Rheumatology key message
COVID-19 should be considered a trigger for treatable autoimmune dermatomyositis with type I interferonopathy.
Dear Editor, A proportion of patients with coronavirus disease 2019 (COVID-19) could develop several myopathic manifestations, including new-onset inflammatory myopathy. Here we describe the first case of anti-nuclear matrix protein 2 (NXP2) antibody-positive DM that developed soon after COVID-19, in which type I interferon-mediated organ toxicity was observed both in cutaneous and muscle specimens.
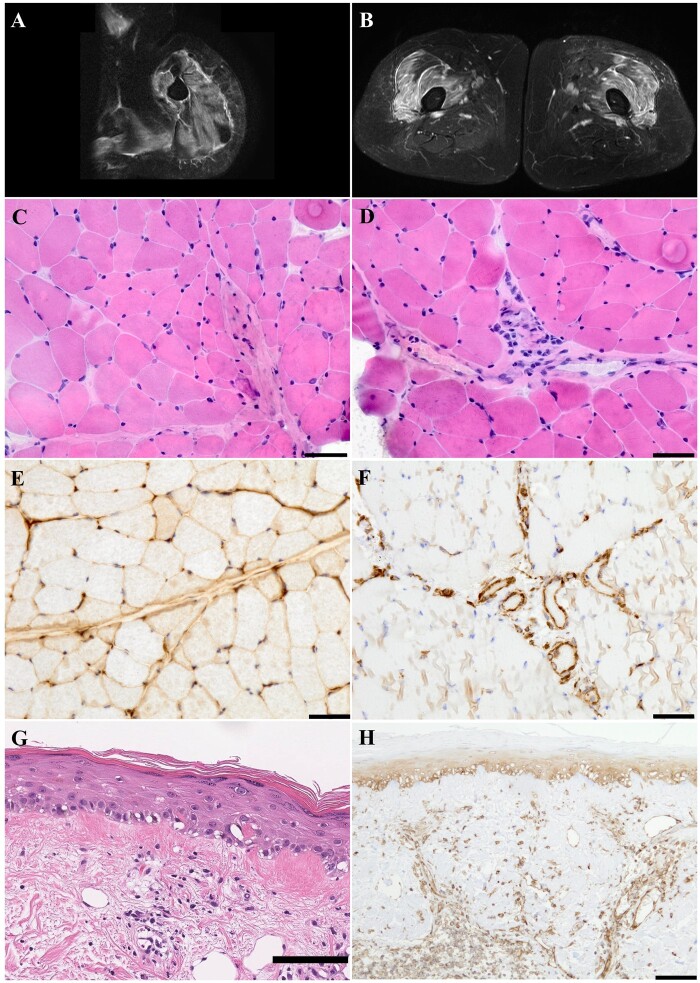
A 64-year-old woman had a 39°C fever without respiratory symptoms for 2 days and tested positive for severe acute respiratory syndrome coronavirus 2 on a real-time polymerase chain reaction test. A month after defervescence, she noticed rashes around the ears and on the face and felt myalgia around the thighs. Another month later, muscle weakness in her neck and arms gradually worsened. On admission, she was found to have itchy erythematous lesions on her forehead, bilateral ears, scalp, and neck, lacking typical heliotrope rash or Gottron’s sign. Periungual telangiectasias were also observed. Subcutaneous oedema was present in the face and proximal limbs. She had muscle weakness of the neck extensor 4, deltoid 4/4-, iliopsoas 3/4, and quadriceps 4/5 based on the Medical Research Council scale. Laboratory tests showed elevated serum creatine kinase (1495 U/l) and D-dimer levels (6.1 µg/ml). Myositis-specific autoantibody for NXP2 was detected by immunoprecipitation and western blotting, but autoantibodies for aminoacyl tRNA synthetase, Mi-2, transcription intermediary factor 1-gamma, and melanoma differentiation-associated gene 5 were not detected. Needle electromyography revealed myopathic state in the right biceps. No malignancy nor interstitial lung disease was detected in systemic computed tomography. Magnetic resonance imaging showed intramuscular hyperintensity on short inversion-time recovery images in the proximal limbs (Fig. 1A and B).
Fig. 1.
Muscle MRI and histopathology of the patient
Axial T2-weighted short tau inversion recovery images of the left upper arm (A) and thigh (B). High signals were diffusely evident in the muscles and fascia of the deltoid, biceps brachii, triceps brachii (A), vastus lateralis and intermedius (B). Sections of the left biceps brachii muscle frozen (C–E) as well as paraffin-embedded (F) and right temporal cutaneous paraffin-embedded sections (G, H) are shown.
Hematoxylin–eosin (HE) staining showed mild perifascicular atrophy (C) and mononuclear cell infiltration around the perimysial vessels (D). Major histocompatibility complex class I was predominantly expressed on the perifascicular sarcolemma (E). Myxovirus resistance A (MxA) was positively stained on the endothelial cells in the perimysial vessels and endomysial capillaries (F). Cutaneous HE staining showed vacuolar interface changes in the dermoepidermal boundary and mononuclear cell infiltration in the superficial dermis (G). MxA was positively stained on the epidermis below the granular layer and the endothelial cells in the dermal vessels (H). Bars = 50 μm (C–F) and 100 μm (G, H).
A biopsy of the left biceps brachii muscle showed mild perifascicular atrophy and perivascular cell infiltration in the perimysium (Fig. 1C and D). On immunostaining, major histocompatibility complex class I was predominantly expressed on the perifascicular sarcolemma (Fig. 1E). Myxovirus resistance A (MxA) was stained positive on the endothelial cells in the perimysial vessels and endomysial capillaries (Fig. 1F). Cutaneous biopsy of the temporal and neck regions revealed vacuolar interface changes in the dermoepidermal boundary, perivascular cell infiltration in the superficial dermis, and mucin deposition and oedema in the dermal reticular layer. MxA was stained positive on the epidermis below the granular layer and dermal vessels (Fig. 1G and H).
Treatment was initiated with a 3-day course of 1000 mg intravenous methylprednisolone followed by oral prednisolone at a dose of 1 mg/kg/day (60 mg/day). After 2 weeks, muscle weakness, erythema and oedema subsided, and serum creatine kinase levels returned to normal. Four months after the therapeutic intervention (tapered prednisolone 25 mg/day and azathioprine 50 mg), muscle weakness of most of the affected muscles improved, except that of the neck extensor 4+ and deltoid 4+/4+, according to the Medical Research Council scale, without apparent disease progression.
To the best of our knowledge, this is the first case of COVID-19-related anti-NXP2 antibody-positive DM. We reviewed four cases of COVID-19-associated DM, including the present case and those reported until July 2021 [1–3]. Among these, only one case had myopathic symptoms simultaneously with COVID-19 [1], whereas the present and other two cases [2, 3] presented cutaneous manifestation simultaneously to 1 month after COVID-19. The manifestation of COVID-19 varied from pyrexia alone to mild respiratory symptoms, and neither was severe enough to require mechanical ventilation. The four cases demonstrated some characteristic features of DM, such as dermatological findings (observed in the present and other two cases [2, 3]); myositis-specific autoantibodies, including anti-NXP2 antibody (detected in the present case), anti- SUMO-1 activating enzyme A antibody [1] and anti-Mi-2 antibody [2]; and histologically positive MxA staining (observed in the present case). All cases that received intravenous methylprednisolone showed an early favourable response.
COVID-19-associated myositis has been reported to be DM due to the common pathogenesis of type I interferon-mediated organ toxicity [4]. MxA is a type I interferon-inducible protein, and its overexpression has been reported in COVID-19-associated myositis [5] as well as autoimmune DM, including anti-NXP2 antibody-positive cases [6]. Considering the delayed onset after COVID-19 and autoantibody positivity in our patient, we postulate that type I interferonopathy played a significant role in the development of her autoimmune DM.
It is unknown whether COVID-19 is associated with the production of anti-NXP2 antibody or its bystander development of autoimmune disease. NXP2 plays important roles in diverse nuclear functions, including RNA metabolism and nuclear architecture maintenance [7]. The expression of NXP2 may be increased and partially localized to the cytoplasm in cases of viral infection [8], may be linked to COVID-19, and may result in an autoimmune response. Further immunomolecular analysis will be required to clarify the exact mechanism of autoantibody production and the role of COVID-19 in the development of DM.
In conclusion, COVID-19 should be considered a possible trigger for autoimmune DM, even in cases with mild respiratory symptoms and delayed-onset myositis.
Acknowledgements
We thank the patient and her family. We are grateful to Mai Kakinuma and Yayoi Aoyama for their technical assistance. The authors would also like to thank Enago (www.enago.jp) for the English language review.
Funding: This work was supported by an Intramural Research Grant (2–5) for Neurological and Psychiatric Disorders of NCNP, Grant-in-Aid for challenging Exploratory Research (20K21563), JSPS KAKENHI Grant Number JP20K16571, and Research on Measures for Intractable Diseases from the Japanese Ministry of Health Labor and Welfare (20FC1036).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Zhang H, Charmchi Z, Seidman RJ et al. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve 2020;62:E57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borges NH, Godoy TM, Kahlow BS. Onset of dermatomyositis in close association with COVID-19 - a first case reported. Rheumatology 2021;60:SI96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ho BVK, Seger EW, Kollmann K, Rajpara A. Dermatomyositis in a COVID-19 positive patient. JAAD Case Rep 2021;13:97–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanboon J, Nishino I. COVID-19-associated myositis may be dermatomyositis. Muscle Nerve 2021;63:E9–10. [DOI] [PubMed] [Google Scholar]
- 5. Manzano GS, Woods JK, Amato AA. Covid-19-associated myopathy caused by type I interferonopathy. N Engl J Med 2020;383:2389–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Zheng Y, Gang Q et al. Perimysial microarteriopathy in dermatomyositis with anti-nuclear matrix protein-2 antibodies. Eur J Neurol 2020;27:514–21. [DOI] [PubMed] [Google Scholar]
- 7. Kimura Y, Sakai F, Nakano O et al. The newly identified human nuclear protein NXP-2 possesses three distinct domains, the nuclear matrix-binding, RNA-binding, and coiled-coil domains. J Biol Chem 2002;277:20611–7. [DOI] [PubMed] [Google Scholar]
- 8. Ver LS, Marcos-Villar L, Landeras-Bueno S, Nieto A, Ortín J. The cellular factor NXP2/MORC3 is a positive regulator of influenza virus multiplication. J Virol 2015;89:10023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]