Abstract
The sterol regulatory element-binding protein 2 (SREBP-2), a transcription factor of the basic helix-loop-helix-leucine zipper (bHLH-Zip) family, is synthesized in the form of a membrane-attached precursor molecule. When cells are deprived of sterols, a two-step proteolytic processing releases the transcriptionally active N-terminal segment of SREBP-2, thereby allowing it to enter the nucleus. In previous studies, we showed that the nuclear import of SREBP-2 occurs via the direct interaction of importin β with the HLH-Zip domain. In this study, in order to more completely understand the intracellular dynamics of SREBP-2, we focused on the manner by which importin β recognizes SREBP-2 at the initial step of the import. It was found that the active form of SREBP-2 exists as a stable dimer in solution and that the substitution of leucine residues for alanine in the leucine zipper motif disrupted the dimerization. It was also demonstrated that this mutant protein did not enter the nucleus either in vivo or in vitro. Solution binding assays, which involved the chemical cross-linking of wild-type or mutated SREBP-2 with importin β, revealed that the import-active complex appeared to be composed of a dimeric form of SREBP-2 and importin β. In addition, the SREBP-2 binding domain of importin β corresponded to an overlapping but not identical region for importin α binding, which may explain how importin β is able to recognize the dimeric HLH-Zip directly. These results indicate that dimerization is a prerequisite process for the nuclear import of SREBP-2 mediated by importin β.
Proteins that are actively transported into or out of the nucleus contain a nuclear localization signal (NLS) or a nuclear export signal, respectively. The shuttling transport receptors largely bind their cargoes via these signals in one side of the nuclear envelope, mediate their translocation through the nuclear pore complex (NPC), and then release them on the other side. An increasing number of signals and their cognate transport receptors have been identified in recent years, and it has been concluded that both import and export pathways are mediated by a related family of transport receptors, which is referred to as the importin β superfamily (for reviews, see references 7, 12, 31, 33, and 34).
The individual family members share the N-terminal motif, which accounts for binding the nuclear pore proteins (nucleoporins) and the small GTPase Ran (37, 51). By interacting with some nucleoporins, each family member translocates through the NPC. The interaction of Ran and the receptor regulates the binding affinity of the cargo-receptor complex. Specifically, complexes of the import receptor and cargo dissociate when Ran-GTP is encountered, and conversely, export receptors require Ran-GTP in order to successfully complex with the export substrates (10, 14, 26, 27, 39). Since the nucleotide exchange factor for Ran, RCC1, is located in the nucleus, while the GTPase-activating protein, RanGAP1, is cytoplasmic, it would be predicted that nuclear Ran exists predominantly as the GTP-bound form and cytoplasmic Ran mainly as the GDP-bound form. Therefore, the nucleus would be favored for releasing the import cargo as well as loading the export cargo. In this respect, the Ran GTPase cycle determines the identity of the nuclear or cytoplasmic compartment, promoting the directionality of transport (20). Indeed, nuclear import and export pathways which are mediated by importin β-related receptors identified thus far are exclusively dependent on the GTPase cycle of Ran (reviewed in references 33 and 54).
Importin β is one of the best characterized import receptors and has been found to be unique in the receptor family because it recognizes nuclear proteins not only via adapter molecules, such as importin α, but also directly. The nuclear import of a classical NLS-containing substrate represents the adapter-dependent pathway. That is, a classical NLS-containing substrate forms a complex with the importin α/β heterodimer, where importin α acts as an adapter which binds the NLS and mediates the interaction with importin β. The resultant heterotrimer docks at the NPC and is translocated through the central channel owing to the direct interaction of importin β with the nucleoporins (reviewed in references 13 and 52). A variation of the adapter-dependent pathway has recently been illustrated by the import of histone H1, in which two of the β-family members, importin 7 and importin β, heterodimerize and serve as an import receptor. Although importin 7 and importin β both contribute to the recognition of H1 and each contacts its distinct domain, the binding of Ran-GTP triggers the release of the importin 7-H1 complex. As a result, importin 7 is considered to be an adapter in the heterotrimer (21).
An adapter-free pathway for nuclear import by importin β was first demonstrated through the finding that the fusion protein, which contains an importin β binding (IBB) domain of importin α, is imported into the nucleus by importin β. This shows that the adapter itself is a potent cargo, of which the IBB domain serves as an NLS to be recognized by importin β (11, 50). Snurportin, which tethers the uracil-rich snRNP (U snRNP) to importin β, is another adapter that carries the IBB domain sequence (17). In addition to adapter molecules, evidence has accumulated in recent years to show that importin β transports a wide variety of cargoes with distinct signals via direct interaction. These involve certain viral proteins, such as human immunodeficiency virus type 1 Tat and Rev, which are characterized by the presence of an arginine-rich NLS (15, 35, 48). Ribosomal protein rpL23a represents another class of substrate, which contains an extremely basic import signal, the so-called BIB domain, and can be imported not only by importin β, but also by at least three other β-like transport receptors (22).
Studies on the import mechanisms utilized by proteins with no canonical NLS have extended our understanding of nuclear transport. Among those is the nuclear import of sterol regulatory element-binding protein 2 (SREBP-2), which we have characterized previously. SREBP-2 is a member of the SREBP family of transcription factors, which contain the basic helix-loop-helix-leucine zipper (bHLH-Zip) motif (reviewed in reference 5). Unlike other bHLH-Zip transcription factors, SREBPs are synthesized as precursors which are bound to the endoplasmic reticulum (ER) membrane and outer nuclear envelope in a hairpin orientation, with the N- and C-terminal segments projecting into the cytoplasm and the hydrophilic loop projecting into the lumen. The cytosolic N-terminal segment includes the bHLH-Zip domain, while the C-terminal regulatory segment interacts with a polytopic membrane protein, which is designated SCAP (SREBP cleavage-activating protein) (41, 42). When cells are deprived of cholesterol, SCAP escorts SREBPs to a post-ER component to reach the Golgi apparatus, where the Site-1 protease (S1P) makes the first cut in the luminal loop, followed by a second cleavage at site 2 within the first membrane-spanning segment (8, 38, 43). This proteolytic processing liberates the transcriptionally active N-terminal fragment, designated as the active form of SREBPs, from the membrane. The active forms of SREBPs enter the nucleus and activate a number of genes which control the synthesis and uptake of cholesterol and unsaturated fatty acids.
We recently reported evidence which shows that the nuclear import of the mature form of SREBP-2 is carried out through the direct interaction with importin β in a Ran-dependent manner. The importin β-binding domain of SREBP-2 was mapped on the HLH-Zip domain, which serves as an NLS (32). It is interesting that the HLH-Zip of SREBP-2 has little similarity with currently identified importin β-binding signals. Therefore, these results raise questions as to whether the HLH-Zip represents a distinct class of importin β substrate and how importin β complexes with and carries these distinct classes of cargoes.
In this study, in an attempt to understand the intracellular behavior of SREBP-2 more comprehensively, we focused on the recognition of the HLH-Zip by importin β at the initial step of import. Using binding assays, import assays, and chemical cross-linking experiments, we herein demonstrate that importin β recognizes the dimerized HLH-Zip via a region that overlaps but is not identical to the importin α binding site. Our results provide direct evidence that HLH-Zip of SREBP-2 serves as a novel type of NLS which becomes functional on dimerization.
MATERIALS AND METHODS
Construction of plasmids and expression and purification of recombinant proteins.
Plasmids encoding Flag-tagged SREBP-2(1–481), green fluorescent protein (GFP)-tagged SREBP-2(343–403), and His-tagged SREBP-2(1–481) [pGEX FL-SREBP2, pGEXGFP-SREBP2(343–403), and pRSETA-SREBP2, respectively] have been described previously (32). The construct for the in vitro transcription and translation of SREBP-2, pET28b-SREBP2, was generated by cloning the XhoI-NotI fragment from pSREBP2(1–481) (44) containing SREBP-2(1–481) coding sequence into the SalI and NotI sites of pET-28b(+) (Novagen). To generate the expression vectors for mutant SREBP-2 bearing triple amino acid substitutions in the leucine zipper, pGEX FL-SREB2/L1.2.3A and pET28b-SREBP2/L1.2.3A, site-directed in vitro mutagenesis was performed by using a QuickChange site-directed mutagenesis kit (Stratagene) with the following oligonucleotide primers: for L380A, 5′-GATTACATCAAATATGCGCAGCAGGTC-3′; for L387A, 5′-GTCAATCATAAAGCGCGCCAGGAGAACATG-3′; and for L394A, 5′-GAGAACATGGTGGCGAAGCTGGCAAATC-3′. The expression vectors which encode the glutathione-S-transferase (GST) chimeras of full-length mouse importin β and importin β fragments (residues 1 to 768, 1 to 643, 1 to 449, 145 to 876, and 448 to 876) have been described previously (25). The construct which encodes GST-importin β(1–602) was derived from pGEX HA-PTAC97 (25) by cutting out the BglII-KpnI fragment, followed by blunting and ligation. To generate the GST-importin β(226–876) expression vector, an amplified fragment encoding importin β(226–448) was inserted into the BamHI and AccI sites of pGEX HA-PTAC97. To construct the plasmid which expresses the IBB domain as a maltose-binding protein (MBP) fusion protein, pMALC2-IBB, mouse importin α(1–65) coding sequence was amplified using the appropriate primer pairs that introduce 5′ BamHI and 3′ SalI sites and cloned into the BamHI and SalI sites of pMAL-c2 (New England Biolabs).
The following proteins were expressed in Escherichia coli and purified as described previously: Flag-SREBP-2, Flag-SREBP-2/L1.2.3A, and GST-NLS-GFP (32); GST-importin β full-length and deletion series (25); hemagglutinin-tagged importin β (18); Ran (16); and p10/NTF2 (47). MBP-IBB protein was produced in E. coli strain BL21 by induction with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 12 h at 20°C and purified using an amylose resin according to the manufacturer's recommendations, followed by dialysis against 20 mM HEPES-KOH (pH 7.3)–110 mM potassium acetate–2 mM dithiothreitol (DTT)–1 μg/ml each aprotinin, leupeptin, and pepstatin. Aliquots of each recombinant protein were frozen in liquid nitrogen and stored at −80°C.
Binding assays using recombinant proteins.
Binding assays using an E. coli lysate which expresses His-SREBP-2 and GST fusion proteins were performed as described previously (32).
To detect the interaction between GST-importin β and GFP–HLH-Zip, 70 pmol of purified GST (≈2 μg) or GST-importin β (≈9 μg) was incubated with 70 pmol of GFP-SREBP-2(343–403) (≈2.5 μg) in binding buffer A (20 mM HEPES-KOH [pH 7.3], 110 mM potassium acetate, 2 mM DTT) to a final volume of 50 μl for 30 min on ice. After centrifugation at 15,000 rpm for 20 min to remove any insoluble materials, the supernatants were incubated with 5 μl of glutathione-Sepharose (Amersham Pharmacia) for 1 h at 4°C on a turntable. After five washes with binding buffer A, the bound proteins were eluted by boiling in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, separated on SDS–10% PAGE, and visualized by Coomassie blue staining. For competition by IBB, binding assays were performed in the presence of 70 pmol (≈4 μg) of purified MBP-IBB.
A binding assay using different concentrations of purified wild-type or mutant Flag-SREBP-2 was performed as follows. Purified Flag-SREBP-2 or Flag-SREBP-2/L1.2.3A (25 nM or 1.6 μM in a total volume of 50 μl) was incubated with either GST or GST-importin β (1 μM) in the presence of bovine serum albumin (BSA) (10 mg/ml) and 5 μl of glutathione-Sepharose beads in binding buffer B (20 mM HEPES-KOH [pH 7.3], 110 mM potassium acetate, 200 mM NaCl, 2 mM DTT) for 20 min at room temperature. After incubation, the beads were collected by centrifugation and washed extensively with binding buffer B, and the bound proteins were eluted by boiling in SDS-PAGE sample buffer. Proteins were separated on SDS–10% PAGE and analyzed by immunoblotting with anti-Flag M2 monoclonal antibody (Kodak) and with anti-GST polyclonal antibodies (Amersham Pharmacia). The probed antibodies were detected by standard methodology using alkaline phosphatase-conjugated secondary antibodies.
Binding assay using in vitro-translated proteins.
[35S]methionine-labeled wild-type and mutant SREBP-2 proteins were synthesized in a rabbit reticulocyte lysate from pET28b-SREBP2 and pET28b-SREBP2/L1.2.3A, respectively, using the TNT system (Promega) according to the manufacturer's instructions. Five microliters of each translation product was incubated with 60 pmol of GST (≈1.6 μg) or GST-importin β (≈7.2 μg) in binding buffer C (20 mM HEPES-KOH [pH 7.3], 50 mM potassium acetate, 2 mM magnesium acetate, 100 mM KCl, 2 mM DTT) at a total volume of 50 μl for 20 min at 30°C. Then 5 μl of glutathione-Sepharose was added to each of the reaction mixtures, followed by further incubation at 4°C for 1 h. After three washes with binding buffer C, the bound proteins were eluted by boiling in SDS-PAGE sample buffer. Half the amount of bound fractions were separated on SDS–10% PAGE and examined by autoradiography.
Microinjection and indirect immunofluoresence.
Microinjection experiments were performed as described previously (32). Briefly, recombinant Flag-SREBP-2 or Flag-SREBP-2/L1.2.3A was microinjected into the cytoplasm of HeLa cells. After incubation for 30 min at 37°C, the cells were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature. To examine the localization of Flag-tagged proteins, the fixed cells were permeabilized with 0.5% Triton X-100 for 5 min at room temperature, incubated with 3% skim milk in PBS for 20 min, and then incubated with a 30-μg/ml solution of monoclonal anti-Flag M2 antibody for 1 h at room temperature. The mouse antibody was detected with Cy3-labeled goat anti-mouse immunoglobulin G (IgG) (Amersham Pharmacia).
In vitro transport assay.
In vitro transport assays were performed as described previously (32). Briefly, digitonin-permeabilized HeLa cells were incubated for 20 min at 30°C with 10 μl of testing solution, which contains 4 pmol of Flag-SREBP-2 or Flag-SREBP-2/L1.2.3A, an ATP regeneration system, and 2% BSA in transport buffer (20 mM HEPES-KOH [pH 7.3], 110 mM potassium acetate, 2 mM magnesium acetate, 5 mM sodium acetate, 0.5 mM EGTA, 2 mM DTT) supplemented with 1 μg each of aprotinin, leupeptin, and pepstatin per ml. Where indicated, cytosol from Ehrlich ascites tumor cells, rabbit reticulocyte lysate, recombinant importin β, recombinant Ran-GDP, recombinant p10 (NTF2), and 0.5 mM GTP were included in the above 10-μl testing solution. Total cytosol from Ehrlich ascites tumor cells was prepared as described previously (19). A rabbit reticulocyte lysate was prepared according to standard protocols. For the competition experiments, 120 pmol of recombinant MBP-IBB was added to the testing solution, which contained 4 pmol of either Flag-SREBP-2 or GST-NLS-GFP, 5 μl of cytosol, and the ATP regeneration system. The import of the Flag-tagged proteins was examined by indirect immunofluorescence using a monoclonal anti-Flag M2 antibody.
Glutaraldehyde cross-linking of recombinant proteins.
To analyze the dimerization of wild-type and mutant SREBP-2 in solution, various concentrations of purified Flag-SREBP-2 or Flag-SREBP-2/L1.2.3A were incubated at 30°C for 20 min in the presence or absence of 0.008% glutaraldehyde in transport buffer. After the addition of 50 mM lysine to quench the cross-linking reaction, the products were precipitated with cold acetone, resolved on a 2 to 15% gradient SDS-polyacrylamide gel, and then analyzed by immunoblotting using the monoclonal anti-Flag M2 antibody and the horseradish peroxidase-2 conjugated goat anti-mouse IgG with the ECL Western blotting detection reagents (Amersham Pharmacia biotech). A sample composed of 70 ng of Flag-tagged protein was loaded in each lane.
Reversible cross-linking of recombinant proteins.
Purified Flag-SREBP-2 or Flag-SREBP-2/L1.2.3A (4.4 μg), alone or together with 6.4 μg of importin β, was incubated in the presence of 0.25 mM dithiobis(succinimidylpropionate) (DSP; Pierce) in 20 mM HEPES-KOH (pH 7.3) containing 200 mM NaCl (total volume, 160 μl) for 30 min at room temperature. The cross-linking reaction was quenched by the addition of 4 μl of 1 M Tris-HCl (pH 7.0), and the incubation was continued for an additional 15 min. The proteins were then precipitated with trichloroacetic acid, washed with acetone, dried, and dissolved in SDS sample buffer in the absence of a reducing agent. After boiling for 5 min, the samples were applied to a 2 to 15% gradient SDS-PAGE gel and stained with a 0.1% aqueous solution of Coomassie brilliant blue. To revert the cross-linking, the appropriate bands were excised, homogenized, and incubated in a twofold volume of elution buffer (15 mM NH4HCO3, 0.025% SDS, 1 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride) at room temperature overnight. The eluted proteins were collected by centrifugation, dried, and dissolved in SDS-PAGE sample buffer containing 100 mM DTT. The samples were boiled for 5 min, applied to SDS–10% PAGE, and visualized by Coomassie staining.
RESULTS
SREBP-2 binding domain of importin β overlaps but is not identical to the importin α binding domain.
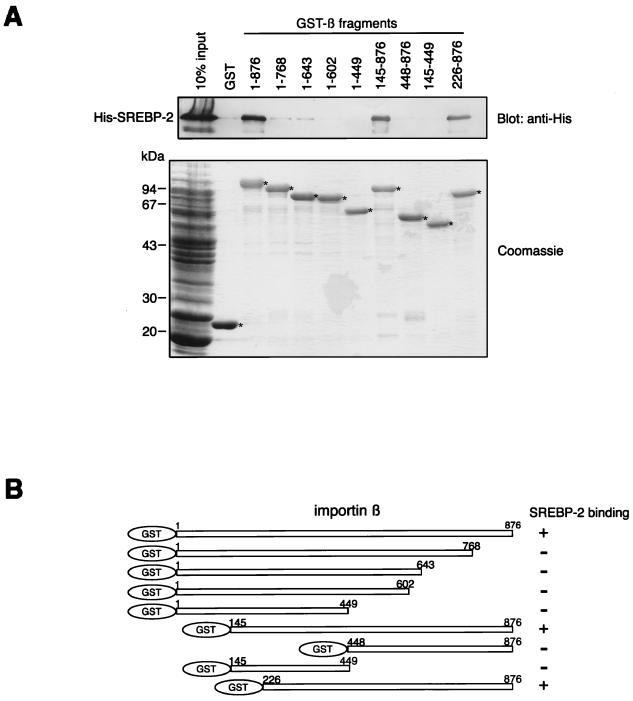
We previously reported that the nuclear import of SREBP-2 occurs via a direct interaction with importin β. The importin β binding domain of SREBP-2 is located in its HLH-Zip region, which shows no apparent sequence similarity with any other importin β binding sequences identified thus far (32). To address the question of how importin β carries different classes of cargoes, we determined the SREBP-2 binding region of importin β. Subsets of N- and C-terminally truncated importin β mutants were produced as GST fusion proteins and then used in binding assays with an E. coli lysate which expresses the N-terminally His-tagged SREBP-2. As shown in Fig. 1A, His-SREBP-2 bound to the full-length importin β(1–876), importin β(145–876), and importin β(226–876), whereas no significant binding of SREBP-2 to other truncated mutants of importin β was observed. Figure 1B schematically depicts the mapping of the SREBP-2 binding domain of importin β. The minimum region required for binding to SREBP-2 is located in importin β(226–876), which covers the importin α binding site and partly overlaps the adjacent NPC binding site as well as the Ran-GTP binding site.
FIG. 1.
Mapping of the SREBP-2 binding domain of importin β. (A) Full-length (1 to 876) and various truncated mutants of importin β were produced as GST fusion proteins and tested for their ability to bind to SREBP-2. Purified GST or GST-importin β fragments (150 pmol) were incubated with 270 μl of E. coli lysate expressing His-SREBP-2 (300 μl final volume). GST fusion proteins were then absorbed to 15 μl of glutathione-Sepharose beads. After extensive washing, the bound proteins were eluted by boiling in SDS-PAGE sample buffer and divided into two equal portions. Each portion was separated by SDS–10% PAGE and analyzed by immunoblotting using the monoclonal anti-penta-His antibody (top) or by Coomassie staining (bottom). E. coli lysate (13 μl) expressing His-tagged SREBP-2 was directly applied to each gel (10% input). Asterisks indicate the positions of the GST and GST-importin β fragments which were absorbed to the glutathione-Sepharose beads (bottom). (B) Schematic representation of the importin β deletion mutants used in this study. All mutants were expressed as GST fusion proteins. Numbers indicate the amino acid position of each importin β fragment.
IBB competes with SREBP-2 for binding to importin β and nuclear import.
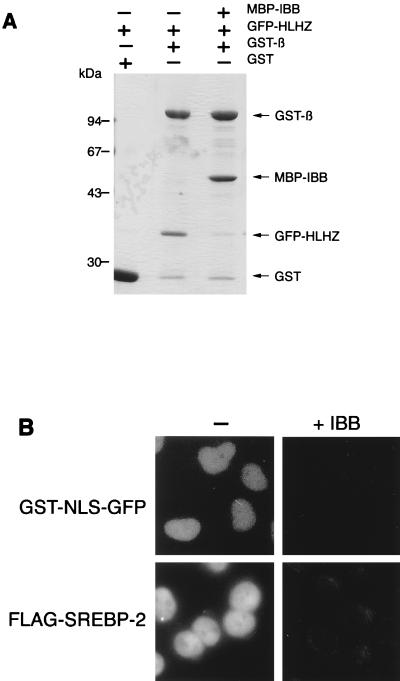
The issue of whether the IBB domain of importin α competes with SREBP-2 for interaction with importin β was then tested. Solution binding assays were performed using the recombinant GFP-HLH-Zip and the immobilized GST-importin β in the absence or presence of recombinant IBB which was fused with MBP at its N terminus (MBP-IBB). Consistent with our previous observation, GFP-HLH-Zip efficiently binds to GST-importin β in the absence of MBP-IBB. In contrast, the binding was competitively inhibited by the addition of an equimolar amount of MBP-IBB (Fig. 2A). Moreover, it was found that an excess amount of MBP-IBB completely blocked the nuclear import of Flag-SREBP-2 as well as that of the simian virus 40 T antigen NLS fused with GST and GFP (GST-NLS-GFP) in digitonin-permeabilized HeLa cells (Fig. 2B).
FIG. 2.
IBB competes with SREBP-2 for binding to importin β and nuclear import. (A) The IBB domain competes for binding of HLH-Zip of SREBP-2 to importin β. GST or GST-importin β (GST-β) (70 pmol) was incubated with 70 pmol of GFP-SREBP-2(343–403) (GFP-HLHZ) in the presence or absence of MBP-IBB (70 pmol). The GST fusion proteins were then recovered on glutathione-Sepharose beads, and the bound proteins were analyzed by SDS–10% PAGE followed by Coomassie staining. (B) Competition with nuclear import by the IBB domain. Nuclear import of GST-NLS-GFP and Flag-SREBP-2 in digitonin-permeabilized HeLa cells was performed by incubating the cells with 10 μl of reaction mixture containing 4 pmol of GST-NLS-GFP or Flag-SREBP-2 in the presence of cytosol from Ehrlich tumor cells and an ATP regeneration system (left panels). For competition by IBB, import assays were carried out in the presence of 120 pmol of MBP-IBB (right panels).
Amino acid substitution of leucine residues in the leucine zipper of SREBP-2 abolishes its nuclear import.
In a previous study, we found that the SREBP-2 leucine zipper domain is essential for binding to importin β and nuclear import (32). Typically, bHLH-Zip proteins, including SREBPs, bind DNA as dimers (36). In terms of structure, the bHLH-Zip motif is comprised of a short stretch of basic residues followed by an HLH domain, which is made up of two α-helices (helix 1 and helix 2) separated by a nonconserved variable loop. The C terminus of helix 2 is extended where a leucine zipper domain (Zip) forms an α-helical dimerization interface. The basic domain is responsible for the sequence-specific binding of DNA, while the HLH-Zip domain mediates the dimerization (reviewed in references 2, 9, and 29). Several investigations have clearly shown that the HLH region of bHLH-Zip proteins alone is not sufficient to generate a stable dimer, whereas a leucine zipper is required to achieve additional dimerization strength and to determine the specificity of dimerization (4, 30, 40). Therefore, it would be of interest to know whether dimerization is a prerequisite for the importin β-mediated nuclear import of SREBP-2.
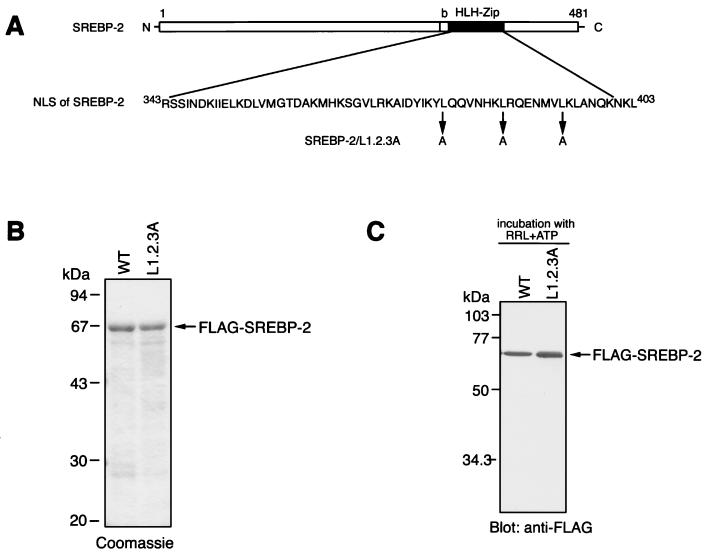
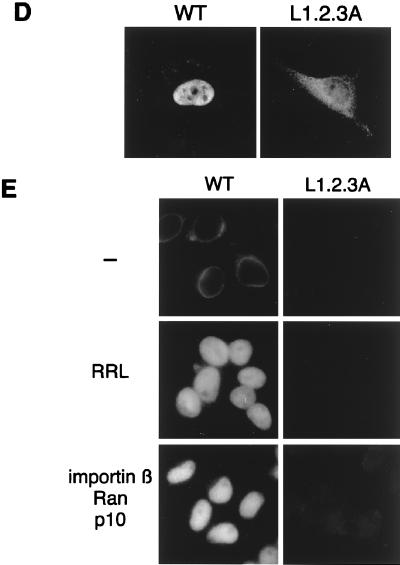
To address this issue, we designed an SREBP-2 mutant in which possible dimerization can be altered. The leucine zipper can be represented as taking the form of a helical wheel, in which the seven amino acids of each repeat are designated by the letters a to g. The leucine residues aligned at position d and the hydrophobic residues at position a lie along the opposing contact surface of the two helices, creating a hydrophobic dimerization interface. The flanking e and g positions are often occupied by charged residues, which may function to stabilize the resulting dimer via electrostatic interactions (29). From this scenario, we constructed a mutant SREBP-2, SREBP-2/L1.2.3A, which has triple substitutions of leucines for the alanines at position d (Fig. 3A), and expressed it in the E. coli as an N-terminally Flag-tagged protein. The SDS-PAGE profile demonstrated that the recombinant Flag-SREBP-2/L1.2.3A protein was purified to near homogeneity of the full-length 67-kDa species as well as the wild-type Flag-SREBP-2 (Fig. 3B). As shown in Fig. 3D, the mutant Flag-SREBP-2/L1.2.3A protein did not accumulate efficiently in the nucleus when microinjected into the cytoplasm of HeLa cells. Furthermore, virtually no import of L1.2.3A protein was observed in the in vitro transport assay, not only when the reticulocyte lysate was used as a source of transport factors, but also in the presence of recombinant importin β, Ran, and p10/NTF2 (Fig. 3E). The immunoblot using an anti-Flag antibody shows that both the wild-type and mutant SREBP-2 proteins were stable under the 20-min incubation at 30°C with the reticulocyte lysate and the ATP regeneration system, suggesting that the import defect of SREBP-2/L1.2.3A is not due to instability of the protein resulting from the substitution of leucine residues (Fig. 3C).
FIG. 3.
Substitution of leucine residues in the leucine zipper of SREBP-2 abolishes its nuclear import. (A) The amino acid sequence of NLS of SREBP-2 (middle) and the positions of the leucines replaced by alanine residues in the SREBP-2/L1.2.3A mutant (bottom) are indicated. A schematic of the overall structure of mature-form SREBP-2 is shown at the top of the figure. NLS of SREBP-2 resides in the HLH-Zip domain (residues 343 to 403). (B) SDS-PAGE profile of purified recombinant Flag-SREBP-2 (WT) and Flag-SREBP-2/L1.2.3A. Purified Flag-SREBP-2 and Flag-SREBP-2/L1.2.3A were subjected to SDS–10% PAGE followed by staining with Coomassie brilliant blue. (C) Recombinant Flag-SREBP-2 (WT) and Flag-SREBP-2/L1.2.3A are stable under incubation with a reticulocyte lysate. Recombinant Flag-SREBP-2 and Flag-SREBP-2/L1.2.3A (0.5 μg each) were incubated for 20 min at 30°C with 8 μl of rabbit reticulocyte lysate and an ATP regeneration system (10 μl final volume). An aliquot of each mixture containing 50 ng of Flag-SREBP-2 or Flag-SREBP-2/L1.2.3A was separated on SDS–10% PAGE and analyzed by immunoblotting with anti-Flag M2 antibody. (D) The L1.2.3A mutant does not accumulate in the nucleus of living cells. Recombinant Flag-SREBP-2 (WT) or Flag-SREBP-2/L1.2.3A (L1.2.3A) (0.5 mg/ml each) was injected into the cytoplasm of HeLa cells. After incubation for 30 min at 37°C, the cells were fixed, and the injected protein was detected by indirect immunofluorescence with an anti-Flag M2 antibody. (E) The L1.2.3A mutant does not accumulate in the nucleus of digitonin-permeabilized cells. In vitro import assays were performed using Flag-SREBP-2 (WT, left panels) or Flag-SREBP-2/L1.2.3A (right panels) as a substrate. Digitonin-permeabilized HeLa cells were incubated with a reaction mixture containing a 0.4 μM concentration of each substrate in the presence of an ATP regeneration system only (—) or together with either rabbit reticulocyte lysate or a combination of recombinant transport factors and the nucleotide at the following concentrations: importin β, 0.4 μM; Ran, 0.4 μM; p10/NTF2, 0.3 μM; and GTP, 0.5 mM. Localization of each substrate was examined by indirect immunofluorescence using a monoclonal anti-Flag M2 antibody.
Leucine-substituted mutant has a reduced ability to importin β binding.
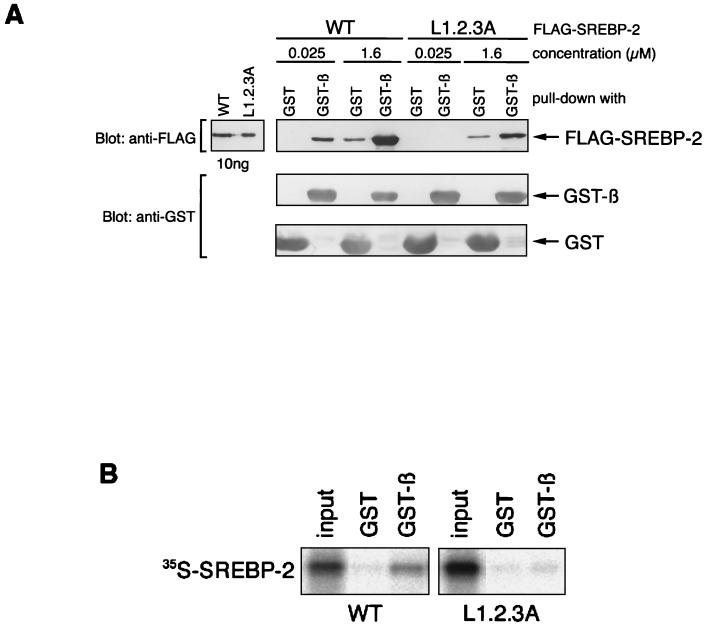
Since the SREBP-2/L1.2.3A mutant appeared to be defective in nuclear import, we next asked whether this mutant had lost its importin β binding ability. To test this possibility, pull-down assays using GST-importin β and low (25 nM) or high (1.6 μM) concentrations of recombinant Flag-SREBP-2 and Flag-SREBP-2/L1.2.3A were performed. As shown in Fig. 4A, wild-type Flag-SREBP-2 bound more strongly to GST-importin β than to GST at both concentrations. In contrast, no interaction of Flag-SREBP-2/L1.2.3A with GST-importin β was detected at the low concentration, whereas a significant level of binding of the L1.2.3A mutant to GST-importin β over nonspecific binding to GST was observed at the high concentration (1.6 μM), although the binding of L1.2.3A to GST-importin β was much weaker than that of wild-type SREBP-2. These results clearly show that the triple substitutions of leucines in the leucine zipper reduced the ability of SREBP-2 to bind to importin β. Therefore, it is plausible that, since the SREBP-2/L1.2.3A mutant cannot bind to importin β under physiological concentrations, it is not imported into the nucleus (refer to Fig. 3). In order to confirm this, we next attempted to examine the binding of SREBP-2 and importin β at conditions closer to physiological. For this, binding assays were performed using immobilized GST-importin β and in vitro-translated 35S-labeled SREBP-2 and SREBP-2/L1.2.3A. As expected, wild-type [35S-]SREBP-2 efficiently bound to GST-importin β, whereas [35S-]SREBP-2/L1.2.3A did not interact with GST-importin β (Fig. 4B).
FIG. 4.
SREBP-2/L1.2.3A mutant has a reduced affinity for importin β. (A) Purified Flag-SREBP-2 (WT) or Flag-SREBP-2/L1.2.3.A (25 nM or 1.6 μM in a total volume of 50 μl) was incubated with GST or GST-importin β (1 μM) for 20 min at room temperature in the presence of 5 μl of glutathione-Sepharose beads. After incubation, the beads were collected by centrifugation followed by extensive washing, and the bound proteins were eluted by boiling in SDS-PAGE sample buffer. Proteins were separated SDS–10% PAGE and analyzed by immunoblotting with anti-Flag M2 monoclonal antibody and anti-GST polyclonal antibodies. Control samples of recombinant Flag-SREBP-2 and Flag-SREBP-2/L1.2.3A (10 ng each) were loaded directly onto the gel. (B) The in vitro-translated L1.2.3A mutant does not bind to immobilized GST-importin β in the reticulocyte lysate. 35S-labeled wild-type (WT) and L1.2.3A mutant SREBP-2 were translated in vitro, and 5 μl of each was incubated with 60 pmol each of GST or GST-importin β in 50 μl of binding buffer C. GST proteins were then absorbed to 5 μl of glutathione-Sepharose beads. After washing three times, the bound proteins were eluted by boiling the beads in SDS-PAGE sample buffer. Half of each eluate was separated on SDS–10% PAGE and analyzed by autoradiography. 35S-labeled SREBP-2 and SREBP-2/L1.2.3A (2.5 μl each) were loaded directly as a control (input).
HLH-Zip of SREBP-2 actually forms a homodimer.
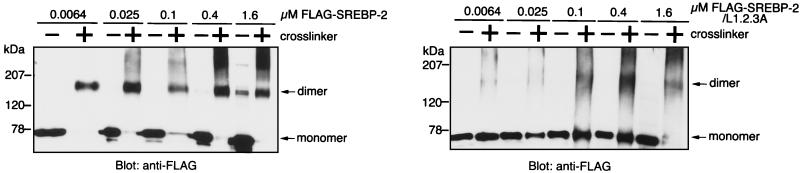
Since the experiments described above indicate that the L1.2.3A mutant has a reduced affinity for importin β, we next attempted to determine which is responsible for interaction with importin β, repeating leucine residues or dimerization mediated by the leucine zipper. To answer this question, the efficiency of homodimerization of wild-type and mutant SREBP-2 was determined using a chemical cross-linking assay. Either Flag-SREBP-2 or Flag-SREBP-2/L1.2.3A recombinant protein was serially diluted and incubated in the presence or absence of glutaraldehyde. The cross-linked products were then separated by SDS-PAGE and analyzed by immunoblotting with the anti-Flag monoclonal antibody. Figure 5 shows that the wild-type Flag-SREBP-2 existed predominantly as a dimer at concentrations ranging from 6.4 nM to 1.6 μM. In addition, at concentrations higher than 0.4 μM, slower-mobility products which appeared to be higher-order oligomers of Flag-SREBP-2 were also observed. In contrast, the L1.2.3A mutant was predominantly in a monomeric form at concentrations ranging from nanomolar to micromolar. Even at concentrations higher than submicromolar, only a small fraction of L1.2.3A was found to be in the dimeric form. These results indicate that SREBP-2 exists as a stable dimer in solution, even in the nanomolar concentration range, whereas the leucine substitutions reduce the feasibility of dimer formation by at least 1,000-fold. Taking these results into consideration, along with the data shown in Fig. 4, it appears that the strength of importin β binding to SREBP-2 parallels the efficiency of dimerization of SREBP-2. This suggests that the homodimerization of SREBP-2 is required for its efficient interaction with importin β.
FIG. 5.
Analysis of the dimerization of wild-type and L1.2.3A mutant SREBP-2 by cross-linking. Recombinant Flag-SREBP-2 or Flag-SREBP-2/L1.2.3A protein was serially diluted to the indicated concentrations and incubated at 30°C for 10 min in the presence (+) or absence (−) of 0.008% glutaraldehyde (cross-linker). Products were precipitated with cold acetone, resolved on 2 to 15% gradient SDS-PAGE, and detected by immunoblotting with the monoclonal anti-Flag M2 antibody.
Importin β preferentially interacts with dimeric form of SREBP-2.
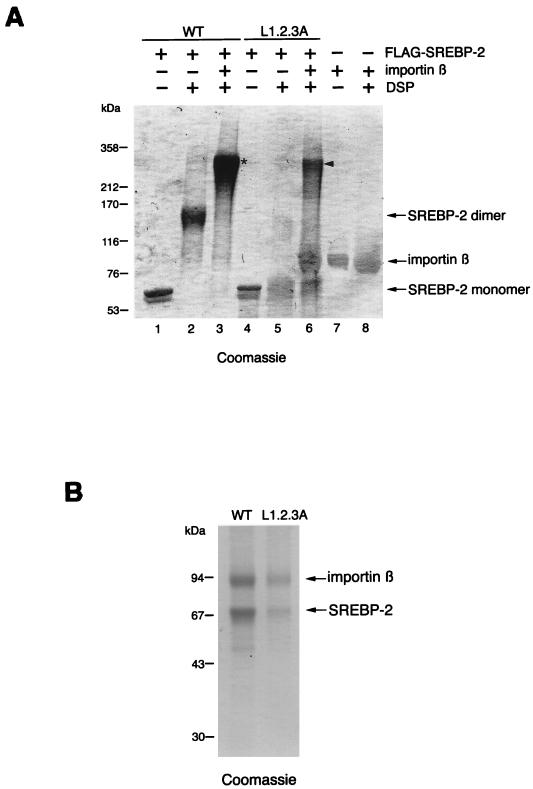
To verify the possibility that importin β interacts favorably with the dimerized SREBP-2, we investigated the stoichiometry of the interaction between SREBP-2 and importin β. For this purpose, chemical cross-linking experiments were performed using the thiol-cleavable cross-linking agent DSP. Initially, 0.4 μM purified Flag-SREBP-2 or FLAG-SREBP-2/L1.2.3A was incubated with DSP in the presence or absence of importin β (0.4 μM). The cross-linked products were separated by SDS-PAGE under nonreducing conditions and visualized by Coomassie staining. Consistent with the results obtained from the glutaraldehyde cross-linking experiments (Fig. 5), wild-type SREBP-2 existed predominantly as a dimer in the absence of importin β, whereas the L1.2.3A mutant was predominantly monomeric and only slightly dimerized. Importin β also did not oligomerize by itself under these conditions. The cross-linking of wild-type SREBP-2 in the presence of importin β resulted in the nearly exclusive formation of an approximately 270-kDa polypeptide (Fig. 6A, asterisk). In contrast, when the L1.2.3A mutant was treated with DSP in the presence of importin β, the monomeric forms of SREBP-2/L1.2.3A and importin β were still evident, while an approximately 270-kDa product was detected instead of the L1.2.3A dimer (Fig. 6A). Next, to assess whether these newly detected ∼270-kDa products contained both SREBP-2 and importin β, the corresponding bands were excised and the products were eluted from the gel slices. The eluted samples were boiled in the presence of a reducing agent to revert cross-linking, and the resulting products were separated on SDS-PAGE under reducing conditions. The Coomassie-stained gel demonstrated that the products contained both importin β and SREBP-2 (Fig. 6B). Densitometric analysis revealed that the molar ratio of importin β to both wild-type SREBP-2 and SREBP-2/L1.2.3A was approximately 1:2, indicating that the complex is composed of two molecules of SREBP-2 and one of importin β. Therefore, it is most likely that importin β preferentially interacts with the dimeric form of wild-type SREBP-2. Collectively, these findings lead to the conclusion that the HLH-Zip of SREBP-2 is a novel class of NLS which is active when in a dimeric form.
FIG. 6.
Importin β preferentially interacts with dimerized SREBP-2. (A) Analysis of the SREBP-2–importin β complex by cross-linking. Purified Flag-SREBP-2 (WT) or Flag-SREBP-2/L1.2.3A (L1.2.3A) at a concentration of 0.4 μM was incubated in the presence (+) or absence (−) of 0.4 μM importin β with 0.25 mM DSP for 30 min at room temperature. Importin β (0.4 μM) alone was also incubated in the presence (+) of DSP. The cross-linked products were resolved on 2 to 15% gradient SDS-PAGE under nonreducing conditions and visualized by Coomassie staining. Flag-SREBP-2, Flag-SREBP-2/L1.2.3A, and importin β (1 μg each) were loaded directly as controls (lanes 1, 4, and 7, respectively). (B) High-mobility cross-linked products actually contain both importin β and SREBP-2. The bands of cross-linked species, which are indicated by an asterisk (lane 3) and an arrowhead (lane 6) in panel A, were excised, and the products were eluted by incubating the gel slices with elution buffer overnight at room temperature. Eluted samples were concentrated and boiled in SDS-PAGE sample buffer containing 100 mM DTT, then resolved on SDS–10% PAGE followed by Coomassie staining. WT, proteins eluted from the cross-linked product indicated by an asterisk in A. L1.2.3A, proteins eluted from the cross-linked product indicated by an arrowhead in A. The intensity of the protein bands was quantified using FluorChem IS-8000 (Alpha Innotech Corporation), and the molar ratio of importin β to SREBP-2 was estimated by dividing the intensity of protein bands by the molecular mass of each protein.
DISCUSSION
The data presented here show how importin β recognizes SREBP-2. The SREBP-2 binding domain corresponds to residues 226 to 876 of importin β, which overlaps but is not identical to the importin α binding domain. Recognition by importin β is dependent on the dimerization of SREBP-2 via its HLH-Zip domain. These findings suggest a scheme for the initial step for the SREBP-2 nuclear import; the primary event involves the dimerization of the mature form of SREBP-2 in the cytoplasm, after which importin β recognizes the dimerized HLH-Zip domain, leading to the formation of a heterotrimeric complex which actively translocates into the nucleus.
Recognition of different classes of cargoes by importin β.
The crystal structure of importin β complexed with IBB and that of an importin β fragment bound to Ran-GppNHp has been solved (6, 49). These studies show that the IBB domain consists of two structurally distinct parts, the N-terminal moiety and the C-terminal helix, each of which interacts with two separate regions of importin β. The C-terminal helix is sufficient for complex formation with importin β, since an IBB fragment which lacks the N-terminal moiety is still able to bind to importin β (6, 50). In addition, it was found that importin β consists of 19 tandemly repeated HEAT motifs, each of which is formed by two α-helices (referred to as A and B helices) connected by an intramotif turn. The repeats are arranged in the form of a right-handed superhelix, with the A helices forming the outer (convex) surface, whereas the B helices, which form the inner (concave) surface, interact directly with the IBB domain and Ran-GTP. The IBB domain interacts with HEAT repeats 7 to 19 of importin β, while Ran interacts within the N-terminal fragment, which contains HEAT repeats 1 to 8. The important structure in terms of explaining how Ran-GTP drives the dissociation of the IBB from importin β is the longest intramotif turn located in HEAT-8, the so-called acidic loop, which is highly acidic and comes into direct contact with both binding partners. The binding of the Ran-GTP and IBB domain to the acidic loop may be mutually exclusive, and in addition, the binding of Ran-GTP to the acidic loop might cause conformational changes in the C-terminal portion of the HEAT repeats, which might loosen the interaction of importin β with the IBB domain.
The importin β fragment with an N-terminal truncation of 225 amino acids retains the ability to bind to SREBP-2, whereas further deletions at the N terminus result in the complete loss of binding ability. Deletions from the C terminus significantly reduce binding activity. Therefore, the SREBP-2 binding domain is located on residues 226 to 876 of importin β (Fig. 1A and B). This domain resides within HEAT repeats 6 to 19, including the importin α binding domain, which corresponds to the C-terminal half of importin β. As expected from this fact, the IBB domain competes with SREBP-2 for binding to importin β (Fig. 2). However, in clear contrast to the IBB, which maintains a high-affinity interaction with importin β(448–876), corresponding to HEAT repeats 11 to 19, SREBP-2 is not capable of binding to this fragment. This suggests that both the 226 to 447 region and the intact C terminus of importin β are critically involved in interactions with SREBP-2 and further implies that the importin β/SREBP-2 complex does not completely mimic the importin β-IBB interaction. The 226 to 447 region corresponds to HEAT repeats 6 to 10, which include both the acidic loop and the long connection between HEAT 7 and 8 formed by elongated helix B7 and an extralong B7-A8 turn, designated the protruding stalk. The function of the protruding stalk is currently unknown, but it might be involved in the recognition of some import cargoes, such as the BIB domain (49). Given this information, it is possible that the release of bound SREBP-2 by Ran-GTP might be the result of interactions with the acidic loop and that the protruding stalk might be involved in the interaction with SREBP-2. Structural analyses of the interaction of SREBP-2 with importin β will be required to solve the question of how importin β binds to different classes of cargoes.
HLH-Zip of SREBP-2 functions as a novel NLS through dimerization.
SREBP-2 forms a stable dimer in solution, which is likely to interact with importin β under physiological conditions (Fig. 4 and 5). Triple substitution of leucines in the leucine zipper of SREBP-2 reduces the efficiency of dimerization by at least 1,000-fold relative to that of the wild type and considerably decreases the nuclear import activity, judging from the results of microinjection and in vitro transport assay using 0.4 μM L1.2.3A protein (Fig. 3 and 5). It is difficult to prove definitively that dimerization is required for importin β binding because the possibility that the mutation affects both dimerization and importin β binding independently cannot be completely excluded. However, several lines of evidence show that importin β preferentially recognizes the dimerized SREBP-2. As expected, a complex of importin β and L1.2.3A is not observed under the physiological conditions (Fig. 4). In contrast, it can be formed in vitro by increasing the concentration of L1.2.3A protein into the micromolar range, at which the L1.2.3A mutant is able to dimerize to some extent (Fig. 4A, 5, and 6A). The molar ratio of L1.2.3A mutant bound to importin β was estimated to be approximately 2:1, which is the same ratio as for wild-type SREBP-2 bound to importin β (Fig. 6B). Furthermore, when higher concentrations (0.8 and 1.6 μM) of the SREBP-2/L1.2.3A mutant are used in the in vitro transport assay, nuclear accumulation of the L1.2.3A mutant can be detected (data not shown), suggesting that the L1.2.3A mutant protein is still active for nuclear import when it forms a dimer. Collectively, these findings strongly suggest that importin β does not recognize a contiguous amino acid stretch in the leucine zipper region, but rather recognizes the structural characteristics of the dimerized SREBP-2 molecules. The requirement for dimerization to achieve recognition is unique for SREBP-2 among the currently identified importin β binding signals.
Regulation of nuclear import of SREBP-2.
Substrate modification by homo- or heterodimerization has been proposed as one of the typical modes of regulating nucleocytoplasmic transport. An example that follows this scheme is the nuclear import of STAT1, which is dependent on the tyrosine phosphorylation that leads to homodimerization, followed by complex formation with NPI-1 (an importin α family member) and importin β (45, 46, 53). Mitogen-activated protein kinase also requires homodimerization for active nuclear import (1, 23). Heterodimerization also seems to be a common mechanism, as transcription factor NF-κB is sequestered in the cytoplasm by interacting with IκB, which is actively exported from the nucleus, the nuclear import of which is triggered by phosphorylation and the resulting degradation of IκB (3, 28, 53). Generally, in these cases, the phosphorylation-dephosphoryaltion reaction is considered critical for dimerization and therefore critical for nuclear import.
In contrast, although SREBP-2 requires dimerization through its HLH-Zip in order to bind to importin β, SREBP-2 is unique in the sense that its nuclear import is regulated by the membrane anchoring-releasing mechanism. That is, the critical trigger for nuclear import is the proteolytic cleavage of precursor SREBP-2 from the Golgi membrane. SREBPs have an atypical tyrosine instead of a conserved arginine in the basic regions, which allows recognition of the sterol regulatory element (ATCACCCCAC) in addition to the canonical E-box motif (ATCACGTGA) (24, 36). This dual DNA-binding specificity distinguishes SREBPs from other bHLH-Zip proteins and is known to be important for lipid metabolism. Therefore, the import pathway in which importin β recognizes only valid dimers may ensure accurate sterol-responsive transcriptional regulation, although there is no evidence at present to show that SREBPs heterodimerize with each other or with any other bHLH-Zip protein.
Assuming that the dimerization of SREBP-2 takes place after the release of SREBP-2 from the membrane, import complex formation is not initiated until the concentration of released mature SREBP-2 increases to a certain level, leading to the response with the threshold to the graded change of intracellular sterol content. Alternatively, it might be possible that precursor SREBP-2s cluster with each other on the membrane and dimerize prior to processing. In the latter scenario, importin β would bind to the HLH-Zip dimer, which is composed of two adjacent precursor SREBP-2s on the membrane, thereby establishing the most rapid transport coinciding with the cleavage. Moreover, by transporting even a small amount of active fragment effectively, this mechanism would ensure a rapid and efficient response to intracellular sterol levels. In either case, our results showing that SREBP-2 alone tends to dimerize in solution even at very low concentrations (Fig. 5) suggest a model in which dimeric HLH-Zip and importin β assemble into a complex independently of other proteins. However, the possibility that complex formation might be regulated by a currently unknown mechanism cannot presently be ruled out. Thus, although we do not know the precise mechanism by which SREBP-2 forms dimers, the nuclear import of SREBP-2, which is dependent on dimerization via its HLH-Zip, should be intensely investigated as a novel type of regulation.
ACKNOWLEDGMENTS
We thank Ryuichiro Sato, University of Tokyo, for valuable discussions.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (B) (no. 11237202), Grant-in-Aid for Scientific Research (B) (no. 12480215), and Grant-in-Aid for COE Research (no. 07CE2006 and 12CE2007) from the Japanese Ministry of Education, Science, Sports and Culture, the Mitsubishi Foundation, and the Human Frontiers Science Program.
REFERENCES
- 1.Adachi M, Fukuda M, Nishida E. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 1999;18:5347–5358. doi: 10.1093/emboj/18.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxevanis A D, Vinson C R. Interactions of coiled coils in transcription factors: where is the specificity? Curr Opin Genet Dev. 1993;3:278–285. doi: 10.1016/0959-437x(93)90035-n. [DOI] [PubMed] [Google Scholar]
- 3.Beg A A, Ruben S M, Scheinman R I, Haskill S, Rosen C A, Baldwin A S., Jr I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 1992;6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 4.Bresnick E H, Felsenfeld G. The leucine zipper is necessary for stabilizing a dimer of the helix-loop-helix transcription factor USF but not for maintenance of an elongated conformation. J Biol Chem. 1994;269:21110–21116. [PubMed] [Google Scholar]
- 5.Brown M S, Goldstein J L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 6.Cingolani G, Petosa C, Weis K, Müller C W. Structure of importin-beta bound to the IBB domain of importin-alpha. Nature. 1999;399:221–229. doi: 10.1038/20367. [DOI] [PubMed] [Google Scholar]
- 7.Corbett A H, Silver P A. Nucleocytoplasmic transport of macromolecules. Microbiol Mol Biol Rev. 1997;61:193–211. doi: 10.1128/mmbr.61.2.193-211.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeBose-Boyd R A, Brown M S, Li W P, Nohturfft A, Goldstein J L, Espenshade P J. Transport-dependent proteolysis of SREBP: relocation of site-1 protease from Golgi to ER obviates the need for SREBP transport to Golgi. Cell. 1999;99:703–712. doi: 10.1016/s0092-8674(00)81668-2. [DOI] [PubMed] [Google Scholar]
- 9.Ferre-D'Amare A R, Burley S K. DNA recognition by helix-loop-helix proteins. Nucleic Acid Mol Biol. 1995;9:285–298. [Google Scholar]
- 10.Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti K G, Fransen J, Grosveld G. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 1997;16:807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Görlich D, Henklein P, Laskey R A, Hartmann E. A 41 amino acid motif in importin-alpha confers binding to importin- beta and hence transit into the nucleus. EMBO J. 1996;15:1810–1817. [PMC free article] [PubMed] [Google Scholar]
- 12.Görlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 13.Görlich D, Mattaj I W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 14.Görlich D, Pante N, Kutay U, Aebi U, Bischoff F R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson B R, Percipalle P. Interactions between HIV Rev and nuclear import and export factors: the Rev nuclear localisation signal mediates specific binding to human importin-beta. J Mol Biol. 1997;274:693–707. doi: 10.1006/jmbi.1997.1420. [DOI] [PubMed] [Google Scholar]
- 16.Hieda M, Tachibana T, Yokoya F, Kose S, Imamoto N, Yoneda Y. A monoclonal antibody to the COOH-terminal acidic portion of Ran inhibits both the recycling of Ran and nuclear protein import in living cells. J Cell Biol. 1999;144:645–655. doi: 10.1083/jcb.144.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber J, Cronshagen U, Kadokura M, Marshallsay C, Wada T, Sekine M, Lührmann R. Snurportin1, an m3G-cap-specific nuclear import receptor with a novel domain structure. EMBO J. 1998;17:4114–4126. doi: 10.1093/emboj/17.14.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imamoto N, Shimamoto T, Kose S, Takao T, Tachibana T, Matsubae M, Sekimoto T, Shimonishi Y, Yoneda Y. The nuclear pore-targeting complex binds to nuclear pores after association with a karyophile. FEBS Lett. 1995;368:415–419. doi: 10.1016/0014-5793(95)00699-a. [DOI] [PubMed] [Google Scholar]
- 19.Imamoto N, Tachibana T, Matsubae M, Yoneda Y. A karyophilic protein forms a stable complex with cytoplasmic components prior to nuclear pore binding. J Biol Chem. 1995;270:8559–8565. doi: 10.1074/jbc.270.15.8559. [DOI] [PubMed] [Google Scholar]
- 20.Izaurralde E, Kutay U, von Kobbe C, Mattaj I W, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jäkel S, Albig W, Kutay U, Bischoff F R, Schwamborn K, Doenecke D, Görlich D. The importin beta/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 1999;18:2411–2423. doi: 10.1093/emboj/18.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jäkel S, Görlich D. Importin beta, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 1998;17:4491–4502. doi: 10.1093/emboj/17.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khokhlatchev A V, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb M H. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 24.Kim J B, Spotts G D, Halvorsen Y D, Shih H M, Ellenberger T, Towle H C, Spiegelman B M. Dual DNA binding specificity of ADD1/SREBP1 controlled by a single amino acid in the basic helix-loop-helix domain. Mol Cell Biol. 1995;15:2582–2588. doi: 10.1128/mcb.15.5.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kose S, Imamoto N, Tachibana T, Shimamoto T, Yoneda Y. Ran-unassisted nuclear migration of a 97-kD component of nuclear pore-targeting complex. J Cell Biol. 1997;139:841–849. doi: 10.1083/jcb.139.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kutay U, Bischoff F R, Kostka S, Kraft R, Görlich D. Export of importin alpha from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- 27.Kutay U, Lipowsky G, Izaurralde E, Bischoff F R, Schwarzmaier P, Hartmann E, Görlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- 28.Latimer M, Ernst M K, Dunn L L, Drutskaya M, Rice N R. The N-terminal domain of IκB alpha masks the nuclear localization signal(s) of p50 and c-Rel homodimers. Mol Cell Biol. 1998;18:2640–2649. doi: 10.1128/mcb.18.5.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Littlewood T D, Evan G I. Helix-loop-helix transcription factors. 3rd ed. New York, N.Y: Oxford University Press; 1998. [Google Scholar]
- 30.Marchetti A, Abril-Marti M, Illi B, Cesareni G, Nasi S. Analysis of the Myc and Max interaction specificity with lambda repressor-HLH domain fusions. J Mol Biol. 1995;248:541–550. doi: 10.1006/jmbi.1995.0241. [DOI] [PubMed] [Google Scholar]
- 31.Mattaj I W, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- 32.Nagoshi E, Imamoto N, Sato R, Yoneda Y. Nuclear import of sterol regulatory element-binding protein-2, a basic helix-loop-helix-leucine zipper (bHLH-Zip)-containing transcription factor, occurs through the direct interaction of importin beta with HLH-Zip. Mol Biol Cell. 1999;10:2221–2233. doi: 10.1091/mbc.10.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakielny S, Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- 34.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 35.Palmeri D, Malim M H. Importin beta can mediate the nuclear import of an arginine-rich nuclear localization signal in the absence of importin alpha. Mol Cell Biol. 1999;19:1218–1225. doi: 10.1128/mcb.19.2.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Párraga A, Bellsolell L, Ferré-D'Amaré A R, Burley S K. Co-crystal structure of sterol regulatory element binding protein 1a at 2.3 Å resolution. Structure. 1998;6:661–672. doi: 10.1016/s0969-2126(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 37.Pemberton L F, Blobel G, Rosenblum J S. Transport routes through the nuclear pore complex. Curr Opin Cell Biol. 1998;10:392–399. doi: 10.1016/s0955-0674(98)80016-1. [DOI] [PubMed] [Google Scholar]
- 38.Rawson R B, Zelenski N G, Nijhawan D, Ye J, Sakai J, Hasan M T, Chang T Y, Brown M S, Goldstein J L. Complementation cloning of S2P, a gene encoding a putative metalloprotease required for intramembrane cleavage of SREBPs. Mol Cell. 1997;1:47–57. doi: 10.1016/s1097-2765(00)80006-4. [DOI] [PubMed] [Google Scholar]
- 39.Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 40.Roman C, Matera A G, Cooper C, Artandi S, Blain S, Ward D C, Calame K. mTFE3, an X-linked transcriptional activator containing basic helix-loop-helix and zipper domains, utilizes the zipper to stabilize both DNA binding and multimerization. Mol Cell Biol. 1992;12:817–827. doi: 10.1128/mcb.12.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai J, Nohturfft A, Cheng D, Ho Y K, Brown M S, Goldstein J L. Identification of complexes between the COOH-terminal domains of sterol regulatory element-binding proteins (SREBPs) and SREBP cleavage-activating protein. J Biol Chem. 1997;272:20213–20221. doi: 10.1074/jbc.272.32.20213. [DOI] [PubMed] [Google Scholar]
- 42.Sakai J, Nohturfft A, Goldstein J L, Brown M S. Cleavage of sterol regulatory element-binding proteins (SREBPs) at site-1 requires interaction with SREBP cleavage-activating protein: evidence from in vivo competition studies. J Biol Chem. 1998;273:5785–5793. doi: 10.1074/jbc.273.10.5785. [DOI] [PubMed] [Google Scholar]
- 43.Sakai J, Rawson R B, Espenshade P J, Cheng D, Seegmiller A C, Goldstein J L, Brown M S. Molecular identification of the sterol-regulated luminal protease that cleaves SREBPs and controls lipid composition of animal cells. Mol Cell. 1998;2:505–514. doi: 10.1016/s1097-2765(00)80150-1. [DOI] [PubMed] [Google Scholar]
- 44.Sato R, Inoue J, Kawabe Y, Kodama T, Takano T, Maeda M. Sterol-dependent transcriptional regulation of sterol regulatory element-binding protein-2. J Biol Chem. 1996;271:26461–26464. doi: 10.1074/jbc.271.43.26461. [DOI] [PubMed] [Google Scholar]
- 45.Sekimoto T, Imamoto N, Nakajima K, Hirano T, Yoneda Y. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J. 1997;16:7067–7077. doi: 10.1093/emboj/16.23.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sekimoto T, Nakajima K, Tachibana T, Hirano T, Yoneda Y. Interferon-γ-dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J Biol Chem. 1996;271:31017–310120. doi: 10.1074/jbc.271.49.31017. [DOI] [PubMed] [Google Scholar]
- 47.Tachibana T, Hieda M, Sekimoto T, Yoneda Y. Exogenously injected nuclear import factor p10/NTF2 inhibits signal- mediated nuclear import and export of proteins in living cells. FEBS Lett. 1996;397:177–182. doi: 10.1016/s0014-5793(96)01180-5. [DOI] [PubMed] [Google Scholar]
- 48.Truant R, Cullen B R. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol Cell Biol. 1999;19:1210–1217. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vetter I R, Arndt A, Kutay U, Görlich D, Wittinghofer A. Structural view of the Ran-Importin beta interaction at 2.3 Å resolution. Cell. 1999;97:635–646. doi: 10.1016/s0092-8674(00)80774-6. [DOI] [PubMed] [Google Scholar]
- 50.Weis K, Ryder U, Lamond A I. The conserved amino-terminal domain of hSRP1 alpha is essential for nuclear protein import. EMBO J. 1996;15:1818–1825. [PMC free article] [PubMed] [Google Scholar]
- 51.Wozniak R W, Rout M P, Aitchison J D. Karyopherins and kissing cousins. Trends Cell Biol. 1998;8:184–188. doi: 10.1016/s0962-8924(98)01248-3. [DOI] [PubMed] [Google Scholar]
- 52.Yoneda Y. How proteins are transported from cytoplasm to the nucleus. J Biochem (Tokyo) 1997;121:811–817. doi: 10.1093/oxfordjournals.jbchem.a021657. [DOI] [PubMed] [Google Scholar]
- 53.Yoneda Y. Nucleocytoplasmic protein traffic and its significance to cell function. Genes Cells. 2000;5:777–787. doi: 10.1046/j.1365-2443.2000.00366.x. [DOI] [PubMed] [Google Scholar]
- 54.Yoneda Y, Hieda M, Nagoshi E, Miyamoto Y. Nucleocytoplasmic protein transport and recycling of Ran. Cell Struct Funct. 1999;24:425–433. doi: 10.1247/csf.24.425. [DOI] [PubMed] [Google Scholar]