Summary
Background
The definition of ‘long-COVID syndrome’ (LCS) is still debated and describes the persistence of symptoms after viral clearance in hospitalized or non-hospitalized patients affected by coronavirus disease 2019 (COVID-19).
Aim
In this study, we examined the prevalence and the risk factors of LCS in a cohort of patients with previous COVID-19 and followed for at least 6 months of follow-up.
Design
We conducted a prospective study including all hospitalized patients affected by COVID-19 at our center of Infectious Diseases (Vercelli, Italy) admitted between 10 March 2020 and 15 January 2021 for at least 6 months after discharge. Two follow-up visits were performed: after 1 and 6 months after hospital discharge. Clinical, laboratory and radiological data were recorded at each visit.
Results
A total of 449 patients were included in the analysis. The LCS was diagnosed in 322 subjects at Visit 1 (71.7%) and in 206 at Visit 2 (45.9); according to the post-COVID-19 functional status scale we observed 147 patients with values 2–3 and 175 with values >3 at Visit 1; at Visit 2, 133 subjects had the score between 2–3 and 73 > 3. In multivariate analysis, intensive care unit (ICU) admission (OR = 2.551; 95% CI = 1.998–6.819; P = 0.019), time of hospitalization (OR = 2.255; 95% CI = 1.018–6.992; P = 0.016) and treatment with remdesivir (OR = 0.641; 95% CI = 0.413–0.782; P < 0.001) were independent predictors of LCS.
Conclusions
Treatment with remdesivir leads to a 35.9% reduction in LCS rate in follow-up. Severity of illness, need of ICU admission and length of hospital stay were factor associated with the persistence of PCS at 6 months of follow-up.
Introduction
The coronavirus disease 2019 (COVID-19) due to the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported as global pandemic by the World Health Organization on the 11 March 2020 and rapidly involved the entire population worldwide.1 Post-viral systemic sequelae are commonly observed after recovery in other coronavirus diseases such as the Middle East respiratory syndrome coronavirus and the SARS-CoV,2 and recently several studies reported data about the persistence of symptoms seemingly attributable to previous COVID-19.3–6 The most frequent reported symptoms were: fever, fatigue, breathlessness, headaches, cough, cognitive blunting (‘brain fog’), anxiety and depression, muscle pains, arthralgias (…).3,7 Risk factors, severity grade of presentation and duration of the post-COVID-19 syndrome are currently subjects for discussion; in some studies, the persistence and severity of symptoms were related with several factors such as need of mechanical ventilation, need of intensive care unit (ICU) support, presence of comorbidities, older age and others,8 whereas in different populations these findings were not confirmed9; this may depend on the higher heterogeneity of the different studies involving population with variables characteristics, severity of illness and time of follow-up. For this reason, some authors proposed a novel definition of this syndrome based mainly on the time of follow-up and the duration of symptoms: acute-post-COVID syndrome, within 12 weeks after hospital discharge, long-post-COVID syndrome (LCS) between 12 and 24 weeks and persistent-PCS after 24 weeks.10 This approach may be interesting because could allow to distinguish the real LCS from non-specific symptoms mainly related to the hospitalization and more frequent in the first visit of follow-up; the presence of LCS after 24 weeks, on the other hand, can be more correlated with the immunological consequence due to the ‘viral trigger’ of SARS-CoV-2 infection.11
The aim of this study was the analysis of prevalence and the risk factors of LCS in a cohort of hospitalized patients affected by COVID-19 and prospectively followed for at least 6 months.
Materials and methods
Study design and definitions
This is a prospective study including all patients affected by COVID-19 and hospitalized from 10 March 2020 to 15 January 2021 at our center of infectious diseases at ‘Saint Andrea Hospital’, Vercelli, Italy, and followed in our ‘post-COVID ambulatory’ for at least 6 months after discharge. Patients were further telephonically contacted by our hospital nurse. The Visits 1 and 2 were performed at about 30 and 180 days after hospital discharge, respectively. At each visit functional status, biochemical analysis, clinical evaluation and patients’ interview. Demographic, clinical, laboratory and radiological data were reported at each visit. Data about the antiviral or supportive treatment were also reported; available antiviral treatment in the first wave of pandemic was: hydroxychloroquine, lopinavir/ritonavir and darunavir/cobicistat; after September 2020 was approved in Italy the use of remdesivir according to the following inclusion criteria: confirmed diagnosis of SARS-CoV-2 infection; radiological confirmation of interstitial pneumonia; onset of symptoms within 10 days from hospital admission, estimated glomerular filtration rate (eGFR) ≥ 30 ml/min, need of low-flow oxygen at the time of admission. Exclusion criteria were: SARS-CoV-2 infection without evidence of interstitial pneumonia, onset of symptoms after 10 days from the hospital admission, eGFR < 30 ml/min, need of non-invasive ventilation (NIV) or orotracheal intubation at the time of admission. All patients were included in this study after acceptance of study protocol and informed consent. The study protocol was approved by the local Ethics Committee: ʽComitato Etico Interaziendale ASL VC’ (4 August 2020; Protocol number: 0026301). This study which involves human participants is in compliance with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in this study.
Study endpoints
The primary endpoint was the evaluation of prevalence and severity of LCS in the enrolled patients at the two different time-points. The level of functional status and the severity of LCS were reported using the ‘post-COVID-19 functional status (PCFS) scale’ which assigns the presence of significant LCS for values >2. Grades 3–4 were referred to significant limitations in everyday life with important functional limitations.12
Statistical analysis
In descriptive statistics, continuous variables were summarized as median (Inter-quartile range (IQR): 25th to 75th percentiles). Categorical variables were described as frequency and percentage. All data were assessed for normality using a Shapiro–Wilk test and categorical data were compared using a Mann–Whitney or Kruskal–Wallis statistical test. To investigate continuous data, a Spearman Rank correlation was utilized. The association was calculated using the χ2-test. Multivariate logistic regression analysis with stepwise forward selection was performed to evaluate the related factors to LCS presence, with P-values of <0.05 as the criteria for model inclusion. All P-values were two-tailed. P < 0.05 was considered statistically significant. Survival analysis was carried out comparing the two-group using the Kaplan–Meier plot and compared with the log-rank (Mantel–Cox) test. Statistical analyses were conducted by using SPSS software package ver. 26.0 (Chicago, IL, USA).
Results
Patients’ selection and baseline characteristics
In the study period, 462 patients were discharged after hospital admission due to COVID-19. A total of 13 subjects were not included in the study for the following reasons: 6 were lost after discharge, 5 died at the long-term care facilities and 2 denied the informed consent. Finally, 449 patients were included in the analysis. Between the Visits 1 and 2, 14 patients (3.1%) were excluded for the following reasons: 2 died, 8 lost at the follow-up, 4 were re-hospitalized. At the Visit 2 included patients were 435. Table 1 was reported the baseline characteristics of the study population. Median age was 65 years; male patients were 362 (78%), the most frequent comorbidities were cardiovascular diseases (14.2%), diabetes (15.8%) and chronic neurological conditions (7.3%). Median time from the onset of symptoms and the hospital admission was 9.4 days; the median time for Visit 1 was 32.5 days, for Visit 2 178.5 days. One hundred and ninety-one subjects received Continuous Positive Airway Pressure (CPAP)/NIV (42%), 62 needed ICU admission (13.8%); median time of hospitalization was 10.5 days. Sixty-nine patients were treated with hydroxychloroquine (15.4%), 28 (6.2%) with lopinavir/ritonavir, 24 (5.3%) with darunavir/cobicistat, 163 (36.3) with remdesivir, 165 without antiviral treatment (36.7%); 390 received a corticosteroid therapy (86.8%). Three hundred and twelve were discharged at home (69.5%), 137 in long-term care facilities (30.5%). Physical rehabilitation was needed in 122 patients (27.2%) after hospital discharge; 4 patients (0.9%) were further re-hospitalized due to different clinical conditions.
Table 1.
Baseline characteristics of the study population
| Characteristics (n = 449 patients) | Values |
|---|---|
| Demographics | |
| Age (median, IQR) | 65 [56–75.5] |
| Male sex (n, %) | 362 (78) |
| BMI (median, IQR) | 24.5 [23.5–26] |
| Comorbidities (n, %) | |
| Cardiovascular disease | 64 (14.2) |
| COPD | 24 (5.3) |
| Chronic kidney disease | 8 (1.7) |
| Diabetes | 71 (15.8) |
| Neurological chronic disease | 33 (7.3) |
| Psychiatric disease | 23 (5.1) |
| Neoplastic disease | 6 (1.3) |
| Days from the onset of symptoms to hospital admission (median, IQR) | 9.4 [6.5–14.5] |
| Median time of follow-up (days) | |
| Visit 1 | 32.5 [30–38.5] |
| Visit 2 | 178.5 [165.5–211.5] |
| Treatment and clinical features | |
| Hydroxychloroquine | 69 (15.4) |
| Lopinavir/ritonavir | 28 (6.2) |
| Darunavir/cobicistat | 24 (5.3) |
| Remdesivir | 163 (36.3) |
| No antiviral therapies | 165 (36.7) |
| Corticosteroids | 390 (86.8) |
| Days of hospitalization (median, IQR) | 10.5 [7–14.5] |
| Need of CPAP/NIV (n, %) | 191 (42) |
| Need of ICU admission (n, %) | 62 (13.8) |
| Discharged at home (n, %) | 312 (69.5) |
| Discharged at long-term care facilities (n, %) | 137 (30.5) |
| Need of oxygen at home (n, %) | 171 (38) |
| Need of rehabilitation after discharge (n, %) | 122 (27.2) |
| Need of rehospitalization after discharge (n, %) | 4 (0.9) |
Clinical outcomes
In Table 2, the clinical outcomes and follow-up data in the study population were reported; abnormal values of C-reactive protein (CRP) were observed in 144 (32%) patients at Visit 1 and in 81 (18.6%) at Visit 2. Elevated ferritin level was observed in 282 patients (62.8%) at Visit 1 and in 135 (31%) at Visit 2; higher d-dimer level was reported in 74 patients (16.5%) at Visit 1 and 31 (7.1%) at Visit 2. Persistence of X-rays chest abnormalities was found in 224 subjects (49.8%) at Visit 1 and 151 (34.7) at Visit 2.
Table 2.
Follow-up data in the post-COVID medical assessment
| Laboratory and radiological examinations, n (%) | Visit 1 (n = 449) | Visit 2 (n = 435) |
| CRP >0.5 mg/l | 144 (32) | 81 (18.6) |
| Ferritin >150 ng/ml | 282 (62.8) | 135 (31) |
| D-dimer >243 ng/ml | 74 (16.5) | 31 (7.1) |
| Lactate dehydrogenase >250 U/l | 61 (13.5) | 18 (4.1) |
| 25-hydroxyvitamin D < 10 mcg/ml | 178 (39.6) | 71 (16.3) |
| Chest X-rays abnormalities | 224 (49.8) | 151 (34.7) |
| Clinical evaluation n, (%) | ||
| Overall PCS n, (%) | 322 (71.7) | 206 (45.9) |
| Systemic symptoms | ||
| Fatigue | 215 (47.9) | 151 (34.7) |
| Myalgias/arthralgias | 181 (40) | 112 (25.7) |
| Fever | 13 (2.9) | 2 (0.4) |
| Headache | 128 (28.5) | 66 (15.1) |
| Pneumological symptoms | ||
| Dyspnea/breathlessness | 228 (50.8) | 166 (38.2) |
| Cough | 134 (29.8) | 87 (20) |
| Chest pain | 129 (28.7) | 89 (20.4) |
| Neurological symptoms | ||
| ‘Brain fog' | 234 (52.1) | 191 (43.9) |
| Dizziness | 88 (19.6) | 13 (2.9) |
| Memory impairment | 186 (41.4) | 155 (35.6) |
| Anosmia | 289 (64.4) | 234 (53.7) |
| Ageusia/dysgeusia | 213 (47.4) | 217 (49.8) |
| Peripheral neuropathy | 133 (29.6) | 78 (17.9) |
| Cardiovascular symptoms | ||
| Tachyarrhytmias | 168 (37.4) | 91 (20.9) |
| Pericarditis/myocarditis | 31 (6.9) | 4 (0.9) |
| Psychiatric symptoms | ||
| Sleeping disorders | 280 (62.4) | 233 (53.6) |
| Post-traumatic stress disorder | 171 (38) | 134 (30.8) |
| Anxiety | 230 (51.2) | 144 (33.1) |
| Major depression | 105 (23.4) | 39 (8.9) |
| Psychosis | 51 (11.3) | 9 (2) |
| Behavior disorder | 23 (5.1) | 6 (1.4) |
| Other referred signs/symptoms | ||
| Weight loss | 186 (41.4) | 102 (23.4) |
| Hair loss | 289 (64.4) | 42 (9.6) |
| Diabetes | 109 (24.3) | 39 (8.9) |
| Hypertension | 116 (25.8) | 61 (14) |
| Psoriasis | 83 (18.5) | 18 (19) |
| Venous thromboembolism | 41 (9.1) | 12 (2.7) |
| Thyroid dysfunction | 66 (20.5) | 35 (16.9) |
The LCS was diagnosed in 322 subjects at Visit 1 (71.7%) and in 206 at Visit 2 (45.9); according to the PCFS scale we observed 147 patients with values 2–3 and 175 with values >3 at Visit 1; at Visit 2 133 subjects had the score between 2–3 and 73 > 3.
The most frequent systemic symptoms at Visit 1 were: fatigue (47.9%), myalgias/arthralgias (40%) and headache (28.5%). The most common pneumological symptoms were: dyspnea/breathlessness (50.8%), cough (29.8%) and chest pain (28.7%). Among neurological symptoms, the persistence of anosmia (64.4%) was the most common self-reported condition followed by ageusia/dysgeusia (47.4%), ‘brain fog’ syndrome (52.1%), memory impairment (41.4%), dizziness (19.6%) and peripheral neuropathy (29.6%). Tachyarrhytmias were the most common cardiological symptoms (37.4%); psychiatric symptoms included: sleep disorders (62.4%), post-traumatic stress disorder (38%), anxiety (51.2%), major depression (23.4%), psychosis (11.3%) and behavioral disorder (5.1%). Other most common referred conditions included: weight loss (41.4%), hair loss (64.4%), diabetes (24.3%), hypertension (25.8%), psoriasis (18.5%) and venous thromboembolism (9.1%). In Table 3, the prescribed therapies for the different clinical condition were reported: the most common were acetaminophen (46.9%) and analgesic (29.1%), beta-blocker for tachycardia (35.4%), anti-hypertensive (24.2%) and benzodiazepines (39.6%).
Table 3.
Prescribed therapies in the cohort study during the follow-up period
| Systemic/general therapies | |
| Analgesic/NSAIDs | 131 (29.1) |
| Acetaminophen | 211 (46.9) |
| Antihistaminic | 27 (6) |
| Prednisone | 113 (25.1) |
| Pneumological | |
| Inhalation steroids/β2 agonists | 124 (27.6) |
| Codeine | 128 (28.5) |
| Cardiovascular | |
| Anti-arrhythmics | 159 (35.4) |
| Anti-hypertensives | 109 (24.2) |
| Low molecular weight heparin (LMWH) | 61 (13.6) |
| Neurological and psychiatric | |
| Pregabalin/gabapentin | 119 (26.5) |
| Benzodiazepines | 178 (39.6) |
| Antidepressants | 75 (16.7) |
| Neuroleptic/mood stabilizers | 31 (6.9) |
Analysis of risk factors for the presence of LCS in the study population
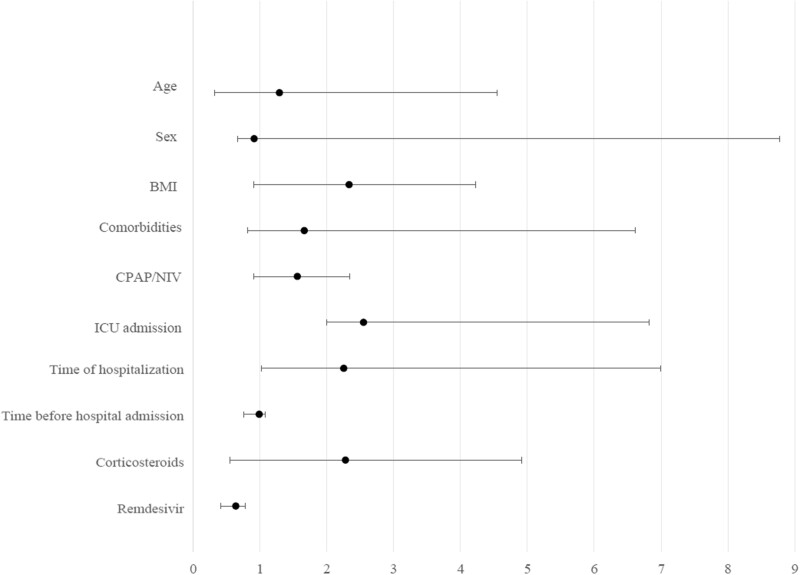
After multivariate adjustment considering the principal baseline parameters, ICU admission (OR = 2.551; 95% CI = 1.998–6.819; P = 0.019), time of hospitalization (OR = 2.255; 95% CI = 1.018–6.992; P = 0.016) and treatment with remdesivir (OR = 0.641; 95% CI = 0.413–0.782; P < 0.001) were independent predictors of LCS (Figure 1).
Figure 1.
Multivariate analysis of the LCS-associated factors.
PCFS scale according to remdesivir treatment in the study population
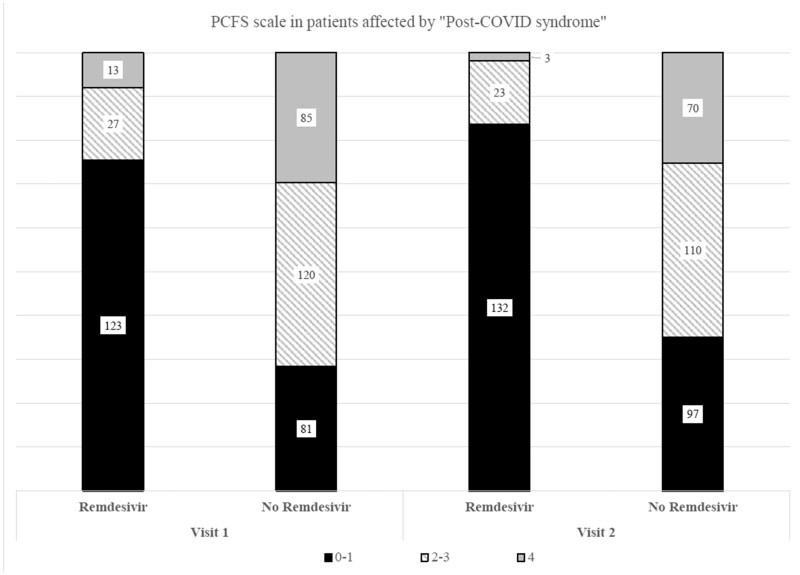
Comparing the group of patients treated with remdesivir against those untreated we observed that at Visit 1, 123 subjects were not affected by LCS vs. 81 without remdesivir treatment. Patients with a score between 2 and 3 were 27 and 120 in the two groups, respectively; patients with a score >3 were 13 and 85, respectively. All the differences in the two groups were statistically significant (P < 0.001; Figure 2).
Figure 2.
PCFS scale in the study population according to remdesivir treatment.
Survival analysis
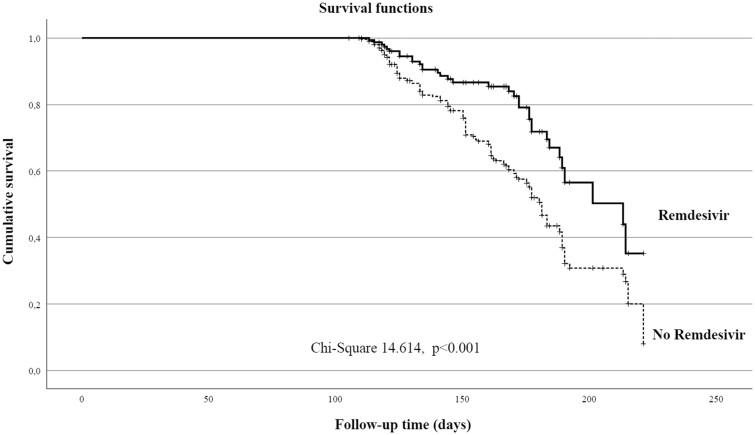
Survival analysis was carried out comparing the patients treated with remdesivir and the control group according to the diagnosis of LCS in the follow-up with significant difference between the two groups (χ2 = 14.614, P < 0.001; Figure 3).
Figure 3.
Survival analysis for LCS presence in the study population according to remdesivir treatment.
Discussion
In this prospective study, we observe a significant presence of LCS in the patients with previous hospitalization due to COVID-19 infection: 71% at the first follow-up visit (1 month) and 45% at the second visit (6 months). These findings are quite similar to other data reported in previous studies, with range due to different characteristics of enrolled patients, time of follow-up and definition of the post-viral syndrome.13
Our multivariate analysis showed that the ICU admission and the hospitalization time were the most important factor related to the LCS; in these subjects, the respiratory symptoms with the need of pneumologist follow-up and the fatigue syndrome were most frequent. Conversely, the antiviral treatment with remdesivir showed a protective effect on the LCS onset; this is to our knowledge the first report about the role of remdesivir treatment in the LCS and the principal reasons can be assumed: first, the patients treated with remdesivir had shorter course of hospitalization and lower rate of the hospital-related symptoms; second, in these patients the risk of CPAP/NIV or ICU admission was lower, with less rate of lung damage; third, the antiviral effect leads to a shorter time of viral replication, with reduction of cytokine syndrome, chronic inflammation and autoimmunity disorders.
A first point of discussion maybe the different ‘post-viral syndrome’ definitions based on the time of diagnosis assessment after clinical recovery, without a current agreement on definition of this syndrome. Although several proposals in LCS definition were available, the most common description was the presence of COVID-19-related symptoms for more than 3 months after the diagnosis of SARS-CoV-2 infection or symptoms’ onset.14 Different definitions such as ‘chronic COVID syndrome, post-COVID, post-acute COVID’ were mainly related to the duration of symptoms and their magnitude; however, can be very different to consider the same definition of LCS in hospitalized or non-hospitalized patients.10 The main difference between the two categories was the time-point of clinical evaluation: in the hospitalized patients, we evaluated the duration of LCS since the hospital discharge, whereas in outpatients this evaluation could be difficult due to the asymptomatic phase of infection or the lack of diagnosis by PCR. Based on our data, we defined all included patients with any symptoms as generical ‘post-COVID’; however, at the first visit within the first month after hospital discharge, a large part of patients suffered from an ‘acute-post-COVID syndrome’, with higher prevalence of symptoms more related to hospitalization than to the SARS-CoV-2 infection: weight loss, myalgias, polyneuropathies, dysphonia, trouble walking, sleep disorders (…); on the other hand, many psychiatric symptoms such as anxiety or depression were related to the hospitalization time, with the prolonged isolation and the ‘death experience’ as main factors causing the post-traumatic stress disorder. Clinical condition as diabetes or hypertension was also strongly related to the time of hospitalization, use of high dose of corticosteroids and CPAP/NIV. We emphasize that a real ‘long-COVID or persistent-COVID’ was observed at the second follow-up visit after a median time of 6 months. This is in our opinion the most relevant clinical condition due to the worsening of the quality of life, the working and social behavior of affected patients. The pathophysiology of these clinical manifestations is related to the long-term tissue damage as direct post-viral syndrome, especially for hearth involvement (tachycardia, myocarditis and pericarditis), lung (dyspnea, cough, chest pain and breathlessness), central nervous system (‘brain fog’, anosmia, ageusia and psychosis) or prolonged inflammation syndrome mainly determinant in ‘fatigue syndrome’, headache, autoimmune diseases, dizziness, fever (…). In our study, we observe that after 6 months of follow-up the fatigue syndrome (34.7%), persistence of breathlessness (38.2%) and ageusia/dysgeusia (49.8%) with anosmia (53.7%) were the most common reported symptoms with a direct worsening of quality of life. Persistence of dyspnea with abnormal X-rays chest was also reported in previous studies,15 but in our population among the 166 subjects with persistent respiratory symptoms only 22 featured the altered diffusion capacity and lung involvement documented with high-resolution computed tomography and required a consequent specialist evaluation. General distress and psychological involvement due to viral infection, hospitalization and emotional consequences have a negative impact on the quality of life and related psychiatric symptoms such as anxiety or depression; the occurrence of psychosis was also reported in a subgroup of patients with longer time of hospitalization, need of mechanical ventilation, treated with higher doses of corticosteroids or benzodiazepines due to psychomotor agitation.
Conclusions
This study presents some strengths: a prospective design, with a well-known hospitalization course; follow-up time longer than most of previous studies (with inclusion of both ‘post-acute-COVID’ and ‘long-COVID’), real-life intervention with clinical evaluation, blood test, radiographs and other relevant tests (this is not a study based only on the telephone interview), the first evidence of a protective role of antiviral treatment with remdesivir; the main limitations were: the single-center analysis, with selection of only hospitalized patients admitted in the infectious disease unit. The observed effect of the remdesivir treatment could also be affected by a ‘temporal bias’ due to the availability in Italy of this drug starting from September 2020; consequently, the major part patients hospitalized during the first wave of pandemic could not benefit from the use of this therapy.
In conclusion, the LCS is a heterogenous syndrome with different definitions and need of a multidisciplinary approach; the role of remdesivir is encouraging and requires further confirmation in other prospective studies.
Author contributions
L.B. conceived the study, performed the analysis, draft the article; V.D. and G.M. contributed to the data entry and clinical management; B.D., C.S. and F.D.P. contributed to patients’ management, first physical examination and blood test. All other Authors actively participated to the final version of this article and followed in first person the enrolled patients.
Data availability
The data analyzed in this study are available from the corresponding author upon reasonable request.
Ethical approval
The study protocol was approved by the local Ethics Committee: ʽComitato Etico Interaziendale ASL VC’ (4/8/2020; Protocol number: 0026301). This study which involves human participants is in compliance with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in this study.
Acknowledgements
We would like to thank the study participants: we shared with them their sufferings, the isolation, the fear of death, the loss of affections and finally the hope of going home. It was an unforgettable experience, for better or for worse. We are also indebted to all the staff of nurses who took care of them day and night with invaluable dedication: Bushi Uejda, Cavagliano Luca, Conti Carola, Danna Ivana, Debernardi Gianna, Del Vecchio Rosa Anna, Fiorentino Vincenza, Germano Sara, Liberti Milena, Mazzucchi Vera, Ronchi Carla, Verri Claudia, Andreo Sara Paola Maria, Bedendo Debora, Bianco Daniela Maria, Concina Fiorella, D’Apolito Maria, Leidi Barbara, Marchitelli Veronica, Moldoveanu Doina, Ragazzoni Stefano, Santamaria Giulia, Ternavasio Alessio and Vattimo Concetta.
Conflict of interest. None declared.
References
- 1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC.. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324:782–93. [DOI] [PubMed] [Google Scholar]
- 2. Ngai JC, Ko FW, Ng SS, To K-W, Tong M, Hui DS.. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010; 15:543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2021; 93:1013–22. [DOI] [PubMed] [Google Scholar]
- 4. Mendelson M, Nel J, Blumberg L, Madhi SA, Dryden M, Stevens W, et al. Long-COVID: an evolving problem with an extensive impact. S Afr Med J 2020; 111:10–2. [DOI] [PubMed] [Google Scholar]
- 5. Garg P, Arora U, Kumar A, Wig N.. The “post-COVID” syndrome: how deep is the damage? J Med Virol 2021; 93:673–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G.. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2021; 76:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. ; COVID-19 BioB Outpatient Clinic Study group. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 2020; 89:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moreno-Pérez O, Merino E, Leon-Ramirez J-M, Andres M, Ramos JM, Arenas-Jiménez J, et al. ; COVID19-ALC research group. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect 2021; 82:378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL.. Defining post-COVID Symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health 2021; 18:2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. ; Sinai Immunology Review Project. Immunology of COVID-19: current State of the Science. Immunity 2020; 52:910–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klok FA, Boon GJAM, Barco S, Endres M, Geelhoed JJM, Knauss S, et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J 2020; 56:2001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korompoki E, Gavriatopoulou M, Hicklen RS, Ntanasis-Stathopoulos I, Kastritis E, Fotiou D, et al. Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. J Infect 2021; 83:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021; 53:737–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Y-M, Shang Y-M, Song W-B, Li Q-Q, Xie H, Xu Q-F, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed in this study are available from the corresponding author upon reasonable request.