Dr Balkhy at the console during a robotic TECAB procedure.
Central Message.
Robotic totally endoscopic coronary bypass is a valuable technique in the current era. The future of this procedure depends on commitments from both our specialty and industry to make it successful.
Feature Editor's Introduction— Robotic totally endoscopic coronary artery bypass grafting represents the most advanced form of coronary surgery. After initial enthusiasm, the technique has not been largely adopted by the surgical community and it is currently performed by only a few technical masters. Dr Husam Balkhy presents a superb review of the technique and an important perspective on its possible future. I'm sure readers will enjoy reading it as much as I did.
Mario Gaudino, MD, PhD, MSCE
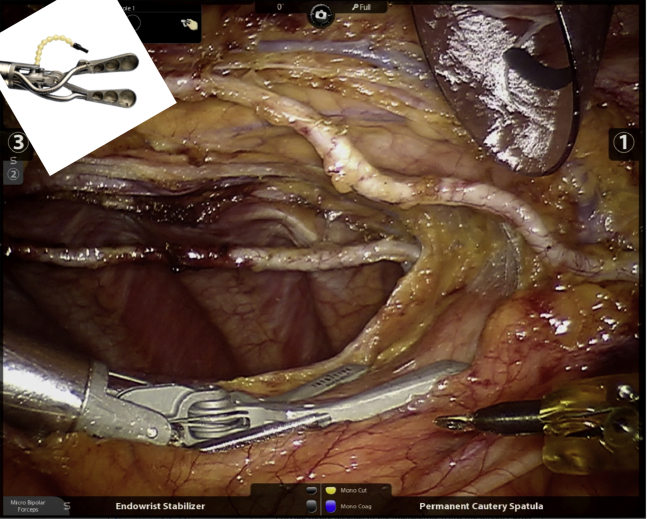
Totally endoscopic coronary artery bypass (TECAB) is the least invasive form of surgical coronary revascularization. Outside of a very small number of experiences, it has only been successfully executed when coupled with robotic technology.1 The possibility of a sternal-sparing, port-only approach to coronary artery bypass grafting (CABG) surgery, which is arguably among the most commonly performed major surgical procedures over the past several decades, was extremely appealing when robotics first became available for clinical use in the late 1990s. Indeed, both of the surgical robot companies that came to life during that early era considered CABG to be their primary target for growth, as demonstrated by the fact that the first preclinical experiments involved coronary anastomoses,2 and among the first cases performed was an arrested heart single vessel TECAB.3 Even the early-generation robotic systems offered a significant improvement over traditional thoracoscopic approaches for executing this highly complex procedure because of the superiority of 3-dimensional vision and instrument dexterity inside the chest. Later generations of the da Vinci robot (Intuitive Surgical, Sunnyvale, Calif) have only improved upon these features by adding a fourth arm, high-definition visualization, more specialized instrumentation, and smart technology for docking and avoiding arm conflicts. During the early to mid-2000s, there was a flurry of activity surrounding TECAB, including a Food and Drug Administration trial and approval,4 as well as increasing program adoption of the technology as multiple groups reported their results with both arrested and beating heart TECAB.5, 6, 7 Unfortunately, and despite improvements in robotic instrumentation, including the introduction in 2008 of an endoscopic suction stabilizer (Endowrist Stabilizer; Intuitive Surgical) (Figure 1) controlled fully by the console surgeon, only a very few dedicated programs persisted in performing this procedure.
Figure 1.
Endowrist Stabilizer (Intuitive Surgical, Sunnyvale, Calif) providing exposure for bilateral internal thoracic artery harvesting. Insert shows components of the stabilizer.
Although a small number of programs continued to publish excellent outcomes using the TECAB approach,8, 9, 10 the majority of surgeons opted to use the robot only for harvesting of 1 internal thoracic artery (ITA) and then creating a left minithoracotomy to perform the left ITA left anterior descending artery anastomosis (robotic minimally invasive direct coronary artery bypass).11 Performing the whole procedure using the robot was proving to be a significant undertaking requiring dedicated teams and a fairly steep learning curve to master the most critical part of the procedure: The anastomosis.12 These factors resulted in low adoption of the TECAB procedure, and when coupled with the rise of off-pump coronary bypass surgery (OPCAB), they also led to a significant increase in research and development efforts in the search for the perfect automated coronary anastomotic device. By the early 2000s, more than 110 patents for end-to-end and end-to-side distal coronary anastomotic devices had been granted.13
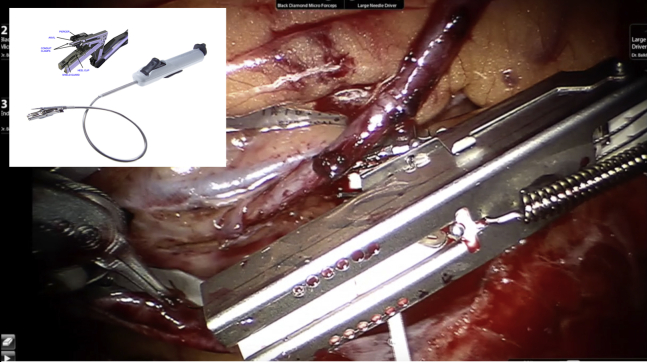
The only device to overcome the regulatory, financial, and clinical hurdles of the time was the C-Port distal anastomotic connector (Aesculap, Tutlingen, Germany), a miniature automated stapling device that was approved for use in Europe during 2002 and cleared for use by the Food and Drug Administration during December 2005. The early data on the first generation of this device were favorable when used in saphenous vein grafts, and when the second-generation C-Port xA (Aesculap) became available during mid-2007, we were the first to use it on a routine basis for ITA grafts and subsequently demonstrated excellent early and midterm patency rates in open sternotomy.14,15 The overwhelming and clear success of this device in OPCAB procedures in our hands led us to use its flexible shafted version (C-Port Flex A; Aesculap) (Figure 2) when we started our robotic TECAB experience during late 2007.
Figure 2.
C-Port Flex A distal anastomotic device (Aesculap, Tutlingen, Germany) after completed anastomosis. Insert shows components of the Flex A.
Having perfected the ability to perform a single-shot automated coronary anastomosis in open cases, our learning curve for robotic TECAB was rendered much less steep. Taking the difficulties of mastering endoscopic suturing with the robotic arms out of the equation and relying on our large OPCAB experience allowed us to quickly overcome the learning curve and take on more challenging cases, expanding from single-vessel left ITA–left anterior descending TECAB to multivessel procedures using bilateral ITA grafts in a relatively short period of time. Having a reliable and reproducible anastomotic coronary device was key in executing these cases off-pump with short to no ischemic times, and a very low conversion rate.16 The establishment of a dedicated team that stayed consistent even during the transition to a new institution was essential in maintaining good outcomes in more than 900 cases to date. Our experience has allowed us to apply this approach in higher-risk candidates,17 including morbidly obese,18 elderly, and redo patients.19
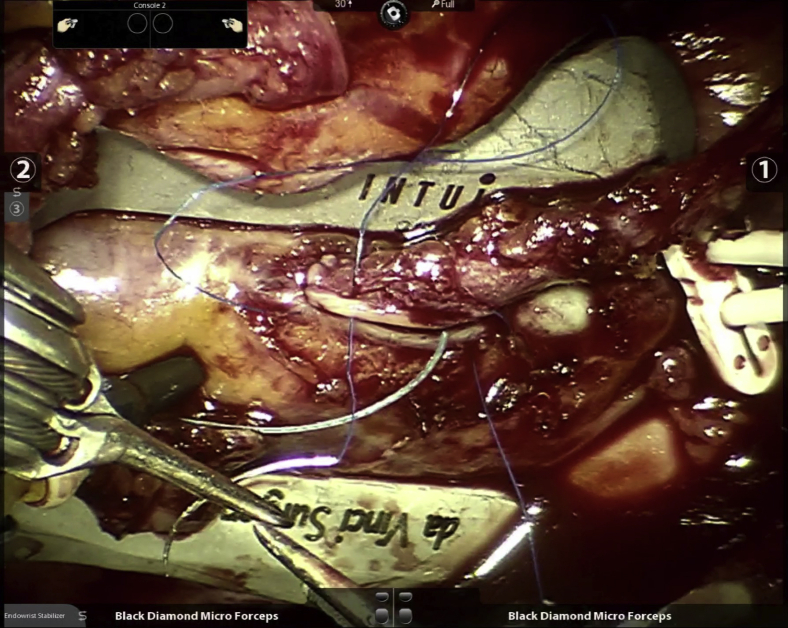
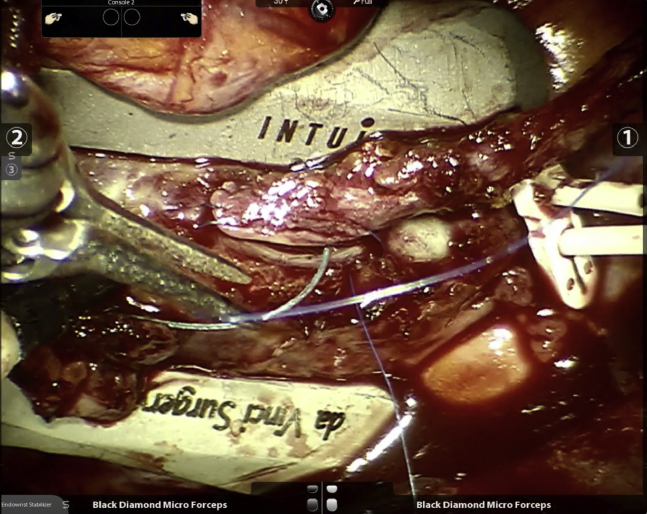
Unfortunately, our enthusiasm for this anastomotic technique was not replicated, save for a small number of programs in the United States that continued to use it sparingly and only in open cases. This, despite a multicenter Food and Drug Administration-mandated postmarket surveillance study that showed superior 12-month vein graft patency using the device when compared with current era historical controls.20 As a result of the lack of widespread use, this device became commercially unavailable during late 2018 and consequently, for the past 3 years we have had to switch to a traditional sutured approach (Figure 3 and Video 1). Another challenge we currently face because of the lack of enthusiasm in the cardiac surgical community for robotic TECAB in general, is that the endoscopic stabilizer and other instruments necessary for this procedure have not been made available for the newer generation of the da Vinci robot (ie, model Xi). Because of this, we find ourselves using the previous-generation device (ie, model Si) to offer this procedure that we continue to perform on a routine and daily basis.
Video 1.
Robotic endoscopic sutured anastomosis to obtuse marginal branch. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00291-1/fulltext.
Figure 3.
Robotic endoscopic sutured anastomosis to obtuse marginal branch.
Other approaches for multivessel nonsternotomy CABG have been introduced and are much more frequently applied than TECAB; however, in our view they are limited by the lack of easy access for harvesting bilateral ITAs, as well as the fact that the larger the patient the larger the thoracotomy by necessity. The robotic approach offers dexterity inside the chest through the same small ports regardless of a patient's body habitus. In our current practice, the only exclusion criteria for robotic TECAB are the need for emergency surgery, severe left ventricle dysfunction, and a fused left chest from previous lung surgery. As we and others have shown, with experience one is able to offer multiarterial bypass with bilateral ITA grafts to a large group of patients, including diabetic and obese patients, as well as redo patients. Our preoperative workup at this point is no different than in patients undergoing open CABG and we perform chest computed tomography scans only in reoperation patients and those with abnormal anatomy (eg, pectus excavatum).
Where Do We Go From Here?
There have been 2 recent meta-analyses of robotic TECAB in the literature. Leonard and colleagues21 studied 17 publications from 2000 to 2017 (including 3721 patients with a mean follow up of 3.3 years) and concluded that robotic TECAB had an acceptable operative risk and early graft patency. Göbölös and colleagues22 studied 19 publications with a total of 2397 patients and concluded that although robotic TECAB was associated with longer operative times, these have come down significantly over the past 20 years, and that operative outcomes were comparable to traditional CABG but that recovery and return to normal activities was significantly better.
Almost every study of sternal-sparing coronary bypass surgery has shown enhanced early outcomes with less perioperative morbidity and shorter recovery time and return to work and normal activities.23 Although these are important factors, especially to our patients who in many cases are self-referred for TECAB, we do not believe that this is the most important benefit of the procedure. In our view, the most important return on our investment in this procedure is the ability to offer patients multiarterial grafting with bilateral ITA grafts, regardless of their risk factors (including insulin-dependent diabetes or obesity). The concept of hybrid coronary revascularization is significantly augmented by the ability to place 2 arterial grafts on the left coronary system that can then be supplemented by percutaneous coronary intervention to the right coronary artery when suitable. Using this strategy of advanced hybrid revascularization in the context of robotic TECAB has allowed us to achieve a low residual Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery score even in patients with multivessel disease. In addition, recent data have shown that placing arterial grafts on the 2 largest left-sided targets may be more important for long-term survival than complete revascularization with a single ITA and saphenous vein grafts.24 We recently reported in a presentation on 544 patients who underwent robotic TECAB at our current institution over the past 7 years of whom 65% had multivessel TECAB and of these 89% were with bilateral ITA grafts. The perioperative morbidity and mortality were comparable to that reported in the literature for traditional CABG surgery, and graft patency in patients undergoing angiography for hybrid revascularization was 97%. Freedom from cardiac mortality and major adverse coronary events were 98% and 93%, respectively, at a mean of 38 months, with the longest follow-up being 7.5 years (Presented at EACTS 2020 virtual meeting on October 8, 2020). Moreover, more than 75% of patients did not use opioid pain medications beyond the first week after surgery and the average time to return to work and normal physical activity was 14 days.
These results have been duplicated by surgeons and teams dedicated to robotic TECAB as outlined above. Unfortunately, there are not enough of these teams to sustain a viable financial model for the necessary technology and instrumentation to be developed and marketed by medical device companies.25 This is a problem, and the main group influenced by it is our patients.
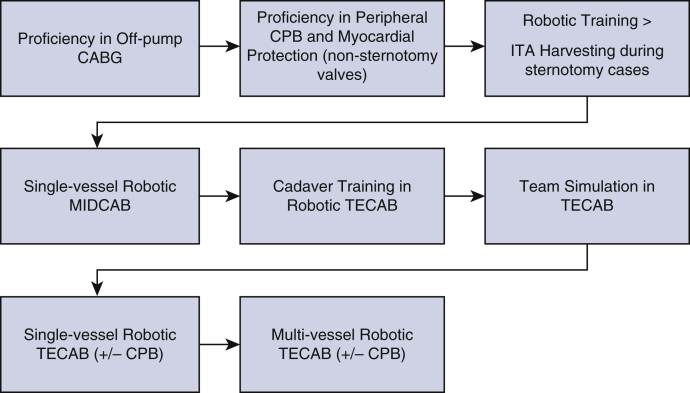
Robotic TECAB is a demanding procedure with what some would call a steep learning curve. But so is all of cardiac surgery. We train young surgeons every day not only on the complexities of the cardiovascular system but also on how to place someone on cardiopulmonary bypass, stop a beating heart, and bring it back to life again. I submit that taking a new medical graduate through the tedious and steep learning curve of becoming a heart surgeon is immensely more complicated than teaching an accomplished coronary surgeon to perform robotic TECAB. What is necessary is a well thought out and stepwise program with guided mastery of a series of skills tailored to each surgeon and team's expertise under the supervision of an experienced robotic device operator. As minimally invasive surgery becomes more and more appealing in this age of increasing percutaneous options to treat cardiac pathology, it is incumbent on heart surgeons to avail themselves of the highly advanced technology that robotics brings to the table. This is nowhere more applicable than in the execution of port-only endoscopic coronary surgery. Other surgical specialties have already come to this realization and have integrated robotics into their residency training programs as a primary teaching objective. Our cardiac surgery societies have also begun to realize the importance of robotics in cardiac surgery and have created task forces and educational programs with funded minifellowships designed to address the growing need for expertise in this field. This is the only way to ensure safe adoption by established surgeons and teams (Figure 4).
Figure 4.
A suggested broad pathway for gradual adoption of robotic totally endoscopic coronary artery bypass (TECAB) for experienced surgeons. The need for a dedicated surgical team and continuous quality control of outcomes are imperative for success. CABG, Coronary artery bypass grafting; CPB, cardiopulmonary bypass; ITA, internal thoracic artery; MIDCAPB, minimally invasive direct coronary artery bypass.
The Future
A new generation of cardiac surgeons has started to become enthusiastic about robotic heart surgery and especially robotic TECAB (Torregrossa, Pettinari, Oosterlinck, Melly, personal communication, October 2019). Their interest is fueled by, among other things, their natural affinity to and adoption of new technology in general and also by what they are seeing in other specialties. Providing young surgeons with the necessary training through exposure to robotics during their residency training and superfellowships that offer intensive hands-on training will be necessary if we are to try and reclaim our position as the most innovative specialty in surgery. Finally, for this to happen there needs to be a rapprochement with industry and a mutual commitment to research and development in the field of robotic cardiac surgical instrumentation, especially as it relates to TECAB, as well as training and education to make this a successful endeavor. New robotic systems are on the horizon and may be the catalyst for a wider interest in the TECAB procedure. We encourage the makers of these new systems to invest in stabilizers, cardiac positioners, and automated coronary staplers to help facilitate the widespread adoption of TECAB. Given renewed surgeon interest and some of the previously mentioned factors, robotic TECAB is in a now or never moment. We owe it to our patients to make it now.
Conflicts of Interest Statement
Dr Balkhy is a proctor for Intuitive Surgical, maker of the da Vinci robot.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Robotic endoscopic sutured anastomosis to obtuse marginal branch. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00291-1/fulltext.
References
- 1.Lee J.D., Srivastava M., Bonatti J. History and current status of robotic TECAB. Circ J. 2012;76:2058–2065. doi: 10.1253/circj.cj-12-0981. [DOI] [PubMed] [Google Scholar]
- 2.Ducko C.T., Stephenson E.R., Jr., Sankholkar S., Damiano R.J., Jr. Robotically Assisted coronary bypass surgery: moving toward a completely endoscopic procedure. Heart Surg Forum. 1999;2:29–37. [PubMed] [Google Scholar]
- 3.Loulmet D., Carpentier A., d'Attellis N., Berrebi A., Cardon C., Ponzio O., et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg. 1999;118:4–10. doi: 10.1016/S0022-5223(99)70133-9. [DOI] [PubMed] [Google Scholar]
- 4.Argenziano M., Katz M., Bonatti J., Srivastava S., Murphy D., Poirier R., et al. Results of the prospective multicenter trial of robotically assisted totally endoscopic CABG. Ann Thorac Surg. 2006;81:1666–1674. doi: 10.1016/j.athoracsur.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava S., Gadasalli S., Agusala M., Kolluru R., Barrera R., Quismundo S., et al. Beating heart totally endoscopic coronary artery bypass. Ann Thorac Surg. 2010;89:1873–1879. doi: 10.1016/j.athoracsur.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Bonatti J., Schachner T., Bonaros N., Oehlinger A., Weidemann D., Ruetzler E., et al. Effectiveness and Safety of Total endoscopic left internal mammary artery bypass graft to the left anterior descending artery. Am J Cardiol. 2009;104:1684–1688. doi: 10.1016/j.amjcard.2009.07.051. [DOI] [PubMed] [Google Scholar]
- 7.De Canniere D., Wimmer-Grienicker G., Cichon R., Gulielmos V., Van Praet F., Seshadri-Kreaden U., et al. Feasibility, safety, and efficacy of TECAB grafting: multi center European experience. J Thorac Cardiovasc Surg. 2007;134:710–716. doi: 10.1016/j.jtcvs.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 8.Bonaros N., Schachner T., Lehr E., Kofler M., Wiedemann D., Hong P., et al. Five hundred cases of robotic totally endoscopic coronary artery bypass grafting: predictors of success and safety. Ann Thorac Surg. 2013;95:803–812. doi: 10.1016/j.athoracsur.2012.09.071. [DOI] [PubMed] [Google Scholar]
- 9.Zaouter C., Imbault J., Labrousse L., Abdelmoumen Y., Coiffic A., Colonna G., et al. Association of robotic totally endoscopic coronary artery bypass graft surgery associated with a preliminary cardiac enhanced recovery after surgery program: a retrospective analysis. J Cardiothorac Vasc Anesth. 2015;29:1489–1497. doi: 10.1053/j.jvca.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Gao C., Yang M., Wu Y., Wang G., Xiao C., Zhao Y., et al. Early and mid-term results of totally endoscopic coronary artery bypass grafting on the beating heart. J Thorac Cardiovasc Surg. 2011;142:843–849. doi: 10.1016/j.jtcvs.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Neshir N., Bakir I., Cassleman F., Degriek I., De Geest R., Wellens F., et al. Robotically enhanced MIDCAB surgery: a winning strategy? J Cardiovasc Surg (Torino) 2007;48:333–338. [PubMed] [Google Scholar]
- 12.Schachner T., Bonaros N., Wiedemann D., Weidinger F., Feuchtner G., Friedrich G., et al. Training surgeons to perform robotically assisted totally endoscopic coronary surgery. Ann Thorac Surg. 2009;88:523–527. doi: 10.1016/j.athoracsur.2009.04.089. [DOI] [PubMed] [Google Scholar]
- 13.Scheltes J.S., Van Andel C.J., Pistecky P.V., Borst C. Coronary anastomotic devices: blood-exposed non-intimal surface and coronary wall stress. J Thorac Cardiovasc Surg. 2003;126:191–199. doi: 10.1016/s0022-5223(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 14.Balkhy H.H., Wann L.S., Arnsdorf S. Early patency evaluation of new distal anastomotic device in internal mammary artery grafts using computed tomography angiography. Innovations (Phila) 2010;5:109–113. doi: 10.1097/IMI.0b013e3181d714ba. [DOI] [PubMed] [Google Scholar]
- 15.Balkhy H.H., Wann S., Arnsdorf S., Maciolek K. Long-term patency evaluation of the Cardica C-Port distal anastomotic device in coronary artery bypass grafting: initial experience in 91 grafts. Innovations. 2009;4:158. [Google Scholar]
- 16.Balkhy H.H., Wann L.S., Krienbring D., Arnsdorf S.E. Integrating coronary anastomotic connectors and robotics toward a totally endoscopic beating heart approach: review of 120 cases. Ann Thorac Surg. 2011;92:821–827. doi: 10.1016/j.athoracsur.2011.04.103. [DOI] [PubMed] [Google Scholar]
- 17.Balkhy H.H., Nisivaco S., Kitahara H., McCrorey M., Patel B. Robotic beating heart TECAB in higher-risk patients: can it be done safely? Innovations. 2018;13:108–113. doi: 10.1097/IMI.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 18.Kitahara H., Patel B., McCrorey M., Nisivaco S., Balkhy H.H. Is robotic beating heart totally endoscopic coronary bypass feasible for BMI > 35 morbidly obese patients? Int J Med Robot. 2018;14:e1911. doi: 10.1002/rcs.1911. [DOI] [PubMed] [Google Scholar]
- 19.Nisivaco S., McCrorey M., Krienbring D., Patel B., Srivastava S., Balkhy H.H. Redo robotic endoscopic beating heart coronary bypass (TECAB) after prior TECAB. Ann Thorac Surg. 2017;104:e417–e419. doi: 10.1016/j.athoracsur.2017.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Balkhy H.H., Patel N., Ramchandani M., Kitahara H., Subramanian V., Augelli N., et al. Multicenter assessment of grafts in coronaries: mid term evaluation of the C-port anastomotic device (The MAGIC Study) Innovations. 2018;13:273–281. doi: 10.1097/IMI.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 21.Leonard J.R., Rahouma M., Abouarab A.A., Schwann A.N., Scuderi G., Lau C., et al. Totally endoscopic coronary artery bypass surgery: a meta-analysis of the current evidence. Int J Cardiol. 2018;261:42–46. doi: 10.1016/j.ijcard.2017.12.071. [DOI] [PubMed] [Google Scholar]
- 22.Göbölös L., Ramahi J., Obeso A., Bartel T., Hogan M., Traina M., et al. Robotic totally endoscopic coronary artery bypass grafting: systematic review of clinical outcomes from the past two decades. Innovations. 2019;14:5–16. doi: 10.1177/1556984519827703. [DOI] [PubMed] [Google Scholar]
- 23.Guenther T.M., Chen S.A., Balkhy H.H., Kiaii B. Robotic coronary artery bypass grafting: the whole 9 yards. Innovations (Phila) 2020;15:204–210. doi: 10.1177/1556984520922931. [DOI] [PubMed] [Google Scholar]
- 24.Bakaeen F.G., Ravichandren K., Blackstone E.H., Houghtaling P.L., Soltesz E.G., Johnston D.R., et al. Coronary artery target selection and survival after bilateral internal thoracic artery grafting. J Am Coll Cardiol. 2020;75:258–268. doi: 10.1016/j.jacc.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Bonatti J., Wallner S., Winkler B., Grabenwöger M. Robotic totally endoscopic coronary artery bypass grafting: current status and future prospects. Expert Rev Med Device. 2020;17:33–40. doi: 10.1080/17434440.2020.1704252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Robotic endoscopic sutured anastomosis to obtuse marginal branch. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00291-1/fulltext.