Abstract
Background
Tocilizumab, an interleukin 6 receptor (IL-6R) antagonist monoclonal antibody, has shown efficacy in patients with coronavirus disease 2019 (COVID-19) pneumonia, but the optimal dose is unknown.
Methods
Patients hospitalized for moderate to severe COVID-19 pneumonia were randomized 1:1 to receive standard of care treatment and 1–2 doses of intravenous tocilizumab 4 mg/kg or 8 mg/kg (open-label). Primary pharmacokinetic and pharmacodynamic end points were serum concentrations of tocilizumab and soluble interleukin 6 receptor (sIL-6R), IL-6, ferritin, and C-reactive protein (CRP), from baseline to day 60. The secondary end point was safety. Key exploratory efficacy end points included clinical status, time to discharge, mortality rate, and incidence of mechanical ventilation.
Results
Of 100 patients randomized, 49 received tocilizumab 4 mg/kg and 48 received 8 mg/kg. In pharmacokinetic and sIL-6R assessments, dose-dependent differences were seen in patients who received 1 or 2 doses of 4 or 8 mg/kg. Serum concentrations of IL-6, ferritin, and CRP and safety outcomes were comparable between groups. Through day 60, serious adverse events were reported in 30.6% and 25.0% of patients in the 4- and 8-mg/kg groups, respectively. Eight patients (16.3%) in the 4-mg/kg group and 6 (12.5%) in the 8-mg/kg group died. Exploratory time-to-event outcomes favored 8 mg/kg within the first 2 weeks.
Conclusions
In patients with moderate to severe COVID-19 pneumonia who received tocilizumab 4 or 8 mg/kg, pharmacokinetic and sIL-6R assessments showed expected dose-dependent effects; pharmacodynamic assessments and safety were comparable, with no new safety signals. Further study is required before a lower dose of tocilizumab can be recommended in patients with COVID-19 pneumonia.
Clinical Trials Registration
Keywords: COVID-19, pharmacodynamics, pharmacokinetics, safety, tocilizumab
In this randomized trial of 97 patients hospitalized for moderate to severe COVID-19 pneumonia who received tocilizumab 4 or 8 mg/kg, pharmacokinetic and soluble IL-6 receptor assessments showed expected dose-dependent effects of tocilizumab. Pharmacodynamic assessments and safety outcomes were comparable between groups.
Coronavirus disease 2019 (COVID-19) has taken an immense toll on human health, with >250 million confirmed cases and >5 million deaths globally [1]. Approximately 20% of patients with COVID-19 experience complications related to severe pneumonia [2], which can progress to acute respiratory distress syndrome and death [3–6]. Elevated levels of proinflammatory cytokines and mediators, such as interleukin 6 (IL-6), C-reactive protein (CRP), and ferritin, are associated with poor clinical outcomes [5, 7–10], which prompted observational studies and clinical trials investigating the safety and efficacy of immunomodulatory treatments early in the pandemic [11, 12].
Tocilizumab is an IL-6 receptor (IL-6R) antagonist monoclonal antibody approved for use in inflammatory diseases, including rheumatoid arthritis and cytokine release syndrome associated with chimeric antigen receptor T-cell therapy [13–15]. Results from randomized controlled trials in patients with rheumatoid arthritis have shown efficacy of tocilizumab at 4- and 8-mg/kg doses [16–19]. Based on resolution of symptoms, tocilizumab was approved at an 8-mg/kg dose for cytokine release syndrome induced by chimeric antigen receptor T-cell therapy, although no formal dose-finding studies were performed [14, 20].
Results from randomized clinical trials of tocilizumab in patients with COVID-19 have been discordant, possibly due to differences in patient populations, coadministered treatments, treatment timing, or the evolving standard of care (SOC) [11]. Most randomized trials and observational studies investigating tocilizumab in patients with COVID-19 pneumonia have evaluated the 8-mg/kg dose [21–28]; however, other studies have shown potential benefits of doses of ≤4 mg/kg [29–31]. In June 2021, the US Food and Drug Administration authorized the emergency use of tocilizumab (8 mg/kg for patients >30 kg) for the treatment of COVID-19 in hospitalized patients receiving systemic corticosteroids and requiring supplemental oxygen, noninvasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) [32].
This phase 2 clinical trial (MARIPOSA) investigated the pharmacokinetics, pharmacodynamics, safety, and efficacy of intravenous tocilizumab at 4- and 8-mg/kg doses in combination with SOC treatment in hospitalized patients for moderate to severe COVID-19 pneumonia.
METHODS
Study Design and Patient Population
This phase 2, open-label, randomized study was conducted at 26 sites in the United States.
We included patients ≥18 years old hospitalized for moderate to severe COVID-19 pneumonia detected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–positive reverse-transcription polymerase chain reaction (RT-PCR) (within 7 days before randomization) and confirmed by chest radiography or computed tomography scan. Severe COVID-19 pneumonia was defined as patients having blood oxygen saturation of ≤93% or partial pressure of oxygen to fraction of inspired oxygen ratio of <300 mm Hg. Patients with moderate pneumonia did not meet the oxygen requirements of severe pneumonia but had evidence of inflammation as indicated by CRP levels >2× the upper limit of normal (ULN). All patients received SOC treatment per local practice, which may have included antiviral treatment, low-dose corticosteroids, and supportive care. Patients who were on invasive ventilation >24 hours, on ECMO, in shock, or with multiorgan failure requiring treatment in an intensive care unit were excluded.
Patient Consent Statement
All patients or their legally authorized representatives gave written or oral informed consent. This study was conducted in accordance with the International Council for Harmonisation E6 guideline for good clinical practice and the Declaration of Helsinki or local regulations, whichever afforded greater patient protection. This trial was approved by trial sites through a central institutional review board or local institutional review board or ethics committee at the study site.
Randomization and Treatments
Patients were randomized 1:1 through permuted-block randomization to receive SOC and 1 dose of tocilizumab 4 or 8 mg/kg (maximum 800 mg) intravenously. An interactive voice or web-based response system provided sites with treatment assignments. Randomization was stratified by pneumonia severity (moderate or severe). An additional infusion (same as initial dose) could be administered 8 to 24 hours after the first if a patient had a sustained fever or clinically significant worsening of signs or symptoms, such as an increased supplemental oxygen requirement.
Outcomes and Assessments
The pharmacokinetic outcomes were serum concentrations of tocilizumab at baseline and days 1, 2, 3, 7, 14, 21, 28, 35, and 60 following administration of tocilizumab 4 or 8 mg/kg. Timing of sampling on day 1 and relative to second infusion are provided in the Supplementary Methods. Tocilizumab concentrations in serum samples were measured by enzyme-linked immunosorbent assay performed at a central laboratory (QPS Netherlands).
The pharmacodynamic outcomes were serum concentrations of soluble IL-6 receptor (sIL-6R), IL-6, ferritin, and CRP at the same time points as above following administration of tocilizumab 4 or 8 mg/kg. The sIL-6R and IL-6 levels were measured using immunoassays validated at QPS. Ferritin was assessed using standard clinical chemistry at local clinical laboratories. CRP was measured using an in vitro diagnostic method validated at PPD, Inc (cobas; Roche Diagnostics).
Key safety outcomes included (1) incidence and severity of adverse events (AEs), with investigator text encoded using the preferred terms in the Medical Dictionary for Regulatory Activities, version 23.0, and severity determined according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0; (2) SARS-CoV-2 viral load over time in nasopharyngeal swab and bronchoalveolar lavage samples (if applicable); (3) time to RT-PCR virus negativity; (4) proportion of patients with any posttreatment infection (as reported by investigator) other than COVID-19; and (5) change from baseline in targeted clinical laboratory test results.
Key exploratory efficacy outcomes included (1) clinical status, as assessed using an ordinal scale ranging from 1 (discharged or ready for discharge) to 7 (death) (Supplementary Table 1) at days 14 and 28; (2) time to hospital discharge or “ready for discharge” (as evidenced by normal body temperature and respiratory rate and stable oxygen saturation on ambient air or ≤2 L of supplemental oxygen) by day 28; (3) mortality rate by day 28; and (4) incidence of mechanical ventilation up to day 28.
Statistical Methods
In healthy volunteers and patients with rheumatoid arthritis, 20–30 individuals per treatment arm was adequate to study between-participant and within-participant pharmacokinetic variability [33]. However, the variability of pharmacokinetic parameters in a heterogeneous population of patients with COVID-19 was unknown; therefore, 50 patients per treatment arm were enrolled. The modified intention-to-treat (mITT) population included all patients who underwent randomization and received either dose of tocilizumab.
The pharmacokinetic analysis population consisted of patients with ≥1 analyzed sample contributing toward the estimation of key parameters. The serum pharmacokinetics of tocilizumab were summarized by estimating the total exposure (area under the curve), maximum concentration, total clearance, and volume of distribution.
Safety assessments were completed for all patients who received any amount of tocilizumab. Efficacy outcomes were exploratory and were evaluated in the mITT population. Time to hospital discharge was compared between the 2 doses using proportional hazards models stratified by moderate vs severe COVID-19 pneumonia. Cause-specific hazards are reported with death as a competing risk. The Cochran-Mantel-Haenszel test, stratified by COVID-19 severity at baseline, was used to assess the between-group difference in mortality and incidence of mechanical ventilation by day 28. Description of subgroup analysis of mortality is provided in the Supplementary Methods.
RESULTS
Patient Disposition and Baseline Characteristics
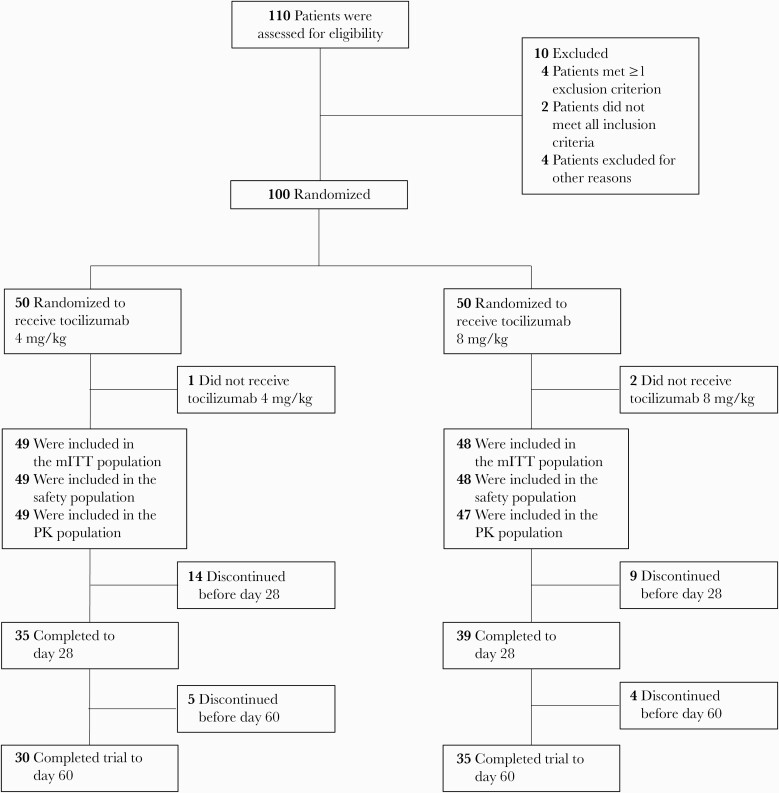
A total of 100 patients were randomized; 3 patients did not receive tocilizumab. In the mITT and safety populations, 49 patients received tocilizumab 4 mg/kg plus SOC (1 dose, n = 37; 2 doses, n = 12) and 48 patients received tocilizumab 8 mg/kg plus SOC (1 dose, n = 39, 2 doses, n = 9; Figure 1). Median time between first and second dose was 18.8 and 19.8 hours in the 4- and 8-mg/kg groups, respectively (Supplementary Table 2). In the 4- and 8-mg/kg groups, 11 patients (22.4%) and 9 patients (18.8%) had moderate disease, respectively, and 38 (77.6%) and 39 (81.3%) patients had severe disease.
Figure 1.
Study flowchart. Abbreviations: mITT, modified intention-to-treat; PK, pharmacokinetic.
The baseline demographic data were generally comparable between groups, with some differences observed for age and race (Table 1). The mean age was 56.8 (standard deviation [SD], 14.3) years in the 4-mg/kg group and 59.8 (SD, 14.6) years in the 8-mg/kg group. In the 4- and 8-mg/kg groups, 20 (40.8%) and 13 (27.1%) patients, respectively, were Black or African American and 15 (30.6%) and 23 (47.9%), respectively, were White. Overall, 11 patients (22.4%) in the 4-mg/kg group and 15 (31.3%) in the 8-mg/kg group were Hispanic or Latino. At baseline, the proportions of patients receiving corticosteroid and all antiviral treatments were comparable between the 4- and 8-mg/kg groups (11 [22.4%] vs 11 [22.9%] and 25 [51.0%] vs 19 [39.6%], respectively). The proportion of patients with previous remdesivir use within 7 days of baseline was comparable between the 4- and 8-mg/kg groups (12 [24.5%] vs 11 [22.9%], respectively; Supplementary Table 3). Postbaseline, differences were observed in the proportion of patients receiving concomitant systemic corticosteroids (12 [24.5%] and 16 [33.3%] in the 4- and 8-mg/kg groups, respectively) and remdesivir (25 [51.0%] and 20 [41.7%], in the 4- and 8-mg/kg groups, respectively).
Table 1.
Baseline Patient Demographics and Disease Characteristics in the Modified Intention-to-Treat Population
| Characteristic | Tocilizumab 4 mg/kg (n = 49) | Tocilizumab 8 mg/kg (n = 48) |
|---|---|---|
| Male sex, No. (%) | 27 (55.1) | 30 (62.5) |
| Age, y, mean (SD) | 56.8 (14.3) | 59.8 (14.6) |
| Weight, kg, mean (SD) | 88.45 (22.6) | 93.95 (27.4) |
| Ethnicity, No. (%)a | ||
| Hispanic or Latino | 11 (22.4) | 15 (31.3) |
| Not Hispanic or Latino | 35 (71.4) | 32 (66.7) |
| Not stated | 3 (6.1) | 0 (0) |
| Unknown | 0 (0) | 1 (2.1) |
| Race, No. (%)a | ||
| American Indian or Alaska Native | 2 (4.1) | 1 (2.1) |
| Asian | 3 (6.1) | 1 (2.1) |
| Black or African American | 20 (40.8) | 13 (27.1) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (2.1) |
| White | 15 (30.6) | 23 (47.9) |
| Multiple | 1 (2.0) | 0 (0) |
| Unknown | 8 (16.3) | 9 (18.8) |
| Smoking history, No. (%) | ||
| Never | 30 (61.2) | 34 (70.8) |
| Current | 3 (6.1) | 2 (4.2) |
| Former | 16 (32.7) | 12 (25.0) |
| Ordinal scale for clinical status, No. (%)b | ||
| 1 | 0 | 1 (2.1) |
| 2 | 8 (16.3) | 5 (10.4) |
| 3 | 16 (32.7) | 11 (22.9) |
| 4 | 20 (40.8) | 24 (50.0) |
| 5 | 5 (10.2) | 4 (8.3) |
| 6 | 0 | 3 (6.3) |
| Disease severity (stratification), No. (%) | ||
| Moderate | 11 (22.4) | 9 (18.8) |
| Severe | 38 (77.6) | 39 (81.3) |
| Corticosteroid use, No. (%) | 11 (22.4) | 11 (22.9) |
| Antiviral treatment, No. (%)c | 25 (51.0) | 19 (39.6) |
| CRP level | ||
| No. of patients | 45 | 42 |
| Median (range), mg/L | 146.6 (5.5–428.2) | 157.2 (4.7–438.2) |
| Ferritin level | ||
| No. of patients | 42 | 38 |
| Median (range), pmol/L | 2013.3 (123.6–30 013.2) | 1958.3 (51.7–21 768.9) |
| IL-6 level | ||
| No. of patients | 41 | 42 |
| Median (range), ng/L | 64.2 (0.0–1820.0) | 68.0 (0.0–2540.0) |
| sIL-6R level | ||
| No. of patients | 45 | 43 |
| Median (range), ng/L | 37 900.0 (17 500.0–69 400.0) | 35 300.0 (18 700.0–60 000.0) |
| Days from first COVID-19 symptom at baseline, median (range) | 8.0 (1.0–20.0) | 9.0 (3.0–68.0) |
| Days from COVID-19 diagnosis, median (range) | 2.0 (0.0–14.0) | 3.0 (0.0–13.0) |
Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; IL, interleukin 6; SD, standard deviation; sIL-6R, soluble interleukin 6 receptor.
Self-reported.
Seven-category ordinal scale: 1, discharged (or ready for discharge as evidenced by normal body temperature and respiratory rate and stable oxygen saturation on ambient air or ≤2 L of supplemental oxygen); 2, non–intensive care unit (ICU) hospital ward (or ready for hospital ward), not requiring supplemental oxygen; 3, non-ICU hospital ward (or ready for hospital ward), requiring supplemental oxygen; 4, ICU or non-ICU hospital ward, requiring noninvasive ventilation or high-flow oxygen; 5, ICU, requiring intubation and mechanical ventilation; 6, ICU, requiring extracorporeal membrane oxygenation or mechanical ventilation and additional organ support; 7, death.
Antiviral treatments included lopinavir-ritonavir, remdesivir, lopinavir, ritonavir, chloroquine, hydroxychloroquine, and hydroxychloroquine sulfate.
Pharmacokinetic Outcomes
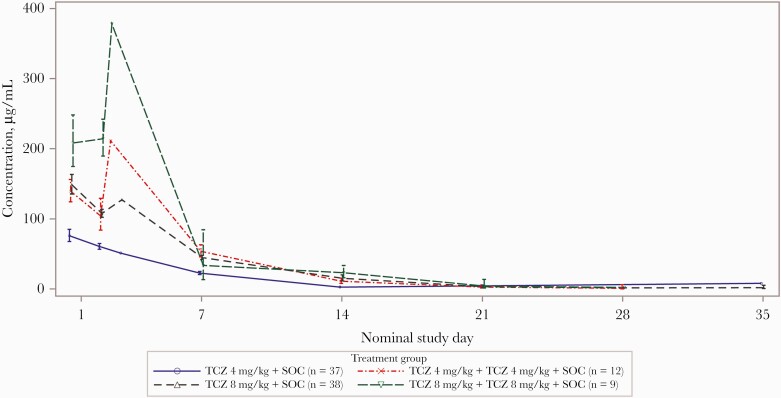
The pharmacokinetic population included 49 patients who received tocilizumab 4 mg/kg and 47 who received tocilizumab 8 mg/kg. Serum tocilizumab concentrations peaked following the end of each infusion and then declined; concentration-time profiles in patients who received two 4-mg/kg doses of tocilizumab were similar to those in patients who received one 8-mg/kg dose (Figure 2).
Figure 2.
Serum tocilizumab concentration over time by 4-mg/kg (1 or 2 doses) vs 8-mg/kg (1 or 2 doses) groups in the pharmacokinetic population. Data points around the nominal time of 1 and 2 days included pre– and post–second infusion measurements for patients receiving a second infusion. Values below the limit of quantitation were set to 0 prior to maximum concentration and missing thereafter. Abbreviations: SOC, standard of care; TCZ, tocilizumab.
Population pharmacokinetic estimates of tocilizumab maximum concentration and area under the curve between days 0 and 28 increased with increasing dose, with patients who received 2 doses of tocilizumab 4 mg/kg having values in the same range as those in patients who received 1 dose of 8 mg/kg (Supplementary Table 4). Linear clearance estimates were similar for 1 and 2 doses of tocilizumab 4 mg/kg and for 1 dose of tocilizumab 8 mg/kg but showed a 26% increase in the geometric mean when 8 mg/kg was administered twice compared with a single administration. The volume of the central compartment was similar for all dose groups.
Pharmacodynamic Outcomes
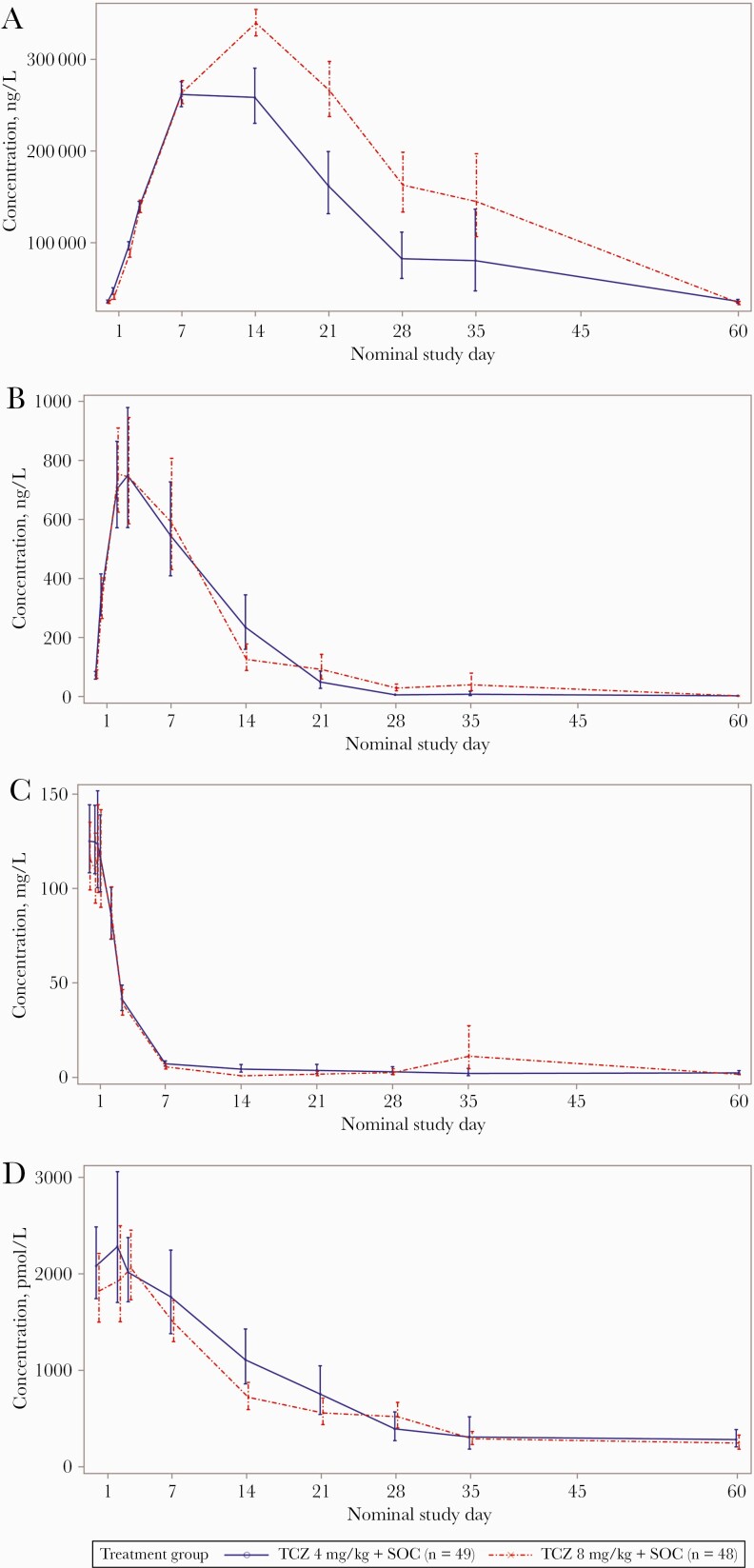
Serum sIL-6R concentrations increased immediately following tocilizumab administration, with a similar rate of sIL-6R–tocilizumab complex production up to day 7 in both groups (Figure 3A). Maximum geometric mean serum sIL-6R concentrations were recorded on day 7 for the 4-mg/kg group and on day 14 for the 8-mg/kg group. Geometric mean serum sIL-6R concentrations were lower in the 4-mg/kg group than in the 8-mg/kg group between days 14 and 35. Interpatient variability was low to moderate over time in both dose groups (Figure 3A).
Figure 3.
Pharmacodynamics of soluble interleukin 6 receptor (A), interleukin 6 (B), C-reactive protein (C), and ferritin (D) in the modified intention-to-treat population. C-reactive protein and ferritin levels presented were from a central laboratory (QPS Netherlands). Values below the limit of quantitation were set to 0 predose and 0.5 times the lower limit of quantitation thereafter. Day 21 samples for 1 patient were collected on actual day 27. Day 60 was assumed as any study completion visit after day 50. Abbreviations: SOC, standard of care; TCZ, tocilizumab.
Serum IL-6 concentrations increased immediately following the tocilizumab infusion, peaking at day 3 for both doses. No notable differences were observed between the 2 doses in the IL-6 serum concentration-time profiles up to day 60 (Figure 3B).
Geometric mean serum CRP concentrations were above the ULN at baseline in both groups. Following tocilizumab administration, the geometric mean concentrations decreased rapidly from baseline to below the ULN at day 7 and remained close to or below the ULN from day 7 to day 60. No notable differences were observed between the 2 doses in the CRP concentration-time profiles up to day 60 (Figure 3C).
Geometric mean serum ferritin concentrations were above the ULN at baseline. Following tocilizumab administration, a marked decrease in serum ferritin concentrations was observed in both groups from approximately day 7 to day 35, with the geometric mean concentrations close to normal range by day 21 (Figure 3D).
Safety Outcomes
Adverse events through day 60 occurred in 57.1% of patients in the 4-mg/kg group and 45.8% in the 8-mg/kg group (Table 2). Serious AEs were reported in 30.6% and 25.0% of patients in the 4- and 8-mg/kg groups, respectively. Eight patients (16.3%) in the 4-mg/kg group and 6 (12.5%) in the 8-mg/kg group died (Table 2 and Supplementary Table 5). Infections were the most common AE by System Organ Class and of special interest (Supplementary Table 6). In the 4-mg/kg group, 10 posttreatment infections were observed in 8 patients (16.3%); in the 8-mg/kg group, 6 posttreatment infections were observed in 5 patients (10.4%) (Table 2). The majority of posttreatment infections were reported as serious; 4 patients (8.2%) experienced 5 serious infections in the 4-mg/kg group, and 4 (8.3%) experienced 4 serious infections in the 8-mg/kg group. The most frequently reported serious infections were events related to sepsis (5 patients [including septic shock and sepsis]) and pneumonia (2 patients). Results of targeted clinical laboratory results, SARS-CoV-2 viral load over time, and time to RT-PCR virus negativity are described in the Supplementary Results.
Table 2.
Adverse Eventsa Through Day 60 in the Safety Population
| Characteristic | Tocilizumab 4 mg/kg (n = 49) | Tocilizumab 8 mg/kg (n = 48) | All Patients (N = 97) |
|---|---|---|---|
| Any AE | |||
| Patients with ≥1 event | 28 (57.1) | 22 (45.8) | 50 (51.5) |
| Total No. of AEs | 63 | 64 | 127 |
| Patients with ≥1 related AE | 2 (4.1) | 1 (2.1) | 3 (3.1) |
| Deaths | 8 (16.3) | 6 (12.5) | 14 (14.4) |
| No. of patients who discontinued the study due to an AE | 0 | 0 | 0 |
| Patients with ≥1 of the following: | |||
| SAE | 15 (30.6) | 12 (25.0) | 27 (27.8) |
| Related SAE | 0 (0) | 1 (2.1) | 1 (1.0) |
| Grade 3–5 event | 16 (32.7) | 13 (27.1) | 29 (29.9) |
| Infection | 8 (16.3) | 5 (10.4) | 13 (13.4) |
| Total No. of events | 10 | 6 | 16 |
| Serious infection | 4 (8.2) | 4 (8.3) | 8 (8.2) |
| Total No. of events | 5 | 4 | 9 |
| Events with incidence of >2% in either group | |||
| Septic shock | 1 (2.0) | 2 (4.2) | 3 (3.1) |
| Pneumonia | 1 (2.0) | 1 (2.1) | 2 (2.1) |
| Sepsis | 1 (2.0) | 1 (2.1) | 2 (2.1) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AE, adverse event; SAE, serious adverse event.
Investigator text for AEs was encoded using the preferred terms in the Medical Dictionary for Regulatory Activities, version 23.0. For frequency counts by preferred term, multiple occurrences of the same AE in an individual were counted only once. For total number of events, multiple occurrences of the same AE in an individual were counted separately.
Exploratory Efficacy Outcomes
No formal statistical comparisons between the 4- and 8-mg/kg groups were conducted for exploratory efficacy outcomes because the main objectives were pharmacokinetic and pharmacodynamic outcomes; at the beginning of the pandemic, there was insufficient information to design a noninferiority trial to compare efficacy of 4 and 8 mg/kg. In the analysis of clinical status, the 7-category ordinal scale distribution favored the 8-mg/kg group from day 2 until day 7, but there were no differences between the groups at day 28 (Supplementary Table 7 and Supplementary Figure 1). A trend for increased progression toward worse clinical status (categories 5 and 6) and death (category 7) was seen up to day 14 for the 4-mg/kg vs 8-mg/kg group, followed by no notable difference between groups at days 21 and 28.
Overall, 39 patients (79.6%) in the 4-mg/kg group and 38 patients (79.2%) in the 8-mg/kg group were discharged or ready to be discharged by day 28, as assessed by hospital records (Supplementary Table 7). In the cause-specific analysis of the time to hospital discharge, the hazard ratio (HR) for hospital discharge favored the 8-mg/kg group (HR, 0.876 [95% confidence interval {CI}, .55–1.40]). The cumulative incidence functions over time for hospital discharge (ordinal scale category 1) indicated a potential benefit in patients with severe COVID-19 pneumonia in the 8-mg/kg group at day 7 (21.1% vs 41.0% in the 4- and 8-mg/kg groups, respectively; Supplementary Figure 2).
The mortality rate at day 28 was higher in the 4- vs 8-mg/kg group (7 patients [14.3%] vs 5 [10.4%], respectively); the weighted difference between the treatment groups was –4.5% (95% CI, –18.2% to 9.2%) (Supplementary Table 7). None of the subgroups showed a clear difference between the 2 treatment groups in the odds ratio for mortality at day 28 (Supplementary Figure 3).
In both groups, the proportion of patients on mechanical ventilation (or ECMO) increased from baseline until day 7 and then declined due to clinical improvement. The incidence of mechanical ventilation to day 28, including mechanical ventilation ongoing at baseline, was 14 patients (28.6%) in the 4-mg/kg group and 15 (31.3%) in the 8-mg/kg group (Supplementary Table 7); the weighted difference was 1.5% (95% CI, –16.4% to 19.5%). In the patients who were not on mechanical ventilation at baseline, the incidence of new mechanical ventilation events to day 28 was 10 patients (22.2%) in the 4-mg/kg group and 8 (19.5%) in the 8-mg/kg group (Supplementary Table 7); the weighted difference was –3.2% (95% CI, –20.6% to 14.1%).
DISCUSSION
In this randomized phase 2 trial of hospitalized patients with moderate to severe COVID-19 pneumonia who received tocilizumab 4 or 8 mg/kg, pharmacokinetic and sIL-6R assessments showed expected dose-dependent effects. Serum sIL-6R concentrations were lower between days 14 and 35 in the 4-mg/kg group compared with the 8-mg/kg group. Similarly, there was a higher clearance of tocilizumab serum concentrations observed in the 8-mg/kg than in the 4-mg/kg group. This difference between the 2 doses is possibly related to an unbalanced distribution of covariates known to have a positive impact on tocilizumab clearance, including body weight [33], with a higher mean body weight in the 8-mg/kg group than the 4-mg/kg group.
Serum sIL-6R concentrations were lower between days 14 and 35 in the 4-mg/kg group than in the 8-mg/kg group; as expected, the duration of target engagement was longer for the 8-mg/kg dose than the 4-mg/kg dose. Otherwise, pharmacodynamic assessments revealed no clear differences between the tocilizumab doses in serum IL-6, CRP, or ferritin concentrations up to day 60.
The safety of tocilizumab 4 mg/kg was comparable to that of 8 mg/kg. No new safety signals were identified. Results of the present MARIPOSA trial are consistent with those of other randomized trials of tocilizumab in COVID-19 [21–28]. Overall mortality rates and frequency of serious AEs in this trial were lower than those in the COVACTA trial [23] but higher than those in the EMPACTA trial [24], possibly due to differences in patient populations and improvements in SOC. Patients enrolled in the COVACTA trial [23] had baseline disease severity ranging from moderate hypoxia to requiring mechanical ventilation and/or ECMO, whereas patients enrolled in the EMPACTA trial [24] were at an earlier disease stage and not receiving noninvasive or invasive mechanical ventilation. In the MARIPOSA trial, patients receiving noninvasive or invasive mechanical ventilation were included, with approximately 10% receiving invasive ventilation. However, patients on invasive ventilation >24 hours, on ECMO, in shock, or with other organ failure were excluded. SOC evolved between the COVACTA, MARIPOSA and EMPACTA trials, with 73% of patients in the EMPACTA trial receiving corticosteroids at baseline [24] compared with 22% of COVACTA patients [23] and 22% of patients in the MARIPOSA trial. The proportion of patients receiving antiviral agents (including remdesivir) was approximately 25% in COVACTA at baseline [23], whereas approximately 45% of patients received antivirals in MARIPOSA and 79% received antivirals in EMPACTA [24]. Finally, in contrast with the global COVACTA and EMPACTA trials, the MARIPOSA trial included only patients in the United States. In the COVACTA, EMPACTA, and MARIPOSA trials, septic shock was the most common serious infection (other than COVID-19 pneumonia), with similar rates in each trial (2.4%, 2.0%, and 3.1%, respectively) [23, 24].
In exploratory efficacy analyses, the 7-category ordinal scale distribution favored the 8-mg/kg group from day 2 until 7, but the significance of this finding is unclear because there was no difference between groups at later time points. The HRs for time to discharge, 28-day mortality rate, and initiation of mechanical ventilation all favored 8 mg/kg. The exploratory nature of these analyses, differences in corticosteroid and remdesivir use between groups, and small sample size warrant caution in interpretation of results.
The open-label design is a limitation of this study; however, pharmacokinetic and pharmacodynamic end points were unlikely to be affected by awareness of tocilizumab dose. The trial was not powered to evaluate efficacy and did not have a placebo arm.
CONCLUSIONS
In this randomized clinical trial of hospitalized patients with moderate to severe pneumonia who received 1–2 doses of tocilizumab 4 or 8 mg/kg, pharmacokinetic assessments and sIL-6R levels showed expected dose-dependent effects, whereas other pharmacodynamic assessments revealed no clear differences between the 2 dose regimens. Safety outcomes were comparable between groups, and no new safety signals were identified. Exploratory efficacy results indicated that further study is required before a lower tocilizumab dose can be recommended in patients with moderate to severe COVID-19 pneumonia.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to thank Christophe Dhalluin and Teresa Gleissl, who assisted with medical data review during the study. Support for third-party writing assistance, provided by Sarah Nordquist, PhD, and Nicola Gillespie, DVM, of Health Interactions, Inc, was provided by Genentech, Inc.
Data sharing. Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available at: https://vivli.org/members/ourmembers/. Details on Roche’s global policy on the sharing of clinical information and how to request access to related clinical study documents can be found at: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Financial support. This study was funded by F. Hoffmann-La Roche Ltd, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Potential conflicts of interest. P. N. K. has served as a consultant for Amgen, Gilead Sciences, GSK, Merck, and Theratechnologies; has received research grants from Gilead Sciences, GSK, Merck, and Eli Lilly; and has stock in Gilead Sciences, GSK, Johnson & Johnson, Merck, and Pfizer. J. H.-S. is an employee of Roche Products Ltd. S. N. and W. S. are employees of F. Hoffmann-La Roche Ltd. Y. F. and L. T. are employees of Genentech, Inc. F. C. and C. J. F. L. were employees of Genentech, Inc, at the time of this study. N. R. N. has received research support from Roche. D. B. G. is on an advisory board for GSK; receives grants from the Chest Foundation and Novartis; and is a site principal investigator for industry-sponsored studies from Roche, Gilead, the National Institute of Allergy and Infectious Diseases, Cyclomedica, and Biopharma. G. A. M. has served as a consultant for Gilead, Merck, Theratechnologies, Janssen, and GSK/ViiV; and has received grant support from Roche, Gilead, Merck, Astellas, Tetraphase, Vanda, and RedHill. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed 10 November 2021.
- 2. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim guidance. https://apps.who.int/iris/handle/10665/331446. Accessed 01 April 2021.
- 3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020; 180:1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 7. Luo X, Zhou W, Yan X, et al. Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin Infect Dis 2020; 71:2174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vargas-Vargas M, Cortés-Rojo C.. Ferritin levels and COVID-19. Rev Panam Salud Publica 2020; 44:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020; 146:128–36.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCreary EK, Meyer NJ.. Covid-19 controversies: the tocilizumab chapter. BMJ 2021; 372:n244. [DOI] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Genentech, Inc. ACTEMRA Prescribing Information. South San Francisco, CA: Genentech, Inc; 2021. [Google Scholar]
- 14. Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018; 23:943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. F. Hoffmann-La Roche. RoActemra. Summary of Product Characteristics. Grenzach-Wyhlen, Germany: Roche; Registration Ltd; 2021. [Google Scholar]
- 16. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis 2016; 75:1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008; 67:1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011; 63:609–21. [DOI] [PubMed] [Google Scholar]
- 19. Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008; 371:987–97. [DOI] [PubMed] [Google Scholar]
- 20. Strohbehn GW, Reid PD, Ratain MJ.. Applied clinical pharmacology in a crisis: interleukin-6 axis blockade and COVID-19. Clin Pharmacol Ther 2020; 108:425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 109:688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021; 181:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 2021; 384:1503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021; 384:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021; 181:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020; 383:2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 2021; 372:n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Lancet 2021: 397:1637–45. [DOI] [PMC free article] [PubMed]
- 29. Kewan T, Covut F, Al–Jaghbeer MJ, et al. Tocilizumab for treatment of patients with severe COVID–19: a retrospective cohort study. EClinicalMedicine 2020; 24:100418100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strohbehn GW, Heiss BL, Rouhani SJ, et al. COVIDOSE: a phase II clinical trial of low-dose tocilizumab in the treatment of noncritical COVID-19 pneumonia. Clin Pharmacol Ther 2021; 109:688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020; 117:10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for ACTEMRA (tocilizumab). https://www.fda.gov/media/150321/download. Accessed 1 December 2021.
- 33. Frey N, Grange S, Woodworth T.. Population pharmacokinetic analysis of tocilizumab in patients with rheumatoid arthritis. J Clin Pharmacol 2010; 50:754–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.