Abstract
OBJECTIVE.
The objective of this study was to report our experience with active surveillance of nonfatty renal masses in a large cohort of patients with lymphangioleiomyomatosis (LAM), correlate their CT features and patterns of growth with histopathology results, and provide guidelines for management.
SUBJECTS AND METHODS.
Yearly CT examinations were performed of 367 women (age range, 21–75 years; mean age, 47 years). For the 31 patients with 37 nonfatty renal masses that were biopsied, excised, or followed for ≥ 5 years, CT enhancement characteristics and patterns of growth were compared with the histopathology results.
RESULTS.
Four of 37 nonfatty renal masses were biopsied without follow-up CT examinations: Two were heterogeneous renal cell carcinomas (RCCs), one was a heterogeneous nonfatty angiomyolipoma (AML), and one was homogeneous nonfatty AML. In the remaining 33 nonfatty renal masses with multiple follow-up CT examinations, two growth patterns were identified. Four showed a continuous increase in size of > 0.5 cm/y in some years, and all four in this first group were heterogeneous and were biopsy-proven RCC. The second group was composed of the remaining 29 masses. These 29 masses showed yearly no change, increase, or decrease in diameter. Eight were heterogeneous, and 21 were homogeneous. Of the masses showing a yearly increase, the increase was < 0.5 cm/y in all except one. In the one exception, the increase followed a decrease. Nine of the 29 masses were biopsied, and all nine were nonfatty renal masses (five homogeneous, four heterogeneous).
CONCLUSION.
Our data provide further evidence in a large prospective study with long-term follow-up that active surveillance is an appropriate strategy in the management of nonfatty renal masses in patients with LAM. Our analysis of the growth patterns reveals duration of growth in addition to growth rate as criteria for biopsy or excision. Biopsy should be reserved for nonfatty renal masses that show sustained growth or growth > 0.5 cm/y during follow-up.
Keywords: active surveillance, lymphangioleiomyomatosis (LAM), nonfatty renal angiomyolipoma (AML), renal cancer, renal cell carcinoma (RCC)
Patients with lymphangioleiomyomatosis (LAM) have a frequency of angiomyolipoma (AML) that varies from 20% to 54% depending on the method of patient or case collection, imaging modality used, diagnostic criteria, and statistics of sampling variation. This frequency of AML is much higher than the reported frequency of 1–3% in the general population [1-3]. Most AMLs are benign tumors that contain variable amounts of muscle, blood vessels, and fat; they are the most common renal mesenchymal neoplasm and originate from perivascular epithelioid cells (PECs) and are also termed “PEComa” [3].
When AMLs contain fat, most can be diagnosed using CT and MRI [4, 5]. However, some AMLs (5% in the general population) contain too little fat to be visible on CT [6]. AMLs with no visible fat that are hyperattenuating relative to the normal renal parenchyma on unenhanced CT have been called “AML with minimal fat,” and AMLs that are hypoattenuating relative to the normal renal parenchyma but with attenuation values not in the range of fatty tissue on unenhanced CT have been termed “AML with diffusely scattered fat” [6]. In this article, we use the term “nonfatty renal mass” to denote any renal mass with no visible fat on CT and the term “nonfatty AML” to denote an AML with no visible fat on CT.
Epithelioid AML is a variant of AML that is characterized by proliferation of predominantly epithelioid cells [7]. On CT, epithelioid AMLs are usually heterogeneous enhancing masses with minimal or no fat and are indistinguishable from nonfatty AML and renal cell carcinoma (RCC) [8]. Although the malignant potential of AMLs has been documented in multiple reports, a report of a large series of 437 consecutively resected AMLs [7] suggests that epithelioid AML is rare (46% of resected AMLs), that its rate of aggressive behavior is very low (5% of patients developed metastasis), and that earlier reports of high rates of metastases and aggressive behavior are likely overestimates due to selection bias [9].
Nonfatty renal masses present a diagnostic dilemma because their differential diagnosis includes RCC and nonfatty AML. The noninvasive assessment of nonfatty renal masses and avoidance of unnecessary surgery are particularly important in patients with LAM because of, first, increased surgical risk in patients with poor lung function due to LAM; second, their potential to develop compromised renal function due to procedures performed to treat hemorrhage; and, third, the increased risk of hemorrhage during biopsy due to the vascular nature of AML [10, 11].
Various CT and MRI features and techniques have been evaluated to differentiate nonfatty AML from RCC including increased attenuation on unenhanced images, enhancement patterns on biphasic CT, pixel mapping, CT texture analysis, and opposed-phase chemical-shift and DWI features on MRI to try to find small amounts of fat [5, 12-17]. Because none of these techniques has high specificity, the CT and MRI diagnosis of nonfatty AML remains problematic. Hence, active surveillance of nonfatty renal masses with follow-up imaging has been recommended with assessment of the presence or absence of growth as the basis for selecting lesions for biopsy or excision [18-27].
In this study, we assess the results of active surveillance of nonfatty renal masses in a large cohort of 367 patients with LAM followed with yearly CT. We evaluate the usefulness of CT features and patterns of growth in differentiating nonfatty AML from RCC and providing a basis for appropriate management. In our analysis, we provide a more thorough assessment of the changes in size than just the presence or absence of enlargement by partitioning the entire time interval of surveillance into a sequence of time intervals of growth, stability, and shrinkage and assess the hypothesis that continued growth serves as a discriminating feature.
Subjects and Methods
Study Group
This prospective study with retrospective review included 367 women (age range, 21–75 years; mean age, 47 years) with the diagnosis of sporadic LAM. The patients were seen between 1995 and 2011 on protocols evaluating the natural history of LAM that included yearly CT of the abdomen and pelvis. The study protocols and consent documents were approved by the institutional review board. All subjects provided written informed consent before enrollment, which included consent for future retrospective analysis. Both the natural history protocols and our current retrospective analysis are compliant with HIPAA.
The diagnosis of sporadic LAM was established by lung biopsy in 231 patients and biopsy of abdominal or pelvic masses in 23 patients. The 113 patients without a biopsy-proven diagnosis had classic clinical histories and CT findings of LAM (i.e., diffusely scattered thin-walled lung cysts in association with renal AMLs or lymphangioleiomyomas, or chylous pleural effusions) [19].
According to protocol design, all patients underwent CT of the abdomen and pelvis. The CT examinations were performed with 5-mm-thick slices after the administration of oral contrast material and before and after the administration of IV contrast material during the portovenous phase (120 mL of iopamidol 61% [Isovue 300, Bracco Diagnostics]). The patients were scanned using various helical CT scanners that included HiSpeed Advantage (single detector), QX/i (4 detectors), HiSpeed (8 detectors), and LightSpeed (16 detectors) (all, GE Healthcare); Definition (32 detectors, Siemens Healthcare); and Brilliance (64 detectors, Philips Healthcare). All CT examinations were performed with automatic tube current modulation or with 240 mA and a 0.8-second rotation time, 120 kV, an FOV of 36 cm, and a pitch of 0.8–1.0. Most patients (360/367, 98%) had yearly follow-up CT examinations.
Biopsies of nonfatty renal masses were performed when the imaging characteristics were suggestive of RCC (growth > 0.5 cm/y, sustained growth, absence of fat, or presence of heterogeneous components suggesting areas of necrosis) or when the patients requested the biopsy because they were apprehensive and unwilling to undergo imaging follow-up. Some patients with radiographically suspicious masses refused biopsy and opted for follow-up because of concerns about the possibility of complications from the procedure.
A board-certified radiologist with more than 20 years of experience with abdominal CT reviewed all images of all patients using a PACS (DirectView, version 5.1, Kodak; Carestream Vue, versions 10.0, 10.1, 11.3, and 12.1.5, Carestream Health).
The CT attenuation, enhancement characteristics, and growth patterns of the nonfatty renal masses were assessed and correlated with biopsy results. Attenuation measurements of the masses were obtained by drawing ROIs at least 0.5 cm in diameter and measuring the mean attenuation in Hounsfield units. The masses were classified as fatty AMLs if they contained portions with an attenuation of less than −10 HU on unenhanced CT and were classified as nonfatty renal masses if they did not. Non fatty renal masses were further classified as homogeneous if they had uniform enhancement > 20 HU compared with the unenhanced images and heterogeneous if they had nonuniform enhancement. We also evaluated if the nonfatty renal masses had calcifications at baseline or if they developed fat or calcification during follow-up.
For each nonfatty renal mass, the largest anteroposterior (AP) and transverse (TRV) diameters were measured with electronic calipers. The craniocaudal (CC) diameter was obtained by counting the number of slices that included the mass and multiplying the number by the slice thickness (5 mm). The geometric mean of these three diameters—that is, the cube root of their product (AP × TRV × CC) ^ 1/3—was used as the measure of the nonfatty renal mass’s size on each CT scan. This value provides an estimate of the diameter of a spherical lesion with the same volume of an elliptical mass with that product of diameters. We used this value (a diameter) instead of the product of diameters (a volume estimate) for two reasons. First, the tumor diameter is more commonly used than tumor volume by radiologists and other medical care providers in describing tumor sizes and their changes over time. Second, the geometric mean diameter allows application of a threshold of significant and insignificant differences in the change of tumor size over time that relates directly to the diameter measurements. For the remainder of this article, the term “diameter” is used to denote “geometric mean diameter.”
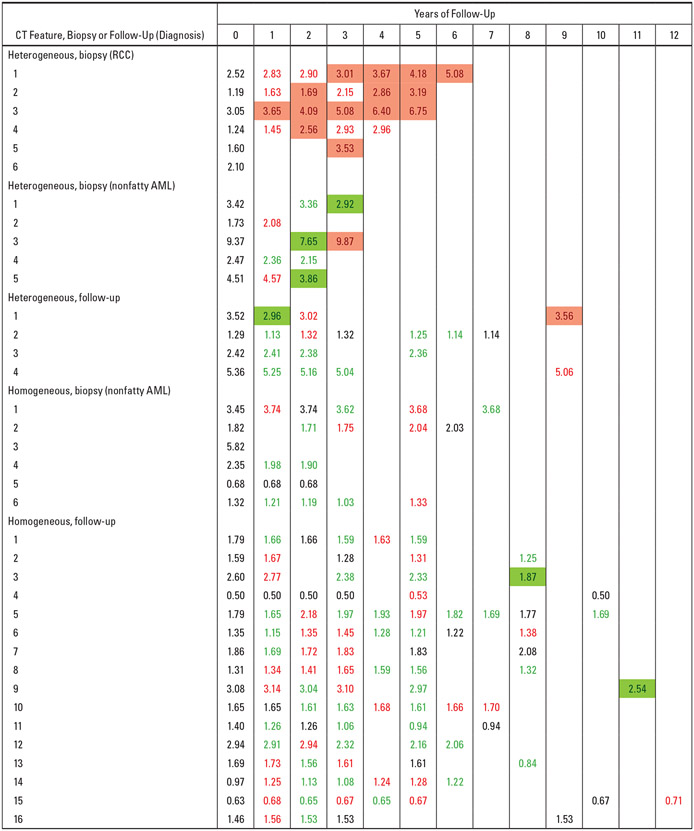
The changes in diameter at baseline and on follow-up CT examinations for the 37 nonfatty renal masses with ≥ 5 years of follow-up or with results from biopsy or excision were cross-tabulated with biopsy results and CT features in Figure 1. In Figure 1, each row corresponds to a nonfatty renal mass, and each column corresponds to a CT examination. The number at the top of each column indicates the number of years after baseline (year 0) when the CT was performed. The masses are grouped in terms of biopsy results and CT characteristic (i.e., heterogeneous vs homogeneous). Entries in Figure 1 are geometric mean diameters in centimeters on each CT examination. The color of the entry indicates direction of change in diameter over time: green indicates intervals of decrease; red, intervals of increase; and black, intervals of no change compared with the prior study. Entries shaded with red indicate significant (> 0.5 cm) increases in diameter and shaded with green indicate significant (> 0.5 cm) decreases in diameters compared with the prior study.
Fig. 1—
Graphic shows geometric mean diameters (in centimeters) on baseline (year 0) and follow-up CT examinations for 37 nonfatty renal masses with either ≥ 5 years of CT follow-up or biopsy results cross-tabulated with CT features (heterogeneous vs homogeneous). Histopathologic results (i.e., renal cell carcinoma [RCC] or nonfatty angiomyolipoma [AML]) are shown for masses that underwent biopsy or excision. Colors show changes in geometric mean diameter since last measurement: black = no change, red = increase, green = decrease. Red shading = significant increase (> 0.5 cm). Green shading = significant decrease (> 0.5 cm).
The diameter on each CT study was compared with the diameter on the immediately preceding CT study and the intertest difference was calculated as follows: (diameter on present CT study – diameter immediately preceding CT study). To capture the details of the growth pattern, we analyzed the intertest data as follows: Each of the intertest interval changes was characterized as an increase, decrease, or no change if the change was positive, negative, or 0, respectively, and was color-coded accordingly as red, green, or black. Adjacent intertest intervals were combined if they had the same sign. This created longer periods of continuous increase, decrease, or no change over multiple intertest intervals and partitioned the total follow-up into a sequence of periods of increase, decrease, or no change in tumor size. A period of increase or decrease was denoted as significant if the diameter changed > 0.5 cm compared with the diameter on the CT performed at the time that marked the beginning of the period (i.e., the CT at the beginning of the first intertest interval in the period).
If an increase or decrease of > 0.5 cm was observed before the end of the period, then this change was marked as a point of significant increase or decrease and the diameter at that point was used as the basis for assessment of the significance of subsequent increases or decreases in the remainder of the period. Thus, a period of continuous increase or decrease may show no significant increase or decrease from beginning to end or may contain intertest intervals of significant increase or decrease.
Statistical Analysis
The Fisher exact test was used to determine the statistical significance of the differences between the prevalence of RCC in the masses with continuous growth and the masses without continuous growth that were biopsied or excised.
Results
One hundred six of the 367 (29%) patients had solid or complex renal masses: 62 (17%) had a fatty renal AML and 44 (12%) had a nonfatty renal mass. Thirty-one of 44 patients with nonfatty renal masses had 37 masses that were biopsied or were followed for at least 5 years (Fig. 1 and Table 1). Seventeen patients had pathologic proof: Four had nephrectomy, two had partial nephrectomy, and 11 had biopsy of the nonfatty renal mass (Table 2).
TABLE 1:
Analysis of Initial Diameter of Nonfatty Renal Masses
| Category | No. of Masses | Initial Diameter (cm) | ||
|---|---|---|---|---|
| Range | Mean | Median | ||
| All masses | 37 | 0.50–9.37 | 2.37 | 1.79 |
| Heterogeneous masses | 15 | 1.19–9.37 | 3.05 | 2.47 |
| Biopsy diagnosis of RCC | 6 | 1.19–3.05 | 1.95 | 1.85 |
| Biopsy diagnosis of AML | 5 | 1.73–9.37 | 4.30 | 3.42 |
| Follow-up CT ≥ 5y | 4 | 1.29–5.36 | 3.15 | 2.97 |
| Homogeneous masses | 22 | 0.50–5.82 | 1.91 | 1.62 |
| Biopsy diagnosis of AML | 6 | 0.68–5.82 | 2.57 | 2.09 |
| Follow-up CT ≥ 5y | 16 | 0.50–3.08 | 1.66 | 1.62 |
Note—RCC = renal cell carcinoma, AML = angiomyolipoma.
TABLE 2:
Cross-Tabulation of CT Features and Histopathologic Results in 17 Nonfatty Renal Masses That Had Biopsy or Nephrectomy and the Growth Pattern Results of 20 Nonfatty Renal Masses Without Biopsy With ≥ 5 Years of CT Follow-Up
| Biopsy | |||
|---|---|---|---|
| CT Feature | RCC | Nonfatty AML | No Sustained Growth at Follow-Up CT for ≥ 5 Years |
| Heterogeneous | 6 | 5 | 4 |
| Homogeneous | 0 | 6 | 16 |
Note—Data are number of masses. RCC = renal cell carcinoma, AML = angiomyolipoma.
After IV contrast administration, 22 of the 37 (59%) masses were homogeneous and 15 (41%) were heterogeneous (Figs. 2 and 3). None of the 37 masses developed fat during follow-up. None of the masses had calcifications at initial diagnosis; one heterogeneous mass developed calcifications after 10 years of follow-up.
Fig. 2—
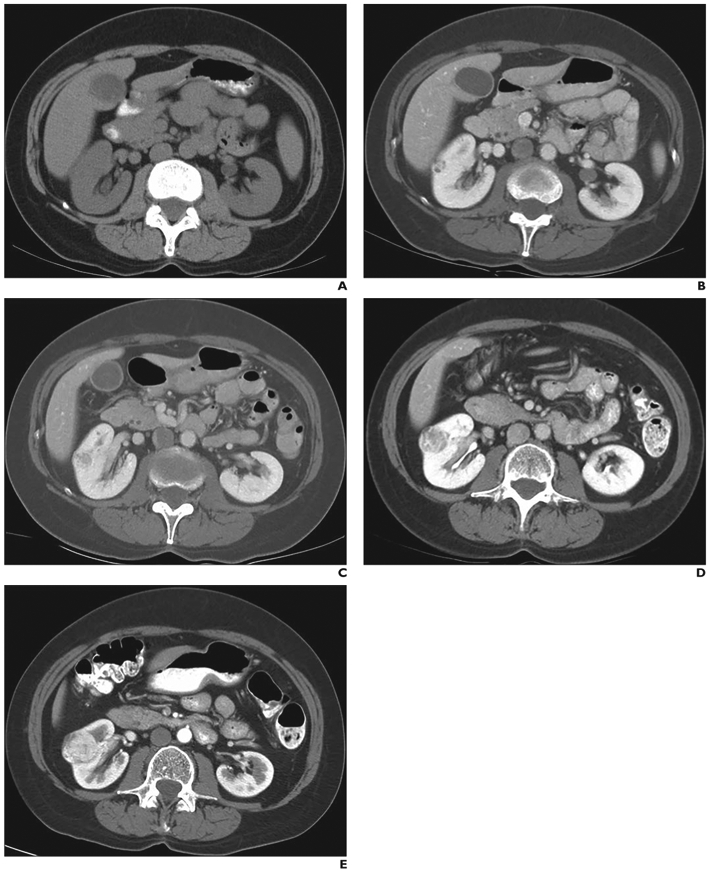
60-year-old woman (patient 2 in heterogeneous, biopsy, renal cell carcinoma [RCC] category in Fig. 1) with severe involvement of lungs with lymphangioleiomyomatosis. Baseline and follow-up abdominal CT images show that geometric mean diameter of heterogeneous renal mass in interpolar region of right kidney progressively increased during follow-up. Mass proved to be RCC at histopathology.
A and B, On baseline unenhanced (A) and enhanced (B) CT images, geometric mean diameter of mass is 1.19 cm.
C, On 1-year follow-up CT image, geometric mean diameter of mass has increased to 1.63 cm.
D, On 3-year follow-up CT image, geometric mean diameter of mass has increased to 2.15 cm.
E, On 5-year follow-up CT image, geometric mean diameter of mass has increased to 3.19 cm.
Fig. 3—
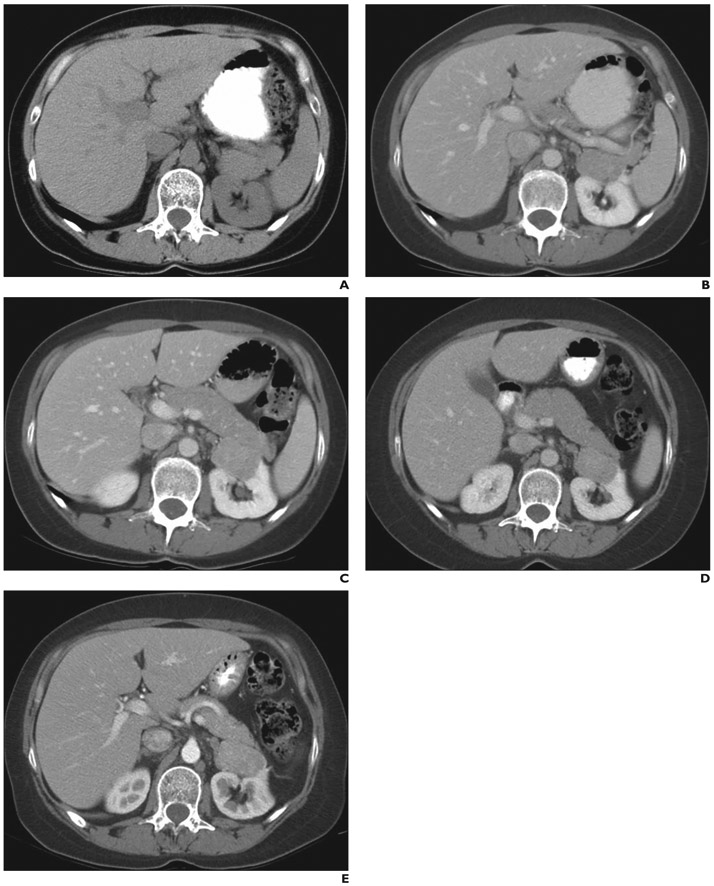
43-year-old woman with severe involvement of lungs with lymphangioleiomyomatosis (patient 1 in homogeneous, biopsy, nonfatty angiomyolipoma [AML] category in Fig. 1). Baseline and follow-up abdominal CT images show homogeneously enhancing mass in upper pole of left kidney. Mass proved to be nonfatty AML at histopathology.
A and B, On baseline unenhanced (A) and enhanced (B) CT images, geometric mean of mass is 3.45 cm.
C, On 1-year follow-up CT image, geometric mean diameter of mass has increased to 3.74 cm.
D, On 3-year follow-up CT image, geometric mean diameter of mass has decreased to 3.62 cm.
E, On 5-year follow-up CT image, geometric mean diameter of mass has increased to 3.68 cm.
As shown in Figure 1, two of 37 nonfatty renal masses were biopsied immediately after CT detection at baseline; one was a heterogeneous RCC (patient 6 in heterogeneous, biopsy, RCC category in Fig. 1), and the second was a homogeneous nonfatty AML (patient 3 in homogeneous, biopsy, nonfatty AML category in Fig. 1). Of the remaining 35 patients, two had masses that were biopsied after only one follow-up CT: One patient had a heterogeneous RCC (patient 5 in the heterogeneous, biopsy, RCC category in Fig. 1), and one patient had a heterogeneous nonfatty AML (patient 2 in the heterogeneous, biopsy, nonfatty AML category in Fig. 1).
The 33 masses evaluated with multiple (i.e., > 2) follow-up CT examinations showed two distinctly different growth patterns. One group of four showed continuous increase in size with > 0.5 cm/y in some years; all four were heterogeneous and biopsy-proven RCC (Fig. 1). The remaining 29 showed interspersed yearly CT findings of no change, increase, or decrease in diameter. All of the yearly increases were < 0.5 cm/y except one. In the one mass that was the exception, the increase followed a decrease. Eight were homogeneous, and 21 were heterogeneous. Nine were biopsy-proven nonfatty AML, four of which were heterogeneous and five, homogeneous.
In the 13 cases with biopsy proof and multiple follow-up measurements (four RCCs and nine nonfatty AMLs), four of four RCCs showed continuous growth and nine of nine nonfatty AMLs showed no continuous growth; this association was significant (p = 0.0014, Fisher exact test) (Fig. 1).
Heterogeneous Nonfatty Renal Masses; Biopsy Results and Patterns of Growth
There were 15 nonfatty renal masses that enhanced heterogeneously (Fig. 1). Eleven of the 15 were biopsied. Of these 11, six were clear cell RCC at histopathology and five were nonfatty AML. One of the six RCCs was biopsied immediately, and the other five had follow-up for 3, 4, 5, 5, and 6 years; all five with follow-up had significant (> 0.5 cm) increases in diameter from baseline (Fig. 1). All four that were biopsied and had multiple (i.e., ≥ 3) follow-up CT examinations showed continuous increases in diameters. All showed > 0.5 cm/y increase in diameter in at least 1 year during follow-up; three of four had continuous significant (> 0.5 cm/y) increases in diameter in multiple (i.e., > 2) yearly follow-up intervals (Fig. 1).
The five histopathologically proven heterogeneous nonfatty AMLs were followed, respectively, for 1, 2, 2, 3, and 3 years (Fig. 1). None of the four that had more than one follow-up showed a continuous increase in diameter, all had a decrease in diameter at some time during follow-up, and three showed a decrease of > 0.5 cm (Fig. 1). One showed a significant increase (2.22 cm) on the third year of follow-up, following a significant decrease (1.72 cm) between baseline and the second year; this increase prompted excision, terminating surveillance and precluding assessment of the duration of increase (patient 3 in the heterogeneous, biopsy, nonfatty AML category in Fig. 1).
Four heterogeneous nonfatty renal masses without biopsy were followed for 5, 7, 9, and 9 years. None showed continuous increase in diameter, and all four showed a decrease in diameter by 1 year, with a decrease of > 0.5 cm/y in one (Fig. 1). One patient did not return for the yearly follow-up in years 3–8 and showed a > 0.5 cm increase at year 9 compared with year 2; however, this increase followed a significant > 0.5 cm decrease in the first year (patient 1 in heterogeneous, follow-up category in Fig. 1).
Homogeneous Nonfatty Renal Masses; Biopsy Results and Patterns of Growth
There were 22 nonfatty renal masses that had homogeneous enhancement (Fig. 1). Six of the 22 were biopsied, and all six proved to be nonfatty AML. One of the six was biopsied immediately. The other five had follow-up for 2, 2, 5, 6, and 7 years. None showed a continuous increase in diameter. Four of five showed a mixed pattern of intervals of < 0.5 cm/y increases or decreases in diameter, and one showed no change. None showed an increase in diameter > 0.5 cm/y or > 0.5 cm over baseline (Figs. 1 and 3).
The 16 of 22 nonfatty renal masses that were not biopsied were followed for ≥ 5 years (range, 5–12 years; mean, 8 years) (Fig. 1). None showed a continuous increase in diameter; all showed a mixed pattern of increases and decreases in diameter. Two showed a decrease in diameter of > 0.5 cm, and all the other changes were < 0.5 cm (Fig. 1). None showed an increase in diameter of > 0.5 cm over baseline or > 0.5 cm/y.
Discussion
In 1998, the Tuberous Sclerosis Complex Consensus Conference recognized renal AML as a useful diagnostic criterion for tuberous sclerosis complex (TSC) but gave no recommendation for follow-up [28]. More recently, the 2012 International Tuberous Sclerosis Complex Consensus Conference provided guidelines for the diagnosis and treatment of renal masses in patients with TSC. These recommendations included yearly surveillance imaging, treatment of growing AMLs that are more than 3 cm in diameter with mechanistic target of rapamycin (mTOR) inhibitors, and biopsy of masses that show growth of > 0.5 cm/y [24-28]. This biopsy recommendation was based on findings from a retrospective study of 12 cases of TSC followed for a median of 4 years with yearly CT or MRI [18]. In that study, three of 52 indeterminate nonfatty renal masses showed rapid growth (> 0.5 cm/y), and one of the three proved to be an RCC; the other two were nonfatty AMLs [18].
Our results provide further evidence in a large cohort of patients of the appropriateness of active surveillance of nonfatty renal masses in patients with LAM. Our analysis of the serial CT follow-up data into sequences of periods of increase, decrease, and no change and our color-coded tabulation of the results in Figure 1 makes visually apparent two distinctly different patterns of growth of nonfatty renal masses. They confirm the value of the presence versus the absence of sustained growth as a basis of the criteria for intervention versus continued surveillance with CT.
The minority of the masses (4/35, 11%) showed significant continuous increase in diameter (> 0.5 cm/y in > 4 consecutive years). All were surgically removed and found to be RCC. Most of the masses (28/35, 80%) showed mixed, interspersed periods of insignificant (≤ 0.5 cm/y) increase or decrease in diameter. A subset of these masses were biopsied or excised, and all were histopathologically nonfatty AML.
There was no case of initial stability or decrease in diameter followed by sustained growth later in the surveillance. In all cases followed for ≥ 5 years, CT findings of stability or a decrease in diameter early in the follow-up predicted continued stability—that is, a benign appearance—later. Thus, CT findings of stability or a decrease in diameter imply continued stability. Moreover, when present, a decrease was apparent early in the surveillance; of the 27 that decreased in diameter during follow-up, 13 (48%) showed a decrease by 1 year, 23 (85%) by 2 years, and all by 4 years.
No case of significant (i.e., > 0.5 cm/y) growth followed by stability was observed in our serial follow-up CT data. However, our identification of sizable, stable nonfatty renal masses implies a pattern of a period of growth followed by a period of quiescence during which our CT examinations were performed. Moreover, the two nonfatty renal masses with growth rates of > 0.5 cm/y observed by Patel et al. [18], that proved to be nonfatty AML, may have been examples of tumors imaged during a period of active growth that would have stabilized later. Gompertzian and other mathematic models of tumor growth explain a growth pattern of active growth followed by stability in terms of dependence of growth rate on energy and metabolic or vascular factors yielding a slowing of growth rate as the tumor enlarges, limiting tumor size, and producing a sigmoid-shaped growth curve [29, 30]. Regardless of the underlying mechanism, the practical implication of the sigmoid growth curve is the possibility of imaging benign masses that ultimately will stabilize after a period of initial growth. This observation mandates the need to follow enlarging lesions to differentiate transient and limited growth from sustained growth and the use of sustained growth in the criteria for recommending biopsy of an enlarging mass.
Our results also show the value of the CT features of nonfatty renal masses in differentiating the groups. All continuously enlarging masses and biopsy-proven RCCs were heterogeneous. Most shrinking or stable masses were homogeneous (i.e., 21 of 28 followed for more than 1 year and 18 of 22 followed for more than 5 years). These findings agree with those in previous reports that homogeneous enhancement is a CT feature of nonfatty AML that is useful in differentiating nonfatty AML from RCC [7, 31-34].
There is a body of reported data on the active surveillance of renal masses in patients without sporadic LAM. In general, the reports show slow growth rates and a lack of correlation between initial size and growth rate [35, 36]; these findings prompted the investigators to conclude that a period of observation with serial imaging can be safely performed in patients who are medically unfit for surgery [35, 36].
These results are similar to ours regarding the finding of a very high prevalence of RCC in growing lesions that were biopsied. In our study, all five masses that showed sustained growth were resected, and 100% were found to be RCC. In a study of surveillance of enhancing renal masses by Kunkle et al. [37], 71 of 106 (67%) masses showed growth, and 32 of the 36 (89%) that were resected were RCCs. In a meta-analysis of 288 lesions by Chawla et al. [35], pathologic confirmation was available in 131 of 286 (46%) cases, and it was confirmed that 120 of 131 (92%) were RCC variants. The rate of malignant lesions in each series included in the meta-analysis was 80–100% [35].
Reported results in patients without LAM differ from ours in patients with LAM in the observed rates of nongrowing nonfatty renal masses and in the probabilities of them being RCCs. In a report by Kunkle et al. [37], 35 of 106 (33%) masses showed no growth or negative growth, and five of the six (83%) masses that were resected were RCC. This result contrasts with the result of our series of 0% frequency of RCC in the nine nonfatty renal masses that showed no sustained growth and were resected. These differences in observed rates of nongrowing, nonfatty renal masses and probabilities of them being RCCs can be explained by the high prevalence of nonfatty AML in patients with LAM and the absence of sustained growth in nonfatty AMLs. The effect of a higher prior probability of non-RCC lesions in our results supports the recommendation of active surveillance a fortiori in patients with LAM.
A major limitation of the study is that most (16/25) of the nonfatty renal masses that did not show significant or continuous growth were not biopsied. Factors that discouraged biopsy of all nonfatty renal masses included the presence of comorbidities in this patient population, presence of multiple nonfatty renal masses, high prevalence of nonfatty AML, and patient reluctance to consent to the procedures. Although all of the masses that were biopsied proved to be nonfatty AML, this limitation precludes confident exclusion of some of the masses being stable RCCs. Regardless of the uncertainty about the histology of these masses, the results suggest that they are benign lesions with no or low potential for metastases and support the policy of no biopsy or excision of stable masses. Another limitation is the small number of biopsy-proven RCCs; this limits assessment of the spectrum of growth patterns of RCC and the sensitivity of criteria based on growth rate. Another limitation was that only one radiologist read all the examinations and performed the measurements.
Ultimately, it may turn out that only a subset of RCCs need be resected; that is, we may learn that some do not metastasize and reach a stable benign pattern. Molecular biomarkers have been discovered that may potentially serve as tools in diagnosing clear cell RCCs; if biomarkers were to be discovered to differentiate tumors that metastasize from those that do not, then the need to resect all tumors to prevent metastasis would be precluded and resection could be restricted to those at risk for metastasis [38, 39]. Discovery of imaging features or other biomarkers that identify the RCC at risk for metastasis would be an important advance and could be included in the surveillance protocol.
In conclusion, our results in a large prospective study with long follow-up confirm the validity of previous recommendations of active surveillance of nonfatty renal masses in patients with LAM. Our analysis of the serial CT follow-up data into sequences of periods of increase, decrease, and no change shows the value of the presence versus absence of sustained growth and the value of growth rate as the bases of criteria for biopsy or excision intervention or continued surveillance. It is not appropriate to biopsy all nonfatty renal masses; those that decrease in diameter or remain stable during follow-up should not be biopsied but should be followed to determine their pattern of growth. Biopsy should be reserved for masses that show sustained growth and significant increase in size during follow-up.
Acknowledgments
We acknowledge the LAM Foundation and the Tuberous Sclerosis Alliance for referring patients for our studies. All research participants gave informed consent before enrollment and participated in a natural history study (NHLBI protocol 95-H-0186), which was approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute.
Supported by the Division of Intramural Research, National Institutes of Health, National Heart, Lung, and Blood Institute.
Footnotes
Based on a presentation at the ARRS 2011 Annual Meeting, Chicago, IL.
References
- 1.Avila NA, Kelly JA, Chu SC, et al. Lymphangioleiomyomatosis: abdominopelvic CT and US findings. Radiology 2000; 216:147–153 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein SM, Newell JD, Adamczyk D, et al. How common are renal angiomyolipomas in patients with pulmonary lymphangioleiomyomatosis? Am J Respir Crit Care Med 1995; 152:2138–2143 [DOI] [PubMed] [Google Scholar]
- 3.Katabatahina VS, Vikram R, Nagar AM, et al. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. RadioGraphics 2010; 30:1525–1540 [DOI] [PubMed] [Google Scholar]
- 4.Bosniak MA, Megibow AJ, Hulnick DH, et al. CT diagnosis of renal angiomyolipoma: the importance of detecting small amounts of fat. AJR 1988; 151:497–501 [DOI] [PubMed] [Google Scholar]
- 5.Israel GM, Hindman N, Hecht E, et al. The use of opposed-phase chemical shift MRI in the diagnosis of renal angiomyolipoma. AJR 2005; 184:1868–1872 [DOI] [PubMed] [Google Scholar]
- 6.Jinzaki M, Silverman SG, Tanimoto A, et al. Angiomyolipoma that do not contain fat attenuation at unenhanced CT. Radiology 2005; 234:311. [DOI] [PubMed] [Google Scholar]
- 7.He W, Cheville JC, Sadow PM, et al. Epithelioid angiomyolipoma of the kidney: pathological features and clinical outcome in a series of consecutively resected tumors. Mod Pathol 2013; 26:1355–1364 [DOI] [PubMed] [Google Scholar]
- 8.Froemming AT, Boland J, Cheville J, Takahashi N, Kawashima A. Renal epithelioid angiomyolipoma: imaging characteristics in nine cases with radiologic-pathologic correlation and review of the literature. AJR 2013; 200:[web]W179–W188 [DOI] [PubMed] [Google Scholar]
- 9.Lei JH, Liu LR, Wei Q, et al. A four-year follow-up study of renal epithelioid angiomyolipoma: a multi-center experience and literature review. Sci Rep 2015; 5:10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu SC, Horiba K, Usuki J, et al. Comprehensive evaluation of 25 patients with lymphangioleiomyomatosis. Am J Respir Crit Care Med 1997; 155:A330 [Google Scholar]
- 11.Oesterling JE, Fishman EK, Goldman SM, et al. The management of renal angiomyolipoma. J Urol 1986; 135:1121–1124 [DOI] [PubMed] [Google Scholar]
- 12.Takahashi N, Leng S, Kitajima K, et al. Small (< 4 cm) renal masses: differentiation of angiomyolipoma without visible fat from renal cell carcinoma using unenhanced and contrast-enhanced CT. AJR 2015; 205:1194–1202 [DOI] [PubMed] [Google Scholar]
- 13.Kim JK, Park SY, Shon JH, et al. Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at biphasic helical CT. Radiology 2004; 230:677–684 [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Kim CS, Jeong JY, et al. Differentiation of clear cell renal cell carcinoma from other subtypes and fat-poor angiomyolipoma by use of quantitative enhancement measurement during three-phase MDCT. AJR 2016; 206:[web]W21–W28 [DOI] [PubMed] [Google Scholar]
- 15.Catalano OA, Samir AE, Sahani DV. Pixel distribution analysis: can it be used to distinguish clear cell carcinomas from angiomyolipomas with minimal fat? Radiology 2008; 247:738–746 [DOI] [PubMed] [Google Scholar]
- 16.Lubner MG, Stabo N, Abel EJ, et al. CT textural analysis of large primary renal cell carcinomas: pretreatment tumor heterogeneity correlates with histologic findings and clinical outcomes. AJR 2016; 207:96–105 [DOI] [PubMed] [Google Scholar]
- 17.Hötker AM, Mazaheri Y, Wibmer A, et al. Use of DWI in the differentiation of renal cortical tumors. AJR 2016; 206:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel U, Simpson E, Kingswood JC, et al. Tuberose sclerosis complex: analysis of growth rates aids differentiation of renal cell carcinoma from atypical or minimal-fat-containing angiomyolipoma. Clin Radiol 2005; 60:665–673 [DOI] [PubMed] [Google Scholar]
- 19.Johnson SR, Cordier JF, Lazor R, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010; 35:14–26 [DOI] [PubMed] [Google Scholar]
- 20.Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013; 49:243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krueger DA, Northrup H; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013; 49:255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kingswood JC, Serglusz J, Belousova ED, et al. The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: subgroup results from the randomized, placebo-controlled, phase 3 trial EXIST-1. Nephrol Dial Transplant 2014; 29:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassouf W, Aprikian AG, Laplante M, et al. Natural history of renal masses followed expectantly. J Urol 2004; 171:111–113 [DOI] [PubMed] [Google Scholar]
- 24.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical stage 1 renal mass. J Urol 2009; 182:1271–1279 [DOI] [PubMed] [Google Scholar]
- 25.American Urological Association website. Guideline for management of the clinical stage 1 renal mass. www.auanet.org/content/media/renalmass09.pdf?CFID=2980781&CFTOKEN=53338937&JSESSIONID=84305943038372E237f85a47377557304155. Accessed January 20, 2012
- 26.Silverman SG, Pearson GD, Seltzer SE, et al. Small (< 3 cm) hyperechoic renal masses: comparison of helical and conventional CT for diagnosing angiomyolipoma. AJR 1996; 167:877–881 [DOI] [PubMed] [Google Scholar]
- 27.Silverman SG, Israel GM, Hertz BR, et al. Management of the incidental renal mass. Radiology 2008; 249:16–31 [DOI] [PubMed] [Google Scholar]
- 28.Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol 1998; 13:624–628 [DOI] [PubMed] [Google Scholar]
- 29.Dang C, Gilewski TA, Surbone A, Norton L. Growth curve analysis. In: Kufe DW, Pollock PE, Weichselbaum RR, eds. Holland-Frei cancer medicine, 6th ed. Hamilton, ON, Canada: BC Decker, 2003:653–662 [Google Scholar]
- 30.West GB, Brown JH, Enquist BJ. A general model for ontogenic growth. Nature 2001; 413:628–631 [DOI] [PubMed] [Google Scholar]
- 31.Obuz F, Karabay N, Secil M, et al. Various radiological appearances of angiomyolipomas in the same kidney. Eur Radiol 2000; 10:897–899 [DOI] [PubMed] [Google Scholar]
- 32.Hosokawa Y, Kinouchi T, Sawai Y, et al. Renal angiomyolipoma with minimal fat. Int J Clin Oncol 2002; 7:120–123 [DOI] [PubMed] [Google Scholar]
- 33.Hafron J, Fogarty JD, Hoenig DM, et al. Imaging characteristics of minimal fat renal angiomyolipoma with histologic correlations. Urology 2005; 66:1155–1159 [DOI] [PubMed] [Google Scholar]
- 34.Milner J, McNeil B, Alioto J, et al. Fat poor renal angiomyolipoma: patient, computerized tomography and histological findings. J Urol 2006; 176:905–909 [DOI] [PubMed] [Google Scholar]
- 35.Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 2006; 175:425–431 [DOI] [PubMed] [Google Scholar]
- 36.Li XS, Yao L, Gong K, et al. Growth pattern of renal cell carcinoma (RCC) in patients with delayed surgical intervention. J Cancer Res Clin Oncol 2012; 138:269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol 2007; 177:849–853 [DOI] [PubMed] [Google Scholar]
- 38.Morrissey JJ, Mellnick VM, Luo J, et al. Evaluation of urine aquaporin-1 and perilipin-2 concentrations as biomarkers to screen for renal cell carcinoma: a prospective cohort study. JAMA Oncol 2015; 1:204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Palma G, Sallustio F, Curci C, et al. The three-gene signature in urinary extracellular vesicles from patients with clear cell renal cell carcinoma. J Cancer 2016; 7:1960–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]