Abstract
Objective:
To determine the extent to which post-discharge feeding behaviors and interactions among caregiver-preterm infant dyads are associated with infant neurodevelopment at one year corrected gestational age (CGA).
Study design:
We studied 119 preterm infants born <34 weeks’ gestation and <1,750 g at birth, and their caregivers, enrolled in the Collaborative Home Infant Monitoring Evaluation (CHIME) with in-person feeding assessments according to the Nursing Child Assessment Feeding Scale (NCAFS) at 39 to 59 weeks post-menstrual age (PMA) that completed Bayley II neurodevelopmental testing at one year CGA.
Results:
Mean ± standard deviation (SD) gestational age was 29.6 ± 2.4 weeks and birth weight was 1260 ± 320 grams. After adjustment for maternal and infant demographics, infant gestational age at birth and discharge and birth weight, mode of infant feeding and caregiver type during the post-discharge NCAFS assessment, overall NCAFS scores were positively associated with higher one-year CGA Bayley Mental Developmental Index (MDI) scores (for each 1 SD increase in overall NCAFS score, MDI increased by 2.8 [95% confidence interval {CI} 0.7, 4.9] points). Among individual NCAFS domains, strongest effects were seen for caregiver responsiveness to infant distress, such that, compared to dyads with domain scores of 11 (highest possible score), the adjusted mean difference in MDI was 8.3 points (95% CI −15.2, −1.4) lower among dyads with scores <9.
Conclusion:
Caregiver-preterm infant feeding interaction and caregiver responsiveness to preterm infant feeding distress were associated with preterm infant Bayley MDI at one-year CGA. Caregiver-infant feeding interaction may represent a modifiable factor to improve the neurodevelopment of at-risk preterm infants.
Keywords: feeding difficulties, feeding styles, feeding behaviors, maternal-infant interaction
Introduction
At the time of discharge from a neonatal intensive care unit (NICU), caregivers, rather than trained NICU staff, assume the primary responsibility for preterm infant feeding. Instruction about feeding is an important task because, across a variety of home environments, caregivers may be ill prepared to manage an array of potential infant feeding difficulties. Feeding difficulties include oral-motor dysfunction, immature coordination of sucking, swallowing, and breathing, avoidant feeding, and poor appetite, which occur in an estimated one-third of preterm infants within the first three months following discharge.[1] Preterm infant feeding skills, including oral-motor skills, gross motor skills involving hand, eye, and mouth, and head and trunk, and communication or social skills to indicate feeding needs to caregivers, remain below the expected developmental level throughout the first year of life[2] and feeding difficulties in the weeks to months following discharge lead to long-standing feeding problems,[3–6] which are associated with slower infant growth[2] and poorer neurodevelopmental and behavioral outcomes.[7, 8] As a consequence, caregivers experience stress and anxiety related to infant feeding, and many experience difficulty recognizing and appropriately responding to post-discharge infant feeding cues.[9] Infant feeding is a frequent means in which caregivers and preterm infants interact following NICU discharge and early caregiver-infant interactions are known to predict developmental outcomes in this population.[10] Despite this, the impact of caregiver-preterm infant feeding behaviors and interactions on preterm infant neurodevelopment are poorly understood.
The Collaborative Home Infant Monitoring Evaluation (CHIME) was a multicenter, prospective longitudinal study of home infant cardiorespiratory monitoring, in which post-discharge caregiver and infant feeding behavior was assessed at 39 to 59 weeks post-menstrual age (PMA) using the Nursing Child Assessment Feeding Scale (NCAFS), and the Bayley II neurodevelopmental test was performed at one year corrected gestational age (CGA). Since the CHIME study included a relatively large number of preterm infants, the study provides a unique opportunity to gain insight into the relationship between post-discharge caregiver-infant feeding behaviors and interactions and subsequent infant neurodevelopment. The purpose of our study was to determine the extent to which measures of post-discharge caregiver-preterm infant feeding behavior and interaction, assessed using the NCAFS, are associated with preterm infant Bayley Mental Developmental Index (MDI) and Psychomotor Index (PDI) at one year CGA.
Methods
Study Sample
Study subjects were enrolled in five clinical sites (Chicago, Cleveland, Honolulu, Los Angeles, and Toledo) as part of the multicenter CHIME study.[11] Enrollment occurred from 1994 to 1998, and the institutional review boards at each study site approved this study. Among the 984 infants enrolled in CHIME, 437 were preterm infants with a birth weight of <1,750 grams and gestational age <34 completed weeks. Additional infant exclusion criteria for the CHIME study included >120 days of life at the time of discharge, current diagnosis of pneumonia confirmed by chest radiography, congenital heart disease (except asymptomatic patent ductus arteriosus, atrial septal defect, or small muscular ventricular septal defect), discharge with continuous oxygen therapy, drug treatment for lung disease (bronchodilators, diuretics, or steroids), gastroesophageal reflux, or seizures, surgical treatment for hydrocephalus, inborn error of metabolism, and/or congenital anomaly or chromosomal abnormality associated with risk for infant death or severe neurodevelopmental morbidity. Caregivers using illicit drugs or parental inability to communicate (non-English or Spanish speakers or no telephone) at the time of hospital discharge were also excluded. For this analysis of caregiver and preterm infant feeding behaviors and interactions and preterm infant neurodevelopmental outcomes, we also restricted our participants to caregiver-preterm infant dyads who completed post-discharge in-person feeding assessments at 39 to 59 weeks PMA and infant neurodevelopmental testing at one year CGA, for a final sample of 119 caregiver-preterm infant dyads.
Main predictor: Caregiver and Preterm Infant Feeding Interactions and Behaviors
Caregiver-preterm infant feeding behaviors and interactions were measured according to the NCAFS,[12] a validated instrument among term and preterm infants.[13] As stipulated in the NCAFS manual, trained observers systematically coded 76 feeding behaviors of caregivers and infants during a single, live, feeding event at 39 to 59 weeks PMA. According to the NCAFS, each feeding behavior was worth 1 point, and caregiver-infant feeding interaction was measured as the overall score, or total number of points achieved during the feeding episode (ranwith 0 to 76).[12] The NCAFS feeding behaviors were also categorized into six domains, with a specified number of feeding behaviors that were observed during the feeding episode. When the specified feeding behavior was noted 1 time or more, a point was given toward that subscale. The four caregiver domains included: 1) sensitivity to cues (0–16 points); 2) response to child’s feeding distress (0–11 points); 3) social-emotional growth fostering (0–14 points); and 4) cognitive growth fostering (0–9 points). The two infant domains included: 1) clarity of cues (0–15 points); and 2) responsiveness to the caregiver (0–11 points). Higher points given on the total score and each subscale represented better feeding performance. No points were given when the specified feeding behavior was not observed or if “negative” behaviors were observed. Study personnel that performed the NCAFS assessments completed training and reliability was achieved as stipulated by Sumner and Spietz, developers of the NCAFS. [12]
In the original CHIME study, NCAFS assessments were planned at 44 and 56 weeks PMA; however, only 57% of caregiver-preterm dyads in our sample had both assessments performed, compared with 15% at 56 week PMA and 27% at 44 week PMA assessments only. Since we believed that assessments at older infant ages would represent more persistent feeding behaviors and would be more relevant to our study question, we used the older NCAFS assessment whenever possible. Specifically, for caregiver-preterm infant dyads with both the 44 and 56 week PMA assessment, we used the 56 week PMA assessment, so that in the final analysis of 119 caregiver-preterm infant dyads with post-discharge NCAFS assessments, 86 dyads had a 56 week PMA assessment and 33 dyads had a 44 week PMA assessment.
Main outcome: Infant neurodevelopment
Neurodevelopment was measured according to the Bayley Scales of Infant Development 2nd edition (BSID-II) at one year CGA[14] by trained research staff. The scales yield a MDI, reflecting memory, language, and problem-solving abilities and a PDI, measuring gross and fine motor control and coordination. Normative data from the scales yield a mean of 100 (standard deviation [SD], 15). Interrater reliability at all the study sites was high (interclass correlation was 0.91 for the MDI and 0.87 for the PDI).[15]
Covariates:
Infant sex, gestational age, weight at birth and discharge, presence of medical morbidities, including receipt of mechanical ventilation, and discharge medications were abstracted from the medical record and/or parent interview. Maternal demographic information and report of maternal smoking and alcohol use during pregnancy was obtained by maternal interview. Mode of infant feeding (breast, bottle, or solid feeding) and caregiver type (mother, father, or other) was recorded at the time of the in-person post-discharge feeding assessments
Statistical Analysis
Differences in participant characteristics that were enrolled in the original CHIME study (n = 437) and those with post-discharge NCAFS testing and Bayley developmental testing at one year CGA (n = 119) were examined, using t-tests for continuous variables and chi-square tests for categorical variables. Next, summary statistics for our main predictors, outcomes, and covariates were examined (Table 1). Multivariable linear regression was used to examine associations of total NCAFS score and NCAFS domain scores (expressed per 1 SD) with Bayley MDI and PDI scores at one year CGA. Models were adjusted for covariates of a priori interest that are known to be related to caregiver or preterm infant feeding behaviors, or infant development that may confound the main associations, which included maternal age, race, education level, and smoking or alcohol use during pregnancy, and infant birth weight, gestational age at birth or discharge, sex, and receipt of mechanical ventilation. Type of caregiver (mother vs. non-mother) and mode of infant feeding (directly breastfed vs. not) at the post-discharge NCAFS assessment were also included as covariates (Figure 1A and 1B).
Table 1.
Characteristics of 119 Caregiver-Preterm Infant Pairs
| Infant characteristics | N = 119 |
|---|---|
| Birth weight, grams | |
| mean (SD) | 1260 (320) |
| median (upper and lower extremes) | 1300 (466,1745) |
|
| |
| Gestational age, weeks | |
| mean (SD) | 29.6 (2.4) |
| median (upper and lower extremes) | 30 (24,34) |
|
| |
| PMA at discharge, weeks | |
| mean (SD) | 36.1 (1.9) |
| median (upper and lower extremes) | 36 (32,41) |
|
| |
| Sex, N (%) | |
| Male | 56 (47) |
| Female | 63 (53) |
|
| |
| Received mechanical ventilation, N (%) | |
| Yes | 85 (71) |
| No | 34 (29) |
|
| |
| Mother characteristics | |
|
| |
| Age at delivery, years | |
| mean (SD) | 29.2 (6.0) |
| median (upper and lower extremes) | 29 (15,43) |
|
| |
| Race/ethnicity, N (%) | |
| White | 54 (46) |
| Black | 23 (20) |
| Hispanic | 16 (14) |
| Asians | 20 (17) |
| Other | 5 (4) |
|
| |
| Education | |
| Did not complete HS or obtain GED | 12 (10) |
| High school/GED | 50 (43) |
| Some college | 29 (25) |
| College or beyond | 26 (22) |
|
| |
| Any smoking during pregnancy, N (%) | |
| Yes | 28 (24) |
| No | 89 (76) |
|
| |
| Any alcohol during pregnancy, N (%) | |
| Yes | 62 (53) |
| No | 55 (47) |
|
| |
| Characteristics of the post-discharge NCAFS Feeding Assessment | |
|
| |
| Infant PMA at the NCAFS Feeding Assessment, weeks | |
| mean (SD) | 53.0 (5.9) |
| median (upper and lower extremes) | 56 (39,59) |
|
| |
| Type of caregiver present, N (%) | |
| Mother | 102 (86) |
| Non-mother | 17 (14) |
|
| |
| Mode of infant feeding, N (%) | |
| Directly breastfed | 13 (11) |
| Did not directly breastfeed | 106 (89) |
|
| |
| Caregiver-Preterm Infant Feeding Interaction (Total NCAFS) | |
| mean (SD) | 62.1 (8.5) |
| median (upper and lower extremes) | 64 (38,76) |
|
| |
| Caregiver Feeding Domains | |
|
| |
| Sensitivity to Cues (0 to 16 points) | |
| mean (SD) | 14.1 (2.0) |
| median (upper and lower extremes) | 15 (7,16) |
|
| |
| Responsive to Distress (0 to 11 points) | |
| mean (SD) | 10.2 (1.3) |
| median (upper and lower extremes) | 11 (4,11) |
|
| |
| Social/Emotional Growth Fostering (0 to 14 points) | |
| mean (SD) | 11.4 (2.2) |
| median (upper and lower extremes) | 12 (6,14) |
|
| |
| Cognitive Growth Fostering (0 to 9 points) | |
| mean (SD) | 6.7 (2.2) |
| median (upper and lower extremes) | 8 (1, 9) |
|
| |
| Preterm Infant Feeding Domains | |
|
| |
| Clarity of Cues (0 to 15 points) | |
| mean (SD) | 12.5 (1.6) |
| median (upper and lower extremes) | 13 (9,15) |
|
| |
| Responsiveness to Caregiver (0 to 11 points) | |
| mean (SD) | 7.3 (2.2) |
| median (upper and lower extremes) | 8 (2,11) |
|
| |
| Characteristics of the one year neurodevelopmental Follow-up | |
|
| |
| Infant PMA at the neurodevelopmental follow-up, weeks | |
| mean (SD) | 92.3 (1.8) |
| median (upper and lower extremes) | 92 (87,96) |
|
| |
| Bayley II MDI | |
| mean (SD) | 97.7 (11.1) |
| median (upper and lower extremes) | 96 (72,125) |
|
| |
| Bayley II PDI | |
| mean (SD) | 94.3 (15.8) |
| median (upper and lower extremes) | 93 (50,149) |
SD = standard deviation, PMA = post-menstrual age, HS = high school, GED = general education development, NCAFS = Nursing Child Assessment Feeding Scale, MDI = mental developmental index; PDI = psychomotor developmental index
Figure 1A.
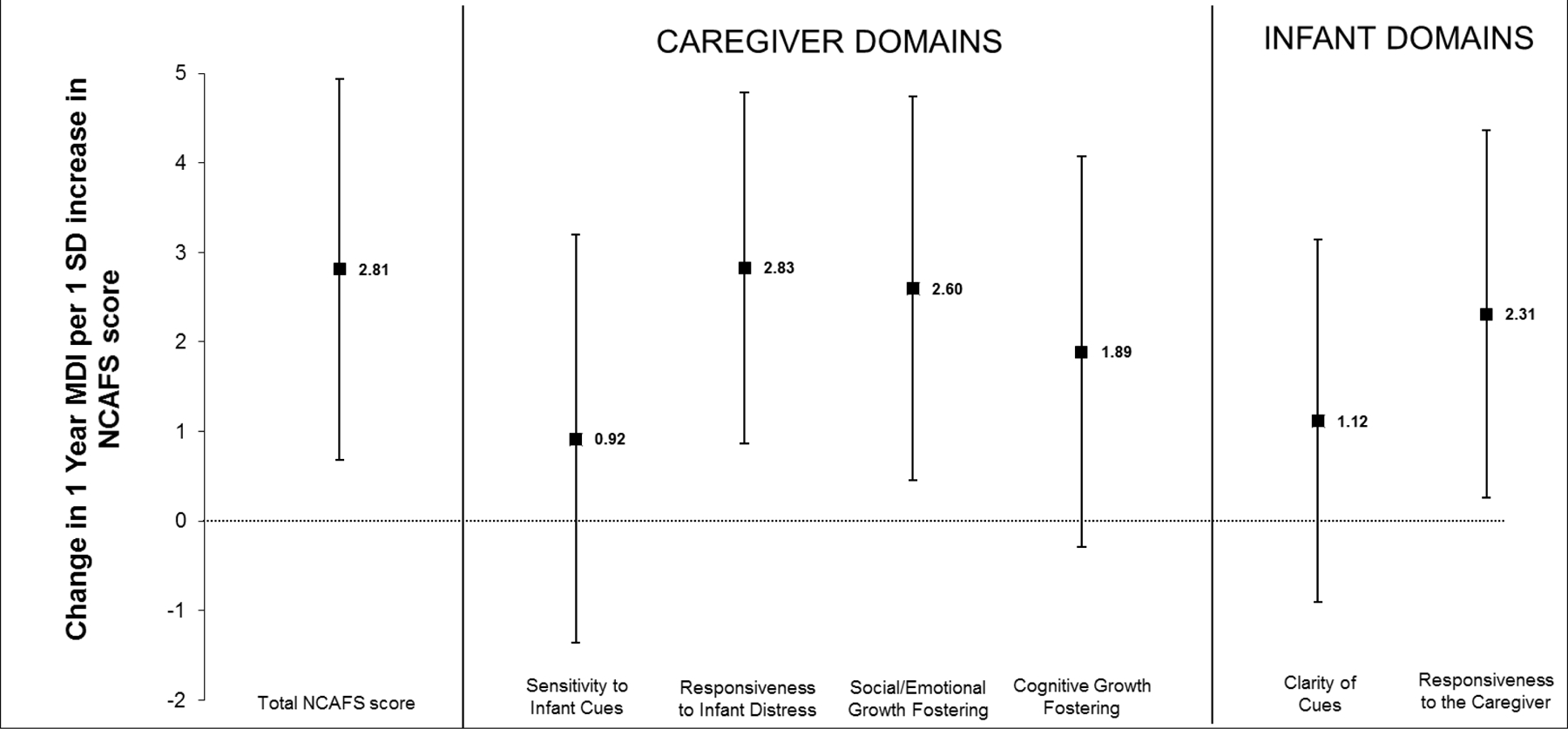
MDI = mental developmental index; NCAFS = Nursing Child Assessment Feeding Scale; SD = standard deviation
Adjusted changes (and 95% confidence intervals) in Bayley II MDI at one year corrected gestational age, associated with a 1 SD increase in total NCAFS score and in the scores for each domain of the NCASF among 119 caregiver-preterm infant pairs are shown.
Linear regression models were adjusted for infant gestational age at birth and discharge, birth weight, gender and receipt of mechanical ventilation; maternal age, race, education status and smoking and alcohol use during pregnancy; and mode of infant feeding (direct breastfeeding or not) and type of caregiver (mother or not) at the post-discharge NCAFS visit. Note, when the confidence interval does not include 0, this indicates p < 0.05.
Figure 1B.
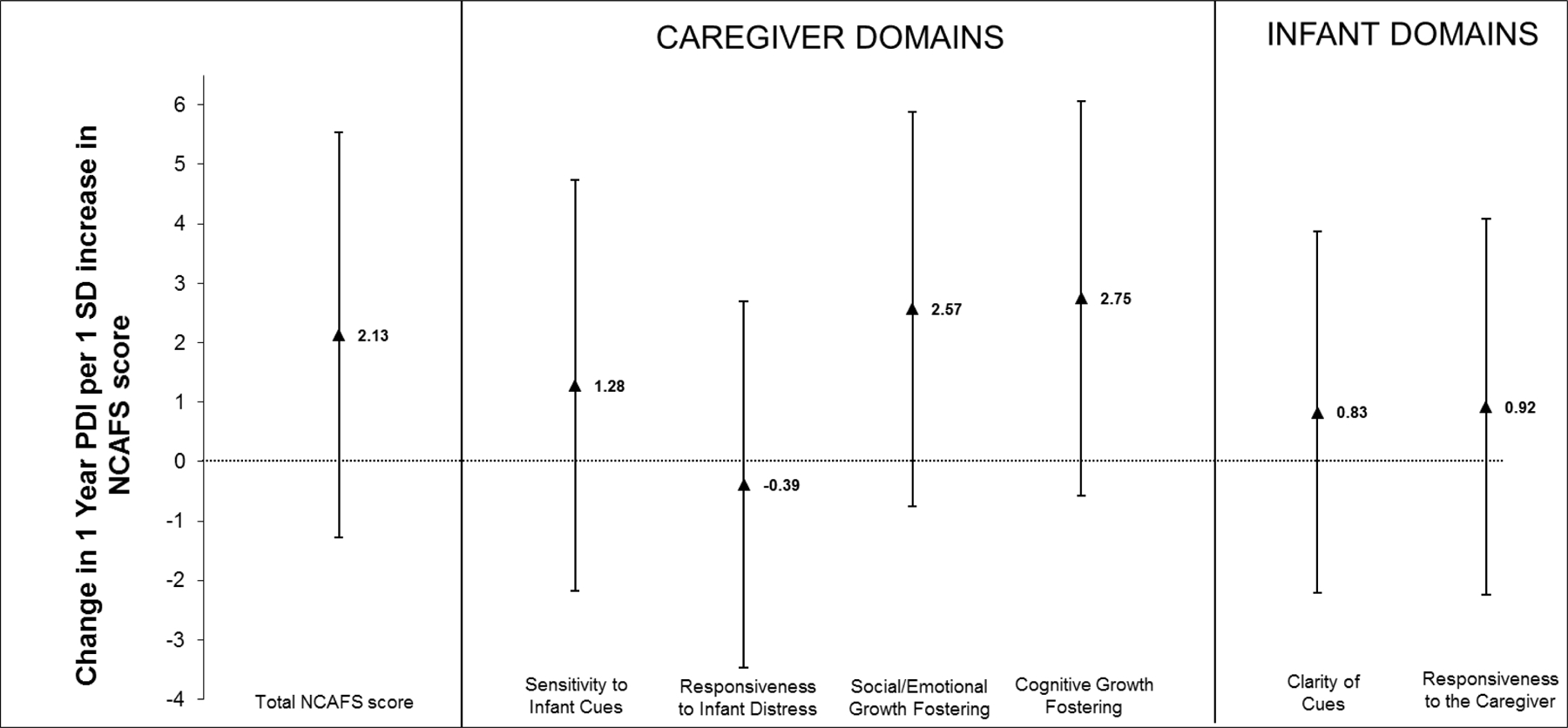
PDI = psychomotor developmental index; NCAFS = Nursing Child Assessment Feeding Scale; SD = standard deviation
Adjusted changes (and 95% confidence intervals) in Bayley II PDI at one year corrected gestational age, associated with a 1 SD increase in total NCAFS score and in the scores for each domain of the NCASF among 119 caregiver-preterm infant pairs are shown.
Linear regression models were adjusted for infant gestational age at birth and discharge, birth weight, gender and receipt of mechanical ventilation; maternal age, race, education status and smoking and alcohol use during pregnancy; and mode of infant feeding (direct breastfeeding or not) and type of caregiver (mother or not) at the post-discharge NCAFS visit. Note, when the confidence interval does not include 0, this indicates p < 0.05.
When examining the distribution of NCAFS scores, we observed that the caregiver responsiveness to infant distress domain (0 to 11 possible points) was skewed and demonstrated a “ceiling” effect, in which 54% of the sample received “perfect scores” of 11 points, with caregivers receiving one point for all 11 possible behaviors. For this reason, additional multiple linear regression analysis using categories of this domain was performed, rather than the continuous score. Our categories were: (1) a group of “perfect scores” of 11 points (64/119; 54%) (reference group); (2) a group with 10 points (32/119; 27%); (3) a group with 9 points (12/119; 10%); and (4) a group with < 9 points (11/119; 9%); range was 2 to 8 points).
Lastly, correlations between the 44- and 56-week NCAFS assessments and a sensitivity analysis using the NCAFS assessment performed at the youngest (rather than the oldest) age available were performed. SAS 9.3 was used to complete our analysis.
Results
The final sample of 119 caregiver-preterm infant pairs differs from the original CHIME cohort of 437 caregiver-preterm infant pairs[11] in the following ways: mothers in our final sample with complete post-discharge data, compared with mothers in the original cohort, were older (29.2 vs. 27.9 years), more often white (46% vs. 35%), more likely to have completed high school or have passed a general education development (GED) test (43% vs. 35%), and more likely to report alcohol use during pregnancy (53% vs. 42%). No differences were found among infant characteristics. Table 1 summarizes sample characteristics of our cohort.
Figure 1A and 1B summarizes multivariate associations between caregiver-preterm infant total NCAFS and individual NCAFS domain scores and preterm infant neurodevelopment. In adjusted models, higher (better) overall NCAFS scores were associated with higher Bayley MDI scores at one year CGA (Figure 1A). For each 1 SD increase in overall NCAFS score, MDI increased by 2.8 [95% confidence interval [CI] 0.7, 4.9] points. Among individual NCAFS domains, all effect estimates were in the direction of better NCAFS domain scores related to higher MDI scores. Associations of two caregiver domains (responsiveness to infant feeding distress, 2.8 [95% CI 0.9, 4.8] and social/emotional growth fostering, 2.6 [95% CI 0.5, 4.7]), and one infant domain (responsiveness to the caregiver, 2.3 [95% CI (0.3, 4.4]) were statistically significant (Figure 1A). No associations were found between NCAFS scores and Bayley PDI at 1 year CGA (Figure 1B).
Figure 2 summarizes multivariate associations between categories of caregiver responsiveness to feeding distress and preterm infant Bayley MDI score at one year CGA. Caregiver-preterm infant dyads with domain scores of 11 points had a mean Bayley MDI of 100, whereas, the mean Bayley MDI of caregiver-preterm infant dyads with domain scores <9 was 93.3 (compared to those with a score of 11, the adjusted difference in MDI was 8.3 points lower [95% CI −15.2, −1.4.]). We did not find associations between categories of caregiver responsiveness to feeding distress and preterm infant PDI scores at one year CGA.
Figure 2.
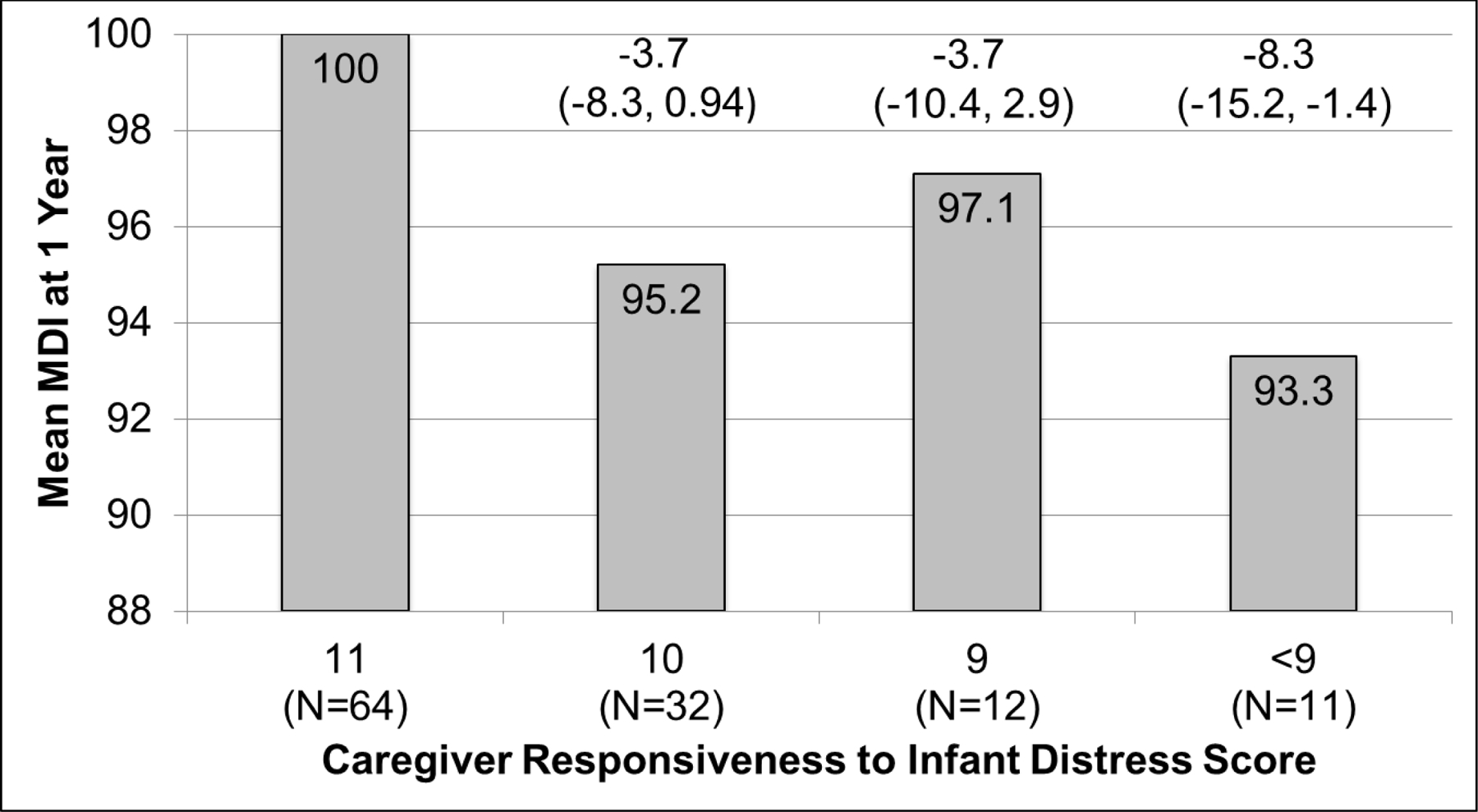
MDI = mental developmental index
Mean MDI at one year corrected gestational age for each category of post-discharge “caregiver responsiveness to infant feeding distress” NCAFS score are shown. Caregiver responsiveness to infant feeding distress domain was categorized as: 11 points (the highest possible score, where caregivers received a point for all 11 behaviors assessed), the reference group, with 54% of study sample; 10 points, (27% of study sample); 9 points (10% of study sample); and < 9 points, 9% of study sample).
The adjusted difference (95% confidence intervals) in mean MDI compared to those with a score of 11 is shown above each bar. Note, when the confidence interval does not include 0, this indicates p < 0.05.
Logistic regression models were adjusted for infant gestational age at birth and discharge, birth weight, gender and receipt of mechanical ventilation; maternal age, race, education status and smoking and alcohol use during pregnancy; and mode of infant feeding (direct breastfeeding or not) and type of caregiver (mother or not) at the post-discharge NCAFS visit.
The correlations between the 44 and 56-week NCAFS assessments ranged from 0.15 to 0.55, with a p-value < 0.05. When we repeated the analysis using the NCAFS assessment performed at the youngest (rather than oldest) age available, we found effect estimates that were in a similar direction, but with wider confidence intervals.
Discussion
In this prospective cohort study of preterm infants following NICU discharge, better caregiver-preterm infant feeding interaction was associated with higher infant Bayley MDI scores at one year CGA. Among individual NCAFS domains, strongest effects were seen among caregiver responsiveness to infant feeding distress. These results suggest that caregiver-preterm infant feeding interactions following NICU discharge may represent a modifiable mechanism to enhance the neurodevelopmental outcome for this at-risk population.
The most common caregivers of preterm infants in the home environment are mothers. Studies examining maternal-infant interactions during play episodes in the first year of life show that adverse interactions are more common among mother-preterm infant dyads[16] and are associated with lower scores on childhood neurodevelopmental testing.[10] In the first weeks to months following hospital discharge, a frequent way that mothers interact with their preterm infants is through infant feeding. During this time period, many preterm infants experience on-going difficulties[1] and mothers feel unequipped and apprehensive in feeding their infants.[9] In our cohort, in which 85% of our caregivers were mothers, mother-preterm infant feeding interaction was associated with infant Bayley MDI scores at one year CGA. We speculate that feeding interactions in the first months following NICU discharge may represent a more global style of maternal-infant interaction that continues throughout infancy and may have important impact on long-term neurodevelopment of at-risk preterm infants. The possibility of providing interventions that may improve mother-preterm infant feeding interaction is supported by White-Traut et al who found improved total NCAFS scores at 6 weeks corrected age among low income mother-preterm infant dyads that received a pre- and post-discharge intervention in which infants received a systematically applied sensory stimulation responsive to infant behavior and mothers received guidance sessions in infant interaction, compared with controls.[17] Hane et al. found improved maternal caregiving behavior during infant holding and feeding episodes among mothers of preterm infants that participated in a NICU-based intervention in which mothers were assisted by a “nurture specialist” in a variety of soothing and calming behaviors designed to improve the emotional connection of mother-infant dyads, compared to controls [18].
Relatively few studies have examined relationships between post-discharge mother and preterm infant feeding behaviors and longer-term child outcomes. Adams-Chapman et al found that ex-preterm infants with severe feeding dysfunction at 18–22 months corrected age were more likely to have lower cognitive and language scores on Bayley III developmental testing.[7] This study differs from ours because their analysis was restricted to infants with severe feeding difficulties, including need for a tube feedings, gagging with feeds, history of aspiration, and swallowing difficulty.[7] Few of the infants in the CHIME study would have met this standard. Our study extends upon these findings, as we additionally demonstrated associations between post-discharge infant, as well as caregiver, feeding behaviors and infant Bayley MDI scores among a “healthier” cohort of preterm infants. In contrast, our findings differ from the trial by Pridham et al randomizing mothers to guidance in optimal feeding behaviors toward their preterm infants in the first year of life following hospital discharge[19]. In this trial, infant neurodevelopment was measured by a 13-item questionnaire at eight months corrected age and worse scores were found in the intervention group. However, neurodevelopment was not the main outcome of this study, but was used to characterize the study sample. Unlike our study, analyses focused on neurodevelopment in the Pridham et al study were not adjusted for covariates.[19]
Taken together, findings of our study and others suggest that investigations of caregiver and preterm infant feeding behaviors that begin in the NICU and develop further in the home environment are warranted. These caregiver-preterm interactions influence neurodevelopment and improvement in feeding behaviors of caregivers and infants may represent modifiable mechanisms to improve further the developmental outcome of at-risk preterm infants. Ideally, future studies would also differentiate adaptive and maladaptive caregiver feeding behaviors toward preterm infants with and without known feeding difficulties and would utilize robust measurements of long-term neurodevelopment.
Our study has several limitations. The feeding behaviors and interactions measurements were limited to post-discharge NCAFS assessments of mainly bottle-fed infants from 39 to 59 weeks PMA only. Thus, we were unable to examine longitudinal tracking of feeding behaviors and interactions over time and impact on infant neurodevelopment. The CHIME study also enrolled infants during the mid to late 1990’s, which raises concern that our findings may not be generalizable to current populations of preterm infants. However, enrollment did occur following the introduction of routine neonatal surfactant use, and the prevalence of major medical morbidities among preterm infants has not dramatically changed.[20, 21] Additionally, the NCAFS scores among our cohort are similar to other more contemporary cohorts,[17] suggesting that our findings may generalize to preterm infants with similar birth weights and gestational ages born today. In addition, neurodevelopmental outcome was assessed only at one year CGA, and measurement of neurodevelopment at older ages are more predictive of longer-term cognitive and physical function.[22] Examination of feeding behaviors during infancy and neurodevelopmental outcomes and older ages may be informative in understanding long-term neurodevelopmental risks. It is also important to note that only approximately 25% of the original CHIME preterm cohort returned for one year developmental follow-up. We have adjusted for the demographic factors that differed between the original and final cohort. Finally, we were not able to control for a number of other factors known to impact infant neurodevelopmental outcomes, including: parental intelligence quotient, quality of the home environment, and parental mental health.[23] These factors, or other unknown factors, may be sources of residual confounding. Despite these limitations, our study represents a step forward understanding the important role of caregiver-preterm infant feeding behaviors and interactions in the home environment and impact on longer term preterm infant neurodevelopment. Improvement in feeding behaviors of caregivers and infants may represent modifiable mechanisms to further improve the neurodevelopment of at-risk preterm infants.
List of abbreviations:
- CGA
Corrected Gestational Age
- CHIME
Collaborative Home Infant Monitoring Evaluation
- MDI
Mental Developmental Index
- NCAFS
Nursing Child Assessment Feeding Scale
- NICU
Neonatal Intensive Care Unit
- PDI
Psychomotor Developmental Index
- PMA
Post-Menstrual Age
Footnotes
The authors have no conflicts of interest to disclose.
References Cited
- [1].DeMauro SB, Patel PR, Medoff-Cooper B, Posencheg M, Abbasi S. Postdischarge feeding patterns in early- and late-preterm infants. Clin Pediatr (Phila) 2011;50:957–62. [DOI] [PubMed] [Google Scholar]
- [2].Pridham K, Steward D, Thoyre S, Brown R, Brown L. Feeding skill performance in premature infants during the first year. Early Hum Dev 2007;83:293–305. [DOI] [PubMed] [Google Scholar]
- [3].Hawdon JM, Beauregard N, Slattery J, Kennedy G. Identification of neonates at risk of developing feeding problems in infancy. Dev Med Child Neurol 2000;42:235–9. [DOI] [PubMed] [Google Scholar]
- [4].Mathisen B, Worrall L, Masel J, Wall C, Shepherd RW. Feeding problems in infants with gastro-oesophageal reflux disease: a controlled study. J Paediatr Child Health 1999;35:163–9. [DOI] [PubMed] [Google Scholar]
- [5].Nelson SP, Chen EH, Syniar GM, Christoffel KK. One-year follow-up of symptoms of gastroesophageal reflux during infancy. Pediatric Practice Research Group. Pediatrics 1998;102:E67. [DOI] [PubMed] [Google Scholar]
- [6].Samara M, Johnson S, Lamberts K, Marlow N, Wolke D. Eating problems at age 6 years in a whole population sample of extremely preterm children. Dev Med Child Neurol 2009;52:e16–22. [DOI] [PubMed] [Google Scholar]
- [7].Adams-Chapman I, Bann CM, Vaucher YE, Stoll BJ. Association between feeding difficulties and language delay in preterm infants using Bayley Scales of Infant Development-Third Edition. J Pediatr 2013;163:680–5 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Motion S, Northstone K, Emond A, and ALSPAC Study Team. Persistent early feeding difficulties and subsequent growth and developmental outcomes. Ambulatory Child Health 2001;7:231–7. [Google Scholar]
- [9].Reyna BA, Pickler RH, Thompson A. A descriptive study of mothers’ experiences feeding their preterm infants after discharge. Adv Neonatal Care 2006;6:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Forcada-Guex M, Pierrehumbert B, Borghini A, Moessinger A, Muller-Nix C. Early dyadic patterns of mother-infant interactions and outcomes of prematurity at 18 months. Pediatrics 2006;118:e107–14. [DOI] [PubMed] [Google Scholar]
- [11].Ramanathan R, Corwin MJ, Hunt CE, Lister G, Tinsley LR, Baird T, et al. Cardiorespiratory events recorded on home monitors: Comparison of healthy infants with those at increased risk for SIDS. JAMA 2001;285:2199–207. [DOI] [PubMed] [Google Scholar]
- [12].Sumner G, Spietz A. NCAST: Caregiver/Parent-Child Interaction Feeding Manual Seattle, Washington: NCAST Publications; 1994. [Google Scholar]
- [13].Barnard KE, Hammond MA, Booth CL, Mitchell SK, Spieker SJ. Measurement and meaning of parent-child interaction. In: Morrison FJ, editor. Applied developmental psychology New York: Academic Press; 1989. p. 39–80. [Google Scholar]
- [14].Bayley N Bayley Scales of Infant Development 2nd Edition ed. San Antonio, Texas: The Psychological Corporation; 1993. [Google Scholar]
- [15].Ratliff-Schaub K, Hunt CE, Crowell D, Golub H, Smok-Pearsall S, Palmer P, et al. Relationship between infant sleep position and motor development in preterm infants. J Dev Behav Pediatr 2001;22:293–9. [DOI] [PubMed] [Google Scholar]
- [16].Muller-Nix C, Forcada-Guex M, Pierrehumbert B, Jaunin L, Borghini A, Ansermet F. Prematurity, maternal stress and mother-child interactions. Early Hum Dev 2004;79:145–58. [DOI] [PubMed] [Google Scholar]
- [17].White-Traut R, Norr KF, Fabiyi C, Rankin KM, Li Z, Liu L. Mother-infant interaction improves with a developmental intervention for mother-preterm infant dyads. Infant Behav Dev 36:694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hane AA, Myers MM, Hofer MA, Ludwig RJ, Halperin MS, Austin J, et al. Family nurture intervention improves the quality of maternal caregiving in the neonatal intensive care unit: evidence from a randomized controlled trial. J Dev Behav Pediatr 2015;36:188–96. [DOI] [PubMed] [Google Scholar]
- [19].Pridham K, Brown R, Clark R, Limbo RK, Schroeder M, Henriques J, et al. Effect of guided participation on feeding competencies of mothers and their premature infants. Research in nursing & health 2005;28:252–67. [DOI] [PubMed] [Google Scholar]
- [20].Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics 2002;110:143–51. [DOI] [PubMed] [Google Scholar]
- [21].Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129:1019–26. [DOI] [PubMed] [Google Scholar]
- [22].Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics 2005;116:333–41. [DOI] [PubMed] [Google Scholar]
- [23].Ronfani L, Vecchi Brumatti L, Mariuz M, Tognin V, Bin M, Ferluga V, et al. The Complex Interaction between Home Environment, Socioeconomic Status, Maternal IQ and Early Child Neurocognitive Development: A Multivariate Analysis of Data Collected in a Newborn Cohort Study. PLoS One 2015;10:e0127052. [DOI] [PMC free article] [PubMed] [Google Scholar]


