Abstract
Background
Data on the safety and efficacy of coronavirus disease 2019 (COVID-19) vaccination in people with a range of primary immunodeficiencies (PIDs) are lacking because these patients were excluded from COVID-19 vaccine trials. This information may help in clinical management of this vulnerable patient group.
Objective
We assessed humoral and T-cell immune responses after 2 doses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccines in patients with PID and functional B-cell defects.
Methods
A double-center retrospective review was performed of patients with PID who completed COVID-19 mRNA vaccination and who had humoral responses assessed through SARS-CoV-2 spike protein receptor binding domain (RBD) IgG antibody levels with reflex assessment of the antibody to block RBD binding to angiotensin-converting enzyme 2 (ACE2; hereafter referred to as ACE2 receptor blocking activity, as a surrogate test for neutralization) and T-cell response evaluated by an IFN-γ release assay. Immunization reactogenicity was also reviewed.
Results
A total of 33 patients with humoral defect were evaluated; 69.6% received BNT162b2 vaccine (Pfizer-BioNTech) and 30.3% received mRNA-1273 (Moderna). The mRNA vaccines were generally well tolerated without severe reactions. The IFN-γ release assay result was positive in 24 (77.4%) of 31 patients. Sixteen of 33 subjects had detectable RBD-specific IgG responses, but only 2 of these 16 subjects had an ACE2 receptor blocking activity level of ≥50%.
Conclusion
Vaccination of this cohort of patients with PID with COVID-19 mRNA vaccines was safe, and cellular immunity was stimulated in most subjects. However, antibody responses to the spike protein RBD were less consistent, and, when detected, were not effective at ACE2 blocking.
Key words: SARS-CoV-2, SARS-CoV-2 vaccination, primary immunodeficiency, ACE2 blocking antibody, SARS-CoV-2 spike protein antibody, antibody deficiency, common variable immunodeficiency, Good syndrome, mAb, SARS-CoV-2 IFN-γ release assay
Abbreviations used: ACE2, Angiotensin-converting enzyme 2; COVID-19, Coronavirus disease 2019; CTLA-4, Cytotoxic T lymphocyte–associated protein 4; CVID, Common variable immunodeficiency; ELISA, Enzyme-linked immunosorbent assay; EUA, Emergency use authorization; IGRA, IFN-γ release assay; IVIG, Intravenous immunoglobulin; mRNA, Messenger RNA; PID, primary immunodeficiency; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Graphical abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains a serious threat to global health and a significant cause of morbidity and mortality, especially in patients with primary immunodeficiencies (PIDs).1 Two safe and effective messenger RNA (mRNA) vaccines targeting the spike protein of SARS-CoV-2 have been granted emergency use authorization (EUA).2 SARS-CoV-2-specific humoral and T-cell responses both contribute to protection against coronavirus disease 2019 (COVID-19) infection.3, 4, 5
Although about 10 million people in the United States are immunocompromised, patients with immunodeficiencies including PIDs were excluded from the SARS-CoV-2 vaccine trials leading up to the EUAs. Thus, data on safety and immune responses to COVID-19 vaccination in recipients with immunodeficiencies and dysregulation syndromes are limited. Recent publications have suggested good tolerance and immunogenicity in patients with PID, but more and larger studies are needed6, 7, 8 that include evaluation of antibody responses that predict protection from infection.
Thirty-three patients with diverse PIDs ranging in age between 19 and 79 years (mean [SD], 50.2 ± 18.35 years) followed at the allergy and immunology clinics at Stanford University and the University of California, San Francisco, were studied. All had received 2 doses of either mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 mRNA vaccines (Table I and see Table E1 in this article's Online Repository at www.jacionline.org ). We focused on the evaluation of safety and efficacy of mRNA vaccination for PID patients with humoral defects, including patients with moderately low to normal levels of B cells and impaired or absent specific antibody responses as well as those with low or absent B cells and globally reduced antibody production. To evaluate the immunogenicity of the vaccine, we measured the spike protein–specific antibody response using a SARS-CoV-2 IgG antibody enzyme-linked immunosorbent assay (ELISA) coating with S1 receptor binding domain (RBD) antigen, with reflex to SARS-CoV-2 angiotensin-converting enzyme 2 (ACE2) receptor blocking activity,9 which correlates well with antibody virus neutralization.10 Spike protein–specific T-cell responses were evaluated using a SARS-CoV-2 IFN-γ release assay (IGRA).11 These assays were performed at Stanford Health Care Clinical Virology Laboratory, a Clinical Laboratory Improvement Amendments–certified laboratory.
Table I.
Subject characteristics and test results
| Subject no. | Age (years) | Sex | PID diagnosis | Antibody deficiency | Ig therapy | SARS-CoV-2 mRNA vaccine | Time between second vaccine dose and serology (weeks) | SARS-CoV-2 spike protein IgG after vaccine | SARS-CoV-2 ACE2 blocking activity | SARS-CoV-2 IGRA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | M | Agammaglobulinemia | Yes | Yes | Pfizer-BioNTech | 4.43 | Negative | — | Positive |
| 2 | 30 | M | XLA | Yes | Yes | Moderna | 4.00 | Negative | — | Positive |
| 3 | 30 | F | CVID | Yes | Yes | Pfizer-BioNTech | 5.86 | Positive | 50-60% | Positive |
| 4 | 32 | F | CVID | Yes | Yes | Pfizer-BioNTech | 8.71 | Negative | — | Positive |
| 5 | 38 | F | CVID | Yes | Yes | Pfizer-BioNTech | 4.14 | Positive | 40-50% | Positive |
| 6 | 40 | M | CVID | Yes | Yes | Moderna | 5.57 | Positive | 40-50% | Positive |
| 7 | 41 | F | CVID | Yes | Yes | Pfizer-BioNTech | 9.14 | Positive | <10% | Positive |
| 8 | 53 | M | CVID | Yes | Yes | Moderna | 9.43 | Negative | — | Positive |
| 9 | 56 | M | CVID | Yes | Yes | Pfizer-BioNTech | 15.00 | Positive | <10% | Negative |
| 10 | 58 | F | CVID | Yes | Yes | Pfizer-BioNTech | 4.86 | Positive | <10% | Positive |
| 11 | 59 | M | CVID | Yes | Yes | Pfizer-BioNTech | 7.00 | Negative | — | Negative |
| 12 | 60 | F | CVID | Yes | Yes | Pfizer-BioNTech | 9.57 | Positive | 30-40% | Positive |
| 13 | 63 | F | CVID | Yes | Yes | Moderna | 9.86 | Positive | 30-40% | Positive |
| 14 | 71 | F | CVID | Yes | Yes | Moderna | 10.71 | Positive | 20-30% | Positive |
| 15 | 72 | M | CVID | Yes | Yes | Moderna | 17.57 | Positive | NA | Positive |
| 16 | 73 | F | CVID | Yes | No | Pfizer-BioNTech | 24.71 | Positive | <10% | Positive |
| 17 | 79 | F | CVID | Yes | Yes | Pfizer-BioNTech | 11.29 | Positive | <10% | Negative |
| 18 | 39 | F | HGG | Yes | Yes | Moderna | 9.57 | Positive | 60-70% | Positive |
| 19 | 55 | F | HGG | Yes | Yes | Pfizer-BioNTech | 6.85 | Negative | — | Positive |
| 20 | 67 | F | HGG | Yes | Yes | Pfizer-BioNTech | 9.43 | Positive | <10% | Positive |
| 21 | 75 | M | HGG | Yes | Yes | Moderna | 16.77 | Negative | — | Negative |
| 22 | 53 | F | SAD | Yes | Yes | Pfizer-BioNTech | 6.57 | Positive | 40-50% | Positive |
| 23 | 74 | F | SAD | Yes | Yes | Moderna | 14.43 | Positive | 10-20% | Positive |
| 24 | 43 | M | GS with HGG | Yes | Yes | Pfizer-BioNTech | 9.86 | Negative | — | Negative |
| 25 | 65 | F | GS with HGG | Yes | Yes | Pfizer-BioNTech | 5.86 | Negative | — | Positive |
| 26 | 68 | F | GS with HGG | Yes | Yes | Moderna | 19.00 | Negative | — | Negative |
| 27 | 70 | F | GS with HGG | Yes | Yes | Pfizer-BioNTech | 19.14 | Negative | — | Negative |
| 28 | 39 | M | Hyper IgM syndrome | Yes | Yes | Pfizer-BioNTech | 15.71 | Negative | — | Positive |
| 29 | 40 | M | Hyper IgM syndrome | Yes | Yes | Pfizer-BioNTech | 13.14 | Negative | — | Positive |
| 30 | 19 | M | CTLA-4 deficiency | Yes | Yes | Pfizer-BioNTech | 6.43 | Negative | — | Positive |
| 31 | 29 | M | PIK3R1 | Yes | Yes | Pfizer-BioNTech | 18.25 | Negative | — | — |
| 32 | 26 | F | Ataxia telangiectasia | Yes | Yes | Pfizer-BioNTech | 5.71 | Negative | — | — |
| 33 | 20 | M | ATP6AP1 gene/immunodeficiency 47 | Yes | Yes | Pfizer-BioNTech | 4.43 | Negative | — | Positive |
GS, Good syndrome; HGG, hypogammaglobulinemia; NA, not applicable; SAD, specific antibody deficiency; XLA, X-linked agammaglobulinemia.
Results and discussion
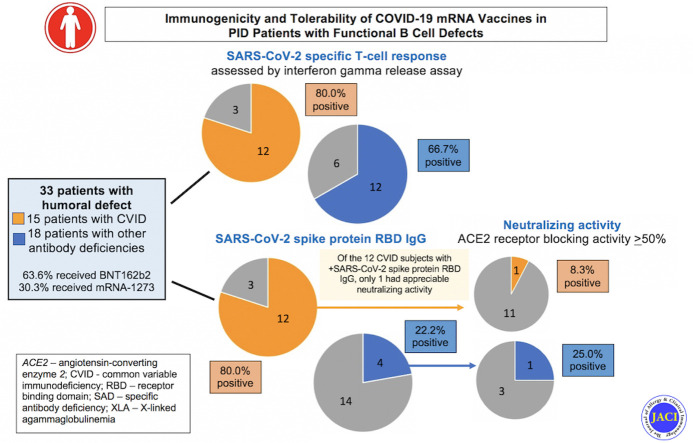
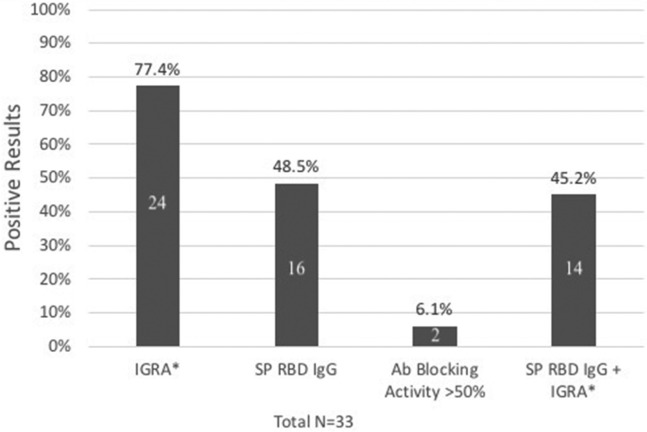
Testing was performed a mean of 10.9 ± 5.3 weeks after the second vaccine dose, and most subjects had positive immune results to some degree (Fig 1 ). Twenty-four (77.4%) of 31 patients had positive IGRA results. About half of our subjects (16 of 33) had detectable RBD-specific IgG responses, but only 2 had an ACE2 receptor blocking activity level of ≥50%. Our subjects had impaired antibody responses as their predominant clinical immunodeficiency, such as common variable immunodeficiency (CVID) (n = 15), hypogammaglobulinemia (n = 4), selective antibody deficiency (n = 2), Good syndrome with absent B cells (n = 4), agammaglobulinemia (n = 2), hyper IgM syndrome (n = 2), PIK3R1 deficiency (n = 1), cytotoxic T lymphocyte–associated protein 4 (CTLA-4) haploinsufficiency (n = 1), and combined immunodeficiency (ataxia telangiectasia, n = 1; ATP6AP1 gene/immunodeficiency 47, n = 1) (Table I). Thirty-two subjects (96.9%) were receiving immunoglobulin replacement therapy. Sixty-nine percent of the patients received the BNT162b2 (Pfizer-BioNTech) vaccine; the remainder received the mRNA-1273 (Moderna) vaccine. Five had a SARS-CoV-2 spike protein IgG level checked before COVID-19 vaccination, which was undetectable in all cases. None of our patients had a known history of SARS-CoV-2 infection before vaccination, and none developed a SARS-CoV-2 infection during the study period. Clinical data for up to 9 months after vaccination are reported. Tolerability/reactogenicity information was gathered through chart review and revealed that the vaccines were well tolerated (Table II ). There were no severe adverse reactions.
Fig 1.
Immunogenicity of the SARS-CoV-2 vaccines in PID patients with functional B-cell defects. SP RBD IgG antibody to the SARS-CoV-2 RBD domain of the spike protein (SP). Antibody blocking activity was ≥50%; ACE2 blocking antibody activity was also ≥50%. Numbers in bars signify number of subjects. Unless otherwise noted, sample size is 33. ∗Denominator is 31.
Table II.
Adverse effects after SARS-CoV-2 vaccination
| Adverse effect | No. (%) |
|---|---|
| Sore arm | 6 (18.2) |
| Fatigue | 4 (12.1) |
| Headache | 5 (15.1) |
| Local reaction/rash | 2 (6) |
| Fever/chills | 1 (3) |
| Myalgias | 1 (3) |
| Neck stiffness | 1 (3) |
| Vertigo/paresthesia | 1 (3) |
| Nausea/vomiting | 1 (3) |
| Flare of enteropathy∗ | 1 (3) |
| Flare of chronic urticaria | 1 (3) |
| Total subjects with symptoms | 14/33 (42) |
Flare occurred 1 week after vaccination.
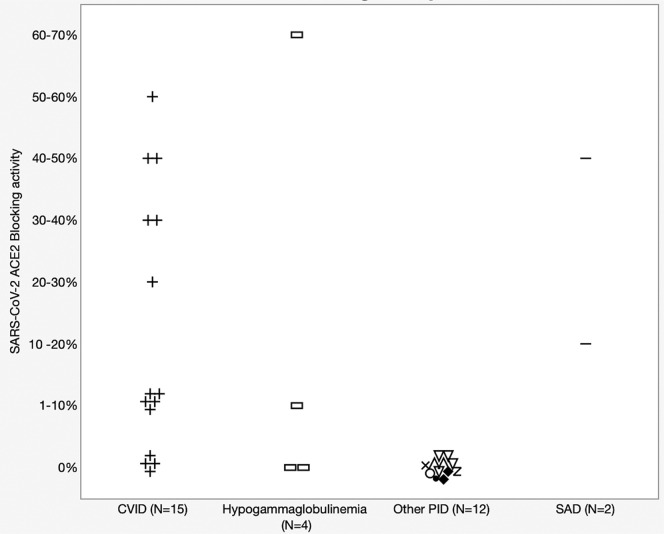
All our patients had an antibody deficiency, which is the most common general category of PID. As expected, our 4 patients harboring inborn errors that markedly impair IgG antibody production (2 with agammaglobulinemia and 2 with hyper IgM syndrome resulting from CD40 ligand deficiency) had negative SARS-CoV-2 IgG antibody results (Table I, Fig 2 ). IGRA results were positive for all 4 of these patients, consistent with the relative selectivity of these immunodefects and their largely sparing T-cell immunity. In our subjects with humoral defects as part of CVID (n = 15), 80% had a positive SARS-CoV-2 spike protein RBD–specific IgG result, and 80% had a positive IGRA result. CVID patients with a positive RBD-specific IgG level had a statistically significant higher average of circulating CD3 T-cell level than those who had a negative result (1317 ± 431.9/μL vs 531 ± 157.0/μL, respectively) (t test P = .029). The mean baseline IgG levels were also higher in CVID patients who had a positive RBD-specific IgG responses than those who did not (364.7 ± 102.0 mg/dL vs 91.0 ± 58.0 mg/dL, respectively (t test P = .004). A striking finding was that only 1 of the 15 CVID patients with a positive RBD IgG-specific antibody response also had functional antibodies that blocked the interaction of the RBD with ACE2, as assessed in the ACE2 blocking antibody assay.9 Because this blocking activity correlates well with antibody that effectively neutralizes SARS-CoV-2 for entry into host cells,10 this indicated that the bodies of most CVID patients did not mount antibody responses that would be protective against SARS-CoV-2 infection.
Fig 2.
SARS-CoV-2 antibody ACE2 blocking activity in 33 PID patients with B-cell functional defect. Patients were subdivided according to different disease categories. “Other PID” includes X-linked agammaglobulinemia (XLA) patients (n = 2), Good syndrome (n = 4), CTLA-4 haploinsufficiency (n = 1), PIK3R1 (n = 1), AT (n = 1), and ATP6AP1 (n = 1). AT, Ataxia telangiectasia.
The responsiveness the disease of patients with PID to COVID-19 vaccination might be potentially difficult to assess if the patient is receiving immunoglobulin replacement therapy with a product that contains SARS-CoV-2 antibody derived from donors who have had SARS-CoV-2 infection, who have received COVID-19 vaccines, or both. We anticipated that this was unlikely to account for the presence of 46.9% of our 32 patients who were receiving immunoglobulin replacement therapy having any spike protein–specific antibody, given the usual lag between seroprevalence in the blood donor population and the specific antibody in manufactured immunoglobulin products.12, 13, 14, 15, 16 To evaluate the potential impact of immunoglobulin therapy on SARS-CoV-2 spike protein RBD–specific humoral responses, we evaluated 2 patients (patients 24 and 32; Table I) for SARS-CoV-2 ACE2 receptor blocking antibody levels before and after intravenous immunoglobulin (IVIG) therapy. For patient 24, both before and after IVIG therapy, ACE2 receptor blocking activity was <10%, and for patient 32, the post-IVIG ACE2 receptor blocking activity minimally changed from <10% before infusion to 14% after infusion. Thus, in these 2 subjects, the IVIG products they received in September 2021 (over 1.5 years since the start of the global COVID-19 pandemic) did not appreciably alter their levels of protective neutralizing antibody.
To our knowledge, this study of PID patients with functional B-cell defects is the first to evaluate the ACE2 receptor blocking activity after 2 doses of the SARS-CoV-2 mRNA vaccines. The receptor blocking activity competition assay evaluates the ability of the antibody in serum or plasma to bind to the spike protein RBD and prevent its interaction with ACE2.9 The level of receptor blocking activity may correlate with antibody-mediated neutralization assays that use viruses pseudotyped with the spike protein.10 Thus, our finding that only 1 of 15 CVID patients had an ACE2 blocking level of ≥50% and that such activity was undetectable in most of these patients raises the possibility that mRNA vaccination may provide minimal protection from SARS-CoV-2 infection for CVID patients. It is also important to consider that the ACE2 receptor blocking assay used the RBD similar to that encoded by the current EUA-approved mRNA vaccines, and protection might be even further reduced with SARS-CoV-2 variants that have amino acid changes in the RBD domain.
Similar to Hagin et al,7 80% of our patients with CVID had a spike protein RBD–specific IgG response. Additionally, 80% of our CVID patients had spike protein–specific T-cell immune response. In the antibody-deficient patients in our cohort, and in contrast to the study of Hagin et al, there was no difference between age or IgG at baseline and a positive SARS-CoV-2 spike protein result, but those with a positive IGRA result were younger, with a mean age of 48.2 ± 17.7 versus 64.3 ± 12.4 years (t test P = .032).
This study has several limitations, including the relatively small size of our cohort and the relatively short period of the vaccine observations. We plan to measure and report on additional data including our patients’ responses to a third vaccine dose, given new recommendations by the US Centers for Disease Control and Prevention for a third mRNA dose in patients with moderate and severe immunodeficiencies.17 We also did not include any patients with hemophagocytic lymphohistiocytosis or autoinflammatory conditions. Additionally, the ability to interpret the clinical significance of individual patient ACE2 receptor blocking activity for providing protection will require additional clinical studies to establish validated cutoff values. The threshold of ACE2 receptor blocking activity of ≥50% for a positive result was chosen for this report, but further studies are needed to more precisely establish protective ranges.
Currently, SARS-CoV-2 mAb therapies are granted EUA for use in older and high-risk individuals, such as some PID patients, for postexposure prophylaxis or infection with SARS-CoV-2. In patients with humoral defects where functional antibody protection is not achieved, either through vaccination or immunoglobulin replacement therapy, it would be reasonable to expand mAb therapy to serve as a prophylactic in this high-risk patient population. In fact, an EUA has recently been requested for a mAb cocktail (AstraZeneca) to serve as preexposure prophylaxis in vulnerable populations, such as the immunocompromised. Studying vaccinated PID patients and their neutralizing antibody may help determine those who can benefit from such prophylactic therapy.
In our cohort of PID patients with functional B-cell defects, mRNA vaccines were well tolerated, and although antibody responses to the spike protein that are associated with protection were not reliably induced in most of our subjects, T-cell responses were elicited in most of our patients. These T-cell immune responses are anticipated to be helpful in limiting virus replication in cases of established infection.5 Further long-term studies will aid in determining effective therapies and recommendations in patients with PID during this SARS-CoV-2 pandemic.
Clinical implications.
mRNA vaccination may be less effective at preventing acquisition of SARS-CoV-2 in our cohort of PID patients with functional B-cell defects. Induction of SARS-CoV-2 spike protein–specific T-cell immunity by vaccination might help reduce disease severity in these patients.
Acknowledgments
We thank the patients and their families for participating in research studies. We would like to thank Cristina R. Crotales (Department of Pathology, Stanford University, Stanford, Calif) for her invaluable technical help.
Footnotes
The last 2 authors contributed equally to this article, and both should be considered senior author.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Methods
X-linked agammaglobulinemia is a humoral immunodeficiency caused by mutations in Bruton tyrosine kinase, a key signal transduction molecule required for B-cell development. Patients have low to absent B cells (most patients have a small number of B cells), and reduced levels of all immunoglobulin classes. CVID is an antibody deficiency syndrome characterized by decreased serum IgG (IgM and IgA are often decreased) with impaired specific antibody responses. CTLA-4 haploinsufficiency is an immunodysregulatory disorder that can present a CVID-like phenotype when hypogammaglobulinemia is present. Immunodeficiency 47 is a complex immunodeficiency syndrome characterized by hypogammaglobulinemia, recurrent bacterial infections, defective glycosylation of serum proteins, and liver disease with neonatal jaundice and hepatosplenomegaly. Good syndrome with immunodeficiency is a rare condition in which thymoma is associated with hypogammaglobulinemia. It is characterized by increased susceptibility to bacterial, viral, and fungal infections, as well as autoimmunity. Most patients have no circulating B cells. X-linked hyper IgM syndrome is a combined immunodeficiency that is characterized by antibody deficiency as well as an impaired ability of T cells to activate monocytes and dendritic cells. This disease is caused by mutations in CD40 ligand, a molecule that is expressed on the surface of activated T cells. CD40 ligand interacts with CD40 on the surface of B cells to activate immunoglobulin class switching (shifting antibody production from IgM to IgG, IgA, or IgE) and to establish B-cell memory. PIK3R1 deficiency is also considered a predominantly antibody deficiency. Patients with X-linked agammaglobulinemia, CVID, Good syndrome, X-linked hyper IgM syndrome, PIK3R1 deficiency, immunodeficiency 47, and CTLA-4 deficiency require uninterrupted immunoglobulin replacement therapy for the antibody deficiency component of their disease (see Table E1).
ELISA to detect anti–SARS-CoV-2 and anti–SARS-CoV antibodies in plasma samples was performed, with ELISA to detect antibodies blocking the binding of ACE2 to RBD. This assay (and its references) was performed at Stanford Health Care Clinical Virology Laboratory, a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory. In brief, 96-well Corning Costar high binding plates (Thermo Fisher Scientific, Waltham, Mass, cat. 9018) were coated with SARS-CoV-2 spike RBD protein in phosphate-buffered saline (PBS) at a concentration of 0.1 μg per well overnight at 4°C. All competition ELISA steps were carried out on the next day at room temperature. Wells were washed 3× with PBS–Tween 20 (PBS-T) and blocked with PBS-T containing 3% nonfat milk powder for 1 hour. Wells were then incubated with plasma samples from our cohort of patients at a dilution of 1:10 in PBS-T containing 1% nonfat milk for 1 hour. Two quality controls (Access SARS-CoV-2 IgG QC, QC1-QC2, 2 levels, cat. C58964, Beckman Coulter, Fullerton, Calif) and 2 blank wells incubated with PBS-T containing 1% nonfat milk were included on each plate. ACE2-mFc diluted to 0.5 μg/mL in 1% nonfat milk powder was added without washing steps and incubated for an additional 45 minutes. After washing 3× with PBS-T, horseradish peroxidase–conjugated goat anti-mouse IgG (Fc specific, cat. 31439, Invitrogen [Thermo Fisher Scientific], 1:10,000 dilution) in PBS-T containing 1% nonfat milk was added and incubated for 45 minutes. Wells were washed 3× with PBS-T and dried by vigorous tapping of plates on paper towels. TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution was added, and the reaction was stopped after 12 minutes by addition of 0.16 mol sulfuric acid. The OD at 450 nanometers was measured with an EMax Plus microplate reader (Molecular Devices, San Jose, Calif). OD values were converted to percentage of blocking using the following formula: 100 × [1 − (sample OD − 0.2)/(QC1 OD − 0.2)], taking into account the background noise of the assay of 0.2 as determined after testing prepandemic control plasma samples. A detailed description is provided in Roltgen et al.E1
The IGRA used here measured IFN-γ released by antigen-specific T cells after overnight stimulation with a commercially available peptide pool consisting of spike, S1, nucleocapsid, and membrane proteins (Miltenyi Biotec, San Diego, Calif).E2 This assay (and its references) was performed at Stanford Health Care Clinical Virology Laboratory, a CLIA-certified laboratory. In brief, freshly collected blood in lithium heparin tube was (1) left unstimulated as negative control; stimulated with (2) peptide pool consisting of spike, S1, nucleocapsid, and membrane proteins (Miltenyi Biotec); or (3) mitogen as positive control. The IFN-γ concentration in the plasma fraction was measured with an automated ELISA instrument (IU/mL). IFN-γ response was defined as peptide stimulated minus unstimulated. The Mann-Whitney U test was used to compare median IFN-γ responses between groups. The Wilcoxon signed-rank test of medians was used to compare differences between paired results. The receiver operating characteristic curve was used to derive an IFN-γ response cutoff at the Youden maximum index value, which assigns equal weight to sensitivity and specificity.
The criteria to establish results were as follows:
-
•
Criteria for positive: Nil is ≤8.0 and SARS-CoV-2 antigen minus nil is ≥0.35.
-
•
Criteria for negative: Nil is ≤8.0 and SARS-CoV-2 antigen minus nil is <0.35 and mitogen-nil is ≥0.5.
-
•
Criteria for indeterminate: (1) Nil is ≤8.0 and SARS-CoV-2 antigen minus nil is <0.35 and mitogen minus nil is <0.5; or (2) nil is >8.
-
•
Criteria for borderline result: Although a borderline range has not been defined, various sources of variability can cause a positive result with SARS-CoV-2 antigen minus nil between 0.35 and 0.7.E3
The IFN-γ measured by IGRA is a signature cytokine for the TH1 subpopulation, while mitogen assays measure a much wider panoply of T-cell–related responses. It is thus expected that only the T cells that can recognize specifically SARS-CoV-2 peptides will produce IFN-γ (∼<1% of the T-cell population).E4
JMP v15 (SAS Institute, Cary, NC) and Microsoft Excel (Microsoft, Redmond, Wash) were used for data analysis and visualization. The chi-square test was performed by JMP v15. Normality of data was determined and established by the Kolmogorov-Smirnov test of normality (https://www.socscistatistics.com/tests/kolmogorov/default.aspx). Two-tailed unpaired t tests were performed by the GraphPad QuickCalcs website (https://www.graphpad.com/quickcalcs/ttest1/?format=C).
Table E1.
Subject characteristics
| Subject no. | Age (years) | Sex | PID diagnosis | ALC | B cells | CD3 T cells | CD4 T cells | CD8 T cells | Immunosuppressant | SARS-CoV-2 spike protein IgG | SARS-CoV-2 ACE2 blocking activity | SARS-CoV-2 IGRA | Baseline IgG | Baseline IgM | Baseline IgA | IgG trough | Genetic information |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | M | Agammaglobulinemia | 980 | 20 | 862 | 666 | 118 | None | Negative | — | Positive | — | 20 | <8 | 738 | PID Invitae panel negative |
| 2 | 30 | M | XLA | 2158 | 0 | 2072 | 928 | 1079 | None | Negative | — | Positive | — | 8 | <8 | 1040 | BTK c.1349+2dup (splice site) |
| 3 | 30 | F | CVID | 1008 | 71 | 796 | 504 | 131 | None | Positive | 50-60% | Positive | 300 | 28.1 | <8 | 876 | PID: VUS in PRKCD and VSP13B |
| 4 | 32 | F | CVID | 467 | 37 | 420 | 266 | 126 | Azathioprine | Negative | — | Positive | — | 26 | 0 | 1127 | Negative WES |
| 5 | 38 | F | CVID | 1788 | 215 | 1570 | 1091 | 411 | Budesonide | Positive | 40-50% | Positive | 335 | 37 | 23 | 977 | PID panel negative |
| 6 | 40 | M | CVID | 1344 | 40 | 981 | 524 | 417 | None | Positive | 40-50% | Positive | 542 | 75 | 90 | 90 | — |
| 7 | 41 | F | CVID | 1566 | 266 | 1237 | 626 | 407 | None | Positive | <10% | Positive | — | — | — | 926 | CVID panel negative |
| 8 | 53 | M | CVID | 774L | 77L | 642L | 317 | 302 | Adalimumab, ustekinumab | Negative | — | Positive | <60 | <11 | <15 | 968 | — |
| 9 | 56 | M | CVID | 3320 | 1162 | 1594 | 1328 | 199 | Tocilizumab | Positive | <10% | Negative | 288 | 15 | <7 | 985 | NOD2 and VUS in ODCK8, JAK3, and TERT |
| 10 | 58 | F | CVID | 1870 | 449 | 1253 | 804 | 411 | None | Positive | <10% | Positive | 361 | 26 | 14 | 818 | — |
| 11 | 59 | M | CVID | 1300 | — | — | — | — | Budesonide, hydrocortisone, vedolizumab | Negative | — | Negative | 132 | 17.4 | 23.3 | 1053 | c.2104C>T (p.Arg702Trp) was identified in NOD2 |
| 12 | 60 | F | CVID | 1887 | 226 | 1189 | 774 | 396 | None | Positive | 30-40% | Positive | 399 | <1 | 32 | 1310 | — |
| 13 | 63 | F | CVID | 1822 | 109 | 1330 | 875 | 474 | Budesonide | Positive | 30-40% | Positive | 413 | 52 | 67 | 1150 | — |
| 14 | 71 | F | CVID | 2982 | 209 | 2117 | 1700 | 388 | None | Positive | 20-30% | Positive | 473 | 33 | 141 | 857 | — |
| 15 | 72 | M | CVID | 2242 | 157 | 1995 | 650 | 1300 | None | Positive | — | Positive | 150 | <5 | <5 | 1020 | — |
| 16 | 73 | F | CVID | 1495 | 194 | 912 | 628 | 254 | Hydroxychloroquine | Positive | <10% | Positive | 377 | 34 | 396 | No Ig therapy | — |
| 17 | 79 | F | CVID | 1066 | 11 | 831 | 725 | 117 | None | Positive | <10% | Negative | 374 | 1110 | 48 | 866 | VUS in RECQL4 |
| 18 | 39 | F | HGG | 947 | 30 | 821 | 442 | 359 | None | Positive | 60-70 | Positive | 658 | 44 | 57 | 916 | — |
| 19 | 55 | F | HGG | 1670 | 117 | 1386 | 1052 | 251 | Hydroxychloroquine, mycophenolate | Negative | — | Positive | 573 | 21 | 115 | 958 | PID panel: VUS FOXP3, ATM, EPG5, AND TTC7A |
| 20 | 67 | F | HGG | 1359 | 82 | 1182 | 761 | 408 | None | Positive | <10% | Positive | 611 | 57 | 79 | 1900 | — |
| 21 | 75 | M | HGG | 1930 | 251 | 1583 | 656 | 965 | None | Negative | — | Negative | 661 | — | — | 1110 | PID panel: VUS ATM |
| 22 | 53 | F | SAD | 1749 | 175 | 1294 | 857 | 367 | None | Positive | 40-50% | Positive | 1080 | 78 | 163 | 1410 | — |
| 23 | 74 | F | SAD | 1600 | 48 | 1312 | 1136 | 176 | None | Positive | 10-20% | Positive | 1130 | 206 | 334 | 1200 | — |
| 24 | 43 | M | GS with HGG | 1579 | 0 | 1,392 | 199 | 1,132 | Everolimus, prednisone | Negative | — | Negative | 259 | 0 | 69 | 754 | — |
| 25 | 65 | F | GS with HGG | 1642 | 0 | 1412 | 755 | 903 | None | Negative | — | Positive | 118 | 22 | 11 | 656 | — |
| 26 | 68 | F | GS with HGG | 858 | 0 | 849 | 223 | 601 | Tacrolimus | Negative | — | Negative | <8 | 705 | <6 | 1034 | — |
| 27 | 70 | F | GS with HGG | 230 | 2 | 177 | 62 | 122 | Cyclosporine, prednisone | Negative | — | Negative | — | — | — | 1203 | — |
| 28 | 39 | M | Hyper IgM syndrome | — | — | — | — | — | None | Negative | — | Positive | — | 301 | 0 | 933 | CD40L: c.491+1G>c (splice donor) |
| 29 | 40 | M | Hyper IgM syndrome | 1340 | 107 | 1179 | 402 | 616 | None | Negative | — | Positive | — | — | — | 854 | CD40L: 530 A>G |
| 30 | 19 | M | CTLA-4 deficiency | 2502 | 500 | 1902 | 1001 | 626 | Sirolimus | Negative | — | Positive | 521 | 31 | 23 | 1030 | CTLA-4: 567+5G>C |
| 31 | 29 | M | PIK3R1 | 795 | 215 | 517 | 231 | 278 | None | Negative | — | — | — | 305 | 0 | 805 | PIK3R1:c.1425+1G.A (splice donor) |
| 32 | 26 | F | Ataxia telangiectasia | 468 | 42 | 295 | 164 | 122 | None | Negative | — | — | 925 | 88 | 45 | 1250 | — |
| 33 | 20 | M | ATP6AP1 gene/immunodeficiency 47 | 1376 | 537 | 743 | 523 | 179 | None | Negative | — | Positive | 40 | 7 | 12 | 708 | ATP6AP1: p.E346K; (c.1036G>A) |
ALC, Absolute lymphocyte count; BTK, Burton tyrosine kinase; CD40L, CD40 ligand; GS, Good syndrome; HGG, hypogammaglobulinemia; VUS, variant of unknown significance; WES, whole exome; XLA, X-linked agammaglobulinemia.
References
- 1.Shields A.M., Burns S.O., Savic S., Richter A.G., UK PIN COVID-19 Consortium COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience. J Allergy Clin Immunol. 2021;147:870–875.e1. doi: 10.1016/j.jaci.2020.12.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley T., Grundberg E., Selvarangan R., LeMaster C., Fraley E., Banerjee D., et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekine T., Perez-Potti A., Rivera-Ballesteros O., Stralin K., Gorin J.B., Olsson A., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delmonte O.M., Bergerson J.R.E., Burbelo P.D., Durkee-Shock J.R., Dobbs K., Bosticardo M., et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J Allergy Clin Immunol. 2021;148:1192–1197. doi: 10.1016/j.jaci.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagin D., Freund T., Navon M., Halperin T., Adir D., Marom R., et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148:739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Squire J., Joshi A. Seroconversion after coronavirus disease 2019 vaccination in patients with immune deficiency. Ann Allergy Asthma Immunol. 2021;127:383–384. doi: 10.1016/j.anai.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roltgen K., Powell A.E., Wirz O.F., Stevens B.A., Hogan C.A., Najeeb J., et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker S.N., Chokkalingam N., Reuschel E.L., Purwar M., Xu Z., Gary E.N., et al. SARS-CoV-2 assays to detect functional antibody responses that block ACE2 recognition in vaccinated animals and infected patients. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01533-20. e01533-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banaei N., Gaur R.L., Pai M. Interferon gamma release assays for latent tuberculosis: what are the sources of variability? J Clin Microbiol. 2016;54:845–850. doi: 10.1128/JCM.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amirthalingam G., Whitaker H., Brooks T., Brown K., Hoschler K., Linley E., et al. Seroprevalence of SARS-CoV-2 among blood donors and changes after introduction of public health and social measures, London, UK. Emerg Infect Dis. 2021;27:1795–1801. doi: 10.3201/eid2707.203167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalakas M.C., Bitzogli K., Alexopoulos H. Anti-SARS-CoV-2 antibodies within IVIg preparations: cross-reactivities with seasonal coronaviruses, natural autoimmunity, and therapeutic implications. Front Immunol. 2021;12:627285. doi: 10.3389/fimmu.2021.627285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farcet MR, Karbiener M, Schwaiger J, Ilk R, Kreil TR. Rapidly increasing SARS-CoV-2 neutralization by intravenous immunoglobulins produced from plasma collected during the 2020 pandemic. J Infect Dis. In press. [DOI] [PMC free article] [PubMed]
- 15.Jin D.K., Nesbitt D.J., Yang J., Chen H., Horowitz J., Jones M., et al. Seroprevalence of anti–SARS-CoV-2 antibodies in a cohort of New York City metro blood donors using multiple SARS-CoV-2 serological assays: implications for controlling the epidemic and “reopening.”. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero C., Diez J.M., Gajardo R. Anti–SARS-CoV-2 antibodies in healthy donor plasma pools and IVIG products. Lancet Infect Dis. 2021;21:765–766. doi: 10.1016/S1473-3099(21)00059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Centers for Disease Control and Prevention COVID-19 vaccines for moderately to severely immunocompromised people. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html Updated October 8, 2021. Available at:
References
- Roltgen K., Powell A.E., Wirz O.F., Stevens B.A., Hogan C.A., Najeeb J., et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan K., Jagannathan P., Pham T.D., Pandey S., Bonilla H.F., Jacobson K., et al. Interferon-gamma release assay for accurate detection of SARS-CoV-2 T cell response. Clin Infect Dis. 2021;73:e3130–e3132. doi: 10.1093/cid/ciaa1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaei N., Gaur R.L., Pai M. Interferon gamma release assays for latent tuberculosis: what are the sources of variability? J Clin Microbiol. 2016;54:845–850. doi: 10.1128/JCM.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., et al. SARS-CoV-2–reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]