Abstract
Background
Wound healing can result in various outcomes, including hypertrophic scar (HTS). Pigs serve as models to study wound healing as their skin shares physiologic similarity with humans. Yorkshire (Yk) and Duroc (Dc) pigs have been used to mimic normal and abnormal wound healing, respectively. The reason behind this differential healing phenotype was explored here.
Methods
Excisional wounds were made on Dc and Yk pigs and were sampled and imaged for 98 days. PCR arrays were used to determine differential gene expression. Vancouver Scar Scale (VSS) scores were given. Re‐epithelialization was analyzed. H&E, Mason's trichrome, and immunostains were used to determine cellularity, collagen content, and blood vessel density, respectively.
Results
Yk wounds heal to a “port wine” HTS, resembling scarring in Fitzpatrick skin types (FST) I‐III. Dc wounds heal to a dyspigmented, non‐pliable HTS, resembling scarring in FST IV–VI. Gene expression during wound healing was differentially regulated versus uninjured skin in 40/80 genes, 15 of which differed between breeds. Yk scars had a higher VSS score at all time points. Yk and Dc wounds had equivalent re‐epithelialization, collagen disorganization, and blood vessel density.
Conclusions
Our findings demonstrate that Dc and Yk pigs can produce HTS. Wound creation and healing were consistent among breeds, and differences in gene expression were not sufficient to explain differences in resulting scar phenotype. Both pig breeds should be used in animal models to investigate novel therapeutics to provide insight into a treatment's effectiveness on various skin types.
Keywords: animal models, burns, Fitzpatrick skin type, hypertrophic scar, wound healing
1. INTRODUCTION
Hypertrophic scarring is a common, undesirable result of burn injury. Hypertrophic scar (HTS) typically presents as a raised, painful, pruritic, and contracted lesion. 1 As a result, it can reduce quality of life by being aesthetically undesirable and functionally impairing. 1 Although HTS can result from burn injury to any Fitzpatrick skin types (I–VI), higher Fitzpatrick skin types (IV, V, and VI) have been identified as a risk factor. 2 , 3 These patients are not only more prone to developing HTS, but also develop more severe HTS. 2 , 3 The etiology of this difference is not clear and has been understudied. Current hypotheses link the difference to increased fibroblast presence or reduced vitamin D‐3 metabolism in darker skin. 4 , 5 , 6
HTS is difficult to study because of the lack of a universally agreed‐upon animal model, especially one that is tunable to different levels of melanin from which the study of chemiexcitation is possible. There is also no perfect model to study HTS itself. Samples from patients are likely the best available representation of HTS. However, since the samples must be taken after a scar is already formed, they provide only a snapshot in time and do not provide insight into the natural history of HTS formation. A number of different animal models have been proposed; however, each has its own pros and cons, and each can be useful depending on the details of the specific research question. One frequently used animal models is the porcine model. 7 In particular, over the last 20 years, the red Duroc (Dc) pig has been reintroduced as a model for human HTS due to its formation of thick, hyperpigmented, contracted scars. 7 Dc pig HTS shares many similarities with human HTS including increased collagen deposition, aberrant expression of insulin‐like growth factor (IGF‐1), transforming growth factor beta 1 (TGF‐β1), versican, decorin, 7 and increased nerve quantity compared to normal, uninjured skin. 8
While the Dc pig is a commonly used model for HTS, the Yorkshire (Yk) pig has been thought inadequate to recapitulate the phenotype of severe scar. The scars produced by Yk pigs have more limited wound contracture and thinner layers of scar tissue and granulation tissue than those seen in Dc HTS. 9 This difference is likely due to the fact that, for un‐identified mechanistic reasons, fibroblasts from Yk skin are less fibroproliferative than Dc pig fibroblasts. 10 Dc pig fibroblasts have been found to be predisposed to myofibroblast differentiation and had impaired myofibroblast migration, leading to hypercellularity and a more contractile phenotype compared to Yk scars. However, a mechanistic reasoning behind these differences in cell properties has not been revealed. 10
Comparing scar formation in the Yk and Dc pigs offers the opportunity to investigate underlying causes of why scars form differently in different skin pigmentation phenotypes. The most effective approach that can be taken to reduce scar formation is through wound healing optimization. 11 Processes that delay or prolong wound healing promote pathologic scar formation. 11 Minimizing the risk of suboptimal wound healing, such as through infection prevention, tension reduction, early wound excision for severe wounds, and utilization of optimal wound dressings can lower the chance of HTS formation. 12 However, in an experimental animal model such as this, where both pig breeds receive the same wound and wound care, reduced scar formation in one breed cannot be attributed to wound healing optimization. Thus, the underlying reason for differences in scar severity can be attributed to something else, possibly differences in melanin. In order to study HTS in the context of chemiexcitation in future work, the model was used here to study HTS at gross, histologic, and molecular levels. Fibroproliferative and antifibroproliferative genes were studied, including genes encoding collagens, chemokines, cytokines, coagulation factors, alpha and beta integrins, growth factors, and matrix metalloproteinases.
Our research group has previously utilized the Dc pig model, instead of the Yk pig model, to generate HTS in multiple research areas. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 However, our data suggests that it is not accurate to purport that Yk pigs do not generate hypertrophic scars. Instead, they generate a different phenotype more representative of how patients with Fitzpatrick skin types I–III heal and scar. In this study, excisional wound healing in Yk and Dc pigs were compared to determine if different pig breeds injured in the same way would result in different scar phenotypes. The results of this study, which serve as an exploration into the two different pig models, can be used to establish a background for future studies on the effect constitutive melanin levels may have on scarring.
2. METHODS
2.1. Animal model
All animal work was approved by the MedStar Health Research Institute's Institutional Animal Care and Use Committee. Paired full thickness excisional wounds (4 × 4 in) were made on the bilateral flanks of Dc and Yk pigs (30–50 kg) as previously described (n = 2 animals, n = 4 wounds/scars each). Details relevant to anesthesia, analgesia, animal monitoring, and pre‐ and postoperative animal care have been previously described. 16 Full thickness wounds were created down to subcutaneous fat with no remaining dermal appendages using three and two passes of the dermatome set to 0.03” in the Dc and Yk pigs, respectively, due to differences in skin thickness. The original intention of this work was to excise the same amount of skin thickness from each of the breeds (0.03”X3); however, the Yk pig skin was thinner than the Dc pig skin. Therefore, in the Yk pig, after 2X0.03” passes of the dermatome, the wound was down to subcutaneous tissue with no dermis at all, and another excision would have resulted in revealing muscle. Therefore, while different thicknesses were excised from each breed, the wounds were down to the same depth, which was full‐thickness, subcutaneous tissue. This methodical detail is important to note because it is known that wounds of differing depths result in scars of differing severity. 9 It is also important in studies with differing breeds and thus skin thickness to judge wound depth based on surgical plane and not total dermatome depth. Wounds were sampled to obtain 3 mm punch biopsies which were stored in All Protect (Qiagen, Valencia, CA) for molecular assays or formalin for histology. Wounds were imaged with digital photography weekly until 42 days post‐injury and then biweekly until day 98. A metric ruler was included in each image to standardize camera distance and contour of the animal. At each check post 100% re‐epithelialization, an occupational therapist (OT) with over 30 years of experience in assigning Vancouver Scar Scale (VSS) scores in burn hypertrophic scar assigned a score for each scar. Due to the fact that the difference in uninjured skin color was apparent upon gross inspection of the animals, the OT could not be blinded to the pig breed during VSS scoring. However, the OT investigator was not informed of the research question and therefore, was not biased towards scoring either breed higher or lower.
2.2. Human subjects
The images of hypertrophic scars of different Fitzpatrick skin types were collected as a part of a prospective, observational clinical trial that was approved by the MedStar Health Research Institute's Institutional Review Board (IRB protocol #00000430). Patient consent was obtained prior to imaging and subjects agreed to the use of their images in published works.
2.3. RNA isolation and PCR arrays
RNA was isolated from tissue biopsies using the RNeasy Fibrous Tissue Mini Kit (Qiagen) according to the manufacturer's protocol. Quantity and quality were assessed using the Nanodrop 2000. RNA was diluted to 50 ng/µL, and 500 ng of RNA was used to create cDNA using the RT2 First Strand Kit (Qiagen) according to the manufacturer's instructions. cDNA and SYBR green were then used to create a mastermix which was pipetted onto an 84‐gene porcine wound healing‐specific RT2 PCR array plate with gene specific primers (Qiagen). Genes with Ct < 35 were excluded from the analysis. The ΔΔCt method was used to obtain fold changes. Genes of interest were normalized to five housekeeping genes as the reference control (gamma‐actin, ACTG1; beta‐2‐microglobulin, B2M; glyceraldehyde 3‐phosphate dehydrogenase, GAPDH; hypoxanthine phosphoribosyltransferase 1, HPRT1; and ribosomal protein L13a, RPL13A). In one analysis, baseline, uninjured skin from Dc pigs were normalized to Yk baseline, uninjured skin to investigate the inherent mRNA expression profiles in the skin (n = 4 Dc, n = 2 Yk). This was critical because in a separate analysis, biopsies from days 7, 14, 21, 28, and 35 were normalized to baseline, uninjured skin as the experimental control. Each animal was normalized to its own baseline (n = 2 Yk pig time courses, n = 4 Dc pig time courses). Due to the sample size, no statistical comparisons were made of the gene expression data between Yk versus Dc at any timepoint.
2.4. Re‐epithelialization
The open wound area on day 0 was calculated using Image J software as previously described (NIH, Bethesda, MD). 24 For each wound, the open wound area was likewise calculated at days 7, 14, 21, 18, 35, 42, 56, 70, 84, and 90 and normalized to the day 0 area to obtain a percentage of open wound area. All images had their scales set prior to calculating area to standardize for camera distance.
2.5. Cellularity and collagen quantification
H&E and Mason's trichrome stains were used to determine cellularity and collagen content of skin, wound, and scar tissue. For each breed, baseline and days 7, 56, and 98 samples were analyzed (n = 4 samples of skin, wound, or scar). At each time point, two distinct images were taken of one section, and were used in Image J to quantify cellularity. The images were imported, converted into a red, green, blue stack, and the red image was used. The threshold was adjusted to obtain distinct labeling of nuclei only for cellularity, and the percentage of the image that stained the nuclei was obtained. A macro was used with the same threshold for all images. Collagen was analyzed qualitatively.
2.6. Immunofluorescence
Blood vessel density was assessed using co‐immunostaining for α‐smooth muscle actin (α‐SMA) and cluster of differentiation factor 31 (CD31) as previously described. 23 Co‐staining was used due to the fact that immature blood vessels such as those in remodeling hypertrophic scars may not have full pericyte coverage that could be identified with α‐SMA alone. 25 Biopsies taken at baseline representing normal skin (n = 4 Dc, n = 4 Yk) and at day 98 representing HTS (n = 4 Dc, n = 4 Yk) were sectioned and stained using primary antibodies for α‐SMA (Abcam, ab7817, 1:250, mouse) and CD31 (Biorbyt, orb10314, 1:500, rabbit) and secondary antibodies (goat anti‐mouse‐CY3 and goat anti‐rabbit‐CY5, Abcam). Two photos of the papillary dermis were taken at 10× magnification for each biopsy. Blood vessel density was calculated by counting the number of vessels and dividing by the area. Secondary antibody controls were stained in parallel and did not show nonspecific staining.
3. RESULTS
3.1. Large, full‐thickness excisional wounds form distinct hypertrophic scar phenotypes in Yorkshire and Duroc pigs
The HTS phenotype of the Yk pig more closely resembles scars observed in patients of Fitzpatrick skin types I–III (Figure 1A, left), while the Dc HTS better approximate the scars seen in patients with Fitzpatrick types skin IV–VI (Figure 1A, right). Excisional wounds in both breeds were fully re‐epithelialized and formed thick, non‐pliable HTS by day 56 post‐excision. The HTS that formed on the Yk pigs were erythematous with a “port wine” coloration and had contracted to a substantially smaller area than that of the original wounds. The scars formed by the Dc pigs, on the other hand, were dyspigmented (both hyper‐ and hypopigmented) and retained a similar size and shape to that of the original wounds (Figure 1B). The wounds on both Yk and Dc pigs had approximately 40% open wound area at day 14 and 5% by day 28 post‐wounding, with no significant differences between Yk and Dc re‐epithelialization at any timepoints (Figure 1C).
FIGURE 1.
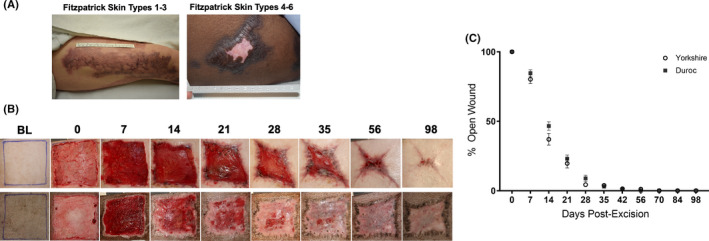
Re‐epithelialization and scar formation in patients and after excisional wounding of Yorkshire and Duroc pigs. Examples of Fitzpatrick I–III scar formation (A, left) and IV–VI (A, right). Photos of wound healing progression after excisional wounding of Yorkshire (top) and Duroc (bottom) pigs (B). Percentage of original wound area remaining open over time (n = 8 Dc and n = 8 Yk) (C)
3.2. Baseline, uninjured skin is different between Yorkshire and Duroc pigs
Seventeen genes were not expressed in baseline‐uninjured skin from both pig breeds (Figure 2, black squares, Ct < 35). When baseline‐uninjured skin from Dc pigs was compared to baseline, uninjured skin from Yk pigs, only 6/84 genes were differently regulated (FC> or <1.5 in at least 3/4 pigs), indicating high similarity between the two breeds. COL1A1, 1A2, and 3A1 were upregulated in Dc versus Yk baseline skin (2.01 ± 1.35, 1.6 ± 1.26, and 1.73 ± 1.35, Figure 2A–D, Wells A10, A11, and A12). CXC chemokine ligand 2 (CXCL2), also known as growth‐regulated protein homolog gamma was upregulated in Dc versus Yk (7.56 ± 3.78, Figure 2A–D, Well B10). EGF was upregulated in Dc versus Yk (3.66 ± 0.50, Figure 2A–D, Well C1). MMP9 was upregulated in Dc versus Yk (1.74 ± 0.87, Figure 2A–D, Well F2).
FIGURE 2.
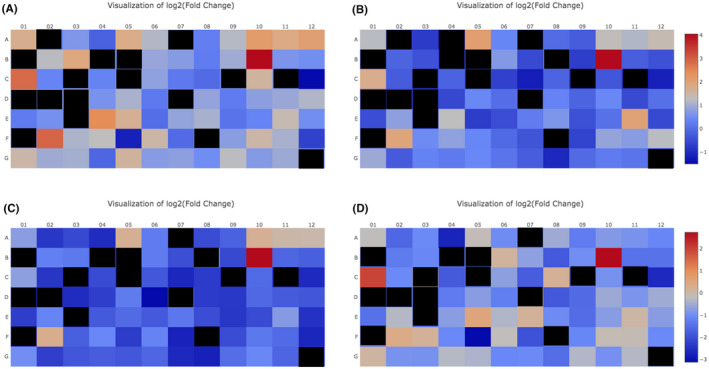
Baseline, uninjured skin from the 2 breeds demonstrate differential gene expression. Baseline, uninjured skin from Dc pigs (n = 4) was compared to baseline, uninjured skin from Yk pigs (n = 2 combined). Dc #1 normalized to Yk combined (A), Dc #2 normalized to Yk combined (B), Dc #3 normalized to Yk combined (C), Dc #4 normalized to Yk combined (D). Gene expression for the 84 genes was plotted as a heat map of the log2 (fold change). Red = upregulation, blue = downregulation. black = Ct < 35, gene not expressed
3.3. Post‐wounding gene expression in Yorkshire and Duroc pigs
Of the 84 genes examined on the PCR Array, 44 genes had Ct values below 35 and were excluded from analysis due to absent/low expression. Of the remaining 40 genes for analysis, all were differentially regulated compared to baseline, uninjured skin at at least one time point (FC> or <2). Post‐wounding changes in gene expression relative to their own baseline were compared between Dc and Yk pigs (n = 4 and n = 2, respectively). Overall, several genes (15/40) showed differences in expression between the two breeds (Figure 3).
FIGURE 3.
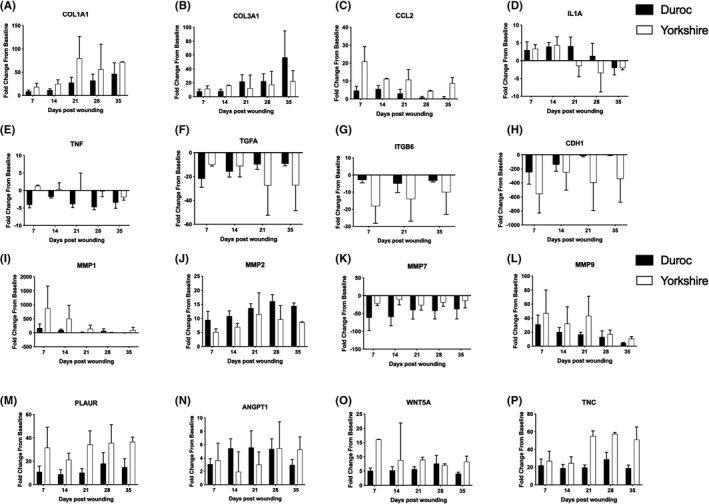
Several genes demonstrated post‐wounding differences in expression from baseline between Yorkshire and Duroc pigs. Samples from days 7, 14, 21, 28, and 35 were compared to baseline, uninjured skin to obtain fold change from baseline. (n = 4 Dc and n = 2 Yk)
Collagen genes that demonstrated differences in expression include COL1A1 and COL3A1. COL1A1, which encodes type I collagen, was increased from baseline, uninjured skin in both species. Yk expression had a larger increase from baseline than Dc expression at all study time points (Figure 3A). The COL3A1 gene, which encodes type III collagen, initially showed a larger increase in expression in Yk (8.12‐ vs. 16.05‐fold increase at D14 in Dc and Yk. respectively), while Dc expression demonstrated a larger fold increase from baseline at later time points (22.19‐ vs. 56.65‐fold increase at D35 in Yk and Dc. respectively) (Figure 3B). For this and all subsequent reports of gene expression changes below, statistical comparisons were not made between Yk and Dc because of the sample size (n = 2 Yk, n = 4 Dc).
CCL2 was the only chemokine gene to demonstrate a difference in expression. Both species demonstrated increased expression relative to baseline at all time points, with a larger fold increase in expression seen in Yk (Figure 3C). Cytokines that showed a difference in expression were IL1A and TNF. Both species initially demonstrated similar increased IL1A expression relative to baseline (3.36‐ vs. 2.97‐fold increase at D7 in Yk and Dc. respectively). Starting at day 21, Yk expression progressively decreased (4.43 at D14, −1.49 at D21, −3.48 at D28, −2.10 at D35). Dc expression decreased at day 28, lagging behind Yk throughout the remainder of the wound healing process (4.07 at D21, 1.35 at D28, −2.07 at D35) (Figure 3D). Yk TNF expression remained close to baseline at all time points. However, Dc expression showed a decrease relative to baseline at all time points (Figure 3E).
Of the studied growth factors, a difference in expression was revealed in TGFA. Both species had a decrease in expression relative to baseline at all time points. A greater fold decrease was initially seen in Dc expression, (−10.05 vs. −21.95 at D7 in Yk and Dc. respectively) while a larger decrease was later seen in Yk expression (−27.34 vs. −9.33 at D35 in Yk and Dc. respectively) (Figure 3F).
ITGB6 was the sole integrin to display a difference in expression. Both species showed a decrease in expression relative to baseline with a greater fold decrease seen in Yk expression (Figure 3G). CHD1 showed decreased expression in both breeds at all time points with a greater fold decrease in the Yk (Figure 3H).
All studied matrix metalloproteinases (MMPs) demonstrated a difference in expression between the two breeds (Figure 3I–L). Expression of MMPs‐1, ‐2, and ‐9 were increased relative to baseline while MMP‐7 was decreased. MMP‐1 showed a greater fold increase in expression in the Yk with values decreasing towards baseline throughout wound healing (882.56 at D7, 94.21 at D35). MMP‐9 also demonstrated larger fold increase in the Yk with a similar trend (46.97 at D7, 10.77 at D35). MMP‐2 had a larger fold increase from baseline in the Dc at all time points. MMP‐7 showed a larger fold decrease relative to baseline in the Dc. The genes PLAUR, TNC, and WNT5a all had a greater fold increase in the Yk than Dc (Figure 3M–O).
There were genes that did not show a difference in expression between Yk and Dc pigs (25/40) (Table 1). Some genes showed similar upregulation compared to baseline (Figure 4A), while others showed similar downregulation compared to baseline (Figure 4B). For the TGFB3 gene, Dc demonstrated a 5.67‐fold increase compared to a 5.79‐fold increase in Yk at day 28. The EGFR gene showed similar downregulation between the two breeds, with a −4.73‐fold change in Dc and −4.74‐fold change in Yk at day 21.
TABLE 1.
Genes that displayed similar fold change in expression from baseline during the wound healing process between Yorkshire and Duroc pigs
| Gene family | Different regulation between breeds? | |
|---|---|---|
| No | Yes | |
| Collagens | COL5A2, COL5A3 COL14A1 | COL1A1, COL3A1 |
| Chemokines/cytokines | CXCL11, CXCL12, IL10 | CCL2, IL1A, TNF |
| Growth factors | EGF, EGFR, FGF2, FGF7, FGF10, HBEGF, TGFB3, VEGFA | TGFA |
| Integrins | ITGA2, ITGA3, ITGA4, ITGA5, ITGA6, ITGB1 | ITGB6 |
| Coagulation | Factor XIII A Chain, Factor III, Serpine 1 | |
| Matrix metalloproteinases | MMP1, MMP2, MMP7, MMP9 | |
| Other | ANGPT1, MET | CDH1, PLAUR, WNT5A, TNC |
FIGURE 4.
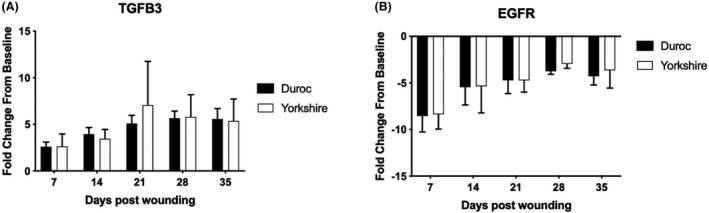
Several genes did not demonstrate post‐wounding differences in expression from baseline between Yorkshire and Duroc pigs. Samples from days 7, 14, 21, 28, and 35 were compared to baseline, uninjured skin to obtain fold change from baseline. (n = 4 Dc and n = 2 Yk)
3.4. HTS in Yorkshire and Duroc pigs is characterized by hypercellularity, disorganized collagen, and increased blood vessel density
H&E staining of biopsies taken during scar formation indicated a significant increase in dermal cellularity at day 7 post‐excision in both breeds (8.53 ± 1.71% at BL vs. 55.41 ± 10.42% at D7, p = .0005 for Yk, 8.20 ± 0.99% at BL vs. 64.03 ± 5.50% at D7, p < .0001 for Dc). While Yk HTS returned to baseline levels of dermal cellularity by day 98, Dc dermal cellularity remained elevated (8.20 ± 0.99% at BL vs. 23.13 ± 3.28% at D98, p = .033) (Figure 5). In both breeds, the presence of disorganized collagen was evident in HTS dermis at days 56 and 98, as indicated by Masson's trichrome stain (Figure 6). Blood vessel density in the papillary dermis was significantly higher at day 98 than at baseline in both breeds (90.95 ± 6.18 vessels/mm2 at BL vs. 159.86 ± 22.30 vessels/mm2 at D98, p = .0430 for Yk, 106.25 ± 10.36 vessels/mm2 at BL vs. 169.62 ± 20.53 vessels/mm2 at D98, p = .0443 for Dc), while there was not a significant difference in blood vessel density between breeds at either time point (Figure 7).
FIGURE 5.
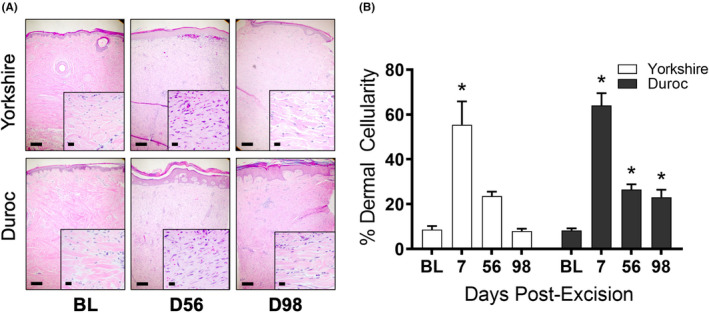
Yorkshire and Duroc dermal cellularity increases during hypertrophic scar development. H&E stains of Yorkshire and Duroc normal skin and HTS at days 56 and 98 post‐excision (A). Percent dermal cellularity of Yorkshire and Duroc skin at baseline and days 7, 56, and 98 post‐excision (B). *p < .05. Scale bars = 200 µm for 5× photos and 20 µm for 40× photos
FIGURE 6.
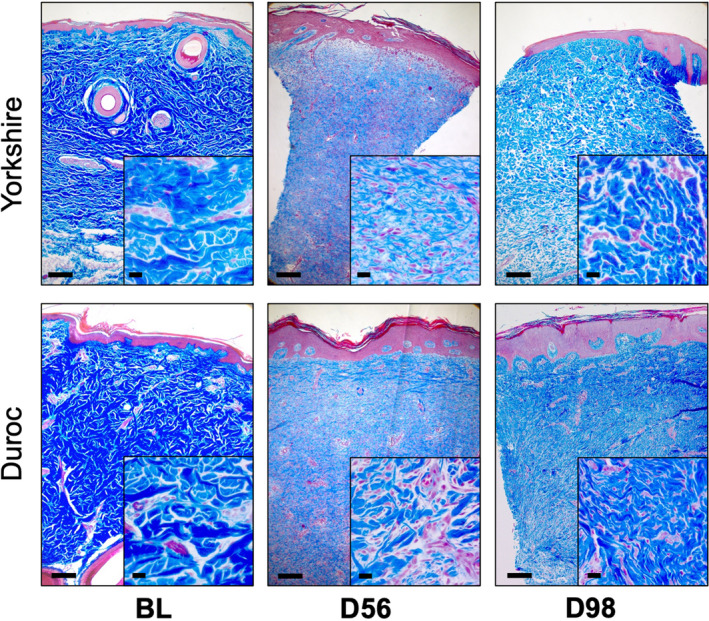
Masson's trichrome stain reflects disorganized collagen in Yorkshire and Duroc hypertrophic scar. Masson's trichrome stain of Yorkshire and Duroc normal skin and HTS at days 56 and 98 post‐excision. Organized collagen appears as dark blue and disorganized collagen appears as pale blue. Scale bars = 200 µm for 5× photos and 20 µm for 40× photos
FIGURE 7.
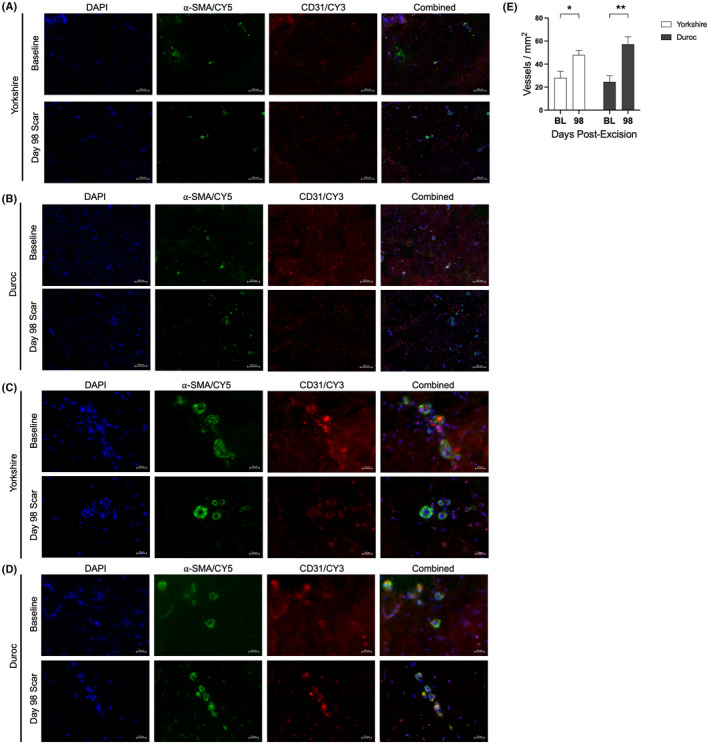
Blood vessel density is elevated in Yorkshire and Duroc hypertrophic scar. Immunofluorescent co‐staining of alpha‐smooth muscle actin and CD31 in Yorkshire (A, C) and Duroc (B, D) baseline skin and HTS at day 98. DAPI = blue; α‐SMA = green, CD31 = red. Scale bar = 100 µm at 10× magnification (A, B) or 20 µm (C, D) at 40× magnification. Dermal microvessel density of Yorkshire and Duroc baseline skin and HTS at day 98 (B) was quantified (E) *p < .05, **p < .01
3.5. The vascularity component of the VSS leads to differences in subjective scoring of Yorkshire versus Duroc HTS
At days 42 and 56 post‐wounding, Yk HTS was more severe than Dc HTS as assessed by the VSS (Figure 8A) (9.63 ± 0.26 vs. 7.25 ± 0.71 for D42, p < .0001). This difference was primarily due to the vascularity parameter of the VSS, where Yk HTS scored consistently higher than Dc (3.0 ± 0 vs. 1.0 ± 0 at D7, p < .0001), despite the lack of a significant difference in dermal vascularity between breeds as determined by histology (Figure 8B). Yk vascularity and overall VSS scores decreased over time as the scars developed (3.0 ± 0 at D42 vs. 2.0 ± 0.38 at D98, p = .015 for vascularity, 9.63 ± 0.26 at D42 vs. 7.57 ± 0.53 at D98, p = .0003 for overall score) (Figure 8C). From days 42 to 98 post‐excision, there were no significant changes in the vascularity or overall VSS score of Dc HTS. From day 70 onwards, the overall VSS scores of Yk and Dc HTS were not significantly different.
FIGURE 8.
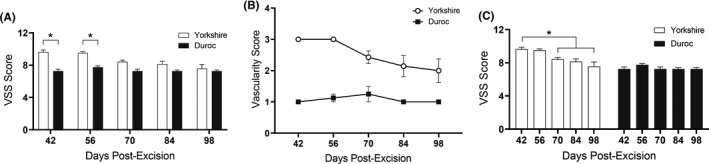
Yorkshire and Duroc scar severity over time as measured by the Vancouver Scar Scale (VSS). Overall VSS scores of Yorkshire and Duroc HTS at days 42, 56, 70, 84, and 98 post‐excision. Results and significance comparisons are given by time point, between breeds (A), or by breed, between time points (C). VSS vascularity scores of Yorkshire and Duroc HTS over time (B). *p < .05
4. DISCUSSION
The underlying mechanistic cause for variations in scar formation in skin with different pigmentation phenotypes has not been well characterized. The current findings demonstrate that both Dc and Yk pigs can produce HTS, with Yk HTS resembling HTS in Fitzpatrick skin types I–III and Dc HTS being similar to HTS in Fitzpatrick skin types IV–VI. Current literature consistently demonstrates that Dc pigs heal with a more fibroproliferative phenotype than Yk pigs, 7 , 26 , 27 but the etiology of this difference is not clearly explained.
Overall, uninjured normal skin in Dc versus Yk pigs has a very similar pattern of gene expression in genes related to wound healing in that 78/84 genes were not different. Despite this similarity, the genes that were differentially expressed were consistent among the four different Dc pigs, and may be important in elucidating the differences in resulting scar phenotypes among breeds. These genes, which were all upregulated, include COL1A1, 1A2, and 3A1; CXCL2; EGF; and MMP9. The upregulation of these genes in uninjured skin from the Dc pig versus Yk pig may play a role in the different response to injury. Additionally, when further studying differences in these six genes during the wound healing timecourse, and evaluating differential regulation between species, it is critical to note that each pig was normalized to its own baseline, which inherently has differing levels of these six genes. Therefore, it is possible that the absolute level of these genes is consistent among the breeds, but the change from their baseline is different. Importantly, only three of the genes identified above were identified as being differentially regulated during wound healing in either pig breed in the second analysis below (COL1A1, COL3A1, and MMP9). The other genes were either not differentially expressed from baseline during wound healing (COL1A2 and CXCL2), or were not differentially regulated between the two pig breeds (EGF).
Gene expression of healing wounds in Yk pigs 24 , 28 and female red Dc pigs has been studied in the past, including papers by Gallant et al. 29 The only overlapping time points between their prior study and the current study are days 14 and 28 post‐wounding because prior work did not necessarily focus on early wounds healing, but incorporated later scar‐related timepoints (days 28, 42, 56, and 70). Gallant did not compare Yk versus Dc gene expression directly, but showed Dc pig gene expression at day 14. Their results largely agreed with the current results which showed upregulation of genes such as COL1A1, COL3A1, MMP1, MMP2, and MMP9. The absolute value of the upregulation compared to normal skin was different in Gallant's paper, but they report their findings in % of normal value, while the current study presents fold change from baseline. Gallant's paper included the study of additional genes that were not studied here including numerous proteoglycans such as biglycan, decorin, and versican. The previous study utilized much smaller wounds (2 cm by 2 cm) compared to our study (10 cm by 10 cm). In both Yk and Dc pigs, wounds were fully re‐epithelialized by day 14, on par with a normal wound healing trajectory. In our present study, 100% re‐epithelialization did not occur until day 42 due to the large size of the wounds. These factors could contribute to the differences in expression observed.
In this study, wound healing optimization was consistent among both breeds and wounds were created similarly. Thus, differences in scar phenotype can be attributed to the difference in breed. Fifteen of the 84 studied genes showed differences in expression between breeds throughout the wound healing process. Changes in COL1A1, COL3A1, CCL2, IL1A, TNF, ITGB6, MMPs 1, 2, 7, 9, and PLAUR genes showed changes consistent with an increased fibroproliferative phenotype in Dc pigs relative to Yk. However, changes in TGFA, CDH1, TNC, and WNT5A indicated the opposite, possibly supporting a theory of dysregulated wound healing in the Dc pig. Additionally, many studied genes (25/40) showed similar expression between the two breeds, and the variations in gene expression are likely not enough to explain the differences in scar formation.
As determined by our histological results, both breeds showed a dramatic increase in dermal cellularity shortly after wound creation, consistent with the cell proliferation, migration, and ECM production and remodeling required for normal wound healing. It is of note, however, that Dc, but not Yk, retained an overabundance of fibroblasts in HTS dermis at day 98—this indicates a difference in apoptotic processes during mid‐to‐late scar formation that may contribute to the more severe fibrosis observed in Dc. Nonetheless, HTS from both breeds retained a high amount of disorganized collagen at day 98, which is a hallmark characteristic of HTS. An interesting difference between scarring in the two breeds was in vascularity as assessed by the VSS. Although histological determination of blood vessel density showed no difference between Dc and Yk HTS at day 98, the VSS consistently rated Yk HTS as more vascular than that of Dc at all timepoints. This contradiction hints that the difference in vascularity VSS scores does not actually reflect a difference in hypervascularity; the discrepancy may result from increased blood flow being easily visible through the light pigmentation of Yk skin, while any redness that would indicate an erythematous and hypervascularized HTS is obscured by the darker and irregular pigmentation of Dc HTS. This is an important observation that highlights the limitations of scar severity and treatment effectiveness measures based solely on macroscopic appearance. In the ongoing and future work, incorporation of quantitative, objective tools to rate differences in scar phenotype are of critical importance. 30 , 31 In addition, when attempting to evaluate scar therapy effectiveness, such tools are even more critical. There is currently no standard operating procedure or consensus among providers on which objective tools should be used and there are known differences in reproducibility and reliability between different devices. 32 Furthermore, it is not known if these tools are effective to the same degree in evaluating these scar parameters in scars from different Fitzpatrick skin types. One such tool is a Mexameter, or similar technologies, which evaluate erythema through the evaluation of “redness” and melanin through the evaluation of brown color. 33 If a Mexameter or similar tool was used here, quantitative evaluations of erythema and vascularity could have been incorporated, however, the technology was not available to us at the time. In addition, here, only one investigator assigned VSS scores, and this observer's calibration and reproducibility was not assessed. This limitation was overcome by their vast, over 30 years of experience in assigning VSS to burn hypertrophic scars. In future work, multiple graders will be used. Another commonly used scale in the evaluation of burn hypertrophic scar is the Patient and Observer Scar Assessment Scale which incorporates the patient's view on metrics such as pain and itch. 34 This scale, or those with similar incorporation of patient feedback, should be used in future human trials, but is not possible in preclinical animal models in pigs.
The healing progression from days 0 to 98 was markedly different between breeds. The Yk wound appeared to close by contracting along two perpendicular axes, resulting in an stellate‐shaped HTS of much smaller size than the original wound, while the Dc HTS remained square and approximately equal in size to the original wound, leaving a much larger area of fibrosis and dyspigmentation. This is most likely the result of differences in the spatiotemporal balance between factors that promote cell proliferation, migration, and ECM production and ones that promote apoptosis and ECM degradation and remodeling. It is possible that these differences are influenced not only by genetics, but additional factors such as the intensity and duration of inflammation and chemiexcitation during healing.
Given that differences in gene expression are not sufficient to explain differences in scar formation between Dc and Yk pigs, we believe that interaction between chemiexcitation and melanin plays a role in this difference. Chemiexcitation has been linked to the development of melanoma through the generation of oxygen and nitrogen free radicals from UV light, which combine to excite electrons in melanin. 35 , 36 This energy is transferred to DNA, creating mutagenic and carcinogenic cyclobutane pyrimidine dimers (CPDs). Inflammation has also been shown to contribute to this process. 35 , 36 Thus, chemiexcitation produced by burn injury inflammation may have differing effects based on the amount of nearby melanin, leading to increased scar formation in Fitzpatrick skin types IV–VI. The association between wound healing and free radical perturbations has been documented, supporting the role of chemiexcitation in scar formation. It has been shown that scarring is associated with nitric oxide free radical production, 37 and chronic production of CPDs occurs throughout the inflammatory process. 35 Mast cell degranulation, which is elevated in HTS, has also been implicated as it generates both nitric oxide and oxygen free radicals. 35 Furthermore, it has been documented that the balance of reactive oxygen species (ROS) and ROS scavengers is perturbed in HTS, 23 with decreased antioxidant activity in addition to elevated free radical production. Differences in skin pigmentation may lead to differences in scar formation through the interaction of chemiexcitation and melanin. If this relationship is borne out further in chemiexcitation research, it opens the door for targeted treatments to reduce the effects of chemiexcitation which may lead to improvements in scar symptoms.
One limitation of this study is the small sample size. This limitation was addressed by including analysis of histological changes and gene expression during wound healing as well as connecting our results to previous research. Data corroboration across several metrics adds strength to conclusions, but these results should be confirmed in additional samples. Another limitation was that only three timepoints were included in scar‐related analyses (baseline, day 56, and day 98). It is known that scar remodeling is a dynamic process that changes over time. Therefore, in future work, additional timepoints should be included to confirm findings. In addition, day 98, which is about 3 months post‐wounding, is on the shorter end of when patients usually follow‐up to burn clinic for scar interventions. The usage of an animal model prevents following these animals for long‐term follow‐ups on the order of years, however, it may be the case that Yk scars and Dc scars differ in the long term as well.
Future work should focus on further exploring the role of chemiexcitation in HTS. Specifically, the results of this study can provide the basis for future work examining free radical changes during wound healing between Dc and Yk pigs to support the role of melanin and chemiexcitation to explain differences in scar formation.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Colton H. Funkhouser analyzed data and contributed to writing the manuscript. Liam D. Kirkpatrick performed bench assays, analyzed data and contributed to manuscript writing. Robert D. Smith performed the animal work and performed bench assays. Lauren T. Moffatt provided project management, acquired grant funding, and edited the manuscript. Jeffrey W. Shupp conceived of the project, interpreted data, acquired grant funding, and edited the manuscript. Bonnie C. Carney helped to conceive of the project, did the animal work, oversaw bench assays, analyzed and interpreted data, and contributed to manuscript writing and editing.
ACKNOWLEDGMENTS
This work was funded in part by R01 AR070851‐01A1.
Funkhouser CH, Kirkpatrick LD, Smith RD, Moffatt LT, Shupp JW, Carney BC. In‐depth examination of hyperproliferative healing in two breeds of Sus scrofa domesticus commonly used for research. Anim Models Exp Med. 2021;4:406–417. doi: 10.1002/ame2.12188
Funding information
This work was funded in part by R01 AR070851‐01A1.
REFERENCES
- 1. Lee HJ, Jang YJ. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci. 2018;19:711. doi: 10.3390/ijms19030711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence JW, Mason ST, Schomer K, Klein MB. Epidemiology and impact of scarring after burn injury: a systematic review of the literature. J Burn Care Res. 2012;33:136‐146. doi: 10.1097/BCR.0b013e3182374452 [DOI] [PubMed] [Google Scholar]
- 3. Visscher MO, Bailey JK, Hom DB. Scar treatment variations by skin type. Facial Plast Surg Clin North Am. 2014;22:453‐462. doi: 10.1016/j.fsc.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 4. Cooke GL, Chien A, Brodsky A, Lee RC. Incidence of hypertrophic scars among African Americans linked to vitamin D‐3 metabolism? J Natl Med Assoc. 2005;97:1004‐1009. [PMC free article] [PubMed] [Google Scholar]
- 5. Hahn JM, Supp DM. Abnormal expression of the vitamin D receptor in keloid scars. Burns. 2017;43:1506‐1515. doi: 10.1016/j.burns.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 6. Lee DE, Trowbridge RM, Ayoub NT, Agrawal DK. High‐mobility group box protein‐1, matrix metalloproteinases, and vitamin D in keloids and hypertrophic scars. Plast Reconstr Surg Glob Open. 2015;3:e425. doi: 10.1097/GOX.0000000000000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seaton M, Hocking A, Gibran NS. Porcine models of cutaneous wound healing. ILAR J. 2015;56:127‐138. doi: 10.1093/ilar/ilv016 [DOI] [PubMed] [Google Scholar]
- 8. Liang Z, Engrav LH, Muangman P, et al. Nerve quantification in female red Duroc pig (FRDP) scar compared to human hypertrophic scar. Burns. 2004;30:57‐64. [DOI] [PubMed] [Google Scholar]
- 9. Engrav LH, Tuggle CK, Kerr KF, et al. Functional genomics unique to week 20 post wounding in the deep cone/fat dome of the Duroc/Yorkshire porcine model of fibroproliferative scarring. PLoS One. 2011;6:e19024. doi: 10.1371/journal.pone.0019024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sood RF, Muffley LA, Seaton ME, et al. Dermal fibroblasts from the red Duroc pig have an inherently fibrogenic phenotype: an in vitro model of fibroproliferative scarring. Plast Reconstr Surg. 2015;136:990‐1000. doi: 10.1097/PRS.0000000000001704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet. 2016;388:1427‐1436. doi: 10.1016/S0140-6736(16)31406-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Travis TE, Mauskar NA, Mino MJ, et al. Commercially available topical platelet‐derived growth factor as a novel agent to accelerate burn‐related wound healing. J Burn Care Res. 2014;35:e321‐e329. doi: 10.1097/BCR.0000000000000013 [DOI] [PubMed] [Google Scholar]
- 13. Mauskar NA, Sood S, Travis TE, et al. Donor site healing dynamics: molecular, histological, and noninvasive imaging assessment in a porcine model. J Burn Care Res. 2013;34:549‐562. doi: 10.1097/BCR.0b013e3182839aca [DOI] [PubMed] [Google Scholar]
- 14. Ghassemi P, Travis TE, Moffatt LT, Shupp JW, Ramella‐Roman JC. A polarized multispectral imaging system for quantitative assessment of hypertrophic scars. Biomed Opt Express. 2014;5:3337‐3354. doi: 10.1364/BOE.5.003337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Travis TE, Mino MJ, Moffatt LT, et al. Biphasic presence of fibrocytes in a porcine hypertrophic scar model. J Burn Care Res. 2015;36:e125‐e135. doi: 10.1097/BCR.0000000000000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Travis TE, Ghassemi P, Ramella‐Roman JC, et al. A multimodal assessment of melanin and melanocyte activity in abnormally pigmented hypertrophic scar. J Burn Care Res. 2015;36:77‐86. doi: 10.1097/BCR.0000000000000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tejiram S, Zhang J, Travis TE, et al. Compression therapy affects collagen type balance in hypertrophic scar. J Surg Res. 2016;201:299‐305. doi: 10.1016/j.jss.2015.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carney BC, Liu Z, Alkhalil A, et al. Elastin is differentially regulated by pressure therapy in a porcine model of hypertrophic scar. J Burn Care Res. 2017;38:28‐35. doi: 10.1097/BCR.0000000000000413 [DOI] [PubMed] [Google Scholar]
- 19. Alkhalil A, Carney BC, Travis TE, et al. Key cell functions are modulated by compression in an animal model of hypertrophic scar. Wounds. 2018;30:353‐362. [PubMed] [Google Scholar]
- 20. Carney BC, Chen JH, Luker JN, et al. Pigmentation diathesis of hypertrophic scar: an examination of known signaling pathways to elucidate the molecular pathophysiology of injury‐related dyschromia. J Burn Care Res. 2019;40:58‐71. doi: 10.1093/jbcr/iry045 [DOI] [PubMed] [Google Scholar]
- 21. Travis TE, Ghassemi P, Prindeze NJ,, et al. Matrix metalloproteinases are differentially regulated and responsive to compression therapy in a red Duroc model of hypertrophic scar. Eplasty. 2018;18:e1. [PMC free article] [PubMed] [Google Scholar]
- 22. Alkhalil A, Carney BC, Travis TE, et al. Dyspigmented hypertrophic scars: beyond skin color. Pigment Cell Melanoma Res. 2019;32:643‐656. doi: 10.1111/pcmr.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carney BC, Chen JH, Kent RA, et al. Reactive oxygen species scavenging potential contributes to hypertrophic scar formation. J Surg Res. 2019;244:312‐323. doi: 10.1016/j.jss.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 24. Wang JF, Olson ME, Reno CR, Kulyk W, Wright JB, Hart DA. Molecular and cell biology of skin wound healing in a pig model. Connect Tissue Res. 2000;41:195‐211. doi: 10.3109/03008200009005290 [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi H, Tsuruchi N, Sugihara K, et al. Expression of alpha‐smooth muscle actin in benign or malignant ovarian tumors. Gynecol Oncol. 1993;48:308‐313. doi: 10.1006/gyno.1993.1054 [DOI] [PubMed] [Google Scholar]
- 26. Zhu KQ, Carrougher GJ, Gibran NS, Isik FF, Engrav LH. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair Regen. 2007;15(Suppl 1):S32‐S39. doi: 10.1111/j.1524-475X.2007.00223.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie Y, Zhu KQ, Deubner H, et al. The microvasculature in cutaneous wound healing in the female red Duroc pig is similar to that in human hypertrophic scars and different from that in the female Yorkshire pig. J Burn Care Res. 2007;28:500‐506. doi: 10.1097/BCR.0B013E318053DAFE [DOI] [PubMed] [Google Scholar]
- 28. Wang JF, Olson ME, Reno CR, Wright JB, Hart DA. The pig as a model for excisional skin wound healing: characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp Med. 2001;51:341‐348. [PubMed] [Google Scholar]
- 29. Gallant CL, Olson ME, Hart DA. Molecular, histologic, and gross phenotype of skin wound healing in red Duroc pigs reveals an abnormal healing phenotype of hypercontracted, hyperpigmented scarring. Wound Repair Regen. 2004;12:305‐319. doi: 10.1111/j.1067-1927.2004.012311.x [DOI] [PubMed] [Google Scholar]
- 30. Nedelec B, Correa JA, Rachelska G, Armour A, LaSalle L. Quantitative measurement of hypertrophic scar: intrarater reliability, sensitivity, and specificity. J Burn Care Res. 2008;29:489‐500. doi: 10.1097/BCR.0b013e3181710869 [DOI] [PubMed] [Google Scholar]
- 31. Nedelec B, Forget NJ, Hurtubise T, et al. Skin characteristics: normative data for elasticity, erythema, melanin, and thickness at 16 different anatomical locations. Skin Res Technol. 2016;22:263‐275. doi: 10.1111/srt.12256 [DOI] [PubMed] [Google Scholar]
- 32. Baumann ME, DeBruler DM, Blackstone BN, et al. Direct comparison of reproducibility and reliability in quantitative assessments of burn scar properties. Burns. 2021;47(2):466‐478. doi: 10.1016/j.burns.2020.07.018 [DOI] [PubMed] [Google Scholar]
- 33. Carney BC, Travis TE, Moffatt LT, et al. Hypopigmented burn hypertrophic scar contains melanocytes that can be signaled to re‐pigment by synthetic alpha‐melanocyte stimulating hormone in vitro. PLoS One. 2021;16:e0248985. doi: 10.1371/journal.pone.0248985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Draaijers LJ, Tempelman FRH, Botman YAM, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113:1960‐1965; discussion 1966‐1967. doi: 10.1097/01.prs.0000122207.28773.56 [DOI] [PubMed] [Google Scholar]
- 35. Brash DE, Goncalves LCP, Bechara EJH; Excited‐State Medicine Working Group . Chemiexcitation and its implications for disease. Trends Mol Med. 2018;24:527‐541. doi: 10.1016/j.molmed.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Premi S, Wallisch S, Mano CM, et al. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347:842‐847. doi: 10.1126/science.1256022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu KQ, Engrav LH, Armendariz R, et al. Changes in VEGF and nitric oxide after deep dermal injury in the female, red Duroc pig‐further similarities between female, Duroc scar and human hypertrophic scar. Burns. 2005;31:5‐10. doi: 10.1016/j.burns.2004.08.010 [DOI] [PubMed] [Google Scholar]


