Abstract
Ciliopathies are a collection of disorders related to cilia dysfunction. Cilia are specialized organelles that project from the surface of most cells. Motile and primary (sensory) cilia are essential structures and have wide ranging functions. Our understanding of the genetics, pathophysiology, and clinical manifestations of motile ciliopathies, including primary ciliary dyskinesia (PCD), has rapidly advanced since the disease was linked to ciliary ultrastructural defects nearly five decades ago. We will provide an overview of different types of cilia, their role in child health and disease, focusing on motile ciliopathies, and describe recent advances that have led to improved diagnostics and may yield therapeutic targets to restore ciliary structure and function.
Keywords: primary ciliary dyskinesia, bronchiectasis, cilia, basal body, dynein
CILIA STRUCTURE AND FUNCTION
Cilia and flagella are evolutionarily conserved structures from simple unicellular algae to humans. Chlamydomonas reinhardtii, a biflagellated single cell organism, has been a powerful model to study motile cilia. The similarities between algal flagella and eukaryotic motile cilia have yielded new insights into the genetics and biological functions of proteins in human cilia. Many genes linked to primary ciliary dyskinesia and other ciliopathies have Chlamydomonas orthologues.
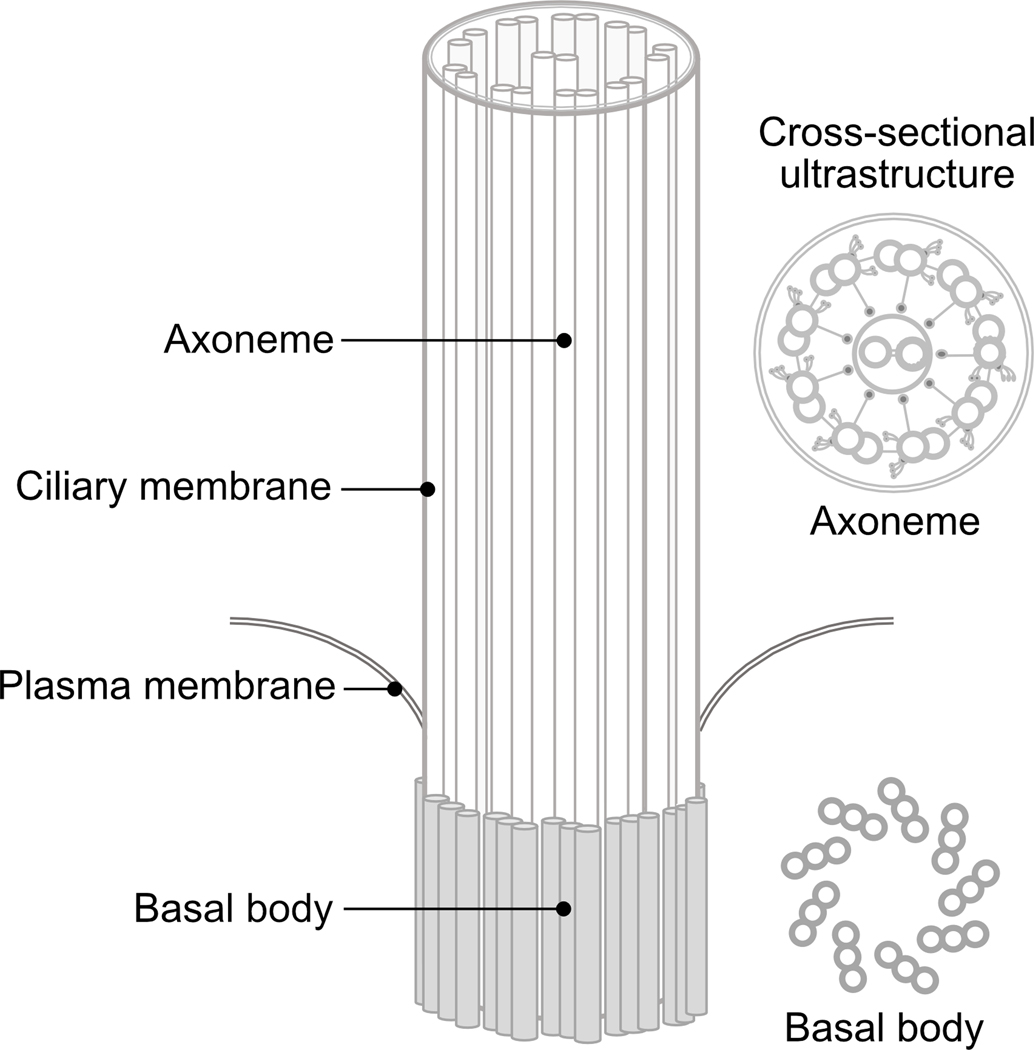
There are two general classes of cilia: motile cilia and immotile cilia. All eukaryotic cilia extend from basal bodies, docked at the surface of the cell (Figure 1). They share similar structures, including the axoneme, a cytoskeletal scaffold that forms their central core1. The axoneme is composed of nine microtubule doublets that extend the length of the cilium, maintaining structural integrity and direct components into and from cilia, using a process known as intra-flagellar transport (IFT). The axoneme is anchored to the cell by a basal body, a protein structure derived from the centriole. Although different types of cilia share a basic structural blueprint (Figure 2), they differ in their function and distribution.
Figure 1.
Schematic diagram of a ciliary axoneme and basal body.
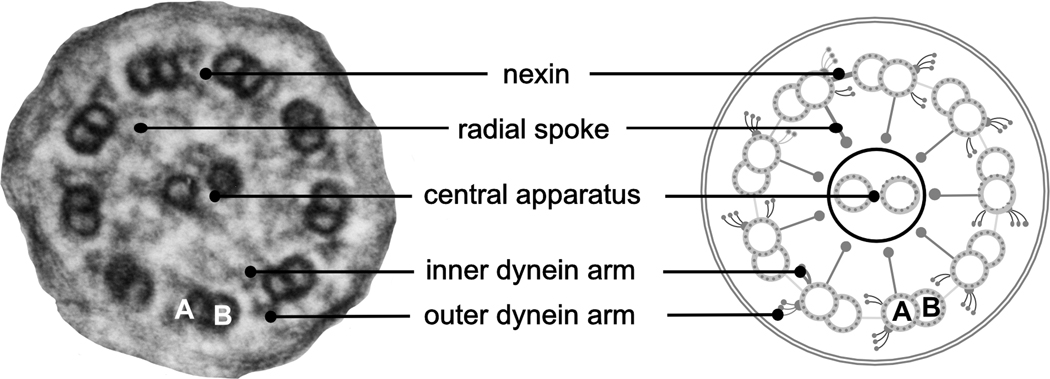
Figure 2.
Electron photomicrograph and diagram showing ultrastructural features of the motile cilium.
Motile cilia are organelles that are found on the apical epithelial surface of upper and lower respiratory tracts. Motile cilia are anatomically and functionally oriented organelles that rhythmically beat, moving fluid, mucus, and trapped bacteria along the epithelial surface. Approximately 200 motile cilia cluster on the surface of airway epithelial cells, and they must undergo a complex process of centrosomal amplification to produce hundreds of basal bodies. Mucociliary clearance is a critical defense of the respiratory tract and is dependent on a highly coordinated ciliary function. Defects in clearance lead to chronic infection and inflammation in the upper and lower respiratory tracts. Normal beat frequency in the airway typically ranges between 8 to 14 Hertz. However, beat frequency can be modulated in response to external stimuli such as changes in redox conditions, infection, or exposure to pollutants, including cigarette smoke2–5. In addition to the respiratory tract, motile cilia are present elsewhere. The brain ependyma and fallopian tubes are lined by motile ciliated cells, and the spermatozoan flagellum have the same core structure and fundamentally similar motility characteristics as cilia.
Motile cilia have multimeric dynein arms that extend from the A microtubule of the outer doublets and attach to the adjacent B microtubule (Figure 2). These elements are critical for motion through adenosine triphosphatase activity. The outer dynein arm generates the force that translates into a sliding motion of two neighboring tubules, whereas the inner dynein arms control ciliary motion through the nexin-dynein regulatory complex, which coordinates activity of various dynein proteins6. Motile cilia also have a central microtubular pair, creating the “9+2” configuration seen on transmission electron micrographs. The central apparatus maintains structural integrity, transfers force during cilia beating, and aligns adjacent cilia, ensuring ciliary motility is maintained in the same direction along the airway. Radial spokes connect the central apparatus and inner dynein arms, sending signals to the dynein regulatory complex to regulate dynein activity 7.
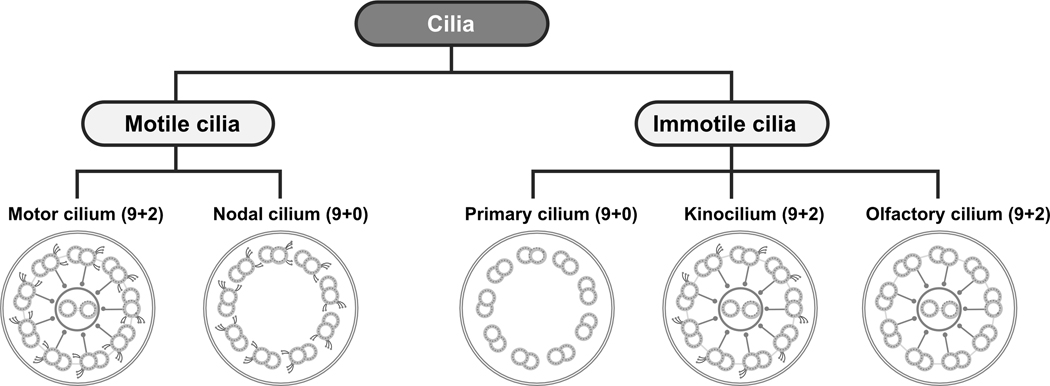
Another motile cilium is only expressed during fetal development. Nodal cilia (Figure 3) are transiently localized to the ventral node of the gastrula, and unlike multi-ciliated cilia on airway epithelial cells, they exist as monocilium and do not have a central pair, creating a “9+0” microtubular arrangement. Because they lack a central pair, their motion is rotatory, thus producing leftward flow of extra-cellular fluid across the surface of the embryonic node, which in turn activates a signaling cascade that establishes left-right sidedness and body laterality8–10. When motile cilia are defective and flow is absent, body laterality occurs at random, and can lead to situs inversus totalis and heterotaxy.
Figure 3.
Schematic diagrams depicting the general classification of motile and immotile cilia based on ultrastructural configuration and function.
In contrast, primary cilia are usually immotile monocilia, present on the surface of many non-dividing differentiated cells. Unlike motile cilia, most primary cilia have a “9+0” microtubule configuration, lacking the central apparatus and dynein arms. There are few exceptions, such as the kinocilium in the cochlea and olfactory cilia (Figure 3). Primary cilia are sensory organelles that sense the extracellular environment and can act as surface mechano- or chemo-receptors. Other primary cilia detect changes in osmolality, light, temperature, and gravity. Furthermore, they have critical roles in normal development and tissue differentiation, and express many essential receptors on their surface, including sonic hedgehog, epidermal growth factor receptor, and platelet derived growth factor receptor11–13. As a result of their ubiquitous distribution, primary ciliary defects are associated with wide-ranging syndromes and diseases that involve multiple systems, referred to as primary ciliopathies (Table). For instance, Bardet-Biedl syndrome (BBS) is a rare, autosomal recessive disorder, caused by defective BBS proteins that localize to the basal body of primary cilia.14, 15 It can present with intellectual disabilities, obesity, retinal degeneration, polycystic kidneys, polydactyly, diabetes mellitus, and cardiovascular anomalies. Other primary ciliopathies include cranioectodermal dysplasia, Sensenbrenner syndrome, short-rib polydactyly, and Jeune syndrome, conditions that have overlapping clinical features, including skeletal dysplasia that result in chest deformities, pulmonary restriction, and respiratory compromise16.
TABLE.
Genes and clinical symptoms associated with selected primary ciliopathies
| Disease or syndrome | Clinical features | Associated genes |
|---|---|---|
| Alstrom syndrome | Obesity, retinitis pigmentosa, diabetes mellitus, hypothyroidism, hypogonadism, skeletal dysplasia, cardiomyopathy, pulmonary fibrosis | ALMS11 |
| Bardet-Biedl syndrome | Obesity, polydactyly, developmental delay, retinitis pigmentosa, renal anomalies, anosmia, hypogonadism, congenital heart disease | ARL6, BBS1–12, CEP290, MKKS, MKS1, MKS3, SDCCAG8, TRIM32, WDPCP |
| Ellis van Creveld syndrome | Chondrodystrophy, polydactyly, ectodermal dysplasia, congenital heart disease | EVC, EVC2 |
| Jeune syndrome | Thoracic cage deformities, renal cysts, retinitis pigmentosa, skeletal dysplasia, polydactyly | DYNC2H1, IFT80, IFT139, IFT140, IFT144, WDR35 |
| Joubert syndrome | CNS anomalies, developmental delay, ataxia, retinitis pigmentosa, polydactyly, cleft lip, cleft palate | ATXN10, AHI1, ARL13B, C5ORF42, CC2D2A, CEP41, CEP290, CORS2, INPP5E, JBTS1, JBTS3, JBTS4, KIF7, NPHP1, NPHP3, RPGRIP1L, TCTN1, TCTN2, TMEM67, TMEM138, TMEM216, TMEM237 |
| Meckel-Gruber syndrome | Renal cysts, polydactyly, developmental delay, CNS anomalies, congenital heart disease, cleft lip, cleft palate | B9D1, B9D2, CC2D2A, CEP290, MKS1–6, MKKS, NPHP3, RPGRIP1L, TCTN2, TMEM67, TMEM216 |
| Nephronophthisis | Renal cysts, interstitial nephritis, hepatic fibrosis, retinitis pigmentosa | ALMS1, ATXN10, CEP290, GLIS2, IFT139, INVS, NEK8, NPHP1–11, TCTN2, TTC21B, TTC8, WDR19, XPNPEP3 |
| Orofaciodigital syndrome type 1 | Polydactyly, syndactyly, cleft lip, cleft palate, brain anomalies, developmental delay, renal cysts | OFD1 |
| Polycystic kidney disease | Early onset renal cysts, hepatic fibrosis | PKHD1 |
Most primary ciliopathies do not affect the function of motile cilia, though there have been rare reports of syndromes that have features of both primary and motile cilia dysfunction17–19. In some families, RPGR mutations, which cause retinitis pigmentosa due to primary cilia dysfunction, can also lead to PCD-like symptoms due motile cilia dysfunction in the airway 18. Both motile and immotile cilia share many proteins and structures, and there are several lines of evidence indicating that the motile cilia have sensory and signaling functions 20.
CLINICAL FEATURES OF PRIMARY CILIARY DYSKINESIA (PCD)
PCD is a rare inherited disease in which genetic mutations impair motile cilia function21. It is widely found across ethnic groups, without racial or gender predilection. The reported frequency of PCD in the general population varies between 1 in 10,000–20,000 live-born children, but some have reported its prevalence as high as 5% in children with repeated respiratory infections22.
Primary ciliary dyskinesia typically presents early in life. Roughly 80% of full-term infants with PCD present with respiratory distress within 24 hours of birth, requiring supplemental oxygen or mechanical ventilator support for days or even weeks. Chest imaging often reveals atelectasis, mainly involving the morphological right middle lobe or lingua. Children with PCD also develop daily, year-round “wet” or productive cough that usually begins under 6-months of age, and is related to impaired mucociliary clearance, which leads to chronic airway infection, inflammation, progressive airway obstruction, and bronchiectasis, even early in life23. Patients with PCD have frequent upper airway involvement, manifested as persistent rhinosinusitis with watery nasal discharge that begins in early infancy 24. Nasal polyposis is relatively uncommon, reported in less than 15% of children25. Middle ear involvement is nearly universal in PCD, with recurrent acute and chronic otitis media. Conductive hearing loss is common, and 75% of children with PCD have some degree of hearing loss. Interestingly, some children have sensorineural or mixed hearing loss26. These chronic respiratory symptoms negatively affect the lives of people with PCD, and delayed diagnosis has been associated with poorer quality of life 27.
Roughly half of all patients with PCD have left-right laterality abnormalities, including situs inversus totalis and heterotaxy syndromes, which can be associated with congenital heart disease, asplenia, or polysplenia28. Male and female infertility or subfertility are other non-respiratory complications, related to sperm dysmotility and ciliary dysfunction in the fallopian tubes, respectively. Rarer manifestations of PCD include prenatal hydrocephalus29 and blindness due to RPGR gene mutations18, 30.
GENETICS OF PRIMARY CILIARY DYSKINESIA
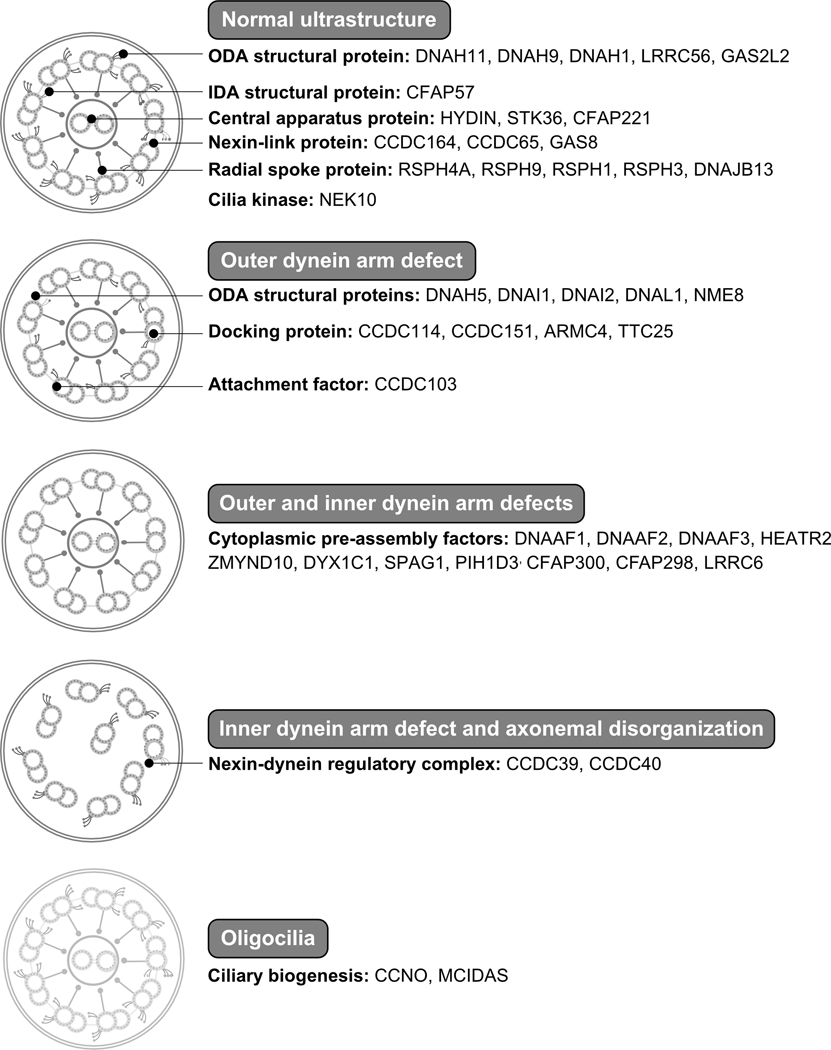
Because of the large number of proteins involved in cilia function and assembly, PCD is genetically heterogeneous, primarily an autosomal-recessive disease, but autosomal-dominant and X-linked inheritance patterns are known. Most of the genes associated with PCD encode proteins that are involved in axonemal motors, structure and regulation, or ciliary assembly and preassembly. The rate of discovery of new genes has accelerated during the past decade, and nearly 50 genes have been linked to disease (Figure 4, available online). Over 70% of all patients tested have biallelic mutations within one of these genes. Through the efforts of international, collaborative consortia, that number will surely rise.
Figure 4.
Classification of ultrastructural defects of the motile ciliary axoneme and genes associated with primary ciliary dyskinesia. IDA: inner dynein arm; ODA: outer dynein arm.
Genetic studies have yielded unexpected insights into the disease. Approximately 30% of people with PCD and ciliary dysmotility have normal ciliary ultrastructure. For example, airway cilia from people who have mutations in DNAH11, which encodes an outer dynein arm protein, appear structurally normal, but have a rapid beat frequency with abnormal waveform31, 32. Individuals who have mutations in genes encoding for dynein regulatory complex proteins may have only subtle ciliary changes which can be missed on transmission electron microscopy33, 34. Recently, a mutation in an inner dynein arm protein was associated with PCD35, with a normal ultrastructure and subtle changes in cilia beat.
Mutations in several genes that encode cytoplasmic proteins have been associated with PCD. Often, these mutations result in both outer and inner dynein arm defects and cilia immotility36–40. Pathogenic mutations in NEK10, a gene that encodes a ciliated cell-specific kinase, results in pathologically shortened motile cilia and impaired mucociliary clearance41. Other mutations can affect cilia orientation, such as mutations in GAS2L2 that cause ciliary disorientation and asynchronous beating42. More commonly, though, ciliary disorientation follows viral infections and airway injury, causing an acquired ciliopathy that impairs mucociliary clearance.
Motile ciliopathies distinct from classical PCD have been described. For instance, people with biallelic mutations in CCNO and MCIDAS, two proteins required for centriole production, have clinical features similar to PCD but are characterized by oligocilia with normal ultrastructure on airway epithelial cell surface. People harboring mutations in these genes are reported to have greater lung disease compared with those who have dynein arm proteins defects 43, 44. Moreover, individuals with MCIDAS mutations have higher incidence of hydrocephalus 43, 45. De novo, single mutations in FOXJ1, a vital transcription factor that regulates cilia gene expression, cause reduced number of motile cilia and are associated with hydrocephalus, recurrent respiratory infections, and laterality defects46.
Finally, PCD exists as a clinical spectrum, and genotype-phenotype associations have emerged. Studies have shown that children who have biallelic mutations in CCDC39 or CCDC40, two genes involved in assembly of the ciliary scaffold and spacing of radial spokes, have greater lung disease and more rapid pulmonary function decline when compared with individuals with dynein arm protein defects47, 48. Conversely, people with RSPH1 defects tend to have milder lung disease, situs solitus, and less middle ear involvement 49.
DIAGNOSING PRIMARY CILIARY DYSKINESIA
Historically, the diagnosis of PCD has been challenging, but newer tools have improved accuracy. Testing should be performed only in patients who have a clinical phenotype consistent with the disease. There are four clinical features that discriminate PCD from other more common respiratory diseases of childhood, which include neonatal respiratory distress in full-term infants, laterality defects, daily non-seasonal nasal congestion, and daily, year-round wet cough that begins before 6 months of age. If two of these findings are present, the sensitivity and specificity for PCD are 80% and 72%, respectively50. Although persistent middle ear effusions and chronic otitis media are common in PCD, the high prevalence of recurrent otitis media in the general pediatric population makes this feature less reliable. The combination of respiratory distress with situs abnormalities recognized in a term infant without congenital heart disease would be consistent with PCD and should prompt the clinician to pursue further evaluation. These clinical features are the basis of published diagnostic algorithms51, 52. Other clinical diagnostic tools have been created and validated to estimate the probability of a positive diagnosis based on clinical features53.
It is important to remember that no single test will diagnose every patient with PCD. Since axonemal ultrastructural defects were first recognized over 40 years ago, transmission electron microscopy to assess axonemal ultrastructure has been the “gold” diagnostic standard. Four ultrastructural defects have been described in primary ciliary dyskinesia: outer dynein arm defects; inner and outer dynein arm defects; microtubular disorganization with inner dynein arm defects; and radial spokes and central apparatus defects. Inner dynein arm defects alone are rarely associated with disease, and to date, only one gene encoding an inner dynein arm protein has been associated with disease35. With advances in genetic tools, the limitations of electron microscopy have become obvious. About 30% of patients with genetically confirmed PCD have normal or near-normal ultrastructure, related to mutations in genes that encode structural proteins that do not affect the integrity of the dynein arms. Moreover, nonspecific changes in ciliary ultrastructure may be observed after airway infections and environmental pollutant exposures, resulting in an erroneous diagnosis of PCD. Recently, British investigators have found that immunofluorescent staining of specific markers can accurately identify abnormal axonemal ultrastructure and may overcome some limitations of electron microscopy54. This approach is frequently used by many PCD centers in Europe, but has not been widely adopted in the United States. With further standardization and optimization of antibody panels, this approach may become a first-line diagnostic test.
Another screening tool arose from the reproducible observation that most people with PCD have reduced nasal nitric oxide levels. Low levels of nasal nitric oxide measurements are sensitive and specific for the diagnosis of PCD in children five years and older, when combined with supportive clinical features55, 56, with sensitivity and specify of 98% and 99%, respectively55. The mechanism by which nitric oxide production is reduced is not entirely understood. Many of the nitric oxide synthase and regulatory enzymes localize to the proximal ciliary axoneme and basal bodies, and their function may be affected when cilia are dysmotile57–59. Despite its usefulness, it is important to note that reduced nasal nitric oxide measurements alone are never sufficient to make the diagnosis52. Indeed, low nasal nitric oxide levels can be found in individuals with cystic fibrosis and primary immunodeficiencies, two conditions that share clinical features with PCD, and must be excluded.
High-speed video microscopy that assess cilia beat frequency and patterns is an alternative diagnostic tool used in Europe60, 61. Although this approach has value, it also has limitations. The technique requires substantial experience and should be performed at specialized center 62. Some centers perform non-standardized ciliary beat analyses using standard brightfield microscopy to determine whether further testing is warranted. This approach can be misleading, however, and should never be used as a screen or diagnostic tool for PCD.
During the last decade, large-scale parallel sequencing of regions of interests and whole exome sequencing have been used to successfully identify candidate genes associated with PCD. These advances have also transformed diagnostic testing for PCD, and there are currently several commercially available gene panels that provide coverage of most known genes associated with PCD36, 63, 64. genetic testing, which has become a first-line diagnostic, is one of many available diagnostic tools, and its use should be limited to patients that fulfill clinical diagnostic criteria for PCD. In some cases, mutations are identified that have no clear effect on the function of the protein, termed variants of unknown significance (VUS). Interpretation of these variants requires experience and often confirmatory functional testing to determine their contribution to disease. Furthermore, it has become evident that different mutations in the same gene may lead to different clinical presentations65. Some children should be referred to specialized PCD centers to confirm or establish the diagnosis.
MANAGEMENT OF PRIMARY CILIARY DYSKINESIA
Despite recent advances, this knowledge has not been translated into disease-specific therapies. Currently, there are no treatments that have been shown to correct or restore cilia function in people with PCD.
Management strategies of children with PCD have been extrapolated largely from other forms of bronchiectasis such as cystic fibrosis. Airway clearance techniques and systemic antibiotics, guided by routine surveillance sputum cultures, are cornerstones of therapy, used to clear purulent secretions, mobilize secretions and control bacterial burden of the lower respiratory tract, particularly during acute exacerbations. Patients with PCD have varying levels of bronchiectasis associated with chronic bacterial overgrowth. Bacterial organisms that are frequently detected include Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa.66, 67. Though data is lacking, there is some evidence that chronic colonization with mucoid P. aeruginosa may be associated with greater lung function decline. 67, 68
A multicenter, European clinical trial examined the efficacy of routine thrice weekly azithromycin as an anti-inflammatory agent in a selected PCD subpopulation69. Macrolide therapy resulted in modest reduction in frequency of respiratory exacerbations, but quality-of-life and pulmonary function measures did not improve. The benefits of other therapies, such as alternate-month inhaled antimicrobials, nebulized hypertonic saline, or mucolytics have not been established.
Management of the sinonasal and middle ear disease is largely derived from other conditions, because large clinical trials are lacking. Myringotomy tubes are frequently placed in infants and young children with PCD, often before the underlying diagnosis is made. Although several studies have reported that myringotomy tube placement improves hearing thresholds, others did not find any improvement. Additionally, prolonged otorrhea and persistent tympanic membrane perforation are relatively common complications70 that have led to recommendations against the routine use of myringotomy tubes in children with PCD. Treatment of sinonasal disease in PCD is largely based on management of chronic rhinosinusitis, consisting of various medical and surgical therapies. Antibiotics are also used to treat upper respiratory tract infections, especially during exacerbations, and in some instances, long-term suppressive antibiotic therapy is used. Occasionally, surgical interventions are required, including adenoidectomy, polypectomy, and functional endoscopic sinus surgery, but usually reserved for cases of failed medical therapy.
SUMMARY
Primary ciliary dyskinesia is a rare inherited disease of the motile cilia, one of a growing collection of genetic disorders known as ciliopathies. Children with PCD have diverse clinical manifestations, and usually present early in life with neonatal respiratory distress, persistent sinonasal and middle ear disease, hearing loss, chronic daily cough, bronchiectasis, and left-right laterality defects. Our understanding of the genetics of PCD has advanced, and nearly 50 different disease-associated genes have been identified, encoding axonemal, cytoplasmic, and regulatory proteins that are involved in the assembly, structure, and function of motile cilia. This knowledge has translated into new insights into the clinical heterogeneity of motile ciliopathies and revolutionized our approach toward diagnostic testing for PCD. This progress has not translated into therapeutics, but undoubtedly new disease targets will be identified and treatments will follow.
Acknowledgments
Financial support and conflicts of interest: The authors were supported by National Institutes of Health (NIH) awards 1K08HL150223 (AH), U54HL096458 (TWF) and R21AI46999 (TWF). The views expressed do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S government.
Abbreviations:
- PCD
primary ciliary dyskinesia
- VUS
variant of unknown significance
Footnotes
Neither author has an actual or perceived conflict of interest concerning the information presented in the paper. Both authors listed on the manuscript have reviewed and approved the content of the submission, and take full responsibility for the information provided.
REFERENCES
- [1].Mitchell DR. The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv Exp Med Biol. 2007;607:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Johnson NT, Villalon M, Royce FH, Hard R, Verdugo P. Autoregulation of beat frequency in respiratory ciliated cells. Demonstration by viscous loading. Am Rev Respir Dis. 1991;144:1091–4. [DOI] [PubMed] [Google Scholar]
- [3].Hirst RA, Sikand KS, Rutman A, Mitchell TJ, Andrew PW, O’Callaghan C. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect Immun. 2000;68:1557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Simet SM, Sisson JH, Pavlik JA, Devasure JM, Boyer C, Liu X, et al. Long-term cigarette smoke exposure in a mouse model of ciliated epithelial cell function. Am J Respir Cell Mol Biol. 2010;43:635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Al-Rawi MM, Edelstein DR, Erlandson RA. Changes in nasal epithelium in patients with severe chronic sinusitis: a clinicopathologic and electron microscopic study. Laryngoscope. 1998;108:1816–23. [DOI] [PubMed] [Google Scholar]
- [6].Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Horani A, Ferkol TW. Advances in the Genetics of Primary Ciliary Dyskinesia: Clinical Implications. Chest. 2018;154:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. [DOI] [PubMed] [Google Scholar]
- [9].Essner JJ, Vogan KJ, Wagner MK, Tabin CJ, Yost HJ, Brueckner M. Conserved function for embryonic nodal cilia. Nature. 2002;418:37–8. [DOI] [PubMed] [Google Scholar]
- [10].Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, et al. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130:1725–34. [DOI] [PubMed] [Google Scholar]
- [11].Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78. [DOI] [PubMed] [Google Scholar]
- [12].Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:261–301. [DOI] [PubMed] [Google Scholar]
- [13].Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, et al. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat Genet. 2002;31:435–8. [DOI] [PubMed] [Google Scholar]
- [15].Mykytyn K, Braun T, Carmi R, Haider NB, Searby CC, Shastri M, et al. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet. 2001;28:188–91. [DOI] [PubMed] [Google Scholar]
- [16].Schmidts M. Clinical genetics and pathobiology of ciliary chondrodysplasias. J Pediatr Genet. 2014;3:46–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jain R, Javidan-Nejad C, Alexander-Brett J, Horani A, Cabellon MC, Walter MJ, et al. Sensory functions of motile cilia and implication for bronchiectasis. Front Biosci (Schol Ed). 2012;4:1088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moore A, Escudier E, Roger G, Tamalet A, Pelosse B, Marlin S, et al. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet. 2006;43:326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hannah WB, DeBrosse S, Kinghorn B, Strausbaugh S, Aitken ML, Rosenfeld M, et al. The expanding phenotype of OFD1-related disorders: Hemizygous loss-of-function variants in three patients with primary ciliary dyskinesia. Mol Genet Genomic Med. 2019;7:e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–9. [DOI] [PubMed] [Google Scholar]
- [22].Chapelin C, Coste A, Reinert P, Boucherat M, Millepied MC, Poron F, et al. Incidence of primary ciliary dyskinesia in children with recurrent respiratory diseases. Ann Otol Rhinol Laryngol. 1997;106:854–8. [DOI] [PubMed] [Google Scholar]
- [23].Magnin ML, Cros P, Beydon N, Mahloul M, Tamalet A, Escudier E, et al. Longitudinal lung function and structural changes in children with primary ciliary dyskinesia. Pediatr Pulmonol. 2012;47:816–25. [DOI] [PubMed] [Google Scholar]
- [24].Bush A, Cole P, Hariri M, Mackay I, Phillips G, O’Callaghan C, et al. Primary ciliary dyskinesia: diagnosis and standards of care. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 1998;12:982–8. [DOI] [PubMed] [Google Scholar]
- [25].Rollin M, Seymour K, Hariri M, Harcourt J. Rhinosinusitis, symptomatology & absence of polyposis in children with primary ciliary dyskinesia. Rhinology. 2009;47:75–8. [PubMed] [Google Scholar]
- [26].Kreicher KL, Schopper HK, Naik AN, Hatch JL, Meyer TA. Hearing loss in children with primary ciliary dyskinesia. Int J Pediatr Otorhinolaryngol. 2018;104:161–5. [DOI] [PubMed] [Google Scholar]
- [27].Pifferi M, Bush A, Di Cicco M, Pradal U, Ragazzo V, Macchia P, et al. Health-related quality of life and unmet needs in patients with primary ciliary dyskinesia. Eur Respir J. 2010;35:787–94. [DOI] [PubMed] [Google Scholar]
- [28].Nakhleh N, Francis R, Giese RA, Tian X, Li Y, Zariwala MA, et al. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation. 2012;125:2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].De Santi MM, Magni A, Valletta EA, Gardi C, Lungarella G. Hydrocephalus, bronchiectasis, and ciliary aplasia. Archives of disease in childhood. 1990;65:543–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ferkol TW, Leigh MW. Ciliopathies: the central role of cilia in a spectrum of pediatric disorders. J Pediatr. 2012;160:366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pifferi M, Michelucci A, Conidi ME, Cangiotti AM, Simi P, Macchia P, et al. New DNAH11 mutations in primary ciliary dyskinesia with normal axonemal ultrastructure. Eur Respir J. 2010;35:1413–6. [DOI] [PubMed] [Google Scholar]
- [32].Bartoloni L, Blouin JL, Pan Y, Gehrig C, Maiti AK, Scamuffa N, et al. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2002;99:10282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Horani A, Brody SL, Ferkol TW, Shoseyov D, Wasserman MG, Ta-shma A, et al. CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia. PLoS One. 2013;8:e72299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Olbrich H, Cremers C, Loges NT, Werner C, Nielsen KG, Marthin JK, et al. Loss-of-Function GAS8 Mutations Cause Primary Ciliary Dyskinesia and Disrupt the Nexin-Dynein Regulatory Complex. Am J Hum Genet. 2015;97:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bustamante-Marin XM, Horani A, Stoyanova M, Charng WL, Bottier M, Sears PR, et al. Mutation of CFAP57, a protein required for the asymmetric targeting of a subset of inner dynein arms in Chlamydomonas, causes primary ciliary dyskinesia. PLoS Genet. 2020;16:e1008691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Horani A, Druley TE, Zariwala MA, Patel AC, Levinson BT, Van Arendonk LG, et al. Whole-exome capture and sequencing identifies HEATR2 mutation as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2012;91:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mitchison HM, Schmidts M, Loges NT, Freshour J, Dritsoula A, Hirst RA, et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat Genet. 2012;44:381–9, S1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Omran H, Kobayashi D, Olbrich H, Tsukahara T, Loges NT, Hagiwara H, et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature. 2008;456:611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tarkar A, Loges NT, Slagle CE, Francis R, Dougherty GW, Tamayo JV, et al. DYX1C1 is required for axonemal dynein assembly and ciliary motility. Nat Genet. 2013;45:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Knowles MR, Ostrowski LE, Loges NT, Hurd T, Leigh MW, Huang L, et al. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am J Hum Genet. 2013;93:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chivukula RR, Montoro DT, Leung HM, Yang J, Shamseldin HE, Taylor MS, et al. A human ciliopathy reveals essential functions for NEK10 in airway mucociliary clearance. Nat Med. 2020;26:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bustamante-Marin XM, Yin WN, Sears PR, Werner ME, Brotslaw EJ, Mitchell BJ, et al. Lack of GAS2L2 Causes PCD by Impairing Cilia Orientation and Mucociliary Clearance. Am J Hum Genet. 2019;104:229–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Boon M, Wallmeier J, Ma L, Loges NT, Jaspers M, Olbrich H, et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Commun. 2014;5:4418. [DOI] [PubMed] [Google Scholar]
- [44].Wallmeier J, Al-Mutairi DA, Chen CT, Loges NT, Pennekamp P, Menchen T, et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet. 2014;46:646–51. [DOI] [PubMed] [Google Scholar]
- [45].Robson EA, Dixon L, Causon L, Dawes W, Benenati M, Fassad M, et al. Hydrocephalus and diffuse choroid plexus hyperplasia in primary ciliary dyskinesia-related MCIDAS mutation. Neurol Genet. 2020;6:e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wallmeier J, Frank D, Shoemark A, Nothe-Menchen T, Cindric S, Olbrich H, et al. De Novo Mutations in FOXJ1 Result in a Motile Ciliopathy with Hydrocephalus and Randomization of Left/Right Body Asymmetry. Am J Hum Genet. 2019;105:1030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Davis SD, Ferkol TW, Rosenfeld M, Lee HS, Dell SD, Sagel SD, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med. 2015;191:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Davis SD, Rosenfeld M, Lee HS, Ferkol TW, Sagel SD, Dell SD, et al. Primary Ciliary Dyskinesia: Longitudinal Study of Lung Disease by Ultrastructure Defect and Genotype. Am J Respir Crit Care Med. 2018. [DOI] [PMC free article] [PubMed]
- [49].Knowles MR, Ostrowski LE, Leigh MW, Sears PR, Davis SD, Wolf WE, et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am J Respir Crit Care Med. 2014;189:707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Leigh MW, Ferkol TW, Davis SD, Lee HS, Rosenfeld M, Dell SD, et al. Clinical Features and Associated Likelihood of Primary Ciliary Dyskinesia in Children and Adolescents. Ann Am Thorac Soc. 2016;13:1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shapiro AJ, Davis SD, Polineni D, Manion M, Rosenfeld M, Dell SD, et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;197:e24–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shapiro AJ, Davis SD, Leigh MW, Knowles MR, Lavergne V, Ferkol T. Limitations of Nasal Nitric Oxide Testing in Primary Ciliary Dyskinesia. Am J Respir Crit Care Med. 2020;202:476–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Behan L, Dimitrov BD, Kuehni CE, Hogg C, Carroll M, Evans HJ, et al. PICADAR: a diagnostic predictive tool for primary ciliary dyskinesia. Eur Respir J. 2016;47:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Frommer A, Hjeij R, Loges NT, Edelbusch C, Jahnke C, Raidt J, et al. Immunofluorescence analysis and diagnosis of primary ciliary dyskinesia with radial spoke defects. American journal of respiratory cell and molecular biology. 2015;53:563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013;10:574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kouis P, Papatheodorou SI, Yiallouros PK. Diagnostic accuracy of nasal nitric oxide for establishing diagnosis of primary ciliary dyskinesia: a meta-analysis. BMC Pulm Med. 2015;15:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jackson CL, Lucas JS, Walker WT, Owen H, Premadeva I, Lackie PM. Neuronal NOS localises to human airway cilia. Nitric Oxide. 2015;44:3–7. [DOI] [PubMed] [Google Scholar]
- [58].Stout SL, Wyatt TA, Adams JJ, Sisson JH. Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem. 2007;55:433–42. [DOI] [PubMed] [Google Scholar]
- [59].Xue C, Botkin SJ, Johns RA. Localization of endothelial NOS at the basal microtubule membrane in ciliated epithelium of rat lung. J Histochem Cytochem. 1996;44:463–71. [DOI] [PubMed] [Google Scholar]
- [60].Raidt J, Wallmeier J, Hjeij R, Onnebrink JG, Pennekamp P, Loges NT, et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respir J. 2014;44:1579–88. [DOI] [PubMed] [Google Scholar]
- [61].Smith CM, Hirst RA, Bankart MJ, Jones DW, Easton AJ, Andrew PW, et al. Cooling of cilia allows functional analysis of the beat pattern for diagnostic testing. Chest. 2011;140:186–90. [DOI] [PubMed] [Google Scholar]
- [62].Konietzko N, Nakhosteen JA, Mizera W, Kasparek R, Hesse H. Ciliary beat frequency of biopsy samples taken from normal persons and patients with various lung diseases. Chest. 1981;80:855–7. [DOI] [PubMed] [Google Scholar]
- [63].Knowles MR, Leigh MW, Ostrowski LE, Huang L, Carson JL, Hazucha MJ, et al. Exome sequencing identifies mutations in CCDC114 as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2013;92:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Onoufriadis A, Paff T, Antony D, Shoemark A, Micha D, Kuyt B, et al. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am J Hum Genet. 2013;92:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Horani A, Ustione A, Huang T, Firth AL, Pan J, Gunsten SP, et al. Establishment of the early cilia preassembly protein complex during motile ciliogenesis. Proc Natl Acad Sci U S A. 2018;115:E1221-E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Alanin MC, Nielsen KG, von Buchwald C, Skov M, Aanaes K, Hoiby N, et al. A longitudinal study of lung bacterial pathogens in patients with primary ciliary dyskinesia. Clin Microbiol Infect. 2015;21:1093 e1–7. [DOI] [PubMed] [Google Scholar]
- [67].Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–67. [DOI] [PubMed] [Google Scholar]
- [68].Wijers CD, Chmiel JF, Gaston BM. Bacterial infections in patients with primary ciliary dyskinesia: Comparison with cystic fibrosis. Chron Respir Dis. 2017;14:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kobbernagel HE, Buchvald FF, Haarman EG, Casaulta C, Collins SA, Hogg C, et al. Efficacy and safety of azithromycin maintenance therapy in primary ciliary dyskinesia (BESTCILIA): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2020;8:493–505. [DOI] [PubMed] [Google Scholar]
- [70].Andersen TN, Alanin MC, von Buchwald C, Nielsen LH. A longitudinal evaluation of hearing and ventilation tube insertion in patients with primary ciliary dyskinesia. Int J Pediatr Otorhinolaryngol. 2016;89:164–8. [DOI] [PubMed] [Google Scholar]