Abstract
The cornea is an avascular, transparent tissue that is essential for visual function. Any disturbance to the corneal transparency will result in a severe vision loss. Due to the avascular nature, the cornea acquires most of the oxygen supply directly or indirectly from the atmosphere. Corneal tissue hypoxia has been noticed to influence the structure and function of the cornea for decades. The etiology of hypoxia of the cornea is distinct from the rest of the body, mainly due to the separation of cornea from the atmosphere, such as prolonged contact lens wearing or closed eyes. Corneal hypoxia can also be found in corneal inflammation and injury when a higher oxygen requirement exceeds the oxygen supply. Systemic hypoxic state during lung diseases or high altitude also leads to corneal hypoxia when a second oxygen consumption route from aqueous humor gets blocked. Hypoxia affects the cornea in multiple aspects, including disturbance of the epithelium barrier function, corneal edema due to endothelial dysfunction and metabolism changes in the stroma, and thinning of corneal stroma. Cornea has also evolved mechanisms to adapt to the hypoxic state initiated by the activation of hypoxia inducible factor (HIF). The aim of this review is to introduce the pathology of cornea under hypoxia and the mechanism of hypoxia adaptation, to discuss the current animal models used in this field, and future research directions.
Keywords: animal model, contact lens wear, cornea, hypoxia, hypoxia adaptation, hypoxia inducible factor (HIF)
The effect of hypoxia on each corneal cell types and the adaptation to hypoxia by the activation of hypoxia inducible factor (HIF) signalling pathway
1. INTRODUCTION
Hypoxia is a state when the oxygen supply does not meet the tissue's needs. Organisms, including mammals, have developed defense mechanisms to survive hypoxia, known as hypoxia adaptation. The hypoxia adaptation process has been studied in numerous tissues and diseases, including ischemia of the heart, brain, and nearly every major organ. Besides ischemia, aggressively malignant tumor 1 and systemic hypoxic status 2 are also the focus of hypoxia adaptation research. The eyes, being a unique and important sensory organ, impact greatly on a person's quality of life. The core concept of any visual system is refraction of the light into a photosensitive array (retina) and further processing into electrochemical signals for the brain neural networks to process and interpret. The eye developed a precise regulatory mechanism for each component to accomplish the visual cycle, a complex and energy‐demanding function in a volume of the organ less than 6 cm3. While efficient, the eye system is vulnerable to hypoxic injury.
The cornea is the second refractory media along the visual pathway (the first being the tear film). It is a transparent dome‐like structure located in the very front of the eye, with the anterior chamber and iris behind it. The cornea comprises 5 layers, namely from the outside to the inside: epithelium, Bowman's membrane, stroma, Descemet's membrane, and endothelium (Figure 1). With maintaining transparency as the major function, the cornea developed several mechanisms: (1) being avascular; (2) maintaining a hydroponic status by keeping the water from leaking in and actively pumping the water out; (3) maintaining an organized structure of the matrix in the stroma. 3 Any disturbance of one of the three mechanisms will cause corneal opacity and consequential visual loss. As an avascular tissue, the cornea is unique in oxygen consumption by acquiring oxygen from the atmosphere. Therefore, any condition separating the cornea from the air will cause corneal hypoxia. In physiological state, corneal oxygen decreases to one third of the original concentration when closing the eyes 4 ; soft contact lens wear is a common etiology of corneal hypoxia. Corneal hypoxia also occurs in pathologies such as infectious keratitis, 5 , 6 limbal stem cell deficiency, 4 and systemic hypoxia such as chronic lung disease. 7
FIGURE 1.
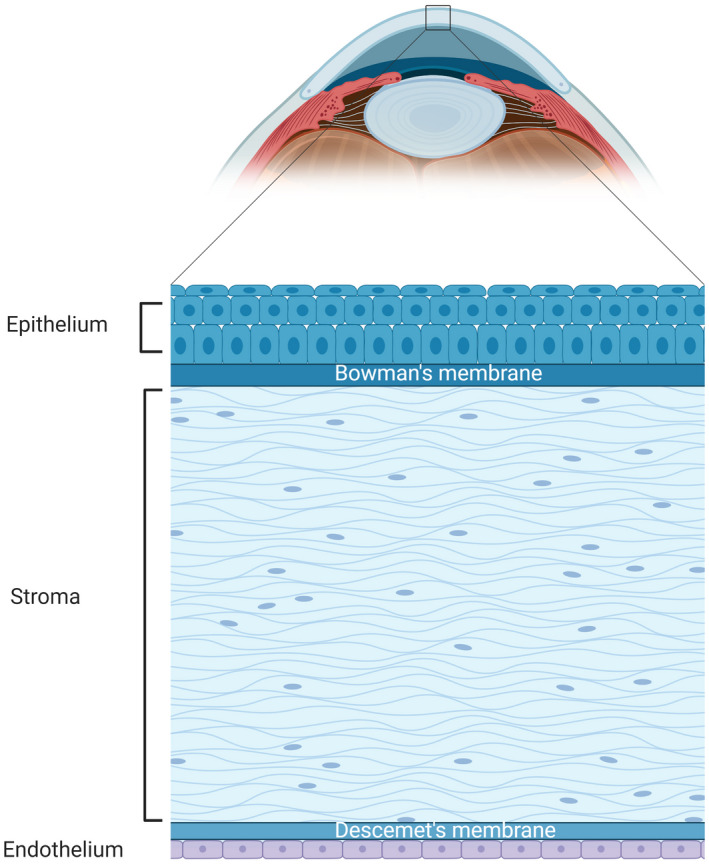
Illustration of an anterior segment of an eyeball (sagittal plane) and detailed illustration of the cornea (bottom panel). The cornea is located at the very front of the eyeball (the arched light blue structure); the darker blue structure next to the cornea on the left is the anterior chamber filled with aqueous humor. The cornea has 5 layers (bottom panel), organized with the top being the outside surface and the button is the inside surface in the illustration. The epithelium is comprises of 5–7 layers of squamous cells at the outside and a monolayer of columnar cells at the inside; the Bowman's membrane is the basement membrane for epithelial cells; the stroma makes up most of the corneal thickness and is composed of highly organized collagen fibers and keratocytes; the Descemet's membrane is the basement membrane of endothelial cells; the endothelium is a monolayer of hexagonal‐shaped endothelial cells, which does not have the potential for proliferation or regeneration
Herein, we reviewed the current animal model used in cornea hypoxia research and the molecular basis of hypoxia and associated adaptation.
2. HYPOXIA IN CORNEA
2.1. Etiology
Hypoxia is a prominent feature of pathological states encountered in inflammation, wounds, and infections (bacteria, fungi, virus, etc.) of the cornea. 5 , 8 , 9 As mentioned above, the cornea requires oxygen primarily from the atmospheric air and secondarily from the aqueous humor for normal metabolic function when eyes are open. In closed eyes, the oxygen is mainly provided by the connecting conjunctiva and the aqueous humor. 10 Due to the avascular nature, a localized oxygen impediment from the embedment 11 , 12 or corneal edema induced by trauma or infection that restricts the availability of the air to the cornea will lead to significant localized corneal hypoxia. 13 In turn, corneal hypoxia induces stroma swelling by increasing the anaerobic metabolism and changing the concentrations of metabolites at the endothelium that regulate water flux into/out of the cornea. 12
Besides the lack of oxygen supply to the cornea, localized hypoxia can also happen in conditions with a sudden increased oxygen demand. For example, hydrogel contact lenses were found to induce acidosis, which increases the corneal oxygen consumption rate by up to 1.8 times that found in normal pH, and in consequence the pH‐regulatory mechanism. 10 , 14 The acute and chronic inflammation induced by mechanical trauma, chemical burn, infection, etc., in corneal tissue often causes the development of hypoxia. Rao et al. reported that inflammatory hypoxia developed in the Herpes stromal keratitis lesions, 6 which is consistent with another study that cellular hypoxia appeared in the acute stage of the alkali burn tracked by pimonidazole staining, a 2‐nitroimidazole, which gets reductively activated in hypoxia cells in vivo and forms stable adducts with thiol groups in proteins, and amino acids. 6 , 15 Depletion of neutrophils significantly reduced the pimonidazole staining cells. 6
Another important cause of corneal hypoxia is systemic hypoxia of the body. In a clinical study involving 114 eyes, hypoxia induced by obstructive sleep apnea (OSA) leads to the reduction of central corneal thickness and endothelial cell density. 7 The stomal acidosis induced by hypoxia may be a cause of corneal thinning. A study reported a significant corneal stromal thinning and loss of keratocytes in hemifacial spasm (HFS) patients with the presence of chronic hypoxia. 16 Noticeably, systemic hypoxia causes the thinning of the stroma, while in the contact lenses wear model, corneal edema is more often observed. Hence, different cellular mechanisms are involved in local corneal tissue hypoxia versus corneal hypoxia secondary to systemic hypoxia of the body.
In summary, corneal hypoxia happens solely or as a complication in multiple corneal pathologies. A better understanding of the process of hypoxia affecting the cornea and the cornea compensating for the lack of oxygen is important for disease prevention and treatment. In the next sections we will discuss the cellular mechanisms of corneal hypoxia.
2.2. Cellular reaction to hypoxia
Cellular hypoxia (0.5%–2% oxygen) can be transient due to the unbalance between the supply and cellular metabolic demands, or chronic due to unresolved tissue edema and inflammation. 8 The cornea comprises the following types of cells: epithelial, endothelial, stromal, and dendritic (Figure 2), and hypoxia damages each cell type differently.
FIGURE 2.
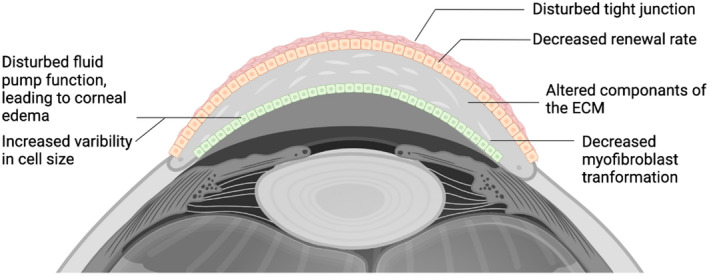
A summary of hypoxia effects on cornea. Hypoxia exposure affects each cell type of the cornea. In epithelial cells, hypoxia disturb the tight junction and decrease the renewal rate of the basal cell layer. In cornea stroma, hypoxia alters the components of the extracellular matrix, and decreases the myofibroblast transformation of the keratocytes. In the endothelium, exposure to hypoxia disturbs the fluid pump function leading to corneal edema; increased variability in cell size has also been observed
2.2.1. Epithelial cells
Corneal epithelial cells form a multilayered epithelium with 5–7 layers of squamous cells at the outside and a monolayer of columnar cells at the inside (Figure 2). The epithelial cells are constantly undergoing mitosis to strengthen the barrier between the cornea and the outside environment. Therefore, oxygen is highly demanded by the epithelium for maintaining the barrier function and homeostatic renewal. Studies have demonstrated that overnight restriction of the oxygen supply from the atmosphere disturbs the renewal homeostasis by increasing the surface cell damage and decreasing the replication of the basal cell layer in both human and rabbit models. 17 , 18 Contact lens wear for 24 h in rabbits induces stem cell markers, ΔNp63α and Ki67, not only in limbal stem cells but also in basal cells throughout the cornea. 19
Besides maintaining the renewal homeostasis, another important factor of corneal epithelial barrier function is the tight junction between epithelial cells. A study using Simian virus 40–immortalized human corneal epithelial (HCE) cells demonstrated a decreased barrier function under 24 h of hypoxia treatment measured by transepithelial resistance. Zonular occludin‐1 (ZO‐1), a cytoplasmic protein associated with tight junctions, the localization of which was disturbed during 24 h of hypoxia. 20 Similar results were demonstrated in a rabbit lens wearing model. 21 Moreover, hypoxia downregulates the expression of Toll‐like receptor (TLR) 4, an important receptor to pathogen damage, weakening the biological barrier function to defeat microbial infections. 22 These results indicate that a short‐term exposure to hypoxia disturbs the epithelium barrier function by disturbing the tight junctions between epithelial cells.
Hypoxia also delays the wound healing process of the cornea epithelium. 23 Wound healing is a more complex process involving (1) the crosstalk between corneal nerves to epithelial cells; (2) the reaction to mediators from injured cells (nucleotides and glutamate); (3) cell migration and proliferation and the reassembly of extracellular matrix. Exposure to hypoxia for 24–48 h exposure to hypoxia attenuates the crosstalk between the corneal nerves and epithelial cells by reducing the transmitter released into the extracellular space. 23 In the same study, acute hypoxia also impairs the cell response to adenosine triphosphate (ATP) and glutamate released by the injured cells, 23 and inhibits migration by decreasing the phosphorylation of a protein paxillin which is crucial for cell adhesion. 23 To summarize, short‐term hypoxia impairs the corneal epithelium function by many mechanisms (Table 1). However, when we trace the source of these affected pathways, they all lead to one core pathogenesis: the altered metabolism pathways.
TABLE 1.
Effect of hypoxia on corneal epithelial cells
| Author | Models | Findings | Duration of hypoxia |
|---|---|---|---|
| Robertson, et al. 19 | Rabbit contact lens wear and telomerase‐immortalized human corneal epithelial cells (hTCEpi) | Reduction of a stem cell marker ΔNp63α in basal cell layer | 24 h |
| Teranishi, et al. 20 | Simian virus 40–immortalized human corneal epithelial (HCE) cells | Decreased transepithelial electrical resistance, decreased ZO‐1 and KGF | 24 h |
| Yanai, et al. 21 | Rabbit lens wear | Disruption of ZO‐1 localization | 24 h |
| Hara, et al. 22 | SV40‐HCEC | Decreased expression of TLR4 and cytokine expression | 48 h |
| Zaidi, et al. 26 | Cultured rabbit and human cornea tissue and primary cells | Increased cystic fibrosis transmembrane conductance regulator leading to Pseudomonas aeruginosa binding | 24–72 h |
| Lee, et al. 23 | human corneal limbal epithelial (HCLE) cell line | Decreased ATP production; attenuated crosstalk between epithelium and cornea nerve; delays wound closure | 24 or 48 h |
| Safvati et al. 27 | Increased expression of numerous angiogenesis mediators | Review | |
| Kosaku et al. 28 | Systemic low oxygen (10%) environment in mice | Maintain normal epithelium structure | 140 days |
One of the fundamental responses of cells to the lack of oxygen is to decrease the portion of aerobic metabolism and increase anaerobic metabolism. Take glucose, the major energy source of the corneal epithelial cells, for example, reactions of the tricarboxylic acid cycle (TCA) will decrease while glycolysis will increase during hypoxia. Since the efficiency of ATP production differs between the two pathways (34–36 mol ATP per 1 mol of glucose for aerobic reaction; 2 mol ATP per 1 mol of glucose for anaerobic reaction), switching to anaerobic metabolism results in a decreased ATP production, as observed by Lee et al. in epithelial cells. 23 The decreased ATP production leads to the impairment of numerous cellular functions that require energy, including ion transportation, Ca2+ homeostasis, and protein synthesis. The imbalance between glycolysis and the TCA cycle also causes accumulation of the product of glycolysis pyruvate acid, which gets further converted to lactic acid. The pH of the cornea tissue could drop from 7.55 ± 0.02 to 7.15 ± 0.04 with 80 min of contact lens wear in early studies, from the accumulated lactic acid and CO2. 24 , 25 The lack of ATP and acidosis processes also cause function deficiency in stromal cells and endothelial cells, which will be reviewed in the next sections.
2.2.2. Stroma
The corneal stroma is the thickest layer of the cornea, composed of highly organized collagen fibers (mainly type II and type V) and keratocytes (also known as fibroblasts). The keratocytes are located in between the layers of collagen fibers. In healthy cornea, the keratocytes secrete collagen and crystallins, a protein that keeps the corneal transparency 29 ; upon injury, the keratocytes either undergo cell death or get activated to regenerate or induce scar formation. 30
Hypoxia is associated with the thinning of the stroma. 31 Studies have shown that hypoxia exposure reduces the production of collagen I, collagen IV, and laminin, 4 while increasing the production of matrix metalloproteinase (MMP)‐1 and MMP‐2. 32 Lee et al. demonstrated that increasing exposure to hypoxia decreased both the sulfation proteoglycans and the expression of sulfatase I mRNA. 33 In a disease that mainly affects the corneal stroma named keratoconus, the alteration of the extracellular matrix is more profound. 32 The level of collagen I and III reduction and the level of MMP‐1/MMP‐2 increase are both significantly more severe compared with normal human corneal stromal fibroblasts. 32
As mentioned above, the keratocytes under stress have two fates: one is undergoing apoptosis, the other is activated to a fusiform shape or transformed to a myofibroblast phenotype. 34 Myofibroblast keratocytes are observed in injured cornea characterized by the expression of alpha smooth muscle actin (αSMA). 35 These cells act as contractors in wound healing and are also responsible for extracellular matrix organization; both processes are crucial for corneal wound healing and transparency. This transformation is believed to be induced by the transforming growth factor beta (TGFβ) released by corneal epithelial cells. 35 Evidence shows that a transient hypoxia exposure for 4 h inhibited the TGFβ1‐induced corneal myofibroblast transformation and αSMA expression. 36 Further analysis showed that hypoxia inhibited the activation of a GTP binding protein RhoA, which is relatively upstream of the TGFβ induced cell signaling pathway. 36 The reduction of myofibroblast transformation has two sides: on one side, it delays the corneal wound healing; on the other side, it may protect the cornea from scarring, which is counter intuitive. In fact, more scarring is observed in conditions when corneal hypoxia is present. Therefore, the relationship between hypoxia, myofibroblast, and corneal scarring needs further investigation (Table 2).
TABLE 2.
Effect of hypoxia on corneal stroma
| Author | Models | Findings | Duration of hypoxia |
|---|---|---|---|
| Tina, et al. 32 | 3D in vitro culture of corneal fibroblasts isolated from healthy cornea stroma and keratoconus in hypoxic environment | Reduction of collagen I and III; Elevation of MMP‐1 and ‐2 expression | 1 week |
| Pramod, et al. 37 | Human corneal fibroblasts cultured in lower oxygen conditions (2%, 0.5%) | Highest extracellular matrix deposition in 2% oxygen tension groups | 14 days |
| Xing, et al. 38 | Bovine corneal stromal cells in hypoxia condition (0.5%) | Upregulation of HIF‐1α and VEGF | 4 h |
2.2.3. Endothelial cell
The endothelium is a single cell layer located at the inner side of the cornea, characterized by a uniformly hexagonal structure. The major function of the endothelial cells is to pump out the water from the cornea stroma, which is crucial for corneal transparency. The fluid pump function is facilitated by ion transportation and carbonic anhydrase (CA). H2O and CO2 in the stroma turn into by CA12; then gets passively transported into the endothelial cells by the co‐transporter (NBC1) down the chemical‐potential gradients created by a 3Na+/2K+‐ATPase on the endothelial cell membrane. The then breaks down to CO2 and H2O by the enzyme CAII, and the CO2 is diffused back to the stroma or anterior chamber. The remaining is transported to the anterior chamber via exchanger. This transportation lowers the osmolarity at the basolateral/stromal interface relative to that in the aqueous humor. Thus, the H2O gets released to the anterior chamber down the osmolarity gradient 12 (Figure 3). When hypoxia occurs, the ion transportation function gets inhibited due to the lack of ATP; and the accumulation of CO2 and lactic acid alters the conversion of to CO2, thus disturbing the fluid pump function of the endothelium, leading to corneal edema. The free water entering the stroma from the damaged corneal epithelium barrier also contributes to the hypoxia‐induced corneal edema. 12
FIGURE 3.
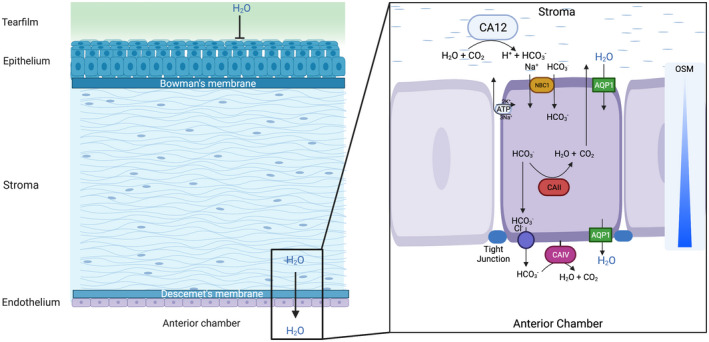
The transportation of free water and ions of the cornea under physiological state. The epithelial cells and the tight junction prevent free water of the tear film from entering the cornea; the endothelial cells create an osmolarity gradient towards the anterior chamber, which allows the free water from the stroma to be “pumped” into the anterior chamber. The osmolarity gradient is facilitated by a group of enzymes and ion channels (left panel). Firstly, the carbonic anhydrase (CA) 12 expressed in the stroma synthesizes , which is then passively transported into the endothelial cells by the co‐transporter (NBC1) down the chemical‐potential gradients created by a 3Na+/2K+‐ATPase. In the cytoplasm of endothelial cells, the breaks down into CO2 and H2O by CAII, then the CO2 diffuses back to the stroma or anterior chamber. The remaining is transported to the anterior chamber via a exchanger. This transportation lowers the osmolarity at the basolateral/stromal interface relative to that in the aqueous humor, allowing the free water to flush into the anterior chamber, possibly via the water channel aquaporin (AQP) 1
Corneal edema has long been observed clinically in corneal hypoxia, and the relationship between hypoxia‐induced corneal edema and the corneal metabolism rate has been well profiled. 39 , 40 Interestingly, in circumstances when only increased glycolysis and lactic acid accumulation are present but not hypoxic (an ultramarathon), the corneal edema also happens. 41 This finding further indicates that the edema caused by hypoxia is due to the increase in glycolysis. Besides edema, clinicians have also observed variation in cell size within the endothelial monolayer (termed polymegathism) in patients with contact lens wear. 42 The polymegathism may be transient or permanent and is believed to be caused by the pH change from lactic acid /CO2 accumulation and the lack of ATP. 42
Another clinical finding of hypoxic endothelial cells is the ‘Belb phenomenon,’ meaning a transient loss of the specular reflection in some endothelial cells during hypoxia exposure. 43 A potential cause of the belb is also believed to be lactic acid accumulation, 44 and the belb is associated with less oxygen transmissibility of contact lenses. 43 Currently, the mechanism of the belb formation is unclear. A possible explanation is that the nuclear area of some endothelial cells becomes edematous during hypoxia, causing the light that reflected from those edematous cells to be shifted to a different direction that the camera/observer could not catch it, leading to the dark appearance of blebs under specular image. 43 However, this hypothesis does not explain why only certain cells undergo the belb phenomenon, and the mechanism of the belb still needs further investigation.
In corneal dendritic cells (DCs), clinical studies have observed an increased number of DCs associated with contact lens wear, 45 but little is known for the hypoxia‐induced cellular function alteration of DCs. As an important player of the innate immune response of the cornea, DCs are responsible for corneal homeostasis. Further studies are required to understand the response of DCs to hypoxia to help understand the reduced barrier function during hypoxia, such as contact lens‐induced infections.
To conclude the sections above, decreased aerobic metabolism and increased anaerobic metabolism is a common pathogenesis shared by almost all types of cells, but leads to cell type‐specific responses.
2.3. Hypoxia‐induced signaling pathways
Although exposure to hypoxia affects the cornea via multiple mechanisms, a study has found that a mild long‐term lack of oxygen (10%) does not affect the normal epithelium structure of the cornea. 28 This result brings up an interesting question: how does the cornea cope with hypoxia?
Hypoxia triggers multiple signaling pathways to adapt cells to the lack of oxygen. Overall, the activation of these signaling pathways leads to altered metabolism, regulation of reactive oxygen species (ROS), angiogenesis, cell cycle progression, or cell death. 46 The key protein facilitating these changes is the hypoxia inducible factor (HIF), a transcription factor that activates upon sensing hypoxia. 47 HIF forms heterodimers composed of α and β subunits when activated. 47 There are three paralogues of the α unit, HIF‐1α, ‐2α, ‐3α; and three isoforms of the β subunits, aryl hydrocarbon receptor nuclear translocator (Arnt)1, ‐2, and ‐3 have been characterized. 48 HIF‐1α and HIF‐2α function synergistically, but each of them has its preference in gene regulation: HIF‐1α is more specific in inducing enzymes involved in glycolysis, 49 while HIF‐2α favors the genes involved in cell cycling and tumor growth. 50 , 51 HIF‐3α is believed to play an autoregulatory role in the HIF pathway that gives negative feedback to HIF1α and HIF2α. 52
HIF accomplishes the oxygen sensing function with the help of several enzymes, including a special group of prolyl hydroxylases (PHDs) and factor inhibiting HIF (FIH‐1). During non‐hypoxic state, PHDs hydroxylate the HIF‐α subunit at the proline residues, leading to proteasomal degradation of HIF‐α subunit; FIH‐1 prevents the binding of an important coactivator p300/Creb‐binding protein (CBP/p300) to the HIF‐α subunit, both of which prevents the activation of the HIF complex. 48 , 53 When exposed to hypoxia, the low oxygen level inactivates PHDs and FIHs, thus the HIF‐α subunit binds to its β subunit and activates the signaling pathway 48 (Figure 4).
FIGURE 4.
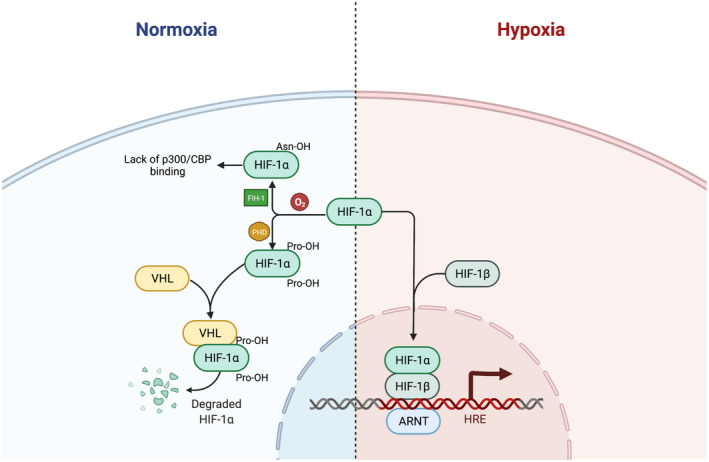
The inhibition and activation of the HIF pathway at different oxygen concentrations. During nonhypoxic state, PHDs hydroxylate the HIF‐α subunit at the proline residues. The hydroxylated HIF‐α binds to VHL, leading to proteasomal degradation of HIF‐α subunit; FIH‐1 prevents the binding of an important coactivator p300/Creb‐binding protein (CBP/p300) to the HIF‐α subunit, both of which prevents the activation of the HIF complex. When exposed to hypoxia, the low oxygen level inactivates PHDs and FIHs, thus the HIF‐α subunit binds to its β subunit and activates the signaling pathway. HIF, hypoxia inducible factor; PHD, prolyl hydroxylase; FIH, factor inhibiting HIF (FIH‐1); VHL, Von Hippel Lindau protein
It has been observed that activated HIF‐1 induces key enzymes in the glycolysis pathway, including aldolase A (ALDA), phosphoglycerate kinase 1 (PGK1), and pyruvate kinase M, all of which contain HIF‐1 binding site in the enhancer of their corresponding gene sequence. 54 Moreover, the target genes of the HIF complex include genes coding for a vascular endothelial growth factor (VEGF), 55 tissue inhibitor of metalloproteinases 1 (TIMP‐1), angiopoietin (ANGPT) that regulates angiogenesis, 55 , 56 CD18 in inflammation pathway, Bcl‐2 and p21/p27 that regulates cell proliferation and cell death, and many more. 57
The HIF signaling pathway was initially studied in erythrocytes and was quickly broadened to other tissues of the body, including the cardiovascular system, nerve system, respiratory system, and cancer. 58 Compared with these tissues, the role of the HIF signaling pathway in cornea has yet to be clarified. To our current knowledge, both HIF‐1α and HIF‐2α are expressed in the corneal tissue. 6 , 59 Low oxygen culture conditions (1% O2) upregulates the protein level of HIF‐1α and VEGF in human corneal epithelial cells. 59 The HIF‐1α signaling pathway was found to play a critical role in regulating VEGF expression in corneal neovascularization, which is a sight‐threatening condition caused by new vessel formation from the limbus and its invasion into the cornea. Silencing Hif1α by shRNA attenuated the corneal angiogenesis both in a contact lens wearing mouse and a rabbit alkali burn model. 60 Intervening the combination between Hif1α/Hif2α and Hifβ by acriflavine treatment decreased the neovascularization during the clinical disease period. 6 , 61 However, inhibition of HIF‐1α did not reduce the VEGF level in the bovine corneal stromal cells under hypoxic culture conditions. 62 Hence, the HIF‐1α‐dependent regulation of VEGF might be cell type specific.
The HIF pathway also plays a protective role in cornea against injuries. HIF‐1α was found to protect stromal cells from apoptosis induced by UV injury. 38 Preconditioning with hypoxia or a PHD inhibitor protects corneal endothelial cells from mechanical injuries. 63 The mechanism of this protective action is unclear, but is possibly due to the expression of anti‐apoptotic genes responsible for enhanced survival, growth, and metabolism of the cells that HIF‐1α promoted. 64
HIF activation was also studied in corneal inflammation. In progressing herpes stromal keratitis lesions, HIF‐1α protein stabilization was observed in infiltrating immune cells, especially the neutrophils. 6 Nuclear localization of HIF‐2α protein was found in the epithelial cells of the infected cornea during the chronic disease period, but not in the naïve and early stage of post‐infection cornea. 6 In addition, HIF‐1α has also been suggested as a master regulator of innate host defenses. 65 Inhibition of HIF‐1α was observed to exacerbate the disease of bacterial keratitis and corneal destruction by compromising the neutrophils functions of bacterial killing, apoptosis and antimicrobial peptides production and increasing pro‐inflammatory cytokine expression. 66
In summary, the HIF pathway has been observed in several pathological processes of the cornea, mainly angiogenesis, injury, and inflammation. However, the regulatory mechanism of the HIF proteins remains fuzzy in the cornea, and the role of HIF in hypoxia secondary to inflammation/injury requires further investigation. In the next section, we are going to introduce the animal models that are currently used in corneal hypoxia studies.
3. CURRENT ANIMAL MODEL
3.1. Contact lens wear model
Contact lens wear is a major model used for the hypoxia study on the ocular surface due to its under‐controlled oxygen transmissibility. Simple as the name implies, this model is built by applying a contact lens to experimental animals to eliminate the oxygen supply from the atmosphere. Several species of contact lens wear models that have been reported, mainly rabbits, mice, and a small number of studies using rats.
3.1.1. Rabbit
Rabbit models of ocular diseases are particularly useful for studying of preclinical drug efficacy, contact lens wear, dry eye syndrome, etc., due to the similar anatomical and biochemical features of humans and rodents, with long life span and larger eye size. 67 In brief, the rabbits were housed in individual cages at 19–23°C room temperature under relative humidity of 30%–50%. To permit lens wear, the nictitating membrane of each rabbits’ eye was excised first. After anesthetization, the nictitating membrane was pulled away from the eye globe with forceps and partially (30%–40%) cut with scissors. 68 , 69 Antibiotic ointment and drops were applied to the eyes of rabbits to prevent infection. After a minimum of 1‐week postoperative quarantine, rabbits were used for contact lens wear. This model is used to evaluate the effects of lower oxygen transmissibility to the corneal epithelium function and structure as well as the bacterial infection (Pseudomonas aeruginosa, etc.). However, the duration of the lens wear in most studies is 24 h or less due to the lens loss in long‐term observation. Hence, a long‐term stable model should be further investigated to observe how the lens affects the cornea during long‐term contact lens wear.
3.1.2. Rodent
The rodent eye is smaller than the rabbit eye. However, due to its lower price and high feasibility, rodent models are also commonly used for ocular diseases, such as corneal neovascularization (NV), corneal scar, corneal transplantation, etc. For example, to induce ocular hypoxia, 3.5 mm lenses were applied onto the mouse's cornea surface followed by a silk suture tarsorrhaphy. 70 The corneal NV scores increased in a time‐dependent manner due to hypoxia. However, the inflammation inflamed by the contact lens after the tarsorrhaphy should be considered since the chronic inflammation may also contribute to corneal NV.
There is no easy explanation of which model to choose when planning a study on corneal hypoxia. Both rabbit and rodent mimic the human cornea well, but other factors such as cost, efficiency, feasibility, and, most importantly, the research question need to be considered.
3.2. Systemic hypoxia models
In contrast to the local hypoxia models, scientists have built systemic hypoxia models to study the corneal hypoxia caused by systemic lack of oxygen. These models use hypoxia chambers of different oxygen concentration to eliminate the oxygen consumption of the entire body. Kosaku et al. put the mice under hypoxia conditions (10% oxygen) to observe how the cornea tissue responds to the long‐term systemic hypoxia. 28 According to their report, long‐term hypoxia caused a decrease in the glycogen granules in the epithelium. 28 However, in the same study, the layer structure and surface microvilli of the corneal epithelium were not disturbed after 140 days (long‐term hypoxia) observation. 28 No obvious clinical manifestations were reported. Compared with obvious clinical manifestations in the local corneal hypoxia models induced by contact lens wear, the 10% oxygen concentration in this model might not be enough to induce serious corneal hypoxia.
Attempts have also been made to build an acute systemic hypoxia model. Scientists created the acute hypoxia by pumping the air from the pressure chamber for 1 min before reaching a pressure of 180 mmHg. Rats were kept under these conditions for 3 min. 71 Apoptosis of both corneal and conjunctival epithelial cells has been found in this model, but no long‐term observation result was reported. 71 However, due to the suffocating nature of this model, more observations need to be made in systemic changes of the body, and whether these changes will affect the cornea. The fluctuation of intraocular pressure should also be considered due to the pressure decrease of the chamber.
To summarize this section, the idea of using a hypoxia chamber to study corneal hypoxia is feasible and positive results have been reported, however, more studies will need to be done to determine the oxygen concentration and the time of exposure.
3.3. Infectious keratitis models
Besides the embedment (contact lens wear, scleral contact lens, etc.) and systemic hypoxia, the corneal inflammation induced by various microbials is the other cause for the corneal hypoxia. For example, Bai et al. established an experimental rat keratitis model induced using P. aeruginosa to detect whether reversing the hypoxia microenvironment by oxygen delivery can improve the antibacterial therapy. 5 In brief, P. aeruginosa (20 μl) was inoculated onto the rat corneas damaged by a needle. After 12 h of infection, the infected rats were used for evaluation. 5 Hypoxic microenvironment was observed in these rats and oxygen supplementation accelerated the healing process of the corneal damage. 5
Another infectious agent commonly used by corneal studies is the herpes simplex virus (HSV). Herpes simplex keratitis (HSK) is a potentially blinding disease caused by HSV infection of the corneal. The reactivation of the virus from latency leads to the recurrent bouts of corneal inflammation and scarring, which cause progressive loss of vision. Rao et al. developed a HSK model by applying 1 × 105 PFU of virus in 3 µl 1 × PBS to the mouse eye and showed the development of hypoxia with progressing HSK lesions. The magnitude of hypoxia correlated with the extent of neutrophils infiltrating the infected corneas. 6
Besides the two models of infectious keratitis, there are a great number of keratitis models related to corneal hypoxia, reviewed by Fleiszig et al. 72 These models are good for studies focusing on hypoxia‐related corneal infection and allow researchers to observe the effect of a longer period of hypoxia exposure.
4. SUMMARY AND PROSPECTS
The cornea is unique in its oxygen supply, with most of the oxygen coming from the atmosphere and the rest coming from the aqueous humor. This makes the etiology of cornea hypoxia different from the rest of the body, where the main cause of hypoxia is due to the lack of blood supply. The animal model of corneal hypoxia is difficult to build due to the unique pathogenesis. The contact lens wearing model has been widely used over the years, but with a better understanding of the relationship between hypoxia and inflammation, the limitation of the contact lens model has become evident. Another factor that should be taken into consideration is the eye‐rubbing behavior induced by ocular surface disturbance in experimental animals. The discomfort in the eye will trigger stress and aggressive behavior, and it is not uncommon to result in a failure of the model. On the contrary, the systemic hypoxic model is a good solution to the eye‐rubbing, but it brings up other problems of exposing every tissue of the body to hypoxia. Further work is required to build a better cornea hypoxic model, and to understand how systemic hypoxia could affect the cornea.
Among the pathologies of cornea under hypoxia, the mechanism of corneal edema induced by hypoxia has been profiled, while other important biological processes are still under‐described. Many of these processes, such as angiogenesis and scar formation, are critical to visual function. A more complete understanding of the HIF pathway and its downstream signal is required, thus enabling the discovery of potential therapeutic targets. More importantly, establishing proper animal models for different hypoxia conditions will accelerate the progress of mechanistic research.
5. AUTHOR CONTRIBUTIONS
Conceptualization: M.Y.; Writing‐Original draft: K.P. and M.Y.; Writing‐Review & Editing: A.L. and M.Y.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
The illustrations used are created using BioRender.com.
Pang K, Lennikov A, Yang M. Hypoxia adaptation in the cornea: current animal models and underlying mechanisms. Anim Models Exp Med. 2021;4:300–310. doi: 10.1002/ame2.12192
Funding information
None.
REFERENCES
- 1. Musah‐Eroje A, Watson S. Adaptive changes of glioblastoma cells following exposure to hypoxic (1% oxygen) tumour microenvironment. Int J Mol Sci. 2019;20(9):2091. doi: 10.3390/ijms20092091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ottolenghi S, Zulueta A, Caretti A. Iron and sphingolipids as common players of (mal)adaptation to hypoxia in pulmonary diseases. Int J Mol Sci. 2020;21(1):307. doi: 10.3390/ijms21010307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanski JJ. Corneal and refractive surgery. In: Bowling B, eds. Clinical Ophthalmology: A Synopsis. Elsevier; 2009:173‐178. doi: 10.1016/b978-0-7020-3135-9.50015-x [DOI] [Google Scholar]
- 4. Onochie OE, Onyejose AJ, Rich CB, Trinkaus‐Randall V. The role of hypoxia in corneal extracellular matrix deposition and cell motility. Anat Rec. 2020;303(6):1703‐1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bai Y, Hu Y, Gao Y, et al. Oxygen self‐supplying nanotherapeutic for mitigation of tissue hypoxia and enhanced photodynamic therapy of bacterial keratitis. ACS Appl Mater Interfaces. 2021;13(29):33790‐33801. [DOI] [PubMed] [Google Scholar]
- 6. Rao P, Suvas S. Development of inflammatory hypoxia and prevalence of glycolytic metabolism in progressing herpes stromal keratitis lesions. J Immunol. 2019;202(2):514‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bojarun A, Vieversyte Z, Jaruseviciene R, Galgauskas S, Asoklis R, Zablockis R. Effect of obstructive sleep apnea on corneal morphological characteristics. Cornea. 2019;38(12):1576‐1581. [DOI] [PubMed] [Google Scholar]
- 8. Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21(5):268‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walmsley SR, Rupp J. Hypoxia and host pathogen responses. Microbes Infect. 2017;19(3):143. doi: 10.1016/j.micinf.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 10. Del Castillo LF, Ramírez‐Calderón JG, Del Castillo RM, Aguilella‐Arzo M, Compañ V. Corneal relaxation time estimation as a function of tear oxygen tension in human cornea during contact lens wear. J Biomed Mater Res B Appl Biomater. 2020;108(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 11. Lin MC, Yeh TN, Graham AD, et al. Ocular surface health during 30‐day continuous wear: rigid gas‐permeable versus silicone hydrogel hyper‐O2 transmitted contact lenses. Invest Ophthalmol Vis Sci. 2011;52(6):3530‐3538. [DOI] [PubMed] [Google Scholar]
- 12. Leung BK, Bonanno JA, Radke CJ. Oxygen‐deficient metabolism and corneal edema. Prog Retin Eye Res. 2011;30(6):471‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim YH, Lin MC, Radke CJ. Central‐to‐peripheral corneal edema during wear of embedded‐component contact lenses. Cont Lens Anterior Eye. 2021;101443. [DOI] [PubMed] [Google Scholar]
- 14. Harvitt DM, Bonanno JA. pH dependence of corneal oxygen consumption. Invest Ophthalmol Vis Sci. 1998;39(13):2778‐2781. [PubMed] [Google Scholar]
- 15. Aguilera K, Brekken R. Hypoxia studies with pimonidazole in vivo. BIO‐PROTOCOL. 2014;4(19): doi: 10.21769/bioprotoc.1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ulusoy DM, Ulusoy EK, Duru Z, Çiçek A. Evaluation of corneal morphology in patients with hemifacial spasm. Eye Contact Lens. 2019;45(4):271‐275. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto K, Ladage PM, Ren DH, et al. Effect of eyelid closure and overnight contact lens wear on viability of surface epithelial cells in rabbit cornea. Cornea. 2002;21(1):85‐90. [DOI] [PubMed] [Google Scholar]
- 18. Ren DH, Petroll WM, Jester JV, Ho‐Fan J, Cavanagh HD. Short‐term hypoxia downregulates epithelial cell desquamation in vivo, but does not increase Pseudomonas aeruginosa adherence to exfoliated human corneal epithelial cells. CLAO J. 1999;25(2):73‐79. [PubMed] [Google Scholar]
- 19. Robertson DM, Zhu M, Wu Y‐C, Cavanagh HD. Hypoxia‐induced downregulation of ΔNp63α in the corneal epithelium. Eye Contact Lens. 2012;38(4):214‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teranishi S, Kimura K, Kawamoto K, Nishida T. Protection of human corneal epithelial cells from hypoxia‐induced disruption of barrier function by keratinocyte growth factor. Invest Ophthalmol Vis Sci. 2008;49(6):2432‐2437. [DOI] [PubMed] [Google Scholar]
- 21. Yanai R, Ko J‐A, Morishige N, Chikama T‐I, Ichijima H, Nishida T. Disruption of zonula occludens‐1 localization in the rabbit corneal epithelium by contact lens‐induced hypoxia. Invest Ophthalmol Vis Sci. 2009;50(10):4605‐4610. [DOI] [PubMed] [Google Scholar]
- 22. Hara Y, Shiraishi A, Ohashi Y. Hypoxia‐altered signaling pathways of toll‐like receptor 4 (TLR4) in human corneal epithelial cells. Mol Vis. 2009;15:2515‐2520. [PMC free article] [PubMed] [Google Scholar]
- 23. Lee A, Derricks K, Minns M, et al. Hypoxia‐induced changes in Ca(2+) mobilization and protein phosphorylation implicated in impaired wound healing. Am J Physiol Cell Physiol. 2014;306(10):C972‐C985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonanno JA, Polse KA. Corneal acidosis during contact lens wear: effects of hypoxia and CO2 . Invest Ophthalmol Vis Sci. 1987;28(9):1514‐1520. [PubMed] [Google Scholar]
- 25. Rivera RK, Polse KA. Effects of hypoxia and hypercapnia on contact lens‐induced corneal acidosis. Optom Vis Sci. 1996;73(3):178‐183. [DOI] [PubMed] [Google Scholar]
- 26. Zaidi T, Mowrey‐McKee M, Pier GB. Hypoxia increases corneal cell expression of CFTR leading to increased Pseudomonas aeruginosa binding, internalization, and initiation of inflammation. Invest Ophthalmol Vis Sci. 2004;45(11):4066‐4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Safvati A, Cole N, Hume E, Willcox M. Mediators of neovascularization and the hypoxic cornea. Curr Eye Res. 2009;34(6):501‐514. [DOI] [PubMed] [Google Scholar]
- 28. Kosaku K, Harada T, Jike T, Tsuboi I, Aizawa S. Long‐term hypoxic tolerance in murine cornea. High Alt Med Biol. 2018;19(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 29. Jester JV, Moller‐Pedersen T, Huang J, et al. The cellular basis of corneal transparency: evidence for “corneal crystallins”. J Cell Sci. 1999;112(Pt 5):613‐622. [DOI] [PubMed] [Google Scholar]
- 30. Fini ME, Stramer BM. How the cornea heals: cornea‐specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24(8 Suppl):S2‐S11. [DOI] [PubMed] [Google Scholar]
- 31. Liesegang TJ. Physiologic changes of the cornea with contact lens wear. CLAO J. 2002;28(1):12‐27. [PubMed] [Google Scholar]
- 32. McKay TB, Hjortdal J, Priyadarsini S, Karamichos D. Acute hypoxia influences collagen and matrix metalloproteinase expression by human keratoconus cells in vitro. PLoS One. 2017;12(4):e0176017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee A, Karamichos D, Onochie OE, et al. Hypoxia modulates the development of a corneal stromal matrix model. Exp Eye Res. 2018;170:127‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. West‐Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38(10):1625‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18(3):311‐356. [DOI] [PubMed] [Google Scholar]
- 36. Xing D, Bonanno JA. Hypoxia reduces TGFbeta1‐induced corneal keratocyte myofibroblast transformation. Mol Vis. 2009;15:1827‐1834. [PMC free article] [PubMed] [Google Scholar]
- 37. Kumar P, Satyam A, Cigognini D, Pandit A, Zeugolis DI. Low oxygen tension and macromolecular crowding accelerate extracellular matrix deposition in human corneal fibroblast culture. J Tissue Eng Regen Med. 2018;12(1):6‐18. [DOI] [PubMed] [Google Scholar]
- 38. Xing D, Sun X, Li J, Cui M, Tan‐Allen K, Bonanno JA. Hypoxia preconditioning protects corneal stromal cells against induced apoptosis. Exp Eye Res. 2006;82(5):780‐787. doi: 10.1016/j.exer.2005.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nguyen T, Soni PS, Brizendine E, Bonanno JA. Variability in hypoxia‐induced corneal swelling is associated with variability in corneal metabolism and endothelial function. Eye Contact Lens. 2003;29(2):117‐125. [DOI] [PubMed] [Google Scholar]
- 40. Stickel TE, Bonanno JA. The relationship between corneal oxygen tension and hypoxic corneal edema. Optometry. 2002;73(10):598‐604. [PubMed] [Google Scholar]
- 41. Moshirfar M, Ding Y, Ronquillo Y, Birdsong OC, Murri MS. Ultramarathon‐induced bilateral corneal edema: a case report and a review of the literature. Ophthalmol Ther. 2018;7(1):197‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Connor CG, Zagrod ME. Contact lens‐induced corneal endothelial polymegathism: functional significance and possible mechanisms. Am J Optom Physiol Opt. 1986;63(7):539‐544. [DOI] [PubMed] [Google Scholar]
- 43. Giasson CJ, Rancourt J, Robillard J, Melillo M, Michaud L. Corneal endothelial blebs induced in scleral lens wearers. Optom Vis Sci. 2019;96(11):810‐817. [DOI] [PubMed] [Google Scholar]
- 44. Holden BA, Williams L, Zantos SG. The etiology of transient endothelial changes in the human cornea. Invest Ophthalmol Vis Sci. 1985;26(10):1354‐1359. [PubMed] [Google Scholar]
- 45. Sindt CW, Grout TK, Critser DB, Kern JR, Meadows DL. Dendritic immune cell densities in the central cornea associated with soft contact lens types and lens care solution types: a pilot study. Clin Ophthalmol. 2012;6:511‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McGarry T, Biniecka M, Veale DJ, Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic Biol Med. 2018;125:15‐24. [DOI] [PubMed] [Google Scholar]
- 47. Wang GL, Semenza GL. Purification and characterization of hypoxia‐inducible factor 1. J Biol Chem. 1995;270(3):1230‐1237. [DOI] [PubMed] [Google Scholar]
- 48. Loboda A, Jozkowicz A, Dulak J. HIF‐1 versus HIF‐2–is one more important than the other? Vascul Pharmacol. 2012;56(5–6):245‐251. [DOI] [PubMed] [Google Scholar]
- 49. Hu C‐J, Wang L‐Y, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia‐inducible factor 1alpha (HIF‐1alpha) and HIF‐2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23(24):9361‐9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gordan JD, Bertout JA, Hu C‐J, Diehl JA, Simon MC. HIF‐2alpha promotes hypoxic cell proliferation by enhancing c‐myc transcriptional activity. Cancer Cell. 2007;11(4):335‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Covello KL, Kehler J, Yu H, et al. HIF‐2alpha regulates Oct‐4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20(5):557‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang S‐L, Wu C, Xiong Z‐F, Fang X. Progress on hypoxia‐inducible factor‐3: Its structure, gene regulation and biological function (Review). Mol Med Rep. 2015;12(2):2411‐2416. [DOI] [PubMed] [Google Scholar]
- 53. Zagórska A, Dulak J. HIF‐1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol. 2004;51(3):563‐585. [PubMed] [Google Scholar]
- 54. Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia‐inducible factor 1. J Biol Chem. 1994;269(38):23757‐23763. [PubMed] [Google Scholar]
- 55. Ozaki H, Yu AY, Della N, et al. Hypoxia inducible factor‐1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40(1):182‐189. [PubMed] [Google Scholar]
- 56. Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia‐inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88(11):2606‐2618. [PubMed] [Google Scholar]
- 57. Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Semenza GL, Agani F, Feldser D, et al. Hypoxia, HIF‐1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123‐130. [DOI] [PubMed] [Google Scholar]
- 59. Zhang Y, Yuan F, Liu L, et al. The Role of the miR‐21/SPRY2 Axis in modulating proangiogenic factors, epithelial phenotypes, and wound healing in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2019;60(12):3854‐3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fu Y‐C, Xin Z‐M. Inhibited corneal neovascularization in rabbits following corneal alkali burn by double‐target interference for VEGF and HIF‐1α. Biosci Rep. 2019;39(1):BSR20180552. doi: 10.1042/BSR20180552 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61. Zeng M, Shen J, Liu Y, et al. The HIF‐1 antagonist acriflavine: visualization in retina and suppression of ocular neovascularization. J Mol Med. 2017;95(4):417‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xing D, Bonanno JA. Hypoxia preconditioning protection of corneal stromal cells requires HIF1alpha but not VEGF. Mol Vis. 2009;15:1020‐1027. [PMC free article] [PubMed] [Google Scholar]
- 63. Bhadange Y, Lautert J, Li S, et al. Hypoxia and the prolyl hydroxylase inhibitor FG‐4592 protect corneal endothelial cells from mechanical and perioperative surgical stress. Cornea. 2018;37(4):501‐507. doi: 10.1097/ico.0000000000001430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Webb JD, Coleman ML, Pugh CW. Hypoxia, hypoxia‐inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell Mol Life Sci. 2009;66(22):3539‐3554. doi: 10.1007/s00018-009-0147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zarember KA, Malech HL. HIF‐1alpha: a master regulator of innate host defenses? J Clin Invest. 2005;115(7):1702‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Berger EA, McClellan SA, Vistisen KS, Hazlett LD. HIF‐1α is essential for effective PMN bacterial killing, antimicrobial peptide production and apoptosis in Pseudomonas aeruginosa keratitis. PLoS Pathog. 2013;9(7):e1003457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zernii EY, Baksheeva VE, Iomdina EN, et al. Rabbit models of ocular diseases: new relevance for classical approaches. CNS Neurol Disord Drug Targets. 2016;15(3):267‐291. [DOI] [PubMed] [Google Scholar]
- 68. Imayasu M, Petroll WM, Jester JV, Patel SK, Ohashi J, Cavanagh HD. The relation between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology. 1994;101(2):371‐388. [DOI] [PubMed] [Google Scholar]
- 69. Ladage PM, Jester JV, Petroll WM, Bergmanson JPG, Cavanagh HD. Vertical movement of epithelial basal cells toward the corneal surface during use of extended‐wear contact lenses. Invest Ophthalmol Vis Sci. 2003;44(3):1056‐1063. [DOI] [PubMed] [Google Scholar]
- 70. Chen P, Yin H, Wang Y, Wang Y, Xie L. Inhibition of VEGF expression and corneal neovascularization by shRNA targeting HIF‐1α in a mouse model of closed eye contact lens wear. Mol Vis. 2012;18:864‐873. [PMC free article] [PubMed] [Google Scholar]
- 71. Akberova SI, Markitantova YV, Ryabtseva AA, Stroeva OG. Hypoxia as pathogenic factor affecting the eye tissues: the selective apoptotic damage of the conjunctiva and anterior epithelium of the cornea. Dokl Biochem Biophys. 2016;467(1):150‐152. [DOI] [PubMed] [Google Scholar]
- 72. Fleiszig SMJ, Kroken AR, Nieto V, et al. Contact lens‐related corneal infection: Intrinsic resistance and its compromise. Prog Retin Eye Res. 2020;76:100804. [DOI] [PMC free article] [PubMed] [Google Scholar]


