Abstract
Myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) is a Cdc42-binding serine/threonine kinase with multiple functional domains. We had previously shown MRCKα to be implicated in Cdc42-mediated peripheral actin formation and neurite outgrowth in HeLa and PC12 cells, respectively. Here we demonstrate that native MRCK exists in high-molecular-weight complexes. We further show that the three independent coiled-coil (CC) domains and the N-terminal region preceding the kinase domain are responsible for intermolecular interactions leading to MRCKα multimerization. N terminus-mediated dimerization and consequent transautophosphorylation are critical processes regulating MRCKα catalytic activities. A region containing the two distal CC domains (CC2 and CC3; residues 658 to 930) was found to interact intramolecularly with the kinase domain and negatively regulates its activity. Its deletion also resulted in an active kinase, confirming a negative autoregulatory role. We provide evidence that the N terminus-mediated dimerization and activation of MRCK and the negative autoregulatory kinase–distal CC interaction are two mutually exclusive events that tightly regulate the catalytic state of the kinase. Disruption of this interaction by a mutant kinase domain resulted in increased kinase activity. MRCK kinase activity was also elevated when cells were treated with phorbol ester, which can interact directly with a cysteine-rich domain next to the distal CC domain. We therefore suggest that binding of phorbol ester to MRCK releases its autoinhibition, allowing N-terminal dimerization and subsequent kinase activation.
Rho GTPases have been shown to regulate a wide range of cellular activities, particularly actin cytoskeleton assembly and organization. Most of the findings described are from studies on Rho and the related proteins Rac and Cdc42 (17). It is well documented that Rho mediates the formation of actin-based stress fibers and focal adhesions induced by lysophosphatidic acid and Rac regulates the formation of platelet-derived growth factor-induced membrane ruffles, whereas Cdc42 is involved in peripheral filopodium formation induced by bradykinin. Many of the downstream activities of these GTPases are mediated through tight regulation of their specific effectors in response to extracellular stimuli (7, 28).
There is evidence that Rho regulates the assembly of stress fibers and focal adhesions through the cooperation of Rho kinase (ROK) and Diaphanous (35, 45). Some other effectors of Rho include protein kinase N, rhotekin, rhophilin, citron, and citron kinase (see reference 7 for a review). Partner of Racl and WASP family verprolin-homologous protein have been implicated in Rac-induced membrane ruffling (33, 43), whereas phosphatidylinositol-4-phosphate-5-kinase has been shown to be involved in Rac-dependent actin filament assembly (41). Cdc42 has been demonstrated to regulate actin cytoskeletal changes through various specific downstream effectors, such as Wiskott-Aldrich syndrome protein and N-Wiskott-Aldrich syndrome protein, in actin polymerization and filopodium formation (6), p21-activated kinase (PAK) in regulation of actomyosin and focal adhesion assembly (31, 39, 48), and IQGAP in regulation of cell-cell junction adhesion (23).
Myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) is another effector of Cdc42 isolated based on their specific interaction (27). Its expression is ubiquitous but is highest in the brain. We previously reported that MRCK potentially acts downstream of Cdc42 in actin cytoskeletal reorganization, particularly Cdc42-mediated peripheral actin formation. In Drosophila, mutations of the gek (a Drosophila homolog of MRCK) locus exhibited abnormal F-actin accumulation and a defect in fertilized-egg production, a phenotype downstream of Drosophila Cdc42 (29). A possible involvement of MRCKα in the regulation of neurite outgrowth in PC12 cells has also been proposed (10), consistent with the view that Cdc42, rac-1, and their effectors are involved in the differentiation of neuronal cells (14, 22).
MRCKα and -β are the two known isoforms of the multidomained serine/threonine protein kinases. ROKs (19, 26, 32) and myotonic dystrophy protein kinase (DMPK) (4, 13) are two other protein kinases closely related to MRCKs. These kinases share homology in their N-terminal kinase domains and, to a certain extent, with other domain arrangements. Interestingly, they all contain a highly conserved stretch of about 70 amino acids N terminal to the kinase domain (termed the leucine-rich domain in DMPK) that is immediately followed by an extended coiled-coil (CC) domain. DMPK is smaller than ROK and MRCK and lacks the various regulatory modules present in the C-terminal halves, except for a membrane association domain (44). Besides the formation of stress fibers and focal adhesions, ROK has also been implicated in other Rho-mediated cellular functions, such as the regulation of intermediate filament assembly (15, 40), neurite remodeling (2, 10, 18), cytokinesis (15, 21), transcription regulation, and cell transformation (11, 38). DMPK is implicated in myotonic dystrophy, an age-related neuromuscular disorder characterized by increasing CTG repeats at the 3′ end of the noncoding sequence. Its expression is most abundant in skeletal and cardiac muscles, where it has been localized to the neuromuscular junction and the intercalated disk (30). The in vivo function and regulation of DMPK are still unclear, but it is predicted to participate in as yet unidentified signal transduction pathways.
It is important to understand how the specific effectors of the GTPases are regulated in order to elucidate the complexity and coordination of the downstream cellular events. In the current work, we analyzed the regulation of MRCKα with regard to its autoregulation and activation. We demonstrate here that native MRCK exists as multimeric complexes. In the inactive state, the kinase is usually kept in a closed conformation, held by the stable interaction between the kinase domain and the negative autoregulatory domain encompassed within the distal CC domain. We also show that the subsequent catalytic activity is dependent on the N terminus-mediated dimerization–transautophosphorylation events. Disruption of the interaction of the distal CC domain and the catalytic domain and treatment of cells with phorbol ester both led to increases in MRCK kinase activities, suggesting that these effects interfere with the formation of a native kinase-autoinhibitory complex, thus allowing dimerization and subsequent kinase activation.
MATERIALS AND METHODS
Construction of MRCKα cDNA constructs.
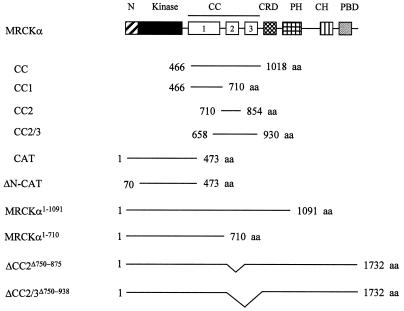
Full-length MRCKα and MRCKα-CAT1–473 in mammalian expression vector pXJ40 containing an N-terminal FLAG or hemagglutinin (HA) tag were constructed as previously described (31). Kinase-dead MRCKα-KDK106A,1–473 was obtained by replacing the N-terminal BamHI/BstXI fragment of MRCKα-CAT1–473 with that of MRCKα-KDK106A previously cloned (27). The N terminus deletion mutant forms MRCKα-ΔN-CAT70–473 and MRCKα-ΔN-KDK106A,70–473 were obtained from BamHI/PstI-digested PCR products of MRCKα-CAT1–473 and MRCKα-KDK106A,1–473 constructs, respectively, with primer 5′-CTGGATCCATGCGCTTACATAGAGAAG-3′ and reverse primer pXJ40. A two-step PCR protocol was used for mutagenesis (27) using VENT polymerase (New England Biolabs) to generate the following point mutations. MRCKα-CATS222L,1–473 was obtained with primer 5′-TGCTAAACGAATATGTC-3′–T7 and a 5′-GATTTTGGTCTTTGTCTGAAG-3′–pXJ40 reverse primer, MRCKα-CATT231A,1–473 was obtained with primer 5′-CCATCTTCCATCAGCTTC-3′–T7 and a 5′-CGCCGTCCAGTCCTCAGTGGCAG-3′–pXJ40 reverse primer, MRCKα-CATS234A,1–473 was obtained with primer 5′-CCACTGAAGCCTGGACCGTTCCATCTTC-3′–T7 and a 5′-CAGTTGGAACTCCAGACTAC-3′–pXJ40 reverse primer, MRCKα-CATS235A,1–473 was obtained with primer 5′-CCATCTTCCATCA GCTCC-3′–T7 and a 5′-AACGGTCCAGTCGGCCGTGGCAGTTGGAACTC-3′–pXJ40 reverse primer, MRCKα-CATT240A,1–473 was obtained with primer 5′-TTCCGGGGAAATGTAGTCTGGAGCTCCAACTGC-3′–T7 and a 5′-ATCCTTCAGGCTATGGAGGAC-3′–pXJ40 reverse primer, MRCKα-CATS245A,1–473 was obtained with primer 5′-TTCCGGGGCAATGTAGTCTGG-3′–T7 and a 5′-ATCCTTCAGGCTATGGAGGAC-3′–pXJ reverse primer, and MRCKα-CATT403A,1–473 was obtained with primer 5′-GCTACTAGTATATGCAAACCCAACAAATG-3′–T7 and a 5′-TGTGTTCTTTCTGATCGGAGC-3′–pXJ40 reverse primer, with pXJ40-FLAG-MRCKα-CAT1–473 used as the template. All mutations were checked by DNA sequencing. A 3.3-kb BamHI fragment of full-length pXJ40-FLAG-MRCKα was ligated to the BamHI-cut pXJ40-FLAG vector to generate the MRCKα1–1091 construct. To obtain pXJ40-FLAG-MRCKα1–710, a 2.1-kb BamHI/EcoRI fragment of full-length pXJ40-FLAG-MRCKα was first ligated to pGEX4T1 and subsequently subcloned into the pXJ40-FLAG vector by BamHI/XhoI digestion. The CC domain pXJ40-FLAG- and HA-MRCKα-CC466–1018 constructs were derived from a 2.9-kb HincII/XmnI partial-digestion fragment of full-length cDNA. It was first ligated in frame to SmaI-cut pGEX4T1 and next subcloned into pXJ40 vectors by BamHI/XhoI digestion. Like MRCKα-CC466–1018, the MRCKα-CC1466–710, MRCKα-CC2710–854 and MRCKα-CC2/3658–930 constructs were all first subcloned in frame in suitable pGEX vectors and later moved into pXJ40 vectors with BamHI/XhoI digestion: MRCKα-CC1466–710 was subcloned from a 732-bp partial HincII/XhoI fragment of MRCKα1–710, MRCKα-CC2710–854 was obtained from a 433-bp EcoRI fragment of full-length cDNA, and MRCKα-CC2/3658–930 was obtained from an 816-bp PvuII fragment of the full-length cDNA. The CC deletion mutant form MRCKα-ΔCC2Δ750–875 was obtained by replacing the wild-type construct with a PstI/NheI-digested PCR product with primers 5′-TTCCCTTCTGGTTTTTTCC-3′–T7 and 5′-GACATGTCAGCTAGACTAG-3′–5′-GTCTCGCTGTCGGCTAG-3′, and MRCKα-ΔCC2/3Δ750–938 was obtained with primers 5′-TTCCCTTCTGGTTTTTTCC-3′–T7 and 5′-AGATCTGAAAAGGTGTAG-3′–5′-GTCTCGCTGTCGGCTAG-3′. A 341-bp PvuII/EcoRV fragment of full-length MRCKα was ligated to the SmaI-cut pGEX-1 vector to generate an MRCKα-CRD construct. A summary of all of the constructs used in this study is shown in Fig. 1.
FIG. 1.
Schematic diagram of the various MRCK constructs used in this study. The conserved N terminus (N), kinase domain (Kinase), CC1, -2, and -3, CRD, pleckstrin homology domain (PH), citron homology domain (CH), and PBD are shown. aa, amino acids.
Gel filtration chromatography.
A Sepharose CL-6B-200 (Pharmacia) column of 1.8 × 45 cm was equilibrated with lysis buffer (25 mM HEPES [pH 7.7], 0.2 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 20 mM β-glycerolphosphate, 1 mM sodium orthovanadate, 0.05% Triton X-100, 5% glycerol) and calibrated with the standard molecular mass markers thyroglobulin (669 kDa), apoferritin (443 kDa), alchohol dehydrogenase (150 kDa), and albumin (66 kDa). Crude rat brain extract prepared in lysis buffer using a Dounce homogenizer was clarified by centrifugation at 100,000 × g for 30 min at 4°C. The soluble supernatant was applied to the CL-6B-200 column at a flow rate of 0.12 ml/min. Fractions of 0.25 ml were collected and subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis. Positions of MRCK and p125FAK were determined by Western blot analysis with respective antibodies.
BS3 cross-linking assay.
COS-7 cells with and without overexpresson of various MRCKα proteins were extracted in lysis buffer. Protein concentrations of the soluble extracts were adjusted to 1 to 3 mg/ml. Cross-linking reactions began with addition of 0.05 to 0.5 mM bis(sulfosuccinimidyl)suberate (BS3; Pierce) at 4°C for 30 min. Reactions were stopped by addition of an equal volume of SDS-PAGE sample buffer. Cross-linked products were detected by immunoblotting with either anti-MRCK antibody (27) or anti-FLAG antibody (Sigma) for detection of the endogenous MRCK and overexpressed FLAG-tagged MRCK proteins, respectively.
Phorbol ester binding assay.
Phorbol ester binding was measured using [20(n)-3H]phorbol-12,13-dibutyrate (PDBu; Amersham). Glutathione S-transferase (GST)-MRCKα-CRD (10 μg) was incubated with 60 nM PDBu (19 Ci/mmol; Amersham Pharmacia) in buffer containing 25 mM Tris (pH 7.5), bovine serum albumin (4 mg/ml), and 2 mM CaCl2, with or without phosphatidylserine at 100 μg/ml, for 30 min at room temperature and then placed on ice for another 30 min. Samples were filtered on prewetted GF/C filter discs (Whatman) and then washed with 5 ml of ice-cold 25 mM Tris (pH 7.5). Filters were dried, and binding activity was quantified using an LKB scintillation counter.
Transfection and immunofluorescence assay.
HeLa and COS-7 cells were cultured in medium containing 10% fetal bovine serum and transfected with various FLAG- and HA-tagged DNA constructs (1 μg/ml) using Lipofectamine (6 μl/ml; GIBCO/BRL) as previously described (26, 31). For immunofluorescence studies, HeLa cells were plated on glass coverslips, transfected, and fixed with 4% paraformaldehyde after 16 h of incubation. An anti-FLAG monoclonal antibody (5 μg/ml; Sigma) and a fluorescein isothiocyanate-conjugated secondary antibody (1:100; Boehringer Mannheim) were used to stain transfected cells. Rhodamine-conjugated phalloidin (1 μg/ml; Sigma) was used to visualize the actin filaments. Stained cells were analyzed with a Hamamatsu C4742-98 digital camera adapted to a Leica fluorescence microscope. Metamorph Imaging software (Universal Imaging Corp.) was used to capture and store images.
Immunoprecipitation and kinase assay.
For immunoprecipitation experiments, cells grown in a 100-mm-diameter dish were transfected and incubated for 16 h before being harvested in lysis buffer. Clarified cell extracts were incubated with anti-FLAG-conjugated agarose beads (Sigma) for 1 to 2 h at 4°C. After extensive washing, the immunoprecipitates were either subjected to kinase assays or boiled for 5 min in SDS-sample buffer and resolved by SDS-PAGE for Western blot analysis. Immunoprecipitation of endogenous MRCK was carried out in a similar way, except that MRCK antibody and protein A-conjugated Sepharose beads (Sigma) were used. Kinase assays were carried out at 30°C for 10 min using GST-MLC2 as the substrate (27) in buffer containing 10 μM [γ-33P]ATP (2,500 Ci/mmol; NEN), 25 mM HEPES (pH 7.3), 25 mM KCl, 5 mM β-glycerolphosphate, 2.5 mM sodium fluoride, 5 mM MgCl2, 1 mM MnCl2, and 0.025% Triton X-100. Autophosphorylation assays were carried out as described above, except that no exogenous substrate was added.
PMA treatment.
Subconfluent HeLa cells with or without serum starvation were treated with phorbol 12-myristate 13-acetate (PMA; Sigma) at 300 ng/ml for 30 min before being harvested in lysis buffer for immunoprecipitation using anti-MRCK antibody for activity measurement. For treatment in a cell-free assay, serum-starved HeLa cells from two 100-mm-diameter dishes were harvested with 50 μl of lysis buffer containing 25 mM HEPES (pH 7.7), 0.15 M NaCl, 5 mM MgCl2, 20 mM β-glycerolphosphate, 1 mM sodium orthovanadate, and 0.05% Triton X-100. Clarified cell extracts were incubated with 0.5 μM PMA in the presence of 2 mM ATP and 2 μg of phosphatidylserine at 30°C for 20 min. The volume of the cell extracts was then adjusted to 0.5 ml with the same lysis buffer before immunoprecipitation for activity measurement as previously described.
RESULTS
Native MRCK exists in high-molecular-weight complexes.
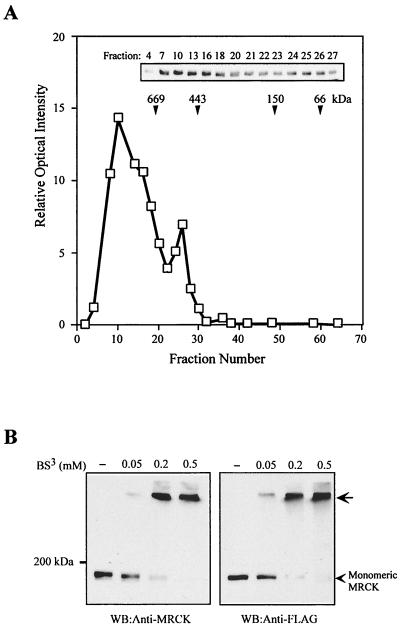
The CC domain of MRCKα (residues 425 to 938) constitutes almost a third of the full-length protein, and it shares some homology with the nonmuscle myosin heavy chain C-terminal CC region. As the myosin heavy chain CC tail has been demonstrated to entwine to form an extended parallel CC for self-assembly (5), we examined the quaternary complexity of native MRCK. Rat brain soluble extract was applied to a CL-6B gel filtration column calibrated with standard molecular mass markers. The position of MRCK in the elution profile was determined by immunoblot analysis with anti-MRCKα antibody. As shown in Fig. 2A, MRCK was largely eluted as two continuous peaks, a major peak of approximately 900 kDa and a smaller, trailing peak of about 500 kDa. No MRCKα migrated in the 190-kDa range, while p125FAK was eluted mostly at 130 kDa under the same experimental conditions (data not shown).
FIG. 2.
Gel filtration chromatography and chemical cross-linking reveal the multimeric nature of MRCK. (A) Rat brain extract was applied onto a Sepharose CL-6B-200 gel filtration column. Fractions were collected, and the relative amount of MRCK in each fraction was determined by Western blotting with anti-MRCK antibody (inset) using a Bio-Rad laser densitometer. The elution profile of the standard molecular mass markers used is indicated by arrowheads. (B) COS-7 cell extracts from untransfected cells or cells expressing plasmid-encoding FLAG-MRCKα were exposed to increasing concentrations of the cross-linking agent BS3 as indicated. Cross-linked endogenous MRCK and overexpressed FLAG-MRCKα were determined by Western blotting (WB) with anti-MRCK or anti-FLAG antibody. The arrow indicates the slow-migrating cross-linked products.
We verified this observation using chemical cross-linking. COS-7 cell extracts prepared from untransfected cells and cells overexpressing FLAG-MRCKα were incubated with increasing concentrations of the cross-linking agent BS3. The presence of multimeric complexes was characterized by the detection of a single slow-migrating band (Fig. 2B). Both endogenous MRCK and overexpressed MRCKα were cross-linked by BS3 in a concentration-dependent manner. The results of gel filtration and chemical cross-linking experiments suggest that native MRCKs exist in a multimeric state.
A putative CC domain and N terminus region facilitate MRCKα multimerization.
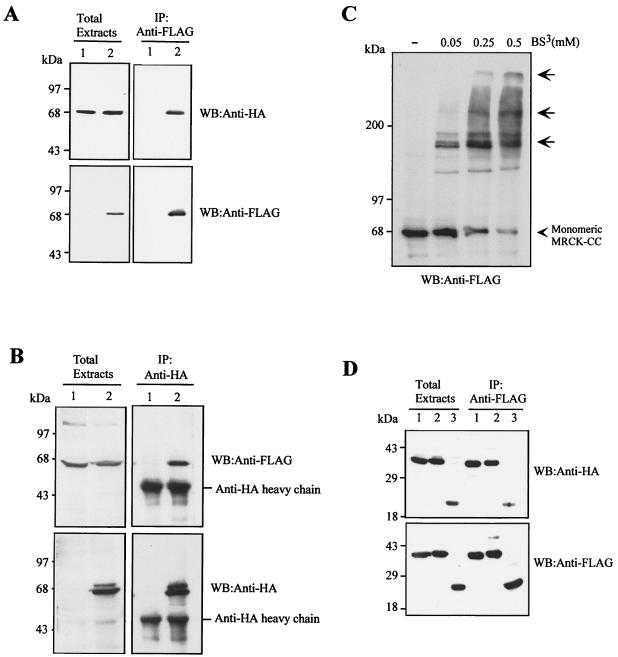
The MRCKα predicted CC domain consists of three discrete blocks with characteristic heptad repeats (Fig. 1; also refer to reference 29). We designated these CC1 (residues 425 to 669), CC2 (a putative leucine zipper, residues 750 to 809), and CC3 (residues 875 to 938). To determine whether these CC domains of MRCKα are responsible for multimerization, we investigated the ability of the whole of the central CC domain to self-associate by coimmunoprecipitation. Extracts of COS-7 cells doubly transfected with the HA- or FLAG-tagged CC domain (HA- or FLAG-MRCKα-CC466–1018) were immunoprecipitated using anti-FLAG antibody. Our results show that HA-MRCKα-CC466–1018 readily associated with FLAG-MRCKα-CC466–1018, as it was coimmunoprecipitated (Fig. 3A). Reverse immunoprecipitation using anti-HA antibody gave similar results (Fig. 3B). Furthermore, addition of BS3 to the cell extract containing overexpressed FLAG-MRCKα-CC466–1018 resulted in the appearance of multimeric cross-linked products (Fig. 3C). We also tested the ability of the smaller individual CC1 and CC2 domains to self-interact. As shown in Fig. 3D, HA-MRCKα-CC1466–710 and HA-MRCKα-CC2710–854 were readily detected in the immunoprecipitates of FLAG-MRCKα-CC1466–710 and FLAG-MRCKα-CC2710–854, respectively. The combined MRCKα-CC2/3 region was also able to self-interact. These homotrophic interactions between the respective individual CC regions were specific, and no heterotrophic interaction between the CC1 and CC2 regions was observed (data not shown). Thus, the CC domains of MRCKα probably entwine to form parallel structures that drive MRCKα oligomerization.
FIG. 3.
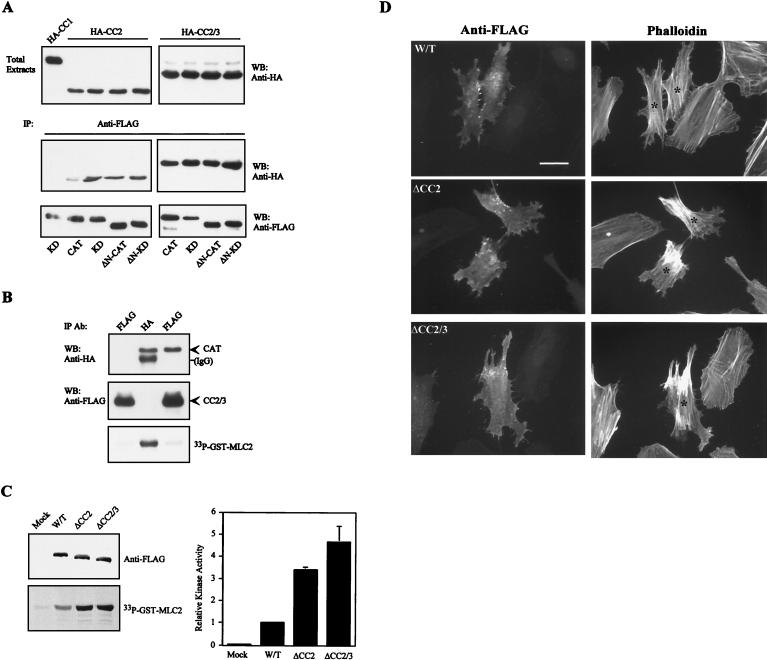
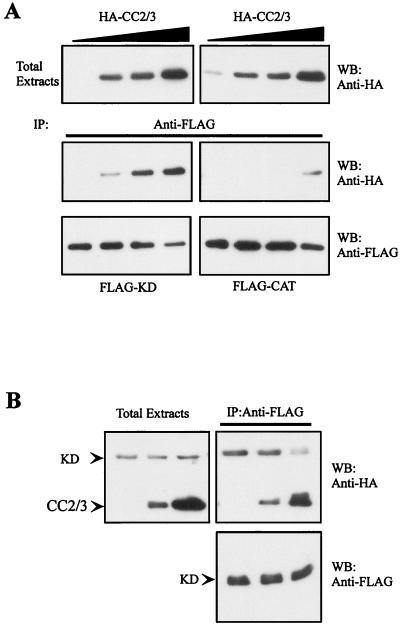
MRCKα CC domains are essential for self-interaction. (A) The HA-tagged MRCKα-CC466–1018 construct was expressed alone (lane 1) or coexpressed with FLAG-tagged MRCKα-CC466–1018 in COS-7 cells (lane 2). Immunoprecipitations (IP) were carried out using anti-FLAG antibody, and the immunoprecipitates recovered were probed with anti-HA or anti-FLAG antibodies. (B) The FLAG-tagged MRCKα-CC466–1018 construct was expressed alone (lane 1) or coexpressed with HA-tagged MRCKα-CC466–1018 in COS-7 cells (lane 2). IP was carried out using anti-HA antibody, and the immunoprecipitates recovered were probed with anti-HA or anti-FLAG antibodies. (C) Extracts from COS-7 cells expressing plasmid-encoded FLAG-MRCKα-CC466–1018 were exposed to increasing concentrations of BS3 as indicated. Cross-linked products were detected by Western blotting (WB) with anti-FLAG antibody. The arrows indicate the slow-migrating cross-linked products. (D) HA- and FLAG-tagged constructs of CC1 (MRCKα-CC1466–710; lane 1), CC2/3 (MRCKα-CC2/3658–930; lane 2), and CC2 (MRCKα-CC2710–854; lane 3) covering different portions of the CC domain were cotransfected into COS-7 cells and subjected to IP with anti-FLAG antibody. The immunoprecipitates recovered were immunoblotted with anti-HA or anti-FLAG antibodies.
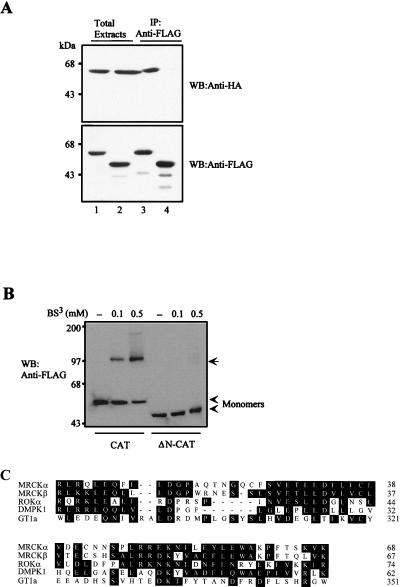
In addition, we also found that the first 70 amino acids of MRCKα could mediate dimerization of the kinase domain. As shown in Fig. 4A, HA-MRCKα-CAT1–473 was readily coimmunoprecipitated with FLAG-MRCKα-CAT1–473 but not with the N terminus deletion mutant construct FLAG-MRCKα-ΔN-CAT70–473. Again in a BS3 cross-linking assay, FLAG-MRCKα-CAT1–473 was readily cross-linked in a concentration-dependent manner (Fig. 4B). By contrast, no cross-linked product was detected with FLAG-MRCKα-ΔN-CAT70–473. In the Western blot, the only major cross-linked product of FLAG-MRCKα-CAT1–473 exhibited a size consistent with the formation of a dimer. This N-terminal sequence stretch preceding the kinase domain is highly conserved among MRCK isoforms and the two closely related kinases ROK and DMPK. It is also noticeably conserved with the C-terminal sequence of a functionally unrelated plant DNA-binding protein, GT1a (Fig. 4C; also refer to reference 24). This motif in GT1a has been shown to be responsible for its oligomerization. Our results provide direct evidence that both the CC domain and the conserved N terminus could play a part in maintaining the quaternary structure of MRCKα through intermolecular interactions.
FIG. 4.
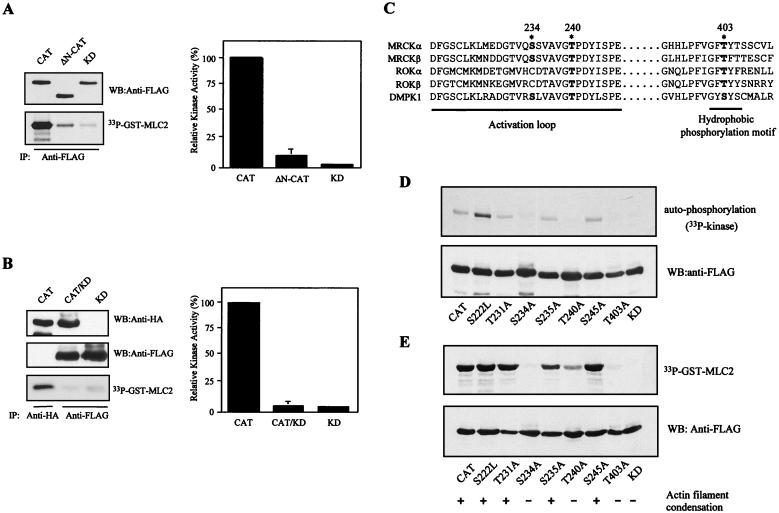
The conserved N-terminal sequence mediates kinase domain dimerization. (A) The FLAG-tagged catalytic domain (MRCKα-CAT1–473; lanes 1 and 3) or ΔN-CAT (MRCKα-ΔN-CAT70–473; lanes 2 and 4) construct was coexpressed with HA-tagged CAT. Immunoprecipitations were carried out using anti-FLAG antibody, and the immunoprecipitates recovered were blotted with anti-HA or anti-FLAG antibody. (B) Extracts from cells expressing the plasmid encoding FLAG-tagged CAT or ΔN-CAT were exposed to increasing concentrations of BS3 as indicated. The arrow indicates the 100-kDa cross-linked products. (C) Sequence alignment of the N-terminal sequences of MRCKα and -β, ROKα, and DMPK1 and the C-terminal sequence of GT1a was performed with the Clustal method (DNASTAR). Conserved residues are boxed in black, and numbers indicate the positions of residues.
N terminus-mediated dimerization and transautophosphorylation are essential for MRCKα catalytic activity.
We next investigated the relationship between N terminus-dependent kinase domain dimerization and kinase activity. As shown in Fig. 5A, deletion of 70 residues from the conserved MRCKα N terminus resulted in essentially complete loss of the kinase activity of FLAG-MRCKα-CAT1–473 toward myosin light chain (MLC2). Similar results were obtained by comparing full-length MRCKα with Δ70N-MRCKα (data not shown). This demonstrated that the N-terminal nonkinase sequence is essential for catalytic activity. A conceivable requirement for dimerization would be intermolecular transautophosphorylation to allow kinase activation (37, 47). To test this, we isolated heterodimers consisting of wild-type HA-MRCKα-CAT1–473 and a kinase-dead kinase domain and tested their activity toward MLC2. If transautophosphorylation is required for activation, pairing up of the wild-type kinase domain with an inactive mutant would result in an inactive dimer (37). Indeed, the heterodimer was as inactive as the homo-FLAG-MRCKα-KDK106A,1–473 dimer (Fig. 5B). Furthermore, constitutively active FLAG-MRCKα-CAT1–473 was unable to phosphorylate the GST-MRCKα-KDK106A,1–473 fusion protein (data not shown), implying that the transautophosphorylation event is likely to take place within the dimer.
FIG. 5.
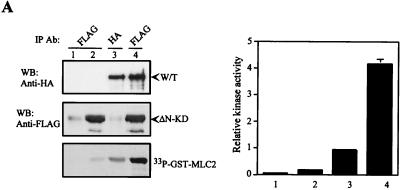
Dimerization and subsequent transautophosphorylation of the kinase domain are required for MRCKα kinase activity. (A) FLAG-CAT, FLAG- ΔN-CAT, and FLAG-KD (MRCK-KDK106A,1–473) were individually overexpressed in COS-7 cells and subjected to immunoprecipitation (IP) using anti-FLAG antibody. Kinase activities were assayed using GST-MLC2 as the substrate (left side). Phosphorylation levels of [33P]GST-MLC2 were quantified on a PhosphorImager, and the means and standard errors of activities relative to that of wild-type CAT (100%) from three independent experiments are shown on the right. (B) COS-7 cells were transfected with HA-CAT, HA-CAT and FLAG-KD (CAT/KD), or FLAG-KD alone. Expressed proteins were immunoprecipitated with either anti-HA antibody or anti-FLAG antibody as indicated. The immunoprecipitated products recovered were assayed for kinase activity using GST-MLC2 as the substrate (left side). The phosphorylation levels quantified are shown as the means and standard errors of activities relative to that of the wild-type CAT domain (100%) from three independent experiments on the right. (C) Comparison of the amino acid sequences in the activation loop and the hydrophobic phosphorylation motif of MRCKα and -β, ROKα and -β, and DMPK1. Potential phosphorylation sites in MRCKα are highlighted by asterisks, and positions are numbered. Boldface letters indicate conserved potential phosphorylation residues found in the related proteins. (D) The overexpressed proteins of all of the MRCKα mutant constructs were immunoprecipitated with anti-FLAG antibody and subjected to in vitro autophosphorylation assays with [γ-33P]ATP or assayed with GST-MLC2 as the substrate in panel E. The mutant constructs were transfected into HeLa cells, and the effects on actin filament arrangements were examined by rhodamine-conjugated phalloidin staining. A plus sign indicates a robust actin filament condensation phenotype, and a minus sign indicates absence of effect (E, bottom). WB, Western blot.
Phosphorylation of key residues within the activation loop and the extended hydrophobic phosphorylation motif provide the means for activation of many protein kinases (9, 34, 36). Autophosphorylation site mapping of recombinant GST-MRCKα-CAT1–473 revealed that the major autophorylation sites reside within two neighboring tryptic peptides, namely, DFGSCLK and LMEDGTVQSSVAVGTPDYISPEILQAMEDGK, that are located within the activation loop of the kinase domain (Fig. 5C). To test the role of specific residues in these peptides, individual serine and threonine residues, together with threonine 403 in the hydrophobic phosphorylation motif, were replaced with alanine (except for serine 222, which was changed to a leucine residue). Three mutations, S234A, T240A, and T403A, strongly affected the in vitro autophosphorylation activity of FLAG-MRCKα-CAT1–473 (Fig. 5D). We noticed that S222L consistently exhibited enhanced autophosphorylation activity relative to the wild type, although its activity toward GST-MLC2 remained unchanged (Fig. 5E). An equivalent residue in Pim-1 serine/threonine kinase has been shown to be critical for its activity (36). The role of this residue in MRCKα is unclear, but it may be involved in modulation of the catalytic property. The mutants with defective autophosphorylation also poorly phosphorylated the GST-MLC2 substrate (Fig. 5D and E). Despite the defects in the phosphorylation properties of these mutant constructs, kinase dimerization was not perturbed (data not shown). Consistent with the biochemical data, overexpression of these three mutant forms in HeLa cells did not elicit the actin filament contraction-condensation phenotype (27) shown by the wild-type kinase domain and another point mutant construct (Fig. 5E and 6B). Taken together, our data suggest that N terminus-mediated dimerization and transautophosphorylation are two events important for kinase activity. It is noteworthy that residues equivalent to S-234, T-240, and T-403 are all present in DMPK, while ROK shows conservation only of residues equivalent to T-240 and T-403 (Fig. 5C).
FIG. 6.
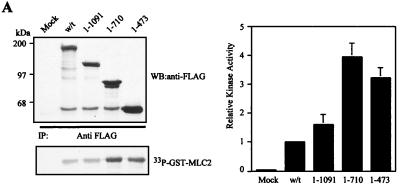
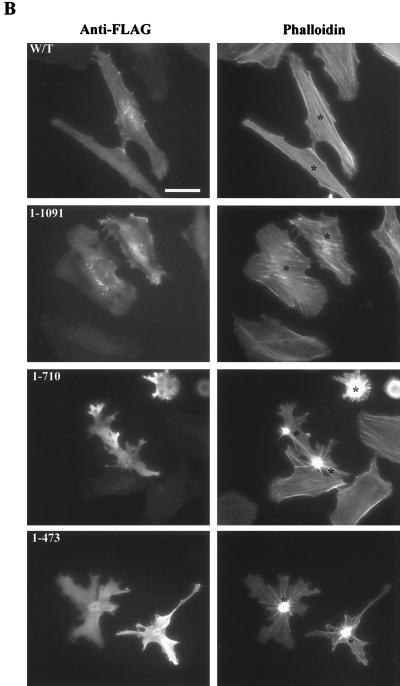
C terminus deletion mutations reveal a potential negative autoregulatory region in MRCKα. (A) pXJ40-FLAG-tagged constructs were transfected into COS-7 cells, and expressed proteins were immunoprecipitated (IP) with anti-FLAG antibody for kinase assay using GST-MLC2 as the substrate (left side). The quantified phosphorylation levels are shown on the right as the means and standard errors of activities relative to the wild-type (w/t) level (taken as 1) from three independent experiments. WB, Western blot. (B) Effects of overexpression of the C terminus deletion mutant constructs on actin filament rearrangement in HeLa cells. Cells were doubly stained with anti-FLAG antibody to show transfected cells (left) and phalloidin to show actin filaments (right). Transfected cells were marked with asterisks. Scale bar = 10 μm.
The kinase domain is negatively regulated by an autoregulatory region within the distal CC domain.
Our previous work showed that the expression of the MRCKα kinase domain in HeLa cells elicited a dramatic actin contraction-condensation effect, while expression of the wild-type construct only resulted in slight enhancement of stress fiber formation (27). This indirectly reflected the fact that full-length MRCKα is catalytically less active than the kinase domain alone. A possible reason for this is the involvement of a negative autoregulation event. To test this assumption, as well as to locate such an autoregulatory region, we compared the activities of four MRCKα constructs of different lengths in the C terminus, namely, MRCKα, MRCKα1–1091, MRCKα1–710, and the kinase domain MRCKα-CAT1–473 (Fig. 1). From the in vitro kinase assays, it is apparent that these four constructs represented two sets of activity (Fig. 6A): full-length FLAG-MRCKα and FLAG-MRCKα1–1091 represent the less active group, while FLAG-MRCKα1–710 and FLAG-MRCKα-CAT1–473 represent the more active species. Overexpression of these constructs in HeLa cells gave similar results (Fig. 6B). Both FLAG-MRCKα and FLAG-MRCKα1–1091 showed enhanced stress fiber formation, while cells transfected with FLAG-MRCKα1–710 and the FLAG-MRCKα-CAT1–473 construct exhibited strong actin contraction-condensation phenotypes. These results suggest that an internal region between residues 710 and 1091 is the negative autoregulatory region responsible for keeping wild-type MRCKα inactive, as its deletion correlates with an acquisition of increases in kinase activities.
Intriguingly, this putative autoregulatory region encompasses the CC2-CC3 (CC2/3) domain and the neighboring cysteine-rich domain (CRD) (Fig. 1). We therefore tested whether the kinase domain could interact with the CC2/3 region. As shown in Fig. 7A, the MRCKα kinase domain was able to bind CC2 and a more pronounced interaction was observed with the larger CC2/3 region. No interaction with CC1 was detectable, by coimmunoprecipitation assays, ruling out nonspecific interactions. The conserved N-terminal sequence is not required for such an association, since its deletion had no effect on the interaction (data not shown).
FIG. 7.
Interaction between the distal CC domains and the kinase domain causes MRCKα kinase inhibition. (A) COS-7 cells were cotransfected with a pXJ40 vector containing HA-CC2 or HA-CC2/3 (Fig. 3), together with each of the various FLAG-tagged MRCKα kinase domain constructs, as indicated (bottom). Immunoprecipitations (IP) were carried out with anti-FLAG antibody, and the IP products were Western blotted (WB) with anti-HA or anti-FLAG antibodies. Expressions of the HA-tagged CC domains in total extracts are shown at the top, and HA-CC1 was used as the negative control. (B) COS-7 cells were cotransfected with plasmids encoding FLAG-CC2/3 alone (left lane), HA-CAT alone (middle lane), and FLAG-CC2/3 together with HA-CAT (right lane). IP were carried out using either anti-FLAG or anti-HA antibodies as indicated, and the products were subjected to kinase assays using GST-MLC2 as the substrate. The immunoprecipitated products recovered were immunoblotted with anti-HA or anti-FLAG antibodies (Ab). IgG, immunoglobulin G. (C) COS-7 cells expressing the FLAG-tagged deletion mutant forms ΔCC2 (MRCKα-ΔCC2Δ750–809 and ΔCC2/3 (MRCKα-ΔCC2/3Δ750–938) were immunoprecipitated with anti-FLAG antibody and assayed for kinase activities toward GST-MLC2 (left). The GST-MLC2 phosphorylation levels shown are means and standard errors of activities relative to that of wild-type (W/T) MRCKα (right). (D) Effects of the expression of CC deletion mutant forms on actin filament rearrangement in HeLa cells. Transfected cells with the various wild-type and mutant constructs were doubly stained with anti-FLAG antibody to show transfected cells (left) and with phalloidin to show actin filaments (right). Transfected cells are marked with asterisks. Scale bar = 10 μM.
To establish unequivocally the CC region as the negative autoregulatory domain, we showed that wild-type kinase domain HA-MRCKα-CAT1–473 coimmunoprecipitated with FLAG-MRCKα-CC2/3 and this specific interaction resulted in inactivation of the catalytic activity toward GST-MLC2 (Fig. 7B). Further support of this was derived by deletion of the CC2 (FLAG-MRCKα-ΔCC2Δ750–875) or CC2/3 (FLAG-MRCKα-ΔCC2/3Δ750–938) region from wild-type MRCKα. Expression of either construct in COS-7 cells resulted in about threefold higher activity (Fig. 7C). Furthermore, overexpression of these deletion constructs in HeLa cells generally produced moderate increases in actin stress fibers (Fig. 7D). This effect is different from those observed with truncated mutant forms which lack the C-terminal regulatory domains. It is possible that the presence of the functional modules (CRD, pleckstrin homology domain, and p21-binding domain [PBD]) in the intact C terminus determines its intracellular distributions and subsequent specific functions. Taken together, our findings show that the CC2/3 region can negatively regulate MRCKα kinase activity and that the intramolecular interaction of the distal CC2/3 region with the kinase domain forms the basis of this inhibition.
N terminus-mediated kinase dimerization and the kinase-distal CC interaction are mutually exclusive processes regulating the catalytic state of MRCKα.
We then examined how the two opposite events, namely, N terminus-dependent dimerization and CC-mediated inhibition, may coordinately regulate MRCKα activity. It was noticed that the interaction between active FLAG-MRCKα-CAT1–473 and HA-MRCKα-CC2 (Fig. 7A, lane 2) was somewhat weaker than that with kinase-inactive proteins such as FLAG-MRCKα-KDK106A,1–473, FLAG-MRCKα-ΔN-CAT70–473, and FLAG-MRCKα-ΔN-KDK106A,70–473. This prompted us to investigate whether the active and inactive kinase domains have differential affinity toward the CC region. To determine this, both FLAG-MRCKα-CAT1–473 and FLAG-MRCKα-KDK106A,1–473 were separately coexpressed with increasing levels of HA-MRCKα-CC2/3 in COS-7 cells, followed by anti-FLAG immunoprecipitation and Western blot analysis with anti-HA antibody to stain for coimmunoprecipitated HA-CC2/3. As shown in Fig. 8A, HA-MRCKα-CC2/3 was only detectable in FLAG-MRCKα-CAT1–473 immunoprecipitate when expressed at higher levels. In contrast, the interaction between inactive FLAG-MRCKα-KDK106A,1–473 and HA-CC2/3 was readily detectable (Fig. 8A). The decline in affinity toward the negative autoregulatory domain in the active kinase implies that the active form is less prone to autoinhibition and, once activated, stays persistently active, possibly as a consequence of autophosphorylation.
FIG. 8.
Kinase dimerization and kinase-CC2/3 interactions are mutually exclusive. (A) Constant amounts of plasmids encoding FLAG-KD or FLAG-CAT were cotransfected with increasing concentrations of an HA-tagged CC2/3 construct (top) in COS-7 cells. Immunoprecipitated (IP) products were obtained with anti-FLAG antibody and Western blotted (WB) with anti-HA or anti-FLAG antibodies. (B) COS-7 cells were triply transfected with constant levels of HA-KD and FLAG-KD plasmids together with increasing concentrations of the HA-CC2/3 construct. The expression levels of HA-MRCKα-KD and HA-MRCKα-CC2/3 are shown on the left.
We therefore tested the possibility that the kinase dimerization process is under the regulation of the negative autoregulatory domain. As shown in Fig. 8B, N terminus-dependent dimerization could be inhibited by interaction between the kinase domain and the CC2/3 region. In this experiment, COS-7 cells were triply transfected with fixed levels of both HA- and FLAG-MRCKα-KDK106A,1–473, together with increasing levels of HA-MRCKα-CC2/3. In the absence or at low concentration of HA-MRCKα-CC2/3, HA-MRCKα-KDK106A,1–473 could be readily detected in anti-FLAG immunoprecipitates, as a result of kinase dimerization. By contrast, in the presence of higher concentrations of HA-MRCKα-CC2/3, HA-MRCKα-KDK106A,1–473 was weakly detectable, which is evidence of a lack of a kinase dimerization event. Taken together, our findings show that N terminus-dependent dimerization and CC-mediated inhibition are two mutually exclusive processes that determine the catalytic state of MRCKα.
Coexpression of a kinase domain mutant capable of disrupting CC-kinase interaction or treatment of cells with PMA caused MRCK kinase activation in vivo.
Our results thus far have revealed that the kinase domain of MRCKα can interact intramolecularly with the negative autoregulatory CC2/3 region to form a closed conformation that is low in catalytic activity. To further substantiate this point, we tested if the disruption of this specific interaction would give rise to active MRCKα. HA-MRCKα was coexpressed with FLAG-MRCKα-ΔN-KDK106A,70–473 (deficient in catalytic activity and N terminus-mediated dimerization) in COS-7 cells and subjected to anti-FLAG immunoprecipitation and kinase assays. FLAG-MRCKα-ΔN-KDK106A,70–473 would be predicted to compete with the kinase domain of HA-MRCKα for interaction with the CC2/3 region, and this, in turn, may lead to an opened conformation with increases in catalytic activity. As shown in Fig. 9A (lane 4), detection of HA-MRCKα in the FLAG-MRCKα-ΔN-KDK106A,70–473 immunoprecipitate provided evidence that FLAG-MRCKα-ΔN-KDK106A,70–473 could indeed compete for the CC2/3 region. Interestingly, we also observed that the coimmunoprecipitated HA-MRCKα (Fig. 9A, lane 4) exhibited threefold higher activity than the control HA-MRCKα immunoprecipitated by anti-HA antibody (Fig. 9A, lane 3). These results support the hypothesis that activation of MRCKα can be achieved by disruption of the preexisting kinase domain-CC interaction.
FIG. 9.
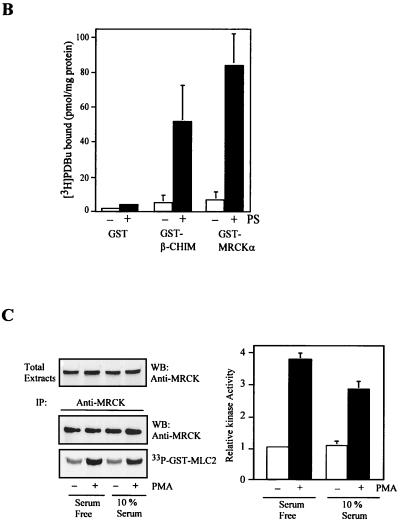
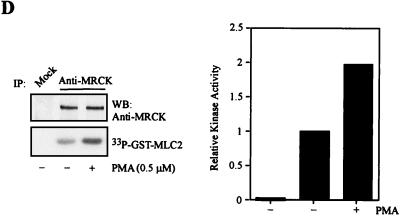
Activation of MRCK by interactive binding to distal CC and CRD. (A) COS-7 cells were transfected with either the pXJ40-HA-MRCKα (lane 1), the FLAG-MRCKα-ΔN-KDK106A,70–473 (lane 2), the HA-MRCKα (lane 3), or the HA-MRCKα plus the FLAG-MRCKα-ΔN-KDK106A,70–473 (lane 4) construct. Immunoprecipitations (IP) were carried out using either anti-HA or anti-FLAG antibody (Ab) as indicated. Immunoprecipitated products were subjected to kinase assay using GST-MLC2 (left). The phosphorylation levels quantified are expressed as means and standard errors of activities relative to that of HA-MRCKα (lane 3; right). W/T, wild type; WB, western blot. (B) Phorbol ester binding for MRCKα-CRD. GST fusion proteins were incubated with [3H]PDBu in the presence or absence of phosphatidylserine (PS) as described in Materials and Methods. The values shown are the means and standard deviations from three independent experiments. (C) Serum-starved and unstarved HeLa cells were treated either with 0.01% dimethylsulfoxide (control) or with PMA at 300 ng/ml in dimethylsulfoxide for 30 min. The endogenous MRCK was immunoprecipitated using an anti-MRCK antibody and subjected to kinase assays using GST-MLC2 as the substrate (left). Quantified GST-MLC2 phosphorylation levels are shown on the right as means and standard errors of activities relative to that of the MRCK immunoprecipitated from control serum-starved cells. (D) Concentrated extracts of serum-starved HeLa cells were preincubated with either 1% dimethylsulfoxide (control) or 0.5 μM PMA in the presence of 2 mM ATP and 2 μg of PS at 30°C for 20 min. The endogenous MRCK was then immunoprecipitated and subjected to kinase assays as previously described (left). Quantified GST-MLC2 phosphorylation levels are shown as means and standard errors of activities relative to that of the control from three independent experiments (right).
It is therefore of interest to identify any physiological activator(s) that may mimic this regulatory catalytic action of MRCKs in vivo. The presence of the PBD and CRD in the C-terminal regulatory half of MRCKs suggests possible roles for Cdc42 and diacylglyerol (DAG). We have previously shown that overexpression of active Cdc42G12V failed to activate overexpressed MRCKα (27). We had also observed a lack of effect of Cdc42G12V on the activity of endogenous MRCKs (data not shown). This is in contrast to PAK, whose activity is dependent on p21 binding (48). The difference between MRCK and PAK in Cdc42-mediated activation can be best explained by the locations of their respective PBDs. The binding of Cdc42-GTP to the PBD of PAK, located adjacent to its autoinhibitory domain, has been shown to release the negative constraint exerted by autoinhibition (25, 48). As for MRCK, the PBD located at the extreme C-terminal end may, possibly, have little or no effect on the negative autoregulatory CC2/3 domain.
The position of MRCK-CRD, in close proximity to the CC2/3 region, suggests that specific ligand interactions at this site may be a better candidate motif for kinase activation. We therefore examined the interaction between MRCKα-CRD and phorbol ester (a DAG analog). As shown in Fig. 9B, [3H]PDBu bound to GST-MRCKα-CRD in a phosphatidylserine-dependent manner, comparable with β-chimaerin, a member of the chimaerin family which is known to interact with and be regulated by phorbol ester (1). We then tested the effect of phorbol ester treatment of cells on endogenous MRCK activity. Immunoprecipitates from HeLa cells exposed to PMA exhibited about threefold higher kinase activity than the control cells cultured both in serum and under serum-free conditions (Fig. 9C). However, cross-linking of BS3 with PMA-treated and control cell extracts did not reveal any differences in the molecular size of the MRCK complex (data not shown), suggesting that PMA treatment did not alter the overall complexity of the kinase. To test if PMA binding is sufficient to induce MRCK activation, a cell-free assay was carried out. As shown in Fig. 9D, the endogenous MRCK immunoprecipitated from serum-starved HeLa cell extracts preincubated with PMA consistently showed about onefold higher activity than the control untreated extracts. The lower extent of activation observed may be due to unoptimized in vitro conditions. Inclusion of recombinant Rho-GAP proteins such as RhoGAP190 and BCR had no obvious effects on the PMA activation of MRCK in this cell-free system (data not shown). This suggests that RhoGTPases are not involved in PMA-dependent kinase activation. Taken together, these findings suggest that MRCK kinase can be activated upon PMA binding, which possibly disrupted the preexisting kinase domain-distal inhibitory CC interaction.
DISCUSSION
In this report, we have shown that native MRCK has the intrinsic capacity of forming oligomers. The elution profile of MRCK from gel filtration chromatography suggests that the majority of MRCK exists as tetrameric complexes (∼900 kDa), with a minor pool of the dimeric form (∼500 kDa). The ability of the entire CC domain to self-interact and the specific interactions observed for the smaller individual CC1 and CC2 domains indicate that MRCKα multimers are formed through parallel intermolecular interaction of the CC domains. We also determined that the conserved N terminus of MRCK is responsible for the dimerization of the kinase domain. Symmetric arrangement or parallel orientation of MRCKα molecules in the complex supports a novel dimerization role of the conserved N-terminal sequence. Our data are consistent with the idea that MRCKs are maintained in a quaternary structure through intermolecular interaction of the CC domain and the conserved N-terminal sequence.
We found that the catalytic activity of MRCKα is critically dependent on N terminus-mediated kinase domain dimerization and the subsequent transautophosphorylation events. This phenomenon is now generally known to be an activation mechanism that is common to many protein kinases. Examples include nonreceptor and receptor tyrosine kinases, as well as serine/threonine kinases. A well-studied case is the binding of a dimeric platelet-derived growth factor to its receptor that induces the formation of a symmetric dimer of the receptor kinase (46), and other examples include Fes (37) and double-stranded-RNA-dependent protein kinase (47), all of which have been reported to depend on dimerization-transautophosphorylation for activation. Despite adopting a similar dimerization-activation mechanism, the specific domains responsible for dimerization in the different kinases appear to be diverse. In the case of MRCK, the unique N-terminal sequence is conserved among MRCKα and -β isoforms, ROK, and DMPK. More importantly, functional conservation as a dimerization motif extends to an unrelated plant DNA-binding protein, GT1a (24). Given the similarity in the domain arrangements, as well as the conservation of the potential phosphorylation sites within the kinase activation loops and the extended hydrophobic phosphorylation motif, it is conceivable that N terminus-mediated kinase dimerization could also be an integral part of ROK and DMPK activation. In support of this, deletion of the N-terminal sequence from ROK resulted in an inactive enzyme (26).
Apart from the multimerization property, the CC2/3 region was also found to act as a negative autoregulatory domain in regulating MRCKα kinase activity by forming a stable complex with the kinase domain. Besides possible blockage of substrate and ATP binding, as described for many kinases (20), our results raise the possibility of an additional mode of inhibition in which the formation of the stable kinase domain-distal CC2/3 interaction prevents N terminus-mediated kinase dimerization-kinase activation from taking place (Fig. 8B). The active MRCKα kinase domain (presumably in the phosphorylated state) exhibited a marked reduction in affinity toward the CC2/3 region (Fig. 8A). These findings imply that the intramolecular kinase domain-CC2/3 interaction and intermolecular N terminus-dependent kinase dimerization are mutually exclusive and that the catalytic state of MRCKα is determined by the interplay of these two events.
We have also identified phorbol ester as an activator of MRCK. The finding of direct phorbol ester binding and activation of MRCK adds the kinase to the growing list of characterized intracellular phorbol ester receptors, such as protein kinase C, DAG kinase, n-chimaerin, Vav, and Ras guanyl nucleotide-releasing protein (12, 16, 42). This has rendered the mechanism of phorbol ester effects on cellular activities more diverse and complicated than first anticipated. However, the exact in vivo function of MRCK in phorbol ester-induced morphological effects is not clear, as dominant negative constructs of MRCK were not effective in blocking PMA-dependent ruffling (data not shown). Further experiments are therefore required to clarify the exact role of MRCK on morphological events induced by phorbol ester.
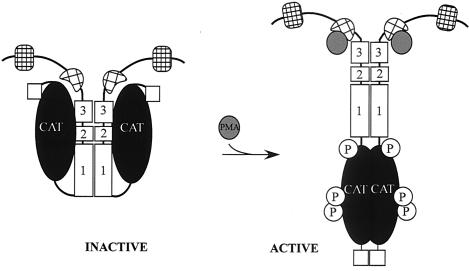
From the observations so far, we propose a model for phorbol ester-dependent activation of MRCK (Fig. 10). When cells are in the resting state, MRCK is kept inactive in a closed conformation by the interaction between the kinase domain and the negative autoregulatory CC2/3 domain. Interaction of PMA with the CRD of MRCK initiates the disruption of the performed kinase domain-distal CC2/3 interaction, releasing the kinase domain to allow N terminus-mediated dimerization and kinase activation. This model is compatible with the recent reports of autoregulation of the related Rho kinase (3) and DMPK (8). The autoinhibitory domain of Rho kinase was mapped within the C-terminal half of the protein, and Rho-GTP binding to the Rho binding site located at the end of the distal CC region has been shown to activate ROK kinase activity, presumably by releasing the constraint of the autoinhibitory event (3). It is of interest that the MRCK's CRD motif responsible for phorbol ester binding is also located at a position similar to that of the Rho binding site in Rho kinase (27). Similarly, a pseudosubstrate-like autoinhibitory domain after the C-terminal CC of DMPK and a correlation of the CC-mediated oligomerization with catalytic activity was recently described (8). It is conceivable that all of these related kinases have adopted a similar mechanism by which to regulate their catalytic activities through inter- and intramolecular interactions, although the exact natures of the interactions may differ. It remains to be seen whether this fundamental regulatory mechanism is conserved among all of these related kinases.
FIG. 10.
Model for regulation of the catalytic activity of MRCKα. The intramolecular interaction between the CC autoinhibitory domain CC2-CC3 and the kinase domain keeps the kinase in a closed, inactive, dimeric structure. Disruption of this interaction (e.g., PMA binding to the CRD or coexpression with a mutant kinase domain) resulted in an open structure that facilitates N terminus-mediated dimerization, autophosphorylation, and subsequent kinase activation.
ACKNOWLEDGMENTS
This work was supported by the Glaxo Singapore Research Fund.
We thank Ed Manser for helpful discussion.
REFERENCES
- 1.Ahmed S, Lee J, Kozma R, Best A, Monfries C, Lim L. A novel functional target for tumor-promoting phorbol esters and lysophosphatidic acid. The p21rac-GTPase activating protein n-chimaerin. J Biol Chem. 1993;268:10709–10712. [PubMed] [Google Scholar]
- 2.Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 3.Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y, Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates Rho-kinase activity. J Biol Chem. 1999;274:32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- 4.Aslanidis C, Jansen G, Amemiya C, Shutler G, Mahadevan M, Tsilfidis C, Chen C, Alleman J, Wormskamp N G M, Vooijs M, Jessica B, Johnson K, Smeets H J M, Lennon G G, Carrano A V, Korneluk R G, Wieringa B, de Jong P J. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992;355:548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson S J, Stewart M. Molecular interactions in myosin assembly. Role of the 28-residue charge repeat in the rod. J Mol Biol. 1992;226:7–13. doi: 10.1016/0022-2836(92)90118-4. [DOI] [PubMed] [Google Scholar]
- 6.Bi E, Zigmond S H. Actin polymerization: where the WASP stings. Curr Biol. 1999;9:R160–R163. doi: 10.1016/s0960-9822(99)80102-x. [DOI] [PubMed] [Google Scholar]
- 7.Bishop A, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- 8.Bush E W, Helmke S M, Birnbaum R A, Perryman M B. Myotonic dystrophy protein kinase domains mediate localization, oligomerization, novel catalytic activity, and autoinhibition. Biochemistry. 2000;39:8480–8490. doi: 10.1021/bi992142f. [DOI] [PubMed] [Google Scholar]
- 9.Chan T O, Rittenhouse S E, Tsichlis P N. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 10.Chen X-Q, Tan I, Leung T, Lim L. The myotonic dystrophy kinase-related Cdc42-binding kinase is involved in the regulation of neurite outgrowth in PC12 cells. J Biol Chem. 1999;274:19901–19905. doi: 10.1074/jbc.274.28.19901. [DOI] [PubMed] [Google Scholar]
- 11.Chihara K, Amano M, Nakamura N, Yano T, Shibata M, Tokui T, Ichikawa H, Ikebe R, Ikebe M, Kaibuchi K. Cytoskeletal rearrangements and transcriptional activation of c-fos serum response element by Rho-kinase. J Biol Chem. 1997;272:25121–25127. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- 12.Ebinu J O, Bottorff D A, Chan E Y W, Stang S L, Dunn R J, Stone J C. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. 1998. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y-H, Pizzuti A, Fenwick G, Jr, King J, Rajnarayan S, Dunne P W, Dubel J, Nasser G A, Ashizawa T, de Jong P J, Wieringa B, Korneluk R, Perryman M B, Epstein H F, Caskey C T. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 14.Gallo G, Letourneau P C. Axon guidance: GTPases help axons reach their targets. Curr Biol. 1998;8:R80–R82. doi: 10.1016/s0960-9822(98)70051-x. [DOI] [PubMed] [Google Scholar]
- 15.Goto H, Kosako H, Tanabe K, Yanagida M, Sakurai M, Amano M, Kaibuchi K, Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- 16.Gulbins E, Coggeshall K M, Baier G, Telford D, Langlet C, Baier-Bitterlich G, Bonnefoy-Berard N, Burn P, Wittinghofer A, Altman A. Direct stimulation of Vav guanine nucleotide exchange activity for Ras by phorbol ester and diglycerides. Mol Cell Biol. 1994;14:4749–4758. doi: 10.1128/mcb.14.7.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 18.Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar W H, Matsumura F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 20.Kemp B E, Parker M W, Hu S, Tiganis T, House C. Substrate and pseudosubstrate interactions with protein kinases: determinants of specificity. Trends Biochem Sci. 1994;19:440–444. doi: 10.1016/0968-0004(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 21.Kosako H, Goto H, Yanagida M, Matsuzawa K, Fujita M, Tomono Y, Okigaki T, Odai H, Kaibuchi K, Inagaki M. Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesis: cleavage furrow-specific phosphorylation of intermediate filaments. Oncogene. 1999;18:2783–2788. doi: 10.1038/sj.onc.1202633. [DOI] [PubMed] [Google Scholar]
- 22.Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodeling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 24.Lam E. Domain analysis of the plant DNA-binding protein GT1a: requirement of four putative α-helices for DNA binding and identification of a novel oligomerization region. Mol Cell Biol. 1995;15:1014–1020. doi: 10.1128/mcb.15.2.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei M, Lu W, Meng W, Parrini M-C, Eck M J, Mayer B J, Harrison S C. Structure of PAK in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 26.Leung T, Chen X-Q, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung T, Chen X-Q, Tan I, Manser E, Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol. 1998;18:130–140. doi: 10.1128/mcb.18.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases: the p21 Ras-related Rho subfamily and its role in phosphorylation signaling pathways. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- 29.Luo L, Lee T, Tsai L, Tang G, Jan L Y, Jan Y-N. Genghis Khan (Gek) as a putative effector for Drosophila Cdc42 and regulator of actin polymerization. Proc Natl Acad Sci USA. 1997;94:12963–12968. doi: 10.1073/pnas.94.24.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda M, Taft C S, Bush E W, Emma H, Bailey W M, Neville H, Perryman M B, Bies R D. Identification, tissue-specific expression, and subcellular localization of the 80- and 71-kDa forms of myotonic dystrophy kinase protein. J Biol Chem. 1995;270:20246–20249. doi: 10.1074/jbc.270.35.20246. [DOI] [PubMed] [Google Scholar]
- 31.Manser E, Huang H-Y, Loo T-H, Chen X-Q, Dong J-M, Leung T, Lim L. Expression of constitutively active α-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine threonine kinase, as a putative target for the small GTP-binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 33.Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millward T, Hess D, Hemmings B A. Ndr protein kinase is regulated by phosphorylation on two conserved sequence motifs. J Biol Chem. 1999;274:33847–33850. doi: 10.1074/jbc.274.48.33847. [DOI] [PubMed] [Google Scholar]
- 35.Nakano K, Takaishi K, Kodama A, Mammoto A, Shiozaki H, Monden M, Takai Y. Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:2481–2491. doi: 10.1091/mbc.10.8.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palaty C K, Kalmar G, Tai G, Oh S, Amankawa L, Affolter M, Aebersold R, Pelech S L. Identification of the autophosphorylation sites of the Xenopus laevis Pim-1 proto-oncogene-encoded protein kinase. J Biol Chem. 1997;272:10514–10521. doi: 10.1074/jbc.272.16.10514. [DOI] [PubMed] [Google Scholar]
- 37.Read R D, Lionberger J M, Smithgall T E. Oligomerization of the Fes tyrosine kinase. Evidence for a coiled-coil domain in the unique N-terminal region. J Biol Chem. 1997;272:18498–18503. doi: 10.1074/jbc.272.29.18498. [DOI] [PubMed] [Google Scholar]
- 38.Sahai E, Ishizaki T, Narumiya S, Treisman R. Transformation mediated by RhoA requires activity of ROCK kinases. Curr Biol. 1999;11:136–145. doi: 10.1016/s0960-9822(99)80067-0. [DOI] [PubMed] [Google Scholar]
- 39.Sells M A, Knaus U G, Bagrodia S, Ambrose D M, Bokoch G M, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 40.Sin W C, Chen X-Q, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol. 1998;18:6325–6339. doi: 10.1128/mcb.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolias K F, Hartwig J H, Ishihara H, Shibasaki Y, Cantley L C, Carpenter C L. Type I alpha-phosphatidylinositol-4-phosphate-5-kinase mediates rac-dependent actin assembly. Curr Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- 42.Topham M K, Prescott S M. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J Biol Chem. 1999;274:11447–11450. doi: 10.1074/jbc.274.17.11447. [DOI] [PubMed] [Google Scholar]
- 43.Van Aelst L, Joneson T, Bar-Sagi D. Identification of a novel Rac1-interacting protein involved in membrane ruffling. EMBO J. 1996;15:3778–3786. [PMC free article] [PubMed] [Google Scholar]
- 44.Waring J D, Haq R, Tamai K, Sabourin L A, Ikeda J E, Korneluk R G. Investigation of myotonic dystrophy kinase isoform translocation and membrane association. J Biol Chem. 1996;271:15187–15193. doi: 10.1074/jbc.271.25.15187. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 46.Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94:277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 47.Wu S, Kaufman R J. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem. 1997;272:1291–1296. doi: 10.1074/jbc.272.2.1291. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Z-S, Manser E, Chen X-Q, Chong C, Leung T, Lim L. A conserved negative regulatory region in αPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]