Abstract
Disability following spinal cord injury is due to failure of axon regeneration, which has been ascribed to environmental factors in the central nervous system and a developmental loss of intrinsic growth capacity in neurons. Recently, the receptor-like protein tyrosine phosphatases, protein tyrosine phosphatase σ (PTPσ) and leukocyte common antigen-related phosphatase (LAR), have been identified as specific receptors for the regeneration-inhibiting matrix molecules chondroitin sulfate proteoglycans (CSPGs). After spinal cord transection in lampreys, axons of the large, identified reticulospinal neurons have heterogeneous regenerative abilities. The bad-regenerating neurons also undergo a delayed form of axotomy-induced apoptosis. In the present study, a lamprey genomic database was used to identify homologs of CSPGs, clone PTPσ and LAR, and examine their mRNA expression. CSPG immunoreactivity was increased significantly near the lesion at 2 weeks post transection, and decreased thereafter. Both receptors were expressed selectively in the bad-regenerating neurons and had overlapping cellular distributions. PTPσ was upregulated with age (LAR was not evaluated). By 2 weeks post transection, neurons expressing PTPσ also showed caspase activation, suggesting apoptosis. The probability of axon regeneration for individual identified neurons was negatively correlated with the expression level of PTPσ in both control and spinal cord–transected lampreys. In an animal 7 weeks post transection, regenerated axons were labeled retrogradely from beyond the transection. PTPσ expression and caspase labeling was seen only in neurons whose axon had not regenerated. These results are consistent with a possible role for PTPσ (and LAR) in both retrograde neuronal death and the poor intrinsic regenerative ability of bad-regenerating neurons.
Keywords: axon regeneration, chondroitin sulfate proteoglycans, apoptosis, caspase
Graphical abstract
The chondroitin sulfate proteoglycans (CSPG) receptors protein tyrosine phosphatase σ (PTPσ) and leukocyte common antigen-related phosphatase (LAR) are expressed selectively in apoptotic “bad-regenerating” neurons, suggesting a role for those receptors in retrograde neuronal death and poor intrinsic regenerative ability. A: Green, activated caspases (marker of apoptosis); magenta, neurons with regenerated axons. B: Cells labeled for PTPσ mRNA.
Much of the permanent disability following spinal cord injury (SCI) is due to failure of axons to regenerate. This has been ascribed to both a developmental reduction in the intrinsic growth capacity of mature neurons (Cai et al., 2001; Hannila and Filbin, 2008; Park et al., 2010) and to environmental factors (David and Aguayo, 1981; Fawcett, 2009; McGee and Strittmatter, 2003; Sharma et al., 2012). Among the environmental factors identified in mammals are several types of inhibitory molecules, including those associated with central nervous system (CNS) myelin (Fournier et al., 2002; Huber et al., 2002; McKerracher et al., 1994; Schachner and Bartsch, 2000; Wang et al., 2002); the glial scar–inhibitory molecules chondroitin sulfate proteoglycans (CSPGs); and the chemorepulsive guidance molecules ephrin and semaphorin (Fawcett and Asher, 1999; Jones et al., 2003; Wahl et al., 2000).
CSPGs are a class of inhibitory extracellular matrix molecules expressed throughout the CNS, consisting of a core protein and one or more sugar side chains. During neuronal development, CSPGs act as negative guidance cues for growth cone navigation, as seen by the axons’ avoidance of CSPG-dense areas (Siebert and Osterhout, 2011). CSPGs that are constituents of the perineuronal nets (PNNs) around many neurons also act as a barrier to axon growth (Bruckner et al., 2000; Deepa et al., 2006). In addition, CSPGs play a crucial role in restricting plasticity in the injured CNS by limiting axon sprouting and synaptogenesis (Galtrey and Fawcett, 2007; Silver and Miller, 2004). When the CNS is damaged in mature animals, glial cells that form the scar around the lesion upregulate production of CSPGs (Fawcett and Asher, 1999; Sharma et al., 2012). Digestion of CSPGs with chondroitinase ABC (ChABC) enhances axon growth and recovery of both motor and sensory function after SCI in mammals (Bradbury et al., 2002; Cafferty et al., 2008; Galtrey and Fawcett, 2007). However, the degree to which the inhibition of axon growth reflects failure of injured axons to regenerate rather than failure of spared axons to sprout collaterals is not clear. Indeed, numerous studies have suggested that blocking extracellular inhibitory influences alone does not allow the majority of injured axons to regenerate and therefore that a limited intrinsic regenerative ability critically underlies regeneration failure in the mature CNS (Liu et al., 2011; Sun and He, 2010).
It is now recognized that a neuron’s intrinsic growth capacity is based on expression, availability, and activation of a large number of cell-autonomous molecules, many of which are involved in signaling the effects of growth-inhibitory and growth-promoting environmental factors (Park et al., 2010; Sharma et al., 2012). The transmembrane receptor-like protein tyrosine phosphatases (RPTPs), protein tyrosine phosphatase sigma (PTPσ) and leukocyte common antigen-related phosphatase (LAR), are functional receptors that bind CSPGs with high affinity and mediate at least some of their inhibitory effects (Fisher et al., 2011; Shen et al., 2009). Two receptors for myelin-associated growth inhibitors, Nogo receptors 1 and 3 (NgR1 and NgR3), also bind to the glycosaminoglycans of CSPGs and may participate in CSPGs’ inhibition of neuronal growth (Dickendesher et al., 2012). After binding to their receptors, the CSPGs that are secreted at the site of SCI are reported to activate the RhoA/ROCK pathway and inhibit Akt (Fisher et al., 2011). ROCK activation leads to phosphorylation of target proteins, including myosin light chain, which mediates cytoskeletal rearrangements and disassembly in neurons, with consequent collapse of growth cones. Akt activation leads to activation of the mammalian target of rapamycin (mTOR) pathway, which results in enhanced protein synthesis and axon regenerative ability (Liu et al., 2011; Park et al., 2008, 2010).
However, it has not been established whether mature, injured CNS axons regenerate using growth cones, and data from the lamprey suggest that the tips of regenerating axons lack filopodia and lamellipodia, have relatively little F-actin, and are tightly packed with neurofilaments, which are excluded from conventional growth cones (Hall et al., 1997; Jin et al., 2009; Lurie et al., 1994; Pijak et al., 1996; Selzer, 2003). This, together with the difficulty in distinguishing axon regeneration from collateral sprouting, make it desirable to determine mechanisms of axon regeneration in simpler animal models, and to verify the effects of specific molecules and pharmacological interventions in axons known to be undergoing true regeneration.
Several characteristics of the lamprey CNS make it a useful model for investigating the neuron-intrinsic factors that determine axon regeneration in the CNS. The nervous system lacks myelin, which makes it translucent and suitable for wholemount examination (Selzer, 1979; Swain et al., 1993, 1994). Among the reticulospinal neurons (RNs) in the lamprey brain, several are very large, and 18 pairs have been identified and characterized with regard to their regenerative abilities. Some are bad regenerators and others good regenerators (Jacobs et al., 1997). This is summarized in Figure 1. During the first 2 weeks post transection, injured reticulospinal axons retract 1–2 mm and then begin to regrow toward the lesion, reaching the transection site by 4 weeks post transection. Recently, we found that the bad regenerators undergo a very delayed form of apoptosis, with caspase activation seen as early as 2 weeks post transection (Barreiro-Iglesias and Shifman, 2012; Hu et al., 2013) and TUNEL positivity appearing as early as 4 weeks post transection, reaching a peak at 12–16 weeks (Shifman et al., 2008). The mechanisms involved are still unclear, but our group reported that after SCI, UNC-5 and neogenin, the chemorepulsive receptors for the guidance molecules netrin and RGM respectively, were upregulated selectively in the poorly regenerating neurons (Shifman and Selzer, 2000; Shifman et al., 2009). Therefore, some of the neuron-intrinsic properties that distinguish “bad regenerators” from “good regenerators” may also contribute to an axotomy-induced process of delayed cell death. In the present study, the experimental advantages of the lamprey nervous system were used to obtain evidence for a relationship between expression of CSPG receptors and both axon regeneration and retrograde cell death.
Figure 1.
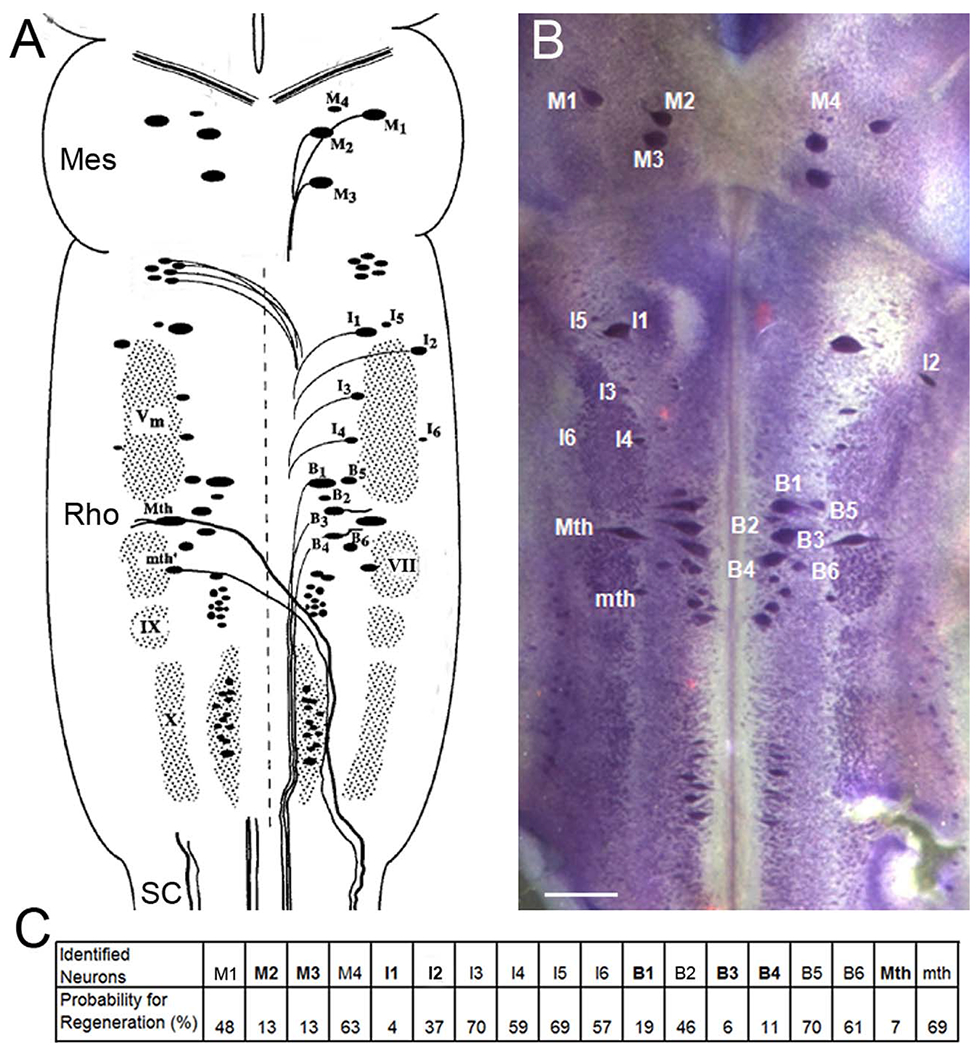
Cytoarchitecture of the identified reticulospinal neurons in lamprey brain. A: The brain of the large larval lamprey is diagrammed to illustrate locations of large individually identified reticulospinal neurons according to the nomenclature of Rovainen (1967) as modified by Swain et al. (1993). These are the Müller and Mauthner neurons, which also will be referred to in subsequent figures. M, mesencephalic Müller cells; I, isthmic Müller cells; B, bulbar Müller cells; Mth, Mauthner cell; mth, auxiliary Mauthner cell; Vm, trigeminal motor nucleus; IX, glossopharyngeal motor nucleus; X, vagal motor nucleus; VII, the facial motor nucleus. A and C are modified from Jacobs et al. (1997), M2, M3, I1, I2, B1, B3, B4, Mth are considered bad regenerators (bolded letters), and the others are good regenerators (Davis and McClellan, 1994; Jacobs et al., 1997). Mes, mesencephalon; Rho, rhombencephalon; SC, spinal cord. B: The brain of a large larval lamprey was stained with toluidine blue to show those identified reticulospinal neurons. C: The probability of axon regeneration for each identified reticulospinal neuron was determined by applying HRP 5 mm caudal to original lesion at 10 weeks after SC transection. Scale bar = 250 μm in B.
A pre-ensemble lamprey genomic database, which provides displays of genomes in the process of being annotated, has been searched carefully. It includes a partial assembly of the sea lamprey genome. Several CSPGs have been identified, including neurocan, brevican, and aggrecan, as well as the CSPG receptors PTPσ and LAR. This raises the possibility that CSPGs and their receptors contribute to the differential regenerative capacities of lamprey RNs. To investigate this possibility, we have cloned lamprey PTPσ and LAR, and studied their expression pattern in lamprey brain at different ages and different times after spinal cord (SC) transection. The results indicate that PTPσ mRNA increases developmentally and selectively expresses in poorly regenerating RNs. The RNs undergoing apoptosis after transection also express PTPσ. Interestingly, PTPσ mRNA seems to start accumulating in the cell body of neurons before they undergo apoptosis. Moreover, the regeneration probability of individual identified RNs negatively correlated with the expression level of PTPσ in both control and SC-transected lampreys and PTPσ was not expressed in neurons with regenerated axons after 7 weeks of recovery. These results suggest that even though the regenerating axons lack true growth cones, PTPσ might contribute to the poor regenerative abilities of the bad-regenerating neurons and/or to their very delayed apoptosis after axotomy.
MATERIALS AND METHODS
Identification of genes for CSPG receptors PTPσ and LAR in a lamprey genomic database and polymerase chain reaction (PCR) cloning
The sea lamprey ensemble database and whole-genome sequencing (WGS) trace database maintained by the National Center for Biotechnology Information (NCBI), National Institutes of Health were used. These databases currently consist of assembled, partially assembled, and raw unassembled sequencing data from the sea lamprey genome. PTPσ sequences from other species, including human, chicken, and zebrafish, were used to query these databases, and contigs found in the lamprey genomic library were confirmed by using the basic local alignment search tool (BLAST) on NCBI servers. These contigs were further analyzed by aligning them with homologous genes of other species. Corresponding domains, such as Ig-like, fibronectin type III (FN III), and catalytic domains, were determined. Then PCR oligonucleotide primers were designed, based on lamprey PTPσ and LAR gene sequences in regions highly identical with those of other species.
Total RNA from lamprey brain and SC was isolated by using Trizol reagent (Invitrogen, Carlsbad, CA). The first-strand cDNA synthesis reaction from total RNA was catalyzed by Superscript III Reverse Transcriptase with oligodT or 8 bp random primers. The synthesized total cDNA served as templates for PCR cloning by using the Expand™ Long Template PCR System (Roche Applied Science, Indianapolis, IN) per the manufacturer’s protocol. Following amplification, PCR fragments of expected size were purified on 1% agarose gels and ligated into the pGEM-T Easy Vector (Promega, Madison, WI). The cloned fragments were sequenced (GENEWIZ, South Plainfield, NJ), analyzed, and compared by BLAST.
Animals and spinal cord transection
Larval sea lampreys (Petromyzon marinus), 11–13 cm in length (4–5 years old), were obtained from streams of Lake Michigan and maintained in freshwater tanks at 16°C until the day of use. To investigate developmental changes of gene expression, three small (6–8 cm; approximately 2–3 years old) larval, two large (12–13 cm; approximately 4–5 years old), and two recently transformed adult lampreys were also included in the studies. The protocol was approved by the Temple University Institutional Animal Care and Use Committee. Lampreys were anesthetized by immersion in saturated aqueous benzocaine for 5 minutes and pinned to a Sylgard (184 silcone elastomer; Dow Corning, Midland, MI) plate filled with ice-cold lamprey Ringer’s solution (110 mM NaCl, 2.1 mM KCl, 2.6 mM CaCl2, 1.8 mM MgCl2, and 10 mM Tris buffer; pH 7.4). Lamprey SCs were completely transected at the level of the 5th gill, and the animals were allowed to recover for 1, 2, 4, 7, and 10 weeks. A total of 86 animals were investigated; 30 were used in CSPG studies (5 in each of the transected groups plus 5 in a control, noninjury group), and another 56 were used in the in situ hybridization (ISH) study of CSPG receptors, as summarized in Table 1. In addition to the wholemount ISH study for PTPσ and LAR, seven animals were used for the developmental expression study of PTPσ, seven for double labeling of activated caspase and PTPσ, and two for double labeling of PTPσ and LAR. Furthermore, to distinguish negative wholemount ISH labeling from false-negative results due to inadequate penetration of probes, ISH was performed on paraffin sections of one animal brain at each time point.
TABLE 1.
Numbers of Animals Used for Each Experiment1
| Experiment | Control | 1 wk | 2 wks | 4 wks | 7 wks | 10 wks | 6–8-cm Larva | 12–13-cm Larva | Adult |
|---|---|---|---|---|---|---|---|---|---|
| CSPG Immunostaining | 5 | 5 | 5 | 5 | 5 | 5 | |||
| PTPσ ISH | 7 | 2 | 8 | 7 | 2 | 2 | |||
| LAR ISH | 1 | 1 | 1 | 1 | 1 | 1 | |||
| PTPσ ISH on paraffin sections | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Caspase + PTPσ ISH | 2 | 2 | 3 | ||||||
| PTPσ ISH | 3 | 2 | 2 | ||||||
| PTPσ + LAR | 1 | 1 |
The times are weeks after spinal cord transection.
Abbreviations: CSPG, chondroitin sulfate proteoglycans; ISH, in situ hybridization; LAR, leukocyte common antigen-related phosphatase; PTPσ, protein tyrosine phosphatase σ.
Antibody characterization and CSPG immunohistochemistry
Two anti-CSPG antibodies (Table 2) were tested for specificity in lamprey SC (Fig. 2), as follows:
TABLE 2.
Primary Antibodies Used in This Study
| Antigen | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| Chondroitin sulfate proteoglycans (CSPGs) | Chick gizzard fibroblasts; specific for the glycosaminoglycan moieties of native CSPGs | Sigma-Aldrich; monoclonal antibody, isotype mouse IgM, Cat. No. C8035 | 1:200 |
| CSPGs | Feline brain proteoglycans; recognizes carbodydrate epitope of native CSPGs | Millipore; monoclonal antibody, isotype mouse IgM, Clone Cat-316, Cat. No. MAB1582 | 1:5,000 |
| Actin | Purified chicken gizzard actin | Chemicon; monoclonal antibody, isotype mouse IgG, Cat. No. MAB1501 | 1:1,500 |
| Digoxigenin | Digoxigenin | Roche Applied Science; polyclonal antibody conjugated to alkaline phosphatase, isotype sheep IgG, Cat. No. 11093274910 | 1:1,000 |
Figure 2.
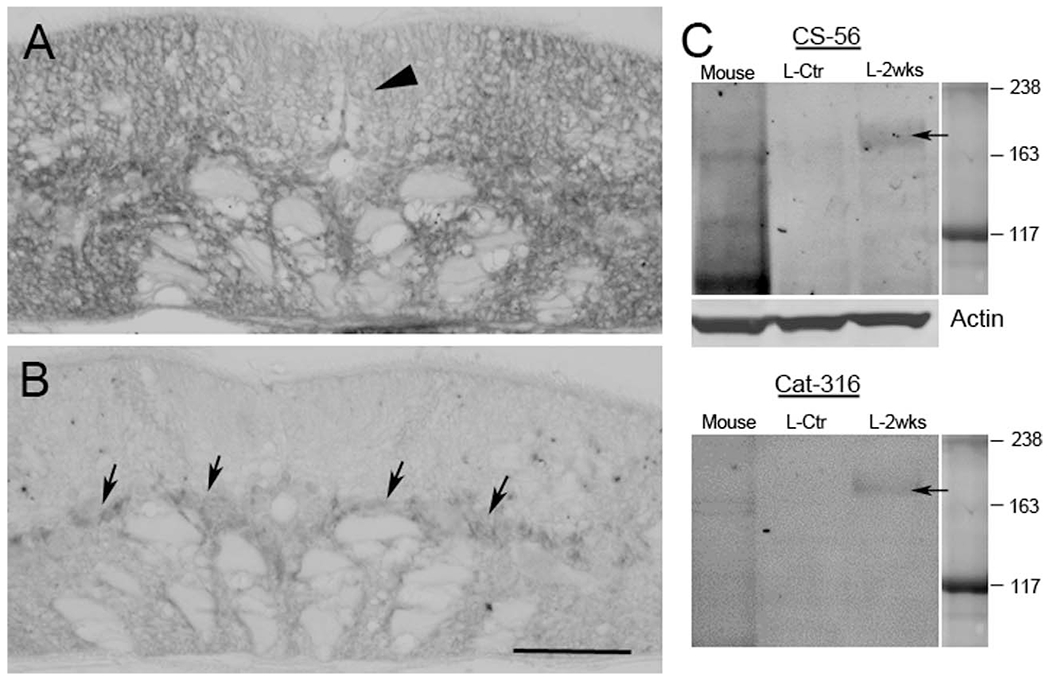
Specificity of CSPG antibodies. A: Transverse SC section from a noninjured lamprey stained with CS-56, showing broad distribution of CSPGs. The dorsal columns (arrowhead), which contain ascending sensory axons, show less intense CSPG staining than other regions. B: After treatment with ChABC, most of the CSPG staining has disappeared. Arrows point to gray matter where most glia and neurons are located. C: Western blots showing that both of the CSPG antibodies, CS-56 and Cat-316, recognize the same band (arrows) in lamprey SC homogenates, and that the level of CSPGs is increased at 2 weeks post transection (L-2weeks) compared with control animals (L-ctr). Mouse brain is used as a positive control. Actin serves as a loading control. Scale bar = 50 μm in B (applies to A,B).
CS-56 is a monoclonal antibody (C8035, Sigma-Aldrich, St. Louis, MO) from ascites fluid from mice immunized with ventral membranes of chicken gizzard fibroblasts, and is specific for the glycosaminoglycan (GAG) moieties of native CSPGs.
Cat-316, a monoclonal antibody (MAB1582, Clone Cat-316, Millipore, Bedford, MA) that recognizes a carbohydrate epitope of CSPGs, was raised against feline brain proteoglycans.
Both antibodies were tested on cross sections of SC in noninjured or 2 weeks post transection lampreys with/without ChABC treatment. Paraffin sections (10 μm) were deparaffinized, rehydrated, and washed in phosphate-buffered saline (PBS) containing 0.2% Tween-20, blocked in 10% fetal bovine serum (FBS) in PBS containing 0.2% Tween-20 for 1 hour and incubated with either the CS-56 (1:200) or Cat-316 (1:5,000) antibody overnight at 4°C. Specimens were washed thoroughly with PBS containing 0.2% Tween-20, blocked as above, and then visualized by incubation in a horseradish peroxidase (HRP)-conjugated anti-mouse IgM secondary antibody (1:200, Santa Cruz Biotechnology, Santa Cruz, CA). The other sections were treated with ChABC (C3667, Sigma-Aldrich), 2 mg/ml in enzyme buffer (100 mM Tris, pH 8.0, 100 mM sodium acetate and 0.02% bovine serum albumin) for 2 hours at 37°C and then washed and stained for CSPGs as above. CSPGs expression was investigated in longitudinal paraffin sections before and after SC transection at different time points. A total of 30 animals were studied, 5 animals from each group: control and at 1, 2, 4, 7, and 10 weeks.
A 2-cm length of lamprey trunk spanning the transection site at the 5th gill was removed and fixed in 4% paraformaldehyde (PFA) in PBS for 3–4 hours at room temperature (RT). The tissues were dehydrated and embedded in paraffin. Serial 10-μm sections were deparaffinized and washed three times in PBS containing 0.2% Tween-20. Sections were blocked with 10% FBS in PBS and incubated at 4°C with Cat-316 overnight. When a large number of samples was involved, Cat-316 was chosen because it can be diluted more than CS-56. The sections were washed and visualized by incubation in goat anti-mouse IgM conjugated with Alexa Fluor 680 (1:500) and mounted in ProLong Gold antifade reagent (Invitrogen). The images were captured by using an epifluorescence microscope (Nikon 80i) under the same parameters. Fluorescence intensity at the transection site, and 0.6 mm caudal and rostral to the transection site, was measured with Nikon Elements image analysis software. Statistical analysis was performed by analysis of variance (ANOVA) and a post hoc two-tailed t-test.
Western blot
The SCs of lamprey were collected from a control animal and one at 2 weeks post transection. A mouse brain was included as a control. The tissues were snap-frozen by liquid nitrogen and homogenized by sonication in cold lysis buffer (C3228, Sigma-Aldrich) supplemented with 1X protease inhibitor cocktail (P8341, Sigma-Aldrich). After brief centrifugation to remove debris, the total protein concentration in supernatants was determined by using Bio-Rad (Hercules, CA) DC protein assay reagents. After 5 minutes of heating at 75°C in loading buffer (NP 0007, Invitrogen) supplemented with reducing reagent (NP 0004, Invitrogen), 30 or 25 μg of SC proteins from each lamprey and 50 μg mouse brain proteins were loaded. Protein was separated in 3–8% NuPAGE® Tris-acetate gradient mini gels (EA0375BOX, Invitrogen). The protein was transferred onto PVDF membrane by using a Bio-Rad transblot apparatus. The membranes were blocked in 5% nonfat dry milk in TBS buffer for 1 hour at RT and probed with different CSPG antibodies (CS-56 or Cat-316) at 4°C overnight. After washes with TBS, the blots were incubated with secondary antibody goat anti-mouse IgM conjugated with Alexa Fluor 488 for 2 hours at RT, scanned with a Typhoon 9400 imager (GE Healthcare Biosciences, Piscataway, NJ), and processed in Adobe (San Jose, CA) Photoshop to adjust contrast and brightness.
Wisteria floribunda lectin labeling on paraffin sections
Although the binding specificity of Wisteria floribunda lectin (WFL) is not completely clear, this lectin appears to bind preferentially to carbohydrate structures and therefore is widely used as a marker for PNNs. To observe whether PNNs also appear in lamprey neurons, as has been observed in mammalian CNS, cross and longitudinal paraffin sections of SC were deparaffinized, washed with PBS, and blocked with 10% FBS/0.5% Tween-20/PBS for 1 hour at RT. After preincubation with 2% BSA/0.05% Tween-20/PBS for 1 hour at RT, the sections were incubated with biotinylated WFL (1:200, L1516, Sigma-Aldrich) in the same solution at 4°C overnight (Giamanco et al., 2010). Sections were washed with PBS and blocked again with the above solution for 30 minutes. For fluorescence staining, sections were incubated with streptavidin conjugated with Alexa Fluor 680 (1:500) in 0.04% BSA/0.05% Tween-20/PBS for 2 hours, washed in PBS, and mounted with VESTASHIELD mounting medium (Vector, Burlingame, CA, H-1000); or sections were incubated with streptavidin conjugated with alkaline phosphatase (AP) in 0.04% BSA/0.05% Tween-20/PBS for 2 hours and washed with PBS followed by SMT buffer (100 mM NaCl, 50 mM MgCl2, 100 mM Tris, pH 9.5, 0.1% Tween-20). The chromogenic reaction was carried out in a solution containing 20 μl of nitro-blue tetrazolium/5-bromo-4-chloro-3-inodolyl phosphate (NBT/BCIP) stock solution (Roche Applied Science) in every 1 ml of SMT on ice in the dark for 10 minutes or until the reaction was completed (staining was monitored under a dissecting microscope). Finally, sections were washed in PBS, dehydrated in serial ethanol solutions, cleared with toluene, and mounted in Permount. Unless otherwise indicated, all procedures were performed at RT.
Double labeling of Wisteria lectin and CSPG antibody
To investigate CSPG distributions and PNNs, double staining of Wisteria lectin and CS-56 was performed. The paraffin cross sections of noninjured SC were rehydrated, washed with PBS, and blocked as described above. The sections were incubated with biotinylated WFL (1:200) and CS-56 antibody (1:200) in the above blocking solution overnight at 4°C, washed, blocked, and visualized by incubation in streptavidin conjugated with Alexa Fluor 680 (1:500) and goat anti-mouse IgM conjugated with Alexa Fluor 594 (1:500). Finally, the sections were washed in PBS and mounted in VESTASHIELD mounting medium.
Riboprobe synthesis
The cloned RPTP cDNA fragments were used as templates for the generation of digoxigenin (Dig) or biotin-labeled antisense riboprobes for ISH on paraffin sections or wholemount brain preparations of control and SC-transected lamprey. We identified three RPTP genes, PTPσ, LAR, and PTPδ; all belong to the type IIa RPTPs. By analogy with mammalian proteins, their features include three extracellular Ig-like and eight extracellular FN III repeats and two intracellular phosphatase domains (Pulido et al., 1995). The lamprey proteins shared ~65% amino acid identity with those of the full-length human proteins, and ~84% identity with the human intracellular catalytic domains. To generate probes specific for the tested genes, we aligned all the cloned RPTP fragments. The low homology (<40 %) regions of the FN III domains were selected for synthesis of probes for PTPσ and LAR, as shown in Figure 3. PTPδ was omitted from the current study because it has not yet been shown to bind to CSPGs. A PCR strategy for rapid generation of template was used. The PCR primers were designed to amplify the targeted region in the FN III domain, and the T7 promoter sequence was added to the 5′ end of the antisense primer. The Dig-labeled antisense RNA probes were constructed from amplified cDNA template, which contained the T7 promoter sequence upstream of the antisense-strand, by using an RNA transcription kit (Stratagene, La Jolla, CA) with Dig RNA-labeling Mix, as recommended by the manufacturer (Roche Applied Science).
Figure 3.

Construction of specific probes for PTPσ and LAR. The sequences of cloned lamprey PTPσ, LAR, and PTPδ genes were aligned, and portions of the less conserved fibronectin (FN) III domain (indicated by the box) were chosen to create templates for construction of LAR and PTPσ probes.
In situ hybridization in wholemounted lamprey brain
For wholemount ISH, the animals were reanesthetized, and the brainstems were removed, pinned flat on Sylgard strips, fixed in 4% PFA for 2 hours, and washed three times in PBS, and then in 70% ETOH at 4°C overnight for immediate use, or kept in a −20°C freezer for future use. ISH was carried out by a modification of the chromogenic method previously described (Swain et al., 1994). Briefly, wholemounted brainstem preparations were washed in PTW (0.1% Tween-20 in PBS) and pre-hybridized at 50–55°C in hybridization solution (50% deionized formamide, 5X standard saline citrate [SSC], 100 mg/ml Torula yeast RNA, 100 mg/ml wheat germ tRNA, 50 mg/ml heparin, 0.1% Tween-20) for 60 minutes, followed by hybridization overnight on a Nutator at 50–55°C in the same solution plus 1 μg/ml Dig-labeled antisense RNA probes for PTPσ or LAR. Specimens were washed in hybridization solution at 55°C followed by room temperature washes in PTW and PBT (0.1% bovine serum albumin, 0.2% Triton X-100 in PBS). AP-conjugated anti-Dig antibody (1:1,000; Roche Applied Science) was applied to tissue overnight at 4°C. The tissue was washed sequentially in PBT and SMT, and the chromogenic reaction was carried out in a solution containing 20 μl of NBT/BCIP stock solution (Roche Applied Science) in every 1 ml of SMT on ice in the dark for 1 hour or until the reaction was completed (staining was monitored under a dissecting microscope). Finally, specimens were washed in PBS, dehydrated in serial ethanol solutions, cleared with toluene, and mounted in Permount.
In situ hybridization on paraffin sections
The wholemount ISH gives excellent overviews of the expression of the target molecules, but questions remain regarding completeness of probe penetration and background staining. To confirm the results in wholemounts, ISH was also performed on paraffin sections taken from one lamprey brain at each time point (control and 1, 2, 4, 7, and 10 weeks). Lampreys were reanesthetized, and the brain and SC as far caudal as the first gill were fixed in 4% PFA for 3–4 hours. Tissues were rinsed in PBS, dehydrated in serial ethanol overnight in a tissue processor, and embedded in paraffin. Horizontal sections (10 μm) were deparaffinized and processed for ISH as described previously (Swain et al., 1994). Briefly, after rehydration, sections were washed in PTW (3X 5 minutes) and prehybridized as above at 50–55°C for 1 hour. The PTPσ or LAR probe was applied to slides at a concentration of 1 μg/ml in hybridization solution, and incubated overnight at 50–55°C. To prevent evaporation, the slides were covered with HybriSlip (Sigma-Aldrich) and kept in a moist chamber. On the next day, the slides were washed in hybridization solution at 55°C (3X 10 minutes), PTW/hybridization solution (1:1) for 10 minutes, PTW at room temperature (3X 10 minutes), and PBT (3X 10 minutes). AP-conjugated Dig antibody diluted 1:1,000 in PBT was applied overnight at 4°C. The sections were washed in PBT (3X 5 minutes) and SMT. The chromogenic reaction was performed as described above for 10–15 minutes. The reaction was stopped in PBS and the slides were dehydrated, cleared, and mounted in Permount.
Correlation of PTPσ expression with regenerative probability
The lamprey brain contains 18 pairs of neurons (36 neurons) that have been individually identified and characterized with regard to the probability that they will regenerate by 10 weeks post transection (Jacobs et al., 1997). To correlate PTPσ expression with regenerative probability, the level of expression of PTPσ mRNA in individual identified neurons was estimated in brainstem wholemounts. A semiquantitative integer scale from 0 to 3 was used to grade the intensity of labeling for PTPσ message, as follows: 0, no label observed; 1, faint staining limited to the perimeter of the perikaryon; 2, faint staining throughout cytoplasm; and 3, intense staining throughout cytoplasm and obscuring the nucleus (Jacobs et al., 1997). To confirm the reproducibility of this scale, scoring was performed independently by two observers, one of whom was blind to the experimental manipulation of the animals. There was a 97% concordance between the two scorers. For each cell type, a PTPσ expression score was calculated by using the total staining intensity score for that type of identified neuron divided by the total maximum possible staining intensity score (i.e., the number of animals × 2 cells of each type per animal × 3 [the maximum density score]) multiplied by 100. The PTPσ expression score for particular neurons was then correlated with the previously determined probability of regeneration for that type of neuron. To determine whether PTPσ expression is induced by transection, seven intact control animals and seven animals at 2 weeks post transection were studied. PTPσ-positive neurons were scored on a 0–3 scale of staining intensity and counted. Expression scores before and after SC transection were compared.
Correlation of PTPσ expression with markers of apoptosis
Previous determinations of regenerative ability have suggested probabilities of regeneration of 3.7–70.4% for individually identified RNs, and 13.8–73.3% for cytoarchitectonically defined neuron groups (Jacobs et al., 1997), as summarized in Figure 1. We have reported that many bad-regenerating neurons die at long times after SC transection (Shifman et al., 2008). Whether an individual neuron will regenerate or undergo apoptotic cell death cannot be predicted before injury, and axotomy may cause changes in PTPσ expression. To correlate PTPσ expression with a molecular marker for apoptosis, neurons were double-labeled for activated polycaspases and expression of PTPσ mRNA. The SCs of five animals were transected at the level of the 5th gill, and the animals were allowed to recover in fresh water tanks from 1 to 2 weeks; another two animals without SC transection were included as controls. The brains were removed and processed to label activated caspases by using fluorochrome-labeled inhibitors of caspases (FLICA) (Barreiro-Iglesias and Shifman, 2012) and then to label PTPσ mRNA by ISH, as described above. To prevent mRNA degradation, all FLICA staining procedures were carried out at 4°C. After removal from the skull, brains were immediately incubated in 300 μl of PBS containing 2 μl of 150X FLICA labeling solution for 1 hour and washed 6X 5 minutes with 1X wash buffer in dark on a nutator. After washes, the posterior and cerebrotectal commissures of the brain were cut along the dorsal midline, pinned flat on a small strip of Sylgard, and fixed in 4% PFA in PBS for 2 hours. After fixation, the brains were washed 3X 15 minutes with PBS (protected from light), mounted wet on glass slides, observed, and photographed (80i, Nikon). They were then washed again with PBS and placed in 70% ethanol overnight at −20°C for ISH. The correlation between potentially apoptotic cells and expression of PTPσ was assessed. To correlate PTPσ expression with actual regrowth, a similar experiment was performed in a lamprey at 7 weeks post transection. A dextran tetramethyl-rhodamine–soaked (DTMR, 10 kDa, 5% in 0.1 M Tris buffer, pH 7.4) Gelfoam plug was inserted into a SC 5 mm caudal to the first transection to label neurons whose axon had actually regenerated and reached to this level. After 1 week when fluorescent tracer had successfully back-filled RNs, the brain was removed and processed for FLICA and ISH for PTPσ as above.
Double labeling of PTPσ and LAR mRNA
To investigate the colocalization of PTPσ and LAR in neurons of the lamprey brain, fluorescence ISH (FISH) was performed. A biotin RNA labeling mix was used for PTPσ probe synthesis. Biotin-PTPσ and Dig-LAR probes were applied to two wholemounted lamprey brains simultaneously: one brain was taken from an unoperated animal as a control, and the other was taken 2 weeks after SC transection. To eliminate autofluorescence, which is typically seen in lamprey brain after SCI, probe signals were detected by sheep anti-Dig antibody labeled with an Alexa Fluor 680 donkey anti-sheep IgG antibody and Alexa Fluor 750 streptavidin; the wavelengths of these signals are distinct from the wavelength range of autofluorescence.
RESULTS
Distribution of CSPGs in lamprey spinal cord
The distribution of CSPGs in a cross section of noninjured SC is shown in Figure 2. The CSPGs were widely distributed in the extracellular matrix, as well as in cell bodies of the gray matter. The level of CSPGs was lower in the dorsal columns (arrowhead in Fig. 2A), where most ascending primary sensory axons are located. After digestion with ChABC, CSPGs staining was greatly reduced (Fig. 2B), indicating that the CS-56 antibody specifically recognizes lamprey CSPGs. Similar results were seen with the Cat-316 antibody, e.g., after treatment of ChABC the staining density was greatly reduced. Staining with 2B6 (1:200, Seikakagaku, Tokyo, Japan, an antibody recognizing digested CSPG stumps) showed an increase in labeling for CSPG stumps after digestion with ChABC (data not shown). The western blots in Figure 2C revealed that these two antibodies recognized bands in same size (arrows), similar to a staining pattern of mouse brain sample. WFL staining in a longitudinal section revealed that lectin reactivity was around neurons and dendrites. This distribution resembled the staining pattern of PNNs described in mammalian CNS (arrows, Fig. 4A). The Wisteria lectin immunostaining colocalized with that for the anti-CSPG antibody CS-56, especially in gray matter in the middle of the SC, where most neurons and glia cells are located (Fig. 4C-E). The level of CSPGs increased at the site of injury following transection, peaked at 2 weeks, and then gradually decreased to control levels by 10 weeks post transection (Fig. 5). CSPG levels in SC beyond 0.6 mm from the transection site appeared unchanged at all survival times compared with control animals (not shown).
Figure 4.
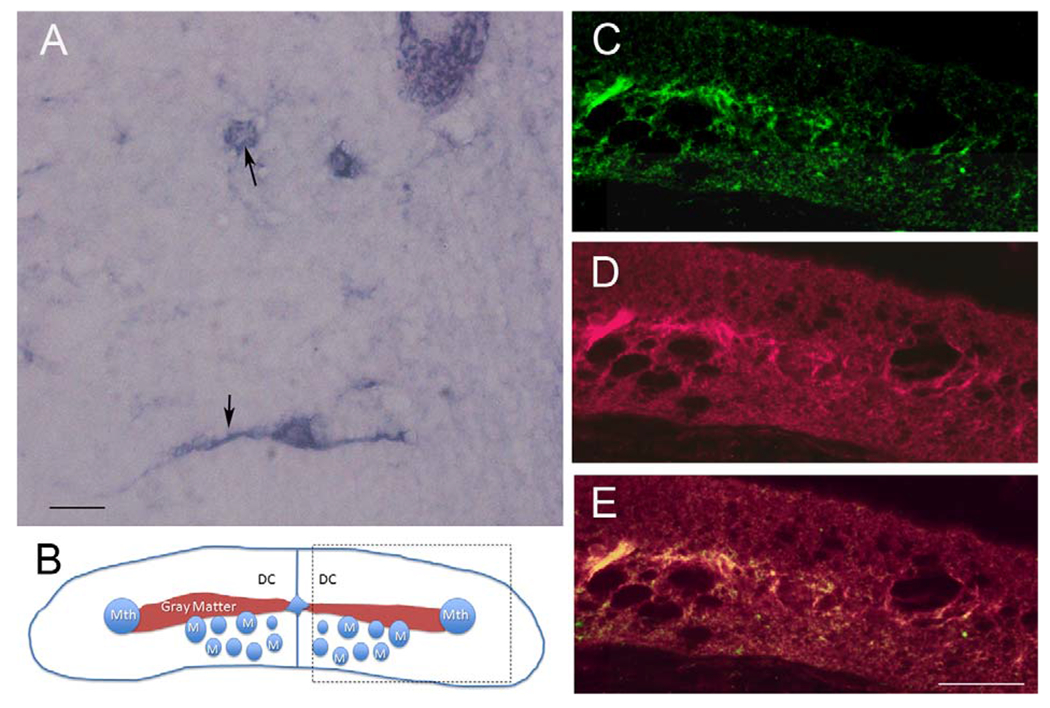
Perineuronal nets are labeled by Wisteria floribunda agglutinin (WFA). A: Lectin staining on an SC longitudinal section, showing WFA reactivity around neurons as well as dendrites (arrows). B: Schematic diagram of a spinal cord cross section. The area within the box represents the part of the cord shown in C-E. Mth, Mauthner axon; M, Müller axon; DC, dorsal columns. C: Transverse section of SC stained with CS-56 for CSPGs. D: The same section stained for WFA. E: Overlap of C and D. Note the higher staining density in the gray matter. Scale bar = 20 μm in A; 50 μm in E (applies to C-E).
Figure 5.
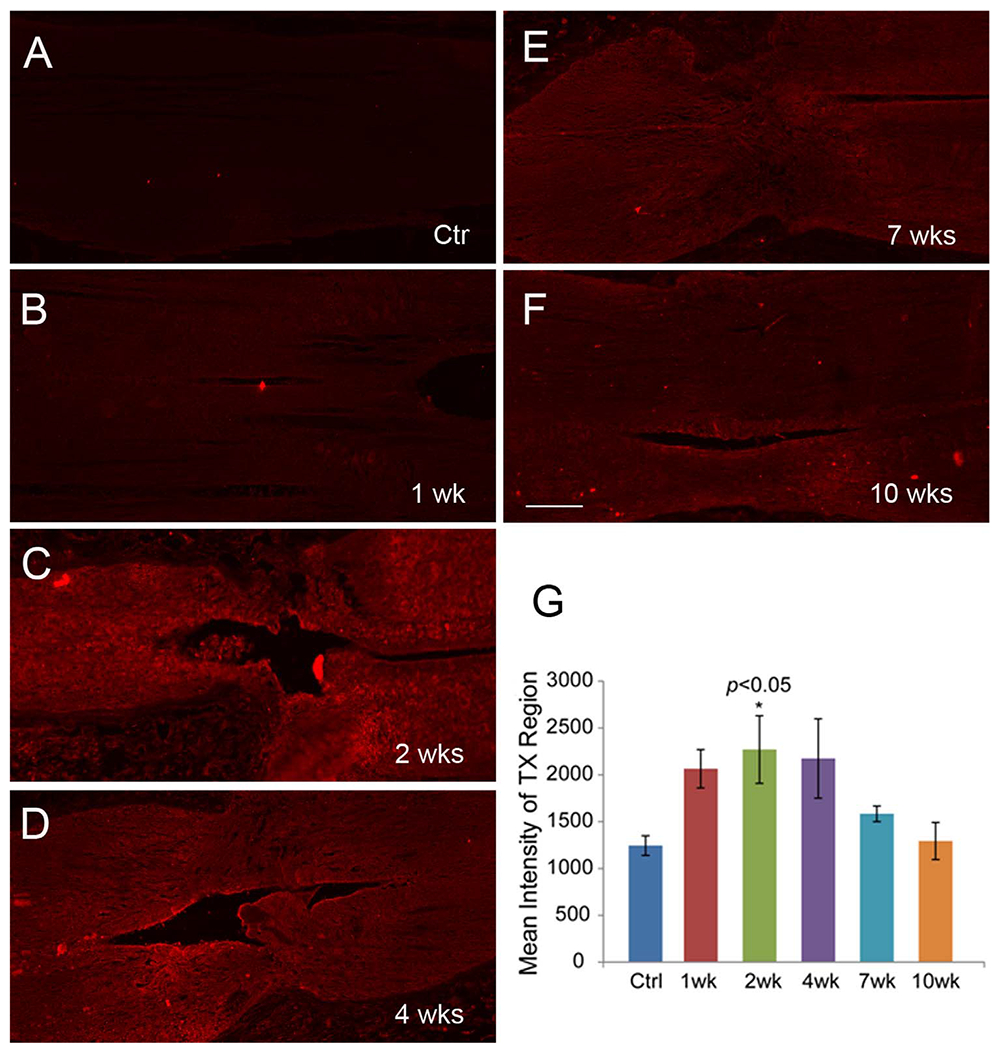
Distribution of CSPGs in the unlesioned control and transected lamprey spinal cords. A-F: CSPG immunostaining with the Cat-316 antibody in longitudinal sections spanning the transection site (arrows) in a control animal and at different post transection times, showing that expression reaches a peak at 2 weeks post transection and declines afterward. G: Intensity analysis of CSPG immunolabeling shows statistically significant increase at 2 weeks post transection compared with control (ANOVA and post hoc two-tailed t-test; n = 5 animals per group). Scale bar = 100 μm in F (applies to A-F).
Lamprey genome has homologs of mammalian CSPG receptors
Sequences from human, chicken, and zebrafish RPTPs were used in BLAST searches to identify many contigs of various lengths in the lamprey genomic database that were homologous to mammalian PTPσ, LAR, and PTPδ. PCR cloning resulted in a total of nine overlapping clones of various lengths, which were assembled with Vector NTI molecular software (Invitrogen). A homology search by BLAST identified three assembled sequences that were homologous to mammalian PTPσ, LAR, and PTPδ, as shown schematically with their putative homologs in Figure 6. Nearly the full-length sequence was obtained for PTPσ (5,437 bp) and LAR (5,856 bp), and a large partial sequence for PTPδ (3,981 bp). All these sequences contain three Ig-like domains, a transmembrane domain, two intracellular phosphatase catalytic domains, and a variable number of FN III domains. The PTPσ and LAR sequences have seven FN III domains, and the PTPδ clone contains only four FN III domains. When all three lamprey sequences were aligned, the two intracellular catalytic domains were the most highly conserved, with over 75% identity; there was an approximately 65% identity in the Ig-like domain, and much less identity in the FN III region (~40%), as shown in Figure 3. This is similar to the sequence similarities among members of the mammalian RPTP family, which in humans show 71% sequence identity in the Ig-like domain, 87% in the catalytic domain, and 47% in the FN III domain.
Figure 6.
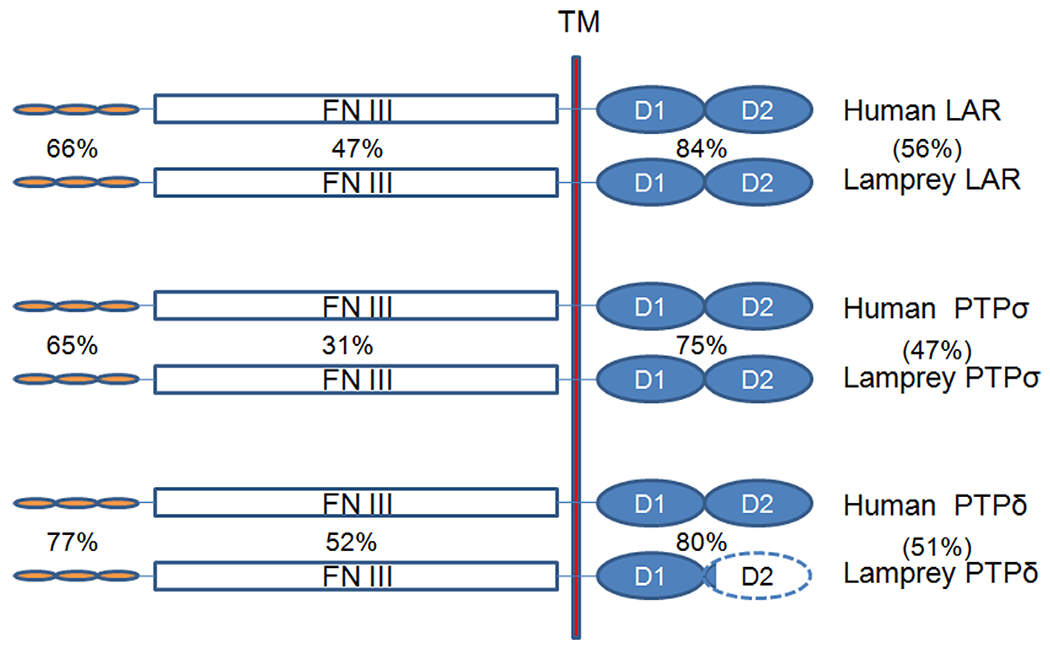
Structure of the RPTPs cloned and sequenced in this study. The percentage of amino acid sequence identity between homologous domains in the lamprey and human proteins are given next to the schematized domains, and the sequence identities for the whole proteins are given in parentheses. Note that lamprey PTPδ is incompletely cloned. The unknown portion of the sequence is indicated by the dashed line. Ig, immunoglobulin-like domain; FN III, fibronectin type III region; TM, transmembrane domain; D, intracellular phosphatase catalytic domain. The FN III regions of lamprey LAR and PTPσ contain seven FN III domains, whereas that of PTPδ contains only four.
PTPσ mRNA is expressed selectively in bad-regenerating neurons
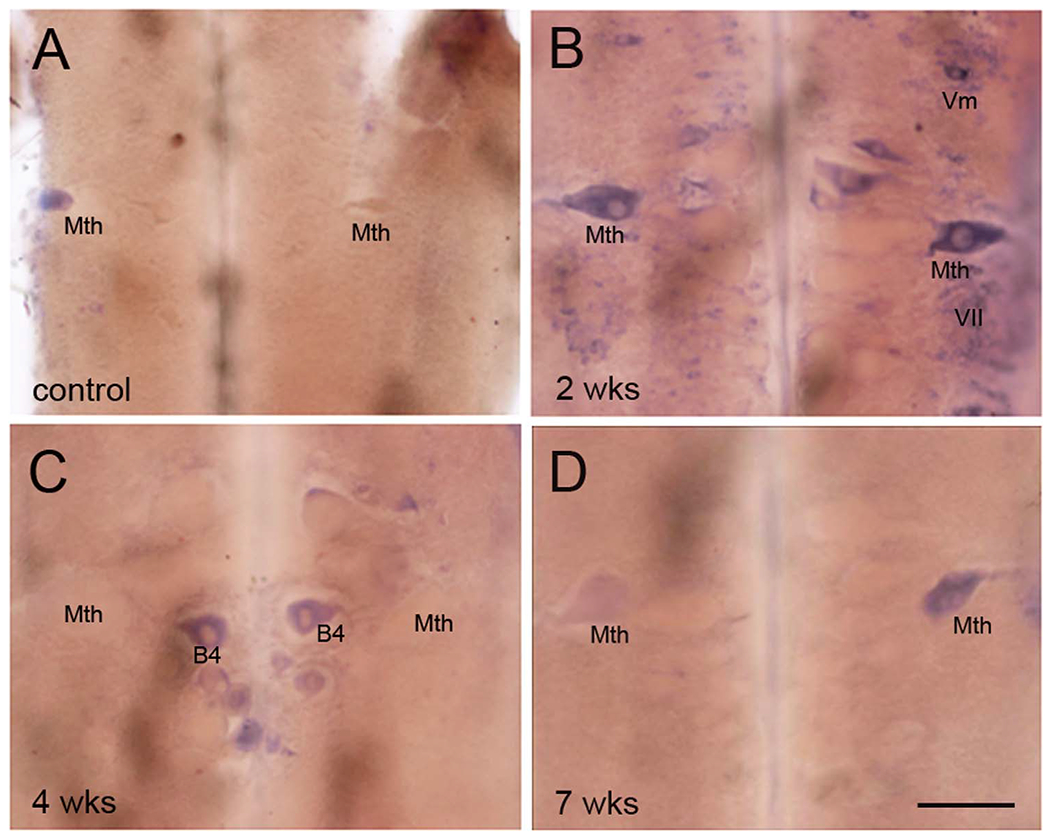
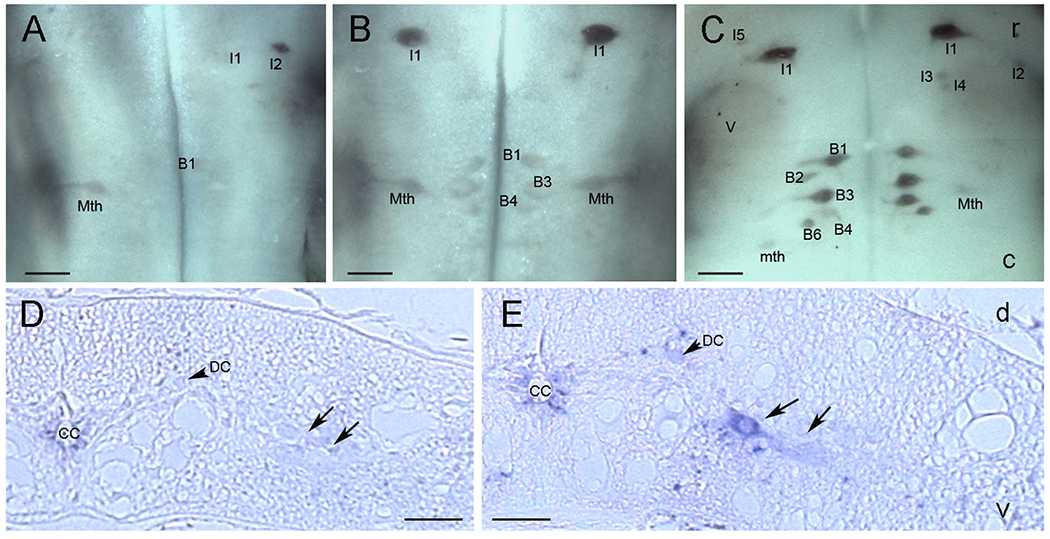
A total of 28 lampreys were investigated for PTPσ mRNA expression on wholemount preparations at different time points after SC transection. LAR mRNA expression was also studied in five animals, including unlesioned control and SC-transected animals up to 10 weeks after injury. PTPσ mRNA was expressed in the brains of unlesioned control animals and in the brains of animals with SC transection, primarily in neurons whose regeneration capacity has been previously reported to be weak (bad regenerators). The PTPσ-positive neurons demonstrated on wholemount (Fig. 7A-D) were also confirmed by ISH on paraffin sections (Fig. 8). {FIG7–8}PTPσ mRNA-positive RNs often included M2 and M3 in the mesencephalon; I1, I2, Mth, B1, B3, and B4 in the rhombencephalon; and additional bad regenerators. In a cross section of SC at 2 weeks post transection (Fig. 7E), PTPσ mRNA was found in some giant axons of RNs (arrows) as well as SC lateral neurons (arrowheads). Projection of individual axons in lamprey SC have been defined by tracing through serial sections (Rovainen et al., 1973). As indicated by arrows, PTPσ mRNA was highly expressed in the axons with poor-regenerative ability (Mth, B3, and I2). This corresponded to the pattern of expression observed in the cell bodies in the brainstem. Note that labeling in the RNs was not always symmetric, i.e., expression is probabilistic, and we have often found that staining intensity could vary greatly between homologous neurons on either side of the brain. This has been true of all transcripts studied thus far in the identified RNs of the lamprey. Similarly, regenerative abilities are not binary, but represent a continuum. Thus, we have often referred to neurons arbitrarily as bad regenerators if their probability of regeneration was less than 40%. By this criterion, I2 would be included in this group because its axon regenerated 37% of the time (Fig. 1C). M1 and B2 are also borderline in their regenerative abilities (48 and 46%, respectively).
Figure 7.
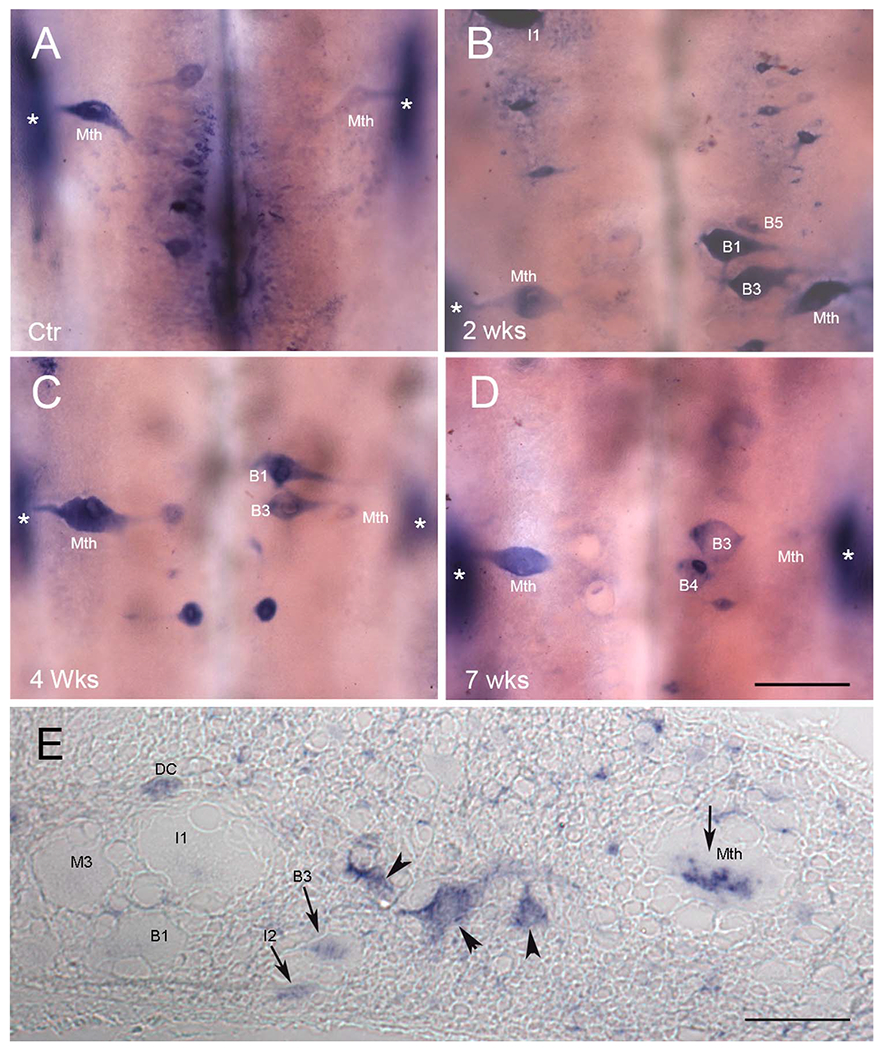
PTPσ mRNA is expressed selectively in bad regenerators. ISH of PTPσ mRNA in whole-mounted lamprey brain shows expression mainly in neurons whose capacity to regenerate axons has previously been reported as weak. Expression was increased after SC transection, concomitantly with the increase in CSPG levels at the transection site. A: Control. B-D; B, 2 weeks, C, 4 weeks, and D, 7 weeks post transection. E: ISH of PTPσ mRNA in a transverse section of SC 2–3 mm rostral to the transection site at 2 weeks post transection. Some individual axons are labeled, based on the description of Rovainen et al. (1973). Arrowheads point to unidentified neurons. Arrows highlight axons that contain mRNA for PTPσ. DC, dorsal cell; Mth, Mauthner axon. In all frames, * indicates the locations of the octavolateralis nuclei, which are out of the focal plane. They expressed PTPσ, probably because they are subject to close axotomy during dissection of the brain at time of removal. See text. Scale bar = 200 μm in D (applies to A-D). Scale bar in E = 80 μm.
Figure 8.
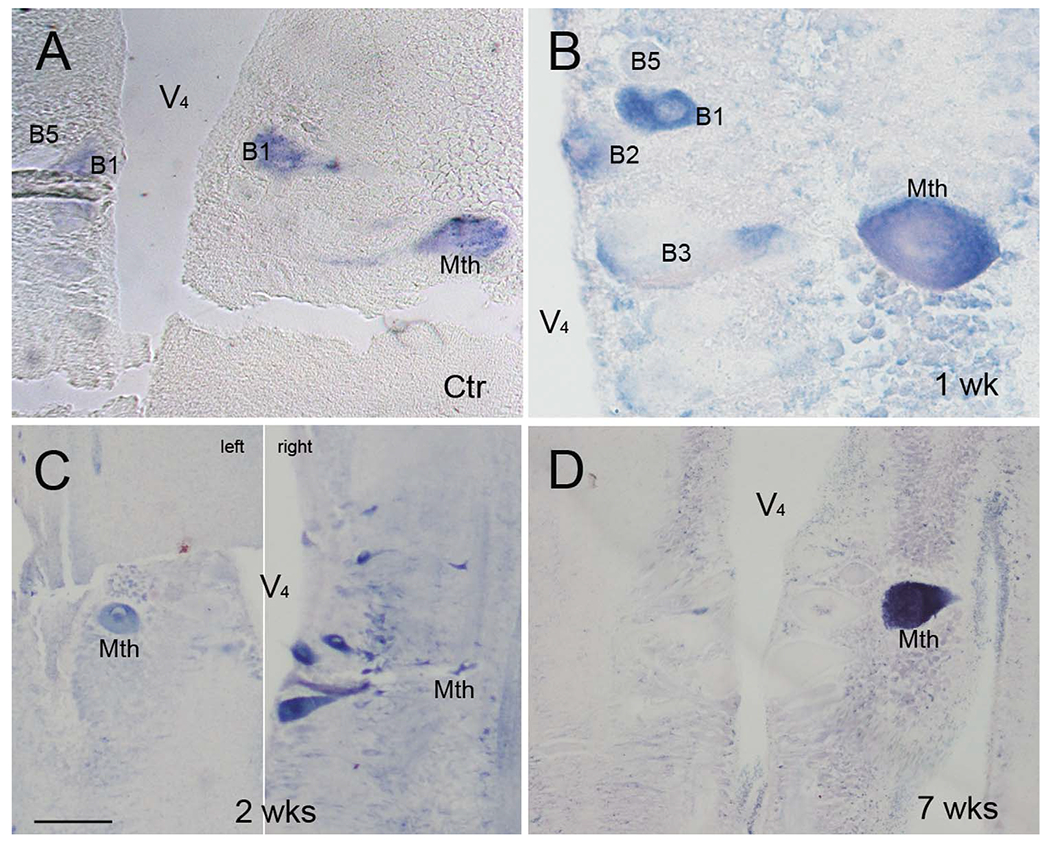
Effect of SC transection on PTPσ mRNA expression in the large reticulospinal neurons. A-D: ISH was performed on 10-μm horizontally sectioned, paraffin-embedded lamprey brains of uninjured control animals, and at 1, 2, and 7 weeks post transection, as indicated. PTPσ mRNA was expressed primarily in bad regenerators, consistent with the pattern observed in wholemount preparations (Fig. 7). Identified neurons are marked in each figure. V44, fourth ventricle. C is comprised of two sections from the same brain, 50 μm apart, to show staining of the Mauthner cell on one side of the brainstem and some of the bulbar Müller cells on the other side. Scale bar = 100 μm in C (applies to A-D).
LAR mRNA was not expressed as strongly as PTPσ but showed a similar distribution pattern (Fig. 9), and was generally also found in poorly regenerating neurons. Previous determinations of regenerative probability in the individually identified RNs ranged from 3.7 to 70.4%, as shown in the histogram of Figure 10A. In this figure the probability of regeneration for each of the 18 pairs of identified neurons is ordered from low (left) to high (right) and is indicated in gray and juxtaposed with corresponding expression levels of PTPσ, indicated in black. The PTPσ expression and regenerative probability of these neurons was inversely correlated (r = −0.79, P < 0.0001; Fig. 10B), and the inverse correlation persisted after SC transection (r = −0.58, P < 0.05; Fig. 10C). To further determine whether a correlation exists between SC transection and PTPσ expression, the total number of PTPσ-positive neurons was counted and compared between control animals and 2 weeks after transection. SC transection slightly enhanced the intensity of expression, and increased the number of cells expressing PTPσ. In seven control animals there were 87 cells expressing PTPσ, compared with 113 cells in seven transected animals, an increase of 30%. However, the increases were not statistically significant (two-tailed Student’s t-test). This result suggests that PTPσ expression is an intrinsic property of poorly regenerating RNs and is consistent with the idea that factors intrinsic to the neuron may contribute to the failure of axonal regeneration after SC transection and determine that a neuron will be a bad regenerator.
Figure 9.
LAR mRNA is expressed and upregulated post transection selectively in reticulospinal neurons known to be bad regenerators. A-D: In wholemount ISH, LAR mRNA expression in (A) a control, noninjured animal, (B) 2 weeks post transaction, (C) 4 weeks post transection, and (D) 7 weeks post transection. The staining pattern is similar to that for PTPσ shown in Figure 7. Scale bar = 200 μm in D (applies to A-D).
Figure 10.
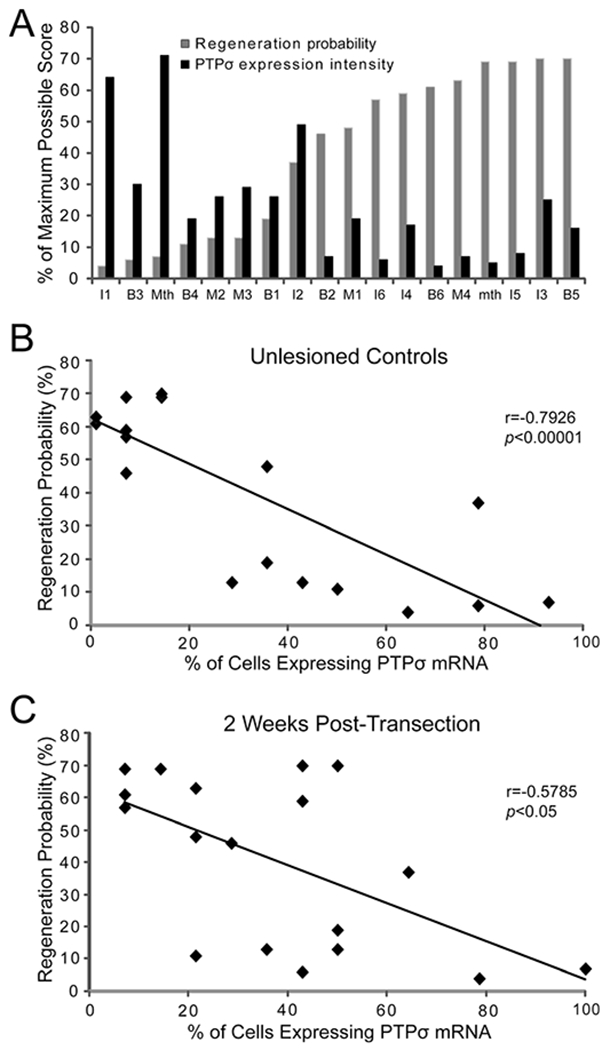
Inverse relationship between PTPσ mRNA expression and probability of axon regeneration. A: Semiquantitative PTPσ expression levels and previously determined regeneration probabilities (Jacobs et al., 1997) of individual identified reticulospinal neurons. Because the difference in expression scores between control animals and those at 2 weeks post transection was not statistically significant, the scores for seven uninjured control animals and seven animals at 2 weeks post transection were pooled (n = 14). The maximum score for any neuron on the semiquantitative scale was 3. Thus the maximum possible cumulative score for the 28 members of any cell type in this pooled sample (2 cells per animal in 14 animals) was 84, and the score for each cell graphed in A was its cumulative score divided by 84. In B and C, the control animals and the animals at 2 weeks post transection were analyzed separately, and the % of each of the reticulospinal neurons expressing PTPσ mRNA was graphed as a function of that cell’s previously determined probability of regeneration Thus for any cell type, the maximum number of cells expressing PTPσ was 14 (2 cells per animal in 7 animals), and the percentage of cells represented in the graphs was the number of labeled cells divided by 14. B: The % of reticulospinal neurons expressing PTPσ in control animals was inversely correlated with the previously determined regeneration probability for each of these cells. C: PTPσ expression 2 weeks post transection was also inversely correlated with regeneration probability.
PTPσ links bad regeneration to apoptosis
The poor regenerative abilities of some of the lamprey reticulospinal axons after SCI is associated with delayed neuronal death by apoptosis (Shifman et al., 2008). The mechanism of the apoptosis has not been fully determined. We stained neurons for activated caspases followed by ISH for PTPσ mRNA. Both stains labeled primarily the poorly regenerating neurons, and neurons that were positive for activated caspases always expressed PTPσ mRNA (Fig. 11). However, the reverse was not true, especially at early times post transection. At 1 week post transection (Fig. 11A,B), some neurons began to show caspase activation, and these same neurons also stained for PTPσ mRNA (not shown for the 1-week animal). However, some neurons slightly expressed PTPσ mRNA (arrows in Fig. 11B) without caspase activation (arrows in Fig. 11A). At 2 weeks post transection, caspases were activated in more neurons compared with 1-week animals (Fig. 11C,D), primarily in poorly regenerating neurons such as I1, M2, B3, B4, and Mth. Again, these neurons also expressed PTPσ mRNA. However, a few neurons that expressed PTPσ mRNA were still negative for activated caspases (arrows in Fig. 11C,D). Of interest, octavolateralis neurons (* in Figs. 7A–D and 11C,D), whose axons were severed close to the perikaryon during dissection of the brain for fixation, also expressed both activated caspases and PTPσ mRNA, suggesting that distance of the site of injury from the perikaryon may be important in determining the latency of caspase activation. The upregulation of PTPσ was specific to the acute neural injury response, because it could be seen in neurons around an accidental injury (not shown) and has not been seen with other riboprobes, e.g., with ISH for neurofilament.
Figure 11.
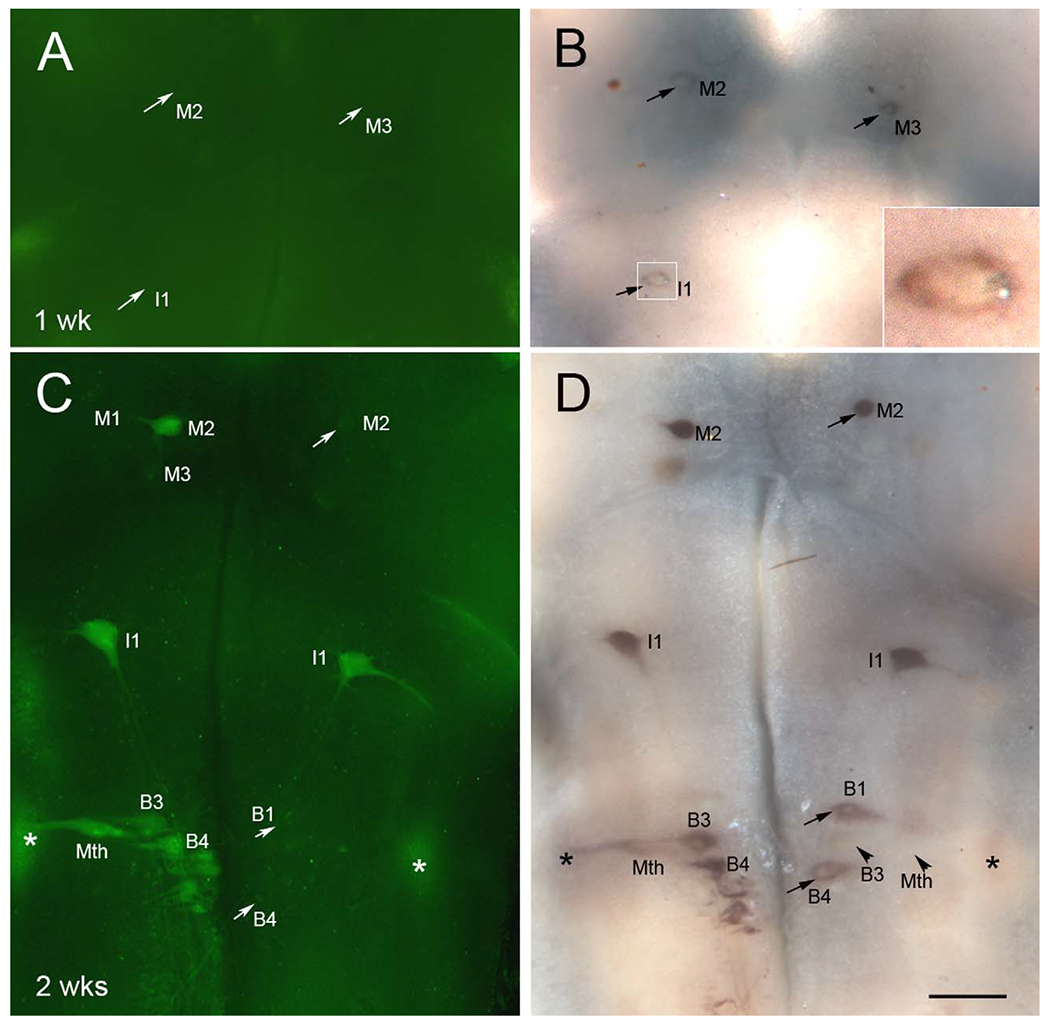
Neurons in which caspases are activated post transection first upregulate PTPσ mRNA expression. A-D: Lamprey brains were stained for activated poly-caspases (A,C) followed by wholemount ISH for PTPσ mRNA (B,D), at 1 week (A,B) and 2 weeks (C,D) after SC transection. Neurons undergoing apoptosis were positive for activated caspases, as shown in C, and also expressed PTPσ mRNA, as shown in D. However, many neurons that were already slightly positive for PTPσ mRNA at 1 week post transection, e.g., M2, M3, and I1 in B, did not yet show caspase activation. Even at 2 weeks post transection, when PTPσ expression was strong, some PTPσ-expressing cells did not show caspase activation, e.g., M2, B1, and B4 in C and D (arrows), although most of these will eventually show caspase activation (Hu et al., 2013), indicating that expression of PTPσ mRNA precedes activation of caspases. In any one animal, some bad regenerators (e.g., B3, Mth, arrowheads in D) showed neither caspase activation nor PTPσ expression. Asterisks in C and D indicate the octavolateralis nucleus, containing the sensory neurons of the lateral line nerve. Insert in B is enlargement of I1. Scale bar = 250 μm in D (applies to A-D).
These results were consistent in all brains examined. They suggest a possible link between expression of PTPσ mRNA and neuronal death, and also suggest that PTPσ mRNA expression may precede activation of caspases. In any given brain, some bad-regenerating neurons showed neither caspase activation nor PTPσ mRNA expression (arrowheads pointing to B3 and Mth in Fig. 11D). It is not clear that they will not regenerate, because all neurons had a finite regenerative probability, even if low (6 and 7% for B3 and Mth, respectively). To correlate PTPσ expression with actual regrowth of those identified RNs, we have labeled neurons whose axon regenerated at least 5 mm caudal to the transection site by backfilling with fluorescent tracer. The brain tissue was processed for FLICA, labeling activated caspases and ISH with PTPσ. The results are shown in Figure 12; again, mRNA of PTPσ selectively existed in the neurons undergoing apoptosis but not in neurons with regenerated axons. The results provided direct evidence that the CSPG receptor PTPσ was selectively expressed in the neurons undergoing apopotosis and revealed the link between PTPσ and neuron death.
Figure 12.
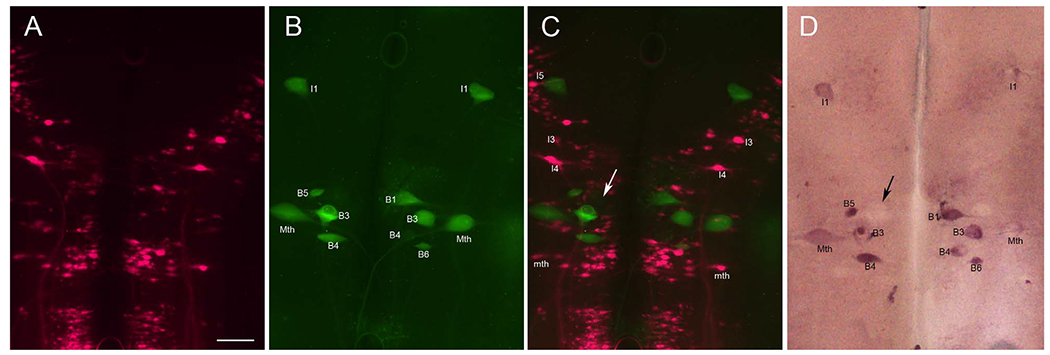
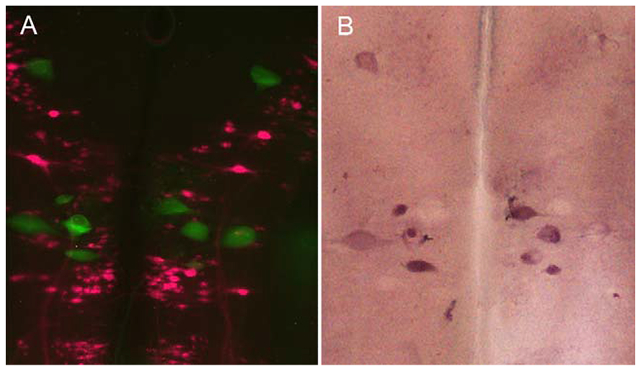
PTPσ mRNA is expressed in neurons undergoing apoptosis but not in regenerating neurons. A: Regenerated spinal-projecting neurons retrogradely labeled with the fluorescent tracer DTMR applied to a second transection placed 5 mm caudal to the original lesion 7 weeks later. This identified those spinal-projecting neurons that had regenerated beyond the transection. B: Reticulospinal neurons (identities indicated) undergoing apoptosis are stained by FLICA in green. C: Overlay of images in A and B. The absence of double-labeled neurons indicates that neurons whose axons had regenerated do not show caspase activation and vice versa. D: ISH for PTPσ reveals that FLICA-positive neurons also expressed PTPσ mRNA. Variations in staining density may indicate different stages of apoptosis. An example is that a swollen B1 neuron was weakly stained with FLICA in C and negative for PTPσ in D. This could reflect that it was in an advanced stage of apoptosis (arrows). Scale bar = 100 μm in A (applies to A-D).
Developmental increase in expression of PTPσ mRNA in lamprey brain
The capacity for axonal regeneration declines with increasing age. Even in the lamprey, which shows substantial regeneration of reticulospinal axons in the adult (Lurie and Selzer, 1991a), regeneration was reported to be less robust in the adult than in the larva (Cohen et al., 1989). The mechanism responsible for this age-dependent loss of intrinsic regeneration ability is elusive. In the present study, PTPσ mRNA and LAR mRNA were selectively expressed in neurons that regenerate poorly. Moreover, when we examined the expression of PTPσ mRNA in lamprey brains and SCs at different ages, the spinal-projecting neurons that expressed PTPσ increased with increasing age (Fig. 13A-C); similar results were also observed in spinal neurons (arrows in Fig. 13D,E). In larval animals, PTPσ mRNA was often expressed in neurons with poor regenerating ability; however, in adults, the majority of RNs expressed PTPσ; expression in good regenerators was either lower or negative, such as in B5, mth, I3, and I4 (Fig. 13C). Thus RPTPs may participate in the developmentally determined loss of neuron-intrinsic regenerative ability.
Figure 13.
Developmental increase in PTPσ mRNA expression in lamprey brain and spinal cord. A: ISH for PTPσ mRNA in the whole-mounted brain of a 9-cm larva (3–4 years old) shows weak PTPσ mRNA expression. B: Increased expression in identified reticulospinal neurons in the brain of a 12-cm larva (4–5 years old). C: Further increase in PTPσ mRNA expression in reticulospinal neurons in the brain of a 13-cm young adult. D: PTPσ mRNA expression in a transverse paraffin section of SC in a 7-cm larva (2–3 years old). E: Increased expression in neurons of SC in a 12-cm larva (4–5 years old). Arrows in D and E point to medium sized neurons; DC, dorsal cell; D, dorsal; V, ventral. Scale bar = 200 μm in A-C; 80 μm in D,E.
Double labeling of PTPσ and LAR mRNA in lamprey brain
Both PTPσ and LAR are CSPG receptors and belong to the type IIa family of RPTPs. In the present study, mRNAs for both of these receptors were expressed selectively in neurons that have poor regenerative ability. To investigate the extent to which expression of PTPσ and LAR mRNA is colocalized in lamprey brain, we carried out double FISH staining. We found that many neurons expressed only PTPσ mRNA and not LAR mRNA (Fig. 14A-C), but expression was colocalized in some neurons, such as the I1 in Figure 14D-F. The link between coexpression and regenerating ability is unknown.
Figure 14.
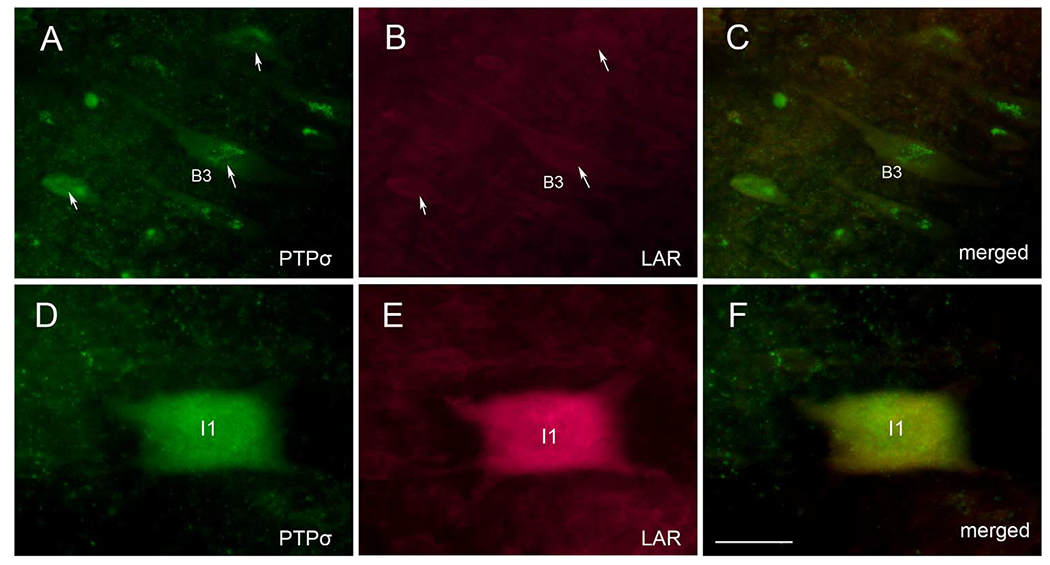
Double labeling for PTPσ and LAR mRNA in lamprey brain 2 weeks after SC transection shows partial overlap. A: ISH for PTPσ. B: ISH for LAR. Arrows point to the neurons that express PTPσ in A but are negative for LAR in B. C: Overlay of A and B suggests absence of overlap among this group of neurons. D: An I1 cell that expresses PTPσ. E: The same neuron expresses LAR. F: Overlay of D and E. Scale bar = 80 μm in F (applies to A-F).
DISCUSSION
Relationship of CSPG location to axon regeneration
CSPGs are major components of the glial scar that contribute to the failure of axonal regeneration after SCI in mammals (Busch and Silver, 2007; Sharma et al., 2012; Silver and Miller, 2004). CSPGs also act during neural development as signals that repel growth cones away from CSPG-abundant areas (Siebert and Osterhout, 2011). In the present study of the lamprey, in which axons regenerate following SCI, we found that levels of CSPG in the glial scar increase to a peak at 2 weeks following complete SC transection, and then return gradually to nearly normal levels by 10 weeks. Interestingly, increased levels of CSPGs persist much longer in mammals, in which axons fail to regenerate after SCI. The time course of CSPG expression in our study is consistent with that of regeneration in the large reticulospinal axons. In animals of the present size, axons retract during the first 1–2 weeks after SC transection, followed by regrowth. Regenerating axon tips enter the scar by 4 weeks, and grow beyond the transection site by 5–7 weeks (Yin and Selzer, 1983). Thus the increase in CSPG expression near the lesion occurs just as axons are beginning their forward growth in the proximal stump, and persists through the period when axons are crossing the lesion and invading the distal stump. This is consistent with a role for CSPGs as inhibitors of true axon regeneration (as distinguished from collateral sprouting by spared axons) in the CNS.
However, the role of the scar in lamprey is different from that in mammalian SC. The glial “scar” in the lamprey provides net positive cues for axon regeneration, because regenerating axons grew preferentially through a hemisection scar rather than around it (Lurie and Selzer, 1991c). Moreover, killing the glial cells in the lesion site eliminated the ability of axons to grow across a transection gap (Lurie and Selzer, 1991b). Glial fibers in control animals or in the SC far from a transection were oriented transversely, but adjacent to the transection site, glial cells sent thickened, longitudinally oriented processes into the lesion blood clot. These longitudinal glial processes preceded the regenerating axons (Lurie et al., 1994). This finding suggests that longitudinally oriented glial fibers may serve as a bridge along which axons can regenerate. This does not mean that the increase in CSPGs in the region of the injury is not inhibitory to axon regeneration. It is widely accepted that even in mammals, the glial scar contains both growth-promoting and growth-inhibiting factors (DiProspero et al., 1997; Muir et al., 2002). Whether an axon grows through a scar would depend on the balance of that axon’s sensitivities to the positive and negative cues, and in the mammalian CNS, the balance for all axons is strongly negative. In the lamprey, by definition, good-regenerating neurons are those for which the balance of effects is positive. In the present study, those neurons that are intrinsically good regenerators (and good survivors) after axotomy most often did not express the CSPG receptors PTPσ and LAR. Therefore, if these receptors mediate a substantial regeneration-inhibiting effect, then failure to express these receptors might be an important reason that the axons of good-regenerator neurons have an overall positive response to the scar with regard to their growth.
Neuron-intrinsic determinants of regeneration
Regenerative failure in the mammalian CNS has been ascribed to both the limited intrinsic growth capacity of postnatal neurons (Cai et al., 2001; Hannila and Filbin, 2008; Park et al., 2010) and environmental factors (David and Aguayo, 1981; Fawcett, 2009; McGee and Strittmatter, 2003). The heterogeneous regenerative abilities of spinal-projecting axons in lamprey present an excellent opportunity to study the mechanisms by which intrinsic factors impact axonal regeneration. It is still unclear whether this heterogeneity is attributable to variable expression of developmentally regulated transcription factors such as the KLF molecules or growth-regulatory molecules. In zebrafish, for example, regenerative success of spinal-projecting neurons in the brain has been correlated positively with upregulation of L1.1 (Becker et al., 1998, 2004) and GAP-43 (Becker et al., 2005) after SCI. In lamprey, UNC-5 expression was inversely correlated with regenerative ability. The upregulation of UNC-5 expression in bad-regenerating neurons begins before axons complete their retraction and begin their forward growth, so this property would seem to be intrinsic to the neuron but not the consequence of lacking regeneration (Shifman and Selzer, 2000). The present results demonstrate the selectivity of RPTP expression in poorly regenerating lamprey reticulospinal neurons, which provide important additional evidence that intrinsic factors play a critical role in determining whether neurons will regenerate. We found that RPTPs were present in poorly regenerating neurons even in the uninjured lamprey, indicating that expression of these molecules is programmed prior to injury and consequently may be a neuron-intrinsic property that correlates with regenerative ability. One important test for RPTPs will be to determine whether blocking their expression enhances axonal regeneration in the SC of lampreys or even in mammals.
Role of CSPG receptors in retrograde neuronal death
Our study focused on PTPσ and LAR, both of which belong to the family of type IIa RPTPs. The results showed that activation of caspases was correlated with PTPσ mRNA expression. PTPσ was expressed before caspases were activated, raising the possibility that PTPσ activation may play a role in activating the apoptosis pathway. Overexpression of LAR has been reported to activate the caspase pathway directly and to induce p53-independent apoptosis in mammalian cell lines, suggesting that LAR contributes to regulation of growth and survival signals (Weng et al., 1998). Although in the present study, we did not examine the correlation between LAR and activation of caspases, because PTPσ and LAR share over 80% sequence identity in their intracellular phosphatase domains, and the ability of LAR to induce apoptosis in cell lines requires the phosphatase domain (Weng et al., 1998, 1999), it is possible that PTPσ also activates a caspase pathway by the same phosphatase-dependent mechanism, and that this accounts for the correlation between PTPσ expression and caspase activation seen in lamprey reticulospinal neurons. Previous studies have demonstrated a relationship between RPTPs and apoptosis, including the observation that inhibitors of PTPs can block apoptosis (Huang et al., 1996; Lund-Johansen et al., 1996; Yang et al., 1996) For example, activation of PTPσ and LAR by SC transection might contribute to cell death by dephosphorylation of proteins such as Akt that are critical for cell survival (Fisher et al., 2011). Moreover, the intracellular domains of all RPTPs, including PTPσ and LAR, contain putative caspase cleavage motifs, which, once cleaved by caspases, would increase their phosphatase activity and promote apoptosis (Halle et al., 2007a,b).
These results are consistent with the idea that LAR and PTPσ act both upstream and downstream in the caspase pathway. The RPTPs are known to dephosphorylate downstream proteins and to regulate a wide range of signaling pathways related to cell adhesion, axon growth, and axon guidance during development (Beltran and Bixby, 2003; Johnson and Van Vactor, 2003; Stoker, 2001, 2005). In contrast to mammalian models, the CSPGs in an injured site reached a peak at 2 weeks post transection in lamprey, when injured axons are just beginning their forward growth but have not yet reached the transection site, and then declined thereafter. Furthermore, PTPσ mRNA was not found in the neurons with regenerated axons in the animal that had recovered for 7 weeks but rather in the neurons undergoing apoptosis (Fig. 11). Our results raise the possibility that CSPGs induce apoptosis as well as modulate regeneration of lamprey CNS neurons through binding to PTPσ and LAR, by mechanisms that are still unknown.
Relevance of age-related increase in CSPG receptor expression to loss of regenerative ability during development
It has long been believed that axons in the adult mammalian CNS fail to regenerate because of a growth-inhibiting environment at the injury site (Schwab and Bartholdi, 1996). However, heterogeneity in the ability of different neurons to regenerate their axons through the same environment, as shown most clearly in the lamprey spinal-projection system (Jacobs et al., 1997), focuses attention on an age-dependent decline in the intrinsic axon growth potential of CNS neurons (Cai et al., 2001; Goldberg et al., 2002; Sun and He, 2010). It is possible that among the neuron-intrinsic properties responsible for this is a decline in the ability to respond to inhibitory environmental cues such as CSPGs, which are known to regulate axon growth during development. The present results show an increase in expression of CSPG receptors during development, which could contribute to age-related loss of regenerative ability and also retrograde neuronal death. Although spinal cord axons regenerated in adult lampreys (Lurie and Selzer, 1991a), this was established without knowing the identities of the regenerating axons and it is not well established how this regeneration compares with that in larval lampreys. It remains to be determined whether the failure of some neurons to regenerate after SCI in lamprey is due to the innate expression of CSPG receptors, whether these receptors act by initiating the apoptotic cascade or by another signaling pathway such as RhoA-ROCK, and whether inhibition of CSPG receptor expression can promote axon regeneration after SCI.
Evidence for CSPG receptor mRNA in axons
We found in situ hybridization signal for PTPσ mRNA in axons, and by comparison with the anatomical studies of Rovainen et al. (1973), the axons appeared to be primarily those of bad regenerators, although we did not attempt to trace them back to their neurons of origin in the brainstem. Protein synthesis has been demonstrated in mammalian axons (Willis and Twiss, 2010). We have performed single-cell PCR on cytosolic microaspirates from the tips of regenerating lamprey axons and found mRNA for several proteins in these axon tips (L. Jin et al., unpublished data). It remains to be determined whether this microaspirated axonal mRNA includes that for PTPσ or LAR, and whether the message is locally translated into protein.
In summary, three RPTP type IIa genes, PTPσ, LAR and PTPδ, were cloned in lamprey and found to be highly homologous to their mammalian counterparts in their Ig and intracellular domains. The CSPG receptors PTPσ and LAR are expressed primarily in neurons that regenerate poorly after SCI and are upregulated developmentally in lamprey brain. In addition, PTPσ mRNA was expressed prior to activation of caspases and not expressed in regenerating neurons, suggesting that it might be involved in a novel form of delayed neuronal death after axotomy, which contributes to the poor regenerative ability of specific neurons in lamprey brain.
ACKNOWLEDGMENTS
Cynthia Laramore helped with GenBank searching and Feisi Liang helped with cloning.
Grant sponsor:
Shriners Research Foundation; Grant number: SHC-85220, R01 NS092876, R01 NS097846 (to M.E.S.).
Footnotes
CONFLICT OF INTEREST STATEMENT
There are no known or potential conflicts of interest.
LITERATURE CITED
- Barreiro-Iglesias A, Shifman MI. 2012. Use of fluorochrome-labeled inhibitors of caspases to detect neuronal apoptosis in the whole-mounted lamprey brain after spinal cord injury. Enzyme Res 2012:835731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Lieberoth BC, Morellini F, Feldner J, Becker T, Schachner M. 2004. L1.1 is involved in spinal cord regeneration in adult zebrafish. J Neurosci 24:7837–7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Bernhardt RR, Reinhard E, Wullimann MF, Tongiorgi E, Schachner M. 1998. Readiness of zebrafish brain neurons to regenerate a spinal axon correlates with differential expression of specific cell recognition molecules. J Neurosci 18:5789–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Lieberoth BC, Becker CG, Schachner M. 2005. Differences in the regenerative response of neuronal cell populations and indications for plasticity in intraspinal neurons after spinal cord transection in adult zebrafish. Mol Cell Neurosci 30:265–278. [DOI] [PubMed] [Google Scholar]
- Beltran PJ, Bixby JL. 2003. Receptor protein tyrosine phosphatases as mediators of cellular adhesion. Front Biosci 8: d87–99. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. 2002. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640. [DOI] [PubMed] [Google Scholar]
- Bruckner G, Grosche J, Schmidt S, Hartig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M. 2000. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol 428:616–629. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. 2007. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol 17:120–127. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Bradbury EJ, Lidierth M, Jones M, Duffy PJ, Pezet S, McMahon SB. 2008. Chondroitinase ABC-mediated plasticity of spinal sensory function. J Neurosci 28: 11998–12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. 2001. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci 21:4731–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AH, Baker MT, Dobrov TA. 1989. Evidence for functional regeneration in the adult lamprey spinal cord following transection. Brain Res 496:368–372. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. 1981. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214:931–933. [DOI] [PubMed] [Google Scholar]
- Davis GR Jr, McClellan AD. 1994. Long distance axonal regeneration of identified lamprey reticulospinal neurons. Exp Neurol 127:94–105. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. 2006. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem 281:17789–17800. [DOI] [PubMed] [Google Scholar]
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. 2012. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci 15:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiProspero NA, Meiners S, Geller HM. 1997. Inflammatory cytokines interact to modulate extracellular matrix and astrocytic support of neurite outgrowth. Exp Neurol 148: 628–639. [DOI] [PubMed] [Google Scholar]
- Fawcett J 2009. Molecular control of brain plasticity and repair. Prog Brain Res 175:501–509. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. 1999. The glial scar and central nervous system repair. Brain Res Bull 49:377–391. [DOI] [PubMed] [Google Scholar]
- Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. 2011. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci 31:14051–14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Gould G, Wang X, Strittmatter SM. 2002. Nogo and the Nogo-66 receptor. Prog Brain Res 137:361–369. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. 2007. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev 54:1–18. [DOI] [PubMed] [Google Scholar]
- Giamanco KA, Morawski M, Matthews RT. 2010. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience 170:1314–1327. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. 2002. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296:1860–1864. [DOI] [PubMed] [Google Scholar]
- Hall GF, Yao J, Selzer ME, Kosik KS. 1997. Cytoskeletal changes correlated with the loss of neuronal polarity in axotomized lamprey central neurons. J Neurocytol 26:733–753. [DOI] [PubMed] [Google Scholar]
- Halle M, Liu YC, Hardy S, Theberge JF, Blanchetot C, Bourdeau A, Meng TC, Tremblay ML. 2007a. Caspase-3 regulates catalytic activity and scaffolding functions of the protein tyrosine phosphatase PEST, a novel modulator of the apoptotic response. Mol Cell Biol 27:1172–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle M, Tremblay ML, Meng TC. 2007b. Protein tyrosine phosphatases: emerging regulators of apoptosis. Cell Cycle 6:2773–2781. [DOI] [PubMed] [Google Scholar]
- Hannila SS, Filbin MT. 2008. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol 209:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhang KG, Selzer ME. 2013. Activated caspase detection in living tissue combined with subsequent retrograde labeling, immunohistochemistry or in situ hybridization in whole-mounted lamprey brains. J Neurosci Methods 220:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TS, Shu CH, Shih YL, Huang HC, Su YC, Chao Y, Yang WK, Whang-Peng J. 1996. Protein tyrosine phosphatase activities are involved in apoptotic cancer cell death induced by GL331, a new homolog of etoposide. Cancer Lett 110:77–85. [DOI] [PubMed] [Google Scholar]
- Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. 2002. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci 22:3553–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AJ, Swain GP, Snedeker JA, Pijak DS, Gladstone LJ, Selzer ME. 1997. Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. J Neurosci 17:5206–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LQ, Zhang G, Jamison C Jr, Takano H, Haydon PG, Selzer ME. 2009. Axon regeneration in the absence of growth cones: acceleration by cyclic AMP. J Comp Neurol 515:295–312. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Van Vactor D. 2003. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev 83:1–24. [DOI] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. 2003. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol 182:399–411. [DOI] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. 2011. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci 34:131–152. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen F, Frey T, Ledbetter JA, Thompson PA. 1996. Apoptosis in hematopoietic cells is associated with an extensive decrease in cellular phosphotyrosine content that can be inhibited by the tyrosine phosphatase antagonist pervanadate. Cytometry 25:182–190. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Selzer ME. 1991a. Axonal regeneration in the adult lamprey spinal cord. J Comp Neurol 306:409–416. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Selzer ME. 1991b. The need for cellular elements during axonal regeneration in the sea lamprey spinal cord. Exp Neurol 112:64–71. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Selzer ME. 1991c. Preferential regeneration of spinal axons through the scar in hemisected lamprey spinal cord. J Comp Neurol 313:669–679. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Pijak DS, Selzer ME. 1994. Structure of reticulospinal axon growth cones and their cellular environment during regeneration in the lamprey spinal cord. J Comp Neurol 344:559–580. [DOI] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. 2003. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci 26:193–198. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. 1994. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13:805–811. [DOI] [PubMed] [Google Scholar]
- Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, Ellis C, Fawcett JW, Rogers JH. 2002. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res 100:103–117. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. 2008. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322:963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Kanter JL, He Z. 2010. PTEN/mTOR and axon regeneration. Exp Neurol 223:45–50. [DOI] [PubMed] [Google Scholar]
- Pijak DS, Hall GF, Tenicki PJ, Boulos AS, Lurie DI, Selzer ME. 1996. Neurofilament spacing, phosphorylation, and axon diameter in regenerating and uninjured lamprey axons. J Comp Neurol 368:569–581. [DOI] [PubMed] [Google Scholar]
- Pulido R, Serra-Pages C, Tang M, Streuli M 1995. The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc Natl Acad Sci U S A 92:11686–11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovainen CM. 1967. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Muller and Mauthner cells. J Neurophysiol 30:1000–1023. [DOI] [PubMed] [Google Scholar]
- Rovainen CM, Johnson PA, Roach EA, Mankovsky JA. 1973. Projections of individual axons in lamprey spinal cord determined by tracings through serial sections. J Comp Neurol 149:193–202. [DOI] [PubMed] [Google Scholar]
- Schachner M, Bartsch U. 2000. Multiple functions of the myelin-associated glycoprotein MAG (siglec-4a) in formation and maintenance of myelin. Glia 29:154–165. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. 1996. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76: 319–370. [DOI] [PubMed] [Google Scholar]
- Selzer ME. 1979. Variability in maps of identified neurons in the sea lamprey spinal cord examined by a wholemount technique. Brain Res 163:181–193. [DOI] [PubMed] [Google Scholar]
- Selzer ME. 2003. Promotion of axonal regeneration in the injured CNS. Lancet Neurol 2:157–166. [DOI] [PubMed] [Google Scholar]
- Sharma K, Selzer ME, Li S. 2012. Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol 237:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. 2009. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326:592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. 2000. Expression of the netrin receptor UNC-5 in lamprey brain: modulation by spinal cord transection. Neurorehabil Neural Repair 14:49–58. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Zhang G, Selzer ME. 2008. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. J Comp Neurol 510:269–282. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Yumul RE, Laramore C, Selzer ME. 2009. Expression of the repulsive guidance molecule RGM and its receptor neogenin after spinal cord injury in sea lamprey. Exp Neurol 217:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR, Osterhout DJ. 2011. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem 119:176–188. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. 2004. Regeneration beyond the glial scar. Nat Rev Neurosci 5:146–156. [DOI] [PubMed] [Google Scholar]
- Stoker AW. 2001. Receptor tyrosine phosphatases in axon growth and guidance. Curr Opin Neurobiol 11:95–102. [DOI] [PubMed] [Google Scholar]
- Stoker AW. 2005. Protein tyrosine phosphatases and signalling. J Endocrinol 185:19–33. [DOI] [PubMed] [Google Scholar]
- Sun F, He Z. 2010. Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr Opin Neurobiol 20:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain GP, Snedeker JA, Ayers J, Selzer ME. 1993. Cytoarchitecture of spinal-projecting neurons in the brain of the larval sea lamprey. J Comp Neurol 336:194–210. [DOI] [PubMed] [Google Scholar]
- Swain GP, Jacobs AJ, Frei E, Selzer ME. 1994. A method for in situ hybridization in wholemounted lamprey brain: neurofilament expression in larvae and adults. Exp Neurol 126: 256–269. [DOI] [PubMed] [Google Scholar]
- Wahl S, Barth H, Ciossek T, Aktories K, Mueller BK. 2000. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J Cell Biol 149:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. 2002. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417:941–944. [DOI] [PubMed] [Google Scholar]
- Weng LP, Yuan J, Yu Q. 1998. Overexpression of the transmembrane tyrosine phosphatase LAR activates the caspase pathway and induces apoptosis. Curr Biol 8:247–256. [DOI] [PubMed] [Google Scholar]
- Weng LP, Wang X, Yu Q. 1999. Transmembrane tyrosine phosphatase LAR induces apoptosis by dephosphorylating and destabilizing p130Cas. Genes Cells 4:185–196. [DOI] [PubMed] [Google Scholar]
- Willis DE, Twiss JL. 2010. Regulation of protein levels in sub-cellular domains through mRNA transport and localized translation. Mol Cell Proteom 9:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Chang J, Gorospe M, Passaniti A. 1996. Protein tyrosine phosphatase regulation of endothelial cell apoptosis and differentiation. Cell Growth Differ 7:161–171. [PubMed] [Google Scholar]
- Yin HS, Selzer ME. 1983. Axonal regeneration in lamprey spinal cord. J Neurosci 3:1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]


