Abstract
Over the last decade the use of measurable residual disease (MRD) diagnostics in adult acute lymphoblastic leukemia (ALL) has expanded from a limited number of study groups in Europe and the United States to a world-wide application. In this review, we summarize the advantages and drawbacks of the current available techniques used for MRD monitoring. Through the use of three representative case studies, we highlight the advances in the use of MRD in clinical decision-making in the management of ALL in adults. We acknowledge discrepancies in MRD monitoring and treatment between different countries, reflecting differing availability, accessibility and affordability.
Keywords: ALL, MRD, acute lymphoblastic leukemia, measurable residual disease, adult
1. INTRODUCTION
Acute lymphoblastic leukemia (ALL) is a clonally heterogeneous malignancy, defined by abnormal differentiation and proliferation of lymphoid precursors. In adults, approximately three quarters of cases develop from precursors of the B-cell lineage (B-ALL), with the remainder of cases consisting of malignant T-cell precursors (T-ALL). Significant strides in the biological understanding have led to cure rates exceeding 90% in the pediatric ALL cohort [1]. The adult setting continues to lag behind: while 70–90% of patients attain a complete remission (CR), relapse occurs in 40–50% of cases. Disease relapse remains a highly difficult clinical challenge, and the survival in this patient group remains poor [2].
In the era of development of new therapeutic options, the use of measurable residual disease (MRD) has never been more imperative. ALL was the first disease entity where MRD assessment was proven to be a fundamental tool to assess the efficacy of induction chemotherapy, to guide therapeutic options and to predict outcomes. MRD is now an established key factor for risk stratification and risk orientated therapy in both the setting of haemopoietic cell transplantation (HCT) and patients treated with chemotherapy alone. MRD allows the identification and quantification of sub-microscopic leukemic levels, and it serves as a predictor of potential relapse, which has been validated in a number of prospective studies.
2. METHODOLOGIES OF MRD DETECTION
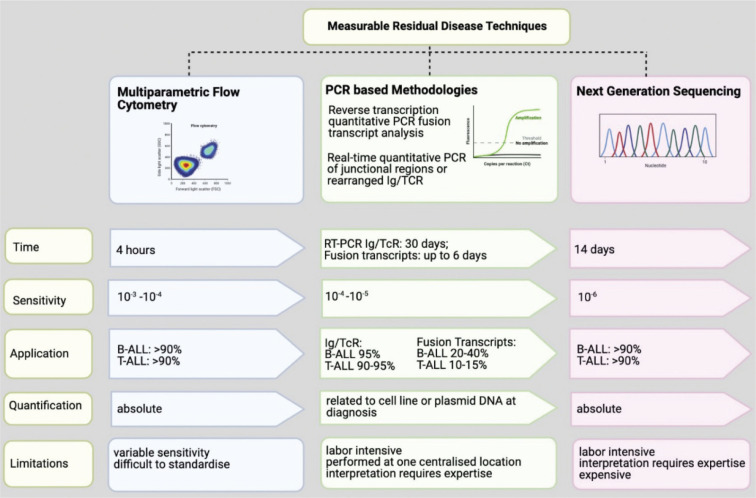
Measurable Residual Disease encompasses a broad spectrum of methodologies (Figure 1), with the ultimate aim being to detect and quantify residual leukemic cells with increased sensitivity, beyond what can be achieved by other available techniques such as morphology. The tissue source of choice for assessment is usually bone marrow (BM) for both B-ALL and T-ALL, despite reports in T-ALL that blood provides a representative population, as opposed to B-ALL where MRD levels in blood are known to markedly underestimate tumor burden [3].
Figure 1.
Key features of MRD detection diagnostics in ALL, including PCR methodologies, multiparameter flow cytometry and next generation sequencing.
For the purpose of this review, we will discuss polymerase chain reaction (PCR) for immunoglobulin (Ig) and T-cell receptor (TCR) gene rearrangements, PCR for fusion genes and transcripts, multiparameter flow cytometry (MPFC) and, next generation sequencing (NGS). A small single-center study claims that MRD data obtained with different techniques has negligible variation: whether by flow cytometry or quantitative PCR of patient-specific Ig/TCR gene rearrangements, the two methods are largely comparable, with 70–80% of samples with MRD levels >10−3 (≤3-fold difference) [4].
2.1. PCR for Ig and TCR Rearrangements
The European Study Group on MRD detection in ALL was established in 2001, and consists of 65 MRD–PCR laboratories across 25 countries. Their main aims were to develop and appraise new MRD strategies, and to develop robust guidelines for molecular analysis, frequency of testing and data interpretation of real-time quantitative PCR (qRT-PCR) MRD data with precise cut-offs to define both sensitivity of the assay and positivity or negativity of follow-up samples.
This is the most frequently used technique to assess MRD in western Europe. Ig or TCR rearrangements are physiological events that occur as a consequence of the somatic rearrangement process, during which a unique receptor sequence is derived through the random insertion and deletion of nucleotides at the junctional sites of V (variable) – D (Diversity) – J (Junction) gene segments. Consequently, during malignant lymphoid proliferation, all leukemic cells will express the same clonal receptor pattern, as they are specific to each patient, providing precise patient specific PCR targets for MRD monitoring [5]. Sensitivities of 10−5 can be achieved using this modality. Although Ig rearrangements are mostly found in B-cells and TCR rearrangements in T-lymphocytes, both B- and T-lineage leukemic cells can display cross-lineage rearrangements, which can be used for MRD evaluation [6].
The process involves identification of molecular markers from genomic DNA at diagnosis, via PCR amplification. These PCR products are usually subjected to heteroduplex analysis to distinguish between polyclonal and clonal leukemic-specific arrangements. By DNA sequencing of the clonal PCR fragments junctional regions are identified and defined. Also, more importantly, the latter allows to develop allele-specific oligonucleotide (ASO) primers used for qRT-PCR in MRD monitoring. MRD quantification of a follow up sample is derived from comparison of measured fluorescence to serial dilutions of diagnostic material in mononuclear cells derived from a pool of healthy donors, from which a standard curve was created. This technology can generate at least one single sensitive molecular probe suitable for MRD analysis in over 90% of pediatric and adult patients. The MRD result is expressed as the logarithmic reduction compared to diagnosis. To ensure the monitoring of possible multiple leukemic clones, at least two ASO primers are usually developed for each patient with a desirable sensitivity of 10−4 or 10−5, allowing sensitivities of 10−4 to 10−6 to be routinely achieved. This method has been standardized by the EuroMRD Consortium [7].
While Ig–TCR rearrangement monitoring is often considered the gold standard of molecular monitoring, there are some known limitations. The first is the quality and quantity of DNA obtained at diagnosis, as this is needed for each subsequent follow-up MRD experiment. The MRD result is documented as the quantification related to the diagnostic tumor load. In addition, the quality has to be adequate to create standard curves for each follow up evaluation. However, clonal evolution of the Ig–TCR rearrangement patterns [8] can occur throughout the disease course, due to secondary rearrangement processes. This can result in loss of PCR targets, mainly in Immunoglobulin Heavy Chain Gene (IGH) and, thus, false negative MRD results.
For approximately 5–10% adult ALL patients, it is not possible to perform MRD assessments, as they do not have leukemic-specific probes, either because of no Ig–TCR rearrangement detected at diagnosis or because the unique VDJ portion or the designed primer is not sufficiently specific or sensitive. Also, of note, some immature cells do not harbor Ig–TCR rearrangements making them unsuitable for analysis. Quantification categories for MRD monitoring include Positive outside quantifiable range (POQR). The latter would lead to clinical scenarios where treatment decisions may be made without a definitive quantification result as the precise tumor burden has not been quantified. In addition, there is much difficulty in precisely defining the amount of residual disease in situations where the disease burden is rather low.
2.2. PCR for Fusion Genes and Transcripts
Between 30% and 40% of ALL patients present with chromosomal translocations derived from fusion genes. Due to their nature as main driver events, present in all leukemic cells, they are ideal targets for fusion transcript analysis, providing excellent markers for MRD monitoring [9]. Unlike the Ig–TCR, this is a leukemia specific approach. The repertoire of chromosomal translocations which occurs in the pediatric cohort versus that of the adult population differs significantly. While ETV6-RUNX1 remains the most common fusion gene found in childhood ALL, BCR-ABL is the most common in adults, increasing with older age and occurring at a rate of 25–30% of cases per year [10,11].
Although DNA-based techniques have been developed [12], most laboratories use RNA to assess BCR-ABL transcript analysis, as it requires a small number of quantitative reverse transcriptase PCR assays, which are able to achieve high sensitivities of one in 105. The same primers can be used for all patients who have the same translocation, at diagnosis and during treatment monitoring, allowing efficient and rapid analysis. Accuracy can be an issue as there is marked patient variability in the number of RNA transcripts per leukemic cell and across different cell types within the leukemic clone. Thereafter, fusion gene levels detected are compared against a standard curve of plasmid DNA containing chimeric transcripts at fixed concentrations. While this technique is not standardized by EuroMRD, there are clear guidelines for quantification of fusion gene transcripts [13].
There are a range of qRT-PCR techniques used by an array of laboratories to determine BCR–ABL levels. Therefore, it is inevitable that there will be marked variation in the reported values. While this variation is not necessarily an issue when managing an individual, in a single center, it does limit the accuracy and makes the comparison of BCR-ABL values between laboratories difficult. An international reporting scale (IS) was established which removes the requirement to determine a baseline value. The IS expresses detectable disease as a percentage and is modelled on the scale used in the seminal IRIS trial [14]. The International BCR-ABL standardization group aims to improve the quality and comparability of BCR-ABL qRT-PCR testing.
2.3. Multiparameter Flow Cytometry Analysis
Globally this is the most accessible technique of MRD detection. Flow cytometry relies on differential antigen expression by B and T lymphoblasts. Immunophenotypic profile is determined through quantification of fluorescence emission by fluorochrome conjugation to specific monoclonal antibodies. This approach for MRD uses a panel of monoclonal antibodies that bind specifically to either surface, cytoplasmic or nuclear antigens to distinguish the clonal leukemic population, which can also be referred to as the leukemia-associated immunophenotype (LAIP). This method requires the initial identification of LAIP at diagnosis. Thereafter, the cell marker profile is then compared to a reference BM sample. There are other MPFC approaches which do not require knowledge of the prior diagnostic immunophenotype [15].
Despite efficient turnaround times, historically this was at a cost of reduced sensitivity in comparison to molecular techniques. However, significant developments and advances in signal processing and software have led to the increased capability of MPFC [16,17], which subsequently had a positive effect on increased sensitivity [18]. Limitations include the need for these samples to be assessed promptly, due to rapid cell death after collection. In addition, regeneration of hematopoiesis can lead to clinical conundrums as immature lymphoid cells can co-express ALL-associated antigens, which can, in fact, lead to false positives [19].
Standardization of MRD techniques form the basis for risk stratification of patients and for assessment of whether MRD-based treatment intervention is associated with improved outcomes. Within Europe, this standardization is supported by the European Commission via the EuroFlow Consortium [20].
2.4. Next Generation Sequencing
Although currently used in very few large centers, NGS may become the methodology of the future. It uses genomic DNA, which undergoes fragmentation, after which oligonucleotides are ligated to the fragment ends to complete the library preparation step. Thereafter, PCR amplification is undertaken, and the produced fragments are sequenced.
To balance for cost and complexity of processing, NGS must be able to exceed current thresholds of sensitivity, and routinely be able to deliver quantification at sensitivities of greater than 10−5, which qRT-PCR can reproducibly do. It provides an excellent platform for clinical cases with low levels of tumor burden. It is estimated that NGS MRD can quantify leukemic burden to a sensitivity level of 10−6 [21]; however, this is dependent on the quantity and quality of DNA available. This increase in sensitivity should theoretically have a positive impact on patient outcomes, given that detection of disease reoccurrence occurs at an earlier time interval. However, whether this will translate into a direct clinical impact, remains to be seen.
Several studies have highlighted the benefits of NGS in the MRD setting. A major one is its ability to re-detect and quantify clonal Ig–TCR sequences initially identified at diagnosis, at subsequent relapses. Most importantly, NGS provides an extensive insight into the residual leukemia burden via all Ig–TCR rearrangements, but also allows examination of entire immune repertoire [22]. In addition, there appears to be a role for MRD–NGS in the context of monitoring post HCT, as a predictor of relapse, due to the marked specificity of this technique [23]. A number of comparative analyses between qRT-PCR and NGS have shown MRD–NGS to be superior at precise prediction of relapse [24].
While at present this is not a routinely used methodology and there are currently no standardized protocols, and it may be some time before it is established as routine in mainstream clinical settings, it not only provides a precise modality to quantify MRD in cases where qRT-PCR is unable to do so, but also serves as an accurate predictor of disease relapse.
3. USE OF MRD IN CLINICAL MANAGEMENT
We discuss the use of MRD in three representative case studies. The first case is a T-ALL; the other two are patients with B-ALL one with Philadelphia negative (Ph−ve) and with the other Philadelphia positive (Ph+ve) disease.
3.1. Case 1: A Patient MRD Negative after Phase 2 Induction
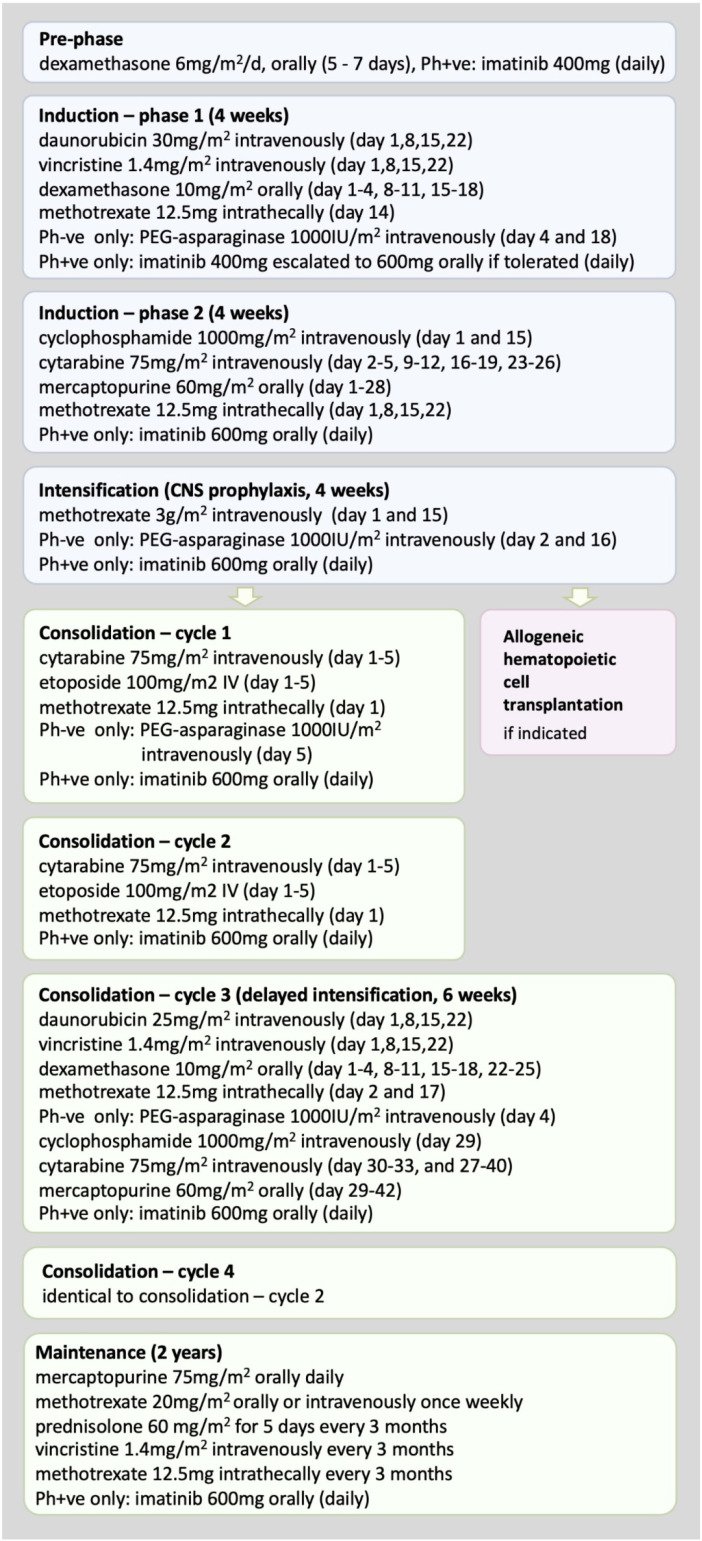
A 33-year-old male with no notable past medical history was diagnosed with T-ALL. The white blood cell (WBC) count at presentation was 143 × 109/L. The patient was commenced on combination chemotherapy as per the UKALL-14 protocol (Figure 2). Hematopoietic cell donor search was initiated, and his sibling was found to be a human leukocyte antigen (HLA) match. The patient achieved CR following phase 1, with no major complications during the treatment course. Thereafter, he completed standard phase-2 induction therapy uneventfully and, while awaiting results of MRD analysis determined by Ig–TCR monitoring, proceeded to high-dose methotrexate intensification. Subsequently, MRD negativity was reported with adequate sensitivity and acceptable quantitative range. The clinical decision was made to continue maintenance chemotherapy on the UKALL-14 protocol, undergoing three monthly MRD assessments.
Figure 2.
Schema of UKALL14 treatment protocol.
T-cell acute lymphoblastic leukemia represents approximately 25% of ALL cases and, although it has historically been associated with poorer outcomes, more recent studies have shown that this paradigm is shifting. The UKALL12/ECOG2993 trial data showed the estimated survival data was comparable for both T-ALL and B-ALL (48% versus 42% respectively, p = 0.07) [25]. Interestingly, the non-transplanted cohort also had similar overall survival (OS) at 5 years between both subtypes (40% versus 40%, p = 1.0).
Allogeneic HCT remains a fundamental component in the management of adult ALL. The UKALL12/ECOG2993 trial, which is the largest to date investigating the role of HCT in T-ALL, compared outcomes of patients with HLA-matched donors who were assigned to allograft versus patients who did not have a suitable donor and were randomized to either continue onto maintenance chemotherapy or an autologous stem cell transplant. The estimated 5-year survival in the T-ALL cohort was reported as 46% for the non-transplanted group and 61% for the transplanted group (p = 0.02), the difference being due to the reduced rate of relapse (51% versus 25%, respectively, p < 0.001) [25]. Furthermore, the reported relapse risk following HCT was similar between T-ALL and B-ALL. The integral value of allogeneic HCT as a part of first line therapy in young and fit patients using unrelated matched, cord blood and haploidentical donors is well described [26–33].
Despite the clear benefits of allogeneic HCT in this setting, the increased rates of non-relapse related mortality (NRM) must be taken into close consideration. Of note, NRM in the T-ALL sibling matched transplanted group from the UKALL12/ECOG2993 trial data was 22% at 5 years, which is in keeping with more recently published series [25,34]. The European Society for Blood and Marrow Transplantation (EBMT) devised a scoring system predicting survival and NRM in patients with chronic myeloid leukemia (CML) in 2011. This was later modified and applied to other hematologic malignancies including ALL [35]. Ultimately, these NRM estimations need to be complemented with clinical expertise and acumen and carefully balanced against the potential relapse risk.
Certain prognostic factors were identified as being integral to the risk stratification of ALL, prior to introduction of MRD monitoring. These included age, WBC at diagnosis, and the presence of characteristic cytogenetic markers [36–40]. For patients with T-ALL, treated on the UKALL12/ECOG2993 trial, there was no significant correlation between diagnostic WBC and survival. However, 96 of 356 patients (27%) with a WBC above 100 × 109/L did have a poorer survival at 5 years than patients with a WBC of less than 100 × 109/L (p = 0.03). Reduced 5-year survival rates were also seen in patients over the age of 35 years and in females (p = 0.004 and 0.05, respectively). Immunophenotypic markers associated with poorer survival included CD1a and CD13 positivity. Key cytogenetic findings such as a complex karyotype, t(11;14) and del(17p) were also associated with a reduced 5-year survival. Mutations in the NOTCH (NOTCH1/FBXW7) pathway in combination with other genetic aberrations have also been associated with improved outcomes in T-ALL [25]. The Group for Research on Adult ALL (GRAALL) reported significantly improved relapse rates and survival in those with NOTCH pathway mutations [41] and, although other groups have confirmed this association, the lack of statistical significance has limited its use in everyday clinical practice thus far [25,42].
The introduction of MRD monitoring has changed risk stratification significantly. The technique was initially introduced in pediatric ALL, and early prospective studies demonstrated its role in guiding treatment decisions [43–45]. These techniques were subsequently applied to adult ALL with promising results in small patient cohorts [46,47]. There have been comparatively fewer studies investigating the role of MRD specifically in T-ALL, likely due to its rarity in comparison to B-ALL. Krampera et al were the first to demonstrate the prognostic value of MRD monitoring in T-ALL. They used flow cytometric identification of a LAIP at fixed time points during the first year of therapy in 53 patients and found it to be a useful predictor of relapse at all measured time points [48]. Patel et al. [49] provided the first large-scale study investigating the role of MRD monitoring in Philadephia-negative adult B-ALL, in the setting of HCT [49]. Bassan et al. [50] were the first to attempt to utilize MRD status to guide treatment decisions in ALL. MRD was evaluated at weeks 10, 16 and 22 using PCR methodologies, achieving a sensitivity of 10−4. Thereafter, those failing to achieve MRD negativity were allografted, whereas MRD-negative patients received maintenance therapy, regardless of any other risk factors. Of note, only 112 of the initial 280 patients were MRD evaluable, of which a small proportion were T-ALL (n = 22). The 5-year disease free survival was significantly higher in MRD negative patients (72% versus 14%), and MRD positivity was reported as the most significant risk factor associated with disease relapse.
The German Multicenter Study Group for Adult ALL (GMALL) study risk-stratified patients. High-risk features included WBC >30 × 109/L in early or mature T-ALL, MLL-AF41 translocation or lack of cytological remission following induction therapy [51]. MRD monitoring was conducted at weeks 10 and 16 by Ig–TCR rearrangement methods. This showed that MRD negativity conferred a significantly higher probability of continuous complete remission (CCR, 74% versus 35%) and survival (80% versus 42%), regardless of risk subtype. This excluded the transplanted patient cohort. MRD-negative patients had a significantly increased chance of CCR and survival compared to MRD positive (70% versus 12%, p = 0.0001 and 81% versus 33%, p = 0.0001, respectively). They concluded that MRD was the only significant predictive factor for both relapse duration and survival, thus demonstrating that allogeneic HCT may be avoided in patients who achieve MRD negativity.
Group for Research on Adult ALL retrospectively evaluated 522 high-risk patients from the GRAALL-2003 and GRAALL-2005 trials, who either received allogeneic HCT, or maintenance therapy, and evaluated their outcomes based on MRD status during first CR [34]. Overall, they found no difference in relapse-free survival and overall survival between the HCT and non-HCT cohort. A subgroup analysis based on MRD status showed that MRD-positive patients had an increased likelihood of relapse-free survival if treatment was consolidated with a HCT (HR 0.4, p = 0.001). These outcomes were mirrored in the analysis of T-ALL patients, but did not achieve statistical significance. Thus, in conclusion, the MRD status was the most important tool to guide treatment decisions in first CR, and allografting should be reserved for those who fail to achieve MRD negativity.
Based on these data, we considered that the risk of relapse was relatively low in our patient who achieved a MRD-negative remission despite a high WBC at presentation. Furthermore, the risk of his NRM was not insignificant as his EBMT score was 2 (age above 20, female donor for male recipient). Therefore, we decided not to proceed to allogeneic HCT. The question remained as to whether he should be managed with chemotherapy alone or with an autologous HCT. Although the UKALL12/ECOG2993 trial showed superior outcomes with chemotherapy in patients with ALL in the intention to treat analysis, this was not observed in the subset of T-ALL patients [25]. This was possibly due to a relatively small number of patients (n = 99) with T-ALL who were randomized. Although the role of autologous transplantation in ALL might be reconsidered with the introduction of maintenance chemotherapy after autologous HCT [52] or in patients with a MRD negative remission [53], we recommended the chemotherapy option. We continue to monitor MRD after each cycle of chemotherapy, three-monthly during the maintenance phase and for 12 months post completion of treatment.
3.2. Case 2: A Patient with MRD Positivity after Phase 2 Induction
A 46-year-old female was diagnosed with B-ALL with a presenting WBC of 25 × 109/L, and was started on UKALL-14 treatment. She achieved CR after phase-1 induction with no significant issues during therapy. Phase-2 induction was complicated by septicemia caused by a fully sensitive Escherichia coli. BM after recovery showed complete morphological remission. The patient commenced intensification therapy but, subsequently, the MRD result was reported as positive by Ig–TCR monitoring, amid the intensification (methotrexate and asparaginase) course. She was found to have a fully HLA-matched unrelated donor. She received two cycles of blinatumomab, and achieved MRD negativity after the first cycle, which was reconfirmed after the second. She then proceeded to total body irradiation (TBI)-based myeloablative matched unrelated donor allograft. Three years after the transplantation she remains in CR with an undetectable MRD.
It is well established that MRD positivity at the end of the second phase of induction chemotherapy is the strongest independent prognostic indicator for disease relapse and poor survival, for which allogenic HCT in ALL patients under this predicament is recommended [34,54]. As in this patient’s case, MRD unfortunately remains detectable in approximately 30–50% of adult ALL patients in CR post-induction chemotherapy [2]. In the UKALL-12 trial, patients with B-ALL who were MRD positive post phase-2 induction had a relative risk of relapse of 8.95 (2.85–28.09)-fold higher than that of MRD negative patients. The 5-year relapse-free survival of MRD positive patients was also shown to be significantly lower; 15% [95% confidence interval (CI) 0–40%] compared to 71% (56–85%) in MRD-negative patients (p = 0.0002) [49]. The largest study to confirm poor prognosis associated with MRD positivity after initial chemotherapy was performed by GMALL and discussed in the previous case [51]. This patient therefore had a very high risk of relapse and needed allogeneic transplantation as supported by multiple studies previously discussed.
However, the MRD status prior to allogeneic HCT is also of great significance, as it correlates with overall patient outcomes and risk of relapse post transplantation. This has been reported in both small retrospective and prospective studies of children and adolescents and, subsequently, in the adult population [55–58]. This finding was further confirmed in a large meta-analysis by Shen et al and on a large registry analysis study performed by the EBMT [59,60]. The latter comprised 2780 patients (median age 38 years, range 18–72), who underwent a first myeloablative allograft in CR between 2000 and 2017. This confirmed that MRD positivity was a significant independent factor for lower overall survival, leukemia-free survival and for higher relapse incidence. This study also concluded that ALL patients, irrespective of their MRD status, benefited from TBI-based conditioning compared to chemotherapy conditioning [60]. Reduction of the risk of relapse is important, as treatment options for post-transplantation relapse are limited. A retrospective registry study which focused on the outcome of ALL patients who relapsed post-HCT, showed the median post-relapse survival was only 5.5 months and the estimated 5-year post relapse survival was 8% [61].
In an attempt to achieve remission prior to subsequent transplantation, we decided to administer blinatumomab to this patient. A phase-2 study investigated the efficacy of this bispecific (CD19/CD3) T-cell engager in MRD-positive B-ALL patients who were either molecularly refractory or had a molecular relapse after intensive chemotherapy. Of the total 21 patients who were treated, 16 (80%) achieved MRD negativity. Of these, 12 originally had refractory disease despite receiving multi-drug induction regimens and high dose consolidation chemotherapy. Therefore, in these patients who expressed molecular resistance to chemotherapy, blinatumomab was the first agent to induce molecular remission [62]. Similarly, a more recent multicenter phase-2 study, which included patients with MRD positivity after at least three cycles of intensive chemotherapy who then achieved a CR, showed high response rates of MRD to blinatumomab. Among the 113 evaluable cases out of the potential 116 enrolled patients who received blinatumomab, 78% were found to have a negative MRD result after just the first cycle of blinatumomab. Two additional patients achieved a complete MRD response after cycle 2; no additional patients achieved a complete MRD response after cycle 3 or cycle 4. MRD-negative patients achieved better survival (38.9 versus 12.5 months; p = 0.002) and relapse free survival (23.6 versus 5.7 months; p = 0.002) compared to those MRD positive [63].
Allogeneic HCT is still necessary after achieving MRD-negative remission in these patients. A large proportion (40–65%) of patients in studies of blinatumomab proceeded to allografting [62,63]. Of those who did not, only a small subset achieved durable long-time responses without transplantation. It was reported that, of the eleven patients in the initial Topp et al. [64] study, who had not received an allogeneic HCT, six remained in continued CR at a median follow-up of 31 months. Similarly, in the larger phase-2 trial, nine (25%) of 36 patients without HCT or chemotherapy after blinatumomab remained in continuous CR, with a median follow-up of 24.0 months (range, 2.8–41.6 months), whereas 36 (49%) of 74 patients with HCT remained in remission [63]. Despite its efficacy in eliminating MRD, global use of blinatumomab remains limited in this indication due to its cost, almost equating to full transplant costs in some countries. It is also not entirely clear if achieving MRD with blinatumomab reduces the relapse risk to that of patients who achieved MRD negativity with chemotherapy alone.
Our patient proceeded to transplantation when in MRD-negative remission and recovered well from the procedure. Although all was done to reduce the risk of recurrence, this risk still remained. Therefore, we monitored her MRD at day +100, and then at three-monthly intervals for 12 months. Although the value of post transplantation monitoring was first demonstrated in 1998 in children, there is little evidence for our schedule. Very early MRD results may not be helpful as they do not allow therapy adjustments, or there can be false positives due to amplification of comparable sequences in regenerated lymphocytes [65]. Investigators in Genoa performed a study of post allogeneic HCT MRD monitoring in 23 adults [46]. Four patients who were negative before transplantation remained so long term. Of the 19 pre-HCT MRD-positive patients, two died before transplantation, three became PCR-negative at first determination within 3 months of HCT and maintained their remission, and 16 were PCR-positive at first determination. Five of them became PCR-negative later (four with chronic graft-versus-host disease and two after donor lymphocyte infusions). Nine patients remained PCR-positive (four remained in remission, and six relapsed). Subsequently, large pediatric studies confirmed the prognostic value of MRD monitoring from day +60 onward [66]. Ideally, MRD monitoring should be accompanied by monitoring of donor chimerisms to guide immunological interventions such as earlier withdrawal of immunosuppression or administration of donor lymphocyte infusions [67]. Administration of blinatumomab maintenance after allogeneic HCT seems to be relatively well tolerated, but it is too early to conclude if this approach would prevent future relapses [68].
3.3. Case 3: A Patient MRD Positive after Allogeneic Transplantation
A 26-year-old female presented with fevers, dyspnea and bony pain. Her full blood count showed a WBC 110 × 109/L and the blood film confirmed the presence of lymphoblasts. Further tests led to diagnosis of Philadelphia positive (Ph+) ALL. She was started on UKALL14 phase-1 induction along with imatinib and achieved a complete morphological remission. She received phase-2 induction and MRD point was undetectable both on MPFC and qRT-PCR for BCR-ABL-1, at the end of this second cycle. She then received a high-dose methotrexate intensification phase. She underwent T-cell replete allogeneic HCT after myeloablative TBI-based conditioning using her HLA identical mother’s cells. She received imatinib maintenance after this with regular MRD monitoring. Twelve months after transplantation her BM qRT-PCR for BCR-ABL-1 became positive (0.035% IS) while on 400 mg of imatinib. No MRD was detectable by BM MPFC, but her qRT-PCR for BCR-ABL-1 was also positive in the blood (0.015% IS). The molecular positivity was confirmed on a second sample, and mutation analysis detected a T315I mutation. Ponatinib was started and she received a donor lymphocyte infusion. Four weeks later, she developed mucocutaneous graft versus host disease, coinciding with her PCR becoming negative. Her GvHD responded to a short course of prednisolone and ciclosporin. Ponatinib was discontinued after 6 months on her request and the ciclosporin dose tapered off over the same duration. Six years after transplantation she remains in molecular remission with limited chronic GvHD for which she uses topical corticosteroids.
As in other subtypes of ALL, the UKALL12/ECOG2993 trial showed superiority of allografting Ph+ patients compared to chemotherapy alone [69]. Addition of imatinib to the treatment regimen in the later stages of the trial improved outcomes and confirmed the superior results achieved with allografting [70]. A number of other studies have shown similar data, reiterating the benefit of allografting, particularly in young and fit adults [71,72]. With the addition of imatinib or other tyrosine kinase inhibitors (TKI), a large proportion of patients achieved a CR and could proceed to allogenic HCT. Introduction of TKIs have also seen a significant proportion of patients achieving MRD negativity prior to transplantation. Foa et al reported that MRD negativity after induction with dasatinib and corticosteroids correlated with disease-free survival [73]. Similar observation was made for patients treated with imatinib and chemotherapy in Japan [74,75]. Investigators in Korea showed that patients who achieved a molecular response after the first cycle of imatinib-based chemotherapy had lower relapse and higher disease-free survival rates after transplantation [76]. Failure to achieve MRD negativity prior to transplantation was associated with an increased risk of relapse in recipients of umbilical cord-blood grafts [77]. Therefore, in patients with detectable MRD, we advocate change to another TKI based on ABL kinase domain mutation analysis and/or addition of blinatumomab [63,78,79]. Allogenic HCT is a potent, long-established strategy, but limited by treatment-related morbidity and mortality, as well as donor availability. Therefore, in older, frail, or unwell patients, and in those who do not wish to undergo an allograft, other strategies should be considered. Investigators in Houston reported a 3-year event-free survival of 70% (95% CI 56–80) in 76 patients with median age of 47 years who had newly diagnosed Ph+ ALL, treated with a combination of ponatinib and intensive chemotherapy. This treatment was not without risk, three patients died from infection, one from a hemorrhage, and two from myocardial infarctions while on treatment [80]. Achieving MRD negativity is prognostically very important in this context; Short et al reported that 51 of 85 patients (60%) who became MRD negative at 3 months into intensive chemotherapy and TKI combination achieved a median survival of 127 months versus 38 months (p = 0.009) and relapse-free survival of 126 months versus 8 months (p = 0.007) compared to patients with detectable MRD. None of these patients received an allograft at first remission and their probability of survival at 4-years was 66% [81].
Patients with Ph+ ALL who achieve MRD negativity may benefit from autologous transplantation. A prospective trial by Chalandon et al. compared the outcome of 134 MRD-negative patients who received allogeneic HCT (n = 90) versus autologous transplantation (n = 29). Their relapse free survival and overall survival did not differ significantly [71]. An EBMT registry retrospective study compared outcomes of 502 patients who underwent myeloablative allografts with 67 who had autologous transplantation after achieving a MRD-negative remission. Probabilities of overall survival and leukemia-free survival after autologous, matched-related and matched-unrelated transplantation were 70% and 70%, 69% and 52%, and, 55% and 60%, respectively. The respective incidence of relapse at 2 years was 47%, 28% and 19% (p = 0.0002) [82].
Although this young patient with high-risk ALL achieved a MRD-negative CR and underwent a relatively uncomplicated allogenic HCT, her risk of relapse remained significant. MRD monitoring using qRT-PCR for BCR-ABL-1 has been routinely used after allogenic HCT transplantation for CML since the 1990s [83]. Using the same techniques, investigators in Seattle showed that detection of MRD after transplantation in patients with Ph+ ALL also identified those destined to have a hematological relapse [84]. As seen in CML, early administration of imatinib upon detection of MRD could prevent relapse in patients who are TKI responsive [85]. Due to reported relative safety of post-transplant administration of imatinib, investigators commonly use imatinib prophylaxis after transplantation in high-risk Ph+ leukemia [86,87]. Subsequent retrospective and prospective studies confirmed effectiveness of prophylactic imatinib in reducing the incidence of positive MRD [88].
Pfeifer et al. published a randomized comparison of prophylactic and MRD-triggered imatinib after allogeneic HCT on behalf of the German study group for ALL. Relapses occurred in two of 26 (8%) patients in the prophylactic arm and in five of 29 (17%) in the MRD-triggered imatinib group. These differences were not significant in this small study, and the probabilities of survival and event-free survival also did not differ significantly. Only 22% of patients received the intended imatinib dose of 600 mg daily, whereas the majority received 400 mg per day, and over 60% of patients in both groups discontinued imatinib prematurely [89]. Our patient did not tolerate imatinib doses higher than 400 mg. In keeping with the EBMT acute leukemia working party recommendations [88], upon detection of MRD we looked for the presence of ABL kinase domain mutations and identified a T315I mutation. Early treatment with ponatinib, a TKI known to be effective in patients with this mutation, was therefore started together with donor lymphocyte infusions.
We observed a discrepancy in the MRD monitoring results between MPFC, which was negative, and qRT-PCR for BCR-ABL-1, which was positive. To investigate this further, we requested an Ig–TCR-based MRD test on this patient, as the relevant primers were obtained at diagnosis. MRD was then reported as negative. Investigators in Prague [12] reported discordance between MRD monitoring using standard Ig–TCR gene rearrangements and detection of genomic breakpoint between BCR and ABL1 genes by DNA-based monitoring. Eight of 32 children with minor BCR-ABL-1 variants and one out of eight with major BCR-ABL-1 variants in different cohorts had >1 log higher levels of BCR-ABL-1 fusion than Ig–TCR rearrangements. This finding may indicate the presence of BCR-ABL-1 in cells other than lymphoblasts, suggesting that these patients may have CML presenting in lymphoid blast crisis. Similar discrepancies between BCR-ABL1 and Ig–TCR MRD monitoring was noted in adult patients [90].
4. MRD AND CHIMERIC ANTIGEN RECEPTOR T-CELLS
Relapse rate is high in adults with ALL. Fortunately, there have been marked developments in therapeutic options for relapsed B-ALL over the past decade, including the use of monoclonal antibodies, bispecific T-cell engagers, and chimeric antigen receptor T-cells (CAR-T). While these options lend themselves to MRD monitoring, the prognostic interpretation of MRD in this setting is not fully understood. CAR-T cells can be either patient- or donor-derived. In the latter case, donor T-cells are manipulated to express a T-cell receptor which exerts an anti-CD19 cytotoxic affect. Clinical studies in both the pediatric and adult setting have shown sustained MRD negativity up to 24 months using multiparameter flow cytometry [91]. Yet, conversely, disease relapse is also reported in MRD-negative patients, primarily due the mechanism of CD19 escape. Hence, while MRD monitoring in this setting is highly informative, its definitive role in this scenario is yet to be clearly defined. It is well evidenced that pre-transplant MRD status strongly correlates with clinical outcomes [55–58]. Therefore, certainly among high-risk stratified patients, subsequent allogenic HCT post CAR-T therapy may provide a promising approach.
5. CONCLUSION
Measurable residual disease assessment has been instrumental in evolving the landscape of ALL management, and has made a striking clinical impact, not only in becoming a key tool in prognostication and risk stratification, but moreover, imperative to clinical treatment decisions. MRD negativity is consistently associated with superior survival outcomes as compared to those of patients with MRD-positive status, and this trend is universally evident across ALL subtypes.
While molecular methods of detecting MRD are well established, and many clinicians regard this methodology as being the gold standard, the use of MPFC due to ease of accessibility and time efficiency is also becoming a more frequently used modality. NGS, which is not currently standardised, may however represent the future of MRD monitoring, given its ability to overcome certain limitations of current standard approaches.
As potential treatment paradigm shift in ALL, in the dawn of available immunotherapeutic options which undoubtedly will further improve MRD negativity rates, the role of MRD is yet to be fully understood in these particular clinical settings. However, it is clear that MRD assessment will certainly play a fundamental role in trial design, moving forward, given its ability to provide early evidence of treatment benefit.
ACKNOWLEDGMENTS
Centre for Haematology at Imperial College London received funding from the National Institute of Health Research (NIHR) Biomedical Research Centre (BRC) based at Imperial College London and Imperial College Healthcare NHS Trust.
Footnotes
Peer review under responsibility of the International Academy for Clinical Hematology
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
All authors contributed to the manuscript.
FUNDING
No financial support was provided.
REFERENCES
- [1].Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–52. doi: 10.1056/NEJMra1400972. https://pubmed.ncbi.nlm.nih.gov/26465987/ [DOI] [PubMed] [Google Scholar]
- [2].Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–50. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- [3].Campana D. Minimal residual disease in acute lymphoblastic leukemia. Semin Hematol. 2009;46:100–6. doi: 10.1053/j.seminhematol.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neale GAM, Coustan-Smith E, Pan Q, Chen X, Gruhn B, Stow P, et al. Tandem application of flow cytometry and polymerase chain reaction for comprehensive detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 1999;13:1221–6. doi: 10.1038/sj.leu.2401459. [DOI] [PubMed] [Google Scholar]
- [5].Cazzaniga G, Biondi A. Molecular monitoring of childhood acute lymphoblastic leukemia using antigen receptor gene rearrangements and quantitative polymerase chain reaction technology. Haematologica. 2005;90:382–90. https://pubmed.ncbi.nlm.nih.gov/15749670/ [PubMed] [Google Scholar]
- [6].Szczepański T, Beishuizen A, Pongers-Willemse MJ, Hählen K, Van Wering ER, Wijkhuijs AJM, et al. Cross-lineage T cell receptor gene rearrangements occur in more than ninety percent of childhood precursor-B acute lymphoblastic leukemias: alternative PCR targets for detection of minimal residual disease. Leukemia. 1999;13:196–205. doi: 10.1038/sj.leu.2401277. [DOI] [PubMed] [Google Scholar]
- [7].van der Velden VHJ, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–11. doi: 10.1038/sj.leu.2404586. [DOI] [PubMed] [Google Scholar]
- [8].Szczepański T, van der Velden VHJ, Raff T, Jacobs DCH, van Wering ER, Brüggemann M, et al. Comparative analysis of T-cell receptor gene rearrangements at diagnosis and relapse of T-cell acute lymphoblastic leukemia (T-ALL) shows high stability of clonal markers for monitoring of minimal residual disease and reveals the occurrence of second T-ALL. Leukemia. 2003;17:2149–56. doi: 10.1038/sj.leu.2403081. [DOI] [PubMed] [Google Scholar]
- [9].Gabert J, Beillard E, van der Velden VHJ, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia – A Europe Against Cancer Program. Leukemia. 2003;17:2318–57. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- [10].Fielding AK. Current treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2010;95:8–12. doi: 10.3324/haematol.2009.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bhojwani D, Pei D, Sandlund JT, Jeha S, Ribeiro RC, Rubnitz JE, et al. ETV6-RUNX1-positive childhood acute lymphoblastic leukemia: improved outcome with contemporary therapy. Leukemia. 2012;26:265–70. doi: 10.1038/leu.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hovorkova L, Zaliova M, Venn NC, Bleckmann K, Trkova M, Potuckova E, et al. Monitoring of childhood ALL using BCR-ABL1 genomic breakpoints identifies a subgroup with CML-like biology. Blood. 2017;129:2771–81. doi: 10.1182/blood-2016-11-749978. [DOI] [PubMed] [Google Scholar]
- [13].Pfeifer H, Cazzaniga G, van der Velden VHJ, Cayuela JM, Schäfer B, Spinelli O, et al. Standardisation and consensus guidelines for minimal residual disease assessment in Philadelphia-positive acute lymphoblastic leukemia (Ph + ALL) by real-time quantitative reverse transcriptase PCR of e1a2 BCR-ABL1. Leukemia. 2019;33:1910–22. doi: 10.1038/s41375-019-0413-0. [DOI] [PubMed] [Google Scholar]
- [14].Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–27. doi: 10.1056/NEJMoa1609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen X, Wood BL. Monitoring minimal residual disease in acute leukemia: technical challenges and interpretive complexities. Blood Rev. 2017;31:63–75. doi: 10.1016/j.blre.2016.09.006. [DOI] [PubMed] [Google Scholar]
- [16].De Rosa SC, Brenchley JM, Roederer M. Beyond six colors: a new era in flow cytometry. Nat Med. 2003;9:112–17. doi: 10.1038/nm0103-112. [DOI] [PubMed] [Google Scholar]
- [17].Preffer F, Dombkowski D. Advances in complex multiparameter flow cytometry technology: applications in stem cell research. Cytometry B Clin Cytom. 2009;76B:295–314. doi: 10.1002/cyto.b.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Denys B, van der Sluijs-Gelling AJ, Homburg C, van der Schoot CE, de Haas V, Philippé J, et al. Improved flow cytometric detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2013;27:635–41. doi: 10.1038/leu.2012.231. [DOI] [PubMed] [Google Scholar]
- [19].Dworzak MN, Gaipa G, Schumich A, Maglia O, Ratei R, Veltroni M, et al. Modulation of antigen expression in B-cell precursor acute lymphoblastic leukemia during induction therapy is partly transient: evidence for a drug-induced regulatory phenomenon. Results of the AIEOP-BFM-ALL-FLOW-MRD-Study Group. Cytometry B Clin Cytom. 2010;78B:147–53. doi: 10.1002/cyto.b.20516. [DOI] [PubMed] [Google Scholar]
- [20].Orfao A, Lopez A, Flores J, Almeida J, Vidriales B, Perez J, et al. Diagnosis of hematological malignancies: new applications for flow cytometry. Hematology (EHA Educ Program) 2006;2:6–13. [Google Scholar]
- [21].Ladetto M, Brüggemann M, Monitillo L, Ferrero S, Pepin F, Drandi D, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia. 2014;28:1299–307. doi: 10.1038/leu.2013.375. [DOI] [PubMed] [Google Scholar]
- [22].Kotrova M, Muzikova K, Mejstrikova E, Novakova M, Bakardjieva-Mihaylova V, Fiser K, et al. The predictive strength of next-generation sequencing MRD detection for relapse compared with current methods in childhood ALL. Blood. 2015;126:1045–7. doi: 10.1182/blood-2015-07-655159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kotrova M, van der Velden VHJ, van Dongen JJM, Formankova R, Sedlacek P, Brüggemann M, et al. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant. 2017;52:962–8. doi: 10.1038/bmt.2017.16. [DOI] [PubMed] [Google Scholar]
- [24].Della Starza I, Nunes V, Cavalli M, De Novi LA, Ilari C, Apicella V, et al. Comparative analysis between RQ-PCR and digital-droplet-PCR of immunoglobulin/T-cell receptor gene rearrangements to monitor minimal residual disease in acute lymphoblastic leukaemia. Br J Haematol. 2016;174:541–9. doi: 10.1111/bjh.14082. [DOI] [PubMed] [Google Scholar]
- [25].Marks DI, Paietta EM, Moorman AV, Richards SM, Buck G, DeWald G, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993) Blood. 2009;114:5136–45. doi: 10.1182/blood-2009-08-231217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–60. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ferrá C, Sanz J, de la Cámara R, Sanz G, Bermúdez A, Valcárcel D, et al. Unrelated transplantation for poor-prognosis adult acute lymphoblastic leukemia: long-term outcome analysis and study of the impact of hematopoietic graft source. Biol Blood Marrow Transplant. 2010;16:957–66. doi: 10.1016/j.bbmt.2010.02.003. [DOI] [PubMed] [Google Scholar]
- [28].Onishi Y, Sasaki O, Ichikawa S, Inokura K, Katsuoka Y, Ohtsuka Ohba R, et al. Favorable outcome of unrelated cord blood transplantation for Philadelphia chromosome–positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2011;17:1093–7. doi: 10.1016/j.bbmt.2011.01.010. [DOI] [PubMed] [Google Scholar]
- [29].Matsumura T, Kami M, Yamaguchi T, Yuji K, Kusumi E, Taniguchi S, et al. Allogeneic cord blood transplantation for adult acute lymphoblastic leukemia: retrospective survey involving 256 patients in Japan. Leukemia. 2012;26:1482–6. doi: 10.1038/leu.2012.11. [DOI] [PubMed] [Google Scholar]
- [30].Segal E, Martens M, Wang HL, Brazauskas R, Weisdorf D, Sandmaier BM, et al. Comparing outcomes of matched related donor and matched unrelated donor hematopoietic cell transplants in adults with B-cell acute lymphoblastic leukemia. Cancer. 2017;123:3346–55. doi: 10.1002/cncr.30737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kumar P, Defor TE, Brunstein C, Barker JN, Wagner JE, Weisdorf DJ, et al. Allogeneic hematopoietic stem cell transplantation in adult acute lymphocytic leukemia: impact of donor source on survival. Biol Blood Marrow Transplant. 2008;14:1394–400. doi: 10.1016/j.bbmt.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Basquiera AL, Berro M, Yantorno S, Castro M, Requejo A, Sorrentino M, et al. Haploidentical transplant in adult patients with acute lymphoblastic leukemia in Argentina: a comparison with matched related and unrelated donors. Bone Marrow Transplant. 2020;55:400–8. doi: 10.1038/s41409-019-0687-x. [DOI] [PubMed] [Google Scholar]
- [33].Han LJ, Wang Y, Fan ZP, Huang F, Zhou J, Fu YW, et al. Haploidentical transplantation compared with matched sibling and unrelated donor transplantation for adults with standard-risk acute lymphoblastic leukaemia in first complete remission. Br J Haematol. 2017;179:120–30. doi: 10.1111/bjh.14854. [DOI] [PubMed] [Google Scholar]
- [34].Dhédin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125:2486–96. doi: 10.1182/blood-2014-09-599894. quiz 2586. [DOI] [PubMed] [Google Scholar]
- [35].Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115:4715–26. doi: 10.1002/cncr.24531. [DOI] [PubMed] [Google Scholar]
- [36].Gaynor J, Chapman D, Little C, McKenzie S, Miller W, Andreeff M, et al. A cause-specific hazard rate analysis of prognostic factors among 199 adults with acute lymphoblastic leukemia: the Memorial Hospital experience since 1969. J Clin Oncol. 1988;6:1014–30. doi: 10.1200/JCO.1988.6.6.1014. [DOI] [PubMed] [Google Scholar]
- [37].Hoelzer D, Thiel E, Löffler H, Büchner T, Ganser A, Heil G, et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood. 1988;71:123–31. https://pubmed.ncbi.nlm.nih.gov/3422030/ [PubMed] [Google Scholar]
- [38].Kantarjian HM, Walters RS, Keating MJ, Smith TL, O’Brien S, Estey EH, et al. Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia. J Clin Oncol. 1990;8:994–1004. doi: 10.1200/JCO.1990.8.6.994. [DOI] [PubMed] [Google Scholar]
- [39].Hussein KK, Dahlberg S, Head D, Waddell CC, Dabich L, Weick JK, et al. Treatment of acute lymphoblastic leukemia in adults with intensive induction, consolidation, and maintenance chemotherapy. Blood. 1989;73:57–63. https://pubmed.ncbi.nlm.nih.gov/2642717/ [PubMed] [Google Scholar]
- [40].Secker-Walker LM, Prentice HG, Durrant J, Richards S, Hall E, Harrison G. Cytogenetics adds independent prognostic information in adults with acute lymphoblastic leukaemia on MRC trial UKALL XA. MRC Adult Leukaemia Working Party. Br J Haematol. 1997;96:601–10. doi: 10.1046/j.1365-2141.1997.d01-2053.x. [DOI] [PubMed] [Google Scholar]
- [41].Ben Abdelali R, Asnafi V, Leguay T, Boissel N, Buzyn A, Chevallier P, et al. Pediatric-inspired intensified therapy of adult T-ALL reveals the favorable outcome of NOTCH1/FBXW7 mutations, but not of low ERG/BAALC expression: a GRAALL study. Blood. 2011;118:5099–107. doi: 10.1182/blood-2011-02-334219. [DOI] [PubMed] [Google Scholar]
- [42].Mansour MR, Sulis ML, Duke V, Foroni L, Jenkinson S, Koo K, et al. Prognostic implications of NOTCH1 and FBXW7 mutations in adults with T-cell acute lymphoblastic leukemia treated on the MRC UKALLXII/ECOG E2993 protocol. J Clin Oncol. 2009;27:4352–6. doi: 10.1200/JCO.2009.22.0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cavé H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer—Childhood Leukemia Cooperative Group. N Engl J Med. 1998;339:591–8. doi: 10.1056/NEJM199808273390904. [DOI] [PubMed] [Google Scholar]
- [44].van Dongen JJM, Seriu T, Panzer-Grümayer ER, Biondi A, Pongers-Willemse MJ, Corral L, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731–8. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- [45].Coustan-Smith E, Behm FG, Sanchez J, Boyett JM, Hancock ML, Raimondi SC, et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet. 1998;351:550–4. doi: 10.1016/S0140-6736(97)10295-1. [DOI] [PubMed] [Google Scholar]
- [46].Miglino M, Berisso G, Grasso R, Canepa L, Clavio M, Pierri I, et al. Allogeneic bone marrow transplantation (BMT) for adults with acute lymphoblastic leukemia (ALL): predictive role of minimal residual disease monitoring on relapse. Bone Marrow Transplant. 2002;30:579–85. doi: 10.1038/sj.bmt.1703659. [DOI] [PubMed] [Google Scholar]
- [47].Mortuza FY, Papaioannou M, Moreira IM, Coyle LA, Gameiro P, Gandini D, et al. Minimal residual disease tests provide an independent predictor of clinical outcome in adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20:1094–104. doi: 10.1200/JCO.2002.20.4.1094. [DOI] [PubMed] [Google Scholar]
- [48].Krampera M, Vitale A, Vincenzi C, Perbellini O, Guarini A, Annino L, et al. Outcome prediction by immunophenotypic minimal residual disease detection in adult T-cell acute lymphoblastic leukaemia. Br J Haematol. 2003;120:74–9. doi: 10.1046/j.1365-2141.2003.03974.x. [DOI] [PubMed] [Google Scholar]
- [49].Patel B, Rai L, Buck G, Richards SM, Mortuza Y, Mitchell W, et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol. 2010;148:80–9. doi: 10.1111/j.1365-2141.2009.07941.x. [DOI] [PubMed] [Google Scholar]
- [50].Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL) Blood. 2009;113:4153–62. doi: 10.1182/blood-2008-11-185132. [DOI] [PubMed] [Google Scholar]
- [51].Gökbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120:1868–76. doi: 10.1182/blood-2011-09-377713. [DOI] [PubMed] [Google Scholar]
- [52].Doubek M, Folber F, Koristek Z, Brychtova Y, Krejci M, Tomiska M, et al. Autologous hematopoietic stem cell transplantation in adult acute lymphoblastic leukemia: still not out of fashion. Ann Hematol. 2009;88:881–7. doi: 10.1007/s00277-009-0700-3. [DOI] [PubMed] [Google Scholar]
- [53].Giebel S, Stella-Holowiecka B, Krawczyk-Kulis M, Gökbuget N, Hoelzer D, Doubek M, et al. Status of minimal residual disease determines outcome of autologous hematopoietic SCT in adult ALL. Bone Marrow Transplant. 2010;45:1095–101. doi: 10.1038/bmt.2009.308. [DOI] [PubMed] [Google Scholar]
- [54].Giebel S, Marks DI, Boissel N, Baron F, Chiaretti S, Ciceri F, et al. Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 2019;54:798–809. doi: 10.1038/s41409-018-0373-4. [DOI] [PubMed] [Google Scholar]
- [55].Knechtli CJ, Goulden NJ, Hancock JP, Grandage VL, Harris EL, Garland RJ, et al. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998;92:4072–9. https://pubmed.ncbi.nlm.nih.gov/9834212/ [PubMed] [Google Scholar]
- [56].Bader P, Hancock J, Kreyenberg H, Goulden NJ, Niethammer D, Oakhill A, et al. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16:1668–72. doi: 10.1038/sj.leu.2402552. [DOI] [PubMed] [Google Scholar]
- [57].Sramkova L, Muzikova K, Fronkova E, Krejci O, Sedlacek P, Formankova R, et al. Detectable minimal residual disease before allogeneic hematopoietic stem cell transplantation predicts extremely poor prognosis in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48:93–100. doi: 10.1002/pbc.20794. [DOI] [PubMed] [Google Scholar]
- [58].Spinelli O, Peruta B, Tosi M, Guerini V, Salvi A, Zanotti MC, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. 2007;92:612–18. doi: 10.3324/haematol.10965. [DOI] [PubMed] [Google Scholar]
- [59].Shen Z, Gu X, Mao W, Yin L, Yang L, Zhang Z, et al. Influence of pre-transplant minimal residual disease on prognosis after Allo-SCT for patients with acute lymphoblastic leukemia: systematic review and meta-analysis. BMC Cancer. 2018;18:755. doi: 10.1186/s12885-018-4670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pavlů J, Labopin M, Niittyvuopio R, Socié G, Yakoub-Agha I, Wu D, et al. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J Hematol Oncol. 2019;12:108. doi: 10.1186/s13045-019-0790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–17. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- [62].Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–8. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- [63].Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–31. doi: 10.1182/blood-2017-08-798322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Topp MS, Goekbuget N, Zugmaier G, Viardot A, Stelljes M, Neumann S, et al. Anti-CD19 BiTE blinatumomab induces high complete remission rate and prolongs overall survival in adult patients with relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL) Blood. 2012;120:670. doi: 10.1182/blood.V120.21.670.670. [DOI] [Google Scholar]
- [65].Fronkova E, Muzikova K, Mejstrikova E, Kovac M, Formankova R, Sedlacek P, et al. B-cell reconstitution after allogeneic SCT impairs minimal residual disease monitoring in children with ALL. Bone Marrow Transplant. 2008;42:187–96. doi: 10.1038/bmt.2008.122. [DOI] [PubMed] [Google Scholar]
- [66].Bader P, Kreyenberg H, von Stackelberg A, Eckert C, Salzmann-Manrique E, Meisel R, et al. Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. J Clin Oncol. 2015;33:1275–84. doi: 10.1200/JCO.2014.58.4631. [DOI] [PubMed] [Google Scholar]
- [67].Terwey TH, Hemmati PG, Nagy M, Pfeifer H, Gökbuget N, Brüggemann M, et al. Comparison of chimerism and minimal residual disease monitoring for relapse prediction after allogeneic stem cell transplantation for adult acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2014;20:1522–9. doi: 10.1016/j.bbmt.2014.05.026. [DOI] [PubMed] [Google Scholar]
- [68].Kebriaei P, Banerjee PP, Ganesh C, Kaplan M, Nandivada V, Cortes AKN, et al. Blinatumomab is well tolerated maintenance therapy following allogeneic hematopoietic cell transplantation for acute lymphoblastic leukemia. Blood. 2019;134:1298. doi: 10.1182/blood-2019-125931. [DOI] [Google Scholar]
- [69].Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113:4489–96. doi: 10.1182/blood-2009-01-199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fielding AK, Rowe JM, Buck G, Foroni L, Gerrard G, Litzow MR, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123:843–50. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chalandon Y, Thomas X, Hayette S, Cayuela JM, Abbal C, Huguet F, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125:3711–19. doi: 10.1182/blood-2015-02-627935. [DOI] [PubMed] [Google Scholar]
- [72].Ravandi F, Othus M, O’Brien SM, Forman SJ, Ha CS, Wong JYC, et al. US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in Philadelphia chromosome positive ALL. Blood Adv. 2016;1:250–9. doi: 10.1182/bloodadvances.2016001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Foà R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood. 2011;118:6521–8. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- [74].Mizuta S, Matsuo K, Maeda T, Yujiri T, Hatta Y, Kimura Y, et al. Prognostic factors influencing clinical outcome of allogeneic hematopoietic stem cell transplantation following imatinib-based therapy in BCR-ABL-positive ALL. Blood Cancer J. 2012;2:e72. doi: 10.1038/bcj.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nishiwaki S, Imai K, Mizuta S, Kanamori H, Ohashi K, Fukuda T, et al. Impact of MRD and TKI on allogeneic hematopoietic cell transplantation for Ph+ALL: a study from the adult ALL WG of the JSHCT. Bone Marrow Transplant. 2016;51:43–50. doi: 10.1038/bmt.2015.217. [DOI] [PubMed] [Google Scholar]
- [76].Lee S, Kim DW, Cho BS, Yoon JH, Shin SH, Yahng SA, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;26:2367–74. doi: 10.1038/leu.2012.164. [DOI] [PubMed] [Google Scholar]
- [77].Tucunduva L, Ruggeri A, Sanz G, Furst S, Cornelissen J, Linkesch W, et al. Impact of minimal residual disease on outcomes after umbilical cord blood transplantation for adults with Philadelphia-positive acute lymphoblastic leukaemia: an analysis on behalf of Eurocord, Cord Blood Committee and the Acute Leukaemia working party of the European group for Blood and Marrow Transplantation. Br J Haematol. 2014;166:749–57. doi: 10.1111/bjh.12970. [DOI] [PubMed] [Google Scholar]
- [78].Foà R, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo MC, et al. Dasatinib–blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383:1613–23. doi: 10.1056/NEJMoa2016272. [DOI] [PubMed] [Google Scholar]
- [79].Couturier MA, Thomas X, Huguet F, Berthon C, Simand C, Hicheri Y, et al. Blinatumomab + ponatinib for relapsed Ph1-positive acute lymphoblastic leukemia: the french experience. Blood. 2018;132:4014. doi: 10.1182/blood-2018-99-111546. [DOI] [Google Scholar]
- [80].Jabbour E, Short NJ, Ravandi F, Huang X, Daver N, DiNardo CD, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. 2018;5:e618–e27. doi: 10.1016/S2352-3026(18)30176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Short NJ, Jabbour E, Sasaki K, Patel K, O’Brien SM, Cortes JE, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128:504–7. doi: 10.1182/blood-2016-03-707562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Giebel S, Labopin M, Potter M, Poiré X, Sengeloev H, Socié G, et al. Comparable results of autologous and allogeneic haematopoietic stem cell transplantation for adults with Philadelphia-positive acute lymphoblastic leukaemia in first complete molecular remission: an analysis by the Acute Leukemia Working Party of the EBMT. Eur J Cancer. 2018;96:73–81. doi: 10.1016/j.ejca.2018.03.018. [DOI] [PubMed] [Google Scholar]
- [83].Pavlů J, Szydlo RM, Goldman JM, Apperley JF. Three decades of transplantation for chronic myeloid leukemia: what have we learned?. Blood. 2011;117:755–63. doi: 10.1182/blood-2010-08-301341. [DOI] [PubMed] [Google Scholar]
- [84].Radich J, Gehly G, Lee A, Avery R, Bryant E, Edmands S, et al. Detection of BCR-ABL transcripts in Philadelphia chromosome-positive acute lymphoblastic leukemia after marrow transplantation. Blood. 1997;89:2602–9. https://pubmed.ncbi.nlm.nih.gov/9116308/ [PubMed] [Google Scholar]
- [85].Wassmann B, Pfeifer H, Stadler M, Bornhaüser M, Bug G, Scheuring UJ, et al. Early molecular response to posttransplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2005;106:458–63. doi: 10.1182/blood-2004-05-1746. [DOI] [PubMed] [Google Scholar]
- [86].Anderlini P, Sheth S, Hicks K, Ippoliti C, Giralt S, Champlin RE. Re: Imatinib mesylate administration in the first 100 days after stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:883–4. doi: 10.1016/j.bbmt.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [87].Carpenter PA, Snyder DS, Flowers MED, Sanders JE, Gooley TA, Martin PJ, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109:2791–3. doi: 10.1182/blood-2006-04-019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Giebel S, Czyz A, Ottmann O, Baron F, Brissot E, Ciceri F, et al. Use of tyrosine kinase inhibitors to prevent relapse after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a position statement of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer. 2016;122:2941–51. doi: 10.1002/cncr.30130. [DOI] [PubMed] [Google Scholar]
- [89].Pfeifer H, Wassmann B, Bethge W, Dengler J, Bornhäuser M, Stadler M, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27:1254–62. doi: 10.1038/leu.2012.352. [DOI] [PubMed] [Google Scholar]
- [90].Clappier E, Kim R, Cayuela JM, Rousselot P, Chalandon Y, Passet M, et al. Persistent BCR-ABL1 clonal hematopoiesis after blast clearance identifies a CML-like subgroup of patients with Philadelphia chromosome-positive (Ph+) ALL: interim results from GRAAPH-2014 trial. Proceedings of the 23rd EHA Annual Congress Stockolm; 2018; Stockholm, Sweden. European Hematology Association; p. S1568. [Google Scholar]
- [91].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]