Abstract
Background
Prokinetic is the first-line conventional treatment for functional dyspepsia (FD) in Asia despite potential adverse events. Chinese herbal medicine (CHM) may be an effective and safe substitution. This network meta-analysis (NMA) aimed to evaluate the comparative effectiveness of different CHM formulae for FD against prokinetics.
Methods
Seven international and Chinese databases were searched from their inception to July 2020 for randomised controlled trials (RCTs) on CHM versus prokinetics. Data from each RCT were first pooled using random-effect pairwise meta-analyses and illustrated as risk difference (RD) or standardised mean difference (SMD) with 95% confidence interval (CI). Random-effect NMAs were then performed to evaluate the comparative effectiveness of CHM formulae and displayed as RD with 95% CI or SMD with 95% credible interval (CrI). The GRADE partially contextualised framework was applied for NMA result interpretation.
Results
Twenty-six unique CHM formulae were identified from twenty-eight RCTs of mediocre quality. Pairwise meta-analyses indicated that CHM was superior to prokinetics in alleviating global symptoms at 4-week follow-up (pooled RD: 0.14; 95% CI: 0.10–0.19), even after trim and fill adjustment for publication bias. NMAs demonstrated that Modified Zhi Zhu Decoction may have a moderate beneficial effect on alleviating global symptoms at 4-week follow-up (RD: 0.28; 95% CI: − 0.03 to 0.75). Xiao Pi Kuan Wei Decoction may have a large beneficial effect on alleviating postprandial fullness (SMD: − 2.14; 95% CI: − 2.76 to 0.70), early satiety (SMD: − 3.90; 95% CI: − 0.68 to − 0.42), and epigastric pain (SMD: − 1.23; 95% CI: − 1.66 to − 0.29). No serious adverse events were reported.
Conclusion
Modified Zhi Zhu Decoction and Xiao Pi Kuan Wei Decoction may be considered as an alternative for patients unresponsive to prokinetics. Confirmatory head-to-head trials should be conducted to investigate their comparative effectiveness against prokinetics.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13020-021-00556-6.
Keywords: Medicine, Chinese traditional, Domperidone, Dyspepsia, Systematic review, Network meta-analysis
Introduction
Functional dyspepsia (FD) is a common gastrointestinal disorder characterised by postprandial fullness, early satiation, epigastric pain, or epigastric burning that is unexplainable by routine investigations [1]. It has a high prevalence of 10–40% among Western countries and a relatively low prevalence of 5–30% among Asian countries, independent of disease definitions [2]. Based on predominant symptoms, FD can be subdivided into diagnostic subtypes of postprandial distress syndrome (PDS, predominant symptoms include postprandial fullness and early satiety) and epigastric pain syndrome (EPS, predominant symptoms include epigastric burning and epigastric pain) [2], with the former subtype being more prevalent in Asia [3].
Current guidelines recommended several conventional treatments for FD. In the 2017 North American clinical guideline [4], proton pump inhibitors (PPIs) are the first-line treatment for both FD subtypes, followed by tricyclic antidepressants (TCAs). PPIs have a relatively high number needed to treat of eleven [5], and their long-term usage is associated with adverse effects such as acute interstitial nephritis, hip fracture, and Clostridium difficile infection [6]. TCAs are associated with adverse events like dry mouth, somnolence, constipation, and urinary retention [4], and some patients tend to avoid TCAs due to the perceived stigma of receiving psychiatric therapy [7]. These imply that the first two treatment options may not help a considerable number of FD patients. In the 2012 Asian Consensus Report on Functional Dyspepsia [8], prokinetics are the first- and second-line treatment for the subtype of PDS and EPS, respectively. Given the relatively higher prevalence of PDS in Asian FD populations, prokinetics, such as domperidone and mosapride, are commonly prescribed for FD patients in China [9] and South Korea [10]. However, recommendations for prokinetics are supported only by very low-quality evidence [11], and certain prokinetics are associated with adverse events ranging from dystonia to life-threatening arrhythmia [4, 12].
Failure of first-line conventional treatment in FD management is not uncommon. For instance, despite the wide use of prokinetics, a study in China revealed that nearly a quarter of FD patients were refractory to conventional treatments [13]. Alternative treatment options for these patients are necessary as they are known to have a longer disease duration, more severe symptom burden, more intense health service utilisation, and higher healthcare-related expenditure [13]. In view of current limitations among guideline-recommended treatments, Chinese herbal medicine (CHM) represents a possible complement or alternative option, especially among patients unresponsive to first-line treatments like PPIs and prokinetics. Indeed, herbal medicine is recommended by the Asian clinical guideline as a potential treatment option after failing a course of 8-week conventional therapy regardless of FD subtypes [8].
Herbal medicine constitutes an important component in many healthcare systems, and strategies for promoting the use of herbal medicine have been outlined by the World Health Organization [14]. CHM is a branch of herbal medicine practice widely adopted in China and other Chinese communities. It refers to the natural medicinal ingredients, including plants, animals, and minerals, and their processed products that are prepared and used under the guidance of Traditional Chinese Medicine (TCM) theories [15]. The cost of herbal medicine for FD management is expected to be low, particularly in regions where herbal medicine has been practised as a tradition [14]. For example, the typical cost for a single-day CHM treatment is only USD5.87 in China [16].
Although a clinical guideline based on expert consensus was published in China on the use of CHM for FD management [17], and it is known to be superior to placebo [18], evidence on the comparative effectiveness of CHM relative to prokinetics has not been synthesised in a systematic manner. Also, as the relative performance of different CHM interventions is unclear, specific recommendations cannot be made to inform routine practice. To clarify the potential role of CHM as an alternative to prokinetics, we explored the comparative effectiveness of different CHM interventions against prokinetics via network meta-analysis (NMA) in this systematic review.
Methods
Literature search
Seven electronic databases were searched from their inception to July 2020 [Additional file 1: Appendix 1]. Four were Chinese databases: Wanfang Data, China National Knowledge Infrastructure, SinoMed, and Index to Taiwan Periodical Literature System. Three were international databases: MEDLINE via Ovid, EMBASE via Ovid, and Cochrane Central Register of Controlled Trials. Validated search filters with high sensitivity for randomised controlled trials (RCTs) were applied for searching MEDLINE and EMBASE [19, 20].
Eligibility criteria
Eligible RCTs must meet the criteria for participants, interventions, controls, and outcomes measures described below, with full-text written in English or Chinese. Systematic reviews, clinical recommendations, conference abstracts, or research protocols were excluded.
Participants
RCTs that recruited adult patients diagnosed with FD based on any editions of the Rome diagnostic criteria were eligible. No restrictions on FD subtypes were placed.
Interventions and comparisons
RCTs comparing orally administered CHM to prokinetics were eligible. Orally administered CHM could be in the form of single herbs, herbal formulae, or proprietary medicine, with components clearly reported. Comparisons between orally administered CHM were also eligible for inclusion. RCTs on cisapride were excluded as it has been withdrawn in most countries due to life-threatening adverse events [8].
Outcomes
RCTs must report the primary outcome of global symptom alleviation. Alleviation of postprandial fullness, early satiety, epigastric pain, and epigastric burning were considered as the secondary outcomes. The primary and secondary outcomes were selected according to current expert recommendations on clinical endpoints for FD trials [21].
Study selection, data extraction, risk of bias assessment, and quality of evidence assessment
Titles, abstracts, and full-texts of all records were screened as per the eligibility criteria after deduplication with EndNote 20. Characteristics and outcome data of eligible studies were then extracted. In classical TCM theories, a diagnostic pattern refers to the summarisation of the cause, nature, and location of the pathological change at a certain stage of disease [14]. It encompasses information on the patient’s clinical signs and symptoms. Considering the significance of diagnostic pattern in TCM, diagnostic pattern(s) of the participants in each included study was extracted, if reported. TCM function(s) of each identified CHM intervention was obtained from the study as well. Risk of bias assessment was performed using the Cochrane Risk-of-Bias Tool for Randomized Trials 2 [22]. Quality of evidence was assessed for pairwise meta-analyses and NMAs using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach [23, 24]. These procedures were performed by two reviewers (Ho and Chan) independently, with disagreements resolved through consensus. Persisted disagreements were settled by a third reviewer (Chung).
Data analyses
Pairwise meta-analysis
To synthesise results of head-to-head comparisons between CHM and prokinetics, random effect pairwise meta-analyses were executed using Review Manager 5.3. Results on the alleviation of global symptoms (rated on dichotomous scale) were pooled and expressed as risk difference (RD) and 95% confidence interval (CI). Results on the alleviation of postprandial fullness, early satiety, epigastric pain, and epigastric burning (rated on continuous scale) were pooled and presented as standardised mean difference (SMD) and 95% CI. All outcome results were pooled as per their length of follow-up [25, 26]. Sensitivity analysis was performed for the primary outcome, comparing pooled results between RCTs with some concerns over risk of bias or at low risk of bias, against those at high risk of bias. Publication bias on the primary outcome was assessed via contour-enhanced funnel plots produced by RStudio 1.3.1073. Trim and fill method was adopted to adjust for publication bias detected [26].
The level of heterogeneity among RCTs was measured with I2 statistics, with I2 < 25%, 25–50%, 50% regarded as low-, moderate-, and high-level heterogeneity, respectively [27]. The following minimally clinically important difference (MCID) values were used to facilitate result interpretation: RD of 0.20 between groups for the primary outcome [23]; and SMD of − 0.50 for the secondary outcomes [23].
Network meta-analysis
NMA combines direct and indirect evidence across a network of interventions in a single analysis, allowing the ranking of interventions based on relative efficacy [26]. Direct evidence refers to results from head-to-head comparisons of two interventions within RCTs (for example, X versus Y and Y versus Z), while indirect evidence is computed via analysing results from comparisons of two interventions via a common comparator (for example, X versus Z via Y) [28].
In this review, random-effect Bayesian NMAs were carried out on RStudio to evaluate the comparative effectiveness of CHM interventions, via specific prokinetics as common comparators. Results on the primary outcome were analysed using binomial likelihood model [29], while results on the secondary outcomes were analysed using normal likelihood model [30]. Dichotomous and continuous outcomes were expressed as RD and risk ratio (RR) with 95% credible interval (CrI) and SMD with 95% CrI, respectively. The ranking of CHM interventions across different outcomes was determined by the probability of specific CHM being at different ranks and expressed using surface under the cumulative ranking curve (SUCRA) [31].
When drawing conclusions, rankings suggested by SUCRA values should be considered together with the effect magnitude of interventions and relevant certainty of evidence [32, 33]. In this review, the GRADE partially contextualised framework was adopted to facilitate the interpretation of NMA results [32]. In this four-step framework, thresholds for small, moderate, and large beneficial effect were first established in accordance with the MCID of different outcomes. For the primary outcome, an RD value of 0.08 represented a small beneficial effect, 0.20 a moderate beneficial effect, and 0.31 a large beneficial effect [34]. Following the method reported in Newcombe et al. [35], RDs were computed from relevant RRs and the expected response (i.e. baseline risk) of FD prokinetic treatment. The baseline risk used for both domperidone and mosapride was 0.42, which was extracted from a meta-analysis on prokinetic response [11], with a 95% CI of 0.38 to 0.46 and of 0.38 to 0.47, respectively, computed using the Wilson score method [36]. For the secondary outcomes, an SMD of − 0.20 represented a small beneficial effect, − 0.50 a moderate beneficial effect, and − 0.80 a large beneficial effect [26].
Secondly, for each outcome, different CHM interventions were categorised into “trivial to no beneficial effect”, “small but important beneficial effect”, “moderate beneficial effect”, or “large beneficial effect” based on point estimates of their relative efficacy against specific prokinetics. Thirdly, the interventions were stratified according to the certainty of evidence supporting their relative efficacy which was graded using the GRADE NMA rating system [23, 24]. Lastly, the consistency between the point estimate and ranking of each intervention was evaluated to finalise the classification of all interventions.
Results
Study selection and characteristics
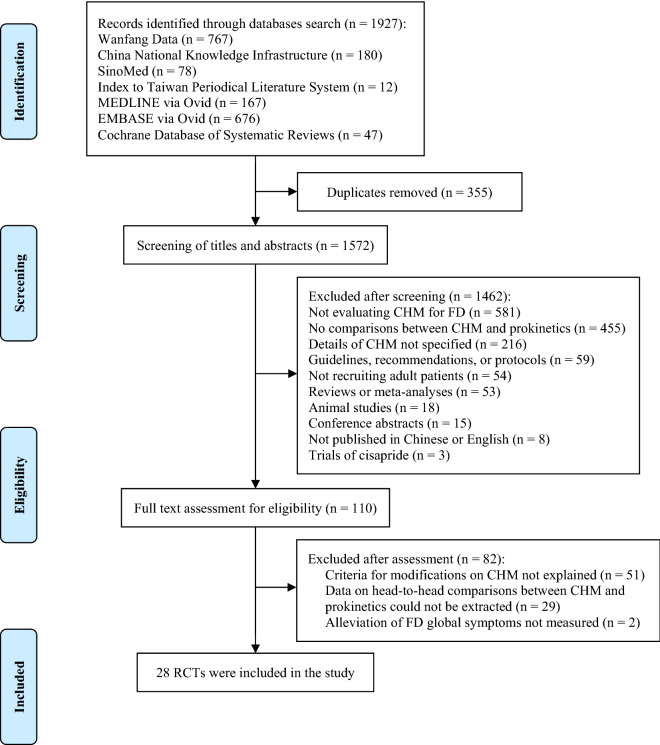
A total of 1,927 citations were yielded from the literature search. 1,572 titles and abstracts were screened after deduplication. 110 potential full-texts then proceeded to further eligibility assessment. Finally, twenty-eight RCTs were included in this study. Selection process is illustrated in Fig. 1.
Fig. 1.
Flow of literature search and selection. CHM Chinese herbal medicine, FD functional dyspepsia, RCT randomised controlled trial
All RCTs were conducted in China, with only one [37] of them published in English, and spanned 2004 to 2019 (Table 1). A total of 2,736 participants took part in the twenty-eight trials, with an average sample size of 98 (range, 56–202). Average age of the participants ranged from 24.0 to 54.6 years. Duration of their FD symptoms ranged from less than one year to over twenty-four years. Participants in twenty-three trials [37–59] were diagnosed by the Rome III criteria, while those in the other five trials [60–64] were diagnosed by the Rome II criteria. Fifteen out of twenty-eight RCTs adopted TCM diagnostic pattern as part of their inclusion criteria (Table 1). Nine unique TCM diagnostic patterns were included: food stagnation [38], liver qi invading the stomach [40, 43], liver depression and spleen deficiency [49, 61, 62], cold-heat complex [42, 46, 64], liver qi depression [44], spleen deficiency and qi stagnation [46, 51, 54], spleen-stomach weakness [48], spleen qi deficiency [56], and spleen deficiency and dampness-heat [57].
Table 1.
Characteristics of included studies
| Reference (Country) |
CHM formula | Prokinetic | Number of participants R/A |
Average age (SD) | FD diagnostic criteria (TCM diagnostic pattern(s), if reported) |
Duration of FD symptoms | Treatment duration (Length of follow-up) |
Outcome |
|---|---|---|---|---|---|---|---|---|
|
Gong [38] (China) |
Liu Wei An Xiao Capsule | Domperidone |
CHM group: 82/82 Prokinetic group: 74/74 |
Not reported |
Rome III criteria (Food stagnation) |
Not reported |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Li [60] (China) |
Liu Wei An Xiao Capsule | Domperidone |
CHM group: 50/50 Prokinetic group: 50/50 |
CHM group: 41.3 Prokinetic group: 38.6 |
Rome II criteria |
CHM group: 3.8 years in average Prokinetic group: 3.2 years in average |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Zhou [39] (China) |
He Wei Decoction | Domperidone |
CHM group: 48/48 Prokinetic group: 48/48 |
CHM group: 37.4 (4.3) Prokinetic group: 36.5 (3.8) |
Rome III criteria | Not reported |
4 weeks (4 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness Alleviation of early satiety Alleviation of epigastric pain |
|
Leng [40] (China) |
He Wei Decoction | Domperidone |
CHM group: 41/41 Prokinetic group: 39/39 |
CHM group: 42.0 (13.0) Prokinetic group: 38.6 (14.2) |
Rome III criteria (Liver qi invading the stomach) |
Not reported |
4 weeks (4 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness Alleviation of early satiety Alleviation of epigastric pain |
|
Gao [41] (China) |
Modified He Gan Decoction | Domperidone |
CHM group: 40/40 Prokinetic group: 40/40 |
CHM group: 41.6 Prokinetic group: 40.1 |
Rome III criteria |
CHM group: 6.1 years in average Prokinetic group: 6.3 years in average |
4 weeks (4 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness |
|
Liu [61] (China) |
Fu An Decoction | Domperidone |
CHM group: 40/40 Prokinetic group: 40/40 |
CHM group: 31.6 (13.1) Prokinetic group: 32.7 (11.3) |
Rome II criteria (Liver depression and spleen deficiency) |
CHM group: 4.8 ± 1.7 years Prokinetic group: 4.8 ± 1.4 years |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Lai [42] (China) |
Xiao Pi Tong Jiang Decoction | Domperidone |
CHM group: 30/30 Prokinetic group: 30/30 |
CHM group: 37.4 (9.8) Prokinetic group: 39.7 (8.9) |
Rome III criteria (Cold-heat complex) |
CHM group: 3.6 ± 1.9 years Prokinetic group: 3.4 ± 1.5 years |
4 weeks (4 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness Alleviation of early satiety Alleviation of epigastric pain Alleviation of epigastric burning |
|
Cai [43] (China) |
Xiao Pi Kuan Wei Decoction | Domperidone |
CHM group: 47/47 Prokinetic group: 47/47 |
CHM group: 35.6 Prokinetic group: 35.9 |
Rome III criteria (Liver qi invading the stomach) |
CHM group: 4.4 ± 0.6 years Prokinetic group: 4.8 ± 0.9 years |
4 weeks (4 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness Alleviation of early satiety Alleviation of epigastric pain Alleviation of epigastric burning |
|
Dong [44] (China) |
Cai Zhu Jie Yu Decoction | Domperidone |
CHM group: 32/32 Prokinetic group: 32/32 |
CHM group: 44.8 (9.5) Prokinetic group: 45.2 (9.8) |
Rome III criteria (Liver qi depression) |
CHM group: 3.7 ± 0.9 years Prokinetic group: 3.5 ± 0.7 years |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Liu [45] (China) |
Zhi Zhu Kuan Zhong Capsule | Domperidone |
CHM group: 97/97 Prokinetic group: 105/105 |
Not reported | Rome III criteria | Not reported |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Liu [46] (China) |
Xiao Pi Decoction | Domperidone |
CHM group: 40/40 Prokinetic group: 40/40 |
Not reported |
Rome III criteria (Spleen deficiency and qi stagnation; cold-heat complex) |
All participants: 0.3–6.0 years |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Ma [47] (China) |
He Wei Xiao Pi Decoction | Domperidone |
CHM group: 66/66 Prokinetic group: 60/60 |
CHM group: 35.8 Prokinetic group: 37.1 |
Rome III criteria | Not reported |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Duan [48] (China) |
Modified Zhi Zhu Decoction | Domperidone |
CHM group: 60/60 Prokinetic group: 60/60 |
Not reported |
Rome III criteria (Spleen-stomach weakness) |
CHM group: 0.5–4.5 years Prokinetic group: 0.5–4.5 years |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Wang [49] (China) |
Shu Gan Jian Pi He Wei Decoction | Domperidone |
CHM group: 50/50 Prokinetic group: 50/50 |
CHM group: 41.5 (13.4) Prokinetic group: 42.6 (11.6) |
Rome III criteria (Liver depression and spleen deficiency) |
CHM group: 2.7 ± 1.0 years Prokinetic group: 2.8 ± 1.2 years |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Li [50] (China) |
Tiao He Gan Pi Xing Qi Decoction | Domperidone |
CHM group: 72/72 Prokinetic group: 56/56 |
All participants: 46.1 (6.7) | Rome III criteria | Not reported |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Huang [51] (China) |
Xiang Su Li Qi Decoction | Domperidone |
CHM group: 30/30 Prokinetic group: 30/30 |
CHM group: 38.9 (6.4) Prokinetic group: 39.5 (6.6) |
Rome III criteria (Spleen deficiency and qi stagnation) |
CHM group: 2.4 ± 0.8 years Prokinetic group: 2.1 ± 0.9 years |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Sheng [52] (China) |
Jian Pi Yi Qi Decoction | Domperidone |
CHM group: 41/41 Prokinetic group: 41/41 |
Not reported | Rome III criteria |
CHM group: 0.5–3.8 years Prokinetic group: 0.5–3.4 years |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Zhao [53] (China) |
Tiao Zhong Xiao Pi Decoction | Domperidone |
CHM group: 60/60 Prokinetic group: 60/60 |
CHM group: 25.0 (1.2) Prokinetic group: 24.0 (1.5) |
Rome III criteria |
CHM group: 2.1 ± 0.6 years Prokinetic group: 2.0 ± 0.4 years |
2 weeks (2 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness Alleviation of early satiety Alleviation of epigastric pain Alleviation of epigastric burning |
|
Liu [54] (China) |
Wu Mo Decoction | Domperidone |
CHM group: 40/40 Prokinetic group: 40/40 |
CHM group: 45.3 (5.3) Prokinetic group: 46.9 (5.1) |
Rome III criteria (Spleen deficiency and qi stagnation) |
CHM group: 2.1 ± 0.6 years Prokinetic group: 2.2 ± 0.4 years |
2 weeks (2 weeks) |
Alleviation of global symptoms |
|
Ma [55] (China) |
Cai Hu Shu Gan Powder | Domperidone |
CHM group: 30/30 Prokinetic group: 30/30 |
CHM group: 42.3 (2.1) Prokinetic group: 41.3 (2.2) |
Rome III criteria |
CHM group: 5.2 ± 3.7 years Prokinetic group: 4.8 ± 3.1 years |
2 weeks (2 weeks) |
Alleviation of global symptoms |
|
Liu [56] (China) |
Wei Kang Ping Decoction | Domperidone |
CHM group: 40/40 Prokinetic group: 40/40 |
CHM group: 54.6 Prokinetic group: 53.8 |
Rome II criteria (Liver depression and spleen deficiency) |
CHM group: 3.5 ± 1.3 years Prokinetic group: 3.4 ± 1.8 years |
2 weeks (2 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness |
|
Wang [57] (China) |
Qi Zhi Wei Tong Granules | Domperidone |
CHM group: 58/58 Prokinetic group: 54/54 |
All participants: 43.2 (12.1) | Rome II criteria | Not reported |
2 weeks (2 weeks) |
Alleviation of global symptoms |
|
Hu [64] (China) |
Ban Xia Xie Xin Decoction | Domperidone |
CHM group: 30/30 Prokinetic group: 30/30 |
CHM group: 37.2 (10.5) Prokinetic group: 39.3 (9.2) |
Rome II criteria (Cold-heat complex) |
CHM group: 19.2 ± 12.1 years Prokinetic group: 17.3 ± 11.4 years |
2 weeks (2 weeks) |
Alleviation of global symptoms |
|
Chen [66] (China) |
Bu Gan Decoction | Mosapride |
CHM group: 28/28 Prokinetic group: 28/28 |
CHM group: 38.1 Prokinetic group: 37.8 |
Rome III criteria (Spleen qi deficiency) |
CHM group: 2.1 years in average Prokinetic group: 2.2 years in average |
4 weeks (4 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness |
|
Wang [57] (China) |
Tiao Wei Xiao Pi Decoction | Mosapride |
CHM group: 33/33 Prokinetic group: 31/31 |
CHM group: 47.9 (12.0) Prokinetic group: 44.8 (12.1) |
Rome III criteria (Spleen deficiency and dampness-heat) |
CHM group: 7.0 ± 5.0 years Prokinetic group: 6.5 ± 5.0 years |
4 weeks (4 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness |
|
Huang [58] (China) |
Da Li Tong Granules | Mosapride |
CHM group: 57/57 Prokinetic group: 57/57 |
Not reported | Rome III criteria | All participants: 0.5–24.0 years |
4 weeks (4 weeks) |
Alleviation of global symptoms |
|
Zheng [59] (China) |
Modified Yue Ju Decoction | Mosapride |
CHM group: 60/60 Prokinetic group: 60/60 |
Not reported | Rome III criteria | Not reported |
6 weeks (6 weeks) |
Alleviation of global symptoms |
|
Liu [60] (China) |
Xiao Pi II | Mosapride |
CHM group: 90/90 Prokinetic group: 90/90 |
CHM group: 42.0 (15.0) Prokinetic group: 43.0 (14.0) |
Rome III criteria | Not reported |
2 weeks (2 weeks) |
Alleviation of global symptoms Alleviation of postprandial fullness Alleviation of epigastric pain |
A Analysed, CHM Chinese herbal medicine, FD functional dyspepsia, R Recruited, SD Standard deviation, TCM Traditional Chinese medicine
Twenty-six unique CHM formulae were studied in the twenty-eight RCTs (Table 2). TCM function(s) of the CHM formulae corresponded to the diagnostic pattern(s) adopted in the fifteen RCTs according to TCM theories. Twenty-one formulae were compared against domperidone in twenty-three RCTs, with Liu Wei An Xiao Capsule [38, 60] and He Wei Decoction [39, 40] studied in two RCTs respectively. Five formulae were compared against mosapride in five RCTs [37, 56–59]. Treatment duration ranged from two to six weeks. Twenty trials [38–52, 56–58, 60, 61] had a length of follow-up of four weeks, while seven [37, 53–55, 62–64] and one [59] trials had a 2-week follow-up and 6-week follow-up, respectively.
Table 2.
Characteristics of included interventions
| Reference | CHM formula (dosage, frequency) |
TCM function(s) of CHM formula | Ingredients of CHM formula | Prokinetics drugs (dosage, frequency) |
Adverse event |
|---|---|---|---|---|---|
| Gong [38] |
Liu Wei An Xiao Capsule (0.5 g powder per capsule, four capsules each time, three times per day) |
Fortifying the spleen and harmonising the stomach Promoting digestion and removing food stagnation |
Radix Inulae, Radix et Rhizoma Rhei, Fructus Chebulae, Rhizoma Kaempferiae, Gypsum Rubrum, and Tronae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
CHM group: 8 cases of frequent bowel movements Prokinetic group: 9 cases, including mild diarrhoea (n = 5), mild headache (n = 2), and insomnia (n = 2) |
| Li [60] |
Liu Wei An Xiao Capsule (0.5 g powder per capsule, four capsules each time, three times per day) |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported | ||
| Zhou [39] |
He Wei Decoction (150 ml decoction, twice per day) |
Soothing the liver and harmonising the stomach | Radix Codonopsis, Radix Bupleuri, Fructus Aurantii Immaturus, Cortex Magnoliae Officinalis, Radix et Rhizoma Glycyrrhizae, Pericarpium Citri Reticulatae, Fructus Amomi Rotundus, and Radix Aucklandiae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Leng [40] |
He Wei Decoction (Weight in granule not reported, three times per day) |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
CHM group: 3 cases, including oral ulcer (n = 2) and diarrhoea (n = 1) Prokinetic group: 1 case of frequent bowel movements |
||
| Gao [41] |
Modified He Gan Decoction (150 ml decoction, once per day) |
Soothing the liver and fortifying the spleen | Radix Angelicae Sinensis, Radix Paeoniae Alba, Radix Codonopsis, Rhizoma Atractylodis Macrocephalae, Poria, Radix Bupleuri, Herba Menthae, Caulis Perillae, Rhizoma Cyperi, Rhizoma Zingiberis Recens, Fructus Jujubae, Radix et Rhizoma Glycyrrhizae, Pericarpium Citri Reticulatae, Massa Medicata Fermentata, and Fructus Amomi |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Liu [61] |
Fu An Decoction (100 ml decoction, twice per day) |
Soothing the liver and fortifying the spleen | Radix Bupleuri, Radix Codonopsis, Radix Paeoniae Alba, Fructus Citri Sarcodactylis, Rhizoma Corydalis. Fructus Toosendan, Radix et Rhizoma Salviae Miltiorrhizae, Rhizoma Atractylodis Macrocephalae, Poria, Radix Aucklandiae, Pericarpium Citri Reticulata, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
CHM group: 1 case of mild diarrhoea Prokinetic group: 4 cases, including diarrhoea (n = 2), skin rash (n = 1), and mild headache (n = 1) |
| Lai [42] |
Xiao Pi Tong Jiang Decoction (150 ml decoction, twice per day) |
Regulating cold and heat | Pinelliae Rhizoma, Radix Scutellariae, Rhizoma Zingiberis, Radix Codonopsis, Radix Curcumae, Cortex Magnoliae Officinalis, Radix Paeoniae Alba, Radix Bupleuri, and Fructus Aurantii |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Cai [43] |
Xiao Pi Kuan Wei Decoction (200 ml decoction, twice per day) |
Soothing the liver and regulating qi | Radix Bupleuri, Massa Medicata Fermentata, Rhizoma Atractylodis Macrocephalae, Flos Inulae, Pericarpium Citri Reticulatae, Rhizoma Pinelliae, Fructus Aurantii Immaturus, Rhizoma Corydalis, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Dong [44] |
Cai Zhu Jie Yu Decoction (Volume of decoction not reported, twice per day) |
Soothing the liver and resolving qi stagnation | Radix Bupleuri, Rhizoma Atractylodis Macrocephalae, Pericarpium Citri Reticulatae Viride, Fructus Aurantii, Fructus Citri Sarcodactylis, Radix Chuanxiong, Massa Medicata Fermentata, Fructus Amomi, Radix Paeoniae Alba, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Liu [45] |
Zhi Zhu Kuan Zhong Capsule (0.43 g powder per capsule, three capsules each time, three times per day) |
Soothing the liver and fortifying the spleen | Rhizoma Atractylodis Macrocephalae, Fructus Gardeniae, Radix Bupleuri, and Fructus Crataegi |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Liu [46] |
Xiao Pi Decoction (200 ml decoction, twice per day) |
Promoting digestion and removing food stagnation Regulating cold and heat |
Fructus Aurantii Immaturus, Poria, Radix Codonopsis, Cortex Magnoliae Officinalis, Rhizoma Coptidis, Pinelliae Rhizoma, Fructus Aurantii, Rhizoma Atractylodis Macrocephalae, Radix et Rhizoma Glycyrrhizae, Radix Aucklandiae, Herba Pogostemonis, Radix et Rhizoma Nardostachyos, Fructus Chebulae, and Fructus Hordei Germinatus |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Ma [47] |
He Wei Xiao Pi Decoction (100 ml decoction, twice per day) |
Fortifying the spleen and harmonising the stomach Promoting digestion and removing food stagnation |
Radix Codonopsis, Poria, Rhizoma Atractylodis Macrocephalae, Fructus Aurantii, Radix Paeoniae Alba, Rhizoma Corydalis, Fructus Toosendan, Rhizoma Cimicifugae, Radix Platycodonis, Herba Pogostemonis, Herba Eupatorii, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Duan [48] |
Modified Zhi Zhu Decoction (Volume of decoction not reported, once per day) |
Fortifying the spleen and tonifying qi Promoting digestion and removing food stagnation |
Fructus Aurantii Immaturus, Fructus Citri Sarcodactylis, Rhizoma Atractylodis Macrocephalae, Rhizoma Dioscoreae, Semen Nelumbinis, Pericarpium Citri Reticulatae, Endothelium Corneum Gigeriae Galli, Rhizoma Cimicifugae, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Wang [49] |
Shu Gan Jian Pi He Wei Decoction (200 ml decoction, twice per day) |
Soothing the liver and regulating qi Fortifying the spleen and harmonising the stomach |
Radix et Rhizoma Salviae Miltiorrhizae, Poria, Rhizoma Atractylodis Macrocephalae, Semen Coicis, Rhizoma Corydalis, Radix Bupleuri, Fructus Aurantii, Radix Paeoniae Alba, Rhizoma Cyperi, Pericarpium Citri Reticulatae, Rhizoma Pinelliae, Fructus Hordei Germinatus, Fructus Crataegi, Massa Medicata Fermentata, Endothelium Corneum Gigeriae Galli, Radix Codonopsis, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Li, 2014 |
Tiao He Gan Pi Xing Qi Decoction (150 ml decoction, twice per day) |
Soothing the liver and spleen Moving qi at the middle energizer |
Rhizoma Atractylodis Macrocephalae, Fructus Aurantii, Radix Bupleuri, Radix Curcumae, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Huang [51] |
Xiang Su Li Qi Decoction (150 ml decoction, twice per day) |
Warming the spleen and stomach Moving qi at the middle energizer |
Radix Astragali, Rhizoma Cyperi, Caulis Perillae, Rhizoma Atractylodis Macrocephalae, Fructus Aurantii, Radix Paeoniae Alba, Radix et Rhizoma Salviae Miltiorrhizae, Radix Aucklandiae, Fructus Amomi, Radix Codonopsis, Poria, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Sheng [52] |
Jian Pi Yi Qi Decoction (100 ml decoction, twice per day) |
Fortifying the spleen and tonifying qi | Radix Codonopsis, Rhizoma Cyperi, Poria, Fructus Jujubae, Radix et Rhizoma Glycyrrhizae, Radix Astragali, Rhizoma Pinelliae, Cortex Magnoliae Officinalis, Fructus Aurantii, Caulis Perillae, and Ramulus Cinnamomi |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
CHM group: no adverse events reported Prokinetic group: 5 cases of mild headache |
| Zhao [53] |
Tiao Zhong Xiao Pi Decoction (Volume of decoction not reported, twice per day) |
Soothing the liver and regulating qi Fortifying the spleen and harmonising the stomach Promoting digestion and removing food stagnation |
Rhizoma Atractylodis Macrocephalae, Fructus Aurantii Immaturus, Radix Bupleuri, Radix Paeoniae Alba, Rhizoma Pinelliae, Rhizoma Coptidis, Fructus Citri Sarcodactylis, Bulbus Lilii, Herba Taraxaci, Fructus Setariae Germinatus, Semen Oroxyli, Flos Rosae Rugosae, Cortex Albiziae, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Liu [54] | Wu Mo Decoction |
Soothing the liver and regulating qi Fortifying the spleen Promoting digestion and removing food stagnation |
Radix Linderae, Lignum Aquilariae Resinatum, Radix Aucklandiae, Semen Arecae, and Radix Codonopsis |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
CHM group: 2 cases, including diarrhoea (n = 1) and dizziness (n = 1) Prokinetic group: 2 cases, including urticaria (n = 1) and dizziness (n = 1) |
| Ma [55] | Cai Hu Shu Gan Powder |
Soothing the liver and resolving qi stagnation Moving qi to relieve pain |
Pericarpium Citri Reticulatae, Radix Bupleuri, Radix Chuanxiong, Rhizoma Cyperi, Fructus Aurantii, Radix Paeoniae Alba, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Liu [62] | Wei Kang Ping Decoction | Soothing the liver and fortifying the spleen | Radix Bupleuri, Fructus Aurantii, Radix Codonopsis, Rhizoma Dioscoreae, Poria, Pericarpium Citri Reticulatae, Rhizoma Acori Tatarinowii, Rhizoma Atractylodis Macrocephalae, Radix Scutellariae, Radix Curcumae, Rhizoma Pinelliae, Radix Aucklandiae, Fructus Hordei Germinatus, Fructus Crataegi, and Massa Medicata Fermentata |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Wang [63] | Qi Zhi Wei Tong Granules |
Soothing the liver and regulating qi Harmonising the stomach to relieve pain |
Radix Bupleuri, Rhizoma Corydalis, Fructus Aurantii, Rhizoma Cyperi, Radix Paeoniae Alba, and Radix et Rhizoma Glycyrrhizae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Hu [64] | Ban Xia Xie Xin Decoction | Regulating cold and heat | Rhizoma Pinelliae, Radix Scutellariae, Rhizoma Coptidis, Radix Codonopsis, Rhizoma Zingiberis, Cortex Magnoliae Officinalis, Fructus Amomi, Radix Paeoniae Alba, Pericarpium Citri Reticulatae, Fructus Jujubae, and Rhizoma Atractylodis Macrocephalae |
Domperidone (10 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Chen [56] | Bu Gan Decoction |
Fortifying the spleen and tonifying qi Promoting digestion and removing food stagnation |
Radix Astragali, Ramulus Cinnamomi, Rhizoma Atractylodis Macrocephalae, Pericarpium Citri Reticulatae, Radix et Rhizoma Asari, Poria, Fructus Amomi, Radix Saposhnikoviae, Rhizoma Pinelliae, Endothelium Corneum Gigeriae Galli, Fructus Hordei Germinatus, Cortex Magnoliae Officinalis, and Radix et Rhizoma Glycyrrhizae |
Mosapride (5 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Wang [57] | Tiao Wei Xiao Pi Decoction |
Soothing the liver and regulating qi Fortifying the spleen Clearing dampness-heat |
Radix Codonopsis, Poria, Rhizoma Atractylodis Macrocephalae, Rhizoma Corydalis, Fructus Toosendan, Herba Taraxaci, Semen Coicis, Caulis Bambusae in Taenia, Fructus Aurantii, Caulis Perillae, Rhizoma Pinelliae, and Radix et Rhizoma Glycyrrhizae |
Mosapride (5 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Huang [58] | Da Li Tong Granules |
Clearing heat and resolving qi stagnation Harmonising the stomach and directing qi downward Promoting digestion and removing food stagnation |
Radix Bupleuri, Radix Aucklandiae, Rhizoma Pinelliae, Fructus Crataegi, Caulis Sinomenii, Rhizoma Corydalis, Fructus Aurantii Immaturus, Pericarpium Citri Reticulatae, Herba Taraxaci, Semen Arecae, Radix Codonopsis, and Massa Medicata Fermentata |
Mosapride (5 mg per tablet, one tablet each time, three times per day) |
CHM group: no adverse events reported Prokinetic group: 1 case of mild diarrhoea |
| Zheng [59] | Modified Yue Ju Decoction |
Soothing the liver and regulating qi Fortifying the spleen and harmonising the stomach Promoting digestion and removing food stagnation |
Rhizoma Cyperi, Radix Aucklandiae, Fructus Aurantii, Cortex Magnoliae Officinalis, Pericarpium Citri Reticulatae, Radix Chuanxiong, Rhizoma Atractylodis, Fructus Gardeniae, and Massa Medicata Fermentata |
Mosapride (5 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
| Liu [37] | Xiao Pi II |
Fortifying the spleen and harmonising the stomach Promoting digestion and removing food stagnation |
Radix Codonopsis, Rhizoma Atractylodis Macrocephalae, Rhizoma Atractylodis, Poria, Rhizoma Pinelliae, Fructus Amomi, Pericarpium Citri Reticulatae, Fructus Aurantii, and Radix et Rhizoma Glycyrrhizae |
Mosapride (5 mg per tablet, one tablet each time, three times per day) |
No adverse events reported |
CHM Chinese herbal medicine, TCM Traditional Chinese medicine
Risk of bias assessment
The overall risk of bias among the included studies was mediocre, with none of them being at low risk, twenty-four having some concerns, and four being at high risk [Additional file 1: Appendix 2]. Those having some concerns [37, 39–44, 46, 48–59, 61–64] did not implement blinding for trial participants, carers, and people delivering interventions. Moreover, they did not report details on allocation sequence generation or provide information on whether the data were analysed following a pre-specified analysis plan. For those at high risk of bias [38, 45, 47, 60], despite limitations described, they did not report details on baseline differences groups.
Pairwise meta-analysis
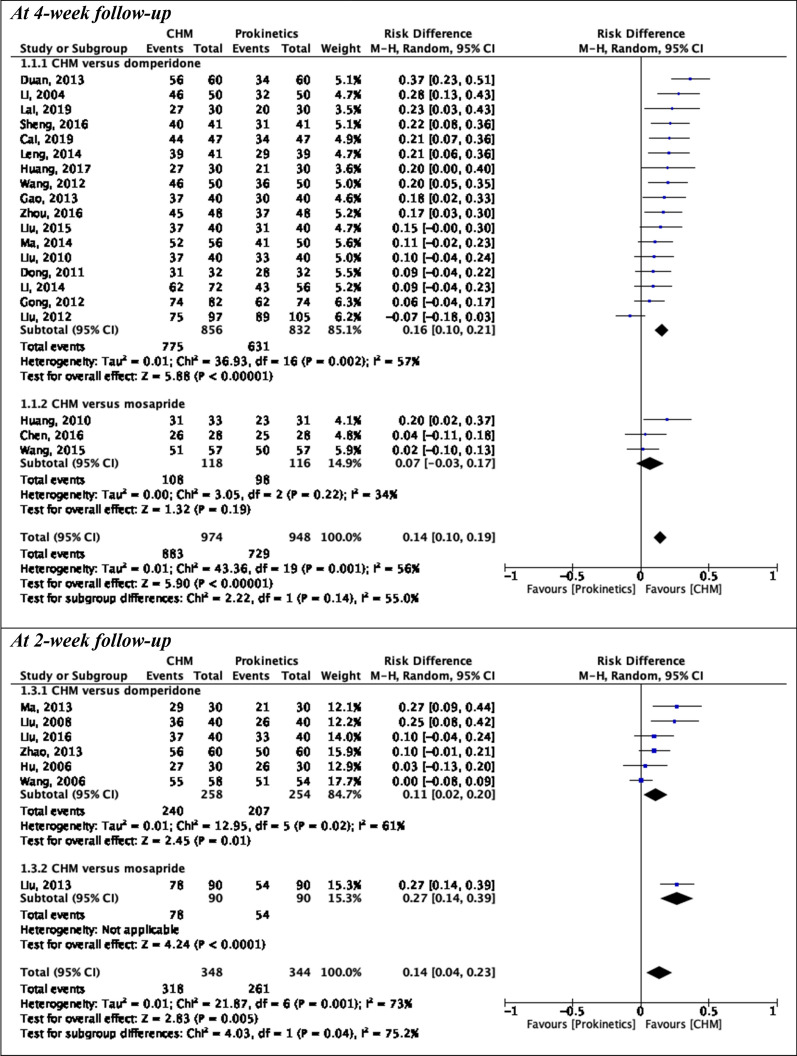
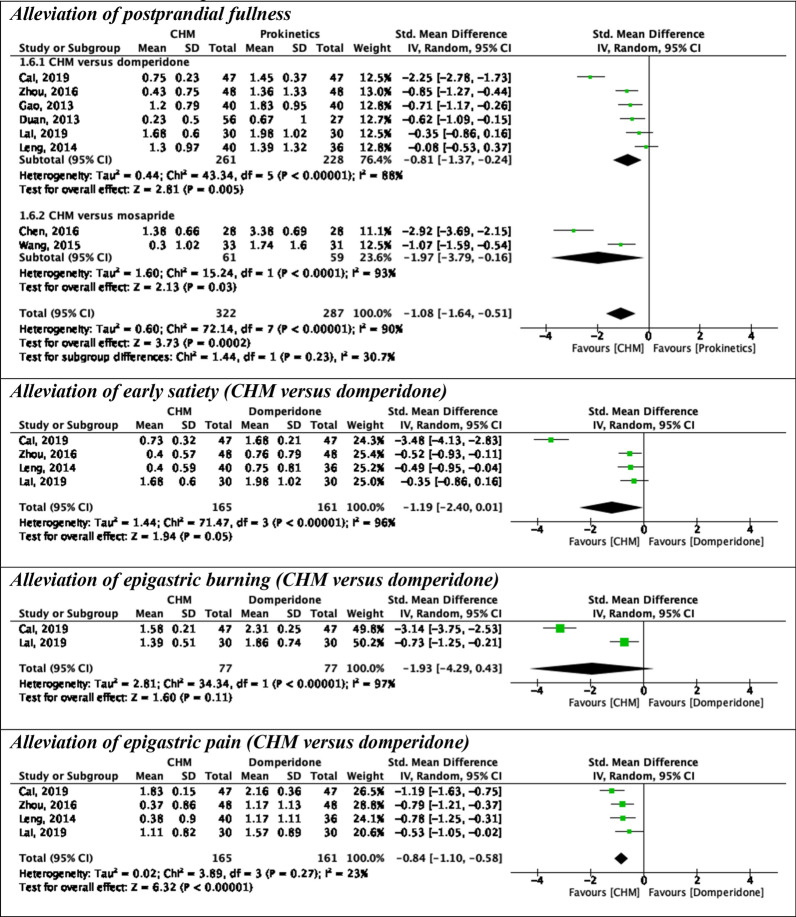
Six pairwise meta-analyses were conducted to compare CHM with prokinetics in alleviating global symptoms, postprandial fullness, early satiety, and epigastric pain (Figs. 2, 3).
Fig. 2.
Pairwise meta-analyses on alleviation of global symptoms at different follow-up periods: Chinese herbal medicine versus prokinetics. CHM Chinese herbal medicine, CI Confidence interval, SD Standard deviation
Fig. 3.
Pairwise meta-analyses on secondary outcomes at 4-week follow-up: Chinese herbal medicine versus prokinetics. CHM Chinese herbal medicine, CI Confidence interval, SD Standard deviation.
Alleviation of global symptoms
Compared to prokinetics, CHM had a stronger effect in alleviating global symptoms at 4-week follow-up (20 RCTs; pooled RD: 0.14; 95% CI: 0.10–0.19; p < 0.00001; I2 = 56%; low-quality of evidence) (Table 3). CHM was also superior to domperidone alone (17 RCTs; pooled RD: 0.16; 95% CI: 0.10–0.21; p < 0.00001; I2 = 57%; low-quality of evidence). Substantial heterogeneity was observed in both results. No significant difference was found between CHM and mosapride (3 RCTs; pooled RD: 0.07; 95% CI: − 0.03 to 0.17; p = 0.19; I2 = 34%; low-quality of evidence). At 2-week follow-up, CHM was more effective than prokinetics (7 RCTs; pooled RD: 0.14; 95% CI: 0.04–0.23; p = 0.005; I2 = 73%; moderate-quality of evidence) and domperidone alone (6 RCTs; pooled RD: 0.11; 95% CI: 0.02–0.20; p = 0.01; I2 = 61%; moderate-quality of evidence). High-level heterogeneity existed in both pooling results. The MCID of 0.20 RD was not met by any comparisons above.
Table 3.
Effect estimates and quality of evidence ratings for comparisons in pairwise meta-analyses on alleviation of global symptoms
| Outcome | Study design (Number of participants) |
Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Pooled result (95% CI) |
Quality |
|---|---|---|---|---|---|---|---|---|
|
Alleviation of global symptoms (4-week follow-up) |
20 RCTs (1924 participants) |
No serious | No serious | No serious | Serious | Strongly suspected |
RD: 0.14 (0.10, 0.19) Trim and fill adjusted RD: 0.10 (0.05, 0.15) RR: 1.21 (1.11, 1.25) |
⨁⨁◯◯ Low |
|
Alleviation of global symptoms (4-week follow-up) (Included only RCTs on CHM versus domperidone) |
17 RCTs (1688 participants) |
No serious | No serious | No serious | Serious | Strongly suspected |
RD: 0.16 (0.10, 0.21) Trim and fill adjusted RD: 0.12 (0.06, 0.17) RR: 1.20 (1.13, 1.28) |
⨁⨁◯◯ Low |
|
Alleviation of global symptoms (4-week follow-up) (Included only RCTs on CHM versus mosapride) |
3 RCTs (234 participants) |
No serious | No serious | No serious | Very serious | Not applicable |
RD: 0.07 (− 0.03, 0.17) RR: 1.08 (0.96, 1.21) |
⨁⨁◯◯ Low |
|
Alleviation of global symptoms (2-week follow-up) |
7 RCTs (692 participants) |
No serious | No serious | No serious | Serious | Not applicable |
RD: 0.14 (0.04, 0.23) RR: 1.18 (1.04, 1.35) |
⨁⨁⨁◯ Moderate |
|
Alleviation of global symptoms (2-week follow-up) (Included only RCTs on CHM versus domperidone) |
6 RCTs (512 participants) |
No serious | No serious | No serious | Serious | Not applicable |
RD: 0.11 (0.02, 0.20) RR: 1.13 (1.01, 1.26) |
⨁⨁⨁◯ Moderate |
CHM Chinese herbal medicine, CI Confidence interval, RCT Randomised controlled trial, RD risk difference, RR risk ratio
Alleviation of postprandial fullness
When compared with prokinetics, CHM showed a stronger effect in alleviating postprandial fullness at 4-week follow-up (8 RCTs; pooled SMD: − 1.08; 95% CI: − 1.64 to − 0.51; p = 0.0002; I2 = 90%; moderate-quality of evidence) (Table 4), with the effect size exceeded the MCID of − 0.50 SMD. CHM was also superior to domperidone (6 RCTs; pooled SMD: − 0.81; 95% CI: − 1.37, − 0.24; p = 0.005; I2 = 88%; low-quality of evidence) and mosapride alone (6 RCTs; pooled SMD: − 1.97; 95% CI: − 3.79, − 0.16; p = 0.03; I2 = 93%; very low-quality of evidence). Substantial heterogeneity was detected in all results.
Table 4.
Effect estimates and quality of evidence ratings for comparisons in pairwise meta-analyses on secondary outcomes at 4-week follow-up
| Outcome | Study design (Number of participants) |
Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Pooled result (95% CI) |
Quality |
|---|---|---|---|---|---|---|---|---|
| Alleviation of postprandial fullness |
8 RCTs (609 participants) |
No serious | Serious | No serious | No serious | Not applicable |
SMD: − 1.08 (− 1.64, − 0.51) |
⨁⨁⨁◯ Moderate |
|
Alleviation of postprandial fullness (Included only RCTs on CHM versus domperidone) |
6 RCTs (489 participants) |
No serious | Serious | No serious | Serious | Not applicable |
SMD: − 0.81 (− 1.37, − 0.24) |
⨁⨁◯◯ Low |
|
Alleviation of postprandial fullness (Included only RCTs on CHM versus mosapride) |
2 RCTs (120 participants) |
No serious | Serious | No serious | Very serious | Not applicable |
SMD: − 1.97 (− 3.79, − 0.16) |
⨁◯◯◯ Very low |
| Alleviation of early satiety |
4 RCTs (326 participants) |
No serious | Serious | No serious | Very serious | Not applicable |
SMD: − 1.19 (− 2.40, 0.10) |
⨁◯◯◯ Very low |
| Alleviation of epigastric burning |
2 RCTs (154 participants) |
No serious | Serious | No serious | Very serious | Not applicable |
SMD: − 1.93 (− 4.29, 0.43) |
⨁◯◯◯ Very low |
| Alleviation of epigastric pain |
4 RCTs (326 participants) |
No serious | No serious | No serious | Very serious | Not applicable |
SMD: − 0.84 (− 1.10, − 0.58) |
⨁⨁◯◯ Low |
A negative SMD indicated an effect favouring Chinese herbal medicine, while a positive SMD indicated an effect favouring prokinetics
CI Confidence interval, RCT Randomised controlled trial, SMD Standardised mean difference
Alleviation of early satiety
There was no significant difference between CHM and domperidone in alleviating early satiety at 4-week follow-up (4 RCTs; pooled SMD: − 1.19; 95% CI: − 2.40 to 0.10; p = 0.05; I2 = 96%; very low-quality of evidence) (Table 4). High-level heterogeneity was observed in this comparison.
Alleviation of epigastric burning
No significant difference was found between CHM and domperidone in alleviating epigastric burning at 4-week follow-up (4 RCTs; pooled SMD: − 1.93; 95% CI: − 4.29 to 0.43; p = 0.11; I2 = 97%; very low-quality of evidence) (Table 4). High-level heterogeneity existed for this meta-analysis.
Alleviation of epigastric pain
CHM was more effective than domperidone in alleviating epigastric pain at 4-week follow-up (4 RCTs; pooled SMD: − 0.84; 95% CI: − 1.10 to − 0.58; p < 0.00001; I2 = 23%; low-quality of evidence), with moderate-level heterogeneity (Table 4). The effect size was higher than the MCID of − 0.50 SMD.
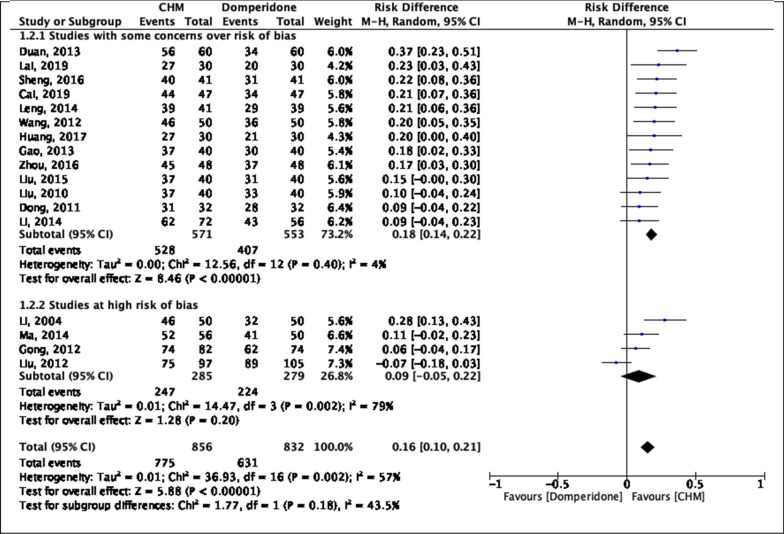
Sensitivity analysis
Sensitivity analysis comparing pooled results from “studies with some concerns over risk of bias” and “studies at high risk of bias” is illustrated in Fig. 4. There was no significant subgroup difference (p = 0.18) between the two groups, implying that the difference in risk of bias level did not influence the pooled results on global symptom alleviation at 4-week.
Fig. 4.
Pairwise meta-analyses on alleviation of global symptoms at 4-week follow-up: Chinese herbal medicine versus domperidone—Subgroup analysis. CHM Chinese herbal medicine, CI Confidence interval
Publication bias assessment
Judging from visual inspection of contour-enhanced funnel plots [Additional file 1: Appendix 3a–3b], evidence of funnel plot asymmetry was observed, indicating the potential presence of publication bias favouring CHM in alleviating global symptoms at 4-week follow-up, as compared to all prokinetics and domperidone alone. After applying the trim and fill adjustment, CHM remained to be superior to prokinetics (adjusted RD: 0.10; 95% CI: 0.05–0.15) and domperidone alone (adjusted RD: 0.12; 95% CI: 0.06–0.17) (Table 3).
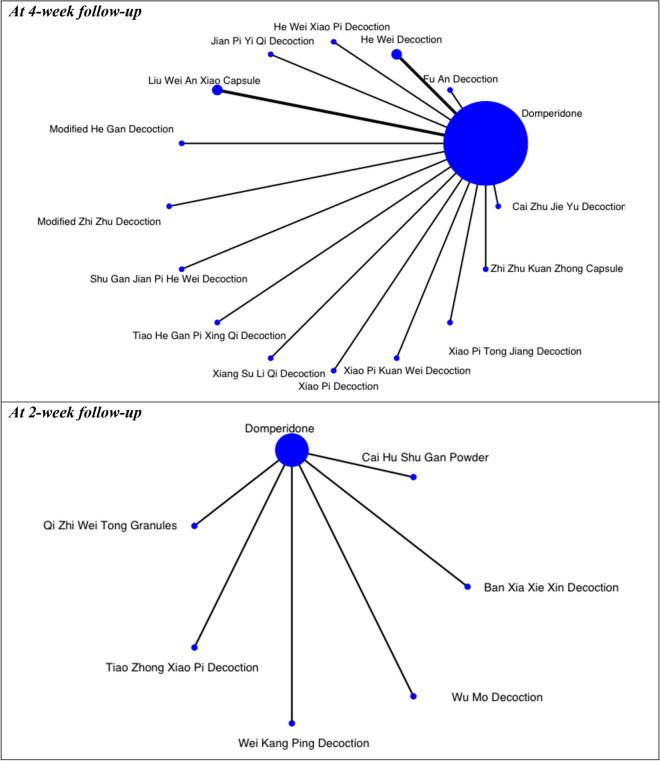
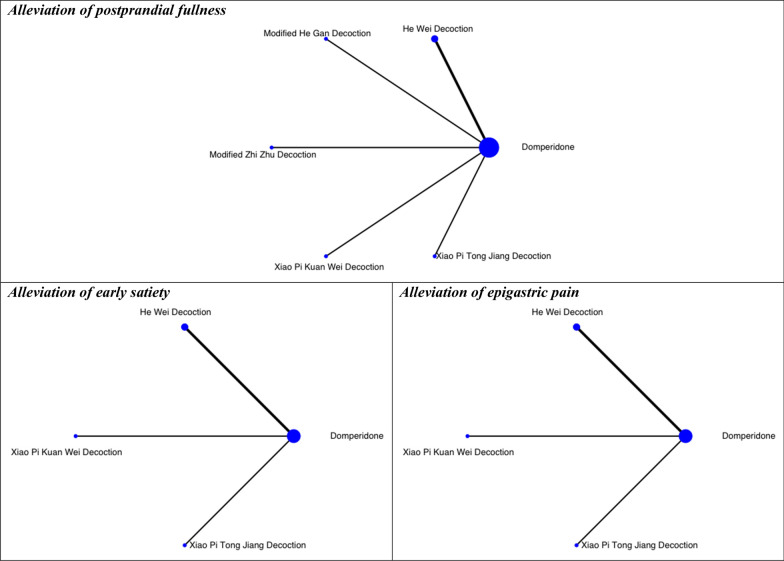
Network meta-analysis
Five star-shaped networks were devised to illustrate comparison networks of CHM formulae in alleviating global symptoms, postprandial fullness, early satiety, and epigastric pain against domperidone (Figs. 5, 6, 7). Another star-shaped network was used to illustrate the comparison network of CHM formulae in alleviating global symptoms against mosapride [Fig. 10]. The quality of evidence supporting each network is illustrated in Additional file 1: Appendix 4–9.
Fig. 5.
Network of comparisons on alleviation of global symptoms at different follow-up periods: Chinese herbal medicine versus domperidone. The width of the lines represents the proportion of the number of trials for each comparison with the total number of trials, and the size of the nodes represents the proportion of the number of randomised patients (sample sizes)
Fig. 6.
Networks of comparisons on secondary outcomes at 4-week follow-up: Chinese herbal medicine versus domperidone. The width of the lines represents the proportion of the number of trials for each comparison with the total number of trials, and the size of the nodes represents the proportion of the number of randomised patients (sample sizes)
Fig. 7.

Network of comparisons on alleviation of global symptoms at 4-week follow-up: Chinese herbal medicine versus mosapride. The width of the lines represents the proportion of the number of trials for each comparison with the total number of trials, and the size of the nodes represents the proportion of the number of randomised patients (sample sizes)
Alleviation of global symptoms
In the NMA of seventeen RCTs, no specific CHM formula was significantly better than domperidone or other CHM formulae in the network in alleviating global symptoms at 4-week follow-up (Table 5). Nevertheless, according to the partially contextualised framework, Modified Zhi Zhu Decoction (RD: 0.28; 95% CI: − 0.03 to 0.75) may have a moderate beneficial effect in alleviating global symptoms at 4-week, comparing to domperidone. It was the best-ranked intervention in the network (SUCRA: 0.85), as supported by low certainty of evidence (Table 6).
Table 5.
Comparative effectiveness of different Chinese herbal medicines: domperidone as comparator
| Alleviation of global symptoms at 4-week follow-up (shown in RD and 95% CI) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZZD | |||||||||||||||
|
0.13 (− 0.19, 0.69) |
XTD | ||||||||||||||
|
0.14 (− 0.17, 0.70) |
0.04 (− 0.22, 0.52) |
XKD | |||||||||||||
|
0.14 (− 0.17, 0.71) |
0.04 (− 0.22, 0.52) |
0.03 (− 0.22, 0.46) |
JYD | ||||||||||||
|
0.15 (− 0.17, 0.72) |
0.05 (− 0.22, 0.53) |
0.03 (− 0.22, 0.48) |
0.03 (− 0.22, 0.48) |
SJHD | |||||||||||
|
0.15 (− 0.17, 0.74) |
0.05 (− 0.22, 0.54) |
0.03 (− 0.22, 0.49) |
0.03 (− 0.22, 0.48) |
0.03 (− 0.23, 0.48) |
XSLD | ||||||||||
|
0.16 (− 0.14, 0.64) |
0.05 (− 0.19, 0.47) |
0.04 (− 0.20, 0.41) |
0.03 (− 0.20, 0.41) |
0.03 (− 0.20, 0.40) |
0.03 (− 0.20, 0.43) |
HWD | |||||||||
|
0.17 (− 0.16, 0.76) |
0.07 (− 0.21, 0.57) |
0.05 (− 0.21, 0.52) |
0.04 (− 0.21, 0.51) |
0.04 (− 0.22, 0.50) |
0.04 (− 0.22, 0.52) |
0.02 (− 0.20, 0.40) |
MHGD | ||||||||
|
0.16 (− 0.13, 0.64) |
0.06 (− 0.19, 0.48) |
0.04 (− 0.19, 0.42) |
0.04 (− 0.19, 0.42) |
0.04 (− 0.20, 0.42) |
0.04 (− 0.20, 0.44) |
0.02 (− 0.20, 0.40) |
0.02 (− 0.20, 0.39) |
LAXC | |||||||
|
0.19 (− 0.15, 0.80) |
0.08 (− 0.20, 0.59) |
0.06 (− 0.21, 0.54) |
0.06 (− 0.21, 0.53) |
0.06 (− 0.21, 0.53) |
0.06 (− 0.21, 0.55) |
0.04 (− 0.19, 0.42) |
0.04 (− 0.22, 0.50) |
0.03 (− 0.20, 0.41) |
XPD | ||||||
|
0.22 (− 0.13, 0.85) |
0.10 (− 0.19, 0.63) |
0.08 (− 0.19, 0.58) |
0.08 (− 0.20, 0.57) |
0.08 (− 0.20, 0.56) |
0.08 (− 0.20, 0.59) |
0.06 (− 0.18, 0.45) |
0.06 (− 0.20, 0.53) |
0.05 (− 0.18, 0.45) |
0.05 (− 0.21, 0.51) |
HXD | |||||
|
0.22 (− 0.13, 0.87) |
0.11 (− 0.19, 0.65) |
0.09 (− 0.19, 0.59) |
0.09 (− 0.20, 0.59) |
0.08 (− 0.20, 0.58) |
0.09 (− 0.20, 0.60) |
0.06 (− 0.18, 0.46) |
0.07 (− 0.20, 0.55) |
0.05 (− 0.18, 0.46) |
0.05 (− 0.21, 0.52) |
0.03 (− 0.22, 0.46) |
TGXD | ||||
|
0.22 (− 0.13, 0.87) |
0.11 (− 0.19, 0.66) |
0.09 (− 0.19, 0.60) |
0.09 (− 0.19, 0.59) |
0.08 (− 0.20, 0.58) |
0.09 (− 0.20, 0.61) |
0.06 (− 0.18, 0.47) |
0.07 (− 0.20, 0.55) |
0.06 (− 0.18, 0.46) |
0.05 (− 0.21, 0.52) |
0.03 (− 0.22, 0.47) |
0.03 (− 0.22, 0.46) |
FAD | |||
|
0.23 (− 0.13, 0.88) |
0.12 (− 0.19, 0.67) |
0.10 (− 0.19, 0.61) |
0.10 (− 0.19, 0.60) |
0.09 (− 0.19, 0.59) |
0.10 (− 0.19, 0.62) |
0.07 (− 0.17, 0.48) |
0.08 (− 0.20, 0.56) |
0.06 (− 0.18, 0.47) |
0.06 (− 0.21, 0.53) |
0.04 (− 0.21, 0.48) |
0.03 (− 0.22, 0.48) |
0.03 (− 0.22, 0.47) |
CZJD | ||
|
0.28 (− 0.03, 0.75) |
0.15 (− 0.11, 0.57) |
0.13 (− 0.11, 0.50) |
0.13 (− 0.11, 0.49) |
0.12 (− 0.11, 0.49) |
0.13 (− 0.11, 0.52) |
0.10 (− 0.07, 0.34) |
0.11 (− 0.12, 0.46) |
0.09 (− 0.07, 0.34) |
0.09 (− 0.13, 0.44) |
0.07 (− 0.14, 0.38) |
0.06 (− 0.14, 0.38) |
0.06 (− 0.14, 0.38) |
0.06 (− 0.15, 0.36) |
DOMP | |
|
0.36 (− 0.07, > 1.00) |
0.23 (− 0.13, 0.89) |
0.20 (− 0.14, 0.81) |
0.20 (− 0.14, 0.81) |
0.20 (− 0.14, 0.79) |
0.20 (− 0.15, 0.83) |
0.17 (− 0.12, 0.66) |
0.17 (− 0.15, 0.76) |
0.16 (− 0.12, 0.64) |
0.16 (− 0.16, 0.72) |
0.13 (− 0.17, 0.65) |
0.12 (− 0.17, 0.65) |
0.13 (− 0.17, 0.65) |
0.12 (− 0.18, 0.63) |
0.05 (− 0.15, 0.36) |
ZZKC |
| Alleviation of global symptoms at 2-week follow-up (shown in RD and 95% CI) | ||||||
|---|---|---|---|---|---|---|
| CHSP | ||||||
|
0.02 (− 0.20, 0.39) |
WKPD | |||||
|
0.11 (− 0.15, 0.53) |
0.11 (− 0.15, 0.53) |
TXD | ||||
|
0.12 (− 0.15, 0.54) |
0.11 (− 0.15, 0.54) |
0.02 (− 0.19, 0.34) |
WMD | |||
|
0.16 (− 0.13, 0.63) |
0.16 (− 0.13, 0.62) |
0.05 (− 0.18, 0.41) |
0.05 (− 0.18, 0.41) |
BXD | ||
|
0.17 (− 0.11, 0.63) |
0.17 (− 0.12, 0.63) |
0.06 (− 0.16, 0.41) |
0.06 (− 0.17, 0.42) |
0.03 (− 0.18, 0.37) |
QWG | |
|
0.16 (− 0.06, 0.50) |
0.16 (− 0.07, 0.49) |
0.06 (− 0.11, 0.29) |
0.06 (− 0.12, 0.3) |
0.02 (− 0.15, 0.26) |
0.01 (− 0.14, 0.22) |
DOMP |
| Alleviation of postprandial fullness at 4-week follow-up (shown in SMD and 95% CrI) | |||||
|---|---|---|---|---|---|
| XKD | |||||
|
− 1.76 (− 2.52, 0.37) |
MHGD | ||||
|
− 2.03 (− 3.01, 2.88) |
– 0.04 (− 0.94, 2.53) |
MZZD | |||
|
− 2.12 (− 2.99, 1.52) |
− 0.27 (− 1.48, 1.55) |
− 0.54 (− 1.37, 1.21) |
HWD | ||
|
− 2.09 (− 3.51, 1.25) |
− 0.24 (− 2.92, 1.6) |
0.31 (− 5.25, 1.45) |
− 0.28 (− 3.94, 2.10) |
XTD | |
|
− 2.14 (− 2.76, 0.70) |
− 0.39 (− 1.55, 0.54) |
− 0.64 (− 2.18, 0.74) |
− 0.12 (− 1.68, 0.80) |
− 0.34 (− 2.28, 3.30) |
DOMP |
| Alleviation of early satiety at 4-week follow-up (shown in SMD and 95% CrI) | |||
|---|---|---|---|
| XKD | |||
|
− 3.27 (− 4.81, 1.95) |
HWD | ||
|
− 3.27 (− 6.34, 0.24) |
− 0.49 (− 4.38, 1.84) |
XTD | |
|
− 3.90 (− 4.68, − 0.42) |
− 0.58 (− 2.69, 2.06) |
− 0.04 (− 2.12, 1.46) |
DOMP |
| Alleviation of epigastric pain at 4-week follow-up (shown in SMD and 95% CrI) | |||
|---|---|---|---|
| XKD | |||
|
− 0.53 (− 1.3, 0.65) |
HWD | ||
|
− 0.23 (− 1.14, 0.36) |
0.01 (− 0.77, 1.03) |
XTD | |
|
− 1.23 (− 1.66, − 0.29) |
− 0.64 (− 1.18, − 0.33) |
− 0.70 (− 1.44, − 0.35) |
DOMP |
BXD Ban Xia Xie Xin decoction, CHSP Cai Hu Shu Gan powder, CI Confidence interval, CrI Credible interval, CZJD Cai Zhu Jie Yu decoction, DOMP Domperidone, FAD Fu An decoction, HWD He Wei decoction, HXD He Wei Xiao Pi decoction, TGXD Tiao He Gan Pi Xing Qi decoction, TXD Tiao Zhong Xiao Pi decoction, JYD Jian Pi Yi Qi decoction, LAXC Liu Wei An Xiao capsule, MHGD Modified He Gan decoction, MZZD Modified Zhi Zhu decoction, QWG Qi Zhi Wei Tong granules, RD Risk difference, SJHD Shu Gan Jian Pi He Wei decoction, SMD Standardised mean difference, WKPD Wei Kang Ping decoction, WMD Wu Mo decoction, XKD Xiao Pi Kuan Wei decoction, XPD Xiao Pi decoction, XSLD Xiang Su Li Qi decoction, XTD Xiao Pi Tong Jiang decoction, ZZKC Zhi Zhu Kuan Zhong capsule
Table 6.
Classification of interventions based on network meta-analysis on alleviating global symptoms at 4-week follow-up: domperidone as comparator
| Classification of intervention | CHM formula | Risk difference (95% CI) | Surface under the cumulative ranking curve | Certainty of evidence+ |
|---|---|---|---|---|
| Moderate beneficial effect# | Modified Zhi Zhu Decoction | 0.28 (− 0.03, 0.75) | 0.85 | Low |
| Small beneficial effect† | Xiao Pi Tong Jiang Decoction | 0.15 (− 0.11, 0.57) | 0.64 | Low |
| Xiao Pi Kuan Wei Decoction | 0.13 (− 0.11, 0.50) | 0.61 | Low | |
| Jian Pi Yi Qi Decoction | 0.13 (− 0.11, 0.49) | 0.60 | Low | |
| Shu Gan Jian Pi He Wei Decoction | 0.12 (− 0.11, 0.49) | 0.59 | Low | |
| Xiang Su Li Qi Decoction | 0.13 (− 0.11, 0.52) | 0.59 | Low | |
| He Wei Decoction | 0.10 (− 0.07, 0.34) | 0.57 | Very low | |
| Modified He Gan Decoction | 0.11 (− 0.12, 0.46) | 0.54 | Low | |
| Liu Wei An Xiao Capsule | 0.09 (− 0.07, 0.34) | 0.53 | Very low | |
| Xiao Pi Decoction | 0.09 (− 0.13, 0.44) | 0.49 | Low | |
| Trivial to no beneficial effect‡ | He Wei Xiao Pi Decoction | 0.07 (− 0.14, 0.38) | 0.42 | Very low |
| Tiao He Gan Pi Xing Qi Decoction | 0.06 (− 0.14, 0.38) | 0.41 | Low | |
| Fu An Decoction | 0.06 (− 0.14, 0.38) | 0.40 | Low | |
| Cai Zhu Jie Yu Decoction | 0.06 (− 0.15, 0.36) | 0.38 | Low | |
| Zhi Zhu Kuan Zhong Capsule | − 0.03 (− 0.19, 0.22) | 0.16 | Very low |
CHM Chinese herbal medicine, CI Confidence interval
#Moderate beneficial effect: 0.31 > risk difference ≥ 0.20
†Small beneficial effect: 0.20 > risk difference ≥ 0.08
‡Trivial to no beneficial effect: risk difference < 0.08
+Quality of evidence ratings for comparisons in network meta-analysis on alleviating global symptoms at 4-week follow-up (domperidone as the comparator). Details refer to Additional file 1: Appendix 4
Similarly, in the NMA of six RCTs, no specific CHM formula was significantly superior to domperidone or other CHM formulae in the network in alleviating global symptoms at 2-week. Under the partially contextualised framework, both Cai Hu Shu Gan Powder (RD: 0.16; 95% CI: − 0.06 to 0.50) and Wei Kang Ping Decoction (RD: 0.16; 95% CI: − 0.07 to 0.49) may have a small beneficial effect in alleviating global symptoms at 2-week, as compared to domperidone. They were the best-ranked intervention in the network (SUCRA: 0.79), as supported by low certainty of evidence (Table 7).
Table 7.
Classification of interventions based on network meta-analysis on alleviating global symptoms at 2-week follow-up: domperidone as the comparator
| Classification of intervention | CHM formula | Risk difference (95% CI) | Surface under the cumulative ranking curve | Certainty of evidence+ |
|---|---|---|---|---|
| Small beneficial effect† | Cai Hu Shu Gan Powder | 0.16 (− 0.06, 0.50) | 0.79 | Low |
| Wei Kang Ping Decoction | 0.16 (− 0.07, 0.49) | 0.79 | Low | |
| Trivial to no beneficial effect‡ | Tiao Zhong Xiao Pi Decoction | 0.06 (− 0.11, 0.29) | 0.50 | Low |
| Wu Mo Decoction | 0.06 (− 0.12, 0.30) | 0.50 | Low | |
| Ban Xia Xie Xin Decoction | 0.02 (− 0.15, 0.26) | 0.36 | Low | |
| Qi Zhi Wei Tong Granules | 0.01 (− 0.14, 0.22) | 0.30 | Low |
CHM Chinese herbal medicine, CI Confidence interval
†Small beneficial effect: 0.20 > risk difference ≥ 0.08
‡Trivial to no beneficial effect: risk difference < 0.08
+Quality of evidence ratings for comparisons in network meta-analysis on alleviating global symptoms at 2-week follow-up (domperidone as the comparator). Details refer to Additional file 1: Appendix 5
In the NMA of three RCTs, no specific CHM formula was significantly more effective than mosapride or other CHM formulae in the network in alleviating global symptoms at 2-week (Table 8). According to the partially contextualised framework, Da Li Tong Granules (RD: 0.12; 95% CI: − 0.05 to 0.35) may have a small beneficial effect in alleviating global symptoms at 2-week, when compared to mosapride. It was the best-ranked intervention in the network (SUCRA: 0.85), as supported by low certainty of evidence (Table 9).
Table 8.
Comparative effectiveness of different Chinese herbal medicines: mosapride as comparator
| Alleviation of global symptoms at 4-week follow-up (shown in RD and 95% CI) | ||||||
|---|---|---|---|---|---|---|
| DLTG | ||||||
|
0.11 (− 0.11, 0.43) |
BGD | |||||
|
0.11 (− 0.10, 0.43) |
0.02 (− 0.15, 0.26) |
TXD | ||||
|
0.12 (− 0.05, 0.35) |
0.02 (− 0.11, 0.19) |
0.01 (− 0.11, 0.17) |
MOSA | |||
BGD Bu Gan Decoction, CI Confidence interval, DLTG Da Li Tong Granules, MOSA Mosapride, RD Risk difference, TXD Tiao Wei Xiao Pi Decoction
Table 9.
Classification of interventions based on network meta-analysis on alleviating global symptoms at 4-week follow-up: mosapride as comparator
| Classification of intervention | CHM formula | Risk difference (95% CI) | Surface under the cumulative ranking curve | Certainty of evidence+ |
|---|---|---|---|---|
| Small beneficial effect† | Da Li Tong Granules | 0.12 (− 0.05, 0.35) | 0.85 | Low |
| Trivial to no beneficial effect‡ | Bu Gan Decoction | 0.02 (− 0.11, 0.19) | 0.44 | Low |
| Tiao Wei Xiao Pi Decoction | 0.01 (− 0.11, 0.17) | 0.39 | Low |
CHM Chinese herbal medicine, CI Confidence interval
†Small beneficial effect: 0.20 > risk difference ≥ 0.08
‡Trivial to no beneficial effect: risk difference < 0.08
+Quality of evidence ratings for comparisons in network meta-analysis on alleviating global symptoms at 4-week follow-up (mosapride as the comparator). Details refer to Additional file 1: Appendix 6
Alleviation of postprandial fullness
In the NMA of six RCTs, no specific CHM formulae were significantly superior to domperidone or other CHM formulae in alleviating postprandial fullness at 4-week (Table 5). However, based on the partially contextualised framework, Xiao Pi Kuan Wei Decoction (SMD: − 2.14; 95% CrI: − 2.76 to 0.70) may have a large beneficial effect on postprandial fullness alleviation at 4-week follow-up. It was the best-ranked intervention in the network (SUCRA: 0.85), and this finding was supported by low certainty of evidence (Table 10).
Table 10.
Classification of interventions based on network meta-analyses on secondary outcomes: domperidone as the comparator
| Outcome | Classification of intervention | CHM formula | Standardised mean difference (95% CI) | Surface under the cumulative ranking curve | Certainty of evidence+ |
|---|---|---|---|---|---|
| Alleviation of postprandial fullness | Large beneficial effect@ | Xiao Pi Kuan Wei Decoction | − 2.14 (− 2.76, 0.70) | 0.85 | Low |
| Moderate beneficial effect# | Modified Zhi Zhu Decoction | − 0.64 (− 2.18, 0.74) | 0.45 | Low | |
| Small beneficial effect† | Modified He Gan Decoction | − 0.39 (− 1.55, 0.54) | 0.48 | Low | |
| Xiao Pi Tong Jiang Decoction | − 0.34 (− 2.28, 3.30) | 0.43 | Low | ||
| Trivial to no beneficial effect‡ | He Wei Decoction | − 0.12 (− 1.68, 0.80) | 0.45 | Very low | |
| Alleviation of early satiety | Large beneficial effect@ | Xiao Pi Kuan Wei Decoction | − 3.90 (− 0.68, − 0.42) | 0.92 | Low |
| Moderate beneficial effect# | He Wei Decoction | − 0.58 (− 2.69, 2.06) | 0.50 | Very low | |
| Trivial to no beneficial effect‡ | Xiao Pi Tong Jiang Decoction | − 0.04 (− 2.12, 1.46) | 0.33 | Low | |
| Alleviation of epigastric pain | Large beneficial effect@ | Xiao Pi Kuan Wei Decoction | − 1.23 (− 1.66, − 0.29) | 0.79 | Low |
| Moderate beneficial effect# | He Wei Decoction | − 0.64 (− 1.18, − 0.33) | 0.63 | Very low | |
| Xiao Pi Tong Jiang Decoction | − 0.70 (− 1.44, − 0.35) | 0.58 | Low |
CHM Chinese herbal medicine, CI Confidence interval
@Large beneficial effect: standardised mean difference ≤ − 0.80
#Moderate beneficial effect: − 0.80 < standardised mean difference ≤ − 0.50
†Small beneficial effect: − 0.50 < standardised mean difference ≤ − 0.20
‡Trivial to no beneficial effect: standardised mean difference > − 0.20
+Quality of evidence ratings for comparisons in network meta-analysis on alleviating postprandial fullness, early satiety, epigastric pain, and epigastric burning, respectively. Details refer to Additional file 1: Appendix 7–9
A negative standardised mean difference indicated an effect favouring Chinese herbal medicine, while a positive standardised mean difference indicated an effect favouring prokinetics
Alleviation of early satiety
In the NMA of four RCTs, Xiao Pi Kuan Wei Decoction was significantly more effective than domperidone in alleviating early satiety at 4-week follow-up (SMD: − 3.90; 95% CrI: − 0.68 to − 0.42) (Table 5). Under the partially contextualised framework, Xiao Pi Kuan Wei Decoction may have a large beneficial effect on early satiety alleviation at 4-week follow-up. It was the best-ranked intervention in the network (SUCRA: 0.92), as supported by low certainty of evidence (Table 10).
Alleviation of epigastric burning
In the NMA of four RCTs, Xiao Pi Kuan Wei Decoction (SMD: − 1.23; 95% CrI: − 1.66 to − 0.29), He Wei Decoction (SMD: − 0.64; 95% CrI: − 1.18 to − 0.33), and Xiao Pi Tong Jiang Decoction (SMD: − 0.70; 95% CrI: − 1.44 to − 0.35) was significantly better than domperidone in alleviating epigastric burning at 4-week (Table 5). According to the partially contextualised framework, Xiao Pi Kuan Wei Decoction may have a large beneficial effect on epigastric burning alleviation at 8-week. It was the best-ranked intervention in the network (SUCRA: 0.79), and the conclusion was supported by low certainty of evidence (Table 10).
Adverse events
No serious adverse events were reported in all included RCTs (Table 2). An RCT on Liu Wei An Xiao Capsule reported most cases of adverse events (n = 8) [38], which were related to frequent bowel movements. Three cases of mild adverse events, including oral ulcer (n = 2) and diarrhoea (n = 1), were recorded in an RCT on He Wei Decoction [40]. Two cases of mild adverse events, including diarrhoea (n = 1) and dizziness (n = 1), were found in the RCT on Wu Mo Decoction [54]. A case of mild diarrhoea was also reported in the RCT on Fu An Decoction [61].
Discussion
Summary of findings
Pairwise meta-analyses showed that CHM was superior to prokinetics in alleviating global symptoms at 2-week and 4-week follow-up, but the magnitude of differences was smaller than relevant MCID values. Although publication bias favouring CHM was detected for the latter outcome, the direction and statistical significance of the result remained unchanged after applying the trim and fill adjustment. Differences exceeding the MCID were observed in other outcomes, with CHM being better than (i) prokinetics in alleviating postprandial fullness and (ii) domperidone alone in alleviating epigastric pain, at 4-week follow-up. These imply that CHM may well serve as an alternative to prokinetics, given the fact that its effectiveness is similar, if not more effective, to the conventional therapy, especially in alleviating postprandial fullness and epigastric pain. Indeed, they are the main symptoms of PDS and EPS respectively.
As interpreted under the partially contextualised framework, NMAs illustrated that Modified Zhi Zhu Decoction may have a moderate beneficial effect on alleviating global symptoms at 4-week follow-up, while Xiao Pi Kuan Wei Decoction may have a large beneficial effect on alleviating postprandial fullness, early satiety, and epigastric pain. In future guideline revisions, Modified Zhi Zhu Decoction and Xiao Pi Kuan Wei Decoction may be recommended as alternative options for FD patients who are unresponsive to prokinetics or opt-out of the treatment due to associated adverse effects. Additional considerations on the implementation will involve the key aspects below.
Implications for practice
Positioning of Chinese herbal medicine in functional dyspepsia clinical guidelines
Current Asian clinical guideline [8] recommends prokinetics as the first-line treatment for the FD diagnostic subtype of PDS and the second-line treatment after PPIs for EPS. Unfortunately, prokinetics have a relatively high number needed to treat of seven to twelve, and evidence supporting their effectiveness was of very low-quality evidence [11]. Specific prokinetics are also related to serious adverse events. For instance, domperidone and cisapride may trigger life-threatening arrhythmia in patients with cardiovascular conditions [4, 12], and metoclopramide may induce dystonia, parkinsonism-type movements, and tardive dyskinesia [4].
Results of this review suggest that CHM may be a potential substitution to prokinetics. Beyond effectiveness and safety, guideline developers should also consider other criteria in the GRADE Evidence to Decision (EtD) framework [65], including acceptability, feasibility, outcome importance, cost-effectiveness, and equity, when preparing guideline updates. Issues on acceptability and feasibility will be discussed briefly.
Acceptability of Chinese herbal medicine among patients with gastrointestinal disorders
CHM is one of the most utilised modalities of traditional, complementary, and integrative medicine (TCIM) worldwide, particularly in Asia [14]. In Taiwan, more than 60% of the population utilised TCM services on a regular basis [66]. 86% of those services involved CHM prescriptions [66]. In Singapore, over three-quarters of the population used TCIM at least once a year, and CHM was the most popular TCIM modality [67]. In both healthcare systems, patients with gastrointestinal disorders constituted a significant portion of CHM users [66, 67]. With a high prevalence of both CHM utilisation and FD in Asia, the acceptability of CHM for FD treatment is likely to be high. The use of CHM may also be accepted by patients in Canada [68] and Australia [69], where TCM practice is statutorily regulated.
Feasibility of Chinese herbal medicine utilisation in interprofessional environment
If CHM is to be included in the next FD clinical guideline, interprofessional collaboration between conventional clinicians and TCM clinicians will be required for the implementation: a conventional clinician may refer a patient unresponsive to prokinetics to a TCM clinician, and vice versa when CHM is found to be ineffective. Different referral mechanisms have been devised to clarify the respective duties and responsibilities of conventional clinicians and TCM clinicians, of which suitability would depend on the clinical context [70]. Potential malpractice liability related to adverse events is a key barrier for collaboration [71]. To address this barrier, pharmacovigilance mechanisms for monitoring potential CHM-related adverse events should be in place to improve confidence in interprofessional collaboration.
Implications for research
Based on the best available evidence, Modified Zhi Zhu Decoction and Xiao Pi Kuan Wei Decoction may be the best CHM formulae for alleviating FD global and individual symptoms (postprandial fullness, early satiety, epigastric pain, and epigastric burning), respectively. Modern pharmacology may help explain their therapeutic effects. In Modified Zhi Zhu Decoction [48], the main ingredients are Aurantii Fructus Immaturus (Zhishi) and Rhizoma Atractylodis Macrocephala (Baizhu). Both herbs contain two flavonoids, naringin and hesperidin, which can be converted to naringenin and hesperitin in human body and may alleviate dyspeptic symptoms through increasing gastrointestinal motility [72, 73]. In Xiao Pi Kuan Wei Decoction [43], the main ingredient of Radix Bupleuri (Chaihu) contains saikosaponin, a component with antidepressant-like effects [74]. It may relieve dyspeptic symptoms via addressing disorder at the brain-gut axis [75]. Aurantii Fructus Immaturus and Rhizoma Atractylodis Macrocephala in the decoction may also help enhance therapeutic effects by increasing the motility of gastrointestinal tract.
In the future, confirmatory head-to-head RCTs should be carried out to further investigate their comparative effectiveness against prokinetics. Trialists should beware of several design aspects when planning such trials:
Patient eligibility
Validated Rome IV diagnostic criteria for FD [1] should be adopted as the eligibility criteria to enable comparisons between and synthesis of similar trials. Patients who remain to be symptomatic after receiving PPIs should be recruited as they are refractory to the current first-line therapy of the North American guideline [4].
Interventions and comparisons
Three-arm RCTs consisting of Modified Zhi Zhu Decoction, Xiao Pi Kuan Wei Decoction, and prokinetics should be performed to allow head-to-head comparisons between the two formulae, and against prokinetics. The choice of prokinetics should comply with local regulatory requirements and safety profile.
Outcome measures
An array of expert-recommended endpoints for FD trials should be adopted to capture outcome changes in a multifaceted manner. Global symptom alleviation evaluated on dichotomous scale allows a global assessment [21], which can be supplemented with assessment on individual symptoms on a seven-point Likert scale [76]. Nepean Dyspepsia Index [77] provides information on changes in disease-specific quality of life [21, 76]. Objective measurements, including gastric emptying scintigraphy, gastric barostat study, and liquid nutrient drink test, may also be conducted to supplement patient-reported outcomes [21, 76]. Furthermore, a longer follow-up period of forty-eight weeks is recommended to allow sufficient assessment on refractory FD, in which symptoms usually wax and wane [76].
To minimise selection bias, trialists should allocate interventions to participants based on random number table or generator and conceal the allocation sequence from research personnel [26]. Blinding of participants and research personnel also helps reduce performance bias and detection bias of RCTs [26]. A priori protocols should be published and followed to avoid outcome reporting bias [26]. Furthermore, to improve the transparency of RCTs, trialists should report their studies in accordance with the CONSORT (Consolidated Standards of Reporting Trials) Extension for CHM Formulas [78].
In this study, specific TCM diagnostic patterns were used in fifteen out of twenty-eight included RCTs as part of the inclusion criteria, despite the absence of gold standards for pattern differentiation [79, 80]. Having said that, when evidence-based differentiation rules are established in the future, clinical trials can be carried out to compare the effectiveness of different CHM formulae that are targeting the same TCM diagnostic patterns [81].
Limitations
This study has several limitations. First, due to the small number of included trials, funnel plots or relevant statistical tests were not conducted for all outcomes to examine the possible presence of publication bias. Second, the consistency of NMA results could not be assessed since no comparisons between CHM formulae were supported by both direct (head-to-head comparisons between CHM formulae) and indirect evidence (comparisons between CHM formulae and prokinetics). Third, sample size of the included RCTs was small and might have influenced the precision of our results. We assessed the degree of imprecision for each outcome under the GRADE framework (Tables 3, 4). The potential impact originating from imprecision has been reflected by downgrading the quality of evidence and certainty of evidence in the GRADE evidence rating for pairwise meta-analyses and NMAs, respectively. Clinicians and policy-makers should consider this information in the decision-making process.
Conclusions
Results from this review suggested that CHM could be a potential alternative to prokinetics as a first-line treatment for FD or a second-line treatment after PPI. Repositioning of CHM in clinical guidelines require thorough discussions on aspects stipulated in the GRADE EtD framework. Confirmatory head-to-head trials are necessary for evaluating the comparative effectiveness of prokinetics, Modified Zhi Zhu Decoction, and Xiao Pi Kuan Wei Decoction.
Supplementary Information
Additional file 1: Appendix 1. Search strategies for databases. Appendix 2. Risk of bias of included studies. Appendix 3. (a) Contour-enhanced funnel plot of 20 included studies (all prokinetics). (b) Contour-enhanced funnel plot of 17 included studies (domperidone only). Appendix 4. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating global symptoms at 4-week follow-up: domperidone as the comparator. Appendix 5. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating global symptoms at 2-week follow-up: domperidone as the comparator. Appendix 6. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating global symptoms at 4-week follow-up: mosapride as the comparator. Appendix 7. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating postprandial fullness: domperidone as the comparator. Appendix 8. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating early satiety: domperidone as the comparator. Appendix 9. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating epigastric pain: domperidone as the comparator.
Acknowledgements
Not applicable.
Abbreviations
- CHM
Chinese herbal medicine
- CI
Confidence interval
- Crl
Credible interval
- EPS
Epigastric pain syndrome
- EtD
Evidence to Decision
- FD
Functional dyspepsia
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- MCID
Minimally clinically important difference
- NMA
Network meta-analysis
- PDS
Postprandial distress syndrome
- PPI
Proton pump inhibitor
- RCT
Randomised controlled trials
- RD
Risk difference
- RR
Risk ratio
- SMD
Standardised mean difference
- SUCRA
Surface under the cumulative ranking curve
- TCIM
Traditional, complementary, and integrative medicine
- TCM
Traditional Chinese Medicine
Authors’ contributions
LH: Investigation, Formal analysis, Writing—original draft, Writing—review & editing; CCWZ: Formal analysis, Writing—Review & Editing; CHLW: Methodology; JCYW: Writing—review & editing; KKHC: Investigation; IXYW: Methodology, Funding acquisition; THL: Writing—review & editing; VCHC: Conceptualisation, Supervision. All authors read and approved the final version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (reference: 81973709) and the Natural Science Foundation of Hunan Province (reference: 2019JJ40348). The funding bodies had no role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stanghellini V, Chan FKL, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology. 2016;150(6):1380–1392. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Enck P, Azpiroz F, Boeckxstaens G, Elsenbruch S, Feinle-Bisset C, Holtmann G, et al. Functional dyspepsia. Nat Rev Dis Primers. 2017;3(1):17081. doi: 10.1038/nrdp.2017.81. [DOI] [PubMed] [Google Scholar]
- 3.Ghoshal UC, Singh R, Chang FY, Hou X, Wong BC, Kachintorn U. Epidemiology of uninvestigated and functional dyspepsia in Asia: facts and fiction. J Neurogastroenterol Motil. 2011;17(3):235–244. doi: 10.5056/jnm.2011.17.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moayyedi P, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112(7):988–1013. doi: 10.1038/ajg.2017.154. [DOI] [PubMed] [Google Scholar]
- 5.Pinto-Sanchez MI, Yuan Y, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;3(3):Cd011194. doi: 10.1002/14651858.CD011194.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kia L, Kahrilas PJ. Therapy: risks associated with chronic PPI use—signal or noise? Nat Rev Gastroenterol Hepatol. 2016;13(5):253–254. doi: 10.1038/nrgastro.2016.44. [DOI] [PubMed] [Google Scholar]
- 7.Yan XJ, Luo QQ, Qiu HY, Ji CF, Chen SL. The impact of stigma on medication adherence in patients with functional dyspepsia. Neurogastroenterol Motil. 2020;33:e13956. doi: 10.1111/nmo.13956. [DOI] [PubMed] [Google Scholar]
- 8.Miwa H, Ghoshal UC, Fock KM, Gonlachanvit S, Gwee KA, Ang TL, et al. Asian consensus report on functional dyspepsia. J Gastroenterol Hepatol. 2012;27(4):626–641. doi: 10.1111/j.1440-1746.2011.07037.x. [DOI] [PubMed] [Google Scholar]
- 9.Study group of Gastrointestinal Motility—Chinese Society of Gastroenterology, Study group of Functional Gastrointestinal Disorders—Chinese Society of Gastroenterology Chinese expert consensus on functional dyspepsia management (2015) (Chinese) Chin J Dig. 2015;36(4):217–228. [Google Scholar]
- 10.Oh JH, Kwon JG, Jung HK, Tae CH, Song KH, Kang SJ, et al. Clinical practice guidelines for functional dyspepsia in Korea. J Neurogastroenterol Motil. 2020;26(1):29–50. doi: 10.5056/jnm19209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pittayanon R, Yuan Y, Bollegala NP, Khanna R, Lacy BE, Andrews CN, et al. Prokinetics for functional dyspepsia: a systematic review and meta-analysis of randomized control trials. Am J Gastroenterol. 2019;114(2):233–243. doi: 10.1038/s41395-018-0258-6. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Mahadeva S, Carbone MF, Lacy BE, Talley NJ. Functional dyspepsia. Lancet. 2020;396(10263):1689–1702. doi: 10.1016/S0140-6736(20)30469-4. [DOI] [PubMed] [Google Scholar]
- 13.Jiang SM, Jia L, Lei XG, Xu M, Wang SB, Liu J, et al. Incidence and psychological-behavioral characteristics of refractory functional dyspepsia: a large, multi-center, prospective investigation from China. World J Gastroenterol. 2015;21(6):1932–1937. doi: 10.3748/wjg.v21.i6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . WHO traditional medicine strategy 2014–2023. Geneva: World Health Organization; 2013. [Google Scholar]
- 15.Xi S, Gong Y. Essentials of Chinese materia medica and medical formulas: new century traditional chinese medicine. 1. Cambridge, MA: Academic Press; 2017. [Google Scholar]
- 16.Wang S, Liu L, Liu J, Miao L, Zhuang Q, Guo N, et al. Characteristics of prescriptions and costs for acute upper respiratory tract infections in Chinese outpatient pediatric patients: a nationwide cross-sectional study. BMC Complement Med Ther. 2020;20(1):346. doi: 10.1186/s12906-020-03141-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Zhao L. Expert consensus on traditional Chinese medicine diagnosis and treatment of functional dyspepsia (2017) (Chinese) CJTCMP. 2017;32(6):2595–2598. [Google Scholar]
- 18.Ho L, Zhong CCW, Wong CHL, Wu JCY, Chan KKH, Wu IXY, et al. Herbal medicine for functional dyspepsia: network meta-analysis of placebo-controlled randomised trials. J Ethnopharmacol. 2022;283:114665. doi: 10.1016/j.jep.2021.114665. [DOI] [PubMed] [Google Scholar]
- 19.McMaster Health Information Research Unit. Search filters for MEDLINE in Ovid Syntax and the PubMed translation. Hamilton, Ontario: McMaster Health Information Research Unit. https://hiru.mcmaster.ca/hiru/HIRU_Hedges_MEDLINE_Strategies.aspx. Accessed 4 Jan 2021
- 20.McMaster Health Information Research Unit. Search Strategies for EMBASE in Ovid Syntax. Hamilton, Ontario: McMaster Health Information Research Unit. https://hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx. Accessed 4 Jan 2021.
- 21.Ang D, Talley NJ, Simren M, Janssen P, Boeckxstaens G, Tack J. Review article: endpoints used in functional dyspepsia drug therapy trials. Aliment Pharmacol Ther. 2011;33(6):634–649. doi: 10.1111/j.1365-2036.2010.04566.x. [DOI] [PubMed] [Google Scholar]
- 22.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Ryan R, Hill S. How to GRADE the quality of the evidence. Melbourne, Australia: La Trobe University. http://cccrg.cochrane.org/author-resources. Accessed 4 Jan 2021.
- 24.Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 25.Gagnier JJ, Morgenstern H, Altman DG, Berlin J, Chang S, McCulloch P, et al. Consensus-based recommendations for investigating clinical heterogeneity in systematic reviews. BMC Med Res Methodol. 2013;13:106. doi: 10.1186/1471-2288-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. London, United Kingdom: The Cochrane Collaboration. https://training.cochrane.org/handbook. Accessed 4 Jan 2021
- 27.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li T, Puhan MA, Vedula SS, Singh S, Dickersin K. The Ad Hoc network meta-analysis methods meeting working G. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med. 2011;9(1):79. doi: 10.1186/1741-7015-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network Meta-analyses. BMC Med Res Methodol. 2019;19(1):196. doi: 10.1186/s12874-019-0829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3(4):285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 31.Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6(1):79. doi: 10.1186/s13643-017-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brignardello-Petersen R, Izcovich A, Rochwerg B, Florez ID, Hazlewood G, Alhazanni W, et al. GRADE approach to drawing conclusions from a network meta-analysis using a partially contextualised framework. BMJ. 2020;371:m3907. doi: 10.1136/bmj.m3907. [DOI] [PubMed] [Google Scholar]
- 33.Brignardello-Petersen R, Florez ID, Izcovich A, Santesso N, Hazlewood G, Alhazanni W, et al. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ. 2020;371:m3900. doi: 10.1136/bmj.m3900. [DOI] [PubMed] [Google Scholar]
- 34.Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing Summary of Findings tables and evidence profiles—continuous outcomes. J Clin Epidemiol. 2013;66(2):173–183. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Newcombe RG, Bender R. Implementing GRADE: calculating the risk difference from the baseline risk and the relative risk. Evid Based Med. 2014;19(1):6–8. doi: 10.1136/eb-2013-101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22(158):209–212. [Google Scholar]
- 37.Liu B, Piao X, Guo L. Effect of herbal formula Xiao Pi-II on functional dyspepsia. J Tradit Chin Med. 2013;33(3):298–302. doi: 10.1016/s0254-6272(13)60168-5. [DOI] [PubMed] [Google Scholar]
- 38.Gong Y, Wang C, Qi S. Liu Wei An Xiao capsule in treating functional dyspepsia with syndrome of food accumulation (Chinese) J Changchun TCM Univ. 2012;28(3):482–483. [Google Scholar]
- 39.Zhou F, Chengye Y. Hewei decoction in the treatment of functional dyspepsia (Chinese) Shaanxi J Tradit Chin Med. 2016;37(5):576–578. [Google Scholar]
- 40.Leng Y, Li Y. Hewei Chongji in treating functional dyspepsia with syndrome of disharmony between liver and stomach: a random controlled trial (Chinese) J Liaoning TCM Univ. 2014;16(8):18–20. [Google Scholar]
- 41.Gao J, Quan H. Clinical obervation on 40 cases of functional dyspepsia treated with modified Hegan decoction (Chinese) Chin Med Modern Distance Edu China. 2013;11(12):27–29. [Google Scholar]
- 42.Lai Y, Liu Y, He Y, Li S, Wang J. Clinical observation on Xiao Pi Tong Jiang decoction in treating functional dyspepsia with syndrome of cold-heat complex (Chinese) Hebei J Tradi Chin Med. 2019;41(2):220–223. [Google Scholar]
- 43.Cai S, F M. Clinical observation on Xiaopi Kuanwei decoction in the treatment of functional dyspepsia with disharmony of liver and stomach (Chinese). Chin Med Modern Distance Edu China. 2019;17(7):74–6.
- 44.Dong Y, Jie L. Clinical observation on 32 cases of Cai Zhu Jie Yu decoction in treating functional dyspepsia with syndrome of liver Qi stagnation (Chinese) Guiding J TCM Pharm. 2011;17(8):26–27. [Google Scholar]
- 45.Liu F, Xe L. Efficacy and safety of Zhizhu Kuanzhong capsule in the treatment of functional dyspepsia and depression symptoms in a multi-centre open randomized controlled trial (Chinese) World Chin Med. 2012;7(6):484–485. [Google Scholar]
- 46.Liu K. Clinical observation on Xiao Pi decoction in treating functional dyspepsia (Chinese) Shanxi J Tradi Chin Med. 2015;31(11):14–15. [Google Scholar]
- 47.Ma W. Clinical observation on He Wei Xiao Pi decoction in treating functional dyspepsia (Chinese) China Health Care Nutr. 2014;5:2800. [Google Scholar]
- 48.Duan G. Clinical observation on modified Zhi Zhu decoction in treating functional dyspepsia (Chinese) Shanxi J Tradi Chin Med. 2013;29(11):12–13. [Google Scholar]
- 49.Wang W. Observation of decoction for dispersing depressed liver-qi, invigorating spleen and regulating stomach on functional dyspepsia (Chinese) Shanxi J Tradi Chin Med. 2012;28(7):12–13. [Google Scholar]
- 50.Li J, Wang S. 72 cases on Tiao He Gan Pi Xing Qi decoction in treating functional dyspepsia (Chinese) Shanxi J Tradi Chin Med. 2014;35(9):1127–1129. [Google Scholar]
- 51.Huang Z, Shen Z. Clinical observation of 30 cases of Xiang Su Li Qi decoction in treating functional dyspepsia with syndome of spleen deficiency and Qi stagnation (Chinese) Hunan J Tradi Chin Med. 2017;33(9):69–70. [Google Scholar]
- 52.Sheng H. 41 cases on Jian Pi Yi Qi decoction in treating functional dyspepsia (Chinese) TCM Res. 2016;29(7):19–20. [Google Scholar]
- 53.Zhao J, Liu G. 60 cases on Tiao Zhong Xiao Pi decoction in treating functional dyspepsia (Chinese) J Gansu TCM Univ. 2013;30(02):49–51. [Google Scholar]
- 54.Liu Z. Efficacy of Wu Mo decoction in treating functional dyspepsia with syndrome of spleen deficiency and Qi stagnation (Chinese) Chin J Med Dev. 2016;29(1):117–118. [Google Scholar]
- 55.Ma H, Zhu Y. Therapeutic efficacy of Chaihu Shugan powder in treatment of functional dyspepsia with Qi depression: a report of 30 cases (Chinese) Hunan J Tradi Chin Med. 2013;29(1):17–19. [Google Scholar]
- 56.Chen Y. 28 cases on Bu Gan decoction in treating functional dyspepsia (Chinese) Hunan J Tradi Chin Med. 2016;32(1):45–46. [Google Scholar]
- 57.Wang H, Long Z, Chen X, Song Y, Dong J. Clinical study on Tiaoweixiaopi decoction in treatment of functional dyspepsia with syndrome of spleen deficiency with dampness-heat (Chinese) Hubei J Tradi Chin Med. 2015;37(11):1–2. [Google Scholar]
- 58.Huang M, Li G, Li H, Nie L. Clinical effect of dalitong granules on 114 cases of functional dyspepsia (Chinese) Sichuan Med J. 2010;31(10):1484–1485. [Google Scholar]
- 59.Zheng B, Pei J, Cai Z. Clinical observation on the effect of Jiaweiyueju decoction in treatment of functional dyspepsia (Chinese) China Mod Doc. 2010;48(29):48–49. [Google Scholar]
- 60.Li X, Lu C. 50 cases on Liu Wei An Xiao capsule in treating functional dyspepsia (Chinese) J Gansu TCM Univ. 2004;21(2):29–30. [Google Scholar]
- 61.Liu J, Lin X. Observation on Fu An decoction in treating functional dyspepsia with syndrome of liver depression and spleen deficiency (Chinese) Hubei J Tradi Chin Med. 2010;32(5):23–24. [Google Scholar]
- 62.Liu J, H Z, Dai Y, Chen J, Lang X. Clinical Research on the Theratmaic Effect of Weikangping Decoction on 40 Cases of Functional Dyspepsia (Chinese). Hebei J Tradi Chin Med. 2008; 30(3):240–2.
- 63.Wang S, Jing G. 58 cases of Qi Zhi Wei Tong granules in treating functional dyspepsia with dysmotility (Chinese) Mod J Integr Trad Chin West Med. 2006;15(21):2930–2931. [Google Scholar]
- 64.Hu H, Zhu D, Zhou H, Xiong X. Clinical research on Ban Xia Xie Xin decoction in treating functional dyspepsia with cold-heat complex (Chinese) J Hunan TCM Univ. 2006;26(01):40–41. [Google Scholar]
- 65.Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. doi: 10.1136/bmj.i2016. [DOI] [PubMed] [Google Scholar]
- 66.Chen FP, Chen TJ, Kung YY, Chen YC, Chou LF, Chen FJ, et al. Use frequency of traditional Chinese medicine in Taiwan. BMC Health Serv Res. 2007;7(1):26. doi: 10.1186/1472-6963-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim MK, Sadarangani P, Chan HL, Heng JY. Complementary and alternative medicine use in multiracial Singapore. Complement Ther Med. 2005;13(1):16–24. doi: 10.1016/j.ctim.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Randall G, Embrett M, Barr N, Vania D. Regulating traditional chinese medicine practitioners and acupuncturists in Ontario, Canada. Health Reform Observer Observatoire Des Réformes De Santé. 2015;3(2).
- 69.The Australian Health Practitioner Regulation Agency. Registration. Canberra, Australia: The Australian Health Practitioner Regulation Agency. https://www.chinesemedicineboard.gov.au/registration.aspx. Accessed 4 Jan 2021
- 70.Chung VCH, Ho LTF, Leung TH, Wong CHL. Designing delivery models of traditional and complementary medicine services: a review of international experiences. Br Med Bull. 2021;137(1):70–81. doi: 10.1093/bmb/ldaa046. [DOI] [PubMed] [Google Scholar]
- 71.Cohen MH, Eisenberg DM. Potential physician malpractice liability associated with complementary and integrative medical therapies. Ann Intern Med. 2002;136(8):596–603. doi: 10.7326/0003-4819-136-8-200204160-00009. [DOI] [PubMed] [Google Scholar]
- 72.Wu H, Jing Z, Tang X, Wang X, Zhang S, Yu Y, et al. To compare the efficacy of two kinds of Zhizhu pills in the treatment of functional dyspepsia of spleen-deficiency and qi-stagnation syndrome: a randomized group sequential comparative trial. BMC Gastroenterol. 2011;11:81. doi: 10.1186/1471-230X-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao H, Chen X, Sun H, Sakurai T, Zhou J, Sun W, et al. Pharmacokinetics-based elucidation on disparity in clinical effectiveness between varieties of Zhi Zhu Wan, a Traditional Chinese Medical formula. J Ethnopharmacol. 2010;128(3):606–610. doi: 10.1016/j.jep.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Yang F, Dong X, Yin X, Wang W, You L, Ni J. Radix Bupleuri: a review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. Biomed Res Int. 2017;2017:7597596. doi: 10.1155/2017/7597596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain–gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61(9):1284–1290. doi: 10.1136/gutjnl-2011-300474. [DOI] [PubMed] [Google Scholar]
- 76.Lacy BE, Talley NJ, Camilleri M. Functional dyspepsia: time to change clinical trial design? Am J Gastroenterol. 2010;105(12):2525–2529. doi: 10.1038/ajg.2010.266. [DOI] [PubMed] [Google Scholar]
- 77.Talley NJ, Haque M, Wyeth JW, Stace NH, Tytgat GN, Stanghellini V, et al. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Aliment Pharmacol Ther. 1999;13(2):225–235. doi: 10.1046/j.1365-2036.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 78.Cheng CW, Wu TX, Shang HC, Li YP, Altman DG, Moher D, et al. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med. 2017;167(2):112–121. doi: 10.7326/M16-2977. [DOI] [PubMed] [Google Scholar]
- 79.Shuldiner SR, Chung VCH, Wu X, Ching J, Ho RST, Cheong PK, et al. Methodological challenges in mapping chinese medicine syndrome with conventional diagnosis: Implications for multi-centre trials in integrative medicine. Eur J Integr Med. 2015;7(4):358–364. [Google Scholar]
- 80.Ho LT, Chung VC, Wong CH, Wu IX, Lan KC, Wu D, et al. Evaluating traditional Chinese medicine diagnostic instruments for functional dyspepsia: systematic review on measurement properties. Integr Med Res. 2021;10(3):100713. doi: 10.1016/j.imr.2020.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chung VCH, Ho RST, Wu X, Wu JCY. Incorporating traditional Chinese medicine syndrome differentiation in randomized trials: Methodological issues. Eur J Integr Med. 2016;8(6):898–904. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. Search strategies for databases. Appendix 2. Risk of bias of included studies. Appendix 3. (a) Contour-enhanced funnel plot of 20 included studies (all prokinetics). (b) Contour-enhanced funnel plot of 17 included studies (domperidone only). Appendix 4. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating global symptoms at 4-week follow-up: domperidone as the comparator. Appendix 5. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating global symptoms at 2-week follow-up: domperidone as the comparator. Appendix 6. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating global symptoms at 4-week follow-up: mosapride as the comparator. Appendix 7. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating postprandial fullness: domperidone as the comparator. Appendix 8. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating early satiety: domperidone as the comparator. Appendix 9. Effect estimates and quality of evidence ratings for comparisons in network meta-analysis on alleviating epigastric pain: domperidone as the comparator.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.