKI-RRT case was diagnosed, with all renal programs submitting weekly data for all AKI-RRT cases. Hospital admissions were subsequently crosschecked with the Canadian Institute for Health Information Discharge Abstract Database, which also allowed determination of medical histories, including comorbidities, based on the International Classification of Diseases 10 diagnostic codes. Each chronic condition was defined based on 5 years of look-back data from the date of SARS-CoV-2 reporting.
The other data sets were: the Ontario Renal Reporting System, also identifying all individuals with AKI-RRT and including ethnicity, collected by data leads in each Program at registration, based on charting by clinical staff who may ask patients or relatives to self-identify ethnicity but who are not mandated to do so [17]; the Ontario Laboratories Information System, providing SARS-CoV-2 test dates and results and laboratory values generally; the Registered Persons Database, providing demographic information and date of death; the Ontario Health Insurance Plan, containing health insurance claims for physician services including dialysis; the Statistics Canada Postal Code Conversion File, linking postal codes to standard geographic areas to derive income quintile; and also the Canadian Index of Multiple Deprivation, a geographically based index used to understand inequalities through various metrics of education, health and society [18]. Ethnocultural composition is a dimension represented in this index and is defined by an area's composition of immigrant populations, including the proportions who are recent immigrants, self-identified visible minorities, born outside of Canada and have no knowledge of Canada's official languages—English and French [18].
Population
All incident AKI-RRT patients registered in the ORN COVID-19 data tool between 10 March 2020 and 31 January 2021, were included. These were people who received acute RRT in association with a diagnosis of SARS-CoV-2 infection, defined as testing positive on a nucleic acid amplification test [19]. The exclusion criteria were missing health card numbers and postal codes, non-Ontario residents, age <18 years, previous kidney transplantation or recent dialysis and no serum creatinine from the previous 3 years, as it was unclear whether those were truly cases of AKI. Patients with chronic kidney disease (CKD) stage 5 were excluded because the degree of insult required for these patients to need RRT is likely much smaller than those with normal renal function or lesser degrees of CKD. Clinical decision making and indications for RRT were determined by individual care givers at each of the hospital/ICU sites.
Statistical analysis
Descriptive statistics included frequency (percentages) for categorical variables and means ± standard deviations or medians and interquartile ranges (IQRs) for continuous variables. Chi-squared test was used to explore the associations of categorical variables between survivors and non-survivors and between waves 1 and 2. We analyzed age >70 years as a categorical variable, thinking that this would be more useful to clinical practitioners because it reflects the intersectional experience of COVID-19 and older age [6]. To explore risk factors associated with mortality, a multivariable logistic regression was used and included sex, age, geographic location, ethnicity, diabetes, type of residence, income quintile, baseline serum creatinine, time of initiation of RRT relative to SARS-CoV-2 diagnosis and the Canadian Index of Multiple Deprivation. Patients with an unknown, missing or other ethnicity were treated as one distinct group and as a factorial variable, with White ethnicity as the reference group. These variables were chosen based on the general literature on risk factors for COVID-19 incidence, morbidity and mortality and on our clinical interest as nephrologists who treat AKI. All statistical analyses were performed using SAS statistical software, with statistical significance set at two-sided P < 0.05.
Ethics approval
The data collection was in accordance with Ontario Health's legislative authority under the Ontario Personal Health Information Protection Act, 2004. This study followed the principles of the declaration of Helsinki.
RESULTS
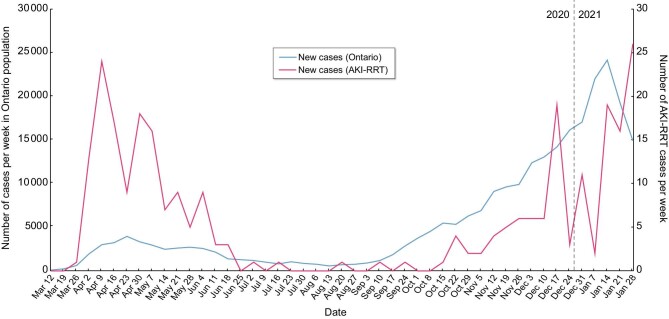
As of 31 January 2021, 271 people had developed COVID-19 related AKI-RRT, representing 0.1% of the 270 180 diagnosed SARS-CoV-2 cases in Ontario, and 2% of the 13 533 hospitalized cases, as of that time (Figure 1). This represents an incidence of 20.3 cases per million population per year [2]. There were 18 people excluded because they had no serum creatinine value recorded in the previous 3 years. Of the 271 patients, 259 had an ICU admission and accounted for 10% of 2490 COVID-19 ICU admissions in Ontario. The 174 patients who died represent 2.8% of COVID-19 deaths in Ontario [2].
Figure 1:
Number of diagnosed new cases of SARS-CoV-2 infection in the general population and of SARS-CoV-2-associated AKI-RRT in Ontario. The relatively lower rates of cases in the general population compared with AKI-RRT cases in the first versus the second wave likely reflects relatively lower availability of SARS-CoV-2 testing in the community during the first wave.
The median age of the 271 people was 65 years, and 11% were aged <50 years, 76% were male and 79% of them were residents of the Greater Toronto Area, where approximately 40% of Ontarians live (Table 1). Overall, 92% lived in a private residence, as distinct from a congregate setting such as a nursing home. By ethnicity, 59% of the patients were either non-White or had an unknown ethnicity. Socioeconomically, 58% lived in the single most ethnocultural quintile of neighborhoods and 51% resided in neighborhoods in the two lowest income quintiles for Ontario (Table 1). Ontario's vaccination eligibility guidelines and the timing of the cases indicate that none of the 271 patients would have been fully or even partially vaccinated.
Table 1.
Characteristics of all 271 people with AKI-RRT at baseline compared with Ontario's general population
| Characteristic | AKI-RRT patients, n = 271, n (%) | Ontario population, n ≈ 14 750 000 (all ages) [20, 21], n ≈ 11 600 000 (adults only) [20] |
|---|---|---|
| Demographics | ||
| Male | 206 (76) | 49% [20] |
| Age group | ||
| 29–49 years | 29 (11) | 51% [15] |
| 50–69 years | 146 (54) | 33% |
| 70+ years | 96 (35) | 16% |
| Patient location | ||
| Toronto area | 215 (79) | 42% [21] |
| Outside Toronto area | 56 (21) | 58% |
| Residency | ||
| Other | 21 (8) | |
| Private residence | 250 (92) | |
| Race | ||
| White | 111 (41) | 68% [15 ] |
| Non-White | 99 (37) | 29% |
| Unknown/missing/other | 61 (23) | 3% |
| Ratio of White to non-White | 1.12 | 2.14 |
| Income quintilea | ||
| 1 | 72 (27) | 20% |
| 2 | 67 (25) | 20% |
| 3 | 53 (20) | 20% |
| 4 | 35 (13) | 20% |
| 5 | 44 (16) | 20% |
| Ethnocultural composition quintileb | ||
| 1 and 2 | 29 (11) | 40% |
| 3 | 32 (12) | 20% |
| 4 | 53 (20) | 20% |
| 5 | 157 (58) | 20% |
| Comorbid conditions | ||
| Diabetes mellitus in adults (age 18+ years) | 129 (48) | 10% [22] |
| Cancer | 25 (9) | |
| Cardiac disease | 54 (20) | |
| CKDc | ||
| eGFR <30 mL/min/1.73 m2 | 10 (4) | |
| eGFR 30–59 mL/min/1.73 m2 | 55 (20) | |
| eGFR 60–89 mL/min/1.73 m2 | 111 (41) | |
| eGFR 90+ mL/min/1.73 m2 | 95 (35) | |
| Baseline serum creatinine (mmol/L), median (IQR) | 84 (71–102) |
Income quintile is a measure of neighborhood socioeconomic status that divides the population into five income groups of equal size. Group 1 lives in the neighborhoods with the lowest incomes and group 5 in those with the highest incomes.
Ethnocultural composition refers to the community makeup of immigrants (i.e. proportions of recent immigrants, of people born outside Canada, of those who self-identify as visible minorities and of those who cannot speak either of Canada's official languages—English and French). The population is divided into five ethnocultural quintiles of equal size. Group 1 lives in the neighborhoods with the greatest degree of ethnocultural composition, and group 5 lives in those with the least [18].
Baseline eGFR within 7 days to 3 years prior to RRT initiation.
eGFR, estimated glomerular filtration rate.
Baseline comorbidities included diabetes in 48%, a much higher rate than in the general Ontario population, cardiac disease in 20% and a previous cancer diagnosis in 9% [22]. The median baseline serum creatinine was 84 µmol/L, but 23% had baseline CKD 3 and 4% had CKD 4 (Table 1).
Treatment received
Of the 271 people, 96% had an ICU stay and >90% received mechanical ventilation. The median (IQR) interval between a first positive SARS-CoV-2 swab and initiating RRT was 9 (3–15) days. However, this varied greatly, with 36% starting within 48 h, 42% within 1 week while in contrast 31% started >2 weeks after their first positive swab (Figure 2). Regarding RRT modality, 42% received sustained low-efficiency dialysis (SLED) and 41% continuous renal replacement therapy (CRRT) at some stage in their course. These include 2.9% who received both. Conventional hemodialysis was used exclusively in 20% and in 51% at some stage in their course. The median time spent on dialysis was 4 days and was 2 weeks or less in 74% of patients and >2 weeks in 26% of patients. The median length of hospital stay was 18 (9–37) days (Table 2). In total, the 271 cases required 3177 patient days of AKI-RRT.
Figure 2:
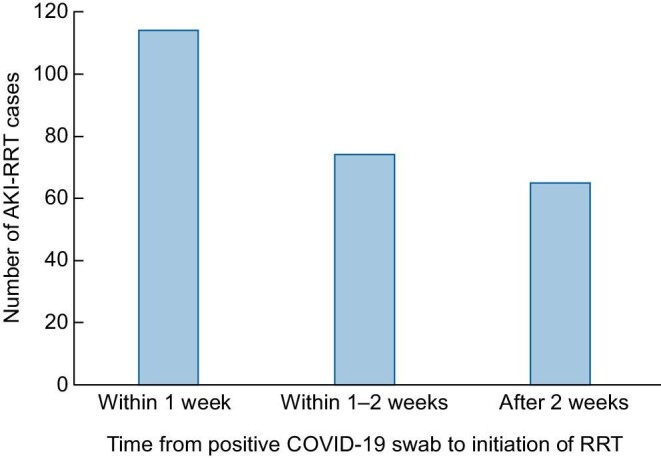
Frequency distribution of all 271 AKI-RRT cases by time between first positive COVID-19 swab and RRT initiation.
Table 2.
Treatments received by the 271 people with AKI-RRT
| AKI-RRT patients, n = 271 | |
|---|---|
| Intensive care admission, n (%) | 259 (96) |
| On ventilator, n (%) | 246 (91) |
| Length of hospital stay (in days), median (IQR) | 18 (9–37) |
| Type of RRT received, n (%)a | |
| Conventional acute hemodialysis | 139 (51) |
| Sustained low efficiency dialysis | 114 (42) |
| Continuous renal replacement therapy | 111 (41) |
| Time to RRT initiation from a positive COVID-19 swab, n (%) | |
| Within 1 week | 114 (42) |
| 1–2 weeks | 74 (27) |
| After 2 weeks | 65 (24) |
| Missing | 18 (7) |
| Median days (IQR) | 9 (3–15) |
| Time on RRT, n (%) | |
| <1 week | 158 (58) |
| 1–2 weeks | 44 (16) |
| >2 weeks | 69 (26) |
| Median days on RRT (IQR) | 4 (0–15) |
Total exceeds 100% as many received more than one RRT modality.
Patient outcomes
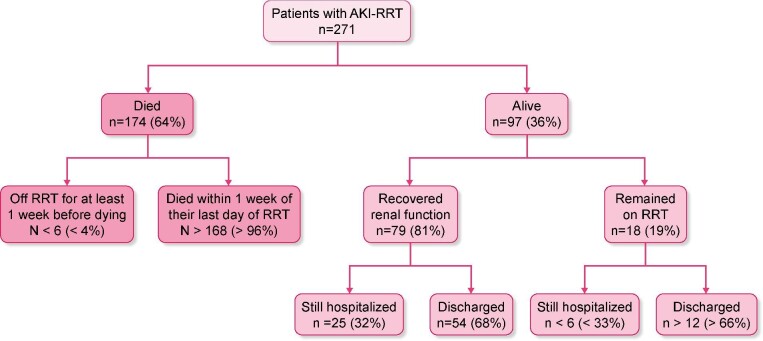
Of these 271 people, 174 (64%) died within 90 days of initiating RRT. Most deaths were soon after RRT initiation, with 31% occurring within 7 days and 45% within 14 days. However, 18 (10%) died >4 weeks after starting RRT (Figure 3). The mortality rate was 58% at 30 days after the start of dialysis and 62% at 60 days. Of the 174 people who died, just 8 (3%) recovered renal function sufficiently to not require RRT for at least 1 week before their death. At 90 days after the start of RRT, 78 of the 97 survivors (80%) were no longer on RRT but 19 (20%) remained RRT-dependent, including 14 who had been discharged from hospital and 30 (31%) were still hospitalized. In total, 31% of AKI-RRT cases had recovered renal function and were discharged, 11% were alive in the hospital but not on RRT and 7% of patients remained on RRT in hospital (Figure 4).
Figure 3:
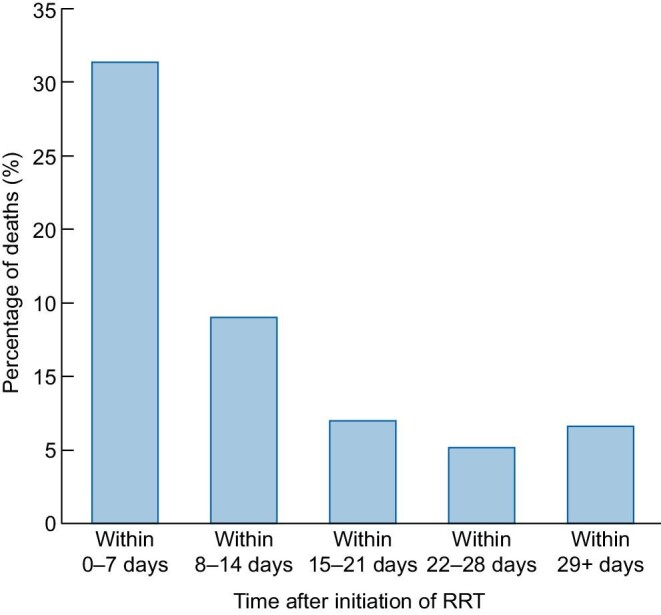
Distribution of the incidence of deaths among 174 AKI-RRT deaths by time after initiation of RRT.
Figure 4:
The 90-day outcomes of 271 COVID-19 patients with AKI-RRT. When number of cases is <6, the exact number cannot be stated for Ontario Health privacy reasons.
Predictors of mortality
The baseline characteristics of the 174 non-survivors and 97 survivors were compared (Table 3). On multivariable analysis, a significantly larger proportion of patients aged 70 years and older died than those aged 29 to 69 years [odds ratio (OR) 2.4, 95% confidence interval (95% CI) 1.3, 4.6] (Table 4). The relationship between age and mortality was also significant when age was analyzed as a continuous variable. There was a trend towards higher mortality in those who initiated RRT >2 weeks after a positive SARS-CoV-2 test as compared with RRT start within 1 week (OR 1.9, 95% CI 1.0, 3.9, P = 0.069). There were no differences between non-survivors and survivors for sex, geographic location, ethnicity, diabetes or other comorbidities, income quintile or baseline serum creatinine, and none for type of RRT received or for time on dialysis. The relationship between ethnicity and mortality was unchanged when those with ‘unknown/missing/other’ ethnicity were omitted from the analysis (See Appendix).
Table 3.
Baseline characteristics and treatments received for the 271 people with AKI-RRT by outcome
| Characteristic | Survivors, n = 97, n (%) | Non-survivors, n = 174, n (%) | P-value |
|---|---|---|---|
| Demographics | |||
| Male | 73 (75) | 133 (76) | 0.828 |
| Age group | 0.006 | ||
| 29–69 years | 73 (75) | 102 (59) | |
| 70+ years | 24 (25) | 72 (41) | |
| Race | 0.853 | ||
| White | 41 (42) | 70 (40) | |
| Non-White | 36 (37) | 63 (36) | |
| Unknown/missing | 20 (21) | 41 (24) | |
| Income quintilea | 0.95 | ||
| 1 | 25 (26) | 47 (27) | |
| 2 | 25 (26) | 42 (24) | |
| 3 | 21 (22) | 32 (18) | |
| 4 | 15 (16) | 20 (12) | |
| 5 | 11 (11) | 33 (19) | |
| Ethnocultural composition quintileb | 0.799 | ||
| 1 and 2 | 11 (11) | 18 (10) | |
| 3 | 10 (10) | 22 (13) | |
| 4 | 19 (20) | 34 (20) | |
| 5 | 57 (59) | 100 (58) | |
| Comorbid conditions | |||
| Diabetes mellitus | 44 (45) | 85 (49) | 0.581 |
| Cancer | 7 (7) | 18 (10) | 0.394 |
| Cardiac disease | 16 (16) | 38 (22) | 0.291 |
| CKDc | 0.996 | ||
| eGFR <59 mL/min/1.73 m2 | 23 (24) | 42 (24) | |
| eGFR 60–89 mL/min/1.73 m2 | 38 (39) | 73 (42) | |
| eGFR 90+ mL/min/1.73 m2 | 36 (37) | 59 (34) | |
| Treatment received | |||
| Intensive care admission | 87 (90) | 172 (99) | <0.001 |
| On ventilator | 82 (85) | 170 (98) | <0.001 |
| Time to RRT start from a positive COVID-19 Swab (days) | 0.083 | ||
| Within 1 week | 44 (45) | 70 (40) | |
| 1–2 weeks | 28 (29) | 46 (26) | |
| After 2 weeks | 15 (16) | 50 (29) | |
| Missing | 10 (10) | 8 (5) | |
| Median (IQR) | 7 (3–13) | 10 (4–16) |
Income quintile is a measure of neighborhood socioeconomic status that divides the population into five income groups of equal size. Group 1 lives in the neighborhoods with the lowest incomes and group 5 in those with the highest incomes.
Ethnocultural composition refers to the community makeup of immigrants (i.e. proportions of recent immigrants, of people born outside Canada, of those who self-identify as visible minorities and of those who cannot speak either of Canada's official languages—English and French). The population is divided into five ethnocultural quintiles of equal size. Group 1 lives in the neighborhoods with the greatest degree of ethnocultural composition, and group 5 lives in those with the least [18].
Baseline eGFR within 7 days to 3 years prior to dialysis start.
P-values were calculated using the Chi-Squared test for analysis of categorical variables. eGFR, estimated glomerular filtration rate.
Table 4.
Multivariable logistic regression predicting risk of mortality
| 95% confidence limits | |||
|---|---|---|---|
| Odds ratio | Lower limit | Upper limit | |
| Sex | |||
| Female | 1.125 | 0.59 | 2.144 |
| Type of residence | |||
| Other | 0.421 | 0.156 | 1.14 |
| Age | |||
| 70+ years | 2.406 | 1.27 | 4.559 |
| Geographic location | |||
| Non-GTA | 1.061 | 0.518 | 2.175 |
| Ethnicity | |||
| Other non-White | 1.13 | 0.596 | 2.144 |
| Unknown/missing | 1.15 | 0.546 | 2.421 |
| Diabetes | |||
| Yes | 1.102 | 0.63 | 1.926 |
| Income quintiles | |||
| 1 to 2 | 1.128 | 0.544 | 2.339 |
| Baseline serum creatinine | |||
| Severe–moderate (eGFR <60) | 0.674 | 0.34 | 1.336 |
| RRT initiation | |||
| After 2 weeks | 1.926 | 0.951 | 3.901 |
| Within 2 weeks | 1.014 | 0.543 | 1.896 |
| Deprivation index | |||
| 4 to 5 | 0.864 | 0.419 | 1.782 |
Reference categories: sex male, private residency, age younger than 70 years, geographic location within the Greater Toronto Area (GTA), ethnicity White, no diabetes, highest three income quintiles, mild–normal (eGFR 60+) baseline serum creatinine, renal replacement therapy within 1 week and the lower 3 deprived quintiles. eGFR, estimated glomerular filtration rate.
Comparison of first and second wave
The incidence of AKI-RRT by time shows that there were two distinct periods for AKI-RRT incidence, corresponding to the two waves of infection seen in the general Ontario community (Figure 1). There was a relatively lower rate of AKI-RRT cases compared to the number of SARS-CoV-2 cases in the general population in the second wave versus the first. The characteristics and course of the 138 people diagnosed during the first wave were compared with the 133 people from the second wave (Table 5). In the second wave, there was a significantly higher proportion of people aged 70 years or older, with cardiac disease (25% versus 15%), and with baseline CKD stages 3 and 4 (29% versus 20%). There was a significantly longer interval between the first positive SARS-CoV-2 swab and RRT initiation in the second wave, with more than twice as high a proportion patients initiating RRT >2 weeks after diagnosis (34% versus 14%) (Table 5). There were no other significant differences between the baseline characteristics, treatments received and outcomes of the patients in the two waves.
Table 5.
Comparison of characteristics of people with AKI-RRT from waves 1 and 2 of the COVID-19 pandemic
| Characteristic | Wave 1, n = 138, n (%) | Wave 2, n = 133, n (%) | P value |
|---|---|---|---|
| Demographics | |||
| Male | 105 (76) | 101 (76) | 0.977 |
| Age group | 0.024 | ||
| 29–69 years | 98 (71) | 77 (58) | |
| 70+ years | 40 (29) | 56 (42) | |
| Comorbid conditions | |||
| Diabetes mellitus | 71 (51) | 58 (44) | 0.196 |
| Cancer | 13 (9) | 12 (9) | 0.910 |
| Cardiac disease | 21 (15) | 33 (25) | 0.048 |
| CKDa | 0.045 | ||
| eGFR <59 mL/min/1.73 m2 | 27 (20) | 38 (29) | |
| eGFR 60–89 mL/min/1.73 m2 | 58 (42) | 53 (40) | |
| eGFR 90+ mL/min/1.73 m2 | 53 (38) | 42 (32) | |
| Treatment received | |||
| ICU admission | 133 (96) | 126 (95) | 0.578 |
| On ventilator | 133 (96) | 119 (90) | 0.047 |
| Time to RRT initiation from positive COVID-19 swab | <0.001 | ||
| Within 1 week | 71 (51) | 43 (32) | |
| 1–2 weeks | 37 (27) | 37 (28) | |
| After 2 weeks | 20 (14) | 45 (34) | |
| Missing | 10 (7) | 8 (6) | |
| Median (IQR), days | 7 (3–12) | 12 (4–16) | |
| Recovery of renal function | 39 (28) | 45 (34) | 0.321 |
| Incidence of deaths by time after initiation of RRT | 0.225 | ||
| Within 0–7 days | 39 (28) | 46 (35) | |
| Within 8–14 days | 18 (13) | 20 (15) | |
| Within 15–28 days | 19 (14) | 14 (11) | |
| After 28 days | 11 (8) | 7 (5) | |
| Total mortality | 87 (63) | 87 (66) |
Baseline eGFR within 7 days to 3 years prior to dialysis start.
The first wave (wave 1) of the pandemic in Ontario comprised the period 1 March to 1 September 2020, and the second wave (wave 2) from 1 September 2020 to 31 January 2021.
P-values were calculated using the Chi-squared test for analysis of categorical variables. eGFR, estimated glomerular filtration rate.
DISCUSSION
In Ontario, 271 patients, representing 2% of hospitalizations, including 259 patients, comprising 10% of ICU admissions due to COVID-19, developed AKI-RRT in the first 11 months of the pandemic. At the 90-day follow-up, 31% of these patients recovered renal function and were discharged, 7% were still on RRT, but 64% had died, demonstrating what a devastating illness this is. Patients aged 70 years and older had an even higher mortality.
These 271 people required 3177 patient-days of acute RRT and so provided an enormous workload for already stressed ICU and nephrology services. This is especially so since the activity was concentrated into two 6-week periods, particularly in the Toronto area, and this put enormous strain on the required human resources, especially because maintenance dialysis units were simultaneously affected by the pandemic [5, 6, 17]. Available CRRT equipment alone would not have been sufficient to provide RRT to all these people, as well as to on-going non-COVID-19 AKI-RRT cases, but many Ontario centers use SLED and conventional acute hemodialysis, and supply of machines for these modalities is relatively abundant.
A number of features stand out from the baseline demographics of the AKI-RRT population. There is a remarkable 76% male preponderance, which is not seen in the general Ontario population infected with SARS-CoV-2 [2]. A similar male preponderance was seen in other COVID-19 AKI-RRT studies and to a lesser degree in other COVID-19 AKI populations [5, 6, 9, 12]. A male preponderance in AKI-RRT of other causes has recently been reported [23]. Almost 50% of the AKI-RRT cohort had baseline diabetes, similar to the 53% noted in the US study but far in excess of the 10% prevalence of diabetes in the Ontario adult population [6, 22]. With regard to ethnicity, 41% of the AKI-RRT population were identified as White, as compared with 68% in the general population of Ontario [11]. However, ethnicity data were missing in 22% of the cohort. The ratio of those identified as White compared with non-White was 1.12 in the AKI-RRT cohort compared with 2.14 in the general population [13]. More strikingly, almost 60% of people with AKI-RRT came from the quintile of neighborhoods with the greatest degree of ethnocultural composition in Ontario and approximately 80% from the two most socioeconomically deprived quintiles [14]. These neighborhoods often have high rates of multigenerational households and of high-density workplaces. All this is consistent with risk factors for SARS-CoV-2 infection and its complications reported elsewhere and emphasizes the socioeconomic and ethnocultural factors underlying so much of the COVID-19 pandemic's worst effects in many countries [17, 24–28]. The need for strategies to protect these vulnerable populations is apparent and, in addition to vaccination, might also include focused workplace and community interventions [17, 26–28].
The mortality rate of 64% and the renal function recovery rate of 31% in this study were both higher than those reported in the US multicenter study, but this may just reflect longer follow-up [6]. The association of older age with mortality was expected [6]. The combination of a 31% rate of persisting hospitalization and a 19% rate of continuing RRT-dependence in 90-day survivors emphasizes the lasting morbidity associated with this condition. The high mortality with COVID-19 AKI-RRT is not much different from that in other types of sepsis-associated AKI-RRT [6, 7, 9]. The absence of any correlation between baseline comorbidities and mortality likely indicates the overwhelming influence of the severity of the infection on outcome in this population and was also seen in the US AKI-RRT study [6].
When comparing AKI-RRT in the two waves of COVID-19, we found a lower rate of AKI-RRT cases than the number of infections in the general population in the second wave versus the first. The decreasing incidence of AKI-RRT and of AKI in general throughout the pandemic has been reported from the USA and Switzerland [29, 30]. The relatively lower rate of AKI-RRT in the second wave may also reflect the lower availability of SARS-CoV-2 testing in the community during the first wave. This suggests that AKI-RRT may be preventable through better initial management of COVID-19. We found in the second wave a higher proportion of people with AKI-RRT aged 70 years or older, with
cardiac disease, and with baseline CKD 3 and 4. We found no other studies comparing AKI-RRT cases between successive COVID-19 waves, although a single-center ICU study in France found no differences between their first two waves in age or co-morbidity [31]. One hypothesis is that improvements in COVID-19 management after the first wave helped reduce AKI-RRT in patients with fewer risk factors, giving greater predominance to those who were more vulnerable with older age, and more comorbidity. The difference in time course to development of AKI-RRT with more than twice as high a proportion of cases occurring >2 weeks after diagnosis of COVID-19 in the second wave is important. It may be that better initial management of COVID-19 is avoiding some of the early AKI-RRT related to fulminant infection, allowing patients to survive longer, but some then develop late onset AKI-RRT due to the cumulative renal insults that occur during a prolonged ICU stay.
Our study has a number of strengths. Our data come from an entire province of >14 million people. The single-payer public funding and delivery of dialysis and acute hospitalization in Ontario makes it possible to report reliably and in detail on the entire population of patients who received AKI-RRT due to COVID-19, in contrast to hospital-based studies, which may not account for all cases in a region [6, 9–12]. This included the small proportion of cases who received AKI-RRT outside the ICU who were not included in other studies [6]. Prospective weekly reporting of cases from the start of the pandemic allowed for more accurate capture of incidence and outcomes over time, including follow-up data on survivors to accurately determine dialysis dependence after 90-day follow-up.
There are several limitations to this study. First, the absence of a detailed database with the characteristics of the general population infected with SARS-CoV-2 precludes detailed comparisons with the AKI-RRT population. Second, the results of this study may not be generalizable to other jurisdictions due to differences in population characteristics, healthcare resources and variant strains of SARS-CoV-2. Third, only studying AKI-RRT patients excludes cases where RRT was theoretically indicated but not received due to decisions by stakeholders in the patient's health.
CONCLUSION
In conclusion, this prospective cohort study comprises all patients with AKI-RRT associated with COVID-19 in Ontario, Canada. We describe a high incidence among critically ill COVID-19 patients and an associated high mortality, especially in older patients, and a high rate of non-recovery of renal function and persisting hospitalization in survivors. The AKI-RRT population is more likely to be male, to have diabetes, to be of non-White ethnicity, and to live in areas with higher rates of ethnocultural and economic deprivation. This study emphasizes socioeconomic and ethnic predisposition to severe COVID-19 and consequent AKI-RRT.
Supplementary Material
ACKNOWLEDGEMENTS
The Ontario Regional Renal Programs and all the associated individuals submitting data each week are thanked for their efforts to serve those with kidney disease. The author(s) acknowledge that data used in this publication were obtained through the Ontario Renal Reporting System and the ORN COVID-19 Data tracker, collected and provided by the Ontario Renal Network, a part of Ontario Health. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health (MOH). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOH is intended or should be inferred. Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI.
Contributor Information
Jian Roushani, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada.
Doneal Thomas, Ontario Renal Network, Ontario Health, Toronto, ON, Canada.
Matthew J Oliver, Ontario Renal Network, Ontario Health, Toronto, ON, Canada; Department of Medicine, University of Toronto, Toronto, ON, Canada.
Jane Ip, Ontario Renal Network, Ontario Health, Toronto, ON, Canada.
Yiwen Tang, Ontario Renal Network, Ontario Health, Toronto, ON, Canada.
Angie Yeung, Ontario Renal Network, Ontario Health, Toronto, ON, Canada.
Leena Taji, Ontario Renal Network, Ontario Health, Toronto, ON, Canada.
Rebecca Cooper, Ontario Renal Network, Ontario Health, Toronto, ON, Canada.
Peter O Magner, Ontario Renal Network, Ontario Health, Toronto, ON, Canada; Division of Nephrology, University of Ottawa, Ottawa, ON, Canada.
Amit X Garg, Ontario Renal Network, Ontario Health, Toronto, ON, Canada; Division of Nephrology, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada.
Peter G Blake, Ontario Renal Network, Ontario Health, Toronto, ON, Canada; Division of Nephrology, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada.
FUNDING
A.X.G. was supported by the Dr Adam Linton Chair in Kidney Health Analytics, and a Clinician Investigator Award from the Canadian Institutes of Health Research. The other authors received no financial support for the research, authorship and/or publication of this article, other than as employees of Ontario Renal Network.
CONFLICT OF INTEREST STATEMENT
D.T., J.I., Y.T., A.Y., L.T. and R.C. are salaried employees of Ontario Renal Network, Ontario Health. M.J.O., P.O.M., A.X.G. and P.G.B. are contracted Medical Leads at Ontario Renal Network, Ontario Health. M.J.O. is the owner of Oliver Medical Management Inc., which licenses Dialysis Management Analysis and Reporting System software. He has received an honorarium for speaking at Baxter Healthcare and participated in Advisory Boards for Janssen and Amgen. P.G.B. has received an occasional honorarium from Baxter Global for speaking engagements.
REFERENCES
- 1. Coronavirus Disease 2019 (COVID-19). https://www.publichealthontario.ca/en/diseases-and-conditions/infectious-diseases/respiratory-diseases/novel-coronavirus (July 2021, date last accessed). [Google Scholar]
- 2. COVID-19: Epidemiologic Summaries from Public Health Ontario. https://covid-19.ontario.ca/covid-19-epidemiologic-summaries-public-health-ontario (July 2021, date last accessed).
- 3. Gabarre P, Dumas G, Dupont Tet al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med 2020; 46: 1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng Y, Luo R, Wang Xet al. The incidence, risk factors, and prognosis of acute kidney injury in adult patients with coronavirus disease 2019. Clin J Am Soc Nephrol 2020; 15: 1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowe B, Cai M, Xie Yet al. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol 2021; 16: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta S, Coca SG, Chan Let al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol 2021; 32: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palevsky PM. COVID-19 and AKI: where do we stand? J Am Soc Nephrol 2021; 32: 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robbins-Juarez SY, Qian L, King KLet al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep 2020; 5: 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan L, Chaudhary K, Saha Aet al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 2021; 32: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doher MP, Torres de Carvalho FR, Scherer PFet al. Acute kidney injury and renal replacement therapy in critically ill COVID-19 patients: risk factors and outcomes: a single-center experience in brazil. Blood Purif 2020; 50: 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fisher R, Clarke J, Al-Arfi Ket al. Provision of acute renal replacement therapy, using three separate modalities, in critically ill patients during the COVID-19 pandemic. An after action review from a UK tertiary critical care centre. J Crit Care 2021; 62: 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eriksson KE, Campoccia-Jalde F, Rysz Set al. Continuous renal replacement therapy in intensive care patients with COVID-19; survival and renal recovery. J Crit Care 2021; 64:125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng JH, Hirsch JS, Hazzan Aet al. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis 2021; 77: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wald R, Bagshaw SM.. COVID-19-associated acute kidney injury: learning from the first wave. J Am Soc Nephrol 2021; 32: 4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ontario [Province] and Canada [Country] (table). Census Profile. 2016 Census. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E (29 November 2017, date last accessed)
- 16. AboutUs. https://www.ontariorenalnetwork.ca/en/about (July 2021, date last accessed).
- 17. Taji L, Thomas D, Oliver MJet al. COVID-19 in patients undergoing long-term dialysis in Ontario. CMAJ 2021; 193: E278–E284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Statistics Canada . The Canadian Index of Multiple Deprivation. Statistics Canada; 2019 [Google Scholar]
- 19. Ontario Ministry of Health . COVID-19 Quick Reference Public Health Guidance on Testing and Clearance. 2020, 0–2
- 20. Varella S. Population Estimate of Ontario Canada in 2020, By Age and Sex. https://www.statista.com/statistics/605960/population-of-ontario-by-age-and-sex/#statisticContainer (July 2021, date last accessed).
- 21. Ontario Demographic Quarterly: Highlights of First Quarter 2020. https://www.ontario.ca/page/ontario-demographic-quarterly-highlights-first-quarter-2020#:~:text=Ontario's%20population%20reached%2014%2C745%2C040%20on,quarter%20of%20the%20previous%20year(23 June 2020, date last accessed)
- 22. 2019 Backgrounder Ontario. Diabetes Canada; 2019 [Google Scholar]
- 23. Neugarten J, Golestaneh L, Kolhe NV. Sex differences in acute kidney injury requiring dialysis. BMC Nephrol 2018; 19: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Udell JA, Behrouzi B, Sivaswamy Aet al. Clinical risk, sociodemographic factors, and SARS-CoV-2 infection over time in Ontario, Canada. medRxiv 2021:2021.2004.2028.21256052; preprint: not peer reviewed [DOI] [PMC free article] [PubMed]
- 25. Greenaway C, Hargreaves S, Barkati Set al. COVID-19: exposing and addressing health disparities among ethnic minorities and migrants. J Travel Med 2020; 27: taaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tai DBG, Shah A, Doubeni CAet al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis 2021; 72: 703–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abedi V, Olulana O, Avula Vet al. Racial, economic, and health inequality and COVID-19 infection in the United States. J Racial Ethnic Health Disparities 2021; 8: 732–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tummalapalli SL, Silberzweig J, Cukor Det al. Racial and neighborhood-level disparities in COVID-19 incidence among patients on hemodialysis in New York city. J Am Soc Nephrol 2021;32: 2048–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diebold M, Martinez AE, Adam KMet al. Temporal trends of COVID-19 related in-hospital mortality and demographics in Switzerland—a retrospective single centre cohort study. Swiss Med Wkly 2021; 151: w20572. [DOI] [PubMed] [Google Scholar]
- 30. Charytan DM, Parnia S, Khatri Met al. Decreasing incidence of acute kidney injury in patients with COVID-19 critical illness in New York city. Kidney Int Rep 2021;6: 916–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Contou D, Fraissé M, Pajot Oet al. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care 2021;25: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.