Abstract
The kidneys are a vital organ that is vulnerable to both acute kidney injury (AKI) and chronic kidney disease (CKD) which can be caused by numerous risk factors such as ischemia, sepsis, drug toxicity and drug overdose, exposure to heavy metals, and diabetes. In spite of the advances in our understanding of the pathogenesis of AKI and CKD as well AKI transition to CKD, there is still no available therapeutics that can be used to combat kidney disease effectively, highlighting an urgent need to further study the pathological mechanisms underlying AKI, CKD, and AKI progression to CKD. In this regard, animal models of kidney disease are indispensable. This article reviews a widely used animal model of kidney disease, which is induced by folic acid (FA). While a low dose of FA is nutritionally beneficial, a high dose of FA is very toxic to the kidneys. Following a brief description of the procedure for disease induction by FA, major mechanisms of FA‐induced kidney injury are then reviewed, including oxidative stress, mitochondrial abnormalities such as impaired bioenergetics and mitophagy, ferroptosis, pyroptosis, and increased expression of fibroblast growth factor 23 (FGF23). Finally, application of this FA‐induced kidney disease model as a platform for testing the efficacy of a variety of therapeutic approaches is also discussed. Given that this animal model is simple to create and is reproducible, it should remain useful for both studying the pathological mechanisms of kidney disease and identifying therapeutic targets to fight kidney disease.
Keywords: acute kidney injury, chronic kidney disease, ferroptosis, fibroblast growth factor 23, folic acid, mitochondria, mitophagy, oxidative stress, pyroptosis
Folic acid induced animal model of kidney disease was reviewed along with the mechanisms of the pathogenesis involved. This model can provide an important platform for the testing of a variety of therapeutic approaches that are designed to fight kidney disease including both acute kidney injury and chronic kidney disease.
1. INTRODUCTION
Kidney disease may be generally classified clinically into two categories: acute kidney injury (AKI) and chronic kidney disease (CKD), both of which are tightly interconnected. 1 , 2 , 3 AKI can often develop in clinical settings in critically ill patients, leading to increased morbidity and mortality. 4 , 5 AKI is manifested by a rapid decline in the glomerular filtration rates (GFRs) 6 and its pathogenesis is complex, involving ischemia, sepsis, drug toxicity, and trauma. 7 If left unmanaged, AKI can develop into CKD, which is characterized by a progressive decrease in GFR, culminating in a gradual loss of renal function. 8 The transition from AKI to CKD can also be hastened by numerous risk factors such as obesity, hypertension, diabetes, and chronic inflammation. 9 , 10 , 11 Currently, there are no effective treatments for either AKI or CKD, stressing a continual need to elucidate the underlying pathological mechanisms of AKI and CKD. In this regard, animal models of kidney disease have been invaluable in that utilization of these animal models not only facilitates our understanding of the pathogenesis of kidney disease, but also provides excellent platforms for disease intervention whereby efficacy of testing compounds or pharmacological agents can be quantitatively assessed. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19
There are numerous animal models that have been used to elucidate the pathological mechanisms of kidney disease. 19 , 20 , 21 Those induced by ischemia, 14 , 22 , 23 lipopolysaccharide, 24 , 25 , 26 , 27 cisplatin, 28 , 29 , 30 arsenic, 31 , 32 , 33 adenine, 34 , 35 , 36 cadmium 15 , 37 , 38 , 39 , 40 and diabetes 41 , 42 , 43 , 44 , 45 , 46 are widely used as animal models of kidney disease. These models have also been used to test the therapeutic effect of a given drug or compound. 19 , 21 , 47 , 48 However, this article will focus on a very popular kidney disease animal model, the folic acid (FA)‐induced rodent model involving the use of both mouse and rat. 49 , 50 , 51 , 52 , 53 A comparison of the FA model with other chemically induced kidney injury animal models is given in Table 1.
TABLE 1.
Comparison of animal models of kidney injury induced by a variety of approaches
| Models | Species | Does range/duration GFR/BUN/Cre | Comments/advantages/disadvantages | Refs. |
|---|---|---|---|---|
| Folic acid | Mouse/rat |
250 mg/kg, 1 time I.P. Injection, 24–48 h AKI BUN: 65–80 AKI BUN: 300–350 (CKD) Cre: 1.2–1.4 (AKI) Cre: 6–7 (CKD) GFR: N.D. |
Reproducible and simple, useful for studying AKI–CKD transition but no clinical correlation | 54, 55 |
| LPS | Mouse/rat |
10–15 mg/kg, single I.P. usually for AKI BUN: 38–45 Cre: 0.5–0.7 GFR: N.D. |
Inexpensive, simple Response may vary between models |
19 |
| Cisplatin | Mouse/rat |
Single I.P. injection with widely ranging dose, 6–20 mg/kg, up to 3 days for AKI BUN: 70–80 Cre: 2.4–2.8; GFR: N.D. |
Reproducible and simple toxic to other organs, high dose needed for AKI induction | 56, 57, 58 |
| Cadmium | Mouse/rat |
1.2–6 mg/kg/day, oral administration or injection up to weeks for CKD induction BUN: 13–15 Cre: 1.4–1.8; GFR: N.D. |
Varying dosage and duration toxic to other organs, epidemiological relevant, single I.P. injection for AKI | 15, 59, 60, 61, 62 |
| Arsenic | Mouse/rat |
Varying dosage I.P injection for AKI induction, chronic drinking for CKD induction BUN: 28–38 Cre: 1.7–1.9; GFR: N.D. |
Varying dosage and duration, toxic to other organs, epidemiological relevant | 63, 64, 65 |
| Adenine | Mouse/rat |
0.15%−0.75% (w/w) in diet, Up to 16 weeks for CKD BUN: 90–120 Cre: 2.8–3.1; GFR; N.D. |
Not for AKI induction, time‐consuming for CKD | 66, 67, 68 |
| Ischemia | Mouse/rat |
30–40 min ischemia, 6–48 h reperfusion, AKI BUN: 160–280 Cre: 0.9–1.5; GFR: N.D. |
Requires surgery, reproducibility maybe an issue, clinical relevant | 69, 70, 71, 72, 73 |
| DKD | Mouse/rat |
Streptozotocin, 60–65 mg/kg single I.P. injection for rats, 30–40 mg/kg 5 injections for mice, type 2 diabetes can be induced by high fat diet‐streptozotocin administration BUN: 25–30 mM Cre: 58–65 µM, GFR: N.D. |
Not for AKI, time‐consuming, duration varies from lab to lab, streptozotocin handled with care, genetic models also available | 42, 74, 75, 76, 77, 78, 79, 80 |
| 5/6 Nx | Mouse/rat |
Invasive surgery required, for CKD induction, at least 1 week duration BUN: 17–19 mM Cre: 45–60 µM; GFR: N.D. |
Infection and kidney bleeding may occur | 81, 82, 83, 84 |
| Nicotine | Mouse/rat |
0.6–2.5 mg/kg I.P. injection up to 4 weeks for CKD induction BUN: 36–45 Cre: 0.75–0.82; GFR: N.D. |
Noninvasive and simple, good model for podocyte injury, requires long term treatment | 85, 86, 87, 88 |
| c‐BSA | Mouse/rat |
50 mg/kg c‐BSA via tail vein injection for up to 5 weeks for CKD induction, c‐BSA dosage and duration could vary BUN: 18–25 Cre: 2.3–2.6; GFR: N.D. |
Good model for membrane glomerulonephritis, chronic treatment required, c‐BSA Needs to be self‐prepared |
89, 90, 91, 92, 93 |
| UUO | Mouse/rat |
7–14 days, longer time for induction of kidney fibrosis BUN: 3.5–4.5 mM Cre: 42–58 µM, GFR: N.D. |
Facile, reproducible, requires surgery, not popular for creating an AKI model | 19, 94, 95, 96, 97, 98 |
This table is not meant to cover all the animal models of kidney injury in the literature. Rather, only popular and widely used animal models are listed. It should also be noted that when rats or mice are used, most investigators choose to use young adult animals aged from 4 to 8 weeks. Therefore, the reported kidney dysfunctional parameters may be different from those derived from old animals. Nonetheless, for a given age group of the same gender in a particular animal species, data may be comparable. For example, in the same lab setting, if every experimental condition is strictly followed, the severity of kidney disease induced by a single injection of FA may be classified based on BUN content as: mild, 40–80 mg/dl; moderate, 100–200 mg/dl; severe, greater than 200 mg/dl. 54 The values shown in the Table for blood BUN and creatinine as well as GFR, if any, are for reference only as these numbers may vary from investigator to investigator.
The unit for BUN and Cre is mg/dl unless otherwise indicated.
Abbreviations: 5/6 Nx, 5/6 nephrectomy; BUN, blood urea nitrogen (mg/dl); c‐BSA, cationic bovine serum albumin; Cre, creatinine (mg/dl); DKD, diabetic kidney disease; GFR, glomerular filtration rate; LPS, lipopolysaccharide; N.D., not determined; UUO, unilateral ureteral obstruction.
It is worth noting that among all the animal models of kidney disease induced by the variety of approaches highlighted in Table 1, the FA‐induced model provides certain advantages that are lacking in other models. First, FA is a vitamin and is not environmentally toxic, therefore routine handling in laboratories does not pose any hazards. Second, unlike ischemic surgery of kidney injury, of FA is administered as a simple injection, which does not require surgery and is noninvasive and animal friendly. Third, unlike the cadmium and cisplatin toxicity models, which induce multiple organ injury, the FA model mainly injures the kidney and has no deleterious effects on other organs. 99 Fourth, depending on the experimental needs, one can investigate AKI or CKD or the AKI–CKD transition using a single injection of FA. 55 Undeniably, the FA‐induced kidney injury model has its own disadvantages. These include the high dose of FA that needs to be injected and the failure as yet to identify a specific biomarker of FA‐induced kidney injury. Moreover, although FA‐induced kidney injury occurs mainly to proximal tubules, 54 , 100 a detailed molecular and biochemical mechanism underlying FA‐induced nephron injury remains to be unraveled. It should be noted that the FA kidney injury model does not mimic patients with membranous nephropathy or glomerulonephritis 101 , 102 or IgG4‐immuned kidney disease. 103 , 104 , 105 , 106
2. FOLIC ACID AND THE KIDNEYS
FA is also known as vitamin B9. 107 , 108 It is a cofactor involved in one‐carbon metabolism that is essential for cellular proliferation and growth. 109 , 110 , 111 FA can be derived from egg yolk, animal livers, leafy vegetables, and yeast. 112 , 113 FA is usually absorbed in the small intestine, and converted intracellularly to tetrahydrofolate by dihydrofolate reductase. 112 , 113 FA deficiency can cause megaloblastic anemia and neural tube defect in the fetus due to its indispensable role in the synthesis of RNA and DNA molecules. 113 , 114 , 115
As a small molecular weight compound, FA or folate is freely filtered by the glomerulus. 109 In fact, little folate renal excretion can be observed under normal folate concentrations and renal reabsorption of folate is nearly 100%. Renal reabsorption of folate is achieved by a high affinity folate receptor (folate receptor 1) that is abundant on the luminal side of proximal tubular epithelial cells. 109 Once folate is bound to the receptor, an endocytosis process occurs which is followed by release of folate via vesicle budding and recycling of the receptor onto the epithelial cell membranes. The released folate is believed to be trapped in endosomal vesicles, as no freely floating folate has been observed in the cytosol. 109 Subsequently, these endosomal vesicles could fuse with the membranes of other organelles and release folate, thereby leading to functional impairment of these organelles. Such is the case for mitochondria which can accumulate folate. 55 It should be noted that non‐endocytosis‐dependent folate transport systems also exist on tubular epithelial membranes but folate receptor‐mediated folate endocytosis is the most well elucidated mechanism. In mice lacking folate receptor due to folate receptor gene knockout, 116 folate clearance is nearly 100% and no reabsorption of folate could be observed, indicating that folate renal toxicity, as well as downstream signaling, is mediated by the folate receptor. 109
As mentioned above, FA can accumulate in larger amounts in the kidney than in other tissues because of the high content of folate receptors in the kidneys. 117 , 118 It is stored as folate derivatives that are cell membrane impermeable. 119 Importantly, while folate distributes in all cellular compartments, mitochondria can take up to 40% of the folate pool, 119 , 120 which can cause mitochondrial oxidative stress and mitochondrial abnormalities. 121 , 122 , 123 , 124 , 125 Moreover, as folate reduction by dihydrofolate reductase to form tetrahydrofolate uses large amounts of NADPH as a reducing power, 110 high levels of folate in the kidneys can severely compromise cellular antioxidative systems that also require NADPH, 126 , 127 leading to aggravated redox imbalance and oxidative stress in this organ. 128 , 129
3. HIGH DOSES OF FA AND RENAL INJURY
While low doses of FA (usually less than 10 mg/day) are beneficial and against oxidative stress, 130 , 131 , 132 , 133 high doses of FA, e.g., 250 mg/day, as widely used in the induction of animal kidney disease, are highly toxic. 134 , 135 A search in the PubMed database indicates that the first report of a renal problem caused by FA was published in 1968, 136 and described renal hypertrophy induced by FA. The first report of kidney injury induced by FA was published in Germany in 1969. 137 These studies led to the concepts of “renal folate toxicity” and “folate nephropathy” in 1970s. 138 , 139 , 140 , 141 , 142 , 143 , 144 Now, the procedures of FA‐induced kidney injury in mice and rats are well established and widely used. As outlined in Figure 1, in both mouse and rat models of acute kidney injury, a single injection of FA at a dosage of 250 mg/kg body weight intraperitoneally can cause AKI, 145 , 146 , 147 resulting in proteinuria and increased blood urea nitrogen (BUN) and creatinine. 148 , 149 AKI can be studied within 72 h of FA administration. 55 If left untreated, CKD will develop and can be studied more than 4 weeks or beyond after FA injection (Figure 1). 55 Multiple injections of a lower dose of FA (125–150 mg/kg body weight) 150 , 151 or a single injection of lower dose of FA (less than 200 mg/kg body weight) can also produce symptoms of kidney disease that can be used to investigate the pathological mechanisms of AKI or CKD. 152 , 153 , 154 Moreover, progression of AKI to CKD can also be investigated after a single high dose FA injection. 55 Therefore, FA‐induced kidney disease can cover AKI, CKD, and the AKI–CKD transition. 54 Additionally, as FA is water‐soluble and the injection is intraperitoneal, the procedure of kidney disease induction is simple and straightforward, without the need for surgery. Importantly, FA‐induced kidney disease can recapitulate the clinical symptoms of human kidney disease and the model is highly reproducible. 128 , 155
FIGURE 1.
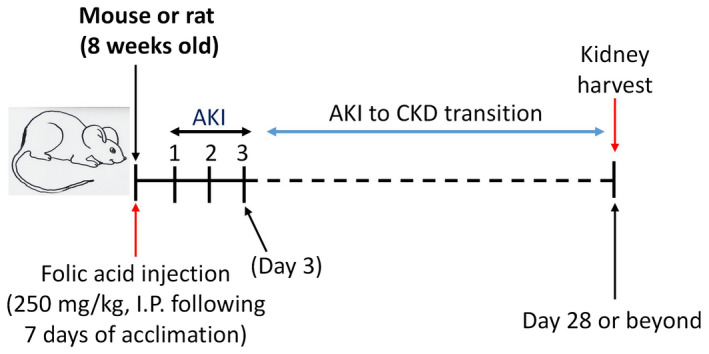
General experimental scheme of folic acid (FA)‐induced acute kidney injury (AKI) and chronic kidney disease (CKD). FA, usually at a dose of 250 mg/kg body weight, is prepared in 300 mM NaHCO3 and injected intraperitoneally. AKI may be investigated within 3 days of FA injection while CKD may be studied up to or beyond 28 days following FA injection
With respect to the FA‐induced AKI–CKD transition, the FA model may provide certain advantages over other models of AKI–CKD transition including the ischemic reperfusion injury model, the cisplatin toxicity mode, the diphtheria toxin model and the aristolochic acid model. As described above, the major advantage of the FA model is the one‐time administration of a high FA concentration, which leads to reproducibility. In contrast, in ischemic reperfusion injury studies of AKI–CKD transition, more ischemic surgeries may be required following the initial surgery, which can cause preconditioning effects and may also result in loss of animals during the study, thereby causing reproducibility issues. 18 The low dose cisplatin model, the diphtheria toxin model, and the aristolochi acid model all require repeated dosing of the animals in order for AKI to progress to CKD. An excellent review of animal models of AKI–CKD transition is provided by Fu et al. 18 Given that the mechanisms underlying AKI–CKD transition still remain elusive, cross‐examination and comparison of different AKI–CKD models may provide comprehensive insights into the mechanisms of AKI–CKD transition. Nonetheless, in the FA‐induced AKI–CKD transition model, it is clear that mitochondrial abnormalities, redox imbalance, oxidative stress, and deranged fatty acid oxidation are involved in AKI–CKD transition. 3 , 55 , 156 , 157
With respect to which site or region in the nephron is vulnerable to FA‐induced damage, it has been well established that FA damage occurs mainly to the proximal tubular epithelial cells (Figure 2). 151 , 158 , 159 , 160 , 161 After FA injection, urinary volume shows a decrease, as does GFR and the filtration fraction. This is followed by an elevation in the concentration of blood urea nitrogen and creatinine. 99 , 100 It should be noted that the concentration of folic acid used for intraperitoneal injection at a dose of 250 mg/kg body weight should not be higher than 12.5 mg/ml, as death of the animals has been observed when 25 mg/ml or 50 mg/ml of folic acid solution was used for AKI induction. 100 For administration doses of folic acid solution at 12.5 mg/ml, the death rate of animals beyond 28 days has not been well documented because the duration of studies after FA injection varies from laboratory to laboratory.
FIGURE 2.
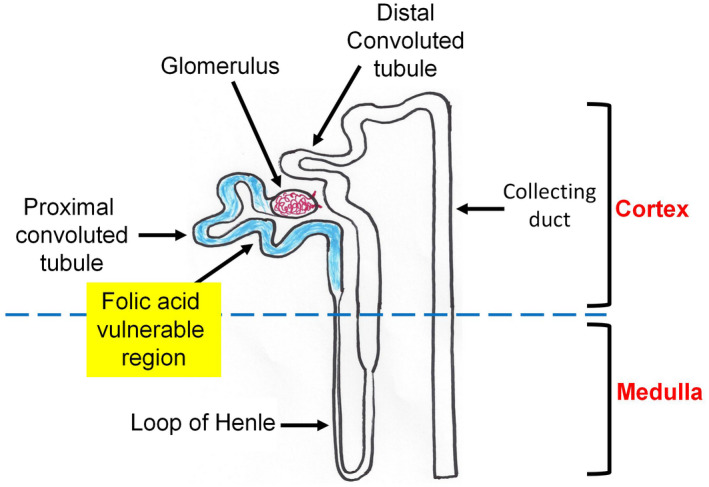
Diagram showing the proximal convoluted tubule in the nephron as the most vulnerable region to folic acid (FA)‐induced damage. The blue highlighted tubule depicts the proximal convoluted region
One question arising herein is that, if FA mainly damages the proximal tubules, then how does this damage lead to the lowered GFRs that have been observed in the FA rodent model. 100 This is likely caused by a tubular‐glomerular interplay response to intratubular pressure created collectively by FA crystallization in the renal tubules, blockage of the proximal tubules, and induction of tubular injury and cell death, 152 , 162 In fact, this tubular‐glomerular response is a well‐known feedback mechanism that also occurs in drug‐induced kidney toxicity 163 , 164 , 165 and ureteral obstruction kidney disease. 166 , 167
It should also be noted that the FA‐induced kidney injury model is only an experimental animal model because high levels of FA have not been observed in patients with CKD or associated with kidney disease progression. Nonetheless, the FA model recapitulates all the human AKI pathologies observed in the clinic. 55 Moreover, the FA model is highly reproducible. 55 In these respects, the FA experimental animal model is similar to streptozotocin‐induced type 1 diabetes animal models, 74 , 75 in that STZ does not exist at high levels in type 1 diabetic patients yet STZ diabetes induction recapitulates many of the clinical manifestations of these patients. As is inherent in all animal models of human diseases, any animal model of kidney disease will serve only as a proxy and will never be identical to human kidney disease.
Despite the inherent drawbacks, the FA model is also clinically relevant because accidental folic acid overdose can occur and cause AKI in humans that shares the major pathological processes of inflammation, fibrosis, cell death and proliferation seen in the FA mouse model. 168 , 169 Another clinical factor that supports the experimental utilization of the FA kidney disease model is use of the broadly employed anti‐cancer drug methotrexate, which is a derivative of folic acid and is highly toxic to the kidneys. 170 , 171
4. MAJOR MECHANISMS OF FA‐INDUCED KIDNEY INJURY
After a high dose of FA administration via IP injection, FA can quickly form crystals in the kidney within renal tubules, followed by acute tubular necrosis, epithelial regeneration, and renal cortical scarring, culminating in renal injury reflected by decreased glomerular filtration rates (GFRs), renal inflammation, 172 , 173 , 174 and renal fibrosis. 175 , 176 While this sequence of events sounds simple, the underlying biochemical and molecular mechanisms are complex and multifaceted. In general, after FA injection, renal hypertrophy occurs, serum BUN and creatinine are elevated, 128 clinical symptoms of acute renal failure such as attenuated alertness, fatigue or lethargy, and bristling of the coat can also be observed. 128 Here, the major mechanisms involved in FA‐induced kidney disease are summarized.
4.1. Oxidative stress
Numerous studies demonstrate renal oxidative stress in the FA‐induced kidney disease model. 55 , 128 , 155 For example, in FA‐AKI mouse model, Gupta et al. 128 found that lipid peroxidation was increased with a decreased level of the reduced form of glutathione. In the meantime, levels of hydrogen peroxide were increased, SOD activity was decreased, and glutathione peroxidase activity was also decreased, so was glutathione‐s‐transferase. These results indicate a redox imbalance status induced by FA injection.
4.2. Ferroptosis
Martin‐Sanchez et al. 177 demonstrated the involvement of ferroptosis in FA induced AKI. When ferroptosis was inhibited by ferrostatin‐1, a ferroptosis inhibitor, renal injury induced by FA could be prevented, together with a decreased occurrence of lipid peroxidation. The authors also found that ferroptosis triggered inflammation in the kidney upon FA injection was also attenuated by ferropstatin‐1 treatment, further demonstrating the role of ferroptosis in FA‐induced AKI. Moreover, when apoptosis or necrosis was targeted, no protection against AKI was observed, indicating that ferroptosis plays a more important role in AKI induced by FA, at least in the authors' experimental settings. It should be noted that other types of cell death such as pyroptosis and apoptosis have also been reported in FA‐induced kidney disease. 178 , 179
4.3. Impairment of mitochondrial bioenergetics
In an elegant study exploring the mechanisms of AKI–CKD transition after FA injection, Aparicio‐Trejo et al. 55 demonstrated that impaired mitochondrial bioenergetics was involved in FA‐induced renal injury. The authors analyzed mitochondrial complex I‐linked respiration using isolated mitochondria and found that state 3 respiration (in the presence of ADP) was decreased at the acute stage of renal injury, but returned to normal after 7 and 14 days, respectively, indicating that decreased complex I‐linked respiration could last up to 7 days. There was also a progressive electron leakage from AKI to CKD, further demonstrating the involvement of mitochondrial uncoupling in kidney disease transition from AKI to CKD. During this process, fatty acid β‐oxidation was also impaired, which may also contribute to the AKI–CKD transition process as well as renal fibrosis. 180 This study demonstrates that impairment of mitochondrial bioenergetics is involved in AKI, CKD, and AKI–CKD transition, further highlighting a key role of mitochondrial dysfunction in FA‐induced kidney disease. 181
4.4. Increased levels of fibroblast growth factor 23 (FGF23)
FGF23 is a protein that regulates phosphate homeostasis and vitamin D metabolism. 182 The content of this protein has been shown to increase rapidly upon FA‐induced AKI. 183 , 184 , 185 This upregulation of FGF23 is likely controlled by interleukin‐6 (IL‐6) as IL‐6 inhibition by dexamethasone abolished FGF23 upregulation in FA‐induced AKI. 185 In contrast, overexpression of IL‐6 could further increase FGF23 levels both in vivo and in vitro. These results demonstrate the involvement of increased FGF23 content in FA‐induced AKI, likely due to dysregulation of phosphate homeostasis and vitamin D metabolism. However, whether there is a link between increased FGF23 and elevated oxidative stress in the FA‐induced AKI model remains elusive at the present time.
4.5. Impaired mitophagy
Mitophagy is a mechanism by which damaged mitochondria are eliminated within a cell after stress challenges. 186 , 187 It is regulated by, among others, PINK1 (PTEN‐induced putative kinase 1) 28 , 188 and autophagy proteins microtubule‐associated protein 1A/1B‐light chain 3I (LC‐3I) and p62 in proximal tubules. 188 , 189 Using rat as an FA‐AKI model, Aparicio‐Trejo et al. 155 demonstrated that PINK1 and p62 were increased 24 h after FA injection with concurrent decreases in LC‐3I and LC‐3II contents, indicating an impaired process of mitophagy. Moreover, the authors also demonstrated a compromised process of mitochondrial fission and fusion process that is regulated by Opa1 and mitofusion‐1, as increased levels of mitochondrial fragments could be clearly detected in the FA‐AKI model. This study suggests that impaired mitophagy and mitochondrial dynamics are involved in FA‐induced AKI. Interestingly, N‐acetylcysteine pretreatment could prevent all these impairments, 155 implying the involvement of oxidative stress in the pathogenesis of AKI‐induced by FA. All the above‐described potential mechanisms of FA‐induced AKI or CKD are schematically represented in Figure 3.
FIGURE 3.
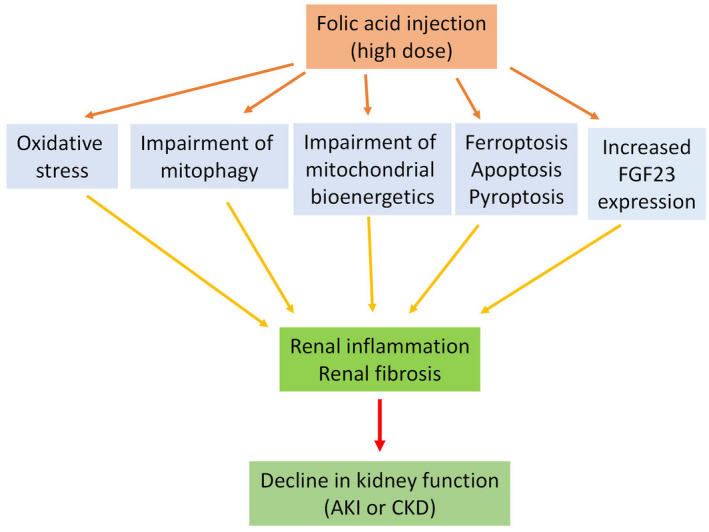
Major pathological mechanisms of folic acid (FA)‐induced acute kidney injury (AKI) and chronic kidney disease (CKD). These include oxidative stress, impairment of mitophagy and mitochondrial bioenergetics, ferroptosis, apoptosis and pyroptosis as well as increased expression of fibroblast growth factor 23 (FGF23). These mechanisms together result in renal inflammation and renal fibrosis, eventually leading to renal dysfunction or kidney disease. Please note that this figure and this article do not mean to exhaust all the mechanisms implicated in FA‐induced kidney disease
Overall and mechanistically, it should be pointed out that FA injection mainly damages the kidney and does not affect other organs, 99 and the damage mainly occurs to the proximal tubules. While it is well established that oxidative damage reflected by enhanced lipid peroxidation and deceased levels of glutathione and antioxidant capacity is the culminating event leading to cell death of tubular epithelial cells including apoptosis, necrosis, and ferroptosis, the upstream signaling processes are multifactorial. These include downregulation of klotho, 177 , 190 and increased expression of FGF21 and FGF23, 183 , 184 , 191 the latter of which is likely regulated by interleukin‐6. 185 FGF21 also relies on beta‐klotho protein to bind fibroblast growth factor receptor to exert its biological function in the kidney. 191 In addition, among the genes affected by FA‐induced kidney injury, c‐myc and c‐fos, involved in initiating cell cycle events, are believed to be the primary response genes. 192 Nonetheless, the exact roles of these response genes in FA‐induced kidney injury remains to be comprehensively evaluated.
5. APPLICATION OF THE FA‐INDUCED KIDNEY DISEASE MODEL IN TESTING THE THERAPEUTIC EFFECTS OF A VARIETY OF PHARMACOLOGICAL COMPOUNDS
In addition to being used to elucidate the pathological mechanisms underlying kidney disease, the FA‐induced animal model of kidney disease, like many other animal models, has also been used to test the therapeutic effects of pharmacological agents, chemicals, and natural compounds. Table 2 lists selectively some of the tests using the FA‐induced animal model of kidney disease as a platform It should be noted that all the listed compounds are at a pre‐clinical stage as the tests of their beneficial effects on kidney disease all involve laboratory animals.
TABLE 2.
FA‐induced animal model of kidney disease as a platform for testing the therapeutic effects of pharmacological agents, chemicals, and natural products
| Compound/or chemical | Model | Mechanism | References |
|---|---|---|---|
| Ancrod | CKD/mouse | Decreased renal fibrosis | 193 |
| Cyclosporine A | AKI/mouse | Decreased apoptosis | 194 |
| Fraxinellone | CKD/mouse | Decreased renal fibrosis | 195 |
| Ibudilast | AKI/mouse | Blocking pyroptosis | 179 |
| Nicorandil | AKI/mouse | Decreased oxidative stress | 196 |
| Curcumin | AKI/rat | Improved kidney structure | 197 |
| Nuciferine | AKI/mouse | Inhibition of ferroptosis | 198 |
| Fluorofenidone | AKI/mouse | Decreased ROS/NLRP3 | 199 |
| Lactoferrin | AKI‐CKD/patients | Autophagy activation | 178 |
| Curcuminoid | AKI/mouse | Inhibition of apoptosis | 190 |
| Nilotinib | AKI/mouse | Hsp70 activation | 200 |
| Salidroside | AKI/mouse | MAPK signaling | 201 |
| Celastrol | AKI/mouse | Increased cannabinoid receptor 2 | 202 |
| Metformin | CKD/mouse | Attenuation of renal fibrosis | 203 |
| Nintedanib | AKI‐CKD/mouse | Decreased renal fibrosis | 153 |
| Melatonin | AKI/mouse | HMGB1 translocation | 151 |
| Tanshinone IIA | AKI/mouse | Attenuation of renal fibrosis | 204 |
| Tanshinone IIA | AKI‐CKD/mouse | Targeting GSK3β | 205, 206 |
| N‐acetylcysteine | AKI/mouse | Increased glutathione | 207 |
| N‐acetylcysteine | AKI/rat | Mitophagy activation | 155 |
| Angiopoietin‐1 | AKI/mouse | Enhancing fibrosis | 208 |
| Anti‐TNF antibody | AKI/mouse | Inhibition of cell death | 209 |
| PFI‐2 | CKD/mouse | Decreased renal fibrosis | 166 |
| Citrus pectin | AKI/mouse | Decreased renal fibrosis | 210 |
| Quercetin | AKI/mouse | Inhibition of ferroptosis | 211 |
| Roxadustat | AKI/mouse | Anti‐ferroptosis | 212 |
Abbreviations: GSK3β, glycogen synthase kinase 3β; HMGB1, high mobility group box 1; PFI‐2, 8‐Fluoro‐N‐(1‐oxo‐1‐(pyrrolidin‐1‐yl)‐3‐(3‐(trifluoromethyl)phenyl)propan‐2‐yl)‐1,2,3,4‐tetrahydroisoquinoline‐6‐sulfonamide hydrochloride.
Relevant to Table 2, all animal models of kidney disease, regardless of the inducers or triggers applied, may end up with increased oxidative damage as a common mechanism that leads to renal inflammation and fibrosis, followed by kidney functional decline reflected by decreased GFR, and increased BUN and creatinine. 213 , 214 Therefore, natural products possessing antioxidant powers, such as those listed in Table 2, could offer potential benefits in treating FA‐induced kidney injury. One caveat is that while the FA‐induced kidney injury model can be used to test numerous natural products, identification of the most potent one would be challenging because testing conditions and experimental designs vary from laboratory to laboratory and no single laboratory can test all the available natural products. It is likely that administration of multiple products that are tolerable will offer synergistic benefits to CKD patients.
6. MISCELLANEOUS
As well as being an experimental tool for elucidating the mechanisms underlying kidney injury, the FA‐induced animal model has also been used for identification of biomarkers of kidney injury. For example, using a proteomic approach Rattanasinganchan et al. reported biomarkers of tubulointerstitial fibrosis from urinary exosomes derived from FA‐treated rats, demonstrating the feasibility of using this model for renal fibrosis biomarker identification. 99 FA‐induced CKD can also cause anemia in mouse. 215 Additionally, in terms of CKD model creation, the FA‐induced model will certainly take less time than does the adenine‐induced CKD model, which requires at least 16 weeks of adenine (0.25%) administration. 68 It should also be noted that while most studies using this FA animal model involve young adult mice or rats, FA‐induced kidney injury in aged animals has also been investigated. Marquez‐Exposito et al. have found that aging can aggravate AKI induced by FA, 216 indicating that age should be factored into an experimental design when the FA‐induced kidney injury model is to be utilized. Future studies using the FA‐induced animal model may also shed light on effects of other risk factors such as hypertension, obesity and diabetes on FA‐induced kidney disease. It is conceivable that such risk factors would also exacerbate FA‐induced kidney injury. Sex‐linked susceptibility of the kidneys to FA‐induced injury, if any, should also be investigated.
It should be emphasized once again that while high doses of FA administered intentionally can cause renal diseases including AKI and CKD, the nutritional and therapeutic value of low levels of FA or purposefully fortified FA supplements cannot be discounted. In fact, given that high levels of blood homocysteine occur in approximately 85% of CKD patients, 217 FA deficiency may serve as a diagnostic indicator and FA administration can slow down the progression of CKD. 217 , 218 , 219 This is due to the mechanism whereby FA is involved in lowering the blood levels of homocysteine by converting it to methionine in a methionine cycle pathway. 220 , 221 High homocysteine is known to pose an independent risk factor for cardiovascular disease. 217 , 222 , 223
7. SUMMARY
High doses of FA can induce both AKI and CKD in mice and rats. This FA‐induced animal model can also be used to study the AKI–CKD transition or progression. The procedure for establishing the model is easy as FA is water soluble and its administration is achieved by intraperitoneal injection. More importantly, the model is reproducible and can recapitulate most, if not all, of the human kidney disease phenotypes. Therefore, this model should continue to play a key role in the field of kidney disease research. In addition, future studies are needed to evaluate any potential cardiovascular disease caused by FA‐induced CKD, and will require analysis of changes in the profiles of blood mineral including phosphate, calcium, and magnesium. Any detrimental effects of FA‐induced kidney disease on other organs such as the liver and the brain will also need to be comprehensively evaluated.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTION
LJY conceived the idea and wrote the paper.
Yan L‐J. Folic acid‐induced animal model of kidney disease. Anim Models Exp Med. 2021;4:329–342. doi: 10.1002/ame2.12194
REFERENCES
- 1. Fiorentino M, Grandaliano G, Gesualdo L, Castellano G. Acute kidney injury to chronic kidney disease transition. Contrib Nephrol. 2018;193:45‐54. doi: 10.1159/000484962 [DOI] [PubMed] [Google Scholar]
- 2. He L, Wei Q, Liu J, et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017;92(5):1071‐1083. doi: 10.1016/j.kint.2017.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang M, Bai MI, Lei J, et al. Mitochondrial dysfunction and the AKI‐to‐CKD transition. Am J Physiol Renal Physiol. 2020;319(6):F1105‐F1116. doi: 10.1152/ajprenal.00285.2020 [DOI] [PubMed] [Google Scholar]
- 4. Al‐Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, Kellum JA. Clinical decision support for in‐hospital AKI. J Am Soc Nephrol. 2018;29(2):654‐660. doi: 10.1681/ASN.2017070765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179‐c184. doi: 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 6. Horne KL, Packington R, Monaghan J, Reilly T, Selby NM. Three‐year outcomes after acute kidney injury: results of a prospective parallel group cohort study. BMJ Open. 2017;7(3):e015316. doi: 10.1136/bmjopen-2016-015316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang J, Bayliss G, Zhuang S. Porcine models of acute kidney injury. Am J Physiol Renal Physiol. 2021;320(6):F1030‐F1044. doi: 10.1152/ajprenal.00022.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313(8):837‐846. doi: 10.1001/jama.2015.0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chintam K, Chang AR. Strategies to treat obesity in patients with CKD. Am J Kidney Dis. 2021;77(3):427‐439. doi: 10.1053/j.ajkd.2020.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamprea‐Montealegre JA, Shlipak MG, Estrella MM. Chronic kidney disease detection, staging and treatment in cardiovascular disease prevention. Heart. 2021;107(16):1282‐1288. doi: 10.1136/heartjnl-2020-318004 [DOI] [PubMed] [Google Scholar]
- 11. Stenvinkel P, Chertow GM, Devarajan P, et al. Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int Rep. 2021;6(7):1775‐1787. doi: 10.1016/j.ekir.2021.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nogueira A, Pires MJ, Oliveira PA. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In Vivo. 2017;31(1):1‐22. doi: 10.21873/invivo.11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thornton MA, Winn R, Alpers CE, Zager RA. An evaluation of the neutrophil as a mediator of in vivo renal ischemic‐reperfusion injury. Am J Pathol. 1989;135(3):509‐515. [PMC free article] [PubMed] [Google Scholar]
- 14. Dare AJ, Bolton EA, Pettigrew GJ, Bradley JA, Saeb‐Parsy K, Murphy MP. Protection against renal ischemia‐reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015;5:163‐168. doi: 10.1016/j.redox.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almeer RS, AlBasher GI, Alarifi S, Alkahtani S, Ali D, Abdel Moneim AE. Royal jelly attenuates cadmium‐induced nephrotoxicity in male mice. Sci Rep. 2019;9(1):5825. doi: 10.1038/s41598-019-42368-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ning YC, Cai GY, Zhuo LI, et al. Beneficial effects of short‐term calorie restriction against cisplatin‐induced acute renal injury in aged rats. Nephron Exp Nephrol. 2013;124(3–4):19‐27. doi: 10.1159/000357380 [DOI] [PubMed] [Google Scholar]
- 17. Shi Q, Lang W, Wang S, et al. Echinacea polysaccharide attenuates lipopolysaccharideinduced acute kidney injury via inhibiting inflammation, oxidative stress and the MAPK signaling pathway. Int J Mol Med. 2021;47(1):243‐255. doi: 10.3892/ijmm.2020.4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z. Rodent models of AKI‐CKD transition. Am J Physiol Renal Physiol. 2018;315(4):F1098‐F1106. doi: 10.1152/ajprenal.00199.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bao YW, Yuan Y, Chen JH, Lin WQ. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res. 2018;39(2):72‐86. doi: 10.24272/j.issn.2095-8137.2017.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh AP, Muthuraman A, Jaggi AS, et al. Animal models of acute renal failure. Pharmacol Rep. 2012;64(1):31‐44. doi: 10.1016/s1734-1140(12)70728-4 [DOI] [PubMed] [Google Scholar]
- 21. Rabe M, Schaefer F. Non‐transgenic mouse models of kidney disease. Nephron. 2016;133(1):53‐61. doi: 10.1159/000445171 [DOI] [PubMed] [Google Scholar]
- 22. Zhu YB, Zhang YP, Zhang J, Zhang YB. Evaluation of vitamin C supplementation on kidney function and vascular reactivity following renal ischemic injury in mice. Kidney Blood Press Res. 2016;41(4):460‐470. doi: 10.1159/000443447 [DOI] [PubMed] [Google Scholar]
- 23. Zhuang S, Lu B, Daubert RA, Chavin KD, Wang L, Schnellmann RG. Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int. 2009;75(3):304‐311. doi: 10.1038/ki.2008.506 [DOI] [PubMed] [Google Scholar]
- 24. Zhang W, Guan Y, Bayliss G, Zhuang S. Class IIa HDAC inhibitor TMP195 alleviates lipopolysaccharide‐induced acute kidney injury. Am J Physiol Renal Physiol. 2020;319(6):F1015‐F1026. doi: 10.1152/ajprenal.00405.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun M, Li J, Mao L, et al. p53 deacetylation alleviates sepsis‐induced acute kidney injury by promoting autophagy. Front Immunol. 2021;12:685523. doi: 10.3389/fimmu.2021.685523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu X, Lu J, Liao Y, et al. Dihydroartemisinin attenuates lipopolysaccharide‐induced acute kidney injury by inhibiting inflammation and oxidative stress. Biomed Pharmacother. 2019;117:109070. doi: 10.1016/j.biopha.2019.109070 [DOI] [PubMed] [Google Scholar]
- 27. Tran M, Tam D, Bardia A, et al. PGC‐1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121(10):4003‐4014. doi: 10.1172/JCI58662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Tang C, Cai J, et al. PINK1/Parkin‐mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury. Cell Death Dis. 2018;9(11):1113. doi: 10.1038/s41419-018-1152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mapuskar KA, Wen H, Holanda DG, et al. Persistent increase in mitochondrial superoxide mediates cisplatin‐induced chronic kidney disease. Redox Biol. 2019;20:98‐106. doi: 10.1016/j.redox.2018.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wen J, Zeng M, Shu Y, et al. Aging increases the susceptibility of cisplatin‐induced nephrotoxicity. Age (Dordr). 2015;37(6):112. doi: 10.1007/s11357-015-9844-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riaz MA, Nisa ZU, Mehmood A, Anjum MS, Shahzad K. Metal‐induced nephrotoxicity to diabetic and non‐diabetic Wistar rats. Environ Sci Pollut Res Int. 2019;26(30):31111‐31118. doi: 10.1007/s11356-019-06022-z [DOI] [PubMed] [Google Scholar]
- 32. Kimura A, Ishida Y, Hayashi T, et al. Interferon‐gamma plays protective roles in sodium arsenite‐induced renal injury by up‐regulating intrarenal multidrug resistance‐associated protein 1 expression. Am J Pathol. 2006;169(4):1118‐1128. doi: 10.2353/ajpath.2006.060024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JY, Eom M, Yang JW, Han BG, Choi SO, Kim JS. Acute kidney injury by arsine poisoning: the ultrastructural pathology of the kidney. Ren Fail. 2013;35(2):299‐301. doi: 10.3109/0886022X.2012.745117 [DOI] [PubMed] [Google Scholar]
- 34. Hussein AM, Eldosoky M, Abdel Malek H, Elshafey M, El Nashar E, Dahab G. Effects of nicorandil on vascular and renal dysfunctions in adenine‐induced nephropathy: possible underlying mechanisms. Gen Physiol Biophys. 2019;38(6):545‐556. doi: 10.4149/gpb_2019034 [DOI] [PubMed] [Google Scholar]
- 35. Liu X, Huang S, Wang F, et al. Huangqi‐Danshen decoction ameliorates adenine‐induced chronic kidney disease by modulating mitochondrial dynamics. Evid Based Complement Alternat Med. 2019;2019:9574045. doi: 10.1155/2019/9574045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feere DA, Velenosi TJ, Urquhart BL. Effect of erythropoietin on hepatic cytochrome P450 expression and function in an adenine‐fed rat model of chronic kidney disease. Br J Pharmacol. 2015;172(1):201‐213. doi: 10.1111/bph.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Almeer RS, Kassab RB, AlBasher GI, et al. Royal jelly mitigates cadmium‐induced neuronal damage in mouse cortex. Mol Biol Rep. 2019;46(1):119‐131. doi: 10.1007/s11033-018-4451-x [DOI] [PubMed] [Google Scholar]
- 38. Chen C, Han X, Wang G, et al. Nrf2 deficiency aggravates the kidney injury induced by subacute cadmium exposure in mice. Arch Toxicol. 2021;95(3):883‐893. doi: 10.1007/s00204-020-02964-3 [DOI] [PubMed] [Google Scholar]
- 39. Chen S, Liu G, Long M, Zou H, Cui H. Alpha lipoic acid attenuates cadmium‐induced nephrotoxicity via the mitochondrial apoptotic pathways in rat. J Inorg Biochem. 2018;184:19‐26. doi: 10.1016/j.jinorgbio.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 40. Edwards JR, Prozialeck WC. Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol. 2009;238(3):289‐293. doi: 10.1016/j.taap.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Al Hroob AM, Abukhalil MH, Alghonmeen RD, Mahmoud AM. Ginger alleviates hyperglycemia‐induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed Pharmacother. 2018;106:381‐389. doi: 10.1016/j.biopha.2018.06.148 [DOI] [PubMed] [Google Scholar]
- 42. Alhaider AA, Korashy HM, Sayed‐Ahmed MM, Mobark M, Kfoury H, Mansour MA. Metformin attenuates streptozotocin‐induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact. 2011;192(3):233‐242. doi: 10.1016/j.cbi.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 43. Ogura Y, Kitada M, Monno I, Kanasaki K, Watanabe A, Koya D. Renal mitochondrial oxidative stress is enhanced by the reduction of Sirt3 activity, in Zucker diabetic fatty rats. Redox Rep. 2018;23(1):153‐159. doi: 10.1080/13510002.2018.1487174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogura Y, Kitada M, Xu J, Monno I, Koya D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD(+)/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging. 2020;12(12):11325‐11336. doi: 10.18632/aging.103410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao P, Yang M, Chen X, Xiong S, Liu J, Sun L. DsbA‐L deficiency exacerbates mitochondrial dysfunction of tubular cells in diabetic kidney disease. Clin Sci. 2020;134(7):677‐694. doi: 10.1042/CS20200005 [DOI] [PubMed] [Google Scholar]
- 46. Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284(6):F1138‐F1144. doi: 10.1152/ajprenal.00315.2002 [DOI] [PubMed] [Google Scholar]
- 47. Becker GJ, Hewitson TD. Animal models of chronic kidney disease: useful but not perfect. Nephrol Dial Transplant. 2013;28(10):2432‐2438. doi: 10.1093/ndt/gft071 [DOI] [PubMed] [Google Scholar]
- 48. Yang HC, Zuo Y, Fogo AB. Models of chronic kidney disease. Drug Discov Today Dis Models. 2010;7(1–2):13‐19. doi: 10.1016/j.ddmod.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martin‐Sanchez D, Fontecha‐Barriuso M, Carrasco S, et al. TWEAK and RIPK1 mediate a second wave of cell death during AKI. Proc Natl Acad Sci U S A. 2018;115(16):4182‐4187. doi: 10.1073/pnas.1716578115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng S, Liu J, Zhao Z, Song R. Role of STAT3/mTOR pathway in chronic kidney injury. Am J Transl Res. 2020;12(7):3302‐3310. [PMC free article] [PubMed] [Google Scholar]
- 51. Guo L, Zhang T, Wang F, et al. Targeted inhibition of Rev‐erb‐alpha/beta limits ferroptosis to ameliorate folic acid‐induced acute kidney injury. Br J Pharmacol. 2021;178(2):328‐345. doi: 10.1111/bph.15283 [DOI] [PubMed] [Google Scholar]
- 52. Chen B, Wang P, Liang X, et al. Permissive effect of GSK3beta on profibrogenic plasticity of renal tubular cells in progressive chronic kidney disease. Cell Death Dis. 2021;12(5):432. doi: 10.1038/s41419-021-03709-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Santos S, Bosch RJ, Ortega A, et al. Up‐regulation of parathyroid hormone‐related protein in folic acid‐induced acute renal failure. Kidney Int. 2001;60(3):982‐995. doi: 10.1046/j.1523-1755.2001.060003982.x [DOI] [PubMed] [Google Scholar]
- 54. Perales‐Quintana MM, Saucedo AL, Lucio‐Gutiérrez JR, et al. Metabolomic and biochemical characterization of a new model of the transition of acute kidney injury to chronic kidney disease induced by folic acid. PeerJ. 2019;7:e7113. doi: 10.7717/peerj.7113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aparicio‐Trejo OE, Avila‐Rojas SH, Tapia E, et al. Chronic impairment of mitochondrial bioenergetics and beta‐oxidation promotes experimental AKI‐to‐CKD transition induced by folic acid. Free Radic Biol Med. 2020;154:18‐32. doi: 10.1016/j.freeradbiomed.2020.04.016 [DOI] [PubMed] [Google Scholar]
- 56. Ewees M‐D, Abdel‐Bakky MS, Bayoumi AMA, et al. Dabigatran mitigates cisplatin‐mediated nephrotoxicity through down regulation of thrombin pathway. J Adv Res. 2021;31:127‐136. doi: 10.1016/j.jare.2020.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perse M. Cisplatin mouse models: treatment, toxicity and translatability. Biomedicines. 2021;9(10):1406. doi: 10.3390/biomedicines9101406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morsy MA, Heeba GH. Nebivolol ameliorates cisplatin‐induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol. 2016;118(6):449‐455. doi: 10.1111/bcpt.12538 [DOI] [PubMed] [Google Scholar]
- 59. Chen Q, Zhang R, Li WM, et al. The protective effect of grape seed procyanidin extract against cadmium‐induced renal oxidative damage in mice. Environ Toxicol Pharmacol. 2013;36(3):759‐768. doi: 10.1016/j.etap.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 60. Claudio SR, Pidone Ribeiro FA, De Lima EC, et al. The protective effect of grape skin or purple carrot extracts against cadmium intoxication in kidney of rats. Pathophysiology. 2019;26(3‐4):263‐269. doi: 10.1016/j.pathophys.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 61. Handan BA, De Moura CFG, Cardoso CM, Santamarina AB, Pisani LP, Ribeiro DA. Protective effect of grape and apple juices against cadmium intoxication in the kidney of rats. Drug Res. 2020;70(11):503‐511. doi: 10.1055/a-1221-4733 [DOI] [PubMed] [Google Scholar]
- 62. Yan L‐J, Allen DC. Cadmium‐induced kidney injury: oxidative damage as a unifying mechanism. Biomolecules. 2021;11(11):1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dutta S, Saha S, Mahalanobish S, Sadhukhan P, Sil PC. Melatonin attenuates arsenic induced nephropathy via the regulation of oxidative stress and inflammatory signaling cascades in mice. Food Chem Toxicol. 2018;118:303‐316. doi: 10.1016/j.fct.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 64. Liu P, Xue Y, Zheng B, et al. Crocetin attenuates the oxidative stress, inflammation and apoptosisin arsenic trioxide‐induced nephrotoxic rats: implication of PI3K/AKT pathway. Int Immunopharmacol. 2020;88:106959. doi: 10.1016/j.intimp.2020.106959 [DOI] [PubMed] [Google Scholar]
- 65. Robles‐Osorio ML, Sabath‐Silva E, Sabath E. Arsenic‐mediated nephrotoxicity. Ren Fail. 2015;37(4):542‐547. doi: 10.3109/0886022X.2015.1013419 [DOI] [PubMed] [Google Scholar]
- 66. Liu X, Deng R, Wei X, et al. Jian‐Pi‐Yi‐Shen formula enhances perindopril inhibition of chronic kidney disease progression by activation of SIRT3, modulation of mitochondrial dynamics, and antioxidant effects. Biosci Rep. 2021;41(10):BSR20211598. doi: 10.1042/BSR20211598 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67. Kim K, Anderson EM, Thome T, et al. Skeletal myopathy in CKD: a comparison of adenine‐induced nephropathy and 5/6 nephrectomy models in mice. Am J Physiol Renal Physiol. 2021;321(1):F106‐F119. doi: 10.1152/ajprenal.00117.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Diwan V, Brown L, Gobe GC. Adenine‐induced chronic kidney disease in rats. Nephrology. 2018;23(1):5‐11. doi: 10.1111/nep.13180 [DOI] [PubMed] [Google Scholar]
- 69. Yan Y, Bai J, Zhou X, et al. P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am J Physiol Cell Physiol. 2015;308(6):C463‐C472. doi: 10.1152/ajpcell.00245.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yan BO, Min S‐J, Xu B, et al. The protective effects of exogenous spermine on renal ischemia‐reperfusion injury in rats. Transl Androl Urol. 2021;10(5):2051‐2066. doi: 10.21037/tau-21-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ohkita M, Hayashi H, Ito K, et al. Preventive effects of grape extract on ischemia/reperfusion‐induced acute kidney injury in mice. Biol Pharm Bull. 2019;42(11):1883‐1890. doi: 10.1248/bpb.b19-00462 [DOI] [PubMed] [Google Scholar]
- 72. Li C, Zheng Z, Xie Y, et al. Protective effect of taraxasterol on ischemia/reperfusion‐induced acute kidney injury via inhibition of oxidative stress, inflammation, and apoptosis. Int Immunopharmacol. 2020;89(Pt A):107169. doi: 10.1016/j.intimp.2020.107169 [DOI] [PubMed] [Google Scholar]
- 73. Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol. 2012;303(11):F1487‐F1494. doi: 10.1152/ajprenal.00352.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu J, Luo X, Thangthaeng N, et al. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes. Biochem Biophys Rep. 2017;11:119‐129. doi: 10.1016/j.bbrep.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu J, Jin Z, Yan LJ. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2017;11:51‐59. doi: 10.1016/j.redox.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tesch GH, Allen TJ. Rodent models of streptozotocin‐induced diabetic nephropathy. Nephrology. 2007;12(3):261‐266. doi: 10.1111/j.1440-1797.2007.00796.x [DOI] [PubMed] [Google Scholar]
- 77. Gajdosik A, Gajdosikova A, Stefek M, Navarova J, Hozova R. Streptozotocin‐induced experimental diabetes in male Wistar rats. Gen Physiol Biophys. 1999;18 Spec No:54‐62. [PubMed] [Google Scholar]
- 78. Chandrasekaran S, Ramajayam N, Pachaiappan P. Ameliorating effect of berbamine on hepatic key enzymes of carbohydrate metabolism in high‐fat diet and streptozotocin induced type 2 diabetic rats. Biomed Pharmacother. 2018;103:539‐545. doi: 10.1016/j.biopha.2018.04.066 [DOI] [PubMed] [Google Scholar]
- 79. Wu J, Luo X, Yan LJ. Two dimensional blue native/SDS‐PAGE to identify mitochondrial complex I subunits modified by 4‐hydroxynonenal (HNE). Methods. Front Physiol. 2015;6:98. doi: 10.3389/fphys.2015.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gao Y, Yang R, Guo L, et al. Qing‐Re‐Xiao‐Zheng formula modulates gut microbiota and inhibits inflammation in mice with diabetic kidney disease. Front Med. 2021;8:719950. doi: 10.3389/fmed.2021.719950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Akan E, Cetinkaya B, Kipmen‐Korgun D, et al. Effects of amnion derived mesenchymal stem cells on fibrosis in a 5/6 nephrectomy model in rats. Biotech Histochem. 2021:1‐14. doi: 10.1080/10520295.2021.1875502 [DOI] [PubMed] [Google Scholar]
- 82. Liu X, Luo D, Huang S, et al. Impaired nicotinamide adenine dinucleotide biosynthesis in the kidney of chronic kidney disease. Front Physiol. 2021;12:723690. doi: 10.3389/fphys.2021.723690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang JS, Tsai PH, Tseng KF, Chen FY, Yang WC, Shen MY. Sesamol ameliorates renal injury‐mediated atherosclerosis via inhibition of oxidative stress/IKKalpha/p53. Antioxidants. 2021;10(10):1519. doi: 10.3390/antiox10101519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tan RZ, Zhong X, Li JC, et al. An optimized 5/6 nephrectomy mouse model based on unilateral kidney ligation and its application in renal fibrosis research. Ren Fail. 2019;41(1):555‐566. doi: 10.1080/0886022X.2019.1627220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim HJ, Park KK, Chung WY, Lee SK, Kim KR. Protective effect of white‐fleshed peach (Prunus persica (L.) Batsch) on chronic nicotine‐induced toxicity. J Cancer Prev. 2017;22(1):22‐32. doi: 10.15430/JCP.2017.22.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lan X, Lederman R, Eng JM, et al. Nicotine induces podocyte apoptosis through increasing oxidative stress. PLoS One. 2016;11(12):e0167071. doi: 10.1371/journal.pone.0167071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ramalingam A, Santhanathas T, Shaukat Ali S, Zainalabidin S. Resveratrol supplementation protects against nicotine‐induced kidney injury. Int J Environ Res Public Health. 2019;16(22):4445. doi: 10.3390/ijerph16224445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Salahshoor MR, Roshankhah S, Motavalian V, Jalili C. Effect of harmine on nicotine‐induced kidney dysfunction in male mice. Int J Prev Med. 2019;10:97. doi: 10.4103/ijpvm.IJPVM_85_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pan P, Wang YJ, Han L, Liu X, Zhao M, Yuan YF. Effects of sodium houttuyfonate on expression of NF‐kappaB and MCP‐1 in membranous glomerulonephritis. J Ethnopharmacol. 2010;131(1):203‐209. doi: 10.1016/j.jep.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 90. Liu L, Xu Q, Zhang L, et al. Fe3O4 magnetic nanoparticles ameliorate albumin‐induced tubulointerstitial fibrosis by autophagy related to Rab7. Colloids Surf B Biointerfaces. 2021;198:111470. doi: 10.1016/j.colsurfb.2020.111470 [DOI] [PubMed] [Google Scholar]
- 91. Song J, Wang Y, Liu C, et al. Cordyceps militaris fruit body extract ameliorates membranous glomerulonephritis by attenuating oxidative stress and renal inflammation via the NF‐kappaB pathway. Food Funct. 2016;7(4):2006‐2015. doi: 10.1039/c5fo01017a [DOI] [PubMed] [Google Scholar]
- 92. Liu Y, Xu X, Xu R, Zhang S. Renoprotective effects of isoliquiritin against cationic bovine serum albumin‐induced membranous glomerulonephritis in experimental rat model through its anti‐oxidative and anti‐inflammatory properties. Drug Des Devel Ther. 2019;13:3735‐3751. doi: 10.2147/DDDT.S213088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang S, Xin H, Li Y, et al. Skimmin, a coumarin from Hydrangea paniculata, slows down the progression of membranous glomerulonephritis by anti‐inflammatory effects and inhibiting immune complex deposition. Evid Based Complement Alternat Med. 2013;2013:819296. doi: 10.1155/2013/819296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang Y, Hao J, Ma X, et al. Huoxue Jiedu Huayu Recipe ameliorates mesangial cell pyroptosis in contralateral kidney of UUO rats. Evid Based Complement Alternat Med. 2020;2020:2530431. doi: 10.1155/2020/2530431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hanifah N, Achmad YF, Humaira A, Salasia SIO. Red ginger‐extract nanoemulsion modulates high blood pressure in rats by regulating angiotensin‐converting enzyme production. Vet World. 2021;14(1):176‐181. doi: 10.14202/vetworld.2021.176-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yu HX, Lin W, Yang K, et al. Transcriptome‐based network analysis reveals hirudin potentiates anti‐renal fibrosis efficacy in UUO rats. Front Pharmacol. 2021;12:741801. doi: 10.3389/fphar.2021.741801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Huang J, Zhang Z, Liu B, et al. Identification of circular RNA expression profiles in renal fibrosis induced by obstructive injury. Ren Fail. 2021;43(1):1368‐1377. doi: 10.1080/0886022X.2021.1979040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Su CT, Jao TM, Urban Z, et al. LTBP4 affects renal fibrosis by influencing angiogenesis and altering mitochondrial structure. Cell Death Dis. 2021;12(10):943. doi: 10.1038/s41419-021-04214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rattanasinganchan P, Sopitthummakhun K, Doi K, et al. A folic acid‐induced rat model of renal injury to identify biomarkers of tubulointerstitial fibrosis from urinary exosomes. Asian Biomed. 2016;10(5):491‐502. [Google Scholar]
- 100. Li X, Zou YU, Fu YY, et al. A‐lipoic acid alleviates folic acid‐induced renal damage through inhibition of ferroptosis. Front Physiol. 2021;12:680544. doi: 10.3389/fphys.2021.680544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Murtas C, Ghiggeri GM. Membranous glomerulonephritis: histological and serological features to differentiate cancer‐related and non‐related forms. J Nephrol. 2016;29(4):469‐478. doi: 10.1007/s40620-016-0268-7 [DOI] [PubMed] [Google Scholar]
- 102. Huart J, Grosch S, Bovy C, Moutschen M, Krzesinski JM. IgG4‐related membranous glomerulonephritis and generalized lymphadenopathy without pancreatitis: a case report. BMC Nephrol. 2017;18(1):139. doi: 10.1186/s12882-017-0561-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mann S, Seidman MA, Barbour SJ, Levin A, Carruthers M, Chen LY. Recognizing IgG4‐related tubulointerstitial nephritis. Can J Kidney Health Dis. 2016;3:34. doi: 10.1186/s40697-016-0126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhang P, Cornell LD. IgG4‐related tubulointerstitial nephritis. Adv Chronic Kidney Dis. 2017;24(2):94‐100. doi: 10.1053/j.ackd.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 105. Jain K, Sengupta M, Basu K, Roychowdhury A, Bandopadhyay M. IgG4 tubulointerstitial nephritis ‐ an uncommon enemy! Indian J Pathol Microbiol. 2021;64(3):556‐558. doi: 10.4103/IJPM.IJPM_687_20 [DOI] [PubMed] [Google Scholar]
- 106. Mbengue M, Goumri N, Niang A. IgG4‐related kidney disease: pathogenesis, diagnosis, and treatment. Clin Nephrol. 2021;95(6):292‐302. doi: 10.5414/CN110492 [DOI] [PubMed] [Google Scholar]
- 107. Lobos P, Regulska‐Ilow B. Link between methyl nutrients and the DNA methylation process in the course of selected diseases in adults. Rocz Panstw Zakl Hig. 2021;72(2):123‐136. doi: 10.32394/rpzh.2021.0157 [DOI] [PubMed] [Google Scholar]
- 108. Goossens JF, Thuru X, Bailly C. Properties and reactivity of the folic acid and folate photoproduct 6‐formylpterin. Free Radic Biol Med. 2021;171:1‐10. doi: 10.1016/j.freeradbiomed.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 109. Samodelov SL, Gai Z, Kullak‐Ublick GA, Visentin M. Renal reabsorption of folates: pharmacological and toxicological snapshots. Nutrients. 2019;11(10):2353. doi: 10.3390/nu11102353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ducker GS, Rabinowitz JD. One‐carbon metabolism in health and disease. Cell Metab. 2017;25(1):27‐42. doi: 10.1016/j.cmet.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lieberman M, Marks AD. Marks' Basic Medical Biochemistry: A clinical Approach. 4th ed. Wolters Kluwer/Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 112. Dipiro JT, Talbet RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A pathophysiological approach. 9th ed. McGraw‐Hill Education; 2014. [Google Scholar]
- 113. Newsholme EA, Leech TR. Functional Biochemistry in Health and Disease. Wiley‐Blackwell; 2009. [Google Scholar]
- 114. Kornberg A, Segal R, Theitler J, Yona R, Kaufman S. Folic acid deficiency, megaloblastic anemia and peripheral polyneuropathy due to oral contraceptives. Isr J Med Sci. 1989;25(3):142‐145. [PubMed] [Google Scholar]
- 115. Reynolds EH. The neurology of folic acid deficiency. Handb Clin Neurol. 2014;120:927‐943. doi: 10.1016/B978-0-7020-4087-0.00061-9 [DOI] [PubMed] [Google Scholar]
- 116. Birn H, Spiegelstein O, Christensen EI, Finnell RH. Renal tubular reabsorption of folate mediated by folate binding protein 1. J Am Soc Nephrol. 2005;16(3):608‐615. doi: 10.1681/ASN.2004080711 [DOI] [PubMed] [Google Scholar]
- 117. Chancy CD, Kekuda R, Huang W, et al. Expression and differential polarization of the reduced‐folate transporter‐1 and the folate receptor alpha in mammalian retinal pigment epithelium. J Biol Chem. 2000;275(27):20676‐20684. doi: 10.1074/jbc.M002328200 [DOI] [PubMed] [Google Scholar]
- 118. Nazki FH, Sameer AS, Ganaie BA. Folate: metabolism, genes, polymorphisms and the associated diseases. Gene. 2014;533(1):11‐20. doi: 10.1016/j.gene.2013.09.063 [DOI] [PubMed] [Google Scholar]
- 119. Fox JT, Stover PJ. Folate‐mediated one‐carbon metabolism. Vitam Horm. 2008;79:1‐44. doi: 10.1016/S0083-6729(08)00401-9 [DOI] [PubMed] [Google Scholar]
- 120. Bailey LB. Folate in Health and Disease. 2nd ed. Taylor & Francis; 2009. [Google Scholar]
- 121. Miguel V, Ramos R, Garcia‐Bermejo L, Rodriguez‐Puyol D, Lamas S. The program of renal fibrogenesis is controlled by microRNAs regulating oxidative metabolism. Redox Biol. 2021;40:101851. doi: 10.1016/j.redox.2020.101851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Justo P, Sanz A, Lorz C, et al. Expression of Smac/Diablo in tubular epithelial cells and during acute renal failure. Kidney Int Suppl. 2003;86:S52‐S56. doi: 10.1046/j.1523-1755.64.s86.10.x [DOI] [PubMed] [Google Scholar]
- 123. Whitaker RM, Wills LP, Stallons LJ, Schnellmann RG. cGMP‐selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. J Pharmacol Exp Ther. 2013;347(3):626‐634. doi: 10.1124/jpet.113.208017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ruiz‐Andres O, Suarez‐Alvarez B, Sanchez‐Ramos C, et al. The inflammatory cytokine TWEAK decreases PGC‐1alpha expression and mitochondrial function in acute kidney injury. Kidney Int. 2016;89(2):399‐410. doi: 10.1038/ki.2015.332 [DOI] [PubMed] [Google Scholar]
- 125. Fontecha‐Barriuso M, Martin‐Sanchez D, Martinez‐Moreno JM, et al. PGC‐1alpha deficiency causes spontaneous kidney inflammation and increases the severity of nephrotoxic AKI. J Pathol. 2019;249(1):65‐78. doi: 10.1002/path.5282 [DOI] [PubMed] [Google Scholar]
- 126. Yan LJ, Rajasekaran NS, Sathyanarayanan S, Benjamin IJ. Mouse HSF1 disruption perturbs redox state and increases mitochondrial oxidative stress in kidney. Antioxid Redox Signal. 2005;7(3–4):465‐471. [DOI] [PubMed] [Google Scholar]
- 127. Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002;21(19):5164‐5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gupta A, Puri V, Sharma R, Puri S. Folic acid induces acute renal failure (ARF) by enhancing renal prooxidant state. Exp Toxicol Pathol. 2012;64(3):225‐232. doi: 10.1016/j.etp.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 129. Yan LJ. NADH/NAD(+) redox imbalance and diabetic kidney disease. Biomolecules. 2021;11(5):730. doi: 10.3390/biom11050730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schneider MP, Schlaich MP, Harazny JM, et al. Folic acid treatment normalizes NOS‐dependence of vascular tone in the metabolic syndrome. Obesity. 2011;19(5):960‐967. doi: 10.1038/oby.2010.210 [DOI] [PubMed] [Google Scholar]
- 131. Hwang SY, Siow YL, Au‐Yeung KKW, House J, O K. Folic acid supplementation inhibits NADPH oxidase‐mediated superoxide anion production in the kidney. Am J Physiol Renal Physiol. 2011;300(1):F189‐F198. doi: 10.1152/ajprenal.00272.2010 [DOI] [PubMed] [Google Scholar]
- 132. Akgun E, Boyacioglu M, Kum S. The potential protective role of folic acid against acetaminophen‐induced hepatotoxicity and nephrotoxicity in rats. Exp Anim. 2021;70(1):54‐62. doi: 10.1538/expanim.20-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shulpekova Y, Nechaev V, Kardasheva S, et al. The concept of folic acid in health and disease. Molecules. 2021;26(12):3731. doi: 10.3390/molecules26123731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Schubert GE. Folic acid‐induced acute renal failure in the the rat: morphological studies. Kidney Int Suppl. 1976;6:S46‐S50. [PubMed] [Google Scholar]
- 135. Doi K, Okamoto K, Negishi K, et al. Attenuation of folic acid‐induced renal inflammatory injury in platelet‐activating factor receptor‐deficient mice. Am J Pathol. 2006;168(5):1413‐1424. doi: 10.2353/ajpath.2006.050634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Taylor DM, Threlfall G, Buck AT. Chemically‐induced renal hypertrophy in the rat. Biochem Pharmacol. 1968;17(8):1567‐1574. doi: 10.1016/0006-2952(68)90216-5 [DOI] [PubMed] [Google Scholar]
- 137. Brade W, Herken H, Merker HJ. [Lesion and regeneration of renal tubule cells following administration of folic acid]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1969;262(2):228‐250. Schadigung und Regeneration renaler Tubuluszellen nach Folsauregabe. [PubMed] [Google Scholar]
- 138. Hsueh W, Rostorfer HH. Chemically induced renal hypertrophy in the rat. Lab Invest. 1973;29(5):547‐555. [PubMed] [Google Scholar]
- 139. Schmidt U, Schlumpf V, Josch W, Dubach UC. Acute renal failure in the rat after folate intoxication: diagnostic value of lactate dehydrogenase and alkaline phosphatase measurements in serum and urine. Clin Nephrol. 1974;2(3):106‐112. [PubMed] [Google Scholar]
- 140. Schmidt U, Dubach U. Acute renal failure in the folate‐treated rat: early metabolic changes in various structures of the nephron. Kidney Int Suppl. 1976;6:S39‐S45. [PubMed] [Google Scholar]
- 141. Searle CE, Blair JA. The renal toxicity of folic acid in mice. Food Cosmet Toxicol. 1973;11(2):277‐281. doi: 10.1016/s0015-6264(73)80494-8 [DOI] [PubMed] [Google Scholar]
- 142. Schubert GE, Welte K, Otten G. Chronic folic acid‐nephropathy. Res Exp Med. 1974;162(1):17‐36. doi: 10.1007/BF01851881 [DOI] [PubMed] [Google Scholar]
- 143. Huguenin ME, Birbaumer A, Brunner FP, et al. An evaluation of the role of tubular obstruction in folic acid‐induced acute renal failure in the rat. A micropuncture study. Nephron. 1978;22(1–3):41‐54. doi: 10.1159/000181422 [DOI] [PubMed] [Google Scholar]
- 144. Kirschbaum BB. Alterations of mitochondrial properties in folate nephropathy. Nephron. 1979;24(6):297‐301. doi: 10.1159/000181740 [DOI] [PubMed] [Google Scholar]
- 145. Nikolic T, Petrovic D, Matic S, et al. The influence of folic acid‐induced acute kidney injury on cardiac function and redox status in rats. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(1):99‐109. doi: 10.1007/s00210-019-01717-z [DOI] [PubMed] [Google Scholar]
- 146. González‐Guerrero C, Morgado‐Pascual JL, Cannata‐Ortiz P, et al. CCL20 blockade increases the severity of nephrotoxic folic acid‐induced acute kidney injury. J Pathol. 2018;246(2):191‐204. doi: 10.1002/path.5132 [DOI] [PubMed] [Google Scholar]
- 147. Kumar D, Singla SK, Puri V, Puri S. The restrained expression of NF‐kB in renal tissue ameliorates folic acid induced acute kidney injury in mice. PLoS One. 2015;10(1):e115947. doi: 10.1371/journal.pone.0115947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Jiang M, Fan J, Qu X, et al. Combined blockade of Smad3 and JNK pathways ameliorates progressive fibrosis in folic acid nephropathy. Front Pharmacol. 2019;10:880. doi: 10.3389/fphar.2019.00880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ye Y, Xu L, Ding H, et al. Pyruvate kinase M2 mediates fibroblast proliferation to promote tubular epithelial cell survival in acute kidney injury. FASEB J. 2021;35(7):e21706. doi: 10.1096/fj.202100040R [DOI] [PubMed] [Google Scholar]
- 150. Newbury LJ, Wang JH, Hung G, Hendry BM, Sharpe CC. Inhibition of Kirsten‐Ras reduces fibrosis and protects against renal dysfunction in a mouse model of chronic folic acid nephropathy. Sci Rep. 2019;9(1):14010. doi: 10.1038/s41598-019-50422-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhu F, Chong Lee Shin OL, Xu H, et al. Melatonin promoted renal regeneration in folic acid‐induced acute kidney injury via inhibiting nucleocytoplasmic translocation of HMGB1 in tubular epithelial cells. Am J Transl Res. 2017;9(4):1694‐1707. [PMC free article] [PubMed] [Google Scholar]
- 152. Jiang K, Ponzo TA, Tang H, Mishra PK, Macura SI, Lerman LO. Multiparametric MRI detects longitudinal evolution of folic acid‐induced nephropathy in mice. Am J Physiol Renal Physiol. 2018;315(5):F1252‐F1260. doi: 10.1152/ajprenal.00128.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Liu F, Wang LI, Qi H, et al. Nintedanib, a triple tyrosine kinase inhibitor, attenuates renal fibrosis in chronic kidney disease. Clin Sci. 2017;131(16):2125‐2143. doi: 10.1042/CS20170134 [DOI] [PubMed] [Google Scholar]
- 154. Burgos‐Silva M, Semedo‐Kuriki P, Donizetti‐Oliveira C, et al. Adipose tissue‐derived stem cells reduce acute and chronic kidney damage in mice. PLoS One. 2015;10(11):e0142183. doi: 10.1371/journal.pone.0142183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Aparicio‐Trejo OE, Reyes‐Fermín LM, Briones‐Herrera A, et al. Protective effects of N‐acetyl‐cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S‐glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic Biol Med. 2019;130:379‐396. doi: 10.1016/j.freeradbiomed.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 156. Zhang X, Agborbesong E, Li X. The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int J Mol Sci. 2021;22(20):11253. doi: 10.3390/ijms222011253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lu M, Wang P, Qiao Y, et al. GSK3beta‐mediated Keap1‐independent regulation of Nrf2 antioxidant response: a molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 2019;26:101275. doi: 10.1016/j.redox.2019.101275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bengatta S, Arnould C, Letavernier E, et al. MMP9 and SCF protect from apoptosis in acute kidney injury. J Am Soc Nephrol. 2009;20(4):787‐797. doi: 10.1681/ASN.2008050515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ortega A, Rámila D, Ardura JA, et al. Role of parathyroid hormone‐related protein in tubulointerstitial apoptosis and fibrosis after folic acid‐induced nephrotoxicity. J Am Soc Nephrol. 2006;17(6):1594‐1603. doi: 10.1681/ASN.2005070690 [DOI] [PubMed] [Google Scholar]
- 160. Fink M, Henry M, Tange JD. Experimental folic acid nephropathy. Pathology. 1987;19(2):143‐149. doi: 10.3109/00313028709077125 [DOI] [PubMed] [Google Scholar]
- 161. Jung JH, Choi JE, Song JH, Ahn SH. Human CD36 overexpression in renal tubules accelerates the progression of renal diseases in a mouse model of folic acid‐induced acute kidney injury. Kidney Res Clin Pract. 2018;37(1):30‐40. doi: 10.23876/j.krcp.2018.37.1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Henry MA, Harris PJ, Naughton RJ, Walker LL, Skinner SL, Tange JD. Filtration failure induced by p‐aminophenol in rats is due to raised intratubular pressure and not changes in glomerular function. Clin Exp Pharmacol Physiol. 1990;17(9):613‐626. doi: 10.1111/j.1440-1681.1990.tb01362.x [DOI] [PubMed] [Google Scholar]
- 163. Hasegawa K. Novel tubular‐glomerular interplay in diabetic kidney disease mediated by sirtuin 1, nicotinamide mononucleotide, and nicotinamide adenine dinucleotide Oshima Award Address 2017. Clin Exp Nephrol. 2019;23(8):987‐994. doi: 10.1007/s10157-019-01719-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Persson AE, Wright FS. Evidence for feedback mediated reduction of glomerular filtration rate during infusion of acetazolamide. Acta Physiol Scand. 1982;114(1):1‐7. doi: 10.1111/j.1748-1716.1982.tb06945.x [DOI] [PubMed] [Google Scholar]
- 165. de Groot T, Sinke AP, Kortenoeven MLA, et al. Acetazolamide attenuates lithium‐induced nephrogenic diabetes insipidus. J Am Soc Nephrol. 2016;27(7):2082‐2091. doi: 10.1681/ASN.2015070796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Liu B, Nie J, Liang H, et al. Pharmacological inhibition of SETD7 by PFI‐2 attenuates renal fibrosis following folic acid and obstruction injury. Eur J Pharmacol. 2021;901:174097. doi: 10.1016/j.ejphar.2021.174097 [DOI] [PubMed] [Google Scholar]
- 167. Tanner GA. Tubuloglomerular feedback after nephron or ureteral obstruction. Am J Physiol. 1985;248(5 Pt 2):F688‐F697. doi: 10.1152/ajprenal.1985.248.5.F688 [DOI] [PubMed] [Google Scholar]
- 168. Ucero ÁC, Berzal S, Ocaña‐Salceda C, et al. A polymeric nanomedicine diminishes inflammatory events in renal tubular cells. PLoS One. 2013;8(1):e51992. doi: 10.1371/journal.pone.0051992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Martin‐Sanchez D, Poveda J, Fontecha‐Barriuso M, et al. Targeting of regulated necrosis in kidney disease. Nefrologia. 2018;38(2):125‐135. doi: 10.1016/j.nefro.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 170. Severin MJ, Campagno RV, Brandoni A, Torres AM. Time evolution of methotrexate‐induced kidney injury: a comparative study between different biomarkers of renal damage in rats. Clin Exp Pharmacol Physiol. 2019;46(9):828‐836. doi: 10.1111/1440-1681.13122 [DOI] [PubMed] [Google Scholar]
- 171. Severin MJ, Torres AM. Time course effects of methotrexate on renal handling of water and electrolytes in rats. Role of aquaporin‐2 and Na‐K‐2Cl‐cotransporter. Toxicol Lett. 2019;311:27‐36. doi: 10.1016/j.toxlet.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 172. Koziolek MJ, Muller G‐A, Zapf A, et al. Role of CX3C‐chemokine CX3C‐L/fractalkine expression in a model of slowly progressive renal failure. Nephrol Dial Transplant. 2010;25(3):684‐698. doi: 10.1093/ndt/gfp602 [DOI] [PubMed] [Google Scholar]
- 173. Rayego‐Mateos S, Morgado‐Pascual JL, Rodrigues‐Diez RR, et al. Connective tissue growth factor induces renal fibrosis via epidermal growth factor receptor activation. J Pathol. 2018;244(2):227‐241. doi: 10.1002/path.5007 [DOI] [PubMed] [Google Scholar]
- 174. Luan J, Fu J, Wang D, et al. miR‐150‐Based RNA interference attenuates tubulointerstitial fibrosis through the SOCS1/JAK/STAT pathway in vivo and in vitro. Mol Ther Nucleic Acids. 2020;22:871‐884. doi: 10.1016/j.omtn.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mullin EM, Bonar RA, Paulson DF. Acute tubular necrosis. An experimental model detailing the biochemical events accompanying renal injury and recovery. Invest Urol. 1976;13(4):289‐294. [PubMed] [Google Scholar]
- 176. Bosch RJ, Woolf AS, Fine LG. Gene transfer into the mammalian kidney: direct retrovirus‐transduction of regenerating tubular epithelial cells. Exp Nephrol. 1993;1(1):49‐54. [PubMed] [Google Scholar]
- 177. Martin‐Sanchez D, Ruiz‐Andres O, Poveda J, et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid‐induced AKI. J Am Soc Nephrol. 2017;28(1):218‐229. doi: 10.1681/ASN.2015121376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hsu YH, Chiu IJ, Lin YF, Chen YJ, Lee YH, Chiu HW. Lactoferrin contributes a renoprotective effect in acute kidney injury and early renal fibrosis. Pharmaceutics. 2020;12(5):434. doi: 10.3390/pharmaceutics12050434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Li X, Zou Y, Fu YY, et al. Ibudilast attenuates folic acid‐induced acute kidney injury by blocking pyroptosis through TLR4‐mediated NF‐kappaB and MAPK signaling pathways. Front Pharmacol. 2021;12:650283. doi: 10.3389/fphar.2021.650283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Chen YY, Chen XG, Zhang S. Druggability of lipid metabolism modulation against renal fibrosis. Acta Pharmacol Sin. 2021. doi: 10.1038/s41401-021-00660-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Stallons LJ, Whitaker RM, Schnellmann RG. Suppressed mitochondrial biogenesis in folic acid‐induced acute kidney injury and early fibrosis. Toxicol Lett. 2014;224(3):326‐332. doi: 10.1016/j.toxlet.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Shimada T, Hasegawa H, Yamazaki Y, et al. FGF‐23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429‐435. doi: 10.1359/JBMR.0301264 [DOI] [PubMed] [Google Scholar]
- 183. Christov M, Waikar SS, Pereira RC, et al. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 2013;84(4):776‐785. doi: 10.1038/ki.2013.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Egli‐Spichtig D, Zhang MYH, Perwad F. Fibroblast growth factor 23 expression is increased in multiple organs in mice with folic acid‐induced acute kidney injury. Front Physiol. 2018;9:1494. doi: 10.3389/fphys.2018.01494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Durlacher‐Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh‐Many T. Interleukin‐6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94(2):315‐325. doi: 10.1016/j.kint.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 186. Zuo Z, Jing K, Wu H, et al. Mechanisms and functions of mitophagy and potential roles in renal disease. Front Physiol. 2020;11:935. doi: 10.3389/fphys.2020.00935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol. 2014;171(8):1917‐1942. doi: 10.1111/bph.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Nguyen TN, Padman BS, Lazarou M. Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends Cell Biol. 2016;26(10):733‐744. doi: 10.1016/j.tcb.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 189. Wang Y, Zhu J, Liu Z, et al. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. 2021;38:101767. doi: 10.1016/j.redox.2020.101767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Córdoba‐David G, Duro‐Castano A, Castelo‐Branco RC, et al. Effective nephroprotection against acute kidney injury with a star‐shaped polyglutamate‐curcuminoid conjugate. Sci Rep. 2020;10(1):2056. doi: 10.1038/s41598-020-58974-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zhou X, Zhang Y, Wang N. Regulation and potential biological role of fibroblast growth factor 21 in chronic kidney disease. Front Physiol. 2021;12:764503. doi: 10.3389/fphys.2021.764503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Calvet JP, Chadwick LJ. Primary and secondary genetic responses after folic acid‐induced acute renal injury in the mouse. J Am Soc Nephrol. 1994;5(6):1324‐1332. doi: 10.1681/ASN.V561324 [DOI] [PubMed] [Google Scholar]
- 193. Craciun FL, Ajay AK, Hoffmann D, et al. Pharmacological and genetic depletion of fibrinogen protects from kidney fibrosis. Am J Physiol Renal Physiol. 2014;307(4):F471‐F484. doi: 10.1152/ajprenal.00189.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wen X, Peng Z, Li Y, et al. One dose of cyclosporine A is protective at initiation of folic acid‐induced acute kidney injury in mice. Nephrol Dial Transplant. 2012;27(8):3100‐3109. doi: 10.1093/ndt/gfr766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zheng B, Yuan M, Wang S, et al. Fraxinellone alleviates kidney fibrosis by inhibiting CUG‐binding protein 1‐mediated fibroblast activation. Toxicol Appl Pharmacol. 2021;420:115530. doi: 10.1016/j.taap.2021.115530 [DOI] [PubMed] [Google Scholar]
- 196. Ezzat DM, Soliman AM, El‐Kashef DH. Nicorandil mitigates folic acid‐induced nephrotoxicity in mice: role of iNOS and eNOS. J Biochem Mol Toxicol. 2021;35(4):e22692. doi: 10.1002/jbt.22692 [DOI] [PubMed] [Google Scholar]
- 197. Mahmoud G, Amer A, Mostafa D. Functional and structural study on the effect of curcumin on folic acid‐induced acute kidney injury in albino rats. Bull Egypt Soc Physiol Sci. 2014;34(2):265‐276. [Google Scholar]
- 198. Li D, Liu B, Fan Y, et al. Nuciferine protects against folic acid‐induced acute kidney injury by inhibiting ferroptosis. Br J Pharmacol. 2021;178(5):1182‐1199. doi: 10.1111/bph.15364 [DOI] [PubMed] [Google Scholar]
- 199. Liao X, Jiang Y, Dai Q, et al. Fluorofenidone attenuates renal fibrosis by inhibiting the mtROS‐NLRP3 pathway in a murine model of folic acid nephropathy. Biochem Biophys Res Commun. 2021;534:694‐701. doi: 10.1016/j.bbrc.2020.11.017 [DOI] [PubMed] [Google Scholar]
- 200. Zaghloul MS, Abdelrahman RS. Nilotinib ameliorates folic acid‐induced acute kidney injury through modulation of TWEAK and HSP‐70 pathways. Toxicology. 2019;427:152303. doi: 10.1016/j.tox.2019.152303 [DOI] [PubMed] [Google Scholar]
- 201. Li R, Guo Y, Zhang Y, Zhang X, Zhu L, Yan T. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF‐kappaB and MAPK signaling pathways. Int J Mol Sci. 2019;20(5):1103. doi: 10.3390/ijms20051103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Tang M, Cao X, Zhang K, et al. Celastrol alleviates renal fibrosis by upregulating cannabinoid receptor 2 expression. Cell Death Dis. 2018;9(6):601. doi: 10.1038/s41419-018-0666-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Yi H, Huang C, Shi Y, et al. Metformin attenuates folic‐acid induced renal fibrosis in mice. J Cell Physiol. 2018;233(9):7045‐7054. doi: 10.1002/jcp.26505 [DOI] [PubMed] [Google Scholar]
- 204. Jiang C, Shao Q, Jin B, Gong R, Zhang M, Xu B. Tanshinone IIA attenuates renal fibrosis after acute kidney injury in a mouse model through inhibition of fibrocytes recruitment. Biomed Res Int. 2015;2015:867140. doi: 10.1155/2015/867140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Jiang C, Zhu W, Shao Q, et al. Tanshinone IIA protects against folic acid‐induced acute kidney injury. Am J Chin Med. 2016;44(4):737‐753. doi: 10.1142/S0192415X16500403 [DOI] [PubMed] [Google Scholar]
- 206. Jiang C, Zhu W, Yan X, et al. Rescue therapy with Tanshinone IIA hinders transition of acute kidney injury to chronic kidney disease via targeting GSK3beta. Sci Rep. 2016;6:36698. doi: 10.1038/srep36698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Wang HZ, Peng ZY, Wen XY, Rimmele T, Bishop JV, Kellum JA. N‐acetylcysteine is effective for prevention but not for treatment of folic acid‐induced acute kidney injury in mice. Crit Care Med. 2011;39(11):2487‐2494. doi: 10.1097/CCM.0b013e31822575fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Long DA, Price KL, Ioffe E, et al. Angiopoietin‐1 therapy enhances fibrosis and inflammation following folic acid‐induced acute renal injury. Kidney Int. 2008;74(3):300‐309. doi: 10.1038/ki.2008.179 [DOI] [PubMed] [Google Scholar]
- 209. Wan B, Hao L, Qiu Y, et al. Blocking tumor necrosis factor‐alpha inhibits folic acid‐induced acute renal failure. Exp Mol Pathol. 2006;81(3):211‐216. doi: 10.1016/j.yexmp.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 210. Kolatsi‐Joannou M, Price KL, Winyard PJ, Long DA. Modified citrus pectin reduces galectin‐3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6(4):e18683. doi: 10.1371/journal.pone.0018683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Wang Y, Quan F, Cao Q, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231‐243. doi: 10.1016/j.jare.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Li X, Zou Y, Xing J, et al. Pretreatment with roxadustat (FG‐4592) attenuates folic acid‐induced kidney injury through antiferroptosis via Akt/GSK‐3beta/Nrf2 pathway. Oxid Med Cell Longev. 2020;2020:6286984. doi: 10.1155/2020/6286984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Gyuraszova M, Gurecka R, Babickova J, Tothova L. Oxidative stress in the pathophysiology of kidney disease: implications for noninvasive monitoring and identification of biomarkers. Oxid Med Cell Longev. 2020;2020:5478708. doi: 10.1155/2020/5478708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Palipoch S. A review of oxidative stress in acute kidney injury: protective role of medicinal plants‐derived antioxidants. Afr J Tradit Complement Altern Med. 2013;10(4):88‐93. doi: 10.4314/ajtcam.v10i4.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Estrela GR, Freitas‐Lima LC, Budu A, et al. Chronic kidney disease induced by cisplatin, folic acid and renal ischemia reperfusion induces anemia and promotes GATA‐2 activation in mice. Biomedicines. 2021;9(7):769. doi: 10.3390/biomedicines9070769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Marquez‐Exposito L, Tejedor‐Santamaria L, Santos‐Sanchez L, et al. Acute kidney injury is aggravated in aged mice by the exacerbation of proinflammatory processes. Front Pharmacol. 2021;12:662020. doi: 10.3389/fphar.2021.662020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Cianciolo G, De Pascalis A, Di Lullo L, Ronco C, Zannini C, La Manna G. Folic acid and homocysteine in chronic kidney disease and cardiovascular disease progression: which comes first? Cardiorenal Med. 2017;7(4):255‐266. doi: 10.1159/000471813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Li Y, Spence JD, Wang X, Huo Y, Xu X, Qin X. Effect of vitamin B12 levels on the association between folic acid treatment and CKD progression: a post hoc analysis of a folic acid interventional trial. Am J Kidney Dis. 2020;75(3):325‐332. doi: 10.1053/j.ajkd.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 219. Capelli I, Cianciolo G, Gasperoni L, et al. Folic acid and vitamin B12 administration in CKD, why not? Nutrients. 2019;11(2):383. doi: 10.3390/nu11020383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab. 2017;14:78. doi: 10.1186/s12986-017-0233-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Skovierova H, Vidomanova E, Mahmood S, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. 2016;17(10):1733. doi: 10.3390/ijms17101733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Cueto R, Zhang L, Shan HM, et al. Identification of homocysteine‐suppressive mitochondrial ETC complex genes and tissue expression profile ‐ novel hypothesis establishment. Redox Biol. 2018;17:70‐88. doi: 10.1016/j.redox.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Angelini A, Cappuccilli ML, Magnoni G, et al. The link between homocysteine, folic acid and vitamin B12 in chronic kidney disease. G Ital Nefrol. 2021;38(4):1–17. PMID: 34469084. [PubMed] [Google Scholar]


