Introduction
The coronavirus disease 2019 (COVID-19) pandemic remains a major cause of morbidity and mortality worldwide. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiologic agent of COVID-19, is predominantly a respiratory virus but impacts many organ systems including the gastrointestinal tract.1 SARS-CoV-2 effectively infects and propagates in intestinal epithelial cells in vitro, and the virus is detectable in stool and the intestinal mucosa of some patients long after clearance from the upper respiratory tract.2, 3
Some medications used to treat gastrointestinal disorders are associated with worse COVID-19 outcomes. In patients with chronic inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, corticosteroid use is associated with an increased risk of severe COVID-19.4 The deleterious effect of immunosuppressive corticosteroids is not unexpected and has been confirmed across non-IBD populations, as well. Early studies also linked mesalamine (5-ASA) and its prodrug sulfasalazine to increased risk of severe COVID-19 in patients with IBD4 and rheumatoid arthritis.5 This negative association was unexpected, as mesalamine has an excellent safety profile and acts primarily in the gastrointestinal tract rather than as a systemic immunosuppressant.4
It is important to note that epidemiology studies are subject to reporting bias and unmeasured confounders, which may lead to evolving conclusions over time.6 The goal of our study was to experimentally address in a controlled manner the relationship between mesalamine and SARS-CoV-2 entry, replication, and/or pathogenesis. To address this, we used primary human intestinal epithelial cells (organoids), a mouse model of COVID-19, and a mouse model of intestinal SARS-CoV-2 entry.
Methods
All study procedures and reagents were approved by the Washington University Institutional Review Board (#202011003) and Animal Studies Committee (Assurance #A-3381-01). Additional experimental details can be found in Supplemental Materials online.
Viruses and Cell Lines
Primary human intestinal cells were cultured as 3D spheroids and 2D monolayers, as previously described.3 We assessed the effect of mesalamine on the expression of the SARS-CoV-2 receptor angiotensin 2–converting enzyme 2 (ACE2) and on cellular proteases that cleave and activate these spike proteins: transmembrane serine protease 2 (TMPRSS2), TMPRSS4, cathepsin B (CTSB), and cathepsin L (CTSL).3, 7 Viral infection was performed with a chimeric vesicular stomatitis virus expressing SARS-CoV-2 spike protein, enhanced green fluorescent protein (VSV-SARS-CoV-2), and SARS-CoV-2 virus derived from an infectious cDNA clone of 2019n-CoV/USA_WA1/2020.3,8
Mice
The K18-ACE2 mice from 8-12 weeks old were treated orally with phosphate buffered saline (PBS) or mesalamine (Millipore Sigma) at 200mg/kg daily. Mice were inoculated intranasally with 1x103 focus-forming units (FFU) of SARS-CoV-2 as previously described.8,9 Mice were weighed daily and killed 7 days postinfection (dpi). For the intestinal viral entry model, after 3 days of treatment, VSV-SARS-CoV-2 was injected into a ligated intestinal loop of anesthetized mice, and tissues were harvested 6 hours postinfection (hpi).
Statistical Analysis
At least 2 experimental replicates were performed for all experiments. Graphs depict means and standard error of the means of biological replicates. Absolute viral copies were compared by Mann-Whitney U test. Gene expression data were compared by 2-way ANOVA. All analyses were performed in RStudio.
Results
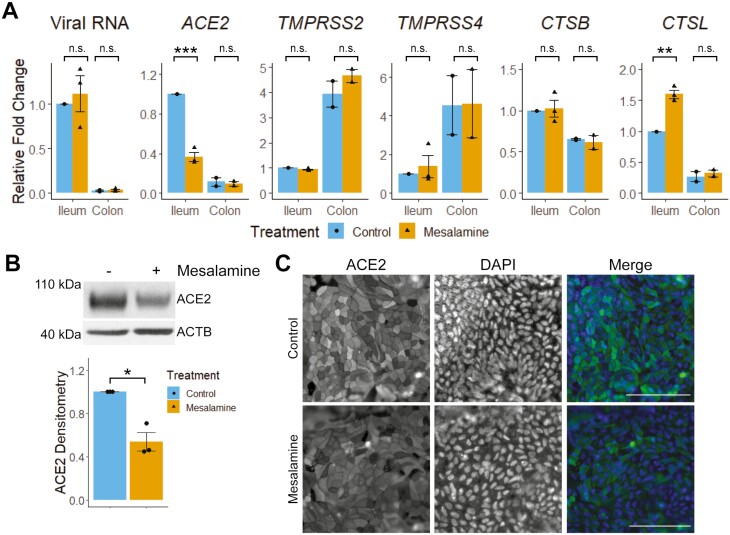
Mesalamine reduces ACE2 expression and increases CTSL expression in human ileal epithelial cells but does not impact SARS-CoV-2 entry. To address the impact of mesalamine on viral entry in human intestine, primary epithelial cells were inoculated with VSV-SARS-CoV-2 with or without mesalamine. Viral entry was greater in ileum-derived epithelial cells vs colon-derived cells (Figure 1A), correlating with the higher ACE2 expression observed in the ileum rather than high TMPRSS2 and TMPRSS4 expression in the colon. Although mesalamine treatment reduced ileal ACE2 expression, it did not alter VSV-SARS-CoV-2 entry. Rather, mesalamine treatment upregulated ileal expression of the lysosomal protease cathepsin L, which mediates S-protein cleavage during endosomal processing of SARS-CoV-2.7 The reduction of ACE2 protein levels by mesalamine was confirmed in uninfected ileum enteroids by immunoblot and immunofluorescence microscopy (Figure 1B and C).
Figure 1.
Mesalamine reduces intestinal ACE2 expression, but does not change VSV-SARS-CoV-2 infectivity. A, Human intestinal epithelial cells were grown as 2D monolayers and treated with 10mM of mesalamine apically 5 hours before administration of VSV-SARS-CoV-2 (~1.0x 105 PFU/mL). After 24 hours, levels of viral RNA and host gene expression were determined by RT-qPCR. B, Human ileal-derived epithelial cells were grown as 3D enteroids and treated with mesalamine for 30 hours, harvested for protein, and assessed for ACE2 by immunoblot. A representative immunoblot (left) and combined densitometry data (right) from all 3 experiments are shown. C, Apical ACE2 expression in 2D human ileal epithelial monolayers treated with vehicle (control) or 10mM of mesalamine for 30 hours. Bar = 100µm. ∗P<.05, ∗∗P<.01, and ∗∗∗P<.001 by Student t test or 2-way ANOVA with Tukey posttest. Abbreviation: n.s., not significant.
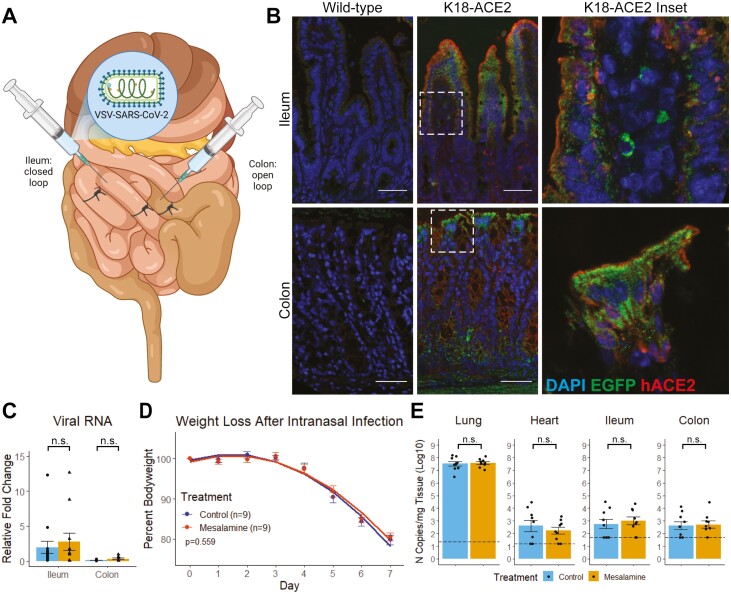
Mesalamine does not increase intestinal SARS-CoV-2 infectivity or morbidity in mouse models of COVID-19. To assess the impact of mesalamine on intestinal viral entry, we developed a mouse model wherein VSV-SARS-CoV-2 is administered to ligated intestinal loops of K18-ACE2 transgenic mice (Figure 2A and B). Three days of mesalamine treatment before virus administration did not alter VSV-SARS-CoV-2 levels (Figure 2C). We used an established mouse model of COVID-19 to study the potential impact of mesalamine on SARS-CoV-2 replication and pathogenesis in vivo. The K18-ACE2 transgenic mice were treated with mesalamine (or vehicle) 3 days before intranasal inoculation with 1x103 FFU of SARS-CoV-2 (2019-nCoV/USA-WA1/2020).8,9 Treatments were continued daily for 7 dpi, and weight loss as a proxy of disease severity was monitored. Mice began losing weight 4 dpi and averaged 19% weight loss by day 7 (Figure 2D). Mice receiving mesalamine lost weight equally compared with mice receiving vehicle control (P=.559 by linear regression). Mesalamine treatment did not change viral load in the lung, heart, or intestinal tissues harvested at 7 dpi (Figure 2E).
Figure 2.
Mesalamine does not change infectivity in a mouse model of intestinal VSV-SARS-CoV-2 Infection. A, Schematic of intestinal loop model of intestinal SARS-CoV-2 infection. Wild-type and K18-hACE2 transgenic mice were gavaged daily with PBS (control) or 200mg/kg of mesalamine for 3 days, then VSV-SARS-CoV-2 was administered into the lumen of a ligated intestinal loop. Tissue was harvested 6 hours postinfection for (B) immunofluorescence and (C) real-time polymerase chain reaction. D and E, K18-ACE2 Tg mice were infected with SARS-CoV-2 (2019-nCoV/USA-WA1/2020) intranasally while receiving daily mesalamine (200mg/kg) or sterile PBS (control) by gavage. D, Weight loss as symptom of COVID-19. E, Viral copy quantification in tissues harvested 7 dpi. Abbreviation: n.s., not significant by 2-way ANOVA with Tukey posttest or Mann-Whitney U test.
Discussion
Mesalamine is used worldwide to treat IBD and rheumatologic disease. Early epidemiologic data associating mesalamine with poor COVID-19 outcomes prompted our group to address the need for experimentally defining how this drug class interacts with SARS-CoV-2 pathogenesis. In the present study, we demonstrate that mesalamine treatment does not enhance SARS-CoV-2 infection in human intestinal epithelial cells in vitro or in mouse models of intestinal SARS-CoV-2. Mesalamine also does not alter viral burden or disease course in a murine model of COVID-19. These data now support newer conclusions from epidemiologic studies that did not find an association between mesalamine and severe COVID-19.6,10
Mesalamine treatment reduced expression of the viral receptor ACE2 while concurrently increasing CTSL expression in human ileum organoids. Prior studies have identified that intestinal ACE2 expression is reduced in active ileal Crohn’s, leading the authors to posit that active IBD may be protective against SARS-CoV-2 infection.11 Although we found that mesalamine also reduced ACE2 expression in vitro, VSV-SARS-CoV-2 infection was unchanged, suggesting a potential role for additional mechanisms of viral entry.12 Since cathepsins have been implicated in endocytic entry of SARS-CoV-2,7 an area of future study will be to determine if upregulation of CTSL expression by mesalamine preferentially enhances spike cleavage in the endosome, thus compensating for reduced ACE2 expression in IBD patients. Overall, these data support the safety of mesalamine use during the COVID-19 pandemic and should offer reassurance to patients with IBD and rheumatologic disorders and their health care providers.
Supplementary Material
Acknowledgments
The authors thank B. Whitener for technical support, the DDRCC AITAC for their expeditious processing of our samples, and the DDRCC PAMOC for cell culture support.
Author Contributions
D.M.A.: study concept and design, collection and assembly of data, data analysis and integration, manuscript writing, and final approval of the manuscript. J.S.: collection and assembly of data, and final approval of the manuscript. L.B.T.: study concept and design, collection and assembly of data, and final approval of the manuscript. M.S.D.: funding, study design and scientific resources, and final approval of the manuscript. S.D.: funding, data analysis and integration, drafting, editing, and final approval of the manuscript. M.A.C.: study concept and design, funding, data analysis and integration, drafting, editing, and final approval of the manuscript.
All data, analytic methods, and study materials will be made available to other researchers upon request.
Funding
This study was supported in part by an Investigator Initiated Research grant from Pfizer (#61798927, D.M.A., S.D., M.A.C.). CCF #648423 (D.M.A.); COVID-19 Fast Grants (S.D.); R01 DK109384R00 AI135031 (S.D); R01 AI157155 (M.S.D.); R01DK109384 (M.A.C) Philanthropic support from the Lawrence C. Pakula MD IBD Innovation Fund at Washington University and www.givinitallforguts.org (D.M.A, M.A.C). Histology and Organoid services were provided by the Washington University Digestive Disease Research Core Center and supported by grant P30DK052574.
Conflicts of Interest
M.S.D. is a consultant for Inbios, Vir Biotechnology, Fortress Biotech, and Carnival Corporation and is on the Scientific Advisory Boards of Moderna and Immunome. M.S.D. has received unrelated funding support in sponsored research agreements from Vir Biotechnology, Kaleido, and Emergent BioSolutions. J.S. and L.B.T. have no conflicts to disclose.
References
- 1. Ding S, Liang TJ.. Is SARS-CoV-2 also an enteric pathogen with potential fecal-oral transmission? A COVID-19 virological and clinical review. Gastroenterology. 2020;159:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Livanos AE, Jha D, Cossarini F, et al. Intestinal host response to SARS-CoV-2 infection and COVID-19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021;160:2435–2450.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zang R, Gomez Castro MF, McCune BT, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5:eabc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strangfeld A, Schäfer M, Gianfrancesco MA, et al. ; COVID-19 Global Rheumatology Alliance. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ungaro RC, Brenner EJ, Agrawal M, et al. Impact of medications on COVID-19 outcomes in inflammatory bowel disease: analysis of over 6,000 patients from an international registry. Gastroenterology. 2021:S0016-5085(21)03490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen RE, Zhang X, Case JB, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winkler ES, Bailey AL, Kafai NM, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan N, Mahmud N, Trivedi C, et al. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut. 2021;70:1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Potdar AA, Dube S, Naito T, et al. Altered intestinal ACE2 levels are associated with inflammation, severe disease, and response to anti-cytokine therapy in inflammatory bowel disease. Gastroenterology. 2021;160:809–822.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Puray-Chavez M, LaPak KM, Schrank TP, et al. Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell. Cell Rep. 2021;36:109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.