ABSTRACT
Lack of awareness of a diagnosis of chronic kidney disease (CKD) in patients and physicians is a major contributor to fueling the CKD pandemic by also making it invisible to researchers and health authorities. This is an urgent matter to tackle if dire predictions of future CKD burden are to be addressed. CKD is set to become the fifth-leading global cause of death by 2040 and the second-leading cause of death before the end of the century in some countries with long life expectancy. Coronavirus disease 2019 (COVID-19) illustrated this invisibility: only after the summer of 2020 did it become clear that CKD was a major driver of COVID-19 mortality, both in terms of prevalence as a risk factor and of the risk conferred for lethal COVID-19. However, by that time the damage was done: news outlets and scientific publications continued to list diabetes and hypertension, but not CKD, as major risk factors for severe COVID-19. In a shocking recent example from Sweden, CKD was found to be diagnosed in just 23% of 57 880 persons who fulfilled diagnostic criteria for CKD. In the very same large cohort, diabetes or cancer were diagnosed in 29% of persons, hypertension in 82%, cardiovascular disease in 39% and heart failure in 28%. Thus, from the point of view of physicians, patients and health authorities, CKD was the least common comorbidity in persons with CKD, ranking sixth, after other better-known conditions. One of the consequences of this lack of awareness was that nephrotoxic medications were more commonly prescribed in patients with CKD who did not have a diagnosis of CKD. Low awareness of CKD may also fuel concepts such as the high prevalence of hypertensive nephropathy when CKD is diagnosed after the better-known condition of hypertension.
Keywords: awareness, chronic kidney disease, hypertensive nephropathy, misdiagnosis, nephrotoxic drugs, nephrotoxicity
THE CONCEPT AND IMPLICATIONS OF CHRONIC KIDNEY DISEASE (CKD)
CKD is diagnosed whenever a decrease in kidney function is assessed as glomerular filtration rate (GFR) or evidence of kidney damage (even with a normal GFR), such as increased albuminuria, abnormal urine sediment or structural abnormalities that persist for >3 months and have implications on health [1]. The GFR and albuminuria thresholds considered to have implications on health are <60 mL/min/1.73 m2 and >30 mg/g of urinary creatinine, respectively. The implications on health include a higher risk of progression to kidney replacement therapy (KRT) requirement, a higher risk of premature all-cause and cardiovascular death and a higher risk of the life-threatening condition of acute kidney injury (AKI). Persons with CKD should be well aware of their condition, as lifestyle changes may be beneficial, certain over-the-counter medications should be avoided or limited, common foods may be lethal and the condition frequently runs in families [2,3]. Physicians should also be aware of a CKD diagnosis, as this will impact the choice of drug prescription and dosing, as well as on the overall management of the patient and family members. Finally, health authorities should be aware of the CKD burden for the purpose of resource allocation and prioritization of research goals.
CKD: A GROWING HEALTH BURDEN
The prevalence of CKD in the adult population has been estimated to be 10–15%, with 850 million people estimated to have CKD globally [4]. The health burden of CKD is growing worldwide. The tip of the iceberg is represented by persons requiring KRT. In Spain, the number of persons on KRT grew by 22% from 2013 to 2019, and at the current rate of growth, the number of persons on KRT will hit 0.23–1.00 million by the end of the century, i.e. 1–4% of the projected population of Spain at that time [5]. Despite the large impact of KRT on health budgets and health managers’ awareness and despite its presence in the general media, among people with CKD, only a minority require KRT. The most common outcome for persons with CKD is premature death without needing KRT. Global Burden of Disease (GBD) data predict that CKD will become the fifth-leading global cause of death by 2040 [6]. In some long-lived countries, such as Spain, CKD will become the second-leading cause of death, after Alzheimer’s, before the end of the century [7]. Thus optimization of CKD diagnosis and treatment in routine clinical practice is needed. A key part of the CKD care optimization process is awareness among physicians, patients, healthcare authorities and the general population of the existence and implications of CKD. Only awareness in patients and physicians will produce the key lifestyle changes and prescription patterns that minimize the long-term negative impacts on kidney function and prevent CKD progression.
THE INVISIBILITY OF CKD IN THE CORONAVIRUS DISEASE 2019 (COVID-19) PANDEMIC
The invisibility of CKD became clear during the CKD pandemic [8]. For months both the lay press and scientific journals emphasized old age, diabetes, hypertension and cardiovascular disease as key risk factors for severe COVID-19. It was not until the summer of 2020 that CKD was shown to be the most common risk factor for severe COVID-19 worldwide and also the second biggest risk factor of COVID-19 death after old age [9, 10]. Apparently CKD was not listed in initial reports of risk factors for severe COVID-19 because it was not diagnosed, despite being present. The lack of awareness of the high risk of patients with CKD, especially those on dialysis, contributed to the high mortality of COVID-19 in this population. Thus, despite efforts by dialysis units to minimize the local exposure of patients and healthcare workers to severe acute respiratory syndrome coronavirus 2, key elements that fell outside the direct control of dialysis facilities, such as transportation to and from dialysis units, were not optimized by the health authorities. As a result, shared transportation to and from dialysis in the absence of masks, which was fully compliant with recommendations of some national governments (e.g. Spanish government), became a key focus of contagion for hemodialysis patients [11,12].
THE INVISIBILITY OF CKD IN SWEDEN
Bosi et al. [13] report on nephrotoxic drug use among patients with CKD in Sweden and the USA. The fact that a researcher can diagnose CKD retrospectively in persons whose physicians were unaware of the diagnosis is striking. The fact that this was the case in almost 80% of CKD patients in the Swedish cohort is alarming for the Swedish healthcare system. Unfortunately, this is likely not a Sweden-only phenomenon. We focus on the Swedish cohort since the US cohort had an automated CKD diagnosis system for reimbursement purposes that did not reflect physician awareness of CKD. Thus data are less clear-cut regarding physician awareness of the condition.
In the Swedish cohort of 57 880 patients with confirmed CKD [two estimated glomerular filtration rate (eGFR) values <60 mL/min/1.73 m2 separated by at least 3 months], CKD was only the sixth most common diagnosis, present in 23% of patients, well below hypertension (82%) and cardiovascular disease (39%) and still below heart failure (28%), cancer (29%) and diabetes (29%) (Figure 1). These findings are even more striking taking into account that a low eGFR is the most common diagnostic criterion for CKD in routine clinical practice, as an assessment of urinary albumin excretion is not a part of routine check-ups as frequent as serum creatinine. Thus we can hypothesize that the non-diagnosis of CKD is even more common among patients having CKD with preserved eGFR, i.e. for CKD categories G1 and G2. These are not isolated data. In a recent report from Japan on 50 091 persons diagnosed with CKD based on a single eGFR value <60 mL/min/1.73 m2, a diagnostic code suggestive of CKD was recorded in only 23% of patients [14].
Figure 1:

Comorbidities diagnosed in a Swedish cohort of patients with CKD, representing clinical conditions that treating physicians were aware of. Inclusion in the cohort required a researcher diagnosis of CKD based on the presence of two eGFR values <60 mL/min/1.73 m2 separated by at least 90 days, as per the Kidney Disease: Improving Global Outcomes definition. Patients on KRT were excluded. Note that among persons included in the cohort because researchers retrospectively diagnosed CKD, the physician in charge diagnosed cancer or diabetes more commonly than CKD.
Consequences of the non-diagnosis of CKD extend well beyond the care of individual patients. In the Swedish cohort of persons with CKD, >90% had a diagnosis of hypertension, almost 4-fold more than those having a diagnosis of CKD [13]. When eventually CKD is diagnosed, a hypertension diagnosis usually precedes the diagnosis of CKD, potentially by years, thus a diagnosis of hypertensive nephropathy can be comfortably made according to textbooks such as UpToDate [15, 16]. Nevermind that a low eGFR had been present for years but did not lead to a CKD diagnosis, or that albuminuria was never assessed and may have been pathological for an even longer period of time.
NEPHROTOXIC DRUG PRESCRIPTION IN PEOPLE WITH CKD
As Bosi et al. [13] point out, there is some discussion related to the concept of nephrotoxic drugs. Some reports considered renin–angiotensin system (RAS) blockers as nephrotoxic drugs. Bosi et al. avoided this confusion by excluding RAS blockers, the most widely used kidney protective medications, from their list of nephrotoxic drugs. During a 1-year period, 20% (Sweden) and 17% (USA) of persons with CKD received at least one nephrotoxic medication, most commonly (10% of persons) a non-steroidal anti-inflammatory drug (NSAID). This was likely an underestimation, as over-the-counter drugs were not assessed. The risk factors for nephrotoxic drug prescription included younger age (<65 years), female gender and milder CKD (CKD G3), as well as provider unawareness of a patient's CKD. The impact of provider unawareness of CKD status was highest for the most commonly prescribed nephrotoxic drug, i.e. NSAIDs. As Bosi et al. [13] point out, for some other drugs in the list the nephrotoxicity potential is still being debated or the known benefits clearly outweigh the risks. However, a correct understanding of the use of potentially nephrotoxic drugs is key to understanding the factors contributing to CKD progression, even if the use of the drug is justified based on the benefit for other organs (Figure 2).
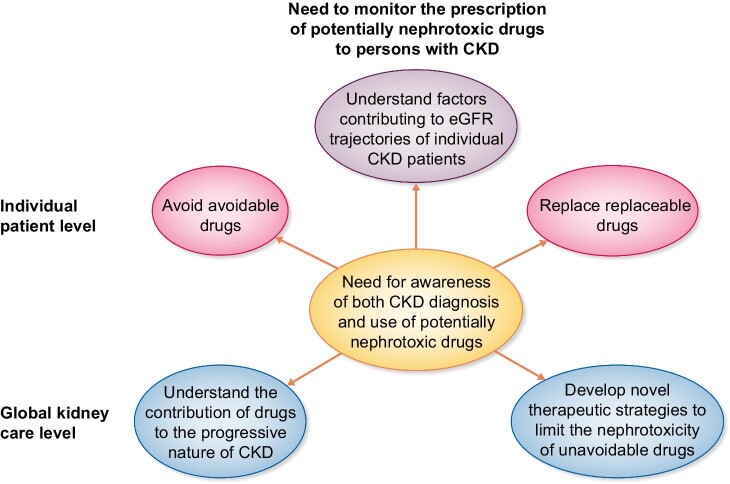
Figure 2:
Need to monitor the prescription of potentially nephrotoxic drugs to persons with CKD. The impact of monitoring clinical practice regarding the prescription of clearly nephrotoxic or potentially nephrotoxic drugs to persons with CKD requires awareness of the CKD diagnosis and may impact both individual patient care as well as the global care for persons with CKD.
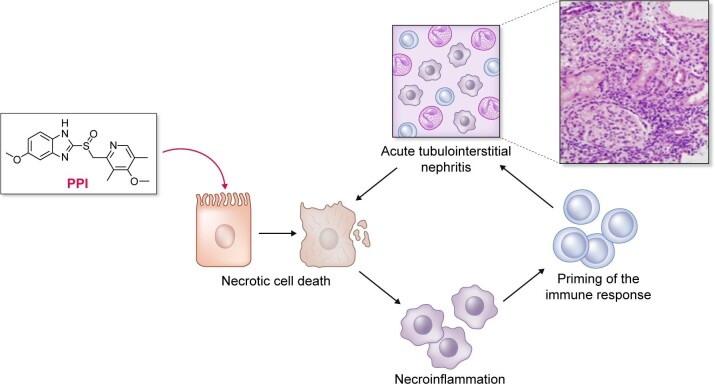
Regarding drugs with debated nephrotoxicity, Bosi et al. [13] further provided a sensitivity analysis considering proton pump inhibitors (PPIs) and vitamin K antagonists (VKAs) as nephrotoxic drugs. The addition of these two groups of drugs increased the prescription of nephrotoxic agents in 48% and 56% of persons with CKD in Sweden and the USA, respectively. If confirmed to be nephrotoxic, PPIs would become the most prescribed nephrotoxic drug for CKD patients (Figure 3). In this regard, the molecular pathways engaged by PPIs that may contribute to nephrotoxicity were recently characterized from a mechanistic point of view in preclinical studies. Thus omeprazole induced dose-dependent necrotic cell death in proximal tubular cells related to a strong oxidative stress response affecting mitochondria and lysosomes [17]. Induction of necrosis may potentially trigger necroinflammation, i.e. the recruitment of inflammatory and immune responses in response to the release of cell contents that may facilitate immune-mediated acute tubulointerstitial nephritis, another feature of PPI nephrotoxicity (Figure 4) [18, 19]. Given the widespread use of PPIs and the increasing life expectancy of the world population, large prospective studies addressing the potential nephrotoxicity of PPIs in different age groups and baseline eGFRs are needed.
Figure 3:
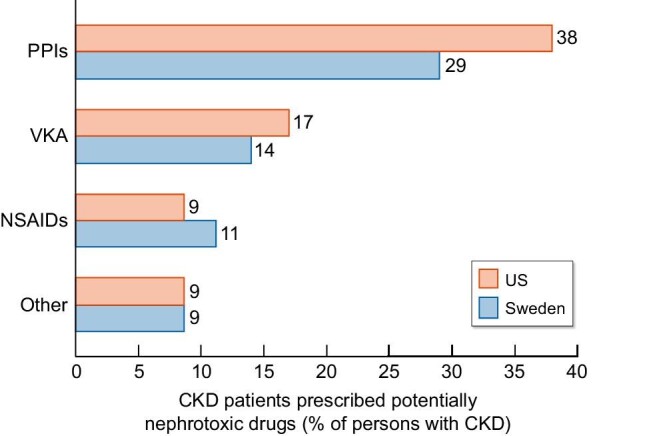
Prescription of potentially nephrotoxic drugs in Swedish and US cohorts of persons with CKD. Data expressed as a percentage of persons prescribed a potentially nephrotoxic drug among the whole cohort of persons with CKD.
Figure 4.
Integration of knowledge regarding preclinical evidence of cytotoxicity of PPIs with epidemiological data linking PPIs to kidney injury.
VKAs have also been associated with faster CKD progression than direct oral anticoagulants [20]. One potential mechanism is repeated episodes of hematuria during periods of over-anticoagulation, leading to heme-mediated tubular cell and podocyte injury [21–23]. In this regard, the combination of PPIs and VKAs, which is frequently observed in routine clinical practice, may theoretically increase their nephrotoxic potential, as microhematuria was associated with more severe acute tubulointerstitial nephritis [24]. Randomized controlled trials evaluating the impact of direct oral anticoagulants versus VKA on kidney function outcomes in patients with CKD would clarify this issue.
Since several guidelines suggest not referring CKD patients to nephrologists until the eGFR falls below 30 mL/min/1.73 m2 or there are signs of alarm, such as pathological albuminuria, primary care physicians should be the prime targets for awareness campaigns. In this regard, primary care physicians prescribed 40–50% of nephrotoxic drugs to persons with CKD [13].
RAISING AWARENESS
This issue of CKJ also presents a manuscript, coauthored by multiple stakeholders in the Spanish kidney disease community, from scientific societies to associations of persons with kidney disease to government agencies, that summarizes local and international data on the burden of CKD [5]. It is aimed at providing a resource for stakeholders seeking to promote awareness of the heath burden of CKD. At the local level, it identifies the lack of awareness of Spanish government agencies funding healthcare research: CKD is the only one among the top 15 global causes of death by 2040 that is not supported by a well-funded Centro de Investigación Biomédica en Red (CIBER) network research structure.
CONCLUSION
Bosi et al. [13] focused on the prescription of nephrotoxic drugs to CKD patients in both Europe and the USA. Beyond the message that there is room for improvement in this regard, a striking piece of information from their article is that there is a systematic bias in electronic health records that makes CKD invisible to health authorities and researchers alike, due the low awareness of the condition by physicians and patients. Thus diagnoses such as hypertension, cardiovascular disease, heart failure and even diabetes and cancer were more common than a diagnosis of CKD in a cohort of people selected for the presence of CKD. The consequences of such invisibility go well beyond inappropriate prescription of nephrotoxic drugs to individual patients with CKD, as CKD is not present in healthcare authority's statistics used to allocate resources and research priorities and CKD is thought to be secondary to hypertension, rather than the other way around, fueling the belief that hypertension is a frequent cause of CKD and hampering research into the causes of CKD. There is an urgent need to address the unawareness of the CKD concept that should start with primary care physicians, who are the gatekeepers of the healthcare system. Increased CKD awareness may also result in increased CKD referrals to nephrologists. In a French healthcare catchment area, it was recently estimated that this would result in the need for 3–17 additional nephrologists per million population (pmp), on top of the 12 nephrologists pmp already available, to fully cover the need for care [25]. Thus, appropriate long-term planning for the increased needs for facilities and personnel is required.
Contributor Information
Sol Carriazo, Instituto de Investigación Sanitaria Fundacion Jimenez Diaz, Madrid, Spain; Red de Investigación Renal (REDINREN), Madrid, Spain.
Priscila Villalvazo, Instituto de Investigación Sanitaria Fundacion Jimenez Diaz, Madrid, Spain.
Alberto Ortiz, Instituto de Investigación Sanitaria Fundacion Jimenez Diaz, Madrid, Spain; Red de Investigación Renal (REDINREN), Madrid, Spain.
CONFLICT OF INTEREST STATEMENT
A.O. has received consultancy or speaker fees or travel support from Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, Bayer, Sanofi-Genzyme, Menarini, Kyowa Kirin, Alexion, Idorsia, Chiesi, Otsuka and Vifor Fresenius Medical Care Renal Pharma and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra AstraZeneca-UAM of chronic kidney disease and electrolytes. A.O. is the Editor-in-Chief of CKJ.
REFERENCES
- 1. Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez Eet al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019; 12: 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandez-Prado R, Esteras R, Perez-Gomez MVet al. Nutrients turned into toxins: microbiota modulation of nutrient properties in chronic kidney disease. Nutrients 2017; 9: 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torra R, Furlano M, Ortiz Aet al. Genetic kidney diseases as an underrecognized cause of chronic kidney disease: the key role of international registry reports. Clin Kidney J 2021; 14: 1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jager KJ, Kovesdy C, Langham Ret al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019; 34: 1803–1805 [DOI] [PubMed] [Google Scholar]
- 5. Ortiz A, Asociación Información Enfermedades Renales Genéticas, European Kidney Patients' Federation et al. RICORS2040: the need for collaborative research in chronic kidney disease. Clin Kidney J 2021; 10.1093/ckj/sfab170 [DOI] [Google Scholar]
- 6. Foreman KJ, Marquez N, Dolgert Aet al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018; 392: 2052–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ortiz A, Sanchez-Niño MD, Crespo-Barrio Met al. The Spanish Society of Nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia 2019; 39: 29–34 [DOI] [PubMed] [Google Scholar]
- 8. ERA-EDTA Council, ERACODA Working Group . Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 2021; 36: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williamson EJ, Walker AJ, Bhaskaran Ket al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark A, Jit M, Warren-Gash Cet al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health 2020; 8: e1003–e1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rincón A, Moreso F, López-Herradón Aet al. The keys to control a COVID-19 outbreak in a haemodialysis unit. Clin Kidney J 2020; 13: 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carriazo S, Kanbay M, Ortiz A.. Kidney disease and electrolytes in COVID-19: more than meets the eye. Clin Kidney J 2020; 13: 274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bosi A, Xu Y, Gasparini Aet al. Use of nephrotoxic medications in adults with chronic kidney disease: parallel cohort studies in Swedish and U.S. routine care. Clin Kidney J 2021; 10.1093/ckj/sfab210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takeuchi M, Shinkawa K, Yanagita Met al. Prevalence, recognition and management of chronic kidney disease in Japan: population-based estimate using a healthcare database with routine health checkup data. Clin Kidney J 2021; 14: 2197–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carriazo S, Vanessa Perez-Gomez M, Ortiz A. Hypertensive nephropathy: a major roadblock hindering the advance of precision nephrology. Clin Kidney J 2020; 13: 504–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mann JFE, Hilgers KF. Clinical features, diagnosis, and treatment of hypertensive nephrosclerosis. https://www.uptodate.com/contents/clinical-features-diagnosis-and-treatment-of-hypertensive-nephrosclerosis (9 July 2020, date last accessed) [Google Scholar]
- 17. Fontecha-Barriuso M, Martín-Sanchez D, Martinez-Moreno JMet al. Molecular pathways driving omeprazole nephrotoxicity. Redox Biol 2020; 32: 101464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mulay SR, Linkermann A, Anders HJ. Necroinflammation in kidney disease. J Am Soc Nephrol 2016; 27: 27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eddy AA. Drug-induced tubulointerstitial nephritis: hypersensitivity and necroinflammatory pathways. Pediatr Nephrol 2020; 35: 547–554 [DOI] [PubMed] [Google Scholar]
- 20. Yao X, Tangri N, Gersh BJet al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2017; 70: 2621–2632 [DOI] [PubMed] [Google Scholar]
- 21. Brodsky SV, Satoskar A, Chen Jet al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis 2009; 54: 1121–1126 [DOI] [PubMed] [Google Scholar]
- 22. Brodsky SV, Nadasdy T, Rovin BHet al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 2011; 80: 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rubio-Navarro A, Sanchez-Niño MD, Guerrero-Hue Met al. Podocytes are new cellular targets of haemoglobin-mediated renal damage. J Pathol 2018;244:296–310 [DOI] [PubMed] [Google Scholar]
- 24. Esteras R, Fox JG, Geddes CCet al. Hematuria is associated with more severe acute tubulointerstitial nephritis. J Clin Med 2020;9:2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torreggiani M, Chatrenet A, Fois Aet al. Unmet needs for CKD care: from the general population to the CKD clinics. How many patients are we missing? Clin Kidney J 2021; 14: 2246–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]