Abstract
Cyclin-dependent kinase 2 (Cdk2) is essential for initiation of DNA synthesis in higher eukaryotes. Biochemical studies in Xenopus egg extracts and microinjection studies in human cells have suggested an additional function for Cdk2 in activation of Cdk1 and entry into mitosis. To further examine the role of Cdk2 in human cells, we generated stable clones with inducible expression of wild-type and dominant-negative forms of the enzyme (Cdk2-wt and Cdk2-dn, respectively). Both exogenous proteins associated efficiently with endogenous cyclins. Cdk2-wt had no apparent effect on the cell division cycle, whereas Cdk2-dn inhibited progression through several distinct stages. Cdk2-dn induction could arrest cells at the G1/S transition, as previously observed in transient expression studies. However, under normal culture conditions, Cdk2-dn induction primarily arrested cells with S and G2/M DNA contents. Several observations suggested that the latter cells were in G2 phase, prior to the onset of mitosis: these cells contained uncondensed chromosomes, low levels of cyclin B-associated kinase activity, and high levels of tyrosine-phosphorylated Cdk1. Furthermore, Cdk2-dn did not delay progression through mitosis upon release of cells from a nocodazole block. Although the G2 arrest imposed by Cdk2-dn was similar to that imposed by the DNA damage checkpoint, the former was distinguished by its resistance to caffeine. These findings provide evidence for essential functions of Cdk2 during S and G2 phases of the mammalian cell cycle.
A substantial body of evidence indicates that DNA synthesis in higher eukaryotes is initiated by activation of cyclin-dependent kinase 2 (Cdk2) (52, 66). Cdk2 associates with cyclin E and is activated shortly before S phase. The actual onset of S phase correlates closely with induction of cyclin A and its binding to Cdk2. Transient transfection of a catalytically inactive form of Cdk2 arrests cells in G1 (74). This arrest was prevented by coexpression of wild-type (wt) Cdk2 (Cdk2-wt) but not other Cdks, suggesting that the mutant abrogates the function of endogenous Cdk2 in a dominant-negative (dn) manner. Similarly, addition of Cdk2 inhibitors or antibodies directed against Cdk2 to Xenopus egg extracts (19), microinjection of antibodies directed against Cdk2, cyclin A, or cyclin E in mammalian cells (54, 56, 73), or mutation of cyclin E in Drosophila (12, 13, 35) can block initiation of DNA synthesis. Candidate substrates of Cdk2 action at the G1-S transition include the retinoblastoma tumor suppressor protein (pRb), CDC6, and NPAT (32, 43, 57, 78, 81). Cdk2 is also implicated in duplication of centrosomes, another important event initiated at the G1/S boundary (26, 41, 46).
Evidence of a more limited scope suggests additional potential roles for Cdk2 in later cell cycle events. The catalytic activity of Cdk2, derived largely from its association with cyclin A, peaks in late S and G2 phases (55, 73). In some Drosophila tissues, mutation of cyclin A blocks mitotic entry in a cyclin B mutant background (36). In Xenopus egg extracts, Cdk2 complexes appear to be required for activation of Cdk1 (Cdc2), independently of Cdk2's role in DNA synthesis (23). In this setting, immunodepletion of Cdk2 or addition of p21WAF1/CIP1 blocks activation of Cdk1. The p21WAF1/CIP1 effect does not appear to result from direct binding to Cdk1, occurs even in the absence of nuclei, and can be rescued by addition of cyclin E-Cdk2 complexes. In HeLa cells, microinjection during S phase of antibodies directed against cyclin A can block cell division, without a gross effect on bromodeoxyuridine (BrdU) incorporation (56). This finding has been supported by two recent microinjection studies in human cells that have provided evidence that Cdk2 may be required to stabilize cyclin B (42) and/or to perform another step required to activate Cdk1 (21).
We have investigated the role of Cdk2 in human cell cycle progression by generating stable clones in which transcription of wt and dn forms of Cdk2 can be efficiently induced. This system permits flow cytometric and biochemical analysis of the effects of these proteins in cells that are proliferating exponentially or are synchronized at specific points in the cell cycle. Using these clones, we found that induction of Cdk2-wt had no apparent cell cycle effect, whereas induction of Cdk2-dn inhibited progression through several distinct phases of the cell cycle.
MATERIALS AND METHODS
Cell culture and transfection.
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) containing 10% fetal bovine serum (Life Technologies), penicillin (100,000 U/liter), streptomycin sulfate (100,000 U/liter), and glutamine (1 mM). Tetracycline (Tet; culture grade; Sigma) was added to the culture medium at 1 to 2 μg/ml to suppress expression of the inducible protein. To allow induction, cells were washed once with phosphate-buffered saline (PBS), treated with trypsin and EDTA, washed off the dish with DMEM, pelleted at 300 × g for 5 min, and replated in the medium without Tet. Semiconfluent U2-OS cells were transfected by the calcium phosphate method, for stable as well as transient transfection (62). For transient expression of Cdk2-wt prior to immunofluorescence, a cytomegalovirus (CMV) vector expressing β-galactosidase (β-Gal; 0.8 μg per 3.5-cm-diameter dish) was cotransfected with either a CMV vector without insert or the same vector expressing Cdk2-wt (2.5 μg) (18, 74). The following methods were used for transfection of other cell types: Lipofectin (2 μl per 6-cm-diameter culture dish; Life Technologies) for HCT 116 cells and Effectene (10 μl per 6-cm-diameter culture dish; Quiagen) for NIH 3T3 mouse fibroblasts. Cells were fixed in methanol-acetone and stained for β-Gal, BrdU, and DNA as described elsewhere (18, 49). For transient expression of Cdk2-wt prior to flow cytometry, a CMV vector expressing CD20 (1.6 μg per 6-cm-diameter dish) (74) was cotransfected with 5 μg of each plasmid.
Plasmid constructs and generation of stable clones.
cDNAs encoding Cdk2-wt and Cdk2-dn (74) were tagged at the carboxy terminus with an eight-amino-acid peptide corresponding to sequences from the hemagglutinin (HA) epitope of influenza virus and cloned behind the Tet operon in plasmid pUHD10-3 (22). A U2-OS osteogenic sarcoma cell clone (U24 [29]) stably transfected with plasmid pUHD15.1, encoding a Tet-sensitive transcription factor (22), was cotransfected with each of the above plasmids and the puromycin-resistant plasmid pBabePuro (51) at a ratio 20:1 (wt/wt). Clones resistant to 1 μg of puromycin per ml were selected in the presence of Tet. Expression of the target protein was assessed by immunofluorescence (49) or by immunoblotting, using antibody 12CA5 directed against the HA tag. About 75% of clones in each transfection produced detectable target protein.
Cell synchronization.
For hydroxyurea (HU) block and release, cells were replated as described above in medium containing 1 mM HU (Sigma) and cultured with or without Tet for 24 h. The cells were washed twice with PBS and cultured in fresh medium with or without Tet. For subsequent nocodazole block, cells were typically replated into nocodazole. In some experiments, nocodazole was added 12 h after the HU release, with similar results. For nocadazole block and release, cells were replated into medium containing 40 ng of nocodazole (Sigma) per ml with or without Tet for 24 h. Cells were gently washed twice with PBS, and the mitotic cells were washed off the plates by pipetting up and down a few times with DMEM. The cells were pelleted at 300 × g for 5 min and replated in medium with or without Tet. For serum starvation of 3T3 cells, cells were incubated in 0.1% serum for 66 h.
Caffeine treatment and irradiation.
For flow cytometry experiments, cells were untreated or treated with caffeine (1 to 10 mM) for 30 min. Some cells were next exposed to 5-Gy irradiation at 2.9 Gy/min from a J. L. Shepard model 30 Mark I 137Cs irradiator. Subsequently, cells were incubated with or without caffeine for another 24 h. Cells with or without Cdk2-dn induction were, similarly, left untreated or treated with 1 to 10 mM caffeine for 24 h. For DNA condensation experiments, cells were synchronized with HU and released from this inhibition for 4 h; 1 mM caffeine was added, and incubation was continued for another 8 h. Cells were then fixed with ice-cold methanol-acetone (1:1) and stained for DNA using bisbenzimide.
Flow cytometry.
Cells were washed with PBS, treated with trypsin-EDTA, washed off the dish with PBS, and centrifuged at 1,500 × g for 5 min (4°C; Eppendorf Microfuge). The cells were resuspended in PBS, fixed by dropwise addition of a 3× volume of ice-cold ethanol (96%), and incubated for at least 2 h at 4°C before staining. Fixed cells were pelleted and stained at 37°C for 30 min with 0.5 ml of a solution containing 0.001% propidium iodide (Sigma) and 250 μg of RNase A per ml. The total cellular DNA content was determined using a Becton Dickinson flow cytometer and ModFit software. Cell aggregates were gated out of the analysis, based on the width of the propidium iodide fluorescence signal. Each profile was compiled from approximately 5,000 gated events. CD20 staining was as described previously (74). A plasmid expressing a farnesylated green fluorescent protein (Clontech) was to mark transfected cells in 3T3 experiments.
Cell extracts.
Total cell extracts were prepared in E1A lysis buffer as described previously (49).
Antibodies.
Immunoblotting and immunoprecipitation were performed as described previously (49). Separation of differentially phosphorylated forms of Cdk1 was performed on 12% polyacrylamide gels. For immunoblotting, we used 1:600 to 1:800 dilutions of a 200-μg/ml solution of antibodies directed against cyclin A (H-432 or BF683), cyclin E (C-19), cyclin B1 (GNS1), cyclin B1 (H-433), Cdk2 (M2), and Cdk1 (C-19 or 17), all from Santa Cruz Biotechnology. Antibody directed against a tyrosine 15-phosphorylated peptide of Cdk1 [phospho-Cdc2 (Tyr15); referred to here as K1Y15-P] was obtained from New England Biolabs. Antibody directed against Chk2 was kindly provided by S. Elledge (Howard Hughes Medical Institute, Baylor College of Medicine) (44). Immunoblotting of proteins containing the HA tag was done with a 1:40 dilution of monoclonal antibody 12CA5 (100 μg/ml). For immunoprecipitations, 50 to 200 μg of cell extract was incubated with 2 to 3 μg of antibody as described previously (49). For immunodepletion of HA-tagged proteins, 3 μg of anti-HA antibody (rat monoclonal clone 3F10; Roche) was used two times, each followed by precipitation with 60 μl of packed protein G-agarose beads (Life Technologies).
Kinase assays.
Anti-cyclin A (H432), anti-cyclin B1 (GNS1), 12CA5, and anti-Cdk2 (M2) were used to immunoprecipitate active kinases. After immunoprecipitation, the beads were washed three times with E1A lysis buffer and once with 1× kinase buffer (25 mM HEPES buffer [pH 7.4], 1 mM phenylmethylsulfonyl fluoride, 0.1 mM sodium vanadate, 10 μg aprotinin/ml, 10 μg of leupeptin/ml) and then incubated with kinase reaction mix (5 μg of histone H1 [Sigma], 0.1 mM ATP, and 0.2 μCi of [γ-32P]ATP in a total volume of 25 μl of 1× kinase buffer) at 30°C for 30 min. The reaction was stopped by addition of 25 μl of 2× sample buffer. The mixture was heated at 90°C for 3 min and centrifuged, and proteins in the supernatant were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% gel).
Sequencing of integrated plasmids.
Two primers close to the cloning site of pUDH10-3 vector were used to amplify integrated Cdk2-dn cDNAs. The upstream primer was 5′-ACCGGGACCGATCCAGCCT-3′, and the downstream primer was 5′-GCATTCTAGTTGTGGTTTGTCC-3′. The products were purified from agarose gels and directly sequenced by automated methods.
Southern blotting.
Genomic DNA was purified from cells as described elsewhere (62). Briefly, 10 μg of each purified genomic DNA was digested overnight by EcoRI and then subjected to electrophoresis in 0.7% agarose. DNA was blotted onto a nylon membrane (Hybond-N+; Amersham) and detected by pUDH10-3 vector labeled by 32P nick translation.
RESULTS
Generation of clones.
To further define the role of Cdk2 in the human cell cycle, we generated cell lines in which expression of Cdk2-wt and Cdk2-dn could be rapidly and strongly induced. We cloned cDNAs for Cdk2-wt and Cdk2-dn into a Tet-regulated expression vector (the Tet-off system [22]). In the Cdk2-dn protein, an asparagine residue is substituted for the aspartic acid residue at position 145 (74). This aspartic acid residue is conserved in all protein kinases and has been implicated in orienting the beta and gamma phosphates of ATP for the phospho-transfer reaction (9, 33, 34). Each cDNA encoded a carboxy-terminal influenza HA epitope tag, to permit identification of the exogenous enzymes.
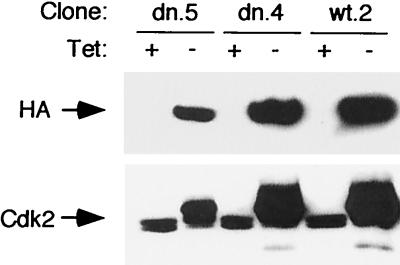
We transfected these constructs into a U2-OS clone (U24 [29, 49]) that expressed a Tet-sensitive transcription activator. We chose U2-OS cells because they have been shown in transient transfection studies to be efficiently arrested by Cdk2-dn (74). In addition, these cells have been shown to support efficient regulation of transcription using the Tet-off system (24, 29, 49). Puromycin-resistant colonies were selected in the presence of Tet, to repress transcription from the target vectors. Similar numbers of colonies were recovered from parallel plates transfected with target vector lacking an insert, suggesting that any leaky expression of the exogenous enzymes that may have occurred did not generally confer a selective disadvantage on clone growth or selection pressure for mutation of the host cells (data not shown). Consistent with this observation, we found little or no expression of the exogenous proteins in the uninduced state (Fig. 1). We selected clones with a range of expression of the exogenous proteins relative to the endogenous enzyme (Fig 1) and identified clones with comparable levels of expression of exogenous wt and dn proteins (e.g., dn.4 and wt.2 [Fig. 1]). Note that induction of Cdk2-dn (Fig. 1, dn.5 lanes) appeared to inhibit formation of the rapidly migrating, active form of Cdk2, suggesting inhibition of endogenous Cdk2 activity (25, 73). The effect of Cdk2-dn on endogenous Cdk2 activity is further addressed below.
FIG. 1.
Induction of Cdk2-wt and Cdk2-dn in U2-OS clones. The designated clones were cultured in the presence (+) or absence (−) of Tet for 3 days. Protein extracts were subjected to immunoblotting with monoclonal antibody 12CA5 directed against the HA epitope tag (top) or a polyclonal antibody directed against Cdk2 (bottom).
Induction of Cdk2-dn imposes S and G2/M phase arrests.
In preliminary studies, we chose a clone with a moderate level of expression (dn.3) to assess whether induction of Cdk2-dn inhibited DNA synthesis, as judged by pulse tritiated thymidine incorporation. Tet withdrawal had no discernible effect on tritiated thymidine incorporation in vector-transfected clones (data not shown) (8, 49). In contrast, Tet withdrawal for 3 days in dn.3 yielded an 85% inhibition of tritiated thymidine incorporation relative to uninduced cells (data not shown). Next, we assessed by flow cytometry the effects of Tet withdrawal on total cellular DNA content in this clone over the same time course. Surprisingly, we observed an increase in the fraction of cells in S and G2/M phases in cells maintained without Tet (data not shown) rather than the expected accumulation of cells in G1 phase (74). The reduced thymidine incorporation that followed Cdk2-dn induction in dn.3 argued against acceleration of G1 progression and indicated that the accumulation of cells in S and G2/M phases was likely caused by cell cycle inhibition. Similar S and G2/M arrests were obtained from a second clone (dn.2 [data not shown]).
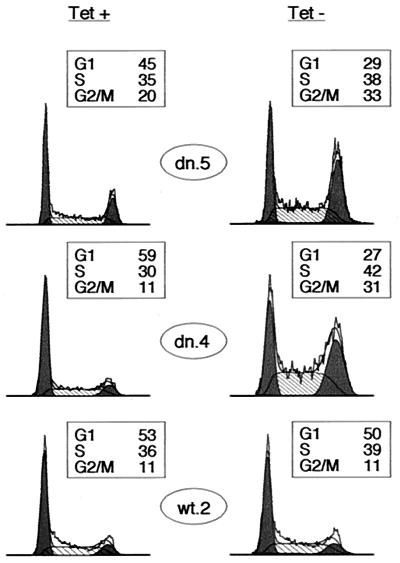
We then examined whether this was a reproducible response in Cdk2-dn-expressing clones. We derived a new set of clones from an independent transfection and selected representative low- and high-expressing clones (dn.5 and dn.4, respectively) for analysis (Fig. 1). Tet withdrawal in each clone again yielded S and G2/M arrests (Fig. 2). (We use the term “arrest,” as opposed to “delay,” throughout this report, without implying that the cells are necessarily permanently arrested.) In contrast, induction of Cdk2-wt had no discernible cell cycle effect (Fig. 2).
FIG. 2.
Cdk2-dn induction preferentially imposes S and G2/M arrests. Dn.5, dn.4, and wt.2 cells were grown in the presence (left) or absence (right) of Tet for 3 days, and the DNA content of the cells was assayed by flow cytometry. DNA content is displayed on the x axis, and cell number is shown on the y axis. For ease of presentation in all figures, DNA content profiles were normalized to the highest peak. G1 and G2/M fractions are shaded black, and S fractions are hatched. Boxed areas show the percentage of cells in each fraction.
Cdk2-dn-mediated G1 arrest.
We sought to reconcile these results with the previously observed G1 arrest mediated by transient transfection of Cdk2-dn in U2-OS cells (74). Because the S and G2/M arrests were seen in every Cdk2-dn-expressing clone and the exogenous enzymes were consistently of the expected size, it seemed unlikely that the Cdk2-dn coding region had undergone rearrangement or mutation during plasmid amplification or integration into genomic DNA. We further excluded these possibilities by performing Southern blotting, PCR amplification, and DNA sequencing of the integrated plasmids from two clones (data not shown).
We noted that simply replating U2-OS cells, which was also done prior to the transient transfections, induces a moderate synchronization in G1 phase (49) (data not shown). In addition, transient transfection is somewhat growth inhibiting (data not shown) and may contribute to cell synchronization in G1. Another potential difference in these experimental settings is that analysis of the transiently transfected cells was gated to the 1 to 5% of cells, with strongest staining for a cotransfected marker protein (74). We therefore reasoned that a G1 arrest might be observed in the stable clones if Cdk2-dn was expressed to high levels during M and/or G1 phases, perhaps mimicking the setting of transient transfection.
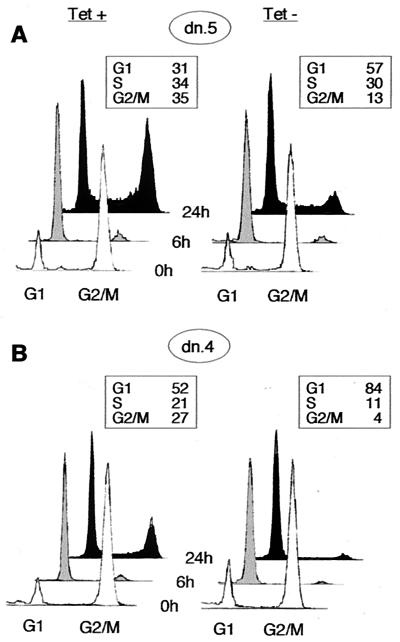
To test this notion, we synchronized cells from clones dn.5 and dn.4 in early mitosis, using the microtubule inhibitor nocodazole. We induced expression of the mutant protein during this period, then released the nocodazole block, and analyzed cellular DNA content at subsequent time intervals by flow cytometry. Cdk2-dn induction yielded a G1 arrest in each clone (Fig. 3). The arrest in the high-expressing clone (dn.4) was as strong as that obtained following transient transfection (Fig. 3 and reference 74). Note that Cdk2-dn did not nonspecifically delay passage through all phases of the cell cycle, because there was no delay in progression through mitosis following release from the nocodazole-mediated arrest (Fig. 3). Furthermore, no mitotic delay was detected in cells examined 1.5 and 3 h after nocodazole release (data not shown). Immunoblotting confirmed that Cdk2-dn was expressed during the nocodazole block (data not shown).
FIG. 3.
Inhibition of progression through G1 phase following induction of Cdk2-dn during a nocodazole block. Dn.5 (low expressor; A) and dn.4 (high expressor; B) cells were incubated with nocodazole for 24 h in the presence or absence of Tet. The mitotic cells were washed off the dish and replated in the absence of nocodazole (0 h; white profiles); cells were collected for flow cytometry at 6 h (grey profiles) and 24 h (black profiles) while maintaining the respective Tet conditions. Boxed areas show the percentage of cells in each fraction at 24 h.
Finally, we tested the effect of transient transfection on the cell cycle distribution of dn.5 cells. We found that cells successfully expressing the transfected marker CD20 were slower to progress through G1 and S phases than either CD20-negative cells exposed to the transfection procedure or untransfected cells (data not shown). This phenomenon was observed in the presence or absence of Cdk2-dn induction, but it particularly enhanced the G1/S arrest imposed by induction of Cdk2-dn (data not shown). We conclude that whereas transient transfection of Cdk2-dn revealed a bona fide role for Cdk2 in the G1/S transition, these experimental conditions masked a more general propensity of Cdk2-dn to arrest progression through S and G2/M phases in U2-OS cells.
Inhibition of S and G2/M phase progression.
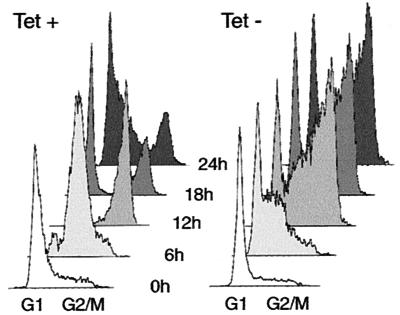
To demonstrate directly that expression of Cdk2-dn results in S and G2/M arrests and to identify favorable settings for biochemical analysis of Cdk2-dn's effects in synchronized cells, we performed experiments using cells synchronized in late G1 and S phases with HU. We determined cellular DNA content at intervals following release of dn.4 cells from this block, with and without induction of Cdk2-dn. The results showed that induced cells were inhibited in passage through S and G2/M phases (Fig. 4). In addition, we noted that 15% of cells with Cdk2-dn induction retained a G1 DNA content, while fewer than 5% of uninduced cells did so following release, further confirming the ability of Cdk2-dn to inhibit G1/S progression. In similar experiments, induction of the wt protein again had no marked effect on cell cycle progression (data not shown).
FIG. 4.
Inhibition of cell cycle progression following induction of Cdk2-dn during an HU block. Dn.4 cells were incubated with HU for 24 h in the presence or absence of Tet. Cells were collected for flow cytometry at 6-h intervals after removal of HU (0 h).
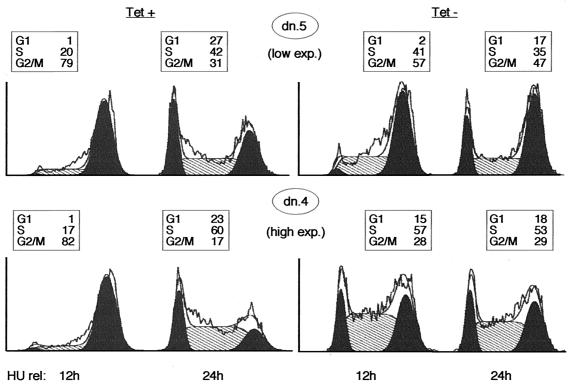
We considered the possibility that the apparent G2/M arrest following induction of the mutant resulted from slow progression through S phase. However, in exponentially growing cells, cells of clones displaying low induced levels of the mutant showed little S-phase arrest, but still accumulated in G2/M (data not shown). We therefore repeated the HU experiment using dn.5, a clone with a lower expression level. As expected, induction of Cdk2-dn in this clone yielded only a modest S-phase arrest, compared to dn.4, but a prominent G2/M arrest (Fig. 5). Likewise, a G1 arrest was not seen in the clone with lower expression of Cdk2-dn (Fig. 5). We conclude that G2/M is the phase most sensitive to Cdk2-dn expression.
FIG. 5.
Induction of lower levels of Cdk2-dn preferentially yields a G2/M arrest, whereas higher levels also yield S and G1 arrests. Dn.5 (low expressor) and dn.4 (high expressor) cells were incubated with HU for 24 h in the presence or absence of Tet. Cells were collected for flow cytometry 12 and 24 h after removal of HU. G1 and G2/M fractions are outlined in black, the S phase fraction is hatched, and the percentage of cells in each fraction is presented in a box above each profile.
DNA damage checkpoint pathways.
Cdk2 is believed to be essential for the firing of DNA replication origins (2, 57, 70). Some regions of the mammalian genome are typically replicated early in S phase, and others are replicated late. It has been documented that in yeast, some origins preferentially fire in early S phase, whereas others fire late (68). The S-phase arrest observed upon induction of Cdk2-dn may therefore reflect a need for Cdk2 within S phase, to fire late-replicating origins (see Discussion). It is less clear what event(s) may be responsible for the accumulation of cells in G2/M phase. Moreover, this is the cell cycle phase most sensitive to expression of Cdk2-dn. We therefore focused on characterizing further the point at which the G2/M arrest occurs.
Given that Cdk2 has been implicated in initiation of DNA synthesis and that Cdk2-dn expression inhibits S-phase progression, we first asked whether the observed G2/M arrest might result from activation of checkpoint pathways that block cell division in response to damaged or unreplicated DNA (16). Some cell death was seen after 3 days of induction of Cdk2-dn, but there was hardly any cell death during the experiments described here (data not shown). p53 is functional in U2-OS cells and is commonly induced by DNA damage (71). We found that p53 levels were unaffected by Cdk2-dn expression (data not shown). p21 levels were moderately increased, but no more so than by induction of Cdk2-wt (data not shown). Chk2 undergoes a shift in mobility on polyacrylamide gels in response to DNA damaging agents in some cells (44). A protein of the expected size that reacts with anti-Chk2 antibodies showed no mobility shift in response to Cdk2-dn expression but also showed no shift with irradiation (data not shown). Thus, these experiments did not provide evidence for Cdk2-dn- induced activation of checkpoint pathways that monitor DNA integrity or replication.
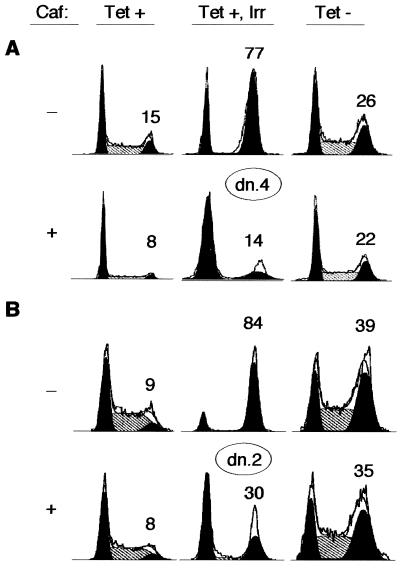
Caffeine is known to be a potent antagonist of checkpoint pathways that monitor damaged and unreplicated DNA in mammalian cells (48, 64, 77). We therefore examined whether caffeine could rescue the S and G2/M arrests mediated by Cdk2-dn. We focused on clones with high levels of Cdk2-dn expression for this analysis, because they display more distinct S and G2/M arrests. As a positive control for caffeine's effects, we exposed uninduced cells to gamma irradiation. Representative flow cytometry results are shown in Fig. 6. Caffeine efficiently prevented the G2/M arrest imposed by irradiation (Fig. 6, Tet +, Irr). In contrast, caffeine only slightly reduced the S and G2/M fractions in cells with Cdk2-dn induction (Fig. 6, Tet −), less so than in control cells without induction or irradiation (Fig. 6, Tet +). Thus, the S and G2/M arrests imposed by Cdk2-dn appear to be relatively resistant to the effects of caffeine.
FIG. 6.
Caffeine fails to rescue the S and G2/M arrests imposed by Cdk2-dn. Dn. 4 (A) and dn.2 (B) cells were incubated in the presence or absence of Tet for 48 h. A third culture maintained in Tet was subjected to 5 Gy of gamma irradiation (Irr) at the end of this time period. Each culture was then incubated in the presence or absence of 1 mM caffeine (Caf) for an additional 24 h and collected for flow cytometry. The percentage of cells in the G2/M fraction is given above each DNA profile.
In addition to its effects on DNA content, caffeine is capable of inducing premature DNA condensation in cells with unreplicated DNA (64, 77). Such an effect would not be evident by flow cytometry. To address this issue, we synchronized cells with HU, with or without Cdk2-dn induction, released the HU block, and examined whether caffeine addition during S phase could increase the fraction of cells with condensed nuclear DNA, assessed by fluorescence microscopy. The results are summarized in Table 1. Caffeine was able to induce premature DNA condensation in 15% of uninduced cells but only 2% of cells with Cdk2-dn induction. We conclude that the S and G2/M arrests imposed by Cdk2-dn are not solely due to activation of caffeine-sensitive checkpoint pathways but may reflect disruption of events required for normal cell cycle progression.
TABLE 1.
Caffeine does not mediate premature DNA condensation in cells with Cdk2-dn inductiona
| Transfection | Condensed nuclei (%)b
|
|
|---|---|---|
| +Tet | −Tet | |
| No caffeine | 5.3 ± 0.9 | 3.2 ± 2.6 |
| Caffeine | 20 ± 2.0 | 5.1 ± 2.4 |
Cells were synchronized with HU, released for 4 h, incubated with 1 mM caffeine for another 8 h, fixed, and stained with bisbenzimide (Hoechst).
Mean ± standard deviation from three independent experiments.
Cdk2-dn associates efficiently with endogenous cyclins.
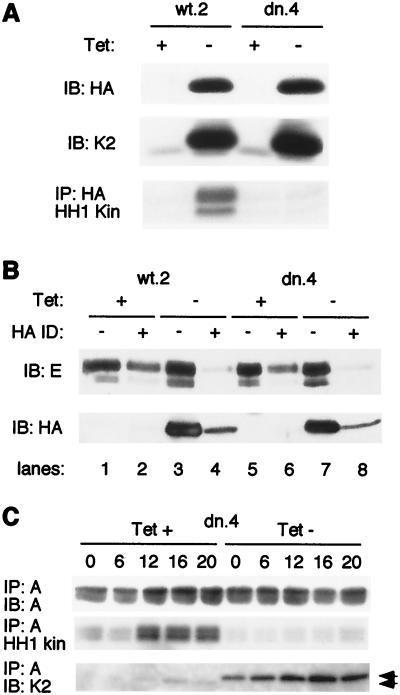
To further characterize the mechanism by which Cdk2-dn exerted its effects, we analyzed the induced protein's association with known cyclin partners. Cyclin E expression is not markedly cell cycle regulated in U2-OS cells (49) (data not shown). We therefore used unsynchronized cells 24 h after induction of wt or dn enzymes to assay association with cyclin E. Immunoblotting with antibodies directed against the HA epitope tag demonstrated that the exogenous proteins were induced to similar levels (Fig. 7A). We immunoprecipitated the exogenous proteins through their HA tags and assayed their associated kinase activity using histone H1 as a substrate. The results confirmed that the wt enzyme was catalytically active whereas the mutant was not (Fig. 7A) (74).
FIG. 7.
Induced Cdk2-wt and Cdk2-dn each associate with the majority of endogenous cyclin A and E, but Cdk2-dn is catalytically inactive. (A) Induced Cdk2-dn is catalytically inactive, whereas induced Cdk2-wt retains catalytic activity. Cells from clones wt.2 and dn.4 were incubated in the presence or absence of Tet for 24 h. Whole-cell extracts were subjected to immunoblotting (IB) with an antibody directed against the HA tag (top), immunoblotting with an antibody directed against Cdk2 (K2; middle), and immunoprecipitation (IP) with an antibody directed against the HA tag, followed by in vitro kinase assays using histone H1 (HH1 Kin) as a substrate (bottom). (B) Induced Cdk2-wt and Cdk2-dn each associate with the majority of endogenous cyclin E. Two successive rounds of immunodepletion (ID) with the HA antibody were performed on the extracts described above. Cyclin E (top) and induced Cdk2 (wt or dn, using the anti-HA antibody; bottom) levels in the extracts were determined by immunoblotting before and after immunodepletion. (C) Induction of Cdk2-dn abolishes most of the endogenous cyclin A-associated kinase activity. Dn.4 cells were synchronized at the G1/S border with HU. The HU was removed, and extracts were prepared from cells at the designated intervals. Cyclin A immunoprecipitates were subjected to immunoblotting with an anti-cyclin A antibody (top), examined for associated kinase activity, using histone H1 as a substrate (middle), or subjected to immunoblotting with an anti-Cdk2 antibody (bottom). Arrowheads, exogenous (upper band) and endogenous Cdk2.
Next, we compared the abilities of the induced enzymes to associate with endogenous cyclins. To estimate the fraction of endogenous cyclin E bound by the induced enzymes, we immunodepleted the induced proteins from the lysates using antibody directed against the HA tag and assayed the level of cyclin E remaining in the supernatant. Immunodepletion effectively removed most of the induced protein (Fig. 7B; compare lane 4 with lane 3 and lane 8 with lane 7). Although some cyclin E was nonspecifically lost from the supernatants due to the procedure itself (lanes 2 and 6), most cyclin E appeared to be bound to the exogenous proteins (lanes 4 and 8). These results suggest that the exogenous wt and dn proteins, respectively, associated with most of the endogenous cyclin E, consistent with their abundance relative to endogenous Cdk2. Consistent with this, cyclin E-associated kinase activity was inhibited by Cdk2-dn induction (data not shown). Because Cdk2-wt induction was without cell cycle effect, the results provide further evidence that the cell cycle inhibition mediated by Cdk2-dn is not due to sequestration of endogenous cyclins per se but also reflects a lack of Cdk2 kinase activity.
We then sought to extend these results by analyzing association of the exogenous enzymes with cyclin A and effects on cyclin A-associated kinase activity. Cyclin A expression and kinase activity are strongly cell cycle regulated in U2-OS cells, peaking in S and G2 phases (49), and the increase in cyclin A-associated Cdk2 activity is primarily responsible for the peak in Cdk2 activity that occurs in late S/G2 phases (55). To control for cell cycle position effects, we induced Cdk2-dn during synchronization of dn.4 cells with HU and prepared cell extracts at intervals following release from this block (see Fig. 4 for flow cytometry profiles of cells treated in this manner). Synchrony was lost in the uninduced cells beyond 20 h, and so we focused our analysis on earlier time points. Induction of Cdk2-dn had little impact on cyclin A expression, assessed either by direct immunoblotting (data not shown) or by immunoprecipitation of cyclin A followed by immunoblotting (Fig. 7C, top). However, cyclin A-associated kinase activity was strongly inhibited (Fig. 7C, middle). This correlated with binding of cyclin A to the exogenous enzyme at the expense of the endogenous (Fig. 7C, bottom). In contrast, induction of Cdk2-wt in wt.2 under similar conditions had no substantial effect on cyclin A-associated kinase activity (data not shown). In conclusion, both Cdk2-wt and Cdk2-dn compete with the endogenous Cdk2 for cyclin binding, but Cdk2-dn lacks kinase activity and blocks the accumulation of cyclin A-associated kinase activity during S and G2/M phases.
G2/M arrest occurs prior to DNA condensation.
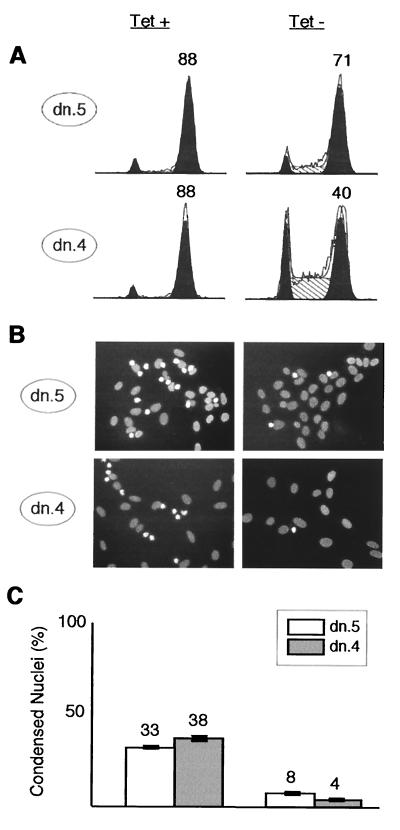
To characterize further the point at which cells are arrested in the G2/M period, we sought to determine whether Cdk2-dn-expressing cells arrested before or after nuclear DNA condensation, a robust marker for prophase, the first stage of mitosis. We synchronized cells with HU, released cells from this block, and added nocodazole, to trap and quantitate cells reaching the mitotic spindle checkpoint. We fixed the cells 24 h after release from HU and stained nuclear DNA with bisbenzimide (Hoechst). A large fraction of U2-OS cells that reach prophase are arrested by nocodazole and show condensed nuclear DNA (65). Flow cytometry showed that most cells without induction were indeed arrested in the presence of nocodazole with a G2/M DNA content (Fig. 8A, left) and that 30 to 40% of cells had fully condensed nuclei (Fig. 8B and C, left). In contrast, even though nearly half of the dn.4 cells and most of dn.5 cells with Cdk2-dn induction achieved a G2/M DNA content (Fig. 8A, right), only 4 and 8%, respectively, had fully condensed nuclear DNA (Fig. 8B and C, right). These data indicate that the cells with induction of Cdk2-dn were arrested prior to prophase.
FIG. 8.
Cdk2-dn arrests cells in G2, prior to DNA condensation. Dn.5 and dn.4 cells were synchronized at the G1/S border by incubation with HU for 24 h in the presence (left) or absence (right) of Tet. HU was then removed, and nocodazole was added; 24 h after HU release, portions of each culture were either collected for flow cytometry or fixed and stained with bisbenzimide. (A) Flow cytometry profiles. G1 and G2/M fractions are outlined in black, S phase fractions are hatched, and the percentage of cells in G2/M is shown above that peak. (B) Representative fields with nuclear DNA stained by bisbenzimide. Interphase nuclei are broad, oval, and pale; early mitotic nuclei trapped by nocodazole are condensed, irregularly shaped, and bright. (C) Quantitation of the results in panel B, expressed as the percentage of nuclei in random high-power fields showing a condensed morphology. The bars depict mean numbers plus or minus ranges from two counts of more than 200 randomly chosen cells per condition. Similar results were obtained in a second experiment (not shown).
We next used this experimental format to examine whether the delay in progression to mitosis imposed by Cdk2-dn could be abrogated by overexpression of Cdk2-wt. Dn.4 cells were cotransfected with a vector expressing β-Gal and either a vector without insert or a vector expressing Cdk2-wt. As discussed previously, this experiment was complicated by the fact that the successfully transfected cells showed an increased fraction of cells arrested at G1 phase and generally slower cell cycle progression; fewer cells progressed to fully condensed DNA under these experimental conditions (data not shown). We therefore scored the fraction of vector- or Cdk2-wt-transfected cells that showed any distinct nuclear condensation, identified by an examiner blinded to the treatment conditions as a marked reduction in nuclear size and/or loss of an oval shape. Cdk2-dn induction reduced the fraction of cells with nuclear condensation by 40% in the vector-transfected population, whereas inclusion of a Cdk2-wt cDNA insert in the transfection vector at least partially abrogated this effect (Table 2). Flow cytometry analyses of similarly treated cells indicated that Cdk2-wt transfection also modestly decreased the fraction of cells that retained G1 and S phase DNA contents and increased the fraction of G2/M cells (data not shown).
TABLE 2.
Transfection of Cdk2-wt rescues the inhibition of DNA condensation mediated by Cdk2-dn inductiona
| Transfection | Condensed nuclei (%)b
|
|
|---|---|---|
| +Tet | −Tet | |
| Vector | 66 ± 6 | 40 ± 10 |
| Cdk2-wt | 73 ± 2 | 65 ± 8 |
Cells were transfected with vector or Cdk2-wt and incubated in medium with or without Tet. The cells were then synchronized with HU, released in the presence of nocodazole for 18 h, fixed, and stained with bisbenzimide.
Mean ± standard deviations from three independent experiments.
Regulation of Cdk1.
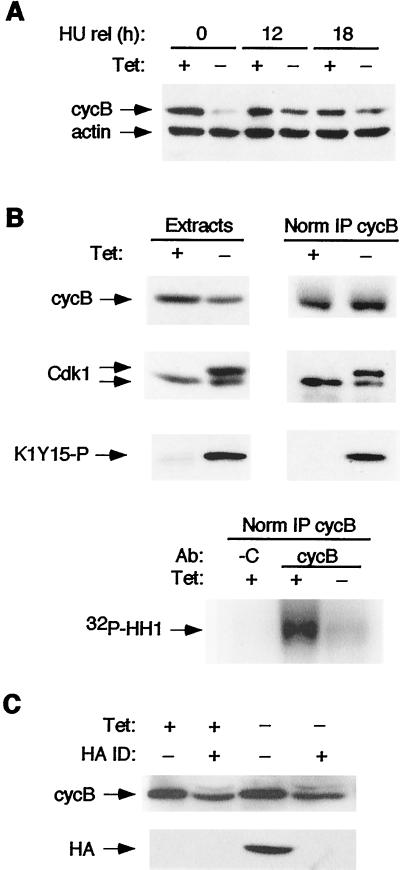
We next examined the effect of Cdk2-dn induction on cyclin B levels and associated kinase activity. Cells were synchronized at the G1/S and then G2/M borders as before (Fig. 8), to minimize cell cycle position effects. We observed that cyclin B levels were reproducibly lower at each stage in cells with Cdk2-dn induction, consistent with recent evidence that hypophosphorylation of pRb and/or inhibition of Cdk2-cyclin A activity at the G1/S transition may decrease cyclin B stability in U2-OS cells (42) (Fig. 9A). However, cyclin B levels actually fell as cells progressed toward G2/M in the absence of Cdk2-dn induction, and the difference in cyclin B levels between uninduced and induced cells narrowed as cells progressed toward G2/M (Fig. 9A). pRb was hyperphosphorylated, as judged by its migration on polyacrylamide gels, with or without Cdk2-dn induction in the G1/S synchronized cells but migrated slightly more rapidly in the extract with Cdk2-dn induction (data not shown). pRb remained largely hyperphosphorylated, with or without Cdk2-dn induction, as the cells progressed toward G2/M (data not shown).
FIG. 9.
Cdk2-dn-expressing cells arrest in G2 phase with moderately reduced levels of cyclin B and greatly reduced activation of Cdk1. Dn.4 cells were synchronized with HU and then nocodazole, with or without Cdk2-dn induction, as described in the legend to Fig. 8. Protein extracts were subjected to immunoblotting, with or without the following immunoprecipitations. (A) Cyclin B (cycB) levels in S and G2/M phases are moderately reduced by Cdk2-dn induction. Extracts were prepared from cells at the end of treatment with HU (0 h) and at 12 h and 18 h after release (rel) into nocodazole and subjected to immunoblotting for cyclin B and actin (loading control). Note that cyclin B levels are strongly reduced by Cdk2-dn induction during the G1/S block but only moderately reduced, compared to uninduced cells, at the G2/M block. (B) Cdk1 activation is inhibited by Cdk2-dn induction. (Top) Immunoblotting was performed on the 18-h extracts (left), normalized by protein content, or cyclin B immunoprecipitates (right), normalized for immunoprecipitated cyclin B (IP cycB), using anti-cyclin B, Cdk1, and K1Y15-P antibodies. (Bottom) Kinase activity associated with the normalized cyclin B immunoprecipitates was assayed using histone H1 (HH1) as a substrate. C denotes immunoprecipitation with a negative control antibody (Ab). (C) Cdk2-dn does not sequester cyclin B. Cdk-2dn was immunodepleted (ID) from the above extracts, and the levels of cyclin B (top) and Cdk2-dn (detected through its HA tag; bottom) remaining in the supernatant were assayed by immunoblotting.
In addition to the modestly lower cyclin B levels in the cells with Cdk2-dn induction, we observed accumulation of Cdk1 in a form that migrated more slowly on polyacrylamide gel electrophoresis (Fig. 9B). Inhibitory phosphorylation on threonine 14 and tyrosine 15 is known to reduce the electrophoretic mobility of Cdk1 (58). We therefore examined whether the slower-migrating form seen in cells with Cdk2-dn induction reacted with an antibody generated against peptide K1Y15-P. Immunoblotting with K1Y15-P antibody yielded a single major band that comigrated with the slower-migrating Cdk1-reactive species (Fig. 9B). Endogenous Cdk2 has an electrophoretic mobility greater than that of Cdk1 (58) (data not shown), but the HA tag on Cdk2-dn causes this protein to migrate at rate similar to that of Cdk1. To confirm that the slowly migrating species reactive with both anti-Cdk1 and anti-K1Y15-P antibodies was not derived from cross-reactivity with Cdk2-dn, we repeated the experiments following Cdk2-dn immunodepletion, using an antibody directed against the HA tag. Depletion of more than 90% of Cdk2-dn (see below) had no effect on the intensity of either the slower-migrating Cdk1-reactive band or the K1Y15-P-reactive band (data not shown). We conclude that these bands represent tyrosine-phosphorylated Cdk1.
The results suggested that Cdk1 activation was likely inhibited in cells with Cdk2-dn induction. Cdk1 was difficult to immunoprecipitate directly, as has been observed by others, but could be precipitated through associated cyclin B. We immunoprecipitated cyclin B from extracts prepared with and without Cdk2-dn induction. Immunoblotting with anti-Cdk1 and anti-K1Y15-P antibodies demonstrated the slowly migrating species, further confirming its identity as tyrosine-phosphorylated Cdk1 (Fig. 9B). We assayed kinase activity associated with the immunoprecipitates using histone H1 as a substrate. Cdk2-dn induction resulted in a strong reduction in cyclin B-associated kinase activity, even after normalizing for the amount of cyclin B immunoprecipitated (Fig. 9B). Similar results were obtained without prior synchronization with HU (data not shown). Somewhat less Cdk1 was present in the immunoprecipitates from Cdk2-dn-expressing cells than would be expected from the level of the protein present in the extracts. Because most of this Cdk1 appears to be tyrosine phosphorylated, an event requiring prior cyclin binding, we infer that the relative defect in immunoprecipitating Cdk1 is likely due to changes in recovery of the complexes or accessibility of the cyclin B epitope. Nonetheless, it is evident that the majority of Cdk1 associated with cyclin B in the cells with Cdk2-dn induction is in the slower migrating form (Fig. 9B). We conclude that the reduced levels of cyclin B-associated kinase activity result both from reduced cyclin B levels and from inhibitory phosphorylation of Cdk1.
We considered the possibility that sequestration of cyclin B by direct binding to Cdk2-dn might contribute to the defect in cyclin B-associated kinase activity. This scenario seemed unlikely, because Cdk2-wt induction should also compete with Cdk1 for binding, yet Cdk2-wt induction had no demonstrable cell cycle effect. Moreover, induction of Cdk2-dn during a nocodazole block had no effect on progression through mitosis, a process dependent on cyclin B-Cdk1 activity. Consistent with this reasoning, we found that immunodepletion of Cdk2-dn from the extracts did not significantly reduce the level of cyclin B remaining in the supernatant, providing further evidence against sequestration of cyclin B by Cdk2-dn (Fig. 9C).
Taken together, these experiments indicate a requirement for Cdk2 in progression through S and G2 phases of the human cell cycle, in addition to its previously described role at the G1/S transition.
Other cell types.
We then examined whether Cdk2-dn could impose S and/or G2 arrests in other cell types. These experiments were complicated by the fact that transient transfection of Cdk2-dn appears to predispose to G1 arrest, potentially outweighing S and G2 arrests mediated in asynchronous cultures. We therefore performed experiments in 3T3 cells synchronized in G1, allowing us to directly assess effects of Cdk2-dn on progression into replicative phases. 3T3 cells were deprived of serum for 66 h, yielding greater than 90% G1/G0 cells (data not shown). Serum was restored, and the cells were cotransfected with a marker plasmid expressing β-Gal and either an empty vector or one expressing Cdk2-dn. Continuous BrdU labeling was used to monitor S-phase progression. Nocodazole was added at 30 h (late S phase [data not shown]) and maintained until 48 h, to trap and quantitate cells reaching the spindle checkpoint. 3T3 cells arrest prior to prophase in response to spindle disruption, apparently due to an intact Chfr checkpoint (65). We therefore released cells from the nocodazole trap for 2 h prior to fixation and assessed the fraction of cells that were able to condense their DNA. Cdk2-dn yielded a dose-dependent reduction in the ability of BrdU-positive cells to undergo DNA condensation (Table 3). In similarly designed flow cytometry experiments in which nocodazole was omitted and cells were fixed 40 h after serum stimulation, Cdk2-dn-transfected cells showed a trend toward increased S-phase fractions and a statistically significant 50% increase in the G2/M fraction (four independent experiments [data not shown]). These results suggest that Cdk2-dn can inhibit S- and G2-phase progression in a nontransformed cell type. Preliminary experiments suggest that Cdk2-dn also mediates S and G2 arrests in HCT 116 colorectal carcinoma cells synchronized with HU (data not shown).
TABLE 3.
Transfection of Cdk2-dn in serum-starved and restimulated 3T3 cells blocks DNA condensation after BrdU incorporationa
| Ratio of expression plasmid to marker plasmid | BrdU-positive nuclei with condensed DNA (%)b
|
|
|---|---|---|
| Vector | Cdk2-dn | |
| 1:1 | 77 ± 1 | 53 ± 9 |
| 2:1 | 75 ± 5 | 39 ± 4 |
| 4:1 | 78 ± 2 | 28 ± 1 |
3T3 cells were synchronized by serum starvation, restimulated with serum (0 h), and cotransfected (between 4 h and 10 h) with a plasmid expressing β-Gal and either an empty vector or one expressing Cdk2-dn. BrdU was added at 10 and 24 h, to ensure continuous labeling of cells in DNA synthesis. Nocodazole was added at 32 h and washed off at 48 h. The cells were fixed at 50 h. A least 100 β-Gal- and BrdU-positive cells were scored for each condition, to determine the fraction with condensed DNA (stained with bisbenzimide).
Means ± standard deviation from four independent experiments for the 2:1 ratio; mean ± range from two independent experiments for the 1:1 and 4:1 ratios.
DISCUSSION
Studies in diverse experimental systems have established that Cdk2 must be activated to initiate DNA synthesis in higher eukaryotes (52, 66). Similarly, increasing evidence suggests that inhibition of Cdk2 is necessary and sufficient to prevent S-phase entry in normal and neoplastic cells (29, 45, 49). Evidence that Cdk2 performs other functions within the replicative cycle has been limited. However, both human Cdk2 and Cdk1 are highly homologous to the single major Cdk in yeast, termed CDC28 in Saccharomyces cerevisiae and Cdc2 in Schizosaccharomyces pombe (17, 38, 53, 72). Either human protein can complement CDC28 mutants that arrest at the G1/S or G2/M transition (17, 38, 47, 53). In addition, recent evidence suggests that CDC28 may drive S-phase progression, including events of semiconservative DNA replication distinct from initiation (11). Based on these observations, Cdk2 may also be expected to regulate multiple steps of cell replication.
To address the role(s) of Cdk2 in the mammalian cell cycle, we generated U2-OS cell clones in which transcription of Cdk2-dn can be efficiently induced. We found that induction of Cdk2-dn could reproduce the G1 arrest observed following its transient transfection (74). However, under standard growth conditions, induction of Cdk2-dn preferentially inhibited progression through S and G2/M phases.
Specificity of the cell cycle effects.
Do the arrests imposed by Cdk2-dn result from defective Cdk2 function? Several lines of evidence support this conclusion. In parallel experiments, induction of Cdk2-wt to levels as high as those achieved for Cdk2-dn did not cause discernible cell cycle effects. Based on the crystal structure of the enzyme, the mutation would not be expected to alter the enzyme's interfaces with known binding partners or substrates (9, 28). In agreement with this prediction, exogenous Cdk2-wt and Cdk2-dn both associated with the majority of cyclin A and E in the cell (Fig. 6), and thus far we have detected no major differences in the composition of wt or dn complexes immunoprecipitated from metabolically labeled cells or sedimented through glycerol gradients (data not shown). We also confirmed that Cdk2-dn did not bind to a significant fraction of cyclin B and did not inhibit progression through mitosis following release from a nocodazole block. Finally, transfection of Cdk2-wt could at least partially prevent the cell cycle arrests imposed by Cdk2-dn induction. A complex scenario can be envisioned in which Cdk2 normally plays no role in G2/M progression, but Cdk2-dn inhibits the G2/M transition by disrupting the function of an essential cyclin A-Cdk1 complex. In this scenario, both the exogenous Cdk2-wt and Cdk2-dn sequester cyclin A from Cdk1 and overexpression of Cdk2-wt artifactually complements the loss of cyclin A-Cdk1 kinase activity, by generating supraphysiologic levels of cyclin A-Cdk2 kinase activity. However, this scenario seems unlikely, because total cellular Cdk2 kinase activity remained near physiologic levels in the setting of Cdk2-wt induction (data not shown), probably because factors other than Cdk2 expression are limiting. In sum, our results suggest that the observed cell cycle inhibition is due to abrogation of Cdk2 function rather than an artifact of Cdk2 overexpression or a gain of function from the Cdk2-dn mutation.
Could the effects of Cdk2-dn on cell cycle progression be mediated indirectly, through activation of DNA damage checkpoint pathways that respond to damaged or unreplicated DNA? Several observations argue against this possibility. p53 levels were not increased by Cdk2-dn expression, and p21 levels were no higher than in cells with Cdk2-wt induction. Migration of Chk2 was also not detectably altered, and caffeine did not rescue the cell cycle arrests. The observation that U2-OS cells are defective in the Chfr spindle checkpoint (65) suggests that this checkpoint pathway is also not involved in the Cdk2-dn-mediated G2 arrest. We have not, of course, ruled out activation of other checkpoint pathways that might be insensitive to caffeine or that might proceed through biochemical events not examined here (61). However, the observation that Cdk2 inhibition can block Cdk1 activation in Xenopus egg extracts in the absence of nuclei (23) further supports the notion that Cdk2 plays a more direct role in mitotic entry.
S-phase arrest.
Cdk2 has not previously been shown to be required for efficient S-phase progression in mammalian cells. Studies have revealed that origins fire at different points throughout S phase in yeasts, and some mammalian genes are preferentially replicated in late S phase, perhaps reflecting the use of late-firing origins (67, 68). Cdk2 can phosphorylate in vitro a number of substrates required for different steps of DNA replication (75). Thus, Cdk2 function may be required during S phase to activate late-firing origins and/or to drive other events of DNA replication (11).
Expression of phosphorylation-resistant pRb mutants during S phase delays DNA synthesis (6, 37, 79), an effect proposed to be mediated by inhibition of cyclin A expression. Consistent with this interpretation, our findings provide evidence that Cdk2 activity drives S-phase progression. On the other hand, it appears unlikely that the effects of Cdk2-dn are mediated through activation of pRb, because we observed only a slight reduction in pRb phosphorylation in HU-synchronized cells with Cdk2-dn induction, and pRb remained hyperphosphorylated throughout S phase following release from the chemical block.
E2F complexes are candidate targets for Cdk2 function during S phase. Phosphorylation by Cdk2 complexes abrogates the DNA binding activity of E2F-DP complexes in vitro (14, 39). Expression of E2F mutants defective for Cdk2 binding can delay progression through S phase, suggesting that inactivation of E2F by Cdk2 may be required to complete S phase (40). However, in our preliminary studies, transfection of a construct expressing a dn mutant of DP-1, the E2F heterodimerization partner, was unable to relieve S-phase inhibition imposed by Cdk2-dn (data not shown). Disruption of Cdk2 function in U2-OS cells through incubation with a peptide derived from the Cdk2 binding domain of E2F1 has been shown to result in apoptosis that is pronounced in serum-starved cells (5). We did not observe substantial cell death until Cdk2-dn had been induced for more than 3 days, perhaps because our cells were maintained continuously in serum.
G2 arrest.
An early experiment in mammalian cells that pointed to a possible role for Cdk2 within replicative phases of the cycle was the observation that microinjection of antibodies directed against cyclin A into S-phase HeLa cells could prevent subsequent cell division (56). This work has been extended by recent microinjection studies in which manipulation of Cdk2 function by several means was found to influence G2/M progression (21, 42). Microinjection of either a phosphorylation-resistant pRb mutant, which repressed cyclin A expression, or p27KIPI in early-S-phase U2-OS cells decreased cyclin B stability (42). Further evidence suggested that cyclin A-Cdk2 complexes could stabilize cyclin B by phosphorylating and inactivating Cdh1, a specificity factor for proteolysis by the anaphase-promoting complex (42). Thus, this work suggests that Cdk2 may contribute to G2/M progression by facilitating accumulation of cyclin B. In a separate study, microinjection of purified cyclin A-Cdk2 complexes in G2-phase HeLa cells was found to accelerate entry into mitosis (21). Injection of cyclin A complexed to an inactive Cdk2 mutant, different from the mutant used in our studies, appeared to slightly delay mitotic entry. A stronger delay was observed following microinjection of the Cdk inhibitory domain of p21WAF1/CIP1. This delay was not noted to alter the intensity of cyclin B immunofluorescent staining but may have been mediated by inhibition of Cdk1, because coinjection of Cdc25 could rescue the effect. Although these studies did not demonstrate directly that Cdk2 is required for entry into mitosis, the results are in good agreement with those presented here.
Several observations suggest that the G2/M arrest observed in our U2-OS cell clones with Cdk2-dn induction occurs within G2 rather than M phase: the arrested cells have uncondensed DNA, low levels of cyclin B-associated kinase activity, and high levels of tyrosine-phosphorylated Cdk1. Furthermore, induction of Cdk2-dn in nocodazole-arrested cells did not interfere with mitosis following removal of the drug. Induction of Cdk2-dn yielded moderately lower cyclin B levels, consistent with the microinjection results in these cells (42). However, we observed that cyclin B levels actually declined as U2-OS cells progressed from S through G2/M in our experimental settings, in the absence of Cdk2-dn induction, raising doubt as to whether stabilization of cyclin B is a critical factor. Cells with Cdk2-dn induction appear to have an additional block to the activation of Cdk1, subsequent to cyclin B binding. These results point to a key role for Cdk2 in mediating entry into mitosis.
Is the defect in mitotic entry in U2-OS cells with Cdk2-dn induction the same as that present in the Cdk2-depleted Xenopus egg extracts and p21WAF1/CIP1-injected HeLa cells? In the Xenopus egg extracts, Cdk2 depletion blocked activation of exogenous cyclin B and appeared to favor inhibitory phosphorylation of Cdk1, a process thought to follow cyclin B binding (23, 50). These results are consistent with our observations in U2-OS cells. The mitotic block in HeLa cells could be rescued with co-injection of Cdc25 or active cyclin B-Cdk1 complexes. Similarly, we have preliminary evidence that adenovirus-mediated expression of a Cdk1 mutant resistant to inhibitory phosphorylation (30) can rescue the G2 block imposed by Cdk2-dn (data not shown). Thus, we believe that the major defect resulting from Cdk2 inhibition in each of these experimental systems is an inability to activate cyclin B-Cdk1 complexes. We suggest that inhibitory phosphorylation of Cdk1 is a default state that is overcome by the peak in Cdk2 kinase activity achieved during G2 phase and/or release of Cdk2 complexes from S-phase substrates. The notion that tyrosine phosphorylation of Cdk1 may limit normal cell cycle progression is supported by the observation that Cdk1 migrated in a slower-migrating form as synchronized leukemia cells traversed G2 (10). Candidate targets of Cdk2 in Cdk1 regulation include CDC25B (10), CDC25C (7), Mik1 (1), Protein phosphatase 2A (27, 31), Pin1 (77), Plx1 (60), and Plkk 1 (60).
G2 versus late S.
We have used the standard operational definition that cells with fully replicated DNA, as judged by flow cytometry, are in G2 or M phase. Whether such cells have actually completed S phase or, instead, retain small amounts of unreplicated DNA or DNA strand breaks cannot be determined. In fact, it has recently been demonstrated that approximately 1% of the genome is replicated within 90 min of mitosis in different mammalian cell types, within the period standardly defined as G2 (76). The replication of specific sequences from autosomes and, in female cells, the inactivated X chromosome appears to occur preferentially at the end of S phase. If, indeed, Cdk2-dn preferentially arrests cells in late S phase, then the blocked steps would appear to be distinct from those performed earlier in S phase. This notion derives from the observation that clones expressing only moderate amounts of Cdk2-dn progress well through early and mid-S phase but still arrest in late S/G2 (Fig. 5).
Implications.
Our results provide strong evidence that Cdk2 is required not only for entry into the cell division cycle but also for efficient progression through S and G2 phases. Our results imply that physiologic cell cycle inhibitors may mediate S and G2 arrests by targeting Cdk2. For example, p21WAF1/CIP1 is induced in response to irradiation and contributes to G2 arrest (3, 15). During the arrest Cdk1 becomes tyrosine phosphorylated, an effect thus far ascribed to other events, such as direct Chk1-mediated inhibition of CDC25 (4, 20, 63). p21WAF1/CIP1 is known to bind primarily to Cdk2 in this setting (59). Our results suggest that inhibition of Cdk2 by p21WAF1/CIP1 may also foster tyrosine phosphorylation of Cdk1. In the same vein, recent experiments suggest that transient overexpression of cyclin E-Cdk2 can relieve the G2/M arrest mediated by transforming growth factor β in Mv1Lu cells (80) and sustained overexpression can contribute to chromosomal instability (69).
ACKNOWLEDGMENTS
B.H. and J.M. contributed equally to this work.
This work was supported in part by a Research Scholar Award from the American Cancer Society (RPG 999-168-01-CCG) and by institutional funds provided to G.H.E. We also acknowledge use of facilities of the Penn Digestive Disease Center, supported by Center Grant P30 DK50306, and the Penn Cancer Center, supported by grants from the NCI and the Markey Charitable Trust.
We thank Amit Maity for help with the caffeine experiments.
REFERENCES
- 1.Baber-Furnari B A, Rhind N, Boddy M N, Shanahan P, Lopez-Girona A, Russell P. Regulation of mitotic inhibitor Mik1 helps to enforce the DNA damage checkpoint. Mol Biol Cell. 2000;11:1–11. doi: 10.1091/mbc.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum B, Nishitani H, Yanow S, Nurse P. Cdc18 transcription and proteolysis couple S phase to passage through mitosis. EMBO J. 1998;17:5689–5698. doi: 10.1093/emboj/17.19.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunz F, Dutriaux A, Lengauer C, Waldman T, Shou S, Brown J, Sedivy J, Kinzler K, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Liu T H, Walworth N C. Association of Chk1 with 14-3-3 proteins is stimulated by DNA damage. Genes Dev. 1999;13:675–685. doi: 10.1101/gad.13.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y N, Sharma S K, Ramsey T M, Jiang L, Martin M S, Baker K, Adams P D, Bair K W, Kaelin W G., Jr Selective killing of transformed cells by cyclin/cyclin-dependent kinase 2 antagonists. Proc Natl Acad Sci USA. 1999;96:4325–4329. doi: 10.1073/pnas.96.8.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew Y P, Ellis M, Wilkie S, Mittnacht S. pRB phosphorylation mutants reveal role of pRB in regulating S phase completion by a mechanism independent of E2F. Oncogene. 1998;17:2177–2186. doi: 10.1038/sj.onc.1202443. [DOI] [PubMed] [Google Scholar]
- 7.Clarke P R, Hoffmann I, Draetta G, Karsenti E. Dephosphorylation of cdc25-C by a type-2A protein phosphatase: specific regulation during the cell cycle in Xenopus egg extracts. Mol Biol Cell. 1993;4:397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai C Y, Enders G H. p16 INK4a can initiate an autonomous senescence program. Oncogene. 2000;19:1613–1622. doi: 10.1038/sj.onc.1203438. [DOI] [PubMed] [Google Scholar]
- 9.De Bondt H L, Rosenblatt J, Jancarik J, Jones H D, Morgan D O, Kim S H. Crystal structure of cyclin-dependent kinase 2. Nature. 1993;363:595–602. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- 10.De Souza C P, Ellem K A, Gabrielli B G. Centrosomal and cytoplasmic Cdc2/cyclin B1 activation precedes nuclear mitotic events. Exp Cell Res. 2000;257:11–21. doi: 10.1006/excr.2000.4872. [DOI] [PubMed] [Google Scholar]
- 11.Duncker B P, Pasero P, Braguglia D, Heun P, Weinreich M, Gasser S M. Cyclin B-Cdk1 kinase stimulates ORC- and Cdc6-independent steps of semiconservative plasmid replication in yeast nuclear extracts. Mol Cell Biol. 1999;19:1226–1241. doi: 10.1128/mcb.19.2.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duronio R J, Brook A, Dyson N, O'Farrell P H. E2F-induced S phase requires cyclin E. Genes Dev. 1996;10:2505–2513. doi: 10.1101/gad.10.19.2505. [DOI] [PubMed] [Google Scholar]
- 13.Duronio R J, O'Farrell P H. Developmental control of the G1 to S transition in Drosophila: cyclin Eis a limiting downstream target of E2F. Genes Dev. 1995;9:1456–1468. doi: 10.1101/gad.9.12.1456. [DOI] [PubMed] [Google Scholar]
- 14.Dynlacht B D, Flores O, Lees J A, Harlow E. Differential regulation of E2F trans-activation by cyclin-cdk2 complexes. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 15.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 16.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 17.Elledge S J, Spottswood M R. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991;10:2653–2659. doi: 10.1002/j.1460-2075.1991.tb07808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enders G H, Koh J, Missero C, Rustgi A K, Harlow E. p16 inhibition of transformed and primary squamous epithelial cells. Oncogene. 1996;12:1239–45. [PubMed] [Google Scholar]
- 19.Fang F, Newport J W. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991;66:731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- 20.Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 21.Furuno N, den Elzen N, Pines J. Human cyclin A is required for mitosis until mid prophase. J Cell Biol. 1999;147:295–306. doi: 10.1083/jcb.147.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guadagno T M, Newport J W. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell. 1996;84:73–82. doi: 10.1016/s0092-8674(00)80994-0. [DOI] [PubMed] [Google Scholar]
- 24.Harkin D P, Bean J M, Miklos D, Song Y H, Truong V B, Englert C, Christians F C, Ellisen L W, Maheswaran S, Oliner J D, Haber D A. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–586. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 25.Higashi H, Suzuki-Takahashi I, Saitoh S, Segawa K, Taya Y, Okuyama A, Nishimura S, Kitagawa M. Cyclin-dependent kinase-2 (Cdk2) forms an inactive complex with cyclin D1 since Cdk2 associated with cyclin D1 is not phosphorylated by Cdk7- cyclin-H. Eur J Biochem. 1996;237:460–467. doi: 10.1111/j.1432-1033.1996.0460k.x. [DOI] [PubMed] [Google Scholar]
- 26.Hinchcliffe E H, Li C, Thompson E A, Maller J L, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann I, Clarke P R, Marcote M J, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J, 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffrey P D, Russo A A, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich N. Mechanism of cdk activation revealed by the structure of a cyclin A-cdk2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H, Chou H S, Zhu L. Requirement of cyclin E-Cdk2 inhibition in p16INK4a-mediated growth suppression. Mol Cell Biol. 1998;18:5284–5290. doi: 10.1128/mcb.18.9.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin P, Gu Y, Morgan D O. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinoshita N, Yamano H, Niwa H, Yoshida T, Yanagida M. Negative regulation of mitosis by the fission yeast protein phosphatase ppa2. Genes Dev. 1993;7:1059–1071. doi: 10.1101/gad.7.6.1059. [DOI] [PubMed] [Google Scholar]
- 32.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 33.Knighton D R, Zheng J H, Ten Eyck L F, Ashford V A, Xuong N H, Taylor S S, Sowadski J M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- 34.Knighton D R, Zheng J H, Ten Eyck L F, Xuong N H, Taylor S S, Sowadski J M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- 35.Knoblich J, Sauer K, Jones L, Richardson H, Saint R, Lehner C. Cyclin E controls S phase progression and its down-regulation during drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 36.Knoblich J A, Lehner C F. Synergistic action of Drosophila cyclins A and B during the G2-M transition. EMBO J. 1993;12:65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knudsen E S, Buckmaster C, Chen T T, Feramisco J R, Wang J Y. Inhibition of DNA synthesis by RB: effects on G1/S transition and S-phase progression. Genes Dev. 1998;12:2278–2292. doi: 10.1101/gad.12.15.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts J M. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 39.Krek W, Ewen M E, Shirodkar S, Arany Z, Kaelin W G, Jr, Livingston D M. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994;78:161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 40.Krek W, Xu G, Livingston D M. Cyclin A-kinase regulation of E2F-1 DNA binding function underlies suppression of an S phase checkpoint. Cell. 1995;83:1149–1158. doi: 10.1016/0092-8674(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 41.Lacey K R, Jackson P K, Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proc Natl Acad Sci USA. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukas C, Sorensen C S, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters J M, Bartek J, Lukas J. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- 43.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuoka S, Huang M, Elledge S. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 45.McConnell B B, Gregory F J, Stott F J, Hara E, Peters G. Induced expression of p16INK4a inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol Cell Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meraldi P, Lukas J, Fry A M, Bartek J, Nigg E A. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat Cell Biol. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- 47.Meyerson M, Enders G, Wu C-L, Su L-K, Gorka C, Nelson C, Harlow E, Tsai L-H. A family of human cdc2-related protein kinases. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michael W M, Ott R, Fanning E, Newport J. Activation of the DNA replication checkpoint through RNA synthesis by primase. Science. 2000;289:2133–2137. doi: 10.1126/science.289.5487.2133. [DOI] [PubMed] [Google Scholar]
- 49.Mitra J, Dai C Y, Somasundaram K, El-Deiry W S, Satyamoorthy K, Herlyn M, Enders G H. Induction of p21WAF1/CIP1 and inhibition of Cdk2 mediated by the tumor suppressor p16INK4a. Mol Cell Biol. 1999;19:3916–3928. doi: 10.1128/mcb.19.5.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 51.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nasmyth K. Viewpoint: putting the cell cycle in order. Science. 1996;274:1643–1645. doi: 10.1126/science.274.5293.1643. [DOI] [PubMed] [Google Scholar]
- 53.Ninomiya-Tsuji J, Nomoto S, Yasuda H, Reed S I, Matsumoto K. Cloning of a human cDNA encoding a CDC2-related kinase by complementation of a budding yeast cdc28 mutation. Proc Natl Acad Sci USA. 1991;88:9006–9010. doi: 10.1073/pnas.88.20.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pagano M, Pepperkok R, Lukas J, Baldin V, Ansorge W, Bartek J, Draetta G. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J Cell Biol. 1993;121:101–111. doi: 10.1083/jcb.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petersen B, Lukas J, Sorensen C, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J, 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poon R Y, Chau M S, Yamashita K, Hunter T. The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 1997;57:5168–5178. [PubMed] [Google Scholar]
- 59.Poon R Y C, Jiang W, Toyoshima H, Hunter T. Cyclin-dependent kinases are inactivated by a combination of p21 and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage. J Biol Chem. 1996;271:13283–13291. doi: 10.1074/jbc.271.22.13283. [DOI] [PubMed] [Google Scholar]
- 60.Qian Y W, Erikson E, Maller J L. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol. 1999;19:8625–8632. doi: 10.1128/mcb.19.12.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruffner H, Jiang W, Craig A G, Hunter T, Verma I M. BRCA1 is phosphorylated at serine 1497 in vivo at a cyclin-dependent kinase 2 phosphorylation site. Mol Cell Biol. 1999;19:4843–4854. doi: 10.1128/mcb.19.7.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 63.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 64.Schlegel R, Pardee A B. Caffeine-induced uncoupling of mitosis from the completion of DNA replication in mammalian cells. Science. 1986;232:1264–1264. doi: 10.1126/science.2422760. [DOI] [PubMed] [Google Scholar]
- 65.Scolnick D M, Halazonetis T D. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature. 2000;406:430–435. doi: 10.1038/35019108. [DOI] [PubMed] [Google Scholar]
- 66.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 67.Shirahige K, Hori Y, Shiraishi K, Yamashita M, Takahashi K, Obuse C, Tsurimoto T, Yoshikawa H. Regulation of DNA-replication origins during cell-cycle progression. Nature. 1998;395:618–621. doi: 10.1038/27007. [DOI] [PubMed] [Google Scholar]
- 68.Simon L, Cedar H. Temporal order of DNA replication. In: DePamphilis M L, editor. DNA replication in eukaryotic cells. Cold Spring Harbor. N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 387–408. [Google Scholar]
- 69.Spruck C H, Won K A, Reed S L. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- 70.Stoeber K, Mills A D, Kubota Y, Krude T, Romanowski P, Marheineke K, Laskey R A, Williams G H. Cdc6 protein causes premature entry into S phase in a mammalian cell-free system. EMBO J. 1998;17:7219–7229. doi: 10.1093/emboj/17.24.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stott F, Bates S, James M, McConnell B, Starborg M, Brookes S, Palmero L, Ryan K, Hara E, Vousden K, Peters G. The alternative product from the human CDKN2A locus, p14ARF, participates in a regulatory feedback loop with p53. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai L-H, Harlow E, Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991;353:174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- 73.Tsai L-H, Lees E, Faha B, Harlow E, Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993;8:1593–1602. [PubMed] [Google Scholar]
- 74.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 75.Weisshart K, Fanning E. Roles of phosphorylation in DNA replication. In: DePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1996. p. 295-330. [Google Scholar]
- 76.Widrow R J, Hansen R S, Kawame H, Gartler S M, Laird C D. Very late DNA replication in the human cell cycle. Proc Natl Acad Sci USA. 1998;95:11246–11250. doi: 10.1073/pnas.95.19.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winkler K E, Swenson K I, Kornbluth S, Means A R. Requirement of the prolyl isomerase Pin 1 for the replication checkpoint. Science. 2000;287:1644–1647. doi: 10.1126/science.287.5458.1644. [DOI] [PubMed] [Google Scholar]
- 78.Zarkowska T, Mittnach S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;272:12738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H, Gavin M, Dahiya A, Postigo A, Ma D, Luo R, Harbour J, Dean D. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell. 2000;101:79–89. doi: 10.1016/S0092-8674(00)80625-X. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H S, Postigo A A, Dean D C. Active transcriptional repression by the Rb-E2F complex mediates G1 arrest triggered by p16INK4a, TGFbeta, and contact inhibition. Cell. 1999;97:53–61. doi: 10.1016/s0092-8674(00)80714-x. [DOI] [PubMed] [Google Scholar]
- 81.Zhao J, Dynlacht B, Imai T, Hori T, Harlow E. Expression of NPAT, a novel substrate of cyclin E-CDK2, promotes S phase entry. Genes Dev. 1998;12:456–461. doi: 10.1101/gad.12.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]