Abstract
Background and Aims
The aim of this study was to examine the risk of new-onset inflammatory bowel disease [IBD] following bariatric surgery.
Methods
We conducted a nationwide population-based prospective cohort study of the entire Danish population 18 to 60 years of age, alive, and residing in Denmark, from 1996 to 2018. Bariatric surgery was included as a time-dependent variable, and Cox proportional hazards regression models were used to estimate hazard ratios [HRs] of IBD. We used a model adjusting for age, sex, and birth cohort and a multifactor-adjusted model additionally including educational status and number of obesity-related comorbidities.
Results
We followed 3 917 843 individuals of whom 15 347 had a bariatric surgery, for development of new-onset IBD. During 106 420 person-years following bariatric surgery, 100 IBD events occurred [incidence rate 0.940/1000 person-years]. During 55 553 785 person-years without bariatric surgery, 35 294 events of IBD occurred [incidence rate 0.635/1000 person-years]. This corresponded to a multifactor-adjusted hazard ratio [HR] of 1.15 (95% confidence interval[CI], 0.94–1.40) for IBD. Multifactor-adjusted HRs of Crohn’s disease [CD] and ulcerative colitis [UC] were 1.85 [95% CI, 1.40–2.44] and 0.81 [95% CI, 0.61–1.08], respectively. Among women, the multifactor-adjusted HR for CD was 2.18 [95% CI, 1.64–2.90]. When limiting the study population to individuals with a diagnosis of overweight/obesity, bariatric surgery remained associated with increased risk of CD, multifactor-adjusted HR 1.59 [95% CI, 1.18–2.13].
Conclusions
This nationwide cohort study shows that bariatric surgery is associated with increased risk of development of new-onset CD, but not of UC. The underlying mechanisms remain elusive.
Keywords: Colitis, ulcerative, Crohn’s disease, gastric bypass
1. Introduction
The incidence of obesity and inflammatory bowel disease [IBD] are both increasing globally.1,2 Patients with IBD, including Crohn’s disease [CD] and ulcerative colitis [UC], often experience a lifelong disease course with relapsing and remitting inflammation of their gut.3–5 The aetiology of IBD remains largely unknown, yet the rapidly changing epidemiology of IBD across time underscores the importance of environmental factors in its pathogenesis.6 The parallel rises in obesity and IBD have spurred studies of the role of obesity-related inflammation in the pathogenesis of IBD.7 IBD has traditionally been associated with weight loss and low body mass index [BMI], but today, the prevalence of overweight and obesity among patients with IBD is similar to that of the general population.7–9 We and others have shown that obesity, a chronic pro-inflammatory state, is associated with an increased risk of developing CD but not UC, though this relationship has not been consistently observed.7,10,11
Bariatric surgery is currently the most effective treatment for severe obesity and its related metabolic disorders, including type 2 diabetes and metabolic syndrome.12,13 In Denmark, the most widely used procedures are Roux-en-Y gastric bypass and sleeve gastrectomy, whereas gastric banding had previously been widely used.14 Whereas the Roux-en-Y gastric bypass is a malabsorptive and restrictive procedure which influences the anatomy of the gastrointestinal tract, sleeve gastrectomy and banding procedures are merely restrictive procedures.15
It has been suggested that bariatric surgery may influence the risk of developing IBD [reviewed by Cañete et al.16]. Potential underlying mechanisms could involve dumping of gastric contents into the small intestine, intestinal dysbiosis, mucosal barrier dysfunction, hormonal changes, or massive release of pro-inflammatory cytokines from fat tissue during rapid weight loss.16 Last, obesity itself may be the real risk factor for IBD, although the current evidence is conflicting.7,16 Development of IBD after bariatric surgery has been described in case reports and in a matched case-control study using a national US medical claims and pharmacy database.16–25 Collectively, these data show that in contrast to the disease incidence in the general population, the incidence of CD seems to be higher than for UC following bariatric surgery.16 In contrast to these data, a recently published retrospective prevalence study using another US claims database reported a lower prevalence of de-novo CD and UC among patients exposed to bariatric surgery.26
The aim of this study was to conduct a population-based prospective cohort study of the risk of new-onset IBD following bariatric surgery.
2. Materials and Methods
2.1. Study population
The study population was identified via the Danish Civil Registration System,27 which contains information on date of birth, sex, and vital status of all Danish citizens. We identified the entire Danish population between 18 and 60 years of age, alive, residing in Denmark and not immigrated to Denmark, from January 1, 1996 to December 31, 2018 [n = 3 937 461]. Individuals with IBD before January 1, 1996 or before their 18th birthday were excluded [n = 18 970].
2.2. Bariatric surgery and inflammatory bowel disease
The exposure, bariatric surgery, was identified via the Danish National Patient Register, which covers public and private hospital contacts for Danish patients.28 We identified all patients who received gastric bypass [SKS code KJDF1], gastric banding [SKS code KJDF2], and gastric sleeve [SKS code KJDF4] during the period. Patients having more than one contact with bariatric surgery were excluded [n = 648].
The outcome, IBD, was also defined based on data from the Danish National Patient Register. The international classification of disease [ICD] 8th and 10th revision codes for CD [563.00–563.09, and K50] and UC [563.19, 569.04, and K51] were used. The diagnoses of IBD in the Danish National Patient Register have been examined and found to be complete [94%] and valid [97% for CD and 90% for UC] when compared with pathology register information.29
2.3. Covariates
Covariates included age, sex, and birth cohort. Birth cohort included three periods: 1] 1936–1958 [individuals turning 60 years within the study period]; 2] 1959–1977 [individuals who did not turn 60 years within the study period]; and 3] 1978–2000 [individuals who were followed from their 18th birthday and who did not turn 60 years within the study period]. Additionally, we included educational status and the following obesity-related comorbidities: type 2 diabetes, hypertension and/or heart disease, sleep apnoea, female infertility, and arthrosis in hip or knee. Educational status was grouped in four categories: 1] primary school and high school; 2] vocational education; 3] short- and medium-length further education; and 4] long-length further education. Information on obesity-related comorbidities was identified via the Danish National Patient Register, the Register of Medicinal Product Statistics,30 and the National Health Insurance Service Register [Supplementary Table 1, available as Supplementary data at ECCO-JCC online]. Finally, treatment with lipid-lowering drugs was included in a sensitivity analysis [Supplementary Table 1].
2.4. Statistical analyses
Individuals free of IBD were followed from January 1, 1996 [if between 18 and 60 years of age] or from their 18th birthday until date of IBD, date of their 60th birthday, date of death, date of emigration, or end of follow-up at December 31, 2018, whichever event occurred first. No individuals were lost during follow-up. We included bariatric surgery status as a time-dependent variable. This means that individuals before surgery contributed risk time in the reference group, and after surgery allocated risk time to the exposure group. The incidence rate of IBD was calculated as the number of IBD cases divided by the person-time. We used Cox proportional hazards regression models with age as underlying time scale to estimate hazard ratios [HRs] of IBD. Sex and birth cohort were time fixed covariates whereas all other covariates were included as time-dependent covariates, updated on daily basis. The proportional hazards assumption was investigated by allowing time-dependent coefficients. For both sex and birth cohort we found these extensions statistically significant; however, including them had only a tiny impact on the estimated results for bariatric surgery. All models included adjustment for age [as the underlying time scale], sex, and birth cohort. A second model additionally included educational status and number of obesity-related comorbidities [0, 1, ≥2]. Additionally, in a sensitivity analysis, treatment with lipid-lowering drugs was included in the model.
All analyses were conducted for IBD and separately for CD and UC to evaluate differential effects on the two subtypes of IBD. When analysing the risk of CD and UC separately, patients diagnosed with both CD and UC on the same date were censored at this date. Further, when analysing risk of CD, patients were censored at date of UC and vice versa. We also stratified by sex to examine whether the risk of IBD was different among women and men. Finally, we did a sensitivity analysis where we limited the study population to individuals who had a diagnosis of overweight/obesity recorded in the Danish National Patient Register. In these analyses of risk of IBD following bariatric surgery, individuals were followed from date of overweight/obesity diagnosis.
2.5. Ethics approval
Data access and linkage permission was obtained from the Danish Data Protection Agency [VD-2018–286, I-Suite no.: 6528]. Approval from an ethics committee is not required for register studies in Denmark.
2.6. Data availability statement
The study is based on data from the Danish nationwide registers [https://sundhedsdatastyrelsen.dk]. The register data are protected by the Danish Act on Processing of Personal Data and are accessed through application to and approval from the Danish Data Protection Agency and the Danish Health Data Authority.
3. Results
We followed 3 917 843 individuals, of whom 15 347 had a bariatric surgery, for development of new-onset IBD. Compared with the unexposed population, patients undergoing bariatric surgery were more likely to be women, have a shorter education, and be affected by more obesity-related comorbidities [Table 1]. Gastric bypass was by far the most used procedure, constituting 90% of surgeries, whereas gastric banding and gastric sleeve accounted for 4% and 6% of surgeries, respectively. Median age at surgery was 41 (interquartile range [IQR]: 34–48) years [Table 1]. When comparing women and men undergoing bariatric surgery, men were more likely to be affected by obesity-related comorbidities [Supplementary Table 2, available as Supplementary data at ECCO-JCC online].
Table 1.
Characteristics of the study population.
| Characteristic | No bariatric surgery | Bariatric surgery |
|---|---|---|
| N [%] | ||
| All | 3 902 496 [100] | 15 347 [100] |
| Women | 1 904 683 [49] | 11 687 [76] |
| Men | 1 997 813 [51] | 3660 [24] |
| Birth cohort | ||
| 1936–1958 | 1 444 706 [37] | 1952 [13] |
| 1959–1977 | 1 211 245 [31] | 9665 [63] |
| 1978–2000 | 1 246 545 [32] | 3730 [24] |
| Type of bariatric surgery | ||
| Gastric bypass | - | 13 827 [90] |
| Gastric banding | - | 583 [4] |
| Gastric sleeve | - | 937 [6] |
| Age median [IQR] years | ||
| Age at cohort entry | 29 [18–45] | 25 [18–32] |
| Age at cohort end | 51 [33–60] | 48 [41–55] |
| Age at surgery | - | 41 [34–48] |
| Person-years of follow-up [%] | ||
| All | 55 553 785 | 106 420 |
| Educationa | ||
| Primary school and high school | 20 883 079 [38] | 37 954 [36] |
| Vocational education | 20 452 993 [37] | 47 241 [45] |
| Short- and medium-length further education | 10 432 992 [19] | 18 988 [18] |
| Long-length further education | 3 384 091 [6] | 1327 [1] |
| Obesity-related comorbidities and drugs | ||
| Type 2 diabetes | 1 186 298 [2] | 18 860 [18] |
| Heart disease | 1 070 614 [2] | 5937 [6] |
| Hypertension | 6 237 415 [11] | 49 654 [47] |
| Sleep apnoea | 321 590 [1] | 8250 [8] |
| Female infertilityb | 1 025 088 [4] | 10 859 [13] |
| Arthrosis in hip or knee | 1 130 006 [2] | 12 132 [11] |
| Lipid-lowering drugs | 1 924 801 [3] | 15 815 [15] |
| Number of obesity-related comorbidities | ||
| 0 | 46 691 323 [84] | 39 957 [38] |
| 1 | 7 058 493 [13] | 37 230 [35] |
| ≥2 | 1 804 969 [3] | 29 233 [27] |
Bariatric surgery, education, and obesity-related comorbidities are included as time-varying covariates in the statistical analyses.
IQR, interquartile range.
a30,611 individuals had missing information on education.
b% of person-years among women.
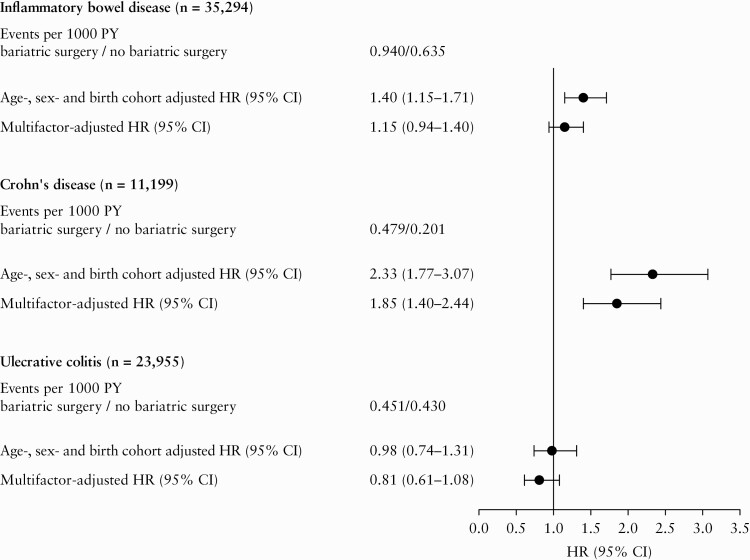
Total follow-up time was 55 660 205 person-years with median length of follow-up of 14.8 [IQR: 7.2–23.0] years. The median follow-up time among those who underwent bariatric surgery was 14.1 [IQR: 12.2‐15.7] years before surgery and 8.0 [IQR: 4.4‐9.2] years after surgery. The incidence rate of death was 2.367/1000 unexposed person-years and 2.547/1000 exposed person-years. During 106 420 person-years following bariatric surgery, 100 events of IBD occurred [incidence rate 0.940/1000 person-years]. The median time from surgery to IBD diagnosis was 4.0 [IQR: 2.0–6.3] years, whereas the median time from surgery to CD and UC diagnosis was 4.2 [IQR: 2.1–6.3] and 3.5 [IQR: 1.7–6.3] years, respectively. During 55 553 785 person-years without bariatric surgery, 35 294 events of IBD occurred [incidence rate 0.635/1000 person-years]. This corresponded to an age-, sex-, and birth cohort-adjusted HR of IBD of 1.40 [95% CI, 1.15–1.71] and a multifactor-adjusted HR of 1.15 [95% CI, 0.94–1.40] [Figure 1].
Figure 1.
Risk of inflammatory bowel disease after bariatric surgery. Multifactor-adjusted hazard ratios were adjusted for age, sex, birth cohort, education, and number of obesity-related comorbidities. Numbers of Crohn’s disease and ulcerative colitis cases do not add up to number of inflammatory bowel disease cases, as 240 patients recorded with both Crohn’s disease and ulcerative colitis on the same date were censored at this date and not included as a case of Crohn’s disease/ulcerative colitis. CI, confidence interval; HR, hazard ratio; PY, person-years.
During the unexposed person-years, the incidence rate for CD was 0.201 events/1000 person-years, whereas the incidence rate for UC was 0.430 events/1000 person-years. Corresponding incidence rates among patients undergoing bariatric surgery were 0.479 and 0.451 events/1000 person-years, respectively. When analysing the risk of CD and UC separately, we found a multifactor-adjusted HR of CD of 1.85 [95% CI, 1.40–2.44], whereas bariatric surgery was not associated with increased risk of UC (HR 0.81 [95% CI, 0.61–1.08]) [Figure 1].
Among the 100 IBD events following bariatric surgery, 90 were observed among women and 10 among men. Incidence rates of IBD following bariatric surgery were 1.095 events/1000 person-years among women and 0.413 events/1000 person-years among men, corresponding to a multifactor-adjusted HR of IBD of 1.31 [95% CI, 1.06–1.61] among women and 0.59 [95% CI, 0.32–1.09] among men [Table 2]. When analysing risk of CD and UC separately among women, we observed a multifactor-adjusted HR for CD of 2.18 [95% CI, 1.64–2.90] and a multifactor-adjusted HR of 0.86 [95% CI, 0.63–1.18] for UC [Table 2]. The low number of IBD events among men precluded separate analysis of CD and UC.
Table 2.
Risk of inflammatory bowel disease after bariatric surgery stratified by sex.
| Bariatric surgery: events/1000 PY | No bariatric surgery: events/1000 PY | Age-, sex- and birth cohort adjusted HR [95% CI] | Multifactor-adjusted HR [95% CI] | |
|---|---|---|---|---|
| Inflammatory bowel disease | ||||
| Women | 1.095 | 0.702 | 1.61 [1.31–1.98] | 1.31 [1.06–1.61] |
| Men | 0.413 | 0.572 | 0.72 [0.39–1.34] | 0.59 [0.32–1.09] |
| Women | ||||
| Crohn’s disease | 0.596 | 0.233 | 2.84 [2.14–3.77] | 2.18 [1.64–2.90] |
| Ulcerative colitis | 0.487 | 0.464 | 1.05 [0.77–1.43] | 0.86 [0.63–1.18] |
Multifactor-adjusted hazard ratios were adjusted for age, sex, birth cohort, education, and number of obesity-related comorbidities.
Cl, confidence interval; HR, hazard ratio; PY, person-years.
A sensitivity analysis including treatment with lipid-lowering drugs in the multifactor-adjusted model showed similar results as compared with the main results: the multifactor-adjusted HRs for IBD, CD, and UC were 1.15 [95% CI, 0.95–1.41], 1.85 [95% Cl, 1.40; 2.44], and 0.81 [95% Cl 0.61; 1.08], respectively. Additionally, we conducted a sensitivity analysis where the study population was limited to individuals who had a diagnosis of overweight/obesity recorded in the Danish National Patient Register [n = 245 882]. This analysis of the risk of IBD following bariatric surgery showed similar results as compared with the main results: the multifactor-adjusted HRs for IBD, CD, and UC were 1.08 [95% CI, 0.88–1.33], 1.59 [95% CI, 1.18–2.13], and 0.80 [95% CI, 0.59–1.07], respectively [Table 3]. Finally, we did not find evidence for differences across calendar time as there was no statistically significant interaction between bariatric surgery and birth cohort on risk of IBD, CD, or UC [p for interaction 0.39, 0.99, and 0.24, respectively].
Table 3.
Risk of inflammatory bowel disease after bariatric surgery among individuals recorded with a diagnosis of overweight/obesity.
| Bariatric surgery: events/1000 PY | No bariatric surgery: events/1000 PY | Age-, sex- and birth cohort adjusted HR [95% CI] | Multifactor-adjusted HR [95% CI] | |
|---|---|---|---|---|
| Inflammatory bowel disease | 0.940 | 0.820 | 1.16 [0.94–1.42] | 1.08 [0.88–1.33] |
| Crohn’s disease | 0.479 | 0.296 | 1.66 [1.24–2.22] | 1.59 [1.18–2.13] |
| Ulcerative colitis | 0.451 | 0.520 | 0.87 [0.65–1.17] | 0.80 [0.59–1.07] |
Multifactor-adjusted hazard ratios were adjusted for age, sex, birth cohort, education, and number of obesity-related comorbidities.
Cl, confidence interval; HR, hazard ratio; PY, person-years.
4. Discussion
In this nationwide prospective cohort study of almost 4 million individuals, of whom approximately 15 000 had a bariatric surgery, we observed an increased risk of development of new-onset CD, but not of UC, after bariatric surgery. Among women undergoing bariatric surgery, the risk of CD was increased over 2-fold.
Our findings of an increased risk of CD after bariatric surgery is in accordance with previous results from case reports16 and a matched case-control study.25 In agreement with the existing data, our prospective cohort study shows that in contrast to the unexposed population where the incidence rate of UC is approximately twice as high as that for CD, the incidence rates of CD and UC are similar among patients who have undergone bariatric surgery. However, our results contrast with a recently published study reporting a lower prevalence of de-novo UC and CD among patients exposed to bariatric surgery.26 This previous study is very different from the present study as it analysed prevalence of IBD after bariatric surgery not taking duration of follow-up or confounders into account.26
A major strength of the present study is its nationwide nature ensuring inclusion of a large and unselected population of patients undergoing bariatric surgery. Also, we were able to adjust for several potential confounders, including age, sex, birth cohort, educational status, and obesity-related comorbidities. The obesity-related comorbidities [type 2 diabetes, hypertension and/or heart disease, sleep apnoea, female infertility, and arthrosis in hip or knee] comprise obesity-related comorbidities stated as indications for bariatric surgery in a national guideline from the Danish Health Authority.31 As we adjusted our analyses for these, it seems unlikely that our results are explained by a higher prevalence of obesity-related comorbidities among patients undergoing bariatric surgery as compared with the unexposed population. In further support of this notion, men generally had more obesity-related comorbidities than women, whereas an increased risk of IBD was observed among women, but not among men.
An important limitation of our study is that we did not have information on lifestyle factors associated with development IBD, such as body mass index [BMI] and smoking. With respect to BMI, we and others have shown that obesity is associated with an increased risk of developing CD, but not UC, though this relationship has not been consistently observed.7,10,11 Further, the relationship between BMI and CD does not seem to be linear, but rather U-shaped, implying that both underweight and obese individuals are at increased risk of CD.10,32,33 To address the potential confounding by BMI, we conducted a sensitivity analysis where the study population was limited to individuals who had a diagnosis of overweight/obesity recorded in the Danish National Patient Register. In accordance with the main analysis, this sensitivity analysis showed higher risk of CD after bariatric surgery, suggesting that the increased risk of CD after bariatric surgery is not simply explained by obesity. However, as we did not have individual-level information on BMI, we still cannot preclude that BMI in the exposed group [bariatric surgery] would be higher than the unexposed group. Our study design, where we included bariatric surgery as a time-dependent variable, may however have counteracted some of the potential confounding by BMI, as exposed individuals contributed unexposed time before their surgery date. Moreover, as we did not have access to measurements of weight loss or adipose tissue changes after bariatric surgery, we could not address the potential link between weight loss/adipose tissue changes following bariatric surgery and development of CD.
The association between smoking and IBD is complicated. Current smoking is associated with an increased risk of CD but a lower risk of UC.6,34 However, former smoking increases risk of UC.6,34 Therefore, it may be speculated that the observed increased risk of CD after bariatric surgery could be explained by a higher prevalence of smoking among patients undergoing bariatric surgery. A Danish prospective study, including all patients [n = 71] scheduled for laparoscopic gastric bypass or gastric sleeve during a continuous period in 2017, examined smoking habits among the referred patients. At referral for bariatric surgery, 17% were current smokers, 35% were previous smokers, and 48% had never smoked.35 These data suggest that the prevalence of smoking is not higher among patients referred to bariatric surgery as compared with the general Danish population with obesity. Based on the Copenhagen General Population Study, we have previously shown that among individuals with BMI ≥30, 15% were current smokers, 45% previous smokers, and 39% never smokers.36 However, smoking habits may change during and after a bariatric surgery. In Denmark, patients who are referred to bariatric surgery are expected to quit smoking at least 6 weeks before surgery, be highly motivated for continuous smoking cessation, and commit to smoking cessation minimum 3 weeks after the surgery.31 However, by use of cotinine urine tests, the aforementioned study showed that despite the mandatory smoking cessation, six of 12 active smokers failed to quit smoking before surgery.35 In any case, smoking cessation, and thereby a lower prevalence of smoking among patients having undergone bariatric surgery, would expectedly associate with a lower risk of CD and a higher risk of UC, which contrasts with our findings. Accordingly, it seems less likely that differences in smoking patterns should account for the observed increased risk of CD after bariatric surgery.
Finally, we cannot rule out that our study could be influenced by misclassification bias. Although the diagnoses of IBD in the Danish National Patient Register have been found to be complete and valid,29 the validity of IBD diagnoses among patients who have undergone bariatric surgery may be different. Indeed, such patients are at increased risk of post-surgical complications which may resemble complications to CD, and it may be that the CD diagnosis recorded in the registers is not true CD, but rather a CD-like disease due to malabsorption and bile acid dysregulation. This could result in lower validity of the CD diagnosis in this patient group. Also, other abdominal surgeries previous to or following bariatric surgery could potentially influence our results.
The observational nature of the present study precludes definite conclusions on potential causal effects. However, underlying mechanisms can involve dumping of gastric contents into the small intestine, intestinal dysbiosis, mucosal barrier dysfunction, hormonal changes, or massive release of pro-inflammatory cytokines from fat tissue during rapid weight loss.16 Whereas the Roux-en-Y gastric bypass is a malabsorptive and restrictive procedure which influences the anatomy of the gastrointestinal tract, the sleeve gastrectomy and banding procedures are merely restrictive procedures.15 In the present study, gastric bypass was by far the most used procedure, constituting 90% of the surgeries, whereas gastric banding and gastric sleeve accounted for 4% and 6% of the surgeries, respectively. Accordingly, we were not able to evaluate differential effects of malabsorptive and restrictive procedures [gastric bypass] compared with restrictive procedures [gastric banding and gastric sleeve]. It thus remains unknown whether leaving the small bowel anatomy in its native state during a purely restrictive surgery, such as the gastric sleeve procedure, would minimise the risk of developing CD. When evaluating risk of IBD among women and men separately, we observed an increased risk of IBD among women, but not among men. However, 76% of the patients undergoing bariatric surgery were women, implying that our statistical power was higher among women than among men. Also, the fact that 90% of the IBD events occurred in women and only 10% occurred in men may reflect a higher incidence of CD among women [in accordance with data from population-based studies from Western countries]37 rather than a biological sex difference for bariatric surgery.
From a clinical point of view, the increased risk of CD following bariatric surgery would not contraindicate bariatric surgery in patients with morbid obesity, as the risks without surgery may be higher than concerns about developing CD after surgery. Yet the increased risk of CD after bariatric surgery is important, as it may pave the way for future studies of the underlying mechanisms, hopefully improving our understanding of the aetiology of IBD. Moreover, future studies should elucidate whether CD arising after bariatric surgery is a specific type of CD. Hence, it would be central to describe how patients present at time of CD diagnosis and whether differences exist in disease location, disease extent, prevalence/types of extra-intestinal manifestations, and disease behaviour, including treatment response. It is also possible that CD diagnosed after bariatric surgery could represent the clinical onset of quiescent disease before surgery, implying that surgery is not a trigger for disease development but for disease worsening. This may be addressed by future studies examining the evidence for existing CD [eg, by measurement of faecal calprotectin or by gastrointestinal endoscopy or radiology [CT/MRI] examinations] before bariatric surgery. If bariatric surgery is a trigger for disease worsening, it may be relevant to rule out quiescent CD before bariatric surgery in patients at high risk of CD, such as patients with familiar disposition to IBD.
In conclusion, in this observational nationwide prospective cohort study, bariatric surgery was associated with an increased risk of development of new-onset CD, but not of UC. Further studies of potential underlying mechanisms for this association will hopefully offer additional insights into IBD pathogenesis.
Supplementary Material
Funding
This work was supported by the Novo Nordisk Foundation [grant number NNF16OC0022586 and NNF17OC0029768] and the Danish Diabetes Association. RCU is supported by an NIH K23 Career Development Award [K23KD111995-01A1]. These had no role in the study design or in the collection, analysis, and interpretation of data.
Conflict of Interest
AE has received research funding from Pfizer, Eli Lilly, Novartis, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation and honoraria as consultant and/or speaker from AbbVie, Almirall, Leo Pharma, Samsung Bioepis, Pfizer, Eli Lilly, Novartis, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, and Janssen Pharmaceuticals. RCU has served as an advisory board member or consultant for Eli Lilly, Janssen, Pfizer, and Takeda; and received research support from AbbVie, Boehringer Ingelheim, and Pfizer. J-FC has served as an advisory board member or consultant for AbbVie, Amgen, Boehringer-Ingelheim, Arena Pharmaceuticals, Celgene, Celltrion, Enterome, Eli Lilly, Ferring Pharmaceuticals, Genentech, Janssen and Janssen, Medimmune, Merck, Nextbiotix, Novartis Pharmaceuticals, Otsuka Pharmaceutical Development and Commercialization, Pfizer, Protagonist, Second Genome, Gilead, Seres Therapeutics, Shire, Takeda, and Theradiag; has served as a speaker for AbbVie, Ferring, Takeda, and Celgene; has stock options in Intestinal Biotech Development and Genefit; and has received research grants from AbbVie, Takeda, and Janssen and Janssen.
Author Contributions
KHA: conceptualisation, funding acquisition, methodology, project administration, visualisation, writing original draft. RKJ: data curation, formal analysis, methodology, writing review and editing. RCU: conceptualisation, writing review and editing. J-FC: conceptualization, writing review and editing. AE: data curation, resources, writing review and editing. MV: conceptualisation, methodology, writing review and editing. TJ: conceptualisation, funding acquisition, project administration, writing review and editing. All authors approved the final draft submitted.
References
- 1. Bixby H, Bentham J, Zhou B, et al. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature 2019;569:260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12:720–7. [DOI] [PubMed] [Google Scholar]
- 3. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 5. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015;12:205–17. [DOI] [PubMed] [Google Scholar]
- 7. Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol 2017;14:110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moran GW, Dubeau MF, Kaplan GG, Panaccione R, Ghosh S. The increasing weight of Crohn’s disease subjects in clinical trials: a hypothesis-generating time-trend analysis. Inflamm Bowel Dis 2013;19:2949–56. [DOI] [PubMed] [Google Scholar]
- 9. Aarestrup J, Jess T, Kobylecki CJ, Nordestgaard BG, Allin KH. Cardiovascular risk profile among patients with inflammatory bowel disease: a population-based study of more than 100 000 individuals. J Crohns Colitis 2019;13:319–23. [DOI] [PubMed] [Google Scholar]
- 10. Harpsøe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 2014;43:843–55. [DOI] [PubMed] [Google Scholar]
- 11. Jensen CB, Ängquist LH, Mendall MA, Sørensen TIA, Baker JL, Jess T. Childhood body mass index and risk of inflammatory bowel disease in adulthood: a population-based cohort study. Am J Gastroenterol 2018;113:694–701. [DOI] [PubMed] [Google Scholar]
- 12. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017;376:1492. [DOI] [PubMed] [Google Scholar]
- 13. Madsbad S, Holst JJ. Bariatric surgery ‐ which procedure is the optimal choice? Lancet 2019;393:1263–4. [DOI] [PubMed] [Google Scholar]
- 14. The Danish Health Data Authority. Number of bariatric surgeries done in Denmark from 2009 and onwards. https://www.Esundhed.Dk/emner/operationer-og-diagnoser/fedmeoperationer. Accessed August 23, 2020.
- 15. Buchwald H, Buchwald JN. Evolution of operative procedures for the management of morbid obesity 1950-2000. Obes Surg 2002;12:705–17. [DOI] [PubMed] [Google Scholar]
- 16. Cañete F, Mañosa M, Clos A, Cabré E, Domènech E. Review article: the relationship between obesity, bariatric surgery, and inflammatory bowel disease. Aliment Pharmacol Ther 2018;48:807–16. [DOI] [PubMed] [Google Scholar]
- 17. Ahn LB, Huang CS, Forse RA, Hess DT, Andrews C, Farraye FA. Crohn’s disease after gastric bypass surgery for morbid obesity: is there an association? Inflamm Bowel Dis 2005;11:622–4. [DOI] [PubMed] [Google Scholar]
- 18. Bernstein GR, Pickett-Blakely O. De novo inflammatory bowel disease after bariatric surgery: a case series and literature review. Dig Dis Sci 2017;62:817–20. [DOI] [PubMed] [Google Scholar]
- 19. Braga Neto MB, Gregory M, Ramos GP, et al. De-novo inflammatory bowel disease after bariatric surgery: a large case series. J Crohns Colitis 2018;12:452–7. [DOI] [PubMed] [Google Scholar]
- 20. Dodell GB, Albu JB, Attia L, McGinty J, Pi-Sunyer FX, Laferrère B. The bariatric surgery patient: lost to follow-up; from morbid obesity to severe malnutrition. Endocr Pract 2012;18:e21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janczewska I, Nekzada Q, Kapraali M. Crohn’s disease after gastric bypass surgery. BMJ Case Rep 2011. PMID: 22693320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korelitz BI, Sonpal N, Schneider J, et al. Obesity/bariatric surgery and Crohn’s disease. J Clin Gastroenterol 2018;52:50–4. [DOI] [PubMed] [Google Scholar]
- 23. Kotze PG, Bremer-Nones R, Kotze LM. Is there any relation between gastric bypass for morbid obesity and the development of Crohn’s disease? J Crohns Colitis 2014;8:712–3. [DOI] [PubMed] [Google Scholar]
- 24. Papakonstantinou AS, Stratopoulos C, Terzis I, et al. Ulcerative colitis and acute stroke: two rare complications after Mason’s vertical banded gastroplasty for treatment of morbid obesity. Obes Surg 1999;9:502–5. [DOI] [PubMed] [Google Scholar]
- 25. Ungaro R, Fausel R, Chang HL, et al. Bariatric surgery is associated with increased risk of new-onset inflammatory bowel disease: case series and national database study. Aliment Pharmacol Ther 2018;47:1126–34. [DOI] [PubMed] [Google Scholar]
- 26. Kochhar GS, Desai A, Syed A, et al. Risk of de-novo inflammatory bowel disease among obese patients treated with bariatric surgery or weight loss medications. Aliment Pharmacol Ther 2020;51:1067–75. [DOI] [PubMed] [Google Scholar]
- 27. Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011;39:22–5. [DOI] [PubMed] [Google Scholar]
- 28. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39:30–3. [DOI] [PubMed] [Google Scholar]
- 29. Fonager K, Sørensen HT, Rasmussen SN, Møller-Petersen J, Vyberg M. Assessment of the diagnoses of Crohn’s disease and ulcerative colitis in a Danish hospital information system. Scand J Gastroenterol 1996;31:154–9. [DOI] [PubMed] [Google Scholar]
- 30. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 31. Sundhedsstyrelsen. Visitation til kirurgisk behandling af svær fedme. Faglig visitationsretningslinje; 2017. [The Danish Health Authority. Admission to surgery for severe obesity. Professional admission guideline 2017. Copenhagen, Denmark: The Danish Health Authority; 2017]. [Google Scholar]
- 32. Mendall M, Harpsøe MC, Kumar D, Andersson M, Jess T. Relation of body mass index to risk of developing inflammatory bowel disease amongst women in the Danish National Birth Cohort. PLoS One 2018;13:e0190600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mendall MA, Jensen CB, Sørensen TIA, Ängquist LH, Jess T. Body mass index in young men and risk of inflammatory bowel disease through adult life: a population-based Danish cohort study. Sci Rep 2019;9:6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc 2006;81:1462–71. [DOI] [PubMed] [Google Scholar]
- 35. Gormsen J, Hjorne F, Helgstrand F. Cotinine test in evaluating smoking cessation at the day of bariatric surgery. Scand J Surg 2020; 109: 265– 68. [DOI] [PubMed] [Google Scholar]
- 36. Winter-Jensen M, Afzal S, Jess T, Nordestgaard BG, Allin KH. Body mass index and risk of infections: a Mendelian randomization study of 101,447 individuals. Eur J Epidemiol 2020;35:347–54. [DOI] [PubMed] [Google Scholar]
- 37. Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-based differences in incidence of inflammatory bowel diseases ‐ pooled analysis of population-based studies from western countries. Gastroenterology 2018;155:1079–89 e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.